Abstract
Introduction:
Hospital-acquired infections (HAIs) after stroke are associated with additional morbidity and mortality, but whether HAIs increase long-term cognitive decline in stroke patients is unknown. We hypothesized that older adults with incident stroke with HAI experience faster cognitive decline than those having stroke without HAI and those without stroke.
Methods:
We performed a longitudinal analysis in the population-based prospective Cardiovascular Health Study. Medicare-eligible participants aged ≥65 years with and without incident stroke had cognition assessed annually. HAIs were assessed by hospital discharge codes. Global cognitive function was assessed annually by the Modified Mini-Mental State Examination (3MSE) and executive function by the Digit Symbol Substitution Test (DSST). We used linear mixed models to estimate the mean decline and 95% confidence intervals (95% CI) for 3MSE and DSST scores by incident stroke and HAI status, adjusted for demographics and vascular risk factors.
Results:
Among 5,443 participants ≥65 years without previous history of stroke, 393 participants had stroke with HAI (SI), 766 had a stroke only (SO), and 4,284 had no stroke (NS) throughout a maximum 9-year followup. For 3MSE, compared with NS participants, SO participants had a similar adjusted mean decline (additional 0.08 points/year, 95% CI: −0.15, 0.31), while SI participants had a more rapid decline (additional 0.28 points/year, 95% CI: 0.16, 0.40). Adjusted mean decline was 0.20 points/year faster (95% CI: −0.05, 0.45) among SI than SO participants. For DSST, compared with NS participants, SO participants had a faster adjusted mean decline (additional 0.17 points/year [95% CI: 0.003, 0.33]), as did SI participants (additional 0.27 points/year [95% CI: 0.19, 0.35]).
Conclusion:
Stroke, when accompanied by HAI, leads to a faster long-term decline in cognitive ability than in those without stroke. The clinical and public health implications of the effect of infection on post-stroke cognitive decline warrant further attention.
Keywords: Hospital-acquired infection, Cognition, Stroke, Epidemiology, Infection, Cardiovascular health study, Cognitive decline
Introduction
Cognitive decline and dementia are common after stroke [1, 2]. In animal models, stroke leads to chronic inflammation in infarcted tissue and other brain regions, contributing to delayed cognitive decline [2]. In humans, systemic infection at the time of stroke is associated with increased immune activation and may correlate with cognitive decline [3–5]. Approximately half of stroke survivors have evidence of neuroinflammation in stroke scars at postmortem decades later [6]. How neuroinflammation increases risk of incident post-stroke dementia is unknown; whether infection at the time of stroke contributes to neuroinflammation and risk of cognitive decline and dementia is uncertain.
Hospital-acquired infections (HAIs) may be caused by viral, bacterial, or fungal pathogens contracted within a hospital setting while hospitalized for a separate illness. Among acute stroke patients, urinary infections and pneumonia are the most frequent etiologies [7]. Brief periods of immunosuppression after a stroke may put patients at higher risk for HAIs, with up to 30% of stroke patients suffering HAI [8, 9].
While short-term consequences of HAIs after stroke are well known, their impact on long-term risk of cognitive decline or dementia is uncertain. Recent studies provide evidence that infection at time of hospitalized stroke is associated with acute neurological deterioration, while others suggest HAI may be associated with a sustained decrease in cognitive function [9, 10]. It remains unclear whether this is due to infection specifically or whether hospitalization alone is associated with cognitive decline [11]. Bidirectional relationships may also exist between infection and cognitive decline [12]. In addition, community-acquired pneumonia after hospitalization may be associated with long-term cognitive decline [13]. We hypothesized that among older adults with incident stroke, those whose stroke hospitalization included an infection had a more rapid cognitive decline after discharge compared with people with stroke hospitalization without infection or no stroke hospitalization.
Methods
Design, Setting, and Participants
The Cardiovascular Health Study (CHS) is a population-based longitudinal study designed to identify coronary heart disease and stroke risk factors in adults aged ≥65 years [14]. Details of the study have been previously published [15, 16]. CHS included 5,888 men and women aged ≥65 years enrolled from 1989–1993 in Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Allegheny County, Pennsylvania. For this analysis, participants were followed through 1998/99, and annual visits allowed for participant’s risk factor data and functional measures to be collected and updated throughout the study [15]. Cardiovascular history and related risk factors were ascertained upon enrollment [17]. Participants with prevalent stroke at study initiation were excluded from this analysis. The institutional review boards at the University of Washington and CHS field centers approved the study; all participants provided written informed consent. Data were provided to Columbia University through a data use agreement. Qualified researchers may request access to the dataset through the Collaborative Health Studies Coordinating Center (https://www.uwchscc.org).
Assessment of Stroke
Clinical stroke was based on standard CHS definitions [18]. History of ischemic or hemorrhagic stroke prior to enrollment was identified at the first visit by participant reports and verified by medical record review. Incident stroke was identified through biannual interviews and confirmed by a stroke adjudication committee reviewing outpatient or inpatient medical records and diagnostic tests [18, 19]. Incident stroke included ischemic and hemorrhagic types. After a confirmed incident of stroke, a participant was considered a stroke participant throughout follow-up.
