Abstract
Using virions harvested from 293T cells stably expressing either low or high levels of surface ICAM-1, we determined that the number of virus-embedded host ICAM-1 proteins is positively influenced by the expression level of ICAM-1 on virus producer cells. Moreover, the increase in virion-bound host cell membrane ICAM-1 led to a concomitant enhancement of virus infectivity when a T-cell-tropic strain of human immunodeficiency virus type 1 (HIV-1) was used. The phenomenon was also seen when primary human cells were infected with virions pseudotyped with the envelope protein from a macrophage-tropic HIV-1 isolate, thus ruling out any envelope-specific effect. We also observed that target cells treated with NKI-L16, an anti-LFA-1 antibody known to increase the affinity of LFA-1 for ICAM-1, were markedly more susceptible to infection with HIV-1 particles bearing on their surfaces large numbers of host-derived ICAM-1 proteins. Given that cellular activation of leukocytes is known to modify the conformational state of LFA-1 and induce ICAM-1 surface expression, it is tempting to speculate that activation of virus-infected cells will lead to the production of HIV-1 particles bearing more host ICAM-1 on their surfaces and that such progeny virions will preferentially infect and replicate more efficiently in activated cells which are prevalent in lymphoid organs.
Viruses are obligate intracellular parasites, and they are thus absolutely dependent on the host cell for essential functions such as the generation of metabolic machinery and protein synthesis. Such a strong dependence on the host cellular machinery is probably linked with the limited genetic resources of viruses. These infectious agents have overcome their genetic limitations by showing a strong adaptation to their host. For example, human immunodeficiency virus type 1 (HIV-1) uses the surface CD4 glycoprotein and chemokine receptors to infect its target cells (1, 19, 21, 22, 24, 25, 27, 43). In addition, due to their high rate of mutations, viruses can adapt themselves to their host by natural selection in an attempt to optimize their life cycle to assure their survival. Enveloped viruses such as HIV-1 acquire their lipid membranes and their own envelope proteins during the process known as budding. A characteristic of the propagation of HIV-1 within its target is the incorporation of several host-encoded proteins during the extrusion of the virus particles from the infected cells. Indeed, the outer surface of HIV-1 has been demonstrated to be composed of numerous host cell membrane constituents including major histocompatibility complex class II (MHC-II) determinants (HLA-DR, -DP, and -DQ), β2-microglobulin, CD43, CD44, CD55, CD59, CD63, CD71, and adhesion receptors such as ICAM-1 and LFA-1 (2, 3, 12, 13, 16, 26, 33, 38, 40, 47–49, 59, 62). The incorporation of these host-derived molecules seems to be a selective process since not all cell surface molecules are found embedded within HIV-1. For example, the transmembrane protein tyrosine phosphatase CD45 is not acquired by newly formed HIV-1 progeny virions (49) despite the fact that it represents the most abundant molecule at the surfaces of leukocytes (65).
Accumulating evidence indicates that virion-bound host proteins are functional and that they seem to confer protection against the harsh environment surrounding HIV-1 (64). More specifically, the neutralizing capacity of sera from HIV-1-infected individuals was enhanced by the addition of anti-LFA-1 antibodies, thus suggesting an important role for this host-encoded glycoprotein in the process of infection (36). In addition, the physical presence of host cell membrane MHC-II, HLA-DR1, and ICAM-1 on HIV-1 has been shown to lead to an enhancement of virus infectivity that is due to the interactions between virion-bound host molecules and their physiological counterreceptors found on the surfaces of target cells (10, 11, 30, 55).
The basis of the present work is founded on several published observations. First, host-derived ICAM-1 is incorporated into nascent HIV-1 progeny viruses and the presence of cell membrane host molecules is also detected in plasma-derived HIV-1 isolates (12, 13, 32, 47, 58). Second, HIV-1 infectivity is significantly increased by the acquisition of host-encoded ICAM-1 (30, 55). Third, target cells are more susceptible to infection with ICAM-1-bearing HIV-1 progeny viruses if they express on their surfaces the natural ligand of ICAM-1, LFA-1, in its activated form (31). Fourth, ICAM-1 is normally expressed in small amounts on peripheral blood leukocytes but is strongly induced by cytokines such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin 1 (IL-1) (15). Fifth, increased levels of TNF-α, IFN-γ, and IL-1 have been observed during the course of HIV-1 infection (51). The major aim of the present study was to assess if the number of virus-acquired host cell membrane ICAM-1 proteins is influenced by the level of ICAM-1 that is expressed on the surfaces of virus producer cells. If so, we were next interested in determining whether virus infectivity was modified by the relative numbers of host-encoded ICAM-1 proteins acquired by newly formed HIV-1 particles. Finally, we have also studied the functional relevance of differences in the levels of virus-embedded host ICAM-1 when infection is carried out with target cells expressing LFA-1 in either the low- or high-affinity conformational state.
Main characteristics of cells used in this study.
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque density gradient centrifugation and were cultured in complete culture medium in the presence of 3 μg of phytohemagglutinin-protein (Sigma, St. Louis, Mo.) per ml and 30 U of recombinant human IL-2 (rhIL-2) per ml for 3 days at 37°C under a 5% CO2 atmosphere prior to viral infection. The Jurkat-tat cell line is a derivative of Jurkat E6.1 cells that stably expresses the HIV-1 Tat protein, and PM1 is a clonal derivative of HUT 78 (14, 45). 293T cells are human embryonic kidney cells which are negative for ICAM-1 expression, while Jurkat-tat and PM1 cells express high levels of LFA-1 on their surfaces (data not shown). Three distinct populations of 293T cells that are either negative for ICAM-1 expression (parental cells termed Null-ICAM-1) or express either low (Lo-ICAM-1) or high (Hi-ICAM-1) levels of surface ICAM-1 were used in this study. These cell lines were obtained by transfecting 293T cells with pCMV-Hygro to obtain Null-ICAM-1 and by cotransfecting 293T cells with pCD1.8, a eukaryotic expression vector containing the entire human ICAM-1 cDNA (63), and pCMV-Hygro (10:1 ratio) to derive ICAM-1-expressing 293T cells. All transfections were performed by a modified version of the calcium phosphate (CaPO4) transfection protocol as described previously (11, 30, 31). Transfected 293T cells were maintained under selective pressure with hygromycin B (400 μg/ml; Calbiochem) for 3 weeks and were next sorted by fluorescence-activated cell sorter analysis with the use of the anti-ICAM-1 RR1/1.1.1 antibody. The Lo-ICAM-1 population was sorted by gating in the low-mean-fluorescence-intensity region, whereas cells with the highest mean fluorescence intensity were kept for the Hi-ICAM-1 population.
