Abstract
Background
Preterm birth is a major complication of pregnancy associated with perinatal mortality and morbidity. Progesterone for the prevention of preterm labour has been advocated.
Objectives
To assess the benefits and harms of progesterone for the prevention of preterm birth for women considered to be at increased risk of preterm birth and their infants.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (14 January 2013) and reviewed the reference list of all articles.
Selection criteria
Randomised controlled trials, in which progesterone was given for preventing preterm birth.
Data collection and analysis
Two review authors independently evaluated trials for methodological quality and extracted data.
Main results
Thirty‐six randomised controlled trials (8523 women and 12,515 infants) were included.
Progesterone versus placebo for women with a past history of spontaneous preterm birth Progesterone was associated with a statistically significant reduction in the risk of perinatal mortality (six studies; 1453 women; risk ratio (RR) 0.50, 95% confidence interval (CI) 0.33 to 0.75), preterm birth less than 34 weeks (five studies; 602 women; average RR 0.31, 95% CI 0.14 to 0.69), infant birthweight less than 2500 g (four studies; 692 infants; RR 0.58, 95% CI 0.42 to 0.79), use of assisted ventilation (three studies; 633 women; RR 0.40, 95% CI 0.18 to 0.90), necrotising enterocolitis (three studies; 1170 women; RR 0.30, 95% CI 0.10 to 0.89), neonatal death (six studies; 1453 women; RR 0.45, 95% CI 0.27 to 0.76), admission to neonatal intensive care unit (three studies; 389 women; RR 0.24, 95% CI 0.14 to 0.40), preterm birth less than 37 weeks (10 studies; 1750 women; average RR 0.55, 95% CI 0.42 to 0.74) and a statistically significant increase in pregnancy prolongation in weeks (one study; 148 women; mean difference (MD) 4.47, 95% CI 2.15 to 6.79). No differential effects in terms of route of administration, time of commencing therapy and dose of progesterone were observed for the majority of outcomes examined.
Progesterone versus placebo for women with a short cervix identified on ultrasound Progesterone was associated with a statistically significant reduction in the risk of preterm birth less than 34 weeks (two studies; 438 women; RR 0.64, 95% CI 0.45 to 0.90), preterm birth at less than 28 weeks' gestation (two studies; 1115 women; RR 0.59, 95% CI 0.37 to 0.93) and increased risk of urticaria in women when compared with placebo (one study; 654 women; RR 5.03, 95% CI 1.11 to 22.78). It was not possible to assess the effect of route of progesterone administration, gestational age at commencing therapy, or total cumulative dose of medication.
Progesterone versus placebo for women with a multiple pregnancy Progesterone was associated with no statistically significant differences for the reported outcomes.
Progesterone versus no treatment/placebo for women following presentation with threatened preterm labour Progesterone, was associated with a statistically significant reduction in the risk of infant birthweight less than 2500 g (one study; 70 infants; RR 0.52, 95% CI 0.28 to 0.98).
Progesterone versus placebo for women with 'other' risk factors for preterm birth Progesterone, was associated with a statistically significant reduction in the risk of infant birthweight less than 2500 g (three studies; 482 infants; RR 0.48, 95% CI 0.25 to 0.91).
Authors' conclusions
The use of progesterone is associated with benefits in infant health following administration in women considered to be at increased risk of preterm birth due either to a prior preterm birth or where a short cervix has been identified on ultrasound examination. However, there is limited information available relating to longer‐term infant and childhood outcomes, the assessment of which remains a priority.
Further trials are required to assess the optimal timing, mode of administration and dose of administration of progesterone therapy when given to women considered to be at increased risk of early birth.
Keywords: Female; Humans; Pregnancy; 17‐alpha‐Hydroxyprogesterone; 17‐alpha‐Hydroxyprogesterone/administration & dosage; 17‐alpha‐Hydroxyprogesterone/adverse effects; Pregnancy, High‐Risk; Premature Birth; Premature Birth/prevention & control; Prenatal Care; Prenatal Care/methods; Progesterone; Progesterone/administration & dosage; Progesterone/adverse effects; Randomized Controlled Trials as Topic
Plain language summary
Prenatal administration of progesterone to prevent preterm birth in women considered to be at risk of having their baby early
Babies who are born before 37 weeks, and particularly those born before 34 weeks, are at greater risk of having problems at birth and complications in infancy. Infants who are born preterm are at greater risk of dying in their first year of life, and of those infants who survive, there is an increased risk of repeated admission to hospital and adverse outcomes including cerebral palsy and long‐term disability. Progesterone is a hormone that reduces contractions of the uterus and has an important role in maintaining pregnancy and is suggested for the prevention of preterm labour. Maternal side‐effects from progesterone therapy include headache, breast tenderness, nausea, cough and local irritation if administered intramuscularly. At present, there is little information available regarding the optimal dose of progesterone, mode of administration, gestation to commence therapy, or duration of therapy.
The review of 36 randomised controlled trials, involving a total of 8523 women considered to be at increased risk of preterm birth, and 12,515 infants, found that where progesterone was given (by injection into the muscle in some studies and as a pessary into the vagina in others), it had beneficial effects, including reducing the risk of the baby dying after birth, suffering complications such as requiring assisted ventilation, necrotising enterocolitis or requiring admission to neonatal intensive care, prolonging the pregnancy, and reducing the chance of neonatal intensive care admission.
Information related to longer‐term infant and childhood outcomes was limited. Overall, the trials included in this review were considered to be of good to fair quality. Further trials are required to assess the optimal timing, mode of administration and dose of administration of progesterone therapy.
Background
Description of the condition
Preterm birth before 37 weeks' gestation is a common problem in obstetric care, with estimates ranging from 5% in several European countries to 18% in some African countries (Blencowe 2012). In Australia, approximately 8% of all infants were born preterm in 2000, with 2.7% of these births occurring prior to 34 weeks' gestation (AIHW 2003). Figures are similar for the United States, with a preterm birth rate of 12.0% (Blencowe 2012). While less than 2% of these infants were born prior to 32 weeks' gestation (Martin 2003), they are at increased risk of complications in infancy, and contribute in excess of 50% of the overall perinatal mortality (AIHW 2003). Infants who are born preterm are at greater risk of dying in their first year of life (Martin 2003), and of those infants who survive, there is an increased risk of repeated admission to hospital (Elder 1999) and adverse outcomes including cerebral palsy and long‐term disability (Hack 1999; Stanley 1992), creating a significant burden upon the community (Kramer 2000).
The 'cause' of preterm labour is multifactorial in origin, and it is important to consider the role of any identifiable risk factors in a woman's pregnancy.
The most significant and consistently identified risk factor for preterm birth, is a woman's history of previous preterm birth (Adams 2000; Bakketeig 1979; Berkowitz 1993; Bloom 2001; Goldenberg 1998; Kaminski 1973; Kistka 2007; Papiernik 1974; Petrini 2005; Robinson 2001). Estimates suggest the rate of recurrent preterm birth in this group of women to be 22.5% (Petrini 2005), a 2.5 times increased risk ratio when compared with women with no previous spontaneous preterm birth (Mercer 1999). For women with a history of a single preterm birth, the recurrence risk in a subsequent pregnancy is approximately 15%, increasing to 32% where there have been two previous preterm births (Carr‐Hill 1985). Information derived from population‐based cohort data suggests that for women who give birth between 20 and 31 weeks' gestation in one pregnancy, 29.3% will give birth prior to 37 weeks in a subsequent pregnancy (Adams 2000). For approximately 10% of these women, the preterm birth will occur at a similar gestational age (Adams 2000; Kistka 2007). In up to 50% of cases of preterm birth, the cause is spontaneous onset of labour or preterm premature rupture of membranes (PPROM) (Hewitt 1988; Mattison 2001; McLaughlin 2002).
Other characteristics in a woman's current pregnancy may place her at increased risk of preterm birth, including women with a short cervix identified by ultrasound assessment, the presence of fetal fibronectin in the vaginal secretions, and presentation with symptoms or signs of threatened preterm labour.
The identification of a short cervix (considered to be less than 2.5cm) on ultrasound examination has been associated with an increased likelihood of preterm birth before 34 weeks’ gestation (Smith 2007). Identification of fetal fibronectin present in cervico‐vaginal secretions has also been proposed as a means of identifying women at risk of preterm birth. Overall, the value of fetal fibronectin in women presenting with symptoms of threatened preterm labour, is a negative test, where women are unlikely to proceed to preterm birth before 34 weeks’ gestation or within seven days of testing (Smith 2007).
Multiple pregnancy is a strong risk factor for preterm birth though the mechanisms may be different to those operating in women with a singleton pregnancy. Up to 50% of women with a twin pregnancy will give birth prior to 37 weeks' gestation (AIHW 2003). The preterm birth risk of early birth before 37 weeks for women with a singleton pregnancy is 6.3% compared with 97% for women with a triplet pregnancy (AIHW 2003).
Description of the intervention
Progesterone may be administered in various forms and by various routes. These different formulations and modes of administration will have different absorption patterns and potentially have differing bio effects. Whilst no teratogenic effects have been described with most progesterones, there is little in the way of long‐term safety data. Maternal side‐effects from progesterone therapy include headache, breast tenderness, nausea, cough and local irritation if administered intramuscularly. At present, there is little information available regarding the optimal dose of progesterone, mode of administration, gestation to commence therapy, or duration of therapy (Greene 2003; Iams 2003).
How the intervention might work
Progesterone has a role in maintaining pregnancy (Haluska 1997; Pepe 1995; Pieber 2001) and is thought to act by suppressing smooth muscle activity in the uterus (Astle 2003; Grazzini 1998). In many animal species, there is a reduction in the amount of circulating progesterone before the onset of labour. While these changes have not been shown to occur in women (Astle 2003; Block 1984; Lopez‐Bernal 2003; Pieber 2001; Smit 1984), it has been suggested that there is a 'functional' withdrawal of progesterone related to changes in the expression of progesterone receptors in the uterus (Astle 2003; Condon 2003; Haluska 2002; Pieber 2001). There have been recent reports in the literature advocating the use of progesterone to reduce the risk of preterm birth (da Fonseca 2003; Meis 2003), rekindling interest that dates back to the 1960s (Le Vine 1964).
This review was modified in 2006, from the original protocol published in The Cochrane Library in Issue 4, 2004, in order to clarify the scope of the review. The title and objectives changed, and the description of participants expanded to include the reason the women were considered to be at increased risk of preterm birth. The primary outcome measure of preterm birth less than 32 weeks' gestation has been changed to preterm birth less than 34 weeks' gestation to be consistent with World Health Organization definitions of preterm birth. Secondary outcome measures reflecting childhood developmental assessment have been added, reflecting the need for ongoing evaluation of children participating in randomised trials.
Why it is important to do this review
Preterm birth and its consequences for women and their babies is a significant health problem in pregnancy and childbirth. While the suppression or prevention of preterm labour should lead to improved survival through a lower incidence of premature delivery, there are theoretical reasons why a fetus may not survive without disability. It is possible that an intrauterine mechanism that would trigger preterm labour could also cause neurological injury to the fetus and that progesterone may prevent labour but not fetal injury. The purpose of this review is to assess the benefits and harms of progesterone administration for the prevention of preterm birth for both women and their infants, when considering the risk factors present for preterm birth.
Objectives
To assess the benefits and harms of progesterone administration for the prevention of preterm birth in women and their infants.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials, in which progesterone was administered for the prevention of preterm birth, subdivided by the reason women were considered to be at risk for preterm birth.
Trials were excluded if:
they utilised quasi‐randomised methodology; cross‐over design;
progesterone was administered for the acute treatment of actual or threatened preterm labour (that is, where progesterone was administered as an acute tocolytic medication); or
progesterone was administered in the first trimester only for preventing miscarriage.
Types of participants
Pregnant women considered to be at increased risk of preterm birth. These reasons include:
past history of spontaneous preterm birth (including preterm prelabour rupture of membranes);
multiple pregnancy;
ultrasound identified short cervical length;
fetal fibronectin testing;
following acute presentation with symptoms or signs of threatened preterm labour (where a tocolytic medication may have been administered);
other reason considered to be at increased risk of preterm birth.
Types of interventions
Administration of progesterone by any route for the prevention of preterm birth.
Types of outcome measures
Primary outcomes
Perinatal mortality
Preterm birth (less than 34 weeks' gestation)
Major neurodevelopmental handicap at childhood follow‐up
Secondary outcomes
Maternal
Threatened preterm labour (as defined by trial authors)
Prelabour spontaneous rupture of membranes
Adverse drug reaction
Pregnancy prolongation (interval between randomisation and birth)
Mode of birth
Number of antenatal hospital admissions
Satisfaction with the therapy
Use of tocolysis
Antenatal corticosteroids (not a prespecified outcome)
Maternal quality of life (not a prespecified outcome)
Infant
Birth before 37 completed weeks
Birth before 28 completed weeks
Birthweight less than the third centile for gestational age
Birthweight less than 2500 g
Apgar score of less than seven at five minutes
Respiratory distress syndrome
Use of mechanical ventilation
Duration of mechanical ventilation
Intraventricular haemorrhage ‐ grades III or IV
Periventricular leucomalacia
Retinopathy of prematurity
Retinopathy of prematurity ‐ grades III or IV
Chronic lung disease
Necrotising enterocolitis
Neonatal sepsis
Fetal death
Neonatal death
Admission to neonatal intensive care unit
Neonatal length of hospital stay
Teratogenic effects (including virilisation in female infants)
Patent ductus arteriosis (not a prespecified outcome)
Child
Major sensorineural disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than ‐2 standard deviations below mean))
Developmental delay (however defined by the authors)
Intellectual impairment
Motor impairment
Visual impairment
Blindness
Deafness
Hearing impairment
Cerebral palsy
Child behaviour
Child temperament
Learning difficulties
Growth assessments at childhood follow‐up (weight, head circumference, length, skin fold thickness)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (14 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of searching carried out for the initial version of the review, please see Appendix 1.