Assessment of Hospital-Acquired Infection
HAIs were assessed based on hospital discharge International Classification of Disease Ninth Revision (ICD-9) codes. Similar to prior published CHS studies, codes were included for HAIs such as ventilator-associated pneumonia, urinary tract infections, sepsis, surgical site infections, and bloodstream infections [20, 21]. All infections commonly obtained during hospitalization that could be identified through ICD-9 codes were included as HAIs in this analysis.
Exposure Group Definitions
Participants were defined as having “No Stroke” (NS, the reference group), “Stroke Only” (SO), or “Stroke and HAI” (SI). HAIs during non-stroke hospitalization were not captured. SO included those individuals who had stroke during study follow-up but no diagnostic codes for HAI during stroke hospitalization. SI included those who had strokes and HAI during stroke admission. Only the first stroke during follow-up was used for exposure definition. Participants who experienced incident stroke contributed cognitive test scores in the NS reference group until the occurrence of stroke, after which they contributed cognitive test scores as SO or SI.
Assessment of Cognitive Function
Global cognitive ability was assessed using the Modified Mini-Mental State Examination (3MSE; scores 0–100, first assessment 1990/91, and 8 annual follow-ups afterward for a maximum follow-up time of 8 years through 1998/99). The 3MSE includes questions assessing memory, orientation, fluency, problemsolving, and ability to follow instructions, providing a global cognitive score between zero (worst) and 100 (best). Processing speed was tested using the Digit Symbol Substitution Test (DSST; score 0–90, first assessment 1989/90, and 9 annual follow-ups afterward, for a maximum follow-up time of 9 years through 1998/99). The DSST consists of a visual-motor task in which, over 90 s, participants match symbols with correct digits according to a key, yielding a measure of information processing speed on a scale of zero (worst) to 90 (best) [15, 16]. From 1995/96 to 1998/99, for participants unable to complete an in-person exam, 3MSE was estimated by the Telephone Interview for Cognitive Status (TICS). Participants were included in the analysis of cognitive decline over time if they had ≥1 cognitive assessment throughout their participation in the CHS period, regardless of whether some years of cognitive scoring were missed on annual examinations. Those without at least one cognitive function score were excluded from the analysis.
Covariate Definitions
Participants reported age, sex, race, education level, and use of cigarettes and alcohol. Medication use was determined annually. Body mass index (BMI) was calculated from height and weight. Creatinine, hemoglobin, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, triglyceride, and glucose were measured in blood samples [22]. History of hypertension was defined as self-reported physician-diagnosed hypertension plus the use of antihypertensive medication or systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. History of dyslipidemia was defined as total cholesterol >240 mg/dL, LDL >160 mg/dL, or HDL <35 mg/dL. Diabetes was defined as the use of insulin or oral hypoglycemic medications, a fasting serum glucose ≥126 mg/dL, or a non-fasting serum glucose ≥200 mg/dL. Coronary heart disease, atrial fibrillation, previous stroke, and congestive heart failure (CHF) were identified by participant or proxy report, confirmed by medical record review, and adjudicated according to standard criteria [18].
Statistical Analysis
We used mixed-effects models to determine whether 3MSE and DSST trajectories over time differed by SI, SO, or NS exposure groups. All participants started as NS at baseline, and change in group status was evaluated as a time-varying covariate [15]. We did not consider recurrent stroke separately. As such, participants could transition from NS to either SO or SI, but they could not switch groups thereafter and remained in that group until the end of follow-up.
Participants were censored at time of death. Age in years was the time scale, centered at 77 years (the average age of cognitive assessments throughout follow-up), with centered year-squared included in the model for acceleration of cognitive decline during aging and centered year exposure group interaction included in the model to estimate differences in cognitive trajectory by SI, SO, or NS groups. A term for centered year-squared exposure group was not statistically significant and was omitted. We considered models unadjusted and adjusted for sex, race, education, smoking status, hypertension, systolic blood pressure, diabetes, BMI, coronary heart disease (CHD), atrial fibrillation, dyslipidemia, and alcohol use, all assessed at baseline. Model-based estimates and mean baseline values of covariates were used to estimate covariate-adjusted mean cognitive score trajectories across age, as done in prior work [16]. In the Results section, annual mean decline and excess annual mean decline are expressed as positive quantities. Confidence intervals (95% CIs) were included with all model-predicted mean 3MSE and DSST score trajectories. Statistical analyses were conducted in STATA version 16 (StataCorp, College Station, TX, USA). Dr. Boehme had full access to all study data and takes responsibility for the integrity of the data analysis.
Results
Over a maximum of eight (3MSE) and 9 years (DSST) of follow-up, incident SI was identified in 393 (7.22%) of the 5,443 participants included in 3MSE analysis, with 766 SO and 4,284 NS (Table 1). Mean length follow-up in the 3MSE analysis was 6.0 years for the cohort overall, including a mean of 2.5 years after incident SI or SO; in the DSST analysis it was 6.3 years for the cohort overall and 2.4 years after incident SI or SO. Compared with NS, SI participants were older and more often female, with fewer current smokers or consumers of alcohol. Lastly, compared with NS participants, SI participants had higher average systolic blood pressure (141.9 vs. 135.2 mm Hg), higher proportion of hypertension (80.2% vs. 58.1%), and higher proportion of diabetes (20.9% vs. 15.5%). Complete baseline characteristics of study participants can be found in Table 1.