Production of virus stocks.
Viral particles differing only by the absence or the presence of host-derived ICAM-1 proteins on their surfaces were produced by CaPO4 transfection of pHXB-Luc (17) or, for pseudotyped virions, of pNL4-3-Luc-E−R+ plus pcDNA-1/Ada-M env, in Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 293T cells as described previously (30). Briefly, a typical transfection experiment was carried out with 10 μg of pHXB-Luc, while for the production of virions pseudotyped with the Ada-M Env protein, we used 2 μg of pNL4-3-Luc-E−R+ and 10 μg of pcDNA-1/Ada-M. Null-ICAM-1 virus preparations were produced with parental 293T cells that do not express ICAM-1, whereas Lo- and Hi-ICAM-1 progeny virions were produced from cells expressing low and high levels of surface ICAM-1, respectively. All virus preparations underwent only one freeze-thaw cycle before the initiation of infection studies. Virus stocks were normalized for virion content by a commercial assay for p24 (Organon Teknika, Durham, N.C.). The standardization on p24 content is based on our previous observation indicating that virus preparations harvested from transfected 293T cells contain minimal amounts of p24 that are not associated with infectious virions (30).
Antibodies and purified proteins.
Anti-ICAM-1 (anti-CD54) antibodies RR1/1.1.1 and R6.5 were provided by Robert Rothlein (Boehringer Ingelheim, Ridgefield, Conn.) (56). LFA-1-activating antibody NKI-L16 (anti-CD11a) was obtained from Carl C. Figdor (University Hospital Nijmegen, Nijmegen, The Netherlands) (42). The ICAM-1 fusion protein (ICAM-1–immunoglobulin G2b [IgG2b]) is composed of the extracellular part of ICAM-1 fused to the hinge region and constant H chain domains 2 and 3 of a mouse IgG2b and was purified by an immunoaffinity column. This fusion protein was supplied by Eric Lundgren (University of Umeå, Umeå, Sweden) and has been described before (37). F105, a human monoclonal antibody directed against a conformational epitope on HIV-1 gp120 mapping to the CD4 binding site, was obtained from Marshall Posner through the AIDS Repository Reagent Program (54). Sheep anti-HIV-1 gp120 was supplied by the AIDS Repository Reagent Program and was purified with a mAbTrap protein G affinity column according to the manufacturer’s instructions (Pharmacia LKB Biotechnology AB, Uppsala, Sweden). Purified recombinant gp120 (rgp120; strain HIV-1IIIB) was obtained from Genentech, Inc. (South San Francisco, Calif.). Sheep anti-HIV-1 gp120 and R6.5 were biotinylated with N-hydroxysuccinimide–long-chain biotin according to the supplier’s instructions (Pierce, Rockford, Ill.). Fluorescein isothiocyanate-conjugated goat anti-mouse IgG was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.).
Virus infection and HIV-1 entry assay.
Similar amounts of each recombinant luciferase-encoding virus stock (5 ng of p24 for Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 virions) were incubated with target cells (105) for 90 min at 37°C. In some experiments, target cells were either left untreated or treated with the LFA-1-activating antibody NKI-L16 at 1 μg/ml for 30 min at 37°C. The cells were next washed with phosphate-buffered saline (PBS), resuspended in 100 μl of complete culture medium supplemented with 30 U of rhIL-2 per ml for PBMCs, and transferred to a 96-well flat-bottom tissue culture plate (Microtest III, Falcon; Becton Dickinson, Lincoln Park, N.J.). Cells were kept for 24 (Jurkat-tat and PM1 cells) or 72 h (PBMCs) at 37°C. After this final incubation, cells were lysed as described previously (11, 30). Luciferase activity was monitored with a microplate luminometer (MLX; Dynex Technologies, Chantilly, Va.). In some experiments, cells were treated with herbimycin A (1 μg/ml) for 60 min at 37°C prior to infection. In this set of experiments, cellular viability was estimated by the MTS assay as described previously (9). Internalization of Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 virions was monitored by a recently described method (46). Briefly, for each sample, 5 × 106 Jurkat-tat cells were washed with PBS and resuspended into 1 ml of an HIV-1 suspension containing 100 ng of p24 in culture medium supplemented with 10% fetal bovine serum (FBS). Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 virus preparations were incubated with target cells for 2.5 h at 37°C. Cells were washed two times with ice-cold PBS and then resuspended in 1 ml of ice-cold Dulbecco modified Eagle medium (DMEM) (without FBS) containing pronase (Boehringer Mannheim, Laval, Quebec, Canada) at 0.1 mg per ml for 5 min at 4°C. Cells were washed immediately with 2 ml of ice-cold DMEM containing 10% FBS and three times with ice-cold PBS to eliminate pronase. Cells were resuspended in 1 ml of complete RPMI medium to which was added 200 μl of disruption buffer (2.5% Triton X-100 in PBS). Cells were agitated for 10 min at room temperature and then stored at −20°C until assayed for p24 content.
Quantitative determination of virion-bound host ICAM-1 and gp120.
The presence of virion-bound ICAM-1 and viral gp120 proteins in our virus preparations was assessed by in-house enzymatic assays. Cell-free Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 virus stocks were pelleted under ultracentrifugation conditions that are sufficient to sediment whole viruses (Heraeus model Contifuge 28RS; 12,000 rpm for 90 min at 4°C) (11). Next, the virus pellets were gently resuspended in PBS in 1/20 of the initial volume and were stored at −20°C until assayed. The virus-embedded host ICAM-1 level was assessed with R6.5 as the coating antibody and biotinylated RR1/1.1.1 as the secondary antibody, while the gp120 level was quantitated with F105 (first antibody) and biotinylated sheep anti-HIV-1 gp120 (second antibody). Proteins were visualized with a streptavidin-horseradish peroxidase conjugate (streptavidin-HRP40; Research Diagnostics Inc., Flanders, N.J.). Appropriate dilutions of rgp120 and ICAM-1-IgG2b were used to produce standard curves.
The level of ICAM-1 surface expression on virus producer cells quantitatively modulates the amount of host-encoded ICAM-1 acquired by HIV-1.