Searching other resources
We also manually cross‐referenced key publications.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Appendix 2.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted third author. We entered data into Review Manager software (RevMan 2012) and checked for accuracy. When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was resolved by discussion or by involving the third author.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; or less than 20% losses to follow‐up);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether studies that included multiple pregnancies accounted appropriately for non‐independence of babies from the same pregnancy in the analysis. There are several ways this can be done, and these studies should present something like an odds ratio adjusted for non‐independence. If adjustment was not done, we assessed the potential for bias i.e. if multiples only made up a small proportion of the total then there is probably not much potential for bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods, if required.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but we may include trials of this type in future updates. If we do, we plan to include cluster‐randomised trials in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in the Cochrane Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not included.
Other unit of analysis issues
The analysis in this review involves multiple pregnancies, therefore, wherever possible, analyses should be adjusted for clustering to take into account the non‐independence of babies from the same pregnancy (Gates 2004). Treating babies from multiple pregnancies as if they are independent, when they are more likely to have similar outcomes than babies from different pregnancies, will overestimate the sample size and give confidence intervals that are too narrow. Each woman can be considered a cluster in multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster trial methods allows calculation of relative risk and adjustment of confidence intervals. Usually this will mean that the confidence intervals get wider. Although this may make little difference to the conclusion of a trial, it avoids misleading results in those trials where the difference may be substantial.
We planned to adjust for clustering in the analyses, wherever possible, and to use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, due to insufficient information in the included trials, we were not able to adjust our analyses. In future updates, if possible, we will adjust for clustering in the analyses.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If 10 or more studies contributed data to meta‐analysis for any particular outcome, we investigated reporting biases (such as publication bias) using funnel plots. We assessed possible asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it. In this version of the review insufficient data were available to allow us to carry out this planned analysis.
Data synthesis
We carried out statistical analysis using the RevMan software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and where we judged the trials’ populations and methods to be sufficiently similar. If we suspected clinical heterogeneity sufficient to expect the underlying treatment effects to differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary provided that we considered an average treatment effect across trials was clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and the clinical implications of treatment effects differing between trials is discussed. If the average treatment effect was not clinically meaningful we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Results were analysed according to the reason women were considered to be at risk of preterm birth, including:
past history of spontaneous preterm birth (including preterm prelabour rupture of membranes);
multiple pregnancy;
ultrasound identified short cervical length;
fetal fibronectin testing;
presentation with symptoms or signs of threatened preterm labour;
other reason for risk of preterm birth.
For analyses where there are high levels of heterogeneity we have provided an estimate of the 95% range of underlying intervention effects (prediction interval).
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it. We planned, where possible, to carry out the following subgroup analyses:
time of treatment commencing (before 20 weeks' gestation versus after 20 weeks' gestation);
route of administration (intramuscular, intravaginal, oral, intravenous);
different dosage regimens (divided arbitrarily into a cumulative dose of less than 500 mg per week and a dose of greater than or equal to 500 mg per week).
All outcomes were considered in subgroup analyses.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012).
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both, with poor‐quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
In the 2006 update, our search strategy identified 22 studies for consideration. Eleven studies met the inclusion criteria stated (Borna 2008; da Fonseca 2003; Facchinetti 2007; Fonseca 2007; Hartikainen 1980; Hauth 1983; Johnson 1975; Meis 2003; O'Brien 2007; Papiernik 1970; Rouse 2007) involving a total of 2714 women and 3452 infants. The study by Northern (Northen 2007) reports the follow‐up of children involved in the Meis study (Meis 2003).
Sixty‐four reports from an updated search in January 2013 have been assessed for this update. Of these 64 reports, an additional 25 studies (33 reports) to the original 11 studies, were included (Aboulghar 2012; Akbari 2009; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; Elsheikhah 2010; Glover 2011; Grobman 2012; Hassan 2011; Ibrahim 2010; Lim 2011; Majhi 2009; Moghtadaei 2008; Ndoni 2010; Norman 2009; Rai 2009; Rode 2011; Rozenberg 2012; Saghafi 2011a; Senat 2012; Serra 2013; Sharami 2010); seven studies (seven reports) were excluded (Abbott 2012; Arikan 2011; Berghella 2010; Chandiramani 2012; Ionescu 2012; Keeler 2009; Rust 2006); two studies (two reports) are ongoing studies (Coomarasamy 2012; van Os 2011); one is an additional report of an ongoing study (Crowther 2007); one additional report was added to each of the following included studies (Combs 2010; Combs 2011; Facchinetti 2007; Lim 2011; Nassar 2007). Two additional reports were identified for the Norman 2009; O'Brien 2007; Rode 2011 and Rouse 2007 included studies. Three additional reports were identified for the Meis 2003 included study.
A total of 36 studies are included in this update.
Included studies
Refer to table Characteristics of included studies for further details.
Use of progesterone in women with a history of prior spontaneous preterm birth
Description of studies
Eleven studies were included involving a total of 1936 women with a past history of spontaneous preterm birth (Akbari 2009; Cetingoz 2011; da Fonseca 2003; Glover 2011; Johnson 1975; Ibrahim 2010; Majhi 2009; Meis 2003; O'Brien 2007; Rai 2009; Saghafi 2011a). Four studies compared weekly intramuscular injection with placebo (Ibrahim 2010; Johnson 1975; Meis 2003) or routine care (Saghafi 2011a); five studies compared daily vaginal progesterone, three with placebo (Cetingoz 2011; da Fonseca 2003; O'Brien 2007) and two with routine care (Akbari 2009; Majhi 2009); and two studies compared daily oral progesterone with placebo (Glover 2011; Rai 2009). Dose of progesterone administered varied from 90 mg daily (O'Brien 2007), to 100 mg daily (Akbari 2009; Cetingoz 2011; da Fonseca 2003; Majhi 2009), to 200 mg daily (Rai 2009), to 400 mg daily (Glover 2011), to 200 mg weekly (Rai 2009), to 250 mg weekly (Johnson 1975; Meis 2003; Saghafi 2011a). Supplementation commenced prior to 20 weeks' gestation in four trials (Glover 2011; Johnson 1975; Meis 2003; O'Brien 2007), and continued up to a gestational age varying from 24 weeks (Johnson 1975; Majhi 2009; Rai 2009), to 28 weeks (da Fonseca 2003), to 34 weeks (Akbari 2009; Cetingoz 2011), to 36 weeks (Ibrahim 2010; Meis 2003), and to 37 weeks (O'Brien 2007; Saghafi 2011a) gestation.
The primary outcomes reported by the trials related to the occurrence of preterm birth prior to 28 weeks' gestation (Rai 2009), 32 weeks' gestation (O'Brien 2007), 34 weeks' gestation (Akbari 2009; Majhi 2009), and 37 weeks' gestation (Akbari 2009; Cetingoz 2011; da Fonseca 2003; Ibrahim 2010; Johnson 1975; Majhi 2009; Meis 2003; Saghafi 2011a). Eight trials involved single centres (Akbari 2009; da Fonseca 2003; Glover 2011; Ibrahim 2010; Johnson 1975; Majhi 2009; Rai 2009; Saghafi 2011a), and two were multicentre trials (Meis 2003; O'Brien 2007), conducted principally from the United States of America (Glover 2011; Johnson 1975; Meis 2003; O'Brien 2007), India (Majhi 2009; Rai 2009), Iran (Akbari 2009; Saghafi 2011a), Egypt (Ibrahim 2010), Istanbul (Cetingoz 2011), and Brazil (da Fonseca 2003). The report by Northen 2007 reports childhood follow‐up of 348 participants in the Meis randomised trial (Meis 2003).
One study (Cetingoz 2011) included a mix of women with a history of prior preterm birth (n = 71) and women with a multiple pregnancy (n = 67) and the results for this study have been analysed separately for the two risk groups.
Use of progesterone in women with a short cervix identified on transvaginal ultrasound examination
Description of studies
Four studies were included involving 1560 women who were identified with a short cervix (various definitions: less than 15 mm (Fonseca 2007); less than 30 mm (Grobman 2012); between 10 and 20mm (Hassan 2011); and less than 25 mm (Rozenberg 2012)) at the time of transvaginal ultrasound examination. One study compared weekly intramuscular injection with placebo (Grobman 2012); one study compared twice weekly intramuscular injection with no treatment (Rozenberg 2012) and two studies compared daily intravaginal progesterone with placebo (Fonseca 2007; Hassan 2011). Dose of progesterone administered varied from 90 mg daily in the morning (Hassan 2011), to 200 mg nightly (Fonseca 2007), to 250 mg weekly (Grobman 2012), to 500 mg twice weekly (Rozenberg 2012). Supplementation commenced from 16 to 22 weeks' gestation in one study (Grobman 2012), from 19 to 23 weeks in another study (Hassan 2011), from 24 to 31 weeks in another study (Rozenberg 2012), and from 24 to 33 completed weeks of gestation in another study (Fonseca 2007).
The primary outcomes reported by the trials related to the occurrence of preterm birth prior to 33 weeks' gestation (Hassan 2011), 34 weeks' gestation (Fonseca 2007), 35 weeks' gestation (Grobman 2012), or 37 weeks' gestation (Grobman 2012) and time from randomisation to delivery in one study (Rozenberg 2012). All trials were multicentre conducted in centres worldwide, including the United Kingdom, USA, France, Greece, Chile and Brazil.
One study (Fonseca 2007) included a mix of singleton and twin pregnancies (226 singleton and 24 twin pregnancies), but due to the small proportion of twin pregnancies in this study, we have analysed all of this data within the short cervix subgroup.
Use of progesterone in women with a multiple pregnancy
Description of studies
Fourteen studies were included involving 3792 women; 11 trials with a twin pregnancy (Aboulghar 2012; Cetingoz 2011; Combs 2011; Elsheikhah 2010; Fonseca 2007; Hartikainen 1980; Norman 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013), two trials with a triplet pregnancy (Caritis 2009; Combs 2010) or one trial with any multiple pregnancy, e.g. twins, triplets or quadruplets (Lim 2011). Six studies compared 250 mg weekly intramuscular progesterone injections with placebo (Caritis 2009; Combs 2010; Combs 2011; Hartikainen 1980; Lim 2011; Rouse 2007); one study compared 1000 mg weekly intramuscular progesterone injections with no treatment (Senat 2012); three studies compared intravaginal progesterone with placebo (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010), one at a daily dose of 100 mg (Cetingoz 2011), one at a daily dose of 200 mg (Elsheikhah 2010) and one at a daily dose of 400 mg Aboulghar 2012); one study compared 90 mg daily intravaginal gel with placebo (Norman 2009); and one study compared 100 mg daily oral progesterone with placebo (Rode 2011). One trial (Serra 2013) consisted of three groups and compared 200 mg daily intravaginal progesterone with 400 mg daily intravaginal progesterone with placebo. Supplementation commenced from 16 to 20 weeks' gestation in three studies (Caritis 2009; Lim 2011; Rouse 2007), from 16 to 22 weeks in one study (Combs 2010), from 16 to 24 weeks in one study (Combs 2011), from 18 to 24 weeks in one study (Aboulghar 2012; Rode 2011), from 24 to 34 weeks in two studies (Cetingoz 2011; Elsheikhah 2010), from 24 weeks' gestation in one study (Norman 2009), and from 28 completed weeks' of gestation in one study (Hartikainen 1980).
The primary outcomes reported by the trials related to the occurrence of preterm birth prior to 34 weeks' gestation (Aboulghar 2012; Cetingoz 2011; Rode 2011; Senat 2012) or 37 weeks' gestation (Aboulghar 2012; Cetingoz 2011; Serra 2013), delivery or fetal loss before 34 weeks' gestation (Norman 2009), delivery or fetal loss before 35 weeks' gestation (Caritis 2009), mean cervical length and mean gestational age at delivery (Elsheikhah 2010), perinatal death (Hartikainen 1980) or composite neonatal morbidity (Combs 2010; Combs 2011; Lim 2011; Senat 2012). Five trials were multicentre conducted in centres worldwide, including the United Kingdom, USA, the Netherlands, Denmark, Austria and France (Caritis 2009; Combs 2010; Combs 2011; Lim 2011; Norman 2009; Rode 2011; Rouse 2007) and four were single‐centre trials conducted in Istanbul, Egypt and Finland (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010; Hartikainen 1980).
Two studies included a mix of women with multiple and singleton pregnancies (Aboulghar 2012; Cetingoz 2011). One study (Aboulghar 2012) included a mix of women with singleton pregnancies (n = 215) and women with a multiple pregnancy (n = 91) all conceived by IVF/ICSI (in vitro fertilisation/intracytoplasmic sperm injection) and the results for this study have been analysed separately for the two risk groups: women at risk of preterm birth for 'other' reasons; and women with a multiple pregnancy. One study (Cetingoz 2011) included a mix of women with a history of prior preterm birth (n = 71) and women with a multiple pregnancy (n = 67) and the results for this study have been analysed separately for the two risk groups.
Use of progesterone in women following symptoms or signs of threatened preterm labour
Description of studies
Six small studies were included involving a total of 505 women presenting with symptoms or signs of threatened preterm labour (Borna 2008; Briery 2011; Combs 2011a; Facchinetti 2007; Ndoni 2010; Sharami 2010). Two studies compared 250 mg weekly progesterone injections with placebo (Briery 2011; Combs 2011a), one study compared vaginal progesterone pessaries on a daily basis (400 mg) with no treatment (Borna 2008), one study compared 341 mg intramuscular progesterone injection every four days with no treatment (Facchinetti 2007), one study had three arms and compared intramuscular progesterone with oral progesterone with placebo (Ndoni 2010) and one study compared vaginal pessaries on a daily basis (200 mg) with placebo pessaries (Sharami 2010). Women presented with symptoms and signs between 24 and 34 weeks' gestation (Borna 2008), between 25 and 33 weeks' gestation (Facchinetti 2007), between 20 and 30 weeks' gestation (Briery 2011), between 23 and 31.9 weeks' gestation (Combs 2011a), between 15 and 22 weeks' gestation (Ndoni 2010) and between 28 and 36 weeks' gestation (Sharami 2010). The primary outcomes reported included the interval from randomisation to birth in one study (Borna 2008), transvaginal ultrasound assessment of cervical length in one study (Facchinetti 2007), gestational age at birth in one study (Briery 2011), prolongation of pregnancy and composite neonatal morbidity in one study (Combs 2011a) and time until delivery and birth before 34 or 37 completed weeks in one study (Sharami 2010). In one study, reported only in abstract form, data relating to outcomes was not reported (Ndoni 2010). One study was a multicentre study conducted in the USA (Combs 2011a) and the remaining five studies were single‐centre studies conducted in Iran, the USA, Italy, and Albania (Borna 2008; Briery 2011; Facchinetti 2007; Ndoni 2010; Sharami 2010).