Table 1.
Baseline characteristics of participants included in 3MSE analysis**
| Characteristic | No stroke (n = 4,284) |
Stroke only (n = 766) |
Stroke with HAI (n = 393) |
|---|---|---|---|
| Self-reported | |||
| Age, years, mean (SD) | 73.2 (5.6) | 74.3 (5.3) | 74.3 (5.4) |
| Male, N (%) | 1,852 (42.8) | 289 (37.7) | 130 (33.1) |
| Black race, N (%) | 681 (15.9) | 117 (15.3) | 62 (15.8) |
| Education, years, N (SD) | 10.9 (2.0) | 10.9 (2.1) | 10.8 (2.1) |
| Smoking, N (%) | |||
| Never | 1,847 (43.1) | 390 (50.9) | 207 (52.7) |
| Former | 1,875 (43.8) | 296 (38.7) | 157 (39.9) |
| Current | 562 (13.1) | 80 (10.4) | 29 (7.4) |
| Any alcohol use, N (%) | 2,284 (53.3) | 358 (46.7) | 179 (45.6) |
| Prevalent disease, N (%) | |||
| Diabetes | 663 (15.5) | 172 (22.5) | 82 (20.9) |
| Hypertension | 2,488 (58.1) | 615 (80.3) | 315 (80.2) |
| Previous stroke | 0 (0) | 0 (0) | 0 (0) |
| Congestive heart failure | 194 (4.5) | 34 (4.4) | 13 (3.3) |
| Coronary heart disease | 833 (19.4) | 158 (20.6) | 80 (20.4) |
| Atrial fibrillation | 1,122 (26.2) | 311 (40.6) | 101 (25.7) |
| Medical measurements | |||
| BMI, mean (SD) | 27.61 (5.2) | 26.6 (4.4) | 26.2 (4.4) |
| Systolic blood pressure, mm Hg, mean (SD) | 135.2 (21.3) | 140.9 (22.9) | 141.9 (22.6) |
| Diastolic blood pressure, mm Hg, mean (SD) | 70.4 (11.3) | 71.7 (11.5) | 73.3 (11.6) |
HAI, hospital-acquired infection; SD, standard deviation; 3MSE, Modified Mini-Mental State Examination; DSST, Digit Symbol Substitution Test.
All percentages in Table 1 are column percentages.
Unadjusted model-predicted mean 3MSE score trajectories for NS participants showed a mean decline of 0.65 points/year (95% CI: 0.60, 0.70; p < 0.0001), plus an additional 0.07 points/year-squared (95% CI: 0.07, 0.08; p < 0.0001), indicating acceleration of cognitive decline during aging. Excess annual decline in mean 3MSE score was an additional 0.13 points/year (SO vs. NS, p = 0.271), 0.31 points/year (SI vs. NS, p < 0.0001), and 0.18 points/ year (SI vs. SO, p = 0.165) (Table 2). Similarly, adjusted model-predicted mean 3MSE score trajectories over advancing age for NS participants showed a mean decline of 0.61 points/year (95% CI: 0.57, 0.66, p < 0.0001) and acceleration of 0.07 points/year-squared (95% CI: 0.06, 0.07 p < 0.0001; Fig. 1; Table 2). Adjusted excess annual decline in mean 3MSE score in SO versus NS was an additional 0.08 points/year (95% CI: −0.15, 0.31; p = 0.463), in SI versus NS was 0.28 points/year (95% CI: 0.16, 0.40; p < 0.0001) and in SI versus SO was 0.20 points/year (95% CI: −0.05, 0.45, p = 0.134; Table 2).
Table 2.
Change in 3MSE score per year of aging in unadjusted and adjusted models
| Unadjusted |
Adjusted* |
|||||
|---|---|---|---|---|---|---|
| estimated 3MSE | 95% CI | p value | estimated 3MSE | 95% CI | p value | |
| Estimated mean 3MSE score at age 77 | ||||||
| No stroke | 90.1 | (89.8, 90.5) | ref | 90.1 | (89.8, 90.4) | ref |
| Stroke only | 90.0 | (88.5, 91.4) | 0.814 | 90.4 | (89.0, 91.7) | 0.770 |
| Stroke and HAI | 88.9 | (88.2, 89.6) | 0.002 | 88.9 | (88.2, 89.5) | <0.0001 |
| Annual mean declinea in 3MSE score | ||||||
| No stroke, per year | 0.65 | (0.60, 0.70) | <0.0001 | 0.61 | (0.57, 0.66) | <0.0001 |
| No stroke, per year-squared | 0.07 | (0.07, 0.08) | <0.0001 | 0.07 | (0.06. 0.07) | <0.0001 |
| Excess annual mean declinea in 3MSE score | ||||||
| Stroke only versus no stroke | 0.13 | (−0.10, 0.37) | 0.271 | 0.08 | (−0.15, 0.31) | 0.463 |
| Stroke with HAI versus no stroke | 0.31 | (0.19, 0.43) | <0.0001 | 0.28 | (0.16, 0.40) | <0.0001 |
| Stroke with HAI versus stroke only | 0.18 | (−0.07, 0.44) | 0.165 | 0.20 | (−0.05, 0.45) | 0.134 |
HAI, hospital-acquired infection; SD, standard deviation; 3MSE, Modified Mini-Mental State Examination.