In an attempt to gain more knowledge on cellular factors affecting the process of incorporation of host cell membrane proteins by progeny HIV-1 particles, we investigated whether the expression level of ICAM-1 can affect the number of virion-embedded host-derived ICAM-1 proteins. To attain this goal, we generated two different 293T cell populations bearing different levels of ICAM-1 by means of stable transfection and cell sorting. This specific cell line was selected because it is recognized as being highly transfectable (53). In addition, a significant advantage of this cell line over lymphoid cells is that virus preparations produced by transfected 293T cells are relatively free of cellular constituents (see below), while purified virus stocks harvested from acutely HIV-1-infected lymphoid cell lines have been reported to be heavily contaminated with microvesicles that cosediment with sucrose gradient-purified virions (6, 35). The parental 293T cells (Null-ICAM-1) are negative for ICAM-1 expression, whereas the mean fluorescence intensities (indicative of the number of ICAM-1 proteins per cell) measured for Lo- and Hi-ICAM-1 293T cells were found to be 12.8 and 44.1, respectively (data not shown).
Progeny viruses were initially produced by transiently transfecting Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 293T cells with pHXB-Luc. We evaluated the relative levels of viral p24 and gp120 proteins, as well as the quantities of virus-embedded host ICAM-1 in such virus preparations. We determined by enzymatic assays that the molar ratios of viral p24 to virus-embedded host ICAM-1 for Lo-ICAM-1 and Hi-ICAM-1 virus stocks were 1:0.001 and 1:0.016, respectively (Table 1). It should be noted that these molar ratios were calculated on the basis of known molecular masses: 24.0 kDa for viral p24 and 90.0 kDa for ICAM-1. Assuming 3,000 p24 molecules per virion (5), this places the numbers of ICAM-1 molecules per Lo-ICAM-1 and Hi-ICAM-1 virion at about 3 and 49, respectively (Table 1). Thus, the number of virus-embedded host ICAM-1 proteins in virions produced by Hi-ICAM-1 cells is 16-fold greater than that in progeny viruses harvested from Lo-ICAM-1 293T cells. We can conclude that the relation between the expression level of ICAM-1 on the surfaces of virus producer cells and the amount of virion-bound ICAM-1 is not linear considering that there is a fourfold increase in ICAM-1 surface expression in Hi-ICAM-1 cells compared to Lo-ICAM-1 cells (mean fluorescence intensity of 44.1 for Hi-ICAM-1 293T cells versus 12.8 for Lo-ICAM-1 293T cells). Interestingly, the molar ratios of viral gp120 to p24 were comparable for Null-ICAM-1, Lo-ICAM-1, and Hi-ICAM-1 virus stocks (data not shown). This last finding suggests that the incorporation of different levels of host-encoded ICAM-1 does not affect the external envelope spike density on HIV-1 particles. Next, the presence of cellular contaminants composed of microvesicles loaded with cellular ICAM-1 proteins was assessed with ultracentrifuged supernatants from mock-transfected Lo-ICAM-1 and Hi-ICAM-1 293T cells. Enzymatic analyses of such samples revealed that pellets from mock-transfected Lo-ICAM-1 and Hi-ICAM-1 293T cells contained 0.3 and 4.8 ng of ICAM-1, respectively, per ml. This indicates that cellular contaminants represent only 5 to 6% of the total host-derived ICAM-1 proteins, which are detected in similar quantities in ultracentrifuged supernatants from Lo-ICAM-1 and Hi-ICAM-1 cells transiently transfected with pHXB-Luc (0.3 versus 5.5 ng/ml for Lo-ICAM-1 and 4.8 versus 79.8 ng/ml for Hi-ICAM-1).
TABLE 1.
Characterization of virus preparations produced by transient transfection of Null-, Lo-, and Hi-ICAM-1 293T cells with pHXB-Luc
| Virus stocka | Amtb (ng/ml) of:
|
Molar ratio (p24/ICAM-1) | No. of ICAM-1 molecules per virion | |
|---|---|---|---|---|
| p24 | ICAM-1 | |||
| Null-ICAM-1 | 3,700 | 0 | NAc | NA |
| Lo-ICAM-1 | 1,600 | 5.5 | 1:0.001 | 3 |
| Hi-ICAM-1 | 1,300 | 79.8 | 1:0.016 | 49 |
Virus stocks were concentrated by ultracentrifugation.
The amounts of viral p24 and host-derived ICAM-1 were assessed by enzymatic assays.
NA, not applicable.
Virus infectivity is enhanced by increasing the amount of virion-bound host ICAM-1.
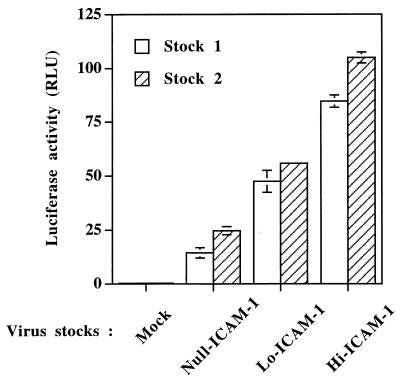
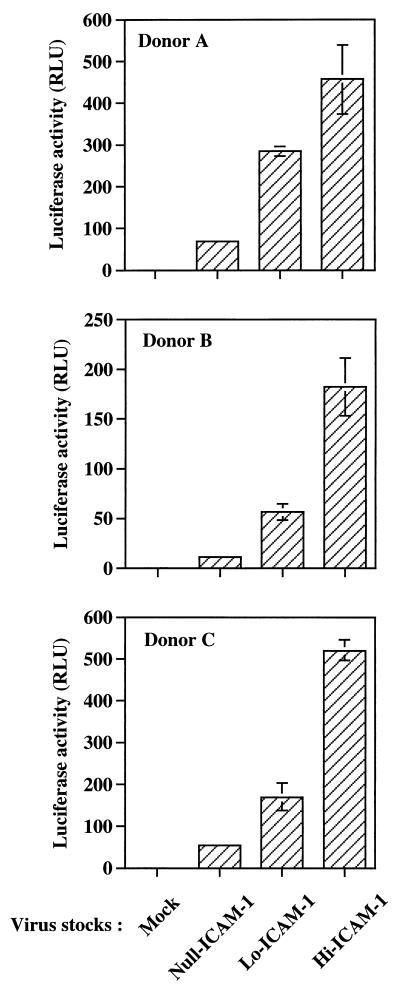
The next logical step was to determine if the observed quantitative changes in the numbers of virus-acquired host ICAM-1 proteins could affect HIV-1 infectivity. First, we inoculated a highly susceptible CD4+ T-lymphoid-cell line (Jurkat-tat) with identical amounts of each virus preparation standardized in terms of p24. It is clear from the results illustrated in Fig. 1 that virus infectivity is increased in a manner that is dependent on the quantity of virion-bound host ICAM-1 proteins. Indeed, Lo-ICAM-1 progeny virions are more infectious than HIV-1 particles devoid of host-encoded ICAM-1 (2.3- to 3.2-fold increase compared to Null-ICAM-1 virions), but the former are less infectious than Hi-ICAM-1 virus particles (4.3- to 5.9-fold increase compared to Null-ICAM-1 virions). However, the observed enhancement of virus infectivity does not seem to correlate in a perfectly linear way with the corresponding level of host cell membrane ICAM-1 proteins physically present within mature HXB-Luc viral entities. A clear example is provided by the fact that only a 2-fold increase is seen when virus infectivities of Hi-ICAM-1 and