Use of progesterone in women at risk of preterm birth for 'other' reasons
Description of studies
Papiernik 1970 recruited 99 women from Paris, France, in a single centred trial, with a 'high preterm risk score'. Women were allocated to receive intramuscular progesterone three times per week or placebo, from 28 to 32 weeks' gestation.
Hauth 1983 involved 168 women from the United States of America who were considered to be at risk of preterm birth due to active military service. Women received 1000 mg of progesterone weekly or placebo, from 16 to 20 weeks' gestation, up until 36 weeks' gestation. The primary outcome for the study related to the incidence of preterm birth at less than 37 weeks' gestation.
Moghtadaei 2008 involved 260 women from Iran, in a single centre trial, who were considered to be at risk of preterm birth due to advanced maternal age (greater than 35 years). Women received weekly injections of 17P (250 mg) starting at 16 to 20 weeks' gestation until 34 weeks or matching placebo. The main outcomes for the study included delivery before 37, 35 or 32 weeks' gestation, hypertension, diabetes, intrauterine growth restriction or side effects at the injection site. Data from this study could not be included in a meta‐analysis because the number of women randomised to each group was not reported in the brief abstract report of the study.
Aboulghar 2012 recruited 313 women from Egypt who were considered to be at high risk of preterm birth because all the pregnancies were conceived by IVF or ICSI. Women received vaginal progesterone 200 mg twice daily from randomisation until delivery or 37 weeks’ gestation or matching placebo. The primary outcomes included preterm birth of singleton and twin pregnancies before 37 completed weeks and before 34 completed weeks. This study contains a mix of singleton pregnancies (n = 215) and twin pregnancies (n = 91) and presented some outcome data separately, as well as for the whole group. The results for this study have been analysed separately for the two risk groups: multiple pregnancies and women at risk for 'other' reasons.
Excluded studies
In total, 16 studies were excluded (Abbott 2012; Arikan 2011; Berghella 2010; Breart 1979; Brenner 1962; Chandiramani 2012; Corrado 2002; Hobel 1986; Ionescu 2012; Keeler 2009; Le Vine 1964; Rust 2006; Suvonnakote 1986; Turner 1966; Walch 2005; Yemini 1985). Three studies were excluded as they used a quasi‐randomised method of treatment allocation (Le Vine 1964; Suvonnakote 1986; Yemini 1985). One study (Hobel 1986) compared an oral progestogen with placebo, but presented outcomes only as percentages. Five studies were excluded as progesterone was administered in the first trimester to prevent miscarriage (Breart 1979; Brenner 1962; Corrado 2002; Turner 1966; Walch 2005), and are covered by the Cochrane review relating to the use of progesterone for prevention of miscarriage (Haas 2008). One study was excluded because progesterone was administered as an acute tocolytic medication (Arikan 2011). A further six studies were excluded because they compared progesterone with cerclage (Abbott 2012; Chandiramani 2012; Ionescu 2012; Keeler 2009; Rust 2006) or compared cerclage with no cerclage (Berghella 2010), and are covered by other Cochrane reviews (Alfirevic 2012; Rafael 2011).
Refer to table Characteristics of excluded studies for further details.
Studies awaiting assessment
There are 11 ongoing studies awaiting assessment (Coomarasamy 2012; Creasy 2008; Crowther 2007; Martinez 2007; Nassar 2007; Norman 2012; Perlitz 2007; Starkey 2008; Swaby 2007; van Os 2011; Wood 2007).
Risk of bias in included studies
The overall quality of the included trials varied from good to fair. Refer to table Characteristics of included studies for further details and to Figure 1; Figure 2, for a summary of 'Risk of bias' assessments.
1.
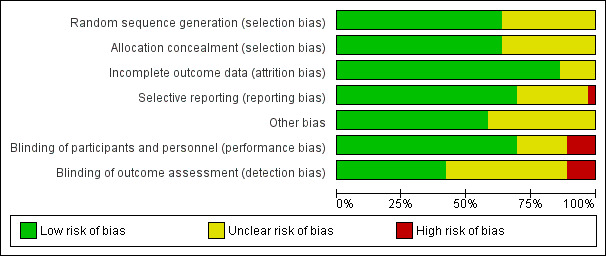
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
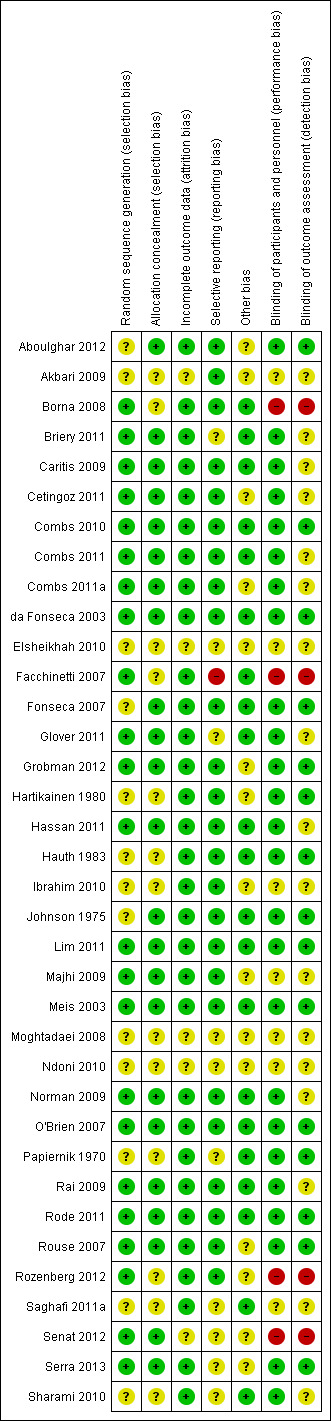
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
While all trials were stated to be randomised and placebo controlled, the method of randomisation was only described in 23 trials (Borna 2008; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Facchinetti 2007; Glover 2011; Grobman 2012; Hassan 2011; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012; Senat 2012; Serra 2013). Allocation concealment was assessed as low risk of bias in 23 trials (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hassan 2011; Johnson 1975; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013); and unclear in 13 trials (Akbari 2009; Borna 2008; Elsheikhah 2010; Facchinetti 2007; Hartikainen 1980; Hauth 1983; Ibrahim 2010; Moghtadaei 2008; Ndoni 2010; Papiernik 1970; Rozenberg 2012; Saghafi 2011a; Sharami 2010).
Blinding
Twenty‐five of the 32 included trials were placebo controlled, with blinding of caregivers and participants (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hartikainen 1980; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Rouse 2007; Serra 2013; Sharami 2010).
Blinding of outcome assessment was evident in 15 of the trials (Aboulghar 2012; Combs 2010; da Fonseca 2003; Fonseca 2007; Grobman 2012; Hartikainen 1980; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; O'Brien 2007; Papiernik 1970; Rode 2011; Rouse 2007; Serra 2013).
Four trials were assessed as high risk of bias for both blinding of caregivers and participants and outcome assessment as no blinding was attempted (Borna 2008; Facchinetti 2007; Rozenberg 2012; Senat 2012).
Incomplete outcome data
Thirty‐one studies were assessed as being at low risk of bias for attrition bias. Thirteen studies reported no losses to follow‐up (Borna 2008; Caritis 2009; Combs 2010; Combs 2011a; Facchinetti 2007; Fonseca 2007; Hartikainen 1980; Hauth 1983; Ibrahim 2010; Majhi 2009; Meis 2003; Papiernik 1970; Saghafi 2011a) and 18 studies reported less than 20% loss to follow‐up (Aboulghar 2012; Briery 2011; Cetingoz 2011; Combs 2011; da Fonseca 2003; Glover 2011; Grobman 2012; Hassan 2011; Johnson 1975; Lim 2011; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012; Serra 2013; Sharami 2010). In five studies reported only in abstract form, it was unclear whether attrition bias was present (Elsheikhah 2010; Grobman 2012; Moghtadaei 2008; Ndoni 2010; Senat 2012) and in one study details were insufficient to make a judgement (Akbari 2009).
Selective reporting
Twenty‐five studies were assessed as being at low risk of bias for selective reporting (Aboulghar 2012; Akbari 2009; Borna 2008; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Grobman 2012; Hartikainen 1980; Hassan 2011; Hauth 1983; Ibrahim 2010; Johnson 1975; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012) as all expected outcomes were reported. One study was assessed as being at high risk of bias, because one of the outcomes was incompletely reported on (Facchinetti 2007) and in one study it was difficult to assess selective reporting based on a translation of the original report (Papiernik 1970). In all the other study reports, it was not possible to determine whether or not selection bias was present (Briery 2011; Elsheikhah 2010; Glover 2011; Moghtadaei 2008; Ndoni 2010; Rozenberg 2012; Saghafi 2011a; Senat 2012; Serra 2013; Sharami 2010).
Other potential sources of bias
Twenty‐one studies were assessed as being at low risk of bias for other potential sources of bias based on baseline characteristics being similar between groups and no other bias apparent (Borna 2008; Briery 2011; Caritis 2009; Combs 2010; Combs 2011; da Fonseca 2003; Facchinetti 2007; Fonseca 2007; Glover 2011; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Saghafi 2011a; Sharami 2010). In the remaining studies, it was not possible to determine whether or not other sources of bias were present (Aboulghar 2012; Akbari 2009; Cetingoz 2011; Combs 2011a; Elsheikhah 2010; Grobman 2012; Hartikainen 1980; Ibrahim 2010; Majhi 2009; Moghtadaei 2008; Ndoni 2010; Rouse 2007; Rozenberg 2012; Senat 2012; Serra 2013).
Assessment of studies that included multiple pregnancies
We assessed whether studies that included multiple pregnancies accounted appropriately for non‐independence of babies from the same pregnancy in the analysis. There were 14 studies that included a multiple pregnancy (Aboulghar 2012; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Elsheikhah 2010; Fonseca 2007; Hartikainen 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013) and in seven studies adjustment appears to have been made in the analysis (Caritis 2009; Combs 2010; Combs 2011; Fonseca 2007; Lim 2011; Norman 2009; Rode 2011). In the remaining seven studies (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010; Hartikainen 1980; Rouse 2007; Senat 2012; Serra 2013), it is not clear that any adjustment was made.
There were insufficient data presented in the trial reports to allow us to carry out necessary adjustment for cluster design effect ourselves and, although in several trials results had already been adjusted, we were not able to present these data in our data and analyses tables because they were not reported in a consistent way.
Effects of interventions
Thirty‐six randomised controlled trials (8523 women and 12,515 infants) in total were included in this review.
Data were only available in a suitable format from 30 randomised controlled trials involving a total of 7561 women and 10,114 infants. Data from these 30 trials contributed data that were included in meta‐analyses. As the aetiology of preterm birth is multifactorial, results are presented according to the reason considered to be at risk for preterm birth (past history of spontaneous preterm birth (including preterm premature rupture of membranes), ultrasound identified short cervical length, multiple pregnancy, prior presentation with threatened preterm labour, and other reason for risk of preterm birth).
Progesterone versus placebo/no treatment for women with a past history of spontaneous preterm birth
Eleven randomised controlled trials involving a total of 1899 women and infants were included in the meta‐analysis.
Primary outcomes
For women administered progesterone during pregnancy, there was a statistically significant reduction in perinatal mortality overall (six studies; 1453 women; risk ratio (RR) 0.50, 95% confidence interval (CI) 0.33 to 0.75), Analysis 1.1. For the primary outcome preterm birth less than 34 weeks' gestation, there was also a statistically significant difference between progesterone when compared with placebo (five studies; 602 women; average RR 0.31, 95% CI 0.14 to 0.69), Analysis 1.2. Substantial heterogeneity was evident for Analysis 1.2 (heterogeneity: Tau² = 0.45, Chi² = 9.15, df = 4 (P = 0.06), I² = 56%) and so a random‐effects model was used. Major neurodevelopmental handicap in childhood was not reported.
1.1. Analysis.
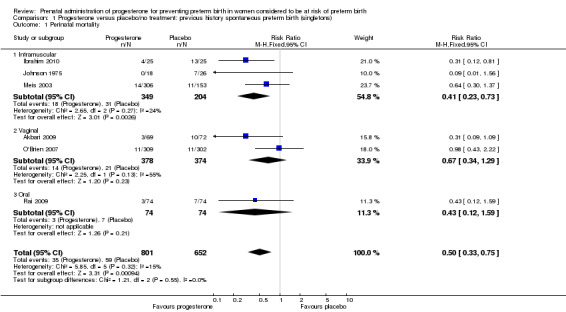
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 1 Perinatal mortality.
1.2. Analysis.
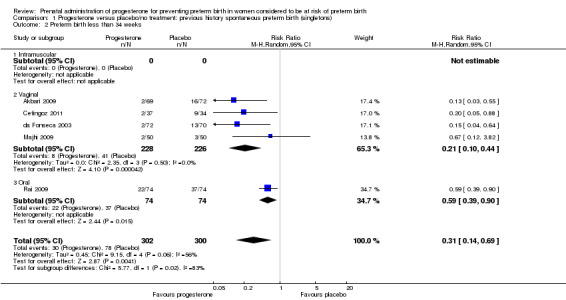
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 2 Preterm birth less than 34 weeks.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, the results showed:
preterm birth less than 37 weeks' gestation (10 studies; 1750 women; average RR 0.55, 95% CI 0.42 to 0.74); considerable heterogeneity was identified, and a random‐effects model was used (heterogeneity: Tau² = 0.11; Chi² = 29.60, df = 9 (P = 0.0005); I² = 70%), Analysis 1.3. This was also evident for the intramuscular subgroup (four studies; 652 women; average RR 0.62, 95% CI 0.52 to 0.75), Analysis 1.3.1. However, for the oral subgroup, no statistically significant differences were observed, Analysis 1.3.3. A funnel plot for this analysis (Figure 3), including the 10 studies was very asymmetrical. This suggests that there may be some important biases or small‐study effects in the set of studies in this analysis and so these results should be viewed with caution.