Adjusted analysis controls for: sex, race, education level, smoking status, hypertension, systolic blood pressure, diabetes, BMI, CHF, CHD, atrial fibrillation, dyslipidemia, and alcohol use.
Annual mean decline and excess annual mean decline are expressed as positive quantities. For example, in the adjusted model, participants without stroke experienced a mean decline in 3MSE score of 0.61 points/year and acceleration in mean decline of an additional 0.07 points/year-squared; and participants after stroke with HAI experienced a mean decline in 3MSE score of 0.28 points/year faster than those without stroke.
Fig. 1.
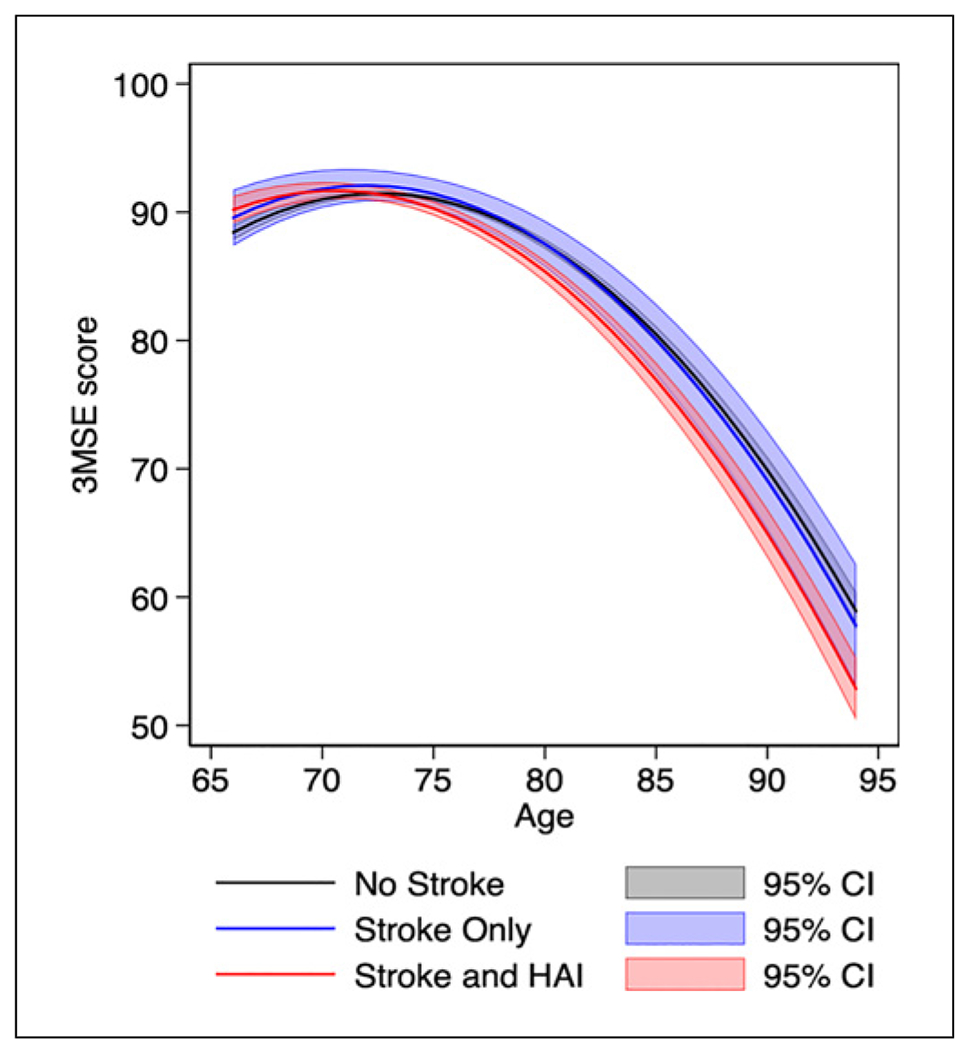
Adjusted model-predicted mean 3MSE score trajectories. The gray line represents 3MSE trajectory without a history of stroke or HAI. The blue line represents 3MSE trajectory after incident stroke without HAI. The red line represents 3MSE trajectory after incident stroke with coexistence of HAI. Gray, blue, and red shadings represent each group’s 95% confidence intervals. The model included 5,443 participants and was adjusted for participant sex, race, education level, smoking status, hypertension, systolic blood pressure, diabetes, BMI, CHD, atrial fibrillation, dyslipidemia, and alcohol use, all assessed at baseline. 3MSE, Modified Mini-Mental State Examination; HAI, hospital-acquired infection; BMI, body mass index; CHD, coronary heart disease.
