Abstract
We tested infectious human immunodeficiency virus type 1 (HIV-1), noninfectious but conformationally authentic inactivated whole HIV-1 virions, and purified gp120 for the ability to induce depletion of CD4+ T cells in human lymphoid tissues ex vivo. Infectious CXCR4-tropic HIV-1, but not matched inactivated virions or gp120, mediated CD4+ T-cell depletion, consistent with mechanisms requiring productive infection.
The critical events in human immunodeficiency virus (HIV) disease occur in human lymphoid tissue, where HIV infection is associated with a cumulatively massive depletion of CD4+ T lymphocytes. Since HIV was first cultured, it has been known that lytic infection can directly kill infected cells in vitro. Other killing mechanisms, both direct and indirect, have also been proposed (1, 4, 5, 7, 11–14, 16, 18–23, 26–30). It remains controversial, however, whether and to what extent uninfected cells are killed. It has been proposed that some indirect cell killing may be mediated by interactions between bystander cells and HIV surface molecules (2, 6, 15, 17, 24, 26, 31). We combined a unique ex vivo culture system and a novel means of inactivating HIV type 1 (HIV-1) to test whether depletion of CD4+ T cells in human lymphoid tissue depends on viral replication or can be triggered by virions or viral proteins, even in the absence of productive infection.
We have shown previously that blocks of human lymphoid tissue cultured ex vivo support productive infection with HIV-1 without exogenous stimulation (8–10). In this system, infection with T-cell/CXCR4-tropic (X4) (3) but not with macrophage/CCR5-tropic (R5) (3) HIV-1 isolates results in CD4+ T-lymphocyte depletion (9). We recently described inactivation of the infectivity of HIV-1 by a novel method which preserves the conformational and functional integrity of virion surface proteins (25). Treatment of virions with 2,2′-dithiodipyridine (aldrithiol-2 [AT-2]; Sigma Chemical Co., St. Louis, Mo.) (100 mM for 1 h; 37°C) inactivates virions by covalent disruption of zinc fingers in the viral nucleocapsid protein (25), required in multiple steps of the viral life cycle. Afterwards, AT-2 is diluted 27,000-fold from treated virions with a centrifugal concentrator with a 500-kDa-cutoff membrane (Amicon, Beverly, Mass.). Experiments with virus-free culture medium spiked with AT-2 showed that the residual concentration of AT-2 did not mediate detectable effects on histoculture cell viability, HIV replication, or B-cell function (as assayed by trypan blue exclusion, HIV p24 enzyme-linked immunosorbent assay [ELISA], or total immunoglobulin G ELISA, respectively.)
We compared X4-tropic infectious virus, AT-2 inactivated virions, and purified gp120 for the ability to deplete CD4+ T cells from ex vivo human tonsil histocultures. HIV-1 strain LAV.04 and purified HIV-1 gp120 (glycosylated or nonglycosylated, from X4 strains LAV.04 and SF2) were obtained through the AIDS Research and Reference Reagent Program, and HIV-1 strain LAI (IIIB) was obtained from the AIDS Vaccine Program (National Cancer Institute, Frederick, Md.). AT-2 treatment eliminated detectable infectivity from virus, demonstrated both by titrations with peripheral blood mononuclear cells, confirming an earlier report (25), and by the finding that tonsil tissues inoculated with inactivated virus did not secrete p24 into culture supernatants (measured by ELISA [AIDS Vaccine Program]).
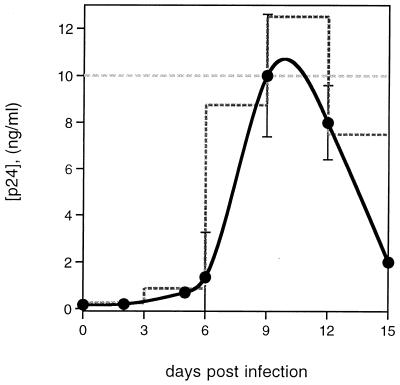
Tonsil cultures were inoculated with either live or inactivated LAV.04 or LAI (IIIB). Infectious virus was applied to 2-mm blocks of human tonsil tissue as a single dose on day 1 of culture, with approximately 400 50% tissue culture infectious doses per block, as described previously (8). The kinetics of HIV replication in this culture system are shown in Fig. 1. Inactivated virus was applied to cultures by either of two protocols. In one, inactivated virus was applied on day 1 and every third day in amounts chosen to approximate the typical growth kinetics for LAV.04 (Fig. 1). Alternatively, inactivated virus was maintained at a constant supernatant concentration (5 to 20 ng of p24/ml) from day 1 of culture (Fig. 1). Results were comparable for the two protocols and for studies using both LAV.04 and LAI (IIIB) viruses.
FIG. 1.