1.3. Analysis.
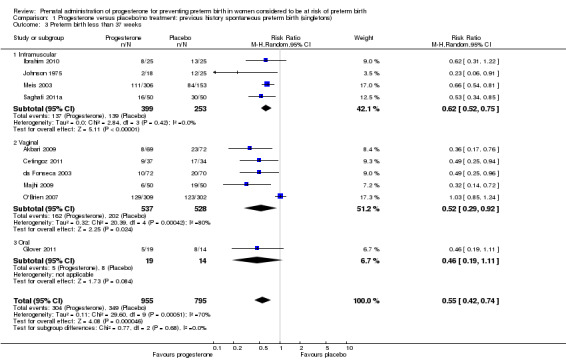
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 3 Preterm birth less than 37 weeks.
3.
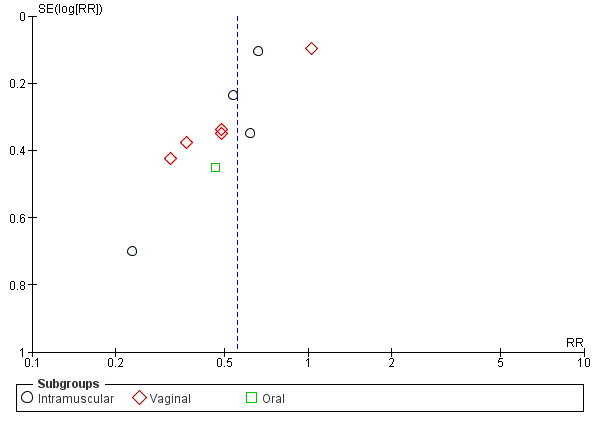
Funnel plot of comparison: 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth, outcome: 1.3 Preterm birth less than 37 weeks.
There was also a statistically significant reduction in the risk of:
infant birthweight less than 2500 g (four studies; 692 infants; RR 0.58, 95% CI 0.42 to 0.79), Analysis 1.9;
use of assisted ventilation (three studies; 633 women; RR 0.40, 95% CI 0.18 to 0.90), Analysis 1.11;
necrotising enterocolitis (three studies; 1170 infants; RR 0.30, 95% CI 0.10 to 0.89), Analysis 1.16;
neonatal death (six studies; 1453 women; RR 0.45, 95% CI 0.27 to 0.76), Analysis 1.20;
admission to neonatal intensive care unit (three studies; 389 women; RR 0.24, 95% CI 0.14 to 0.40), Analysis 1.33.
1.9. Analysis.
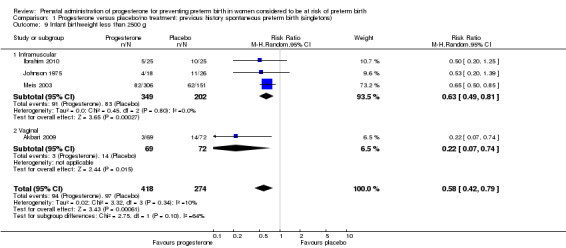
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 9 Infant birthweight less than 2500 g.
1.11. Analysis.
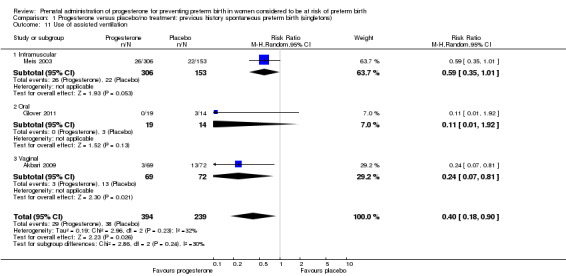
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 11 Use of assisted ventilation.
1.16. Analysis.
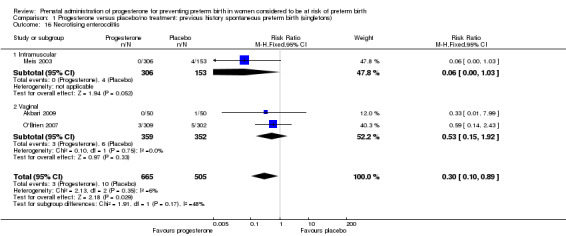
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 16 Necrotising enterocolitis.
1.20. Analysis.
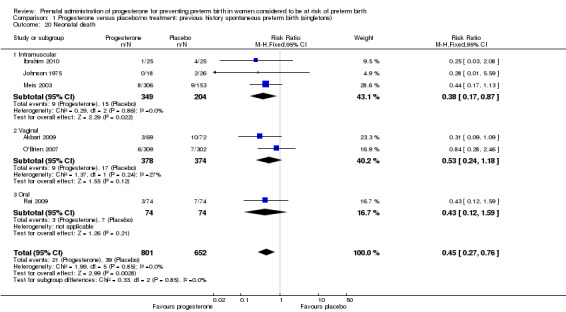
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 20 Neonatal death.
1.33. Analysis.
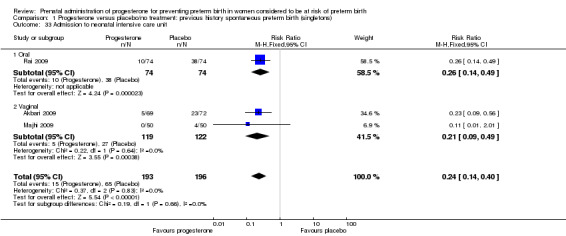
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 33 Admission to neonatal intensive care unit.
For infant outcomes Apgar score less than seven at five minutes Analysis 1.32, respiratory distress syndrome Analysis 1.10, intrauterine fetal death Analysis 1.19, intraventricular haemorrhage (all grades) Analysis 1.12, intraventricular haemorrhage (grade III or IV) Analysis 1.13, periventricular leucomalacia Analysis 1.14, retinopathy of prematurity Analysis 1.15, neonatal sepsis Analysis 1.17, patent ductus arteriosus Analysis 1.18, intrauterine fetal death Analysis 1.19, or neonatal length of hospital stay Analysis 1.34, there were no statistically significant differences identified.
1.32. Analysis.
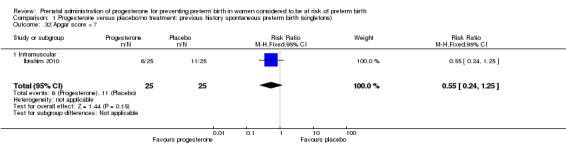
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 32 Apgar score < 7.
1.10. Analysis.
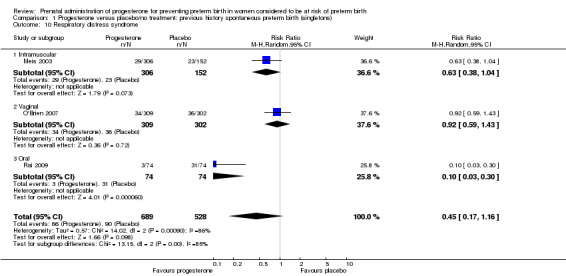
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 10 Respiratory distress syndrome.
1.19. Analysis.
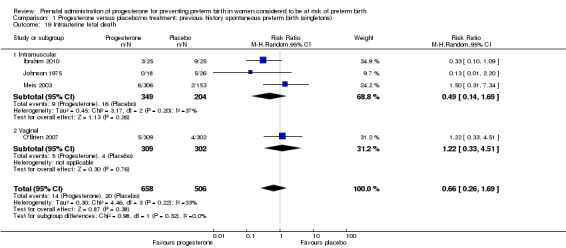
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 19 Intrauterine fetal death.
1.12. Analysis.
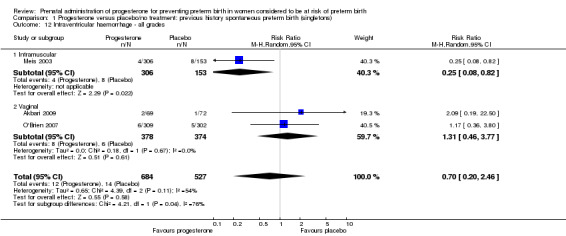
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 12 Intraventricular haemorrhage ‐ all grades.
1.13. Analysis.
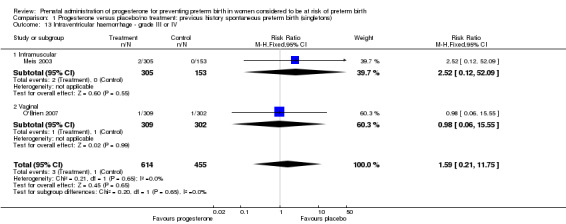
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 13 Intraventricular haemorrhage ‐ grade III or IV.
1.14. Analysis.
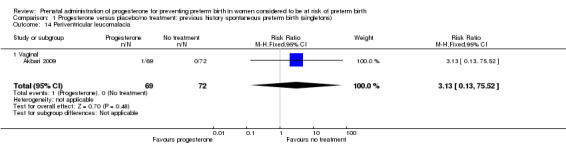
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 14 Periventricular leucomalacia.
1.15. Analysis.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 15 Retinopathy of prematurity.
1.17. Analysis.
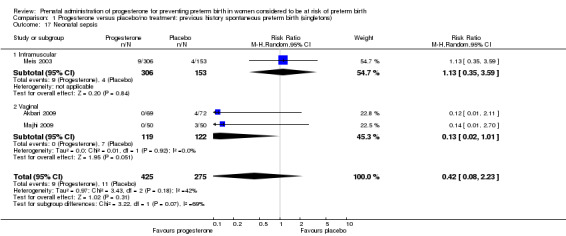
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 17 Neonatal sepsis.
1.18. Analysis.
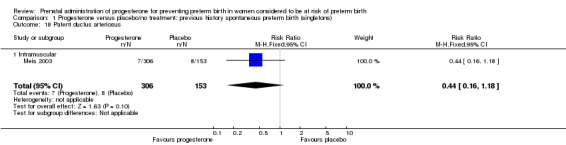
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 18 Patent ductus arteriosus.
1.34. Analysis.
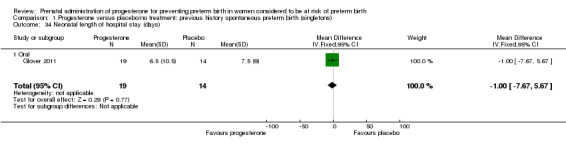
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 34 Neonatal length of hospital stay (days).
Secondary maternal outcomes
For women administered progesterone during pregnancy, when compared with placebo, there was a statistically significant increase in:
pregnancy prolongation in weeks (one study; 148 women; mean difference (MD) 4.47, 95% CI 2.15 to 6.79), Analysis 1.31.
1.31. Analysis.
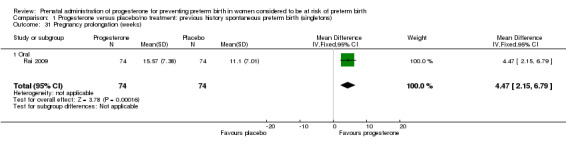
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 31 Pregnancy prolongation (weeks).
There were no statistically significant differences for the outcomes threatened preterm labour Analysis 1.4, spontaneous vaginal birth Analysis 1.5, adverse drug reaction Analysis 1.30, caesarean birth Analysis 1.6, use of antenatal corticosteroids Analysis 1.7, or the use of antenatal tocolysis Analysis 1.8.
1.4. Analysis.
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 4 Threatened preterm labour.
1.5. Analysis.
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 5 Spontaneous vaginal delivery.
1.30. Analysis.
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 30 Adverse drug reaction.
1.6. Analysis.
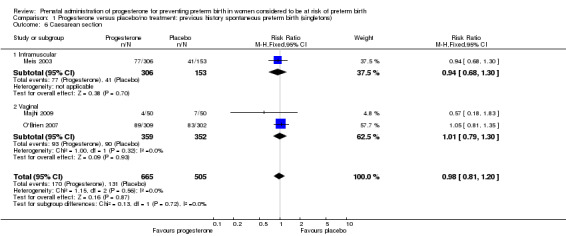
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 6 Caesarean section.
1.7. Analysis.
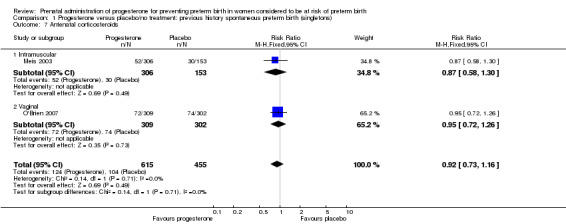
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 7 Antenatal corticosteroids.
1.8. Analysis.
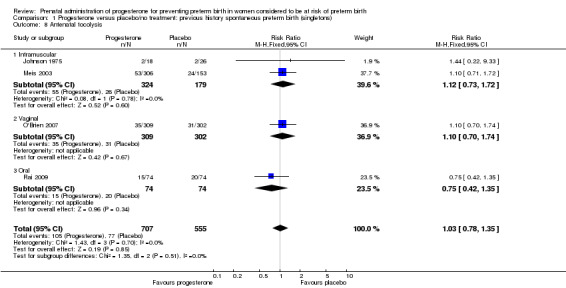
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 8 Antenatal tocolysis.
Secondary childhood outcomes
There were no statistically significant differences identified for the outcomes developmental delay Analysis 1.21, intellectual impairment Analysis 1.22, motor impairment Analysis 1.23, visual impairment Analysis 1.24, hearing impairment Analysis 1.25, cerebral palsy Analysis 1.26, learning difficulties Analysis 1.27, height less than fifth centile Analysis 1.28 , weight less than the fifth centile Analysis 1.29, infant weight at six, 12 and 24 months' follow‐up Analysis 1.34; Analysis 1.35; Analysis 1.36, infant length (cm) at six, 12 and 24 months' follow‐up Analysis 1.38; Analysis 1.39; Analysis 1.40, and infant head circumference (cm) at six, 12 and 24 months' follow‐up Analysis 1.41; Analysis 1.42; Analysis 1.43.
1.21. Analysis.
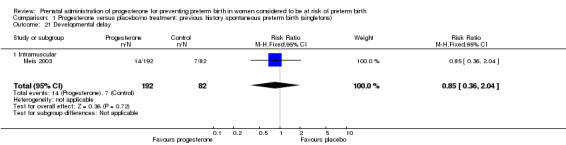
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 21 Developmental delay.