Unadjusted model-predicted mean DSST score trajectories for NS participants showed a mean decline of 0.65 points/year (95% CI: 0.61, 0.68 p < 0.0001) and acceleration of 0.04 points/year-squared (95% CI: 0.04, 0.05, p < 0.0001). Based on these trajectories, the excess annual decline in mean DSST score was an additional 0.18 points/year (SO vs. NS, p = 0.025), 0.28 points/year (SI vs. NS, p < 0.0001), and 0.09 points/year (SI vs. SO, p = 0.312) (Table 3). Similarly, adjusted model-predicted mean DSST score trajectories over advancing age for NS participants showed a mean decline of 0.63 points/ year (95% CI: 0.57, 0.66, p < 0.0001) and acceleration of 0.04 points/year-squared (95% CI: 0.04, 0.05 p < 0.0001; Fig. 2; Table 3). Examination of model-adjusted excess annual decline in mean DSST score in SO versus NS demonstrated an additional 0.17 points/year (95% CI: 0.003, 0.33, p = 0.046), while SI versus NS was 0.27 points/year (95% CI: 0.19, 0.35, p < 0.0001) and SI versus SO was 0.10 points/year (95% CI: −0.08, 0.27, p = 0.288; Table 3).
Table 3.
Change in DSST score per year of aging in unadjusted and adjusted models
| Unadjusted |
Adjusted* |
|||||
|---|---|---|---|---|---|---|
| estimated DSST | 95% CI | p value | estimated DSST | 95% CI | p value | |
| Estimated mean DSST score at age 77 | ||||||
| No stroke | 37.4 | (36.9, 37.7) | ref | 37.3 | (36.9, 37.6) | ref |
| Stroke only | 35.5 | (33.8, 37.3) | 0.042 | 36.2 | (34.6, 37.6) | 0.211 |
| Stroke and HAI | 36.0 | (35.2, 36.9) | 0.004 | 35.9 | (35.2, 36.6) | 0.001 |
| Annual mean declinea in DSST score | ||||||
| No stroke, per year | (0.65) | (0.61, 0.68) | <0.0001 | 0.63 | (0.57, 0.66) | <0.0001 |
| No stroke, per year-squared | (0.04) | (0.04, 0.05) | <0.0001 | (0.04) | (0.04, 0.05) | <0.0001 |
| Excess annual mean declinea in DSST score | ||||||
| Stroke only versus no stroke | 0.18 | (0.02, 0.35) | 0.025 | 0.17 | (0.003, 0.33) | 0.046 |
| Stroke with HAI versus no stroke | 0.28 | (0.19, 0.36) | <0.0001 | 0.27 | (0.19, 0.35) | <0.0001 |
| Stroke with HAI versus stroke only | (0.09) | (−0.08, 0.26) | 0.312 | (0.10) | (−0.08, 0.27) | 0.288 |
HAI, hospital-acquired infection; SD, standard deviation; DSST, Digit Symbol Substitution Test.
Adjusted analysis controls for: sex, race, education level, smoking status, hypertension, systolic blood pressure, diabetes, BMI, CHF, CHD, atrial fibrillation, dyslipidemia, and alcohol use.
Annual mean decline and excess annual mean decline are expressed as positive quantities. For example, in the adjusted model, participants without stroke experienced a mean decline in DSST score of 0.63 points/year and acceleration in mean decline of an additional 0.04 points/year-squared; and participants after stroke with HAI experienced a mean decline in DSST score of 0.27 points/year faster than those without stroke.
Fig. 2.
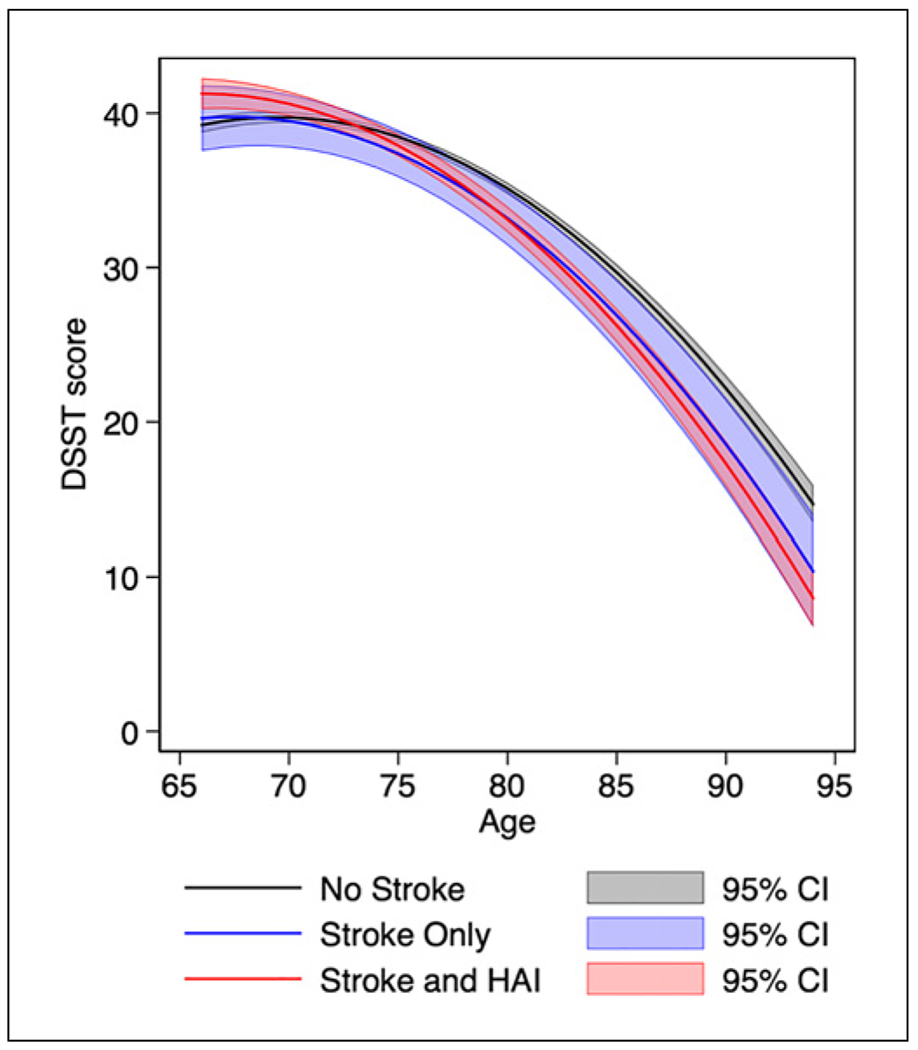
Adjusted model-predicted mean DSST score trajectories. The gray line represents DSST trajectory without a history of stroke or HAI. The blue line represents DSST trajectory after incident stroke without HAI. The red line represents DSST trajectory after incident stroke with coexistence of HAI. Gray, blue, and red shadings represent each group’s 95% confidence intervals. The model included 5,443 participants and was adjusted for participant sex, race, education level, smoking status, hypertension, systolic blood pressure, diabetes, BMI, CHD, atrial fibrillation, dyslipidemia, and alcohol use, all assessed at baseline. DSST, Digit Symbol Substitution Test; HAI, hospital-acquired infection; BMI, body mass index; CHD, coronary heart disease.
Discussion