Lo-ICAM-1 virus stocks are compared, whereas Hi-ICAM-1 virions incorporate 16-fold more host ICAM-1 proteins than Lo-ICAM-1 progeny viruses (49 ICAM-1 molecules for Hi-ICAM-1 versus 3 for Lo-ICAM-1). The positive correlation between virion-bound host ICAM-1 and HIV-1 infectivity is not an isolated phenomenon since this observation was made with Null-, Lo-, and Hi-ICAM-1 virus preparations originating from two independent transfections (stocks 1 and 2). The observation that Null-, Lo-, and Hi-ICAM-1-purified virus preparations contain comparable levels of gp120 per virion (data not shown) demonstrates that the increase of HIV-1 infectivity is not due to variations in the gp120 content but rather to the number of virion-embedded host cell membrane ICAM-1 proteins (Table 1). To more closely parallel physiological conditions, similar experiments were conducted with mitogen-stimulated PBMCs isolated from three healthy donors as the targets. The infection of primary mononuclear cells with Null-, Lo-, and Hi-ICAM-1 virus stocks led to an effect on virus infectivity that was still positively modulated by the amount of virion-bound host ICAM-1 (Fig. 2). More specifically, Lo-ICAM-1 virions were 3.1- to 4.6-fold more infectious than Null-ICAM-1 HIV-1 particles, while Hi-ICAM-1 viruses were 6.8- to 14.8-fold more infectious than isogenic progeny viruses devoid of host-encoded ICAM-1 (Null-ICAM-1). The exact mechanism(s) responsible for such a wide variation in the enhancement of virus infectivity for the Hi-ICAM-1 HIV-1 particles (6.8- to 14.8-fold) is undefined, but it might be linked with differences between donors with regard to the level of surface expression of LFA-1, the natural counterreceptor of ICAM-1.
FIG. 1.
The number of virion-bound host ICAM-1 proteins positively affects HIV-1 infectivity. Jurkat-tat cells were infected with standardized amounts of virus stocks composed of Null-, Lo-, and Hi-ICAM-1 HIV-1 particles produced by two independent transfection experiments (5 ng of p24). Cells were incubated for 90 min at 37°C and then washed with PBS. Thereafter, the cells were further incubated for 24 h at 37°C. Finally, cells were lysed and luciferase activity, expressed in RLU was monitored as described in Materials and Methods. Results are the means ± standard errors of the means for quadruplicate samples and are representative of three independent experiments. Negative controls consisted of uninfected Jurkat-tat cells. Luciferase activity values for mock-infected cells were 0.35 ± 0.03 RLU for stock 1 and 0.37 ± 0.04 RLU for stock 2.
FIG. 2.
The positive effect on HIV-1 infectivity mediated by the amount of virion-bound host ICAM-1 is also seen in primary human cells. Similar amounts of Null-, Lo-, and Hi-ICAM-1 virus preparations were used to inoculate freshly isolated PBMCs from three different healthy donors (5 ng of p24). Virions and target cells were incubated together for 90 min at 37°C and washed with PBS, and incubation at 37°C was pursued for an additional 72 h. Next, cells were lysed and luciferase activity was monitored as described in Materials and Methods. Results are the means ± standard errors of the means for quadruplicate samples and are representative of three independent experiments. Negative controls consisted of mock-infected PBMCs. Luciferase activity values for mock-infected cells were 0.44 ± 0.07 RLU for donor A, 0.41 ± 0.02 RLU for donor B, and 0.43 ± 0.03 RLU for donor C.
The enhancement of virus infectivity conferred by host-derived ICAM-1 is not due to signal transduction through LFA-1 but rather is linked with an increase in the process of virus entry.
A previous study has reported that LFA-1 possesses signaling properties, as it leads to activation of phospholipase C-γ1 (41). In order to verify whether virion-bound ICAM-1 could generate signaling in the target cells that could upregulate virus expression, target cells were pretreated with herbimycin A, a potent tyrosine kinase inhibitor which has been shown to abrogate LFA-1-mediated signal transduction events (41). Virus infectivity was increased by the presence of host-derived ICAM-1 despite treatment with herbimycin A (data not shown). These data suggest that the positive effect of host-encoded ICAM-1 on virus infectivity is not the result of a postinfection LFA-1-mediated intracellular signaling event. A virus entry assay was next performed to determine if virus-acquired host ICAM-1 could modify the early steps in the virus infection cycle. Proteolytic enzymes (pronase) were used to eliminate noninternalized virus particles attached to the cell surface. Indeed, treatment of cells with pronase has been recently shown to remove cell surface CD4, thus eliminating any virions attached to its primary receptor (46). This virus entry assay showed that the percentage of progeny viruses entering susceptible cells was correlated with the number of virion-bound host ICAM-1 proteins. The percentages of virus entry for Null-, Lo-, and Hi-ICAM-1 virions were 30.2, 37.5, and 46.8%, respectively (the percentages were determined by dividing the p24 content after pronase digestion by the total p24 content in the absence of pronase treatment). It can thus be concluded that virus-acquired host cell membrane ICAM-1 proteins are positively modulating the early stages of the virus infection process.
Expression of a high-affinity LFA-1 form strongly enhanced the susceptibility of target cells to infection by Hi-ICAM-1 virions.