Inactivated and infectious HIV in cultures of human lymphoid tissue ex vivo. The solid line shows accumulation of virus in culture medium of tissue productively infected with HIV-1 LAV.04. Data are the average of 18 experiments. Infection with HIV-1 IIIB in four experiments gave a similar average curve and resulted in similar CD4+ T-cell depletions. The stepped dashed line shows the addition of inactivated virus to culture medium every third day to approximate the productive infection curve. The straight dashed line shows the addition of a constant amount of inactivated virus to culture medium every third day to approximate the peak of productive infection.
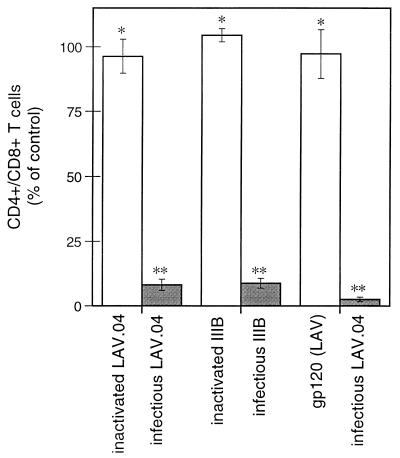
CD4+ T-cell depletion was assessed by the CD4+/CD8+ ratio among T lymphocytes, determined by flow cytometry of cells recovered mechanically from tissue blocks (9). Infection with untreated virus resulted in CD4+ T-cell depletion greater than 90% relative to uninfected controls (Fig. 2), whereas no depletion (95% confidence) was observed in cultures exposed to AT-2-treated virus. Similar results were obtained even when inactivated virus was added at a 100-fold excess over peak concentrations in matched infected cultures (data not shown).
FIG. 2.
Depletion of CD4+ T lymphocytes in lymphoid tissue ex vivo. Shaded bars represent tissues exposed to infectious X4 isolate LAV.04 or LAI (IIIB). Open bars represent tissues exposed to inactivated virus or to gp120. Tissues were analyzed on day 15 of culture, and results are expressed as a percentage relative to uninfected controls from the same donors. Bars represent averages (± standard error of the mean) of data from three to eight experiments, each using tissue from different donors. Measurements in each experiment were based on pooling of 12 to 18 identically treated tissue blocks. ∗, no significant difference from uninfected control (95% confidence); ∗∗, significant difference from uninfected control (P < 0.01).
Results with gp120 were similar to those obtained with inactivated virus. In these experiments, gp120 was incubated with tissues at a concentration of 8 pM, a level found in supernatants of typical infected cultures at peak virus replication. After 15 days of continuous exposure to gp120 from X4 strain LAV.04, no significant CD4+ T-cell depletion was seen (Fig. 2). Incubations with glycosylated and nonglycosylated gp120 from the X4 strain SF2 gave similar results. Moreover, no depletion was seen when gp120 was added to a background of live infection with the R5 virus isolate SF162, which itself does not deplete CD4+ T cells (9).
Inactivation of HIV-1 by AT-2 results in noninfectious virus that is capable of authentic binding to and fusion with target cell membranes but incapable of initiating reverse transcription (25). Such virions should be capable of mediating many of the interactions with cell membranes presumed to be responsible for indirect mechanisms of CD4+ T-cell loss (2, 6, 7, 12, 27). It is conceivable that indirect mechanisms of cell killing require concentrations of virus higher than those examined here and that such higher concentrations might occur in microenvirionments directly adjacent to productively infected cells. However, no depletion of CD4+ T cells was observed, even with exposure to a 100-fold-increased concentration of inactivated virus, which simulated retention of the equivalent of 3 days of peak virus production entirely within the tissue. The absence of CD4+ T-cell depletion under even these extreme conditions strongly implies that CD4+ T-cell killing requires productive HIV infection in ex vivo tonsil histocultures and, by inference, in human lymphoid tissue in vivo.
Acknowledgments
We gratefully acknowledge the technical assistance of Doug Schneider and Gabriela Vasquez in preparing the inactivated virus. We are also grateful to Svetlana Glushakova for advice and to Joshua Zimmerberg for encouragement and support.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000, and by the NASA/NIH Center for Three-Dimensional Tissue Culture.