1.22. Analysis.
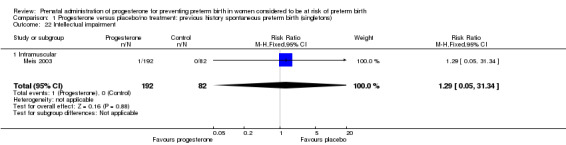
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 22 Intellectual impairment.
1.23. Analysis.
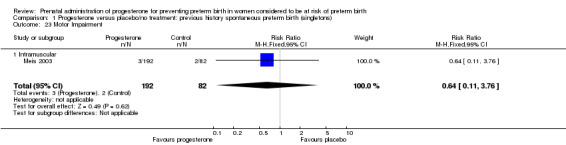
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 23 Motor Impairment.
1.24. Analysis.
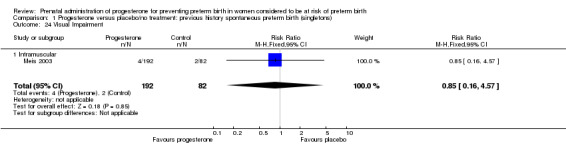
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 24 Visual Impairment.
1.25. Analysis.
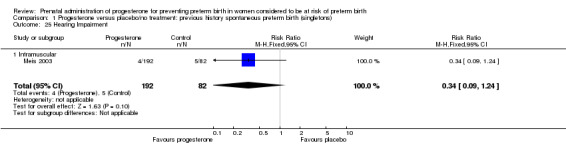
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 25 Hearing Impairment.
1.26. Analysis.
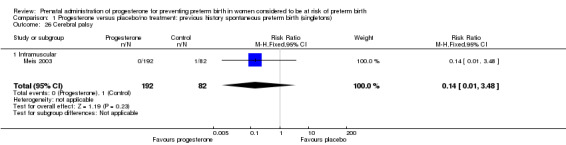
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 26 Cerebral palsy.
1.27. Analysis.
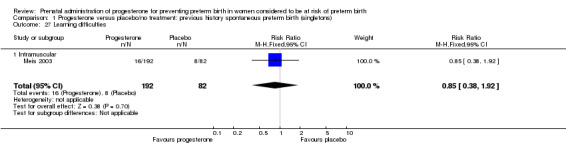
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 27 Learning difficulties.
1.28. Analysis.
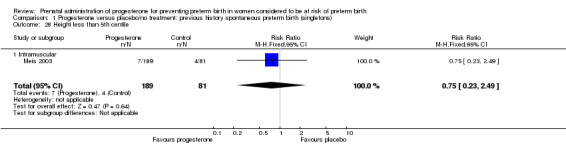
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 28 Height less than 5th centile.
1.29. Analysis.
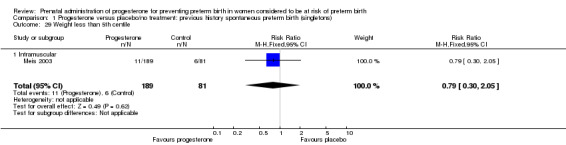
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 29 Weight less than 5th centile.
1.35. Analysis.
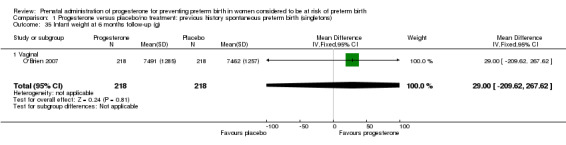
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 35 Infant weight at 6 months follow‐up (g).
1.36. Analysis.
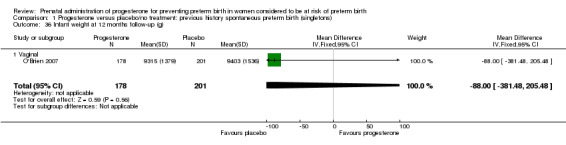
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 36 Infant weight at 12 months follow‐up (g).
1.38. Analysis.
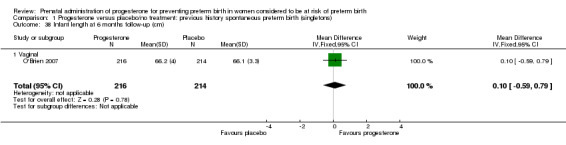
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 38 Infant length at 6 months follow‐up (cm).
1.39. Analysis.
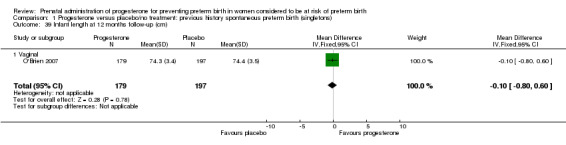
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 39 Infant length at 12 months follow‐up (cm).
1.40. Analysis.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 40 Infant length at 24 months follow‐up (cm).
1.41. Analysis.
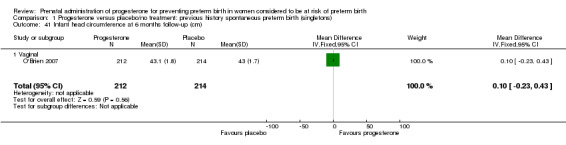
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 41 Infant head circumference at 6 months follow‐up (cm).
1.42. Analysis.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 42 Infant head circumference at 12 months follow‐up (cm).
1.43. Analysis.
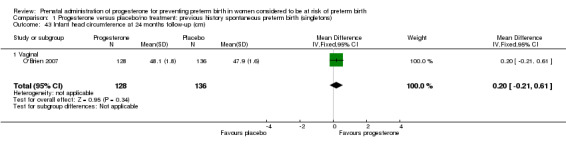
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 43 Infant head circumference at 24 months follow‐up (cm).
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses where possible for all outcomes and found no differential effect on the majority of outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal versus oral). However, for respiratory distress syndrome, the subgroup analysis indicated a differential effect between the different routes of administration (test for subgroup differences: P = 0.001, I² = 84.8%, Analysis 1.10), although only one trial was included in each subgroup of intramuscular versus vaginal versus oral, Analysis 1.10.
We performed subgroup analysis to investigate the differential effect of time of commencement of supplementation (prior to 20 weeks' gestation versus after 20 weeks' gestation) where outcome data allowed, and found no subgroup differences (test for subgroup differences: P = 0.28, I² = 15.9%, Analysis 2.1).
2.1. Analysis.
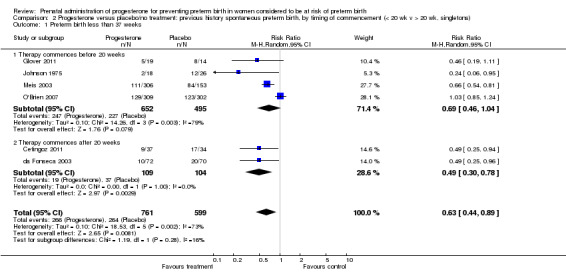
Comparison 2 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 1 Preterm birth less than 37 weeks.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 mg versus greater than 500 mg) and found no differential effect for the majority of outcomes examined: Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.9; Analysis 3.10; Analysis 3.11; Analysis 3.12. However, for intraventricular haemorrhage (all grades), the subgroup analysis indicated a differential effect between the different doses of progesterone (test for subgroup differences: P = 0.04, I² = 76.2%, Analysis 1.38), although only one trial was included in each subgroup of intramuscular versus vaginal versus oral, Analysis 1.10.
3.1. Analysis.
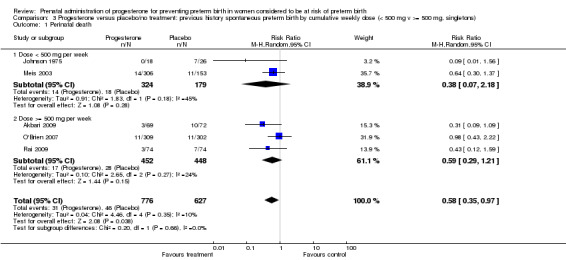
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 1 Perinatal death.
3.2. Analysis.
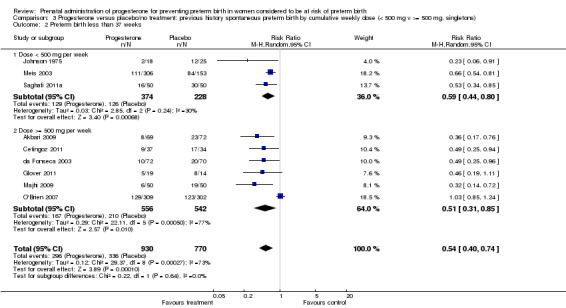
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 2 Preterm birth less than 37 weeks.
3.3. Analysis.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 3 Threatened preterm labour.
3.4. Analysis.
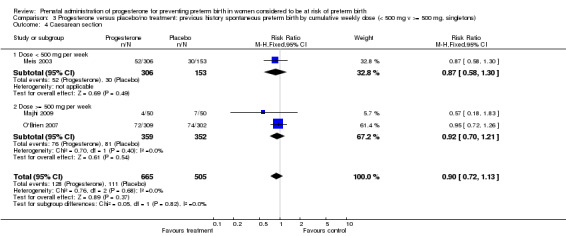
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 4 Caesarean section.
3.5. Analysis.
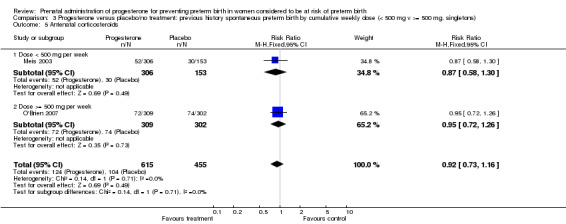
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 5 Antenatal corticosteroids.
3.6. Analysis.
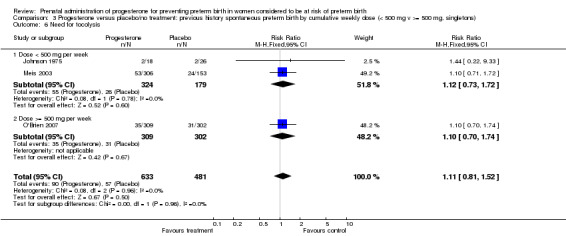
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 6 Need for tocolysis.
3.7. Analysis.
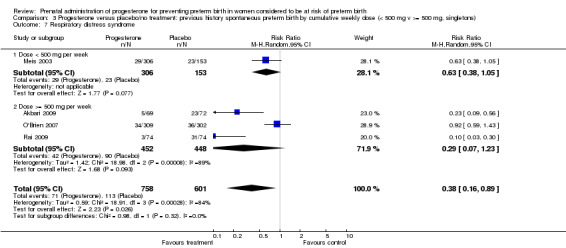
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 7 Respiratory distress syndrome.
3.9. Analysis.
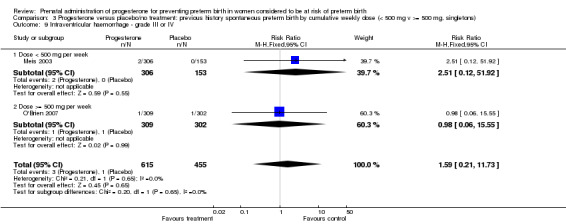
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 9 Intraventricular haemorrhage ‐ grade III or IV.
3.10. Analysis.
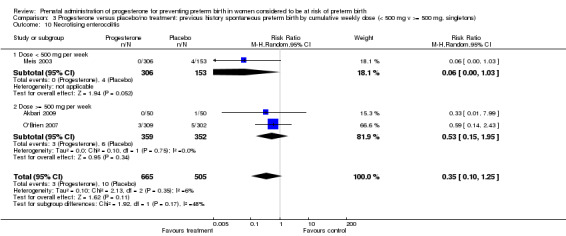
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 10 Necrotising enterocolitis.
3.11. Analysis.
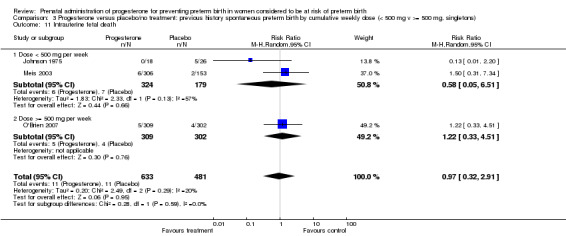
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 11 Intrauterine fetal death.
3.12. Analysis.
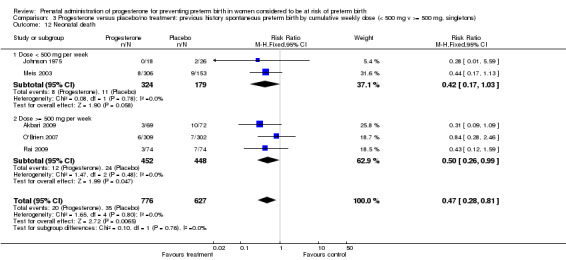
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 12 Neonatal death.
Progesterone versus placebo for women with a short cervix identified on ultrasound
Four randomised controlled trials involving a total of 1556 women and infants were included in the meta‐analysis.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcome perinatal death, there were no statistically significant differences identified when compared with placebo, Analysis 4.1. Women administered progesterone were significantly less likely to have a preterm birth at less than 34 weeks' gestation (two studies; 438 women; RR 0.64, 95% CI 0.45 to 0.90), Analysis 4.2. Major neurodevelopmental handicap in childhood was not reported.
4.1. Analysis.
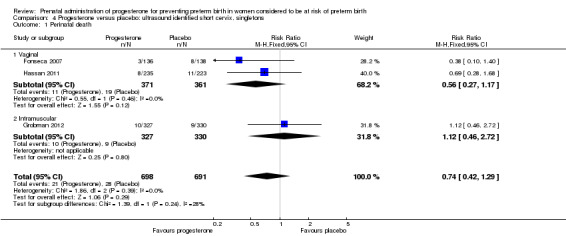
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 1 Perinatal death.
4.2. Analysis.
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 2 Preterm birth less than 34 weeks.