It has been previously reported that the susceptibility to infection with ICAM-1-bearing HIV-1 particles is increased upon surface expression of LFA-1 in a conformational state of high affinity for ICAM-1 (31). It was thus of interest to determine whether this cellular factor could differentially modulate the process of infection with Lo- and Hi-ICAM-1 virions. The conformational change of LFA-1 was accomplished by treating target cells with anti-LFA-1 monoclonal antibody NKI-L16 (42). In Jurkat-tat cells, treatment with NKI-L16 has no noticeable effect on the process of infection with Null-ICAM-1 progeny viruses, while the susceptibility of target cells to infection with Lo-ICAM-1 HIV-1 particles was slightly enhanced (1.8-fold increase) by modifying the conformational state of LFA-1 (70.5 versus 39.1 relative light units [RLU]) (Table 2). In sharp contrast to this observation, the expression of an activated form of LFA-1 was found to dramatically affect cellular susceptibility to infection with Hi-ICAM-1 virions. Indeed, a 7.3-fold increase in the level of infection with Hi-ICAM-1 virions was seen by changing the conformational state of LFA-1 on the surfaces of Jurkat-tat cells (535.1 RLU with NKI-L16 versus 73.3 RLU without NKI-L16). Interestingly, target cells bearing LFA-1 in the conformational state of high affinity for ICAM-1 were found to be 26-fold more susceptible to infection by Hi-ICAM-1 progeny viruses than to infection by Null-ICAM-1 HIV-1 particles (535.1 RLU with Hi-ICAM-1 versus 20.8 RLU with Null-ICAM-1). These data were confirmed with another cell line since the susceptibility of PM1 cells to infection by Null-ICAM-1 particles was unaffected by treating PM1 cells with NKI-L16, while a similar NKI-L16 treatment resulted in slight and strong increases in cellular susceptibility to infection with Lo- and Hi-ICAM-1 virions, respectively. To determine whether the same phenomenon can also occur in human primary cells, we infected PBMCs from a healthy donor with standardized amounts of Null-, Lo-, and Hi-ICAM-1 virus stocks. In agreement with our previous results obtained with T-lymphoid-cell lines, cellular susceptibility to infection by Null-ICAM-1 virions was unaffected by the conformational state of LFA-1 while treatment with NKI-L16 resulted in a 1.9-fold increase in susceptibility to infection with Lo-ICAM-1 HIV-1 (87.6 versus 47.3 RLU). Again, cellular susceptibility to infection with Hi-ICAM-1 progeny virions was markedly affected by modifying the conformational state of LFA-1 since treatment of PBMCs with the LFA-1-activating antibody led to a 6.9-fold increase (994.6 versus 143.8 RLU). The enhancement of cellular susceptibility to virus infection is even more substantial, reaching a 54-fold increase when the levels of infection of PBMCs treated with NKI-L16 produced by Hi- and Null-ICAM-1 virus preparations were compared (994.6 RLU for Hi-ICAM-1 versus 18.5 RLU for Null-ICAM-1).
TABLE 2.
Lymphoid and primary cells expressing an activated form of surface LFA-1 are much more susceptible to infection by Hi-ICAM-1 progeny viruses
| Target cells | NKI-L16 treatment | Luciferase activity (RLU) upon infection with indicated virus stocka
|
||
|---|---|---|---|---|
| Null-ICAM-1 | Lo-ICAM-1 | Hi-ICAM-1 | ||
| Jurkat-tat | No | 26.2 ± 0.5 | 39.1 ± 3.7 | 73.3 ± 4.0 |
| Yes | 20.8 ± 3.0 | 70.5 ± 1.9 | 535.1 ± 33.6 | |
| PM1 | No | 1.4 ± 0.2 | 2.1 ± 0.2 | 3.3 ± 0.2 |
| Yes | 1.4 ± 0.2 | 2.8 ± 0.5 | 7.8 ± 0.8 | |
| PBMCs | No | 17.9 ± 1.1 | 47.3 ± 5.2 | 143.8 ± 10.2 |
| Yes | 18.5 ± 1.1 | 87.6 ± 8.9 | 994.6 ± 195.2 | |
Virus stocks were prepared as described in the text. Results are the means ± standard errors of the means for quadruplicate samples and are representative of three independent experiments. Luciferase activity values for mock-infected cells ranged between 0.37 ± 0.02 and 0.68 ± 0.08 RLU.
Infectivity of a primary macrophage-tropic HIV-1 isolate is enhanced by increasing the amount of virion-bound host ICAM-1.
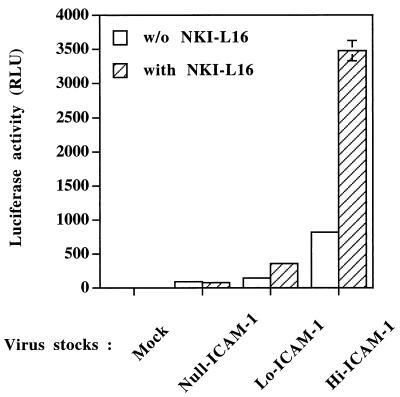
All the previous experiments were carried out with a T-cell line-adapted laboratory strain of HIV-1 (HXB-Luc, which is derived from HXB-2D). We next sought to determine whether the enhancement of virus infectivity due to virus-incorporated host ICAM-1 can be influenced by the nature of the viral envelope glycoprotein. This possibility was evaluated by infecting human PBMCs with Null-, Lo-, and Hi-ICAM-1 progeny virions pseudotyped with the envelope from Ada-M, a primary macrophage-tropic strain of HIV-1 (34). An increase in virus infectivity, which correlates with the level of ICAM-1 on the surfaces of the virus producer cells, was observed for Lo-ICAM-1 and Hi-ICAM-1 virus preparations compared to Null-ICAM-1 virions (Fig. 3). Again, expression of the high-affinity LFA-1 form further increased the susceptibility of PBMCs to infection with Lo-ICAM-1 and Hi-ICAM-1 progeny virions bearing the Ada-M envelope but not susceptibility to infection with Null-ICAM-1 viruses.
FIG. 3.
The increase in virus infectivity mediated by the amount of virion-bound host ICAM-1 is also seen in PBMCs infected with a primary macrophage-tropic HIV-1 isolate. PBMCs were either left untreated or treated with NKI-L16 for 30 min at 37°C prior to infection with similar numbers of Null-, Lo-, and Hi-ICAM-1 virions pseudotyped with the envelope protein from Ada-M (5 ng of p24). Viruses were incubated with PBMCs for 90 min at 37°C, and cells were washed with PBS. The cells were kept in culture for 72 h before luciferase activity was monitored. Results are the means ± standard errors of the means for quadruplicate samples and are representative of three independent experiments. Negative controls consisted of mock-infected PBMCs. Luciferase activity values for mock-infected cells were 0.38 ± 0.11 RLU for untreated cells and 0.27 ± 0.02 RLU for cells treated with NKI-L16.