In this longitudinal study of older adults, cognitive decline was more rapid after stroke accompanied by HAI than in those without stroke; however, cognitive decline was found to be similar between stroke patients both with and without HAI. These findings held for both global cognition and processing speed (3MSE and DSST, respectively), although they were stronger for global cognition. Concerning stroke unaccompanied by infection, measures of global cognition did not show impressively more rapid cognitive decline than non-stroke individuals, while measures of processing speed did. Our results provide indirect evidence that immunostimulation related to infection may play a significant role in cognitive decline after stroke. Our results are consistent with those from prior studies suggesting that pro-inflammatory immune signatures in peripheral blood in days after stroke are associated with a cognitive decline between 3 and 12 months after stroke, as well as studies that indicate infections contribute to functional outcomes after stroke [23, 24].
Our findings are important as they demonstrate the potential long-term implications of preventable HAI in this population, trajectories largely unknown previously. These findings are particularly important in the current aging population, as the risk of death from stroke has declined in recent years, while at the same time demonstrating the number of survivors with cerebral compromise, dementia, and cognitive dysfunction has increased [25]. Recent observational studies provide evidence that stroke is associated with acute cognitive decline, delayed chronic cognitive decline, and worse long-term cognitive outcomes over 6 years; providing cognitive trajectories for stroke patients with and without HAI will be important for clinicians to determine possible short- and long-term needs for functional independence [26].
Infections themselves have also been independently associated with immediate and sustained declines in cognitive function, demonstrating the need to discern the effect stroke and infection can play, both separately and simultaneously, on cognitive decline in vulnerable patient populations [10]. Shah et al. [12] have described a possible bidirectional relationship between cognitive function and infection. A study by Morton et al. [27] demonstrated that infection occurring at delayed intervals after a stroke, during long-term follow-up, increases the risk of a subsequent diagnosis of dementia. Our study expands on these results to include patients with infection at the time of their hospitalization for stroke, demonstrating that presence of HAIs during hospitalized stroke may contribute to accelerated long-term cognitive decline.
Clinical implications of our results require further research. While accurately predicting who will experience HAI during a hospitalization is challenging, our findings provide support for aggressive prevention of infection among patients receiving hospitalized stroke care, particularly with HAIs showing to be relatively common among patients >65 years of age (10–12%) [28, 29]. Physicians and healthcare administrators can use these findings to promote the priority of proper sanitation and HAI-preventative protocols. This heightened sensitivity to the risk of infection may be particularly relevant during and after the coronavirus pandemic, given the recognition that the severe acute respiratory syndrome coronavirus 2019 may be associated with an increased risk of both stroke and cognitive impairment [30, 31]. Additionally, during the heightened infection risk over the 6 months post-stroke, greater use of telemedicine could also help in the early identification of other infections during clinical follow-up [26, 32]. These findings may also help reduce the costs associated with a prolonged hospital stay, worse cognitive projections, and higher delirium risks seen in those with HAI and stroke early on [33].