Secondary infant outcomes
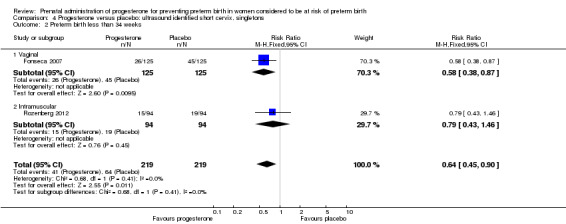
For women administered progesterone during pregnancy, for the outcome preterm birth at less than 37 weeks' gestation, there were no statistically significant differences identified when compared with placebo, Analysis 4.12. However, women administered progesterone were significantly less likely to have a preterm birth at less than 28 weeks' gestation (two studies; 1115 women; RR 0.59, 95% CI 0.37 to 0.93), Analysis 4.13.
4.12. Analysis.
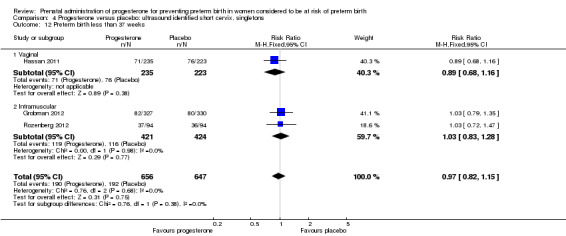
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 12 Preterm birth less than 37 weeks.
4.13. Analysis.
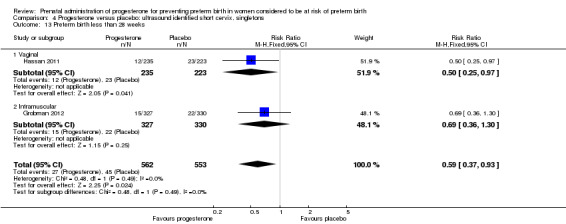
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 13 Preterm birth less than 28 weeks.
For infant outcomes infant birthweight less than 2500 g Analysis 4.14, respiratory distress syndrome Analysis 4.15, Apgar score less than seven at five minutes Analysis 4.16, need for assisted ventilation Analysis 4.17, intraventricular haemorrhage (all grades) Analysis 4.18, intraventricular haemorrhage (grades III or IV) Analysis 4.19, periventricular leucomalacia Analysis 4.20, retinopathy of prematurity Analysis 4.21, necrotising enterocolitis Analysis 4.22, neonatal sepsis Analysis 4.23, intrauterine fetal death Analysis 4.24, neonatal death Analysis 4.25 or admission to neonatal intensive care unit Analysis 4.26, there were no statistically significant differences identified.
4.14. Analysis.
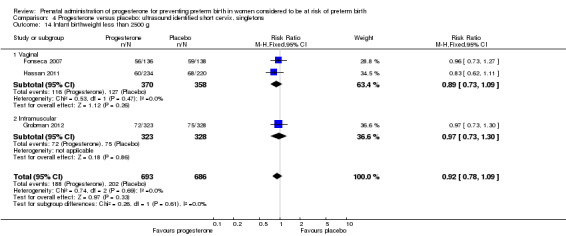
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 14 Infant birthweight less than 2500 g.
4.15. Analysis.
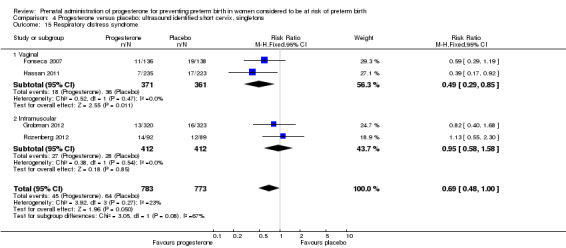
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 15 Respiratory distress syndrome.
4.16. Analysis.
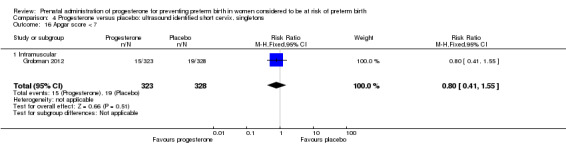
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 16 Apgar score < 7.
4.17. Analysis.
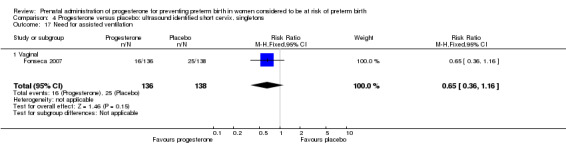
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 17 Need for assisted ventilation.
4.18. Analysis.
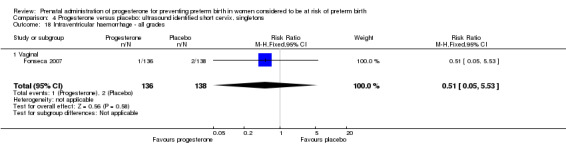
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 18 Intraventricular haemorrhage ‐ all grades.
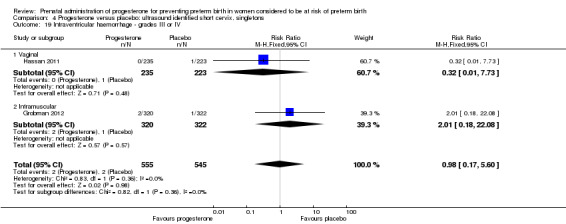
4.19. Analysis.
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 19 Intraventricular haemorrhage ‐ grades III or IV.
4.20. Analysis.
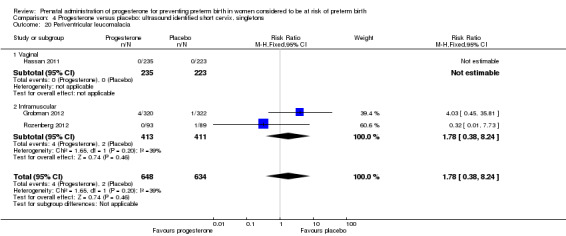
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 20 Periventricular leucomalacia.
4.21. Analysis.
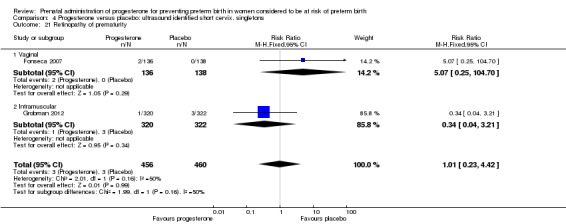
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 21 Retinopathy of prematurity.
4.22. Analysis.
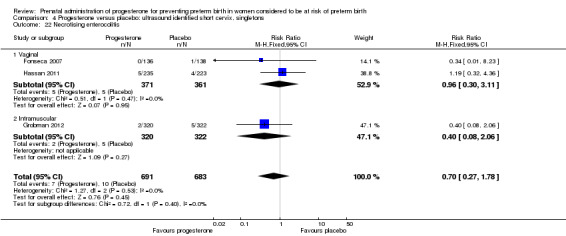
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 22 Necrotising enterocolitis.
4.23. Analysis.
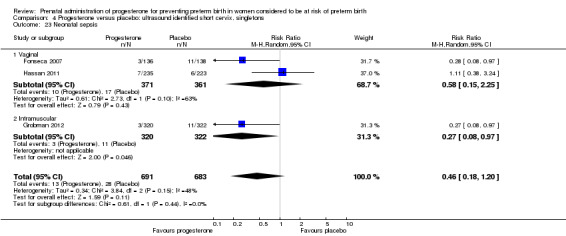
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 23 Neonatal sepsis.
4.24. Analysis.
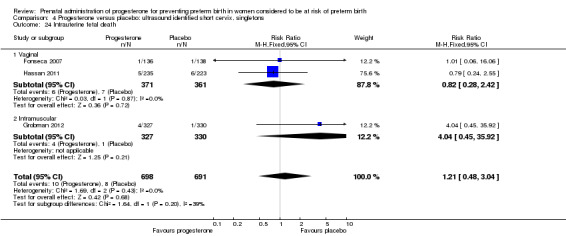
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 24 Intrauterine fetal death.
4.25. Analysis.
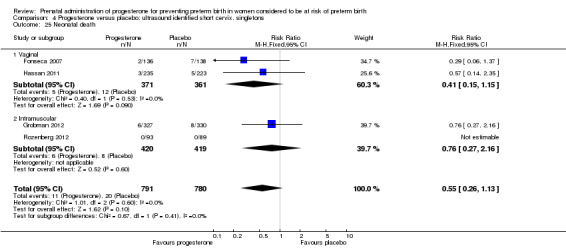
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 25 Neonatal death.
4.26. Analysis.
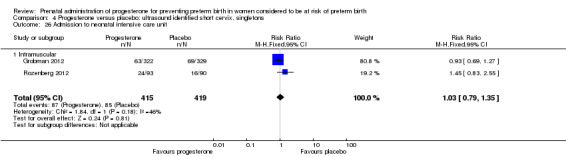
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 26 Admission to neonatal intensive care unit.
Secondary maternal outcomes
Women administered progesterone were significantly more likely to experience the adverse drug reaction urticaria (one study; 654 women; RR 5.03, 95% CI 1.11 to 22.78), Analysis 4.7. For all other maternal outcomes, threatened preterm labour Analysis 4.3, prelabour spontaneous rupture of membranes Analysis 4.4, adverse drug reactions (any, injection site, nausea) Analysis 4.5; Analysis 4.6; Analysis 4.8, pregnancy prolongation Analysis 4.9, caesarean section Analysis 4.10, or antenatal tocolysis Analysis 4.11, there were no statistically significant differences identified.
4.7. Analysis.
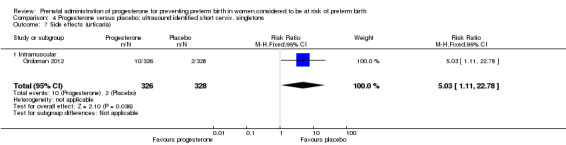
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 7 Side effects (urticaria).
4.3. Analysis.
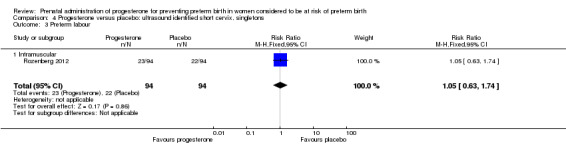
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 3 Preterm labour.
4.4. Analysis.
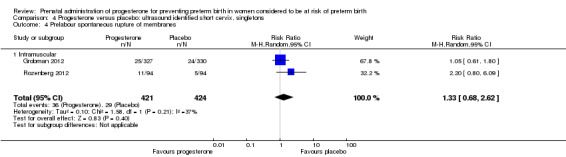
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 4 Prelabour spontaneous rupture of membranes.
4.5. Analysis.
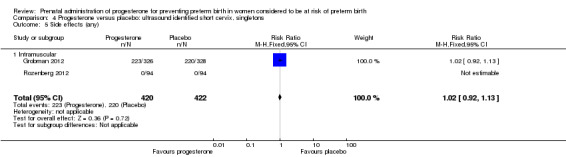
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 5 Side effects (any).
4.6. Analysis.
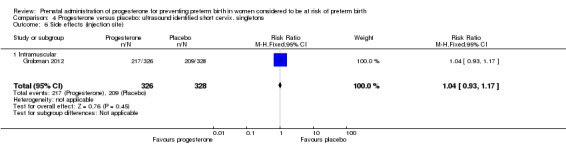
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 6 Side effects (injection site).
4.8. Analysis.
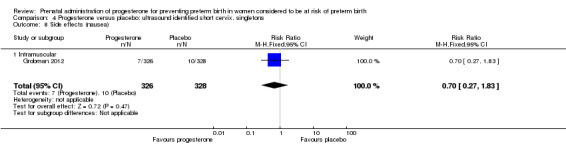
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 8 Side effects (nausea).
4.9. Analysis.
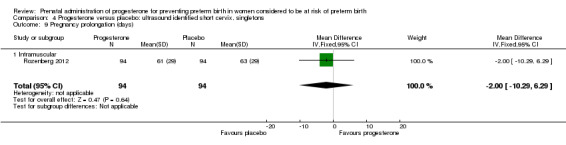
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 9 Pregnancy prolongation (days).
4.10. Analysis.
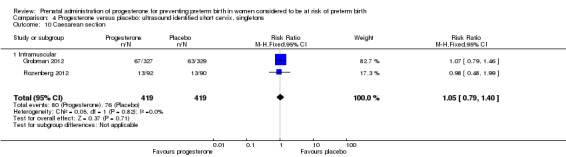
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 10 Caesarean section.
4.11. Analysis.
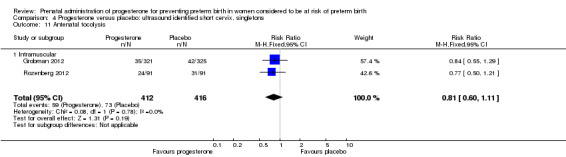
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 11 Antenatal tocolysis.
Secondary childhood outcomes
None of the secondary childhood outcomes were reported.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses where possible for all outcomes and found no differential effect on the outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal), Analysis 4.20; Analysis 4.21; Analysis 4.23. It was not possible to assess the effect of gestational age at commencing therapy.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 mg versus greater than 500 mg) and found no differential effect for the two outcomes examined: Analysis 5.1; Analysis 5.2.
5.1. Analysis.
Comparison 5 Progesterone versus placebo: ultrasound identified short cervix, singletons by cumulative weekly dose (<500 mg v >=500 mg), Outcome 1 Periventricular leucomalacia.
5.2. Analysis.
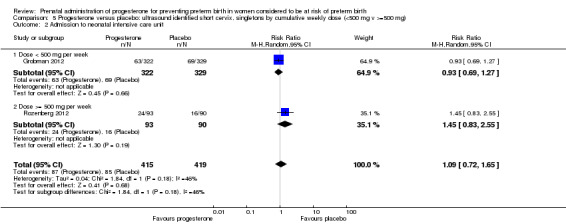
Comparison 5 Progesterone versus placebo: ultrasound identified short cervix, singletons by cumulative weekly dose (<500 mg v >=500 mg), Outcome 2 Admission to neonatal intensive care unit.
Progesterone versus placebo for women with a multiple pregnancy
Ten randomised controlled trials involving a total of 3395 women and 6178 infants were included in the meta‐analysis.