REFERENCES
- 1.Ameisen J C. Programmed cell death (apoptosis) and AIDS pathogenesis. AIDS Res Hum Retroviruses. 1994;10:S3–S5. [PubMed] [Google Scholar]
- 2.Banda N K, Bernier J, Kurahara D K, Kurrle R, Haigwood N, Sekaly R P, Finkel T H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger E A, Doms R W, Fenyo E-M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 4.Casella C R, Finkel T H. Mechanisms of lymphocyte killing by HIV. Curr Opin Hematol. 1997;4:24–31. doi: 10.1097/00062752-199704010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Clerici M, Sarin A, Berzofsky J A, Landay A L, Kessler H A, Hashemi F, Hendrix C W, Blatt S P, Rusnak J, Dolan M J, Coffman R L, Henkart P A, Shearer G M. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS. 1996;10:603–611. doi: 10.1097/00002030-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 7.Garry R F. Potential mechanisms for the cytopathic properties of HIV. AIDS. 1989;3:683–694. doi: 10.1097/00002030-198911000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Glushakova S, Baibakov B, Margolis L B, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 9.Glushakova S, Baibakov B, Zimmerberg J, Margolis L B. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res Hum Retroviruses. 1997;13:461–471. doi: 10.1089/aid.1997.13.461. [DOI] [PubMed] [Google Scholar]
- 10.Glushakova S, Grivel J C, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis L B. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–349. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 11.Golding H, Robey F A, Gates F T, Linder W, Beining P R, Hoffman T, Golding B. Identification of homologous regions in HIV-1 gp41 and human MHC class II beta 1 domain. I. Monoclonal antibodies against the gp41-derived peptide and patients’ sera react with native HLA class II antigens, suggesting a role for autoimmunity in the pathogenesis of AIDS. J Exp Med. 1988;167:914–923. doi: 10.1084/jem.167.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gougeon M L, Laurent-Crawford A G, Hovanessian A G, Montagnier L. Direct and indirect mechanisms mediating apoptosis during HIV infection: contribution to in vivo CD4 T-cell depletion. Sem Immunol. 1993;5:187–194. doi: 10.1006/smim.1993.1022. [DOI] [PubMed] [Google Scholar]
- 13.Hoxie J A, Alpers J D, Rachowski J L, Huebner K, Haggarty B S, Cedarbaum A J, Reed J C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986;234:1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- 14.Imberti L, Sottini A, Bettinardi A, Puoti M, Primi D. Selective depletion in HIV infection of T-cells that bear specific T-cell receptor V beta sequences. Science. 1991;254:860–862. doi: 10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- 15.Kameoka M, Kimura T, Zheng Y H, Suzuki S, Fujinaga K, Luftig R B, Ikuta K. Protease-defective, gp120-containing human immunodeficiency virus type 1 particles induce apoptosis more efficiently than does wild-type virus or recombinant gp120 protein in healthy donor-derived peripheral blood T cells. J Clin Microbiol. 1997;35:41–47. doi: 10.1128/jcm.35.1.41-47.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent-Crawford A G, Krust B, Müller S, Rivire Y, Rey-Cuille M A, Bechet J M, Montagnier L, Hovanessian A G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;189:695–715. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 17.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 18.Leonard R, Zagury D, Disportes I, Bernard J, Zagury J F, Gallo R C. Cytopathic effect of human immunodeficiency virus in T4 cells is linked to the last stage of virus infection. Proc Natl Acad Sci USA. 1988;85:3570–3574. doi: 10.1073/pnas.85.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lifson J D, Reyes G R, McGrath M S, Stein B S, Engleman E G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986;232:1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- 20.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 21.Pauza C D, Galinde J E, Richman D D. Reinfection results in accumulation of unintegrated viral DNA in cytopathic and persistent human immunodeficiency virus type 1 infection of CEM cells. J Exp Med. 1990;172:1035–1042. doi: 10.1084/jem.172.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popovic M, Sarngadharan M G, Read E. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 23.Purvis S F, Jacobberger J W, Sramkoski R M, Patki A H, Lederman M M. HIV type 1 Tat protein induces apoptosis and death in Jurkat cells. AIDS Res Hum Retroviruses. 1995;11:443–450. doi: 10.1089/aid.1995.11.443. [DOI] [PubMed] [Google Scholar]
- 24.Rasheed S, Gottlieb A A, Garry R F. Cell killing by ultraviolet-inactivated human immunodeficiency virus. Virology. 1986;154:395–400. doi: 10.1016/0042-6822(86)90465-4. [DOI] [PubMed] [Google Scholar]
- 25.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W, Jr, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q, Arthur L O, Henderson L E, Lifson J D. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sodroski J, Haseltine W, Kowalski M. Role of the human immunodeficiency virus type I envelope glycoprotein in cytopathic effect. Adv Exp Med Biol. 1991;300:200–201. doi: 10.1007/978-1-4684-5976-0_12. [DOI] [PubMed] [Google Scholar]
- 27.Sodroski J, Goh W C, Rosen C, Campbell K, Haseltine W A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 28.Somasundaran M, Zapp M L, Beattie L K, Pang L, Byron K S, Bassell G J, Sullivan J L, Singer R H. Localization of HIV RNA in mitochondria of infected cells: potential role in cytopathogenicity. J Cell Biol. 1994;126:1353–1360. doi: 10.1083/jcb.126.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temin H M. Mechanisms of cell killing/cytopathic effects by nonhuman retroviruses. Rev Infect Dis. 1988;10:399–405. doi: 10.1093/clinids/10.2.399. [DOI] [PubMed] [Google Scholar]
- 30.Terai C, Kornbluth S, Pauza C D, Richman D D, Carson D A. Apoptosis is a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Investig. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]