In summary, the expression level of ICAM-1 on the surfaces of virus producer cells was found to influence the relative number of host-encoded ICAM-1 proteins that are acquired by budding HIV-1 particles (Table 1). Indeed, we noticed that the level of ICAM-1 on the surfaces of virus producer cells directly influenced the number of virion-bound ICAM-1 proteins in a nonlinear manner. The functional significance of this observation was next investigated by infecting Jurkat-tat, PM1, and primary human cells with Null-, Lo-, and Hi-ICAM-1 HXB-Luc particles. We observed that the presence of increasing numbers of virus-embedded host ICAM-1 proteins led to a concomitant enhancement of virus infectivity (Fig. 1 and 2). The increase in virus infectivity for Lo-ICAM-1 and Hi-ICAM-1 viruses compared to that for the Null-ICAM-1 virus was not due to any LFA-1-mediated signaling events. Based on our previous observation (30) and on results from the present virus entry assay (see the data above), it can be stated that the marked increase in virus infectivity is due to interactions between virus-incorporated host ICAM-1 and LFA-1 proteins expressed on the surfaces of target cells. LFA-1 is the physiological counterreceptor for ICAM-1 and is a member of the integrin family that is primarily expressed on lymphocytes, granulocytes, monocytes, and macrophages, with elevated levels on memory T cells (60). The affinity constant determined for the binding of multimeric ICAM-1 to LFA-1 is similar to the strong binding constant for the gp120-CD4 interaction (Kd = 4 nM) (44, 67). Therefore, it is not surprising to discover that virion-bound host ICAM-1 enhances HIV-1 infectivity through its positive action on the process of virus entry, i.e., by favoring attachment and/or entry. It has been shown that the whole process leading to HIV-1 entry is sequential (61). Indeed, a series of events has to take place in order to achieve virus internalization. The additional interaction between virus-embedded ICAM-1 and cell surface LFA-1 may result in the stabilization of the viral entity on the surface of the target cell. The final result will be an increase in the efficiency of the whole virus entry process.
LFA-1 can be found in two distinct states of affinity for ICAM-1, the low- and high-affinity conformational states of LFA-1. This conformational modification can be induced by several agents, including phorbol esters, chemoattractants, and antibodies specific for surface receptors such as the T-cell receptor–CD3 complex, CD2, and MHC II (23). Treatment with the anti-LFA-1 NKI-L16 monoclonal antibody has also been reported to induce a similar conformational change of LFA-1 because this antibody binds to a peripheral domain and opens a link site for ICAM-1 (42). We then assessed if this property of LFA-1 can result in the modulation of the susceptibility of target cells to infection with Lo- and Hi-ICAM-1 HXB-Luc particles by treating target cells with NKI-L16. Cellular susceptibility to infection by Hi-ICAM-1 HXB-Luc virions was found to be markedly accentuated when target cells carried LFA-1 on their surfaces in the conformational state of high affinity for ICAM-1 (Table 2).
The external envelope glycoprotein of HIV-1 (gp120) is known to play an essential role in the viral replicative cycle, including a role in tropism (T cell- or macrophage-tropic), replication kinetics, and syncytium induction (18, 28). Since the first part of our experiments was performed with T-cell-tropic (X4 virus) HXB-2D, and considering the crucial role played by the viral gp120 in the pathogenesis of HIV-1 infection, we considered that the inclusion in our study of a viral strain with a macrophage-tropic (R5) phenotype was of critical importance. Similar observations were made when Null-, Lo-, and Hi-ICAM-1 virus preparations pseudotyped with the envelope protein from the R5 Ada-M isolate were used (Fig. 3). The physiological relevance of this finding is great based on the concept that macrophage-tropic strains are preferentially transmitted between individuals and predominate during the asymptomatic phase of HIV-1 infection, which generally lasts several years (4).
It can be argued that these data were collected by using progeny HIV-1 particles produced by transient transfection of human embryonic kidney cells, a cell type that does not serve as a natural reservoir for HIV-1. However, our technical approach is validated by the fact that the quantitative determination of virus-acquired host proteins cannot be accomplished for virus preparations produced by lymphoid cells because these are known to be heavily contaminated with cellular proteins (6, 35). In this regard, we estimated that the molar ratios of HIV-1 p24 to host cell membrane ICAM-1 proteins detected within purified Lo- and Hi-ICAM-1 HXB-Luc particles were 1:0.001 and 1:0.016, respectively (Table 1). These figures are much lower than those previously observed by Capobianchi et al., who have calculated a molar ratio for viral p24 proteins to virion-bound host ICAM-1 proteins of 1:0.14 (13). Such a wide difference might be attributed to their use of a T-lymphoid-cell line to produce sucrose gradient-purified virus preparations. A recent report has shown that all uninfected T-cell lines tested (H9, CEM-SS, DAUDI, and MOLT-3) secreted large amounts of microvesicles that banded at the same density as that for HIV-1 when fractionated by sucrose gradient centrifugation (6). The same study has demonstrated that mitogen-stimulated human PBMCs also produce microvesicles loaded with cellular proteins. It should be noted that we have established that Lo- and Hi-ICAM-1 293T cells secrete relatively minimal quantities of such microvesicles, therefore indicating that these cells represent an appropriate tool to quantitatively evaluate virion-associated cellular proteins.
The precise mechanism(s) responsible for the incorporation of foreign constituents by nascent viral entities is still undefined. However, results from the present study allow us to speculate about the factor(s) responsible for the insertion of at least host-encoded ICAM-1 molecules into newly formed HIV-1 particles. It can be postulated that the incorporation of host cell membrane ICAM-1 molecules into budding virions is probably not due to a direct physical interaction between ICAM-1 and a viral protein present within the mature particle. This assumption is based on the fact that Null-, Lo-, and Hi-ICAM-1 virus stocks originated from the same genetic source (HXB-Luc or pNL4-3-Luc-E−R+) and the only difference between virus preparations was the producer cells (Null-, Lo-, or Hi-ICAM-1 293T cells). A possible scenario to explain the acquisition of host proteins by HIV-1 is that nascent virions emerge from specific sites at the cellular membrane that would be loaded with patches composed of host cell components such as ICAM-1. This postulate is based primarily on previous studies that have showed that the budding of HIV-1 is restricted to a certain localized region of the cell membrane (20, 39). The fact that copolarization of ICAM-1 and budding viral entities were observed at the site of cell-to-cell contact during HIV-1-induced syncytium formation supports this idea (26). This would explain why ICAM-1 is incorporated in a nonlinear manner into the virus envelope.