One trial (Serra 2013) consisted of three groups: progesterone 200 mg versus progesterone 400 mg versus placebo. This trial has been analysed as separate pair‐wise comparisons, with separate analyses for progesterone 200 mg versus placebo (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.5; Analysis 6.9; Analysis 6.10; Analysis 6.11; Analysis 6.12; Analysis 6.13; Analysis 6.14; Analysis 6.21; Analysis 6.24; Analysis 6.25; Analysis 6.26) and progesterone 400 mg versus placebo (Analysis 6.27; Analysis 6.28; Analysis 6.29; Analysis 6.30; Analysis 6.31; Analysis 6.32; Analysis 6.33; Analysis 6.34; Analysis 6.35; Analysis 6.36; Analysis 6.37; Analysis 6.38; Analysis 6.39; Analysis 6.40; Analysis 6.41; Analysis 6.42; ; Analysis 7.1; Analysis 7.2; Analysis 7.3).
6.1. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 1 Perinatal death.
6.2. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 2 Preterm birth less than 34 weeks.
6.3. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 3 Preterm PROM.
6.5. Analysis.
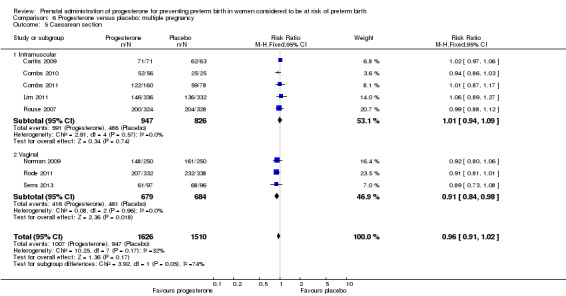
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 5 Caesarean section.
6.9. Analysis.
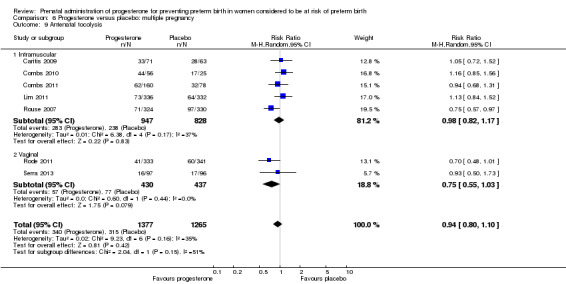
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 9 Antenatal tocolysis.
6.10. Analysis.
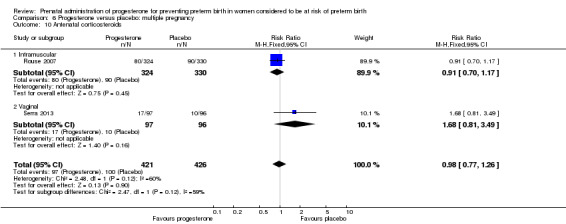
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 10 Antenatal corticosteroids.
6.11. Analysis.
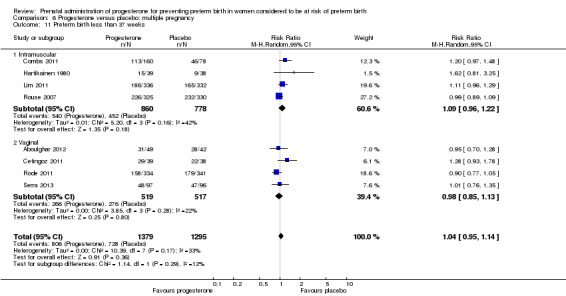
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 11 Preterm birth less than 37 weeks.
6.12. Analysis.
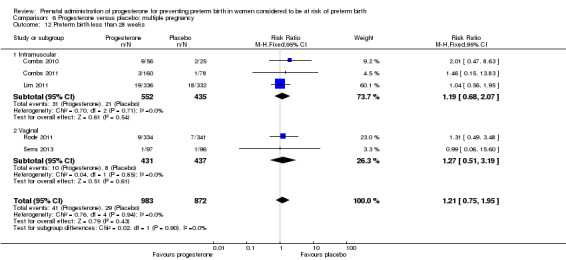
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 12 Preterm birth less than 28 weeks.
6.13. Analysis.
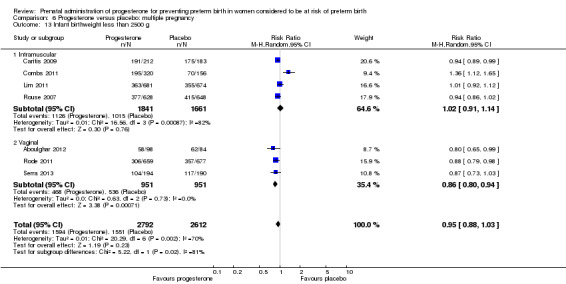
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 13 Infant birthweight less than 2500 g.
6.14. Analysis.
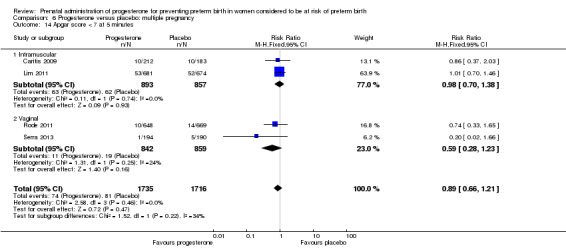
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 14 Apgar score < 7 at 5 minutes.
6.21. Analysis.
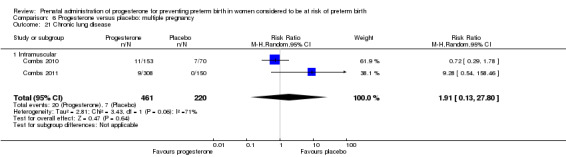
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 21 Chronic lung disease.
6.24. Analysis.
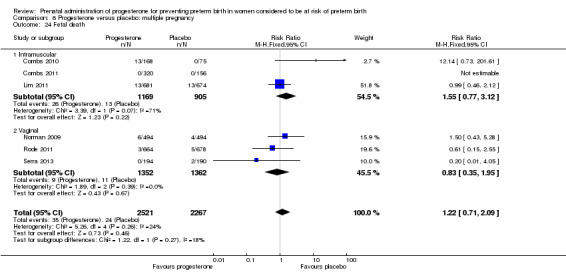
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 24 Fetal death.
6.25. Analysis.
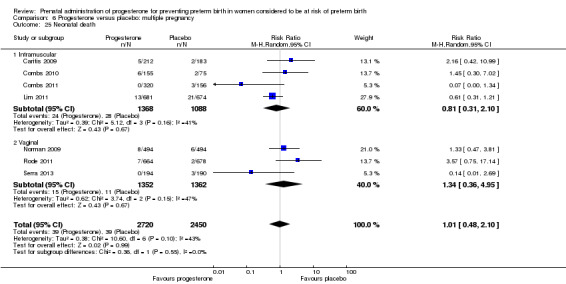
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 25 Neonatal death.
6.26. Analysis.
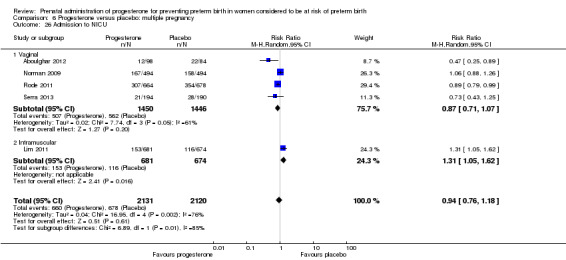
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 26 Admission to NICU.
6.27. Analysis.
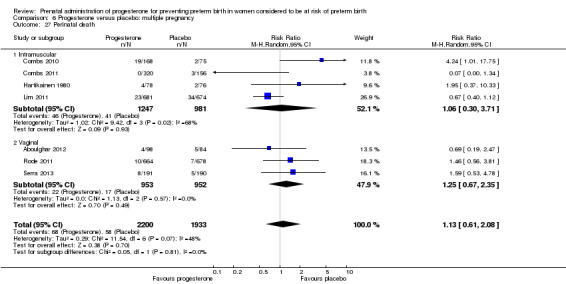
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 27 Perinatal death.
6.28. Analysis.
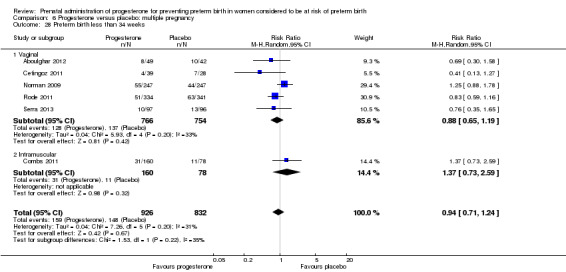
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 28 Preterm birth less than 34 weeks.
6.29. Analysis.
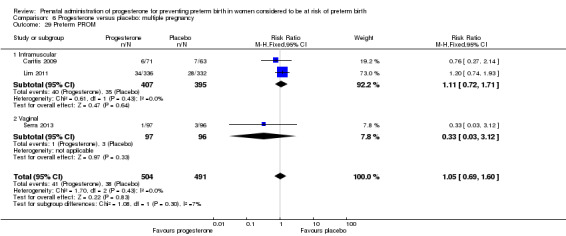
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 29 Preterm PROM.
6.30. Analysis.
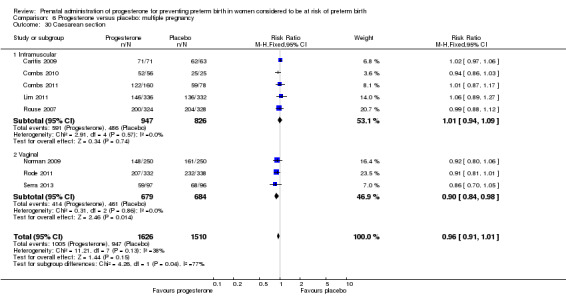
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 30 Caesarean section.
6.31. Analysis.
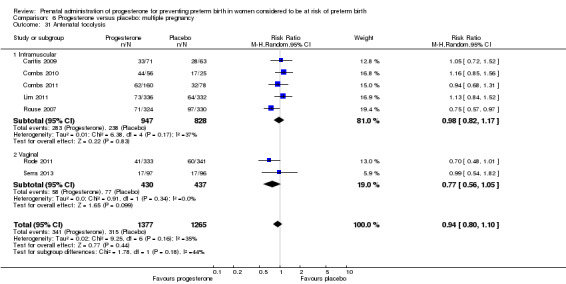
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 31 Antenatal tocolysis.
6.32. Analysis.
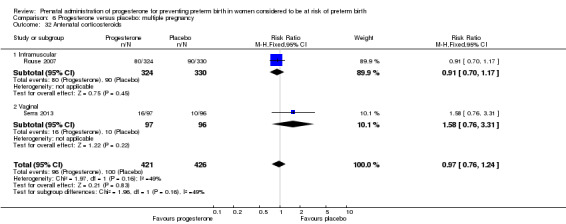
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 32 Antenatal corticosteroids.
6.33. Analysis.
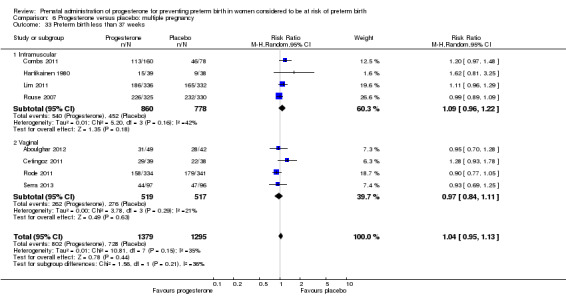
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 33 Preterm birth less than 37 weeks.
6.34. Analysis.
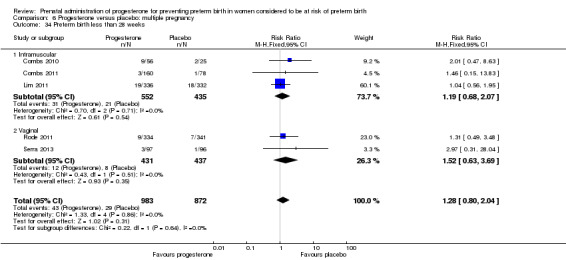
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 34 Preterm birth less than 28 weeks.
6.35. Analysis.
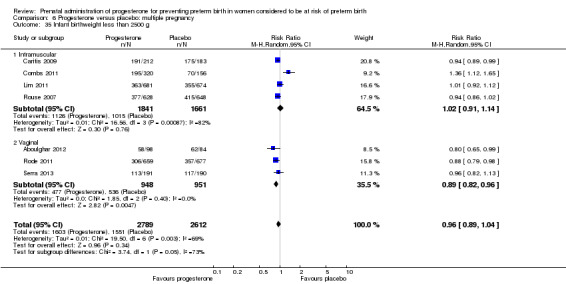
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 35 Infant birthweight less than 2500 g.
6.36. Analysis.
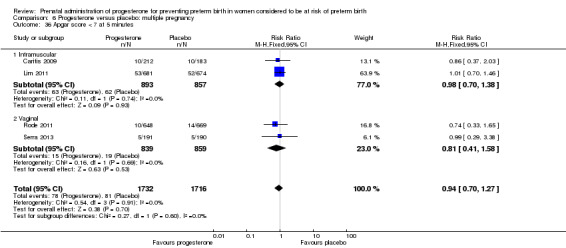
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 36 Apgar score < 7 at 5 minutes.
6.37. Analysis.
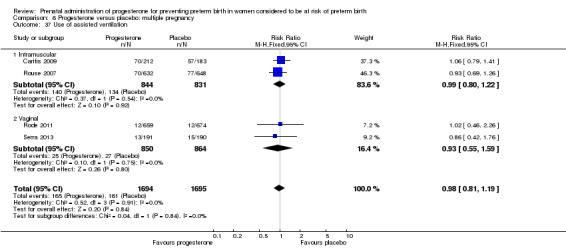
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 37 Use of assisted ventilation.
6.38. Analysis.
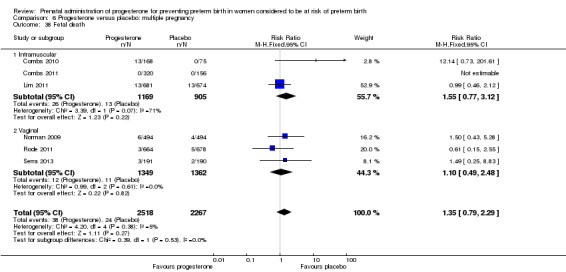
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 38 Fetal death.