Our study has several methodologic strengths. First, we excluded participants with prevalent stroke at enrollment. Second, we had a large sample size with nearly a decade-long annual cognitive follow-up, with an average 2.5 years of cognitive follow-up after stroke, allowing us to graph meaningful model-predicted trajectories related to our objectives. Third, our study included hundreds of stroke events and included cognitive follow-up before and after each incident stroke event. Lastly, we modeled cognitive trajectories using time-varying variables for incident stroke.
However, there are limitations to the present study, and therefore, results need to be interpreted cautiously. First, HAIs were defined through ICD-9 codes throughout the CHS, which may be subject to misclassification, though studies have found these codes to adequately identify and classify inpatient HAIs in general [34–38]. Second, we lacked information from CHS on the length of stay and were unable to account for the severity, location, or volume of stroke, all of which can impact HAI risk and level of cognitive impairment after stroke. We did not consider data on recurrent stroke. Third, data on stroke subtypes were unavailable for adequate assessment of their interaction with cognitive decline. Residual confounding is a possibility. Fourth, selection bias may be a possibility, as those experiencing incident stroke or experiencing cognitive decline may be assumed to be more likely to miss annual cognitive testing and therefore not contribute their averages to cognitive scoring in grouped analyses; attenuation of the association between stroke and cognitive decline may be present to a certain degree as a result. In addition, we could not eliminate the possibility of unmeasured confounders contributing to the risk of cognitive decline, including depression, thyroid disease, undiagnosed dementia, etc. Similarly, participants with prior dementia were not excluded at baseline; however, we preferred to estimate the associations of stroke and infection with cognitive trajectories amongst all participants, regardless of initial cognitive diagnoses. Lack of a non-stroke HAI group prevented direct comparison of cognitive decline against SI cognitive trajectories. While a great strength, utilizing two separate cognitive function tests (3MSE and DSST) to provide a clearer understanding of decline over time for both physicians and patients, separate and joint effects of stroke and infection on cognitive decline may differ across different cognitive domains and should be interpreted as such [39–42].
Conclusion
This longitudinal study suggests that stroke patients with HAI, but not stroke patients without HAI, have more decline in long-term global cognition than those without stroke. Stroke patients with HAI also have a greater long-term decline in executive function than those without stroke, and they may have a greater decline in executive function than stroke patients without HAI. Further research is required to confirm the role of infection on post-stroke cognitive decline and dementia, identify mechanisms, and address potential therapeutic implications.
Acknowledgments
No other persons have made substantial contributions to this manuscript.
Funding Sources
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Statement of Ethics
The presented research complies with COPE guidelines. This study protocol was reviewed and approved by the Institutional Review Boards at the University of Washington and the Cardiovascular Health Study, approval number STUDY00000109/CR00006343 on May 31, 2022. All participants provided written informed consent for participation in this study.
Conflict of Interest Statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Availability Statement
Access to the Cardiovascular Health Study data is available and may be requested at https://biolincc.nhlbi.nih.gov/studies/chs/. Further inquiries can be directed to the corresponding author.
References
- 1.Ivan C, Seshadri S, Beiser A, Au R, Kase C, Kelly-Hayes M, et al. Dementia after stroke: the framingham study. Stroke. 2004;35(6):1264–8. [DOI] [PubMed] [Google Scholar]
- 2.Doyle K, Quach L, Solé M, Axtell R, Nguyen T, Soler-Llavina G, et al. B-Lymphocyte-Mediated delayed cognitive impairment following stroke. J Neurosci. 2015;35(5):2133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. 2015;3(1):61–9. [DOI] [PubMed] [Google Scholar]
- 4.Chiu M. Systemic infections may cause cognitive deterioration and neurodegeneration. Crit Care Med. 2014;42(5):1282–3. [DOI] [PubMed] [Google Scholar]
- 5.Tate J, Snitz B, Alvarez K, Nahin R, Weissfeld L, Lopez O, et al. Infection hospitalization increases risk of dementia in the elderly. Crit Care Med. 2014;42(5):1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mena H, Cadavid D, Rushing E. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. 2004;108(6):524–30. [DOI] [PubMed] [Google Scholar]
- 7.George A, Boehme A, Siegler J, Monlezun D, Fowler B, Shaban A, et al. Hospital-acquired infection underlies poor functional outcome in patients with prolonged length of stay. ISRN Stroke. 2013;2013:312348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme A, Kulick E, Canning M, Alvord T, Khaksari B, Omran S, et al. Infections increase the risk of 30-day readmissions among stroke survivors. Stroke. 2018;49(12):2999–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme A, Kumar A, Dorsey A, Siegler J, Aswani M, Lyerly M, et al. Infections present on admission compared with hospital-acquired infections in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2013;22(8):e582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gracner T, Agarwal M, Murali K, Stone P, Larson E, Furuya E, et al. Association of infection-related hospitalization with cognitive impairment among nursing home residents. JAMA Netw Open. 2021;4(4):e217528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathews S, Arnold S, Epperson C. Hospitalization and cognitive decline: can the nature of the relationship be deciphered? Am J Geriatr Psychiatry. 2014;22(5):465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah F, Pike F, Alvarez K, Angus D, Newman A, Lopez O, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188(5):586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard T, Self W, Edwards K, Grijalva C, Zhu Y, Williams D, et al. Long-term cognitive impairment after hospitalization for community-acquired pneumonia: a prospective cohort study. J Gen Intern Med. 2018;33(6):929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried L, Borhani N, Enright P, Furberg C, Gardin J, Kronmal R, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. [DOI] [PubMed] [Google Scholar]