It is well known that secondary lymphoid organs such as lymph nodes constitute a natural viral reservoir in HIV-1-infected patients. Interestingly, a fairly high proportion of immune cells residing within the lymph nodes are in an activated state since antigen-specific immune responses primarily take place in these peripheral lymphoid tissues (52). This is exemplified by the nodal migration of activated CD4+ T lymphocytes during a natural antigenic response (57). It is well established that the replication of HIV-1 is tightly linked to the proliferative state of the cells and that cellular activation is thus necessary for a productive HIV-1 infection of CD4+ T lymphocytes. Indeed, HIV-1 particles have the ability to bind to and fuse with both quiescent and activated CD4+ T cells (29, 69). However, in nondividing T lymphocytes, HIV-1 is unable to complete a full replicative life cycle (7, 8, 68, 70). The intense cellular activation observed in the lymphoid organs will also lead to the upregulation of ICAM-1 expression and consequently, as shown in this study, to a higher number of host-derived ICAM-1 proteins acquired by budding HIV-1 particles. The observation that activated germinal-center B cells secrete TNF-α, a proinflammatory cytokine known to induce ICAM-1 expression (66), supports this concept. In addition, cellular activation will also lead to surface expression of the activated conformational state of LFA-1. This conformational modification of LFA-1 can alter the host cell-virus interaction, as shown by our previous work (31) and the present data indicating that cellular susceptibility to infection with Hi-ICAM-1 virions is markedly enhanced by the expression on target cells of the activated form of LFA-1. The activated cellular populations living within the lymph nodes represent thus an ideal cellular environment to facilitate the initial process of virus infection and to achieve more productive virus replication events. Results from clinical studies confirm that lymphoid organs constitute preferential anatomical sites for HIV-1 replication, as 5 to 10 times more virus-infected cells were found in the lymphoid organs (lymph nodes, adenoids, and tonsils) than in the peripheral blood of HIV-1-infected individuals (50).
There are now many reasons to believe that host cell membrane constituents acquired by HIV-1 play a critical role in the virus life cycle (64). The data we have presented here provide supplementary evidence of the functionality of cellular proteins embedded within mature HIV-1 particles. On the basis of our results, we propose a model in which cellular activation will lead to the production of virions bearing high numbers of host-encoded ICAM-1 proteins. The activated cellular state will also result in the expression of LFA-1 in a conformational state of high affinity for ICAM-1 on the surfaces of target cells. The combined action of these two factors will ultimately increase the efficiency of the process of virus infection. Hence, by using the activated cells of the immune system, HIV-1 is able to increase its propagation rate in strategic tissues such as the secondary lymphoid organs.
Acknowledgments
We thank M. Dufour for excellent technical assistance in flow cytometric studies. We are grateful to D. Baltimore for pHXB-Luc, M. Emmerman for pCMV-Hygro, N. Landau for pNL4-3-Luc-E−R+ and pcDNA-1/Ada-M env, T. Springer for pCD1.8, W. C. Greene for 293T cells, R. Rothlein for RR1/1.1.1 and R6.5 antibodies, and C. C. Fidgor for NKI-L16 antibodies. We are indebted to the NIH AIDS Research and Reference Reagent Program for kindly providing the following items: PM1 and Jurkat-tat cells and the anti-gp120 F105 antibody.
This work was supported by a grant to M.J.T. from the Medical Research Council of Canada (MRC) (grant MT-14438). M.J.T. is the recipient of a scholarship award from the Fonds de la Recherche en Santé du Québec. J.-S.P. and J.-F.F. hold Ph.D. fellowships from the Natural Sciences and Engineering Research Council of Canada and the MRC, respectively.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Arthur L O, Bess J W J, Sowder II R C, Benveniste R E, Mann D L, Cherman J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implication for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 3.Benkirane M, Blanc-Zouaoui D, Hirn M, Devaux C. Involvement of human leukocyte antigen class I molecules in human immunodeficiency virus infection of CD4-positive cells. J Virol. 1994;68:6332–6339. doi: 10.1128/jvi.68.10.6332-6339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:S3–S16. [PubMed] [Google Scholar]
- 5.Bess J W, Powell P J, Issaq H J, Schumack L J, Grimes M K, Henderson L E, Arthur L O. Tightly bound zinc in human immunodeficiency virus type 1, human T-cell leukemia virus type 1, and other retroviruses. J Virol. 1992;66:840–847. doi: 10.1128/jvi.66.2.840-847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bess J W J, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;15:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttke T M, McCubrey J A, Owen T C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods. 1993;157:233–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- 10.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 11.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantin R, Fortin J-F, Tremblay M. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology. 1996;218:372–381. doi: 10.1006/viro.1996.0206. [DOI] [PubMed] [Google Scholar]
- 13.Capobianchi M R, Fais S, Castilletti C, Gentile M, Ameglio F, Dianzani F. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J Infect Dis. 1994;169:886–889. doi: 10.1093/infdis/169.4.886. [DOI] [PubMed] [Google Scholar]
- 14.Caputo A, Sodroski J G, Haseltine W A. Constitutive expression of HIV-1 Tat protein in human Jurkat T cells using a BK virus vector. J Acquired Immune Defic Syndr. 1990;3:372–379. [PubMed] [Google Scholar]
- 15.Carlos T M, Harlan J M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 16.Castilletti C, Capobianchi M R, Fais S, Abbate I, Ficociello B, Ameglio F, Fei P C, Santini S M, Dianzani F. HIV type 1 grown on interferon γ-treated U937 cells shows selective increase in virion-associated intercellular adhesion molecule 1 and HLA-DR and enhanced infectivity for CD4-negative cells. AIDS Res Hum Retroviruses. 1995;11:547–553. doi: 10.1089/aid.1995.11.547. [DOI] [PubMed] [Google Scholar]
- 17.Chen B K, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 19.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G L, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 20.Chrystie I L, Almeida J D. Further studies of HIV morphology by negative staining. AIDS. 1988;2:459–464. doi: 10.1097/00002030-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4(T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 22.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 23.Diamond M S, Springer T A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 24.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 25.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 26.Fais S, Capobianchi M R, Abbate I, Catilletti C, Gentile M, Fei P C, Ameglio F, Dianzani F. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with co-polarization of intercellular adhesion molecules (ICAM-1) and HIV-1 viral matrix protein. AIDS. 1995;9:329–335. [PubMed] [Google Scholar]