6.39. Analysis.
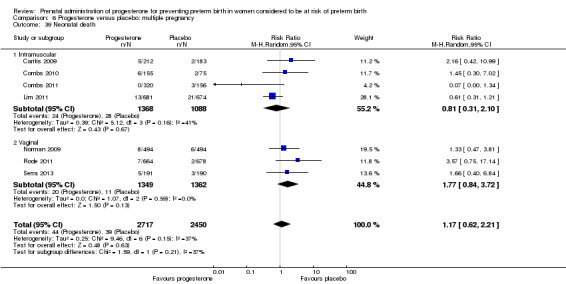
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 39 Neonatal death.
6.40. Analysis.
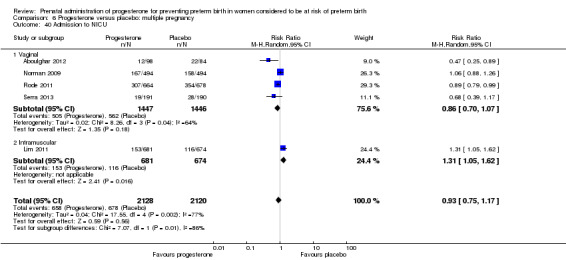
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 40 Admission to NICU.
6.41. Analysis.
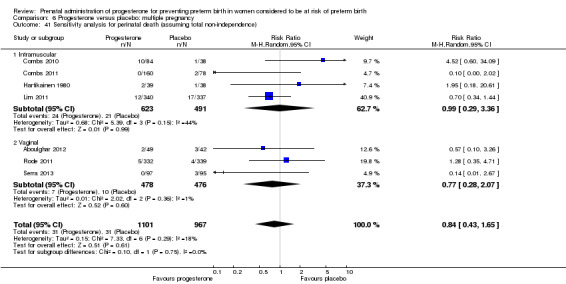
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 41 Sensitivity analysis for perinatal death (assuming total non‐independence).
6.42. Analysis.
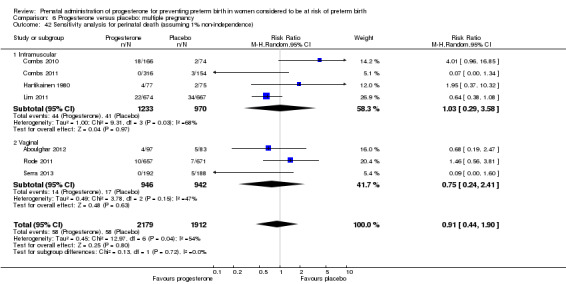
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 42 Sensitivity analysis for perinatal death (assuming 1% non‐independence).
7.1. Analysis.
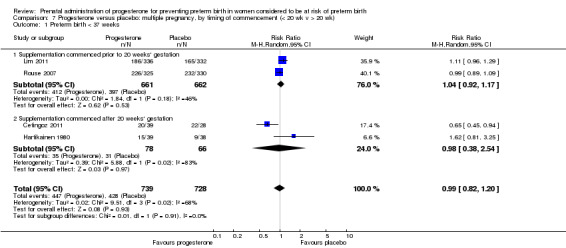
Comparison 7 Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk), Outcome 1 Preterm birth < 37 weeks.
7.2. Analysis.
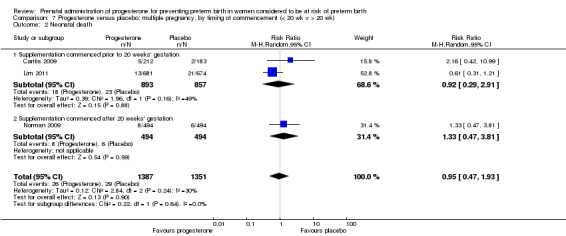
Comparison 7 Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk), Outcome 2 Neonatal death.
7.3. Analysis.
Comparison 7 Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk), Outcome 3 Admission to NICU.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcomes perinatal death Analysis 6.1, Analysis 6.27, and preterm birth less than 34 weeks' gestation Analysis 6.2; Analysis 6.28, there were no statistically significant differences identified when compared with placebo. Major neurodevelopmental handicap in childhood was not reported.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, there were no statistically significant differences identified in the risk of birth before 37 Analysis 6.11; Analysis 6.33 or 28 weeks Analysis 6.12; Analysis 6.34, infant birthweight less than 2500 g Analysis 6.13; Analysis 6.35, Apgar score less than seven at five minutes Analysis 6.14; Analysis 6.36, respiratory distress syndrome Analysis 6.15, need for ventilation Analysis 6.16; Analysis 6.37, intraventricular haemorrhage Analysis 6.17; Analysis 6.18, periventricular leucomalacia Analysis 6.19, retinopathy of prematurity Analysis 6.20, chronic lung disease Analysis 6.21, necrotising enterocolitis Analysis 6.22, neonatal sepsis Analysis 6.23, fetal death Analysis 6.24; Analysis 6.38, neonatal death Analysis 6.25; Analysis 6.39, or admission to neonatal intensive care unit (NICU) Analysis 6.26; Analysis 6.40. Due to extreme heterogeneity, we did not combine data from trials for the outcomes neonatal length of hospital stay or patent ductus arteriosus.
6.15. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 15 Respiratory distress syndrome.
6.16. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 16 Use of assisted ventilation.
6.17. Analysis.
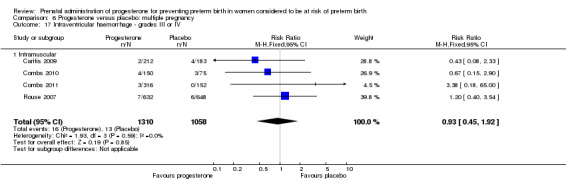
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 17 Intraventricular haemorrhage ‐ grades III or IV.
6.18. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 18 Intraventricular haemorrhage ‐ all grades.
6.19. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 19 Periventricular leucomalacia.
6.20. Analysis.
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 20 Retinopathy of prematurity.
6.22. Analysis.
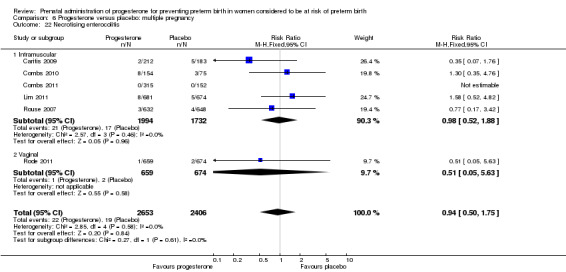
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 22 Necrotising enterocolitis.
6.23. Analysis.
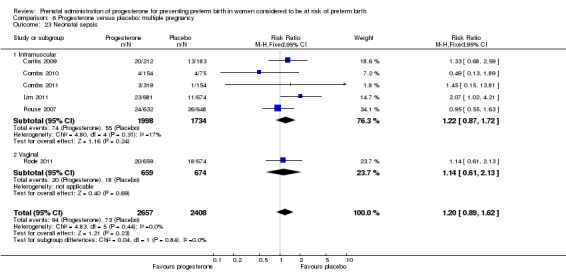
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 23 Neonatal sepsis.
Secondary maternal outcomes
For women administered progesterone during pregnancy, when compared with placebo, there were no statistically significant differences identified in any of the following maternal outcomes: prelabour spontaneous rupture of membranes Analysis 6.3, adverse drug reaction Analysis 6.4, caesarean section Analysis 6.5, spontaneous birth Analysis 6.6, assisted birth Analysis 6.7, satisfaction with the therapy Analysis 6.8, antenatal tocolysis Analysis 6.9, or antenatal corticosteroids Analysis 6.10.
6.4. Analysis.
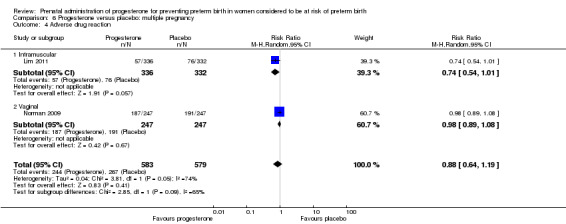
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 4 Adverse drug reaction.
6.6. Analysis.
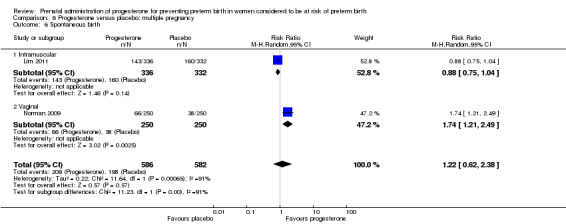
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 6 Spontaneous birth.
6.7. Analysis.
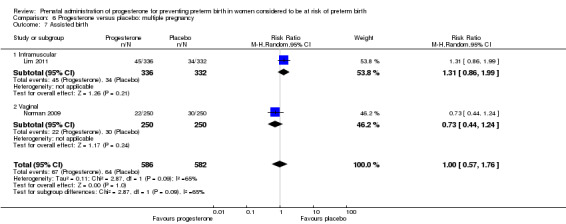
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 7 Assisted birth.
6.8. Analysis.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 8 Satisfaction with therapy.
Secondary childhood outcomes
None of the secondary childhood outcomes were reported.
Sensitivity analyses to account for multiple pregnancies
For multiple pregnancies we had planned to analyse neonatal data as 'clusters' to account for dependency between twins and triplets. We anticipated that the degree of dependence between twins would vary at the outcome level i.e. in the case of preterm birth the dependency (ICC) is likely to be high, whereas in some outcomes, such as perinatal death or morbidity, the ICC may be much lower. However, there were insufficient data presented in the trial reports to allow us to carry out necessary adjustment for cluster design effect ourselves and, although in several trials results had already been adjusted, we were not able to present these data in our data and analyses tables because they were not reported in a consistent way.
For the primary outcome perinatal death, we therefore carried out a sensitivity analysis assuming two extremes. In the first we assumed complete dependence between infants in multiple pregnancies, i.e. we assumed outcomes were the same for all infants within that pregnancy; in this case the effective sample size for twin pregnancies would be the total number of women rather than infants, and for infant outcomes all event rates and sample sizes were therefore divided by two. In the second sensitivity analyses we assumed very limited dependence (1%) and in this case event rates and sample sizes were divided by 1.01. Results from sensitivity analyses were very similar to those from the unadjusted analyses although the effect estimates changed slightly due to rounding up of event rates and sample sizes, and the 95% CIs were generally slightly wider. Adjusted analyses showed that for women administered progesterone during pregnancy, for the primary outcome perinatal death, there were no statistically significant differences identified when compared with placebo. (Data not shown, available from the authors on request).
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses and found no differential effect on the majority of outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal). However, for spontaneous birth, infant birthweight less than 2500 g and admission to NICU, the subgroup analyses indicated a differential effect between the different routes of administration (test for subgroup differences: P = 0.0008, I² = 91.1%, Analysis 6.6; P = 0.02, I² = 80.8% Analysis 6.13; P = 0.009, I² = 85.5%, Analysis 6.26) although it must be noted that in Analysis 6.6; Analysis 6.26, some of the subgroups contained only one trial.
We performed subgroup analysis to investigate the differential effect of time of commencement of supplementation (prior to 20 weeks' gestation versus after 20 weeks' gestation) where outcome data allowed, and found no subgroup differences for the outcomes examined, Analysis 7.1; Analysis 7.2; Analysis 7.3.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 per week mg versus greater than 500 mg per week) and found no differential effect for the majority of outcomes examined, Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5; Analysis 8.6; Analysis 8.7. However, for infant birthweight less than 2500 g and admission to NICU, the subgroup analyses indicated a differential effect between the cumulative doses of progesterone (test for subgroup difference: P = 0.02, I² = 80.8%, Analysis 8.5; P = 0.009, I² = 85.5%, Analysis 8.8).
8.1. Analysis.
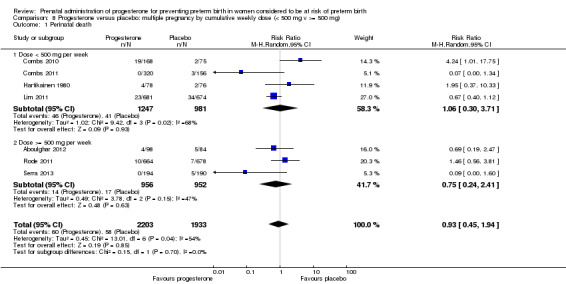
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 1 Perinatal death.
8.2. Analysis.
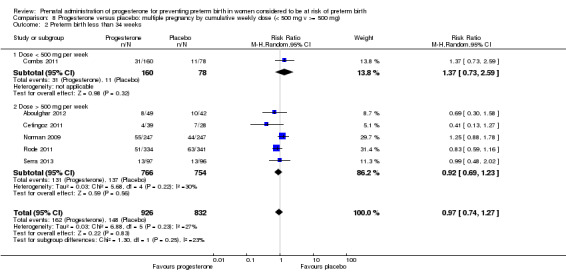
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 2 Preterm birth less than 34 weeks.
8.3. Analysis.
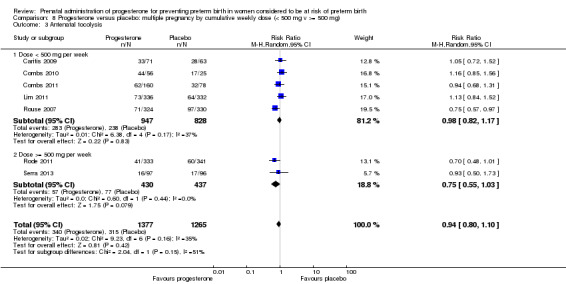
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 3 Antenatal tocolysis.
8.4. Analysis.
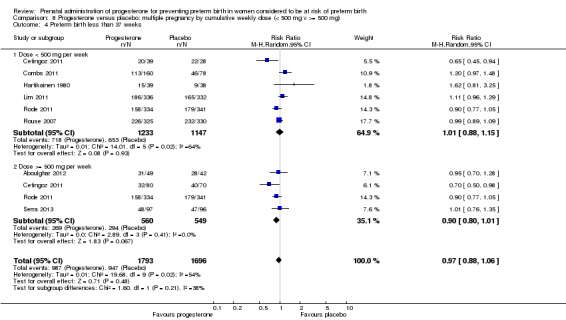
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 4 Preterm birth less than 37 weeks.
8.5. Analysis.