- 15.Hammond C, Blades N, Chaudhry S, Dodson J, Longstreth W, Heckbert S, et al. Long-term cognitive decline after newly diagnosed heart failure: longitudinal analysis in the CHS (cardiovascular health study). Circ Heart Fail. 2018;11(3):e004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thacker E, McKnight B, Psaty B, Longstreth W, Sitlani C, Dublin S, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81(2):119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Psaty B, Kuller L, Bild D, Burke G, Kittner S, Mittelmark M, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–7. [DOI] [PubMed] [Google Scholar]
- 18.Longstreth W, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56(3):368–75. [DOI] [PubMed] [Google Scholar]
- 19.Kuller L, Arnold AM, Psaty BM, Robbins JA, O’Leary DH, Tracy RP, et al. 10-year followup of subclinical cardiovascular disease and risk of coronary heart disease in the cardiovascular health study. Arch Intern Med. 2006;166(1):71–8. [DOI] [PubMed] [Google Scholar]
- 20.Corrales-Medina V, Taljaard M, Yende S, Kronmal R, Dwivedi G, Newman A, et al. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia in elderly adults. Am Heart J. 2015;170(2):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkind M, Carty C, O’Meara E, Lumley T, Lefkowitz D, Kronmal R, et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke. 2011;42(7):1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushman M, Cornell E, Howard P, Bovill E, Tracy R. Laboratory methods and quality assurance in the cardiovascular health study. Clin Chem. 1995;41(2):264–70. [PubMed] [Google Scholar]
- 23.Tsai A, Berry K, Beneyto M, Gaudilliere D, Ganio E, Culos A, et al. A year-long immune profile of the systemic response in acute stroke survivors. Brain. 2019;142(4):978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker K, Zierath D, Kunze A, Fecteau L, Lee B, Skerrett S. The contribution of antibiotics, pneumonia and the immune response to stroke outcome. J Neuroimmunol. 2016;295–6:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allan L, Rowan E, Firbank M, Thomas A, Parry S, Polvikoski T, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134(Pt 12):3716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine D, Galecki A, Langa K, Unverzagt F, Kabeto M, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton C, Forbes H, Pearce N, Smeeth L, Warren-Gash C. Association between common infections and incident post-stroke dementia: a cohort study using the clinical practice research datalink. Clin Epidemiol. 2020;12:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz MJ, Roghmann MC. Healthcare-associated infections in the elderly: what’s new. Curr Opin Infect Dis. 2016;29(4):388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristina ML, Spagnolo AM, Giribone L, Demartini A, Sartini M. Epidemiology and prevention of healthcare-associated infections in geriatric patients: a narrative review. Int J Environ Res Public Health. 2021;18(10):5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers J, Chesney E, Oliver D, Pollak T, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7(7):611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakil S, Emmons-Bell S, Rutan C, Walchok J, Navi B, Sharma R, et al. Stroke among patients hospitalized with COVID-19: results from the American heart association COVID-19 cardiovascular disease registry. Stroke. 2022;53(3):800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pendlebury S, Rothwell P; Oxford Vascular Study. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18(3):248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkind M, Boehme A, Smith C, Meisel A, Buckwalter M. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51(10):3156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins T, Deshpande A, Zilberberg M, Lindenauer P, Imrey P, Yu P, et al. Assessment of the accuracy of using ICD-9 diagnosis codes to identify pneumonia etiology in patients hospitalized with pneumonia. JAMA Netw Open. 2020;3(7):e207750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanathan R, Leavell P, Stockslager G, Mays C, Harvey D, Duane T. Validity of international classification of diseases, ninth revision, clinical modification (ICD-9-CM) screening for sepsis in surgical mortalities. Surg Infect. 2014;15(5):513–6. [DOI] [PubMed] [Google Scholar]
- 36.Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294(21):2712–9. [DOI] [PubMed] [Google Scholar]
- 38.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–13. [DOI] [PubMed] [Google Scholar]
- 39.Ip EH, Pierce J, Chen SH, Lovato J, Hughes TM, Hayden KM, et al. Conversion between the Modified Mini-Mental State Examination (3MSE) and the Mini-Mental State Examination (MMSE). Alzheimers Dement. 2021;13(1):e12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377–83. [DOI] [PubMed] [Google Scholar]
- 42.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to the Cardiovascular Health Study data is available and may be requested at https://biolincc.nhlbi.nih.gov/studies/chs/. Further inquiries can be directed to the corresponding author.