- 27.Feng Y, Broder C C, Kennedey P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 28.Fenyö E M, Morfeldt-Manson L, Chiodi F, Lind B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folks T, Kelly J, Benn S, Kinter A, Justement J, Gold J, Redfield R, Sell K W, Fauci A S. Susceptibility of normal human lymphocytes to infection with HTLV-III/LAV. J Immunol. 1986;136:4049–4053. [PubMed] [Google Scholar]
- 30.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fortin J-F, Cantin R, Tremblay M. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger H W D, Dierich M P. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–1620. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Gelderblom H, Reupke H, Winkel T, Kunze R, Pauli G. MHC-antigens: constituents of the envelopes of human and simian immunodeficiency viruses. Z Naturforsch. 1987;42:1328–1334. doi: 10.1515/znc-1987-11-1230. [DOI] [PubMed] [Google Scholar]
- 34.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A S, Burke D S, Skillman D, Meltzer M S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gluschankof P, Mondor I, Gelderblom H S, Sattentau Q J. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 36.Gomez M B, Hildreth J E K. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–4632. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedman H, Lundgren E. Regulation of LFA-1 activity in human B cells. J Immunol. 1992;149:2295–2299. [PubMed] [Google Scholar]
- 38.Henderson L E, Sowder R, Copeland T D, Oroszlan S, Arthur L O, Robey W G, Fischinger P J. Direct identification of class II histocompatibility DR proteins in preparations of human T-cell lymphotropic virus type III. J Virol. 1987;61:629–632. doi: 10.1128/jvi.61.2.629-632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hockley D J, Wood R D, Jacobs J P, Garrett A J. Electron microscopy of human immunodeficiency virus. J Gen Virol. 1988;69:2455–2469. doi: 10.1099/0022-1317-69-10-2455. [DOI] [PubMed] [Google Scholar]
- 40.Hoxie J, Fitzharris T P, Yougbar P R, Matthews D M, Rackowski J L, Radka S F. Nonrandom association of cellular antigens with HTLV-III virions. Hum Immunol. 1987;18:39–52. doi: 10.1016/0198-8859(87)90111-x. [DOI] [PubMed] [Google Scholar]
- 41.Kanner S B, Grosmaire L S, Ledbetter J A, Damle N K. β2-Integrin LFA-1 signaling through phospholipase C-γ1 activation. Proc Natl Acad Sci USA. 1993;90:7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keizer G D, Visser W, Vliem M, Figdor C C. A monoclonal antibody (NKI-L16) directed against a unique epitope on the α-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988;140:1393–1400. [PubMed] [Google Scholar]
- 43.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 44.Lasky L, Nakamura G, Smith D H, Fernie C, Shimasaki C, Patzer E, Berman P, Gregory T, Capon D J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987;50:975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- 45.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maréchal V, Clavel F, Heard J-M, Schwartz O. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merloo T, Sheikh M A, Bloem A C, de Ronde A, Schutten M, van Els C A C, Roholl P J M, Joling P, Goudsmit J, Schuurman H-J. Host cell membrane proteins on human immunodeficiency virus type 1 after in vitro infection of H9 cells and blood mononuclear cells. J Gen Virol. 1993;74:129–135. doi: 10.1099/0022-1317-74-1-129. [DOI] [PubMed] [Google Scholar]
- 48.Merloo T H, Parmentier H K, Osterhaus A D M E, Goudsmit J, Schuurman H-J. Modulation of cell surface molecules during HIV-1 infection of H9 cells. An immunoelectron microscopic study. AIDS. 1992;6:1105–1116. doi: 10.1097/00002030-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Orentas R J, Hildreth J E K. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 50.Pantaleo G, Graziosi C, Butini L, Pizzo P A, Schnittman S M, Kotler D P, Fauci A S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pantaleo G, Graziosi C, Fauci A S. The immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 52.Parrot D M, Wilkinson P C. Lymphocyte locomotion and migration. Prog Allergy. 1981;28:193–284. [PubMed] [Google Scholar]
- 53.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8932–8936. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Posner M R, Cavacini C A, Emes C L, Power J, Bym R. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J Acquired Immune Defic Syndr. 1993;6:7–14. [PubMed] [Google Scholar]
- 55.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothlein R, Dustin M L, Marlin S D, Springer T A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 57.Rouse R V, Ledbetter J A, Weissman I L. Mouse lymph node germinal centers contain a selected subset of T cells—the helper phenotype. J Immunol. 1982;128:2243–2246. [PubMed] [Google Scholar]
- 58.Saarloos M-N, Sullivan B L, Czerniewski M A, Parameswar K D, Spear G T. Detection of HLA-DR associated with monocytotropic, primary, and plasma isolates of human immunodeficiency virus type 1. J Virol. 1997;71:1640–1643. doi: 10.1128/jvi.71.2.1640-1643.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Atkinson J P, Spear G T. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders M E, Makgoba M W, Sharrow S O, Stephany D, Springer T A, Young H A, Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-γ production. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 61.Sattentau Q J. CD4 activation of HIV fusion. Int J Cell Cloning. 1992;10:323–332. doi: 10.1002/stem.5530100603. [DOI] [PubMed] [Google Scholar]
- 62.Schols D, Pauwels R, Desmyter J, De Clerk E. Presence of class II histocompatibility DR proteins on the envelope of human immunodeficiency virus demonstrated by FACS analysis. Virology. 1992;189:374–376. doi: 10.1016/0042-6822(92)90719-6. [DOI] [PubMed] [Google Scholar]
- 63.Staunton D E, Marlin S D, Stratowa C, Dustin M L, Springer T. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin families. Cell. 1988;52:925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- 64.Tremblay M J, Fortin J-F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 65.Trowbridge I A, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 66.van de Stolpe A, Caldenhoven E, Stade B G, Koenderman L, Raaijmakers J A M, Johnson J P, van der Saag P T. 12-O-Tetradecanoylphorbol-13-acetate- and tumor necrosis factor α-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. J Biol Chem. 1994;269:6185–6192. [PubMed] [Google Scholar]
- 67.Woska J R J, Morelock M M, Jeanfavre D D, Bormann B-J. Characterization of molecular interactions between intercellular adhesion molecule-1 and leukocyte function-associated antigen-1. J Immunol. 1996;158:4680–4685. [PubMed] [Google Scholar]
- 68.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 69.Zack J A, Cann A J, Lugo J P, Chen I S Y. HIV-1 production from infected peripheral blood T cells after HTLV-I induced mitogenic stimulation. Science. 1988;240:1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]
- 70.Zack J A, Haislip A M, Krogstad P, Chen I S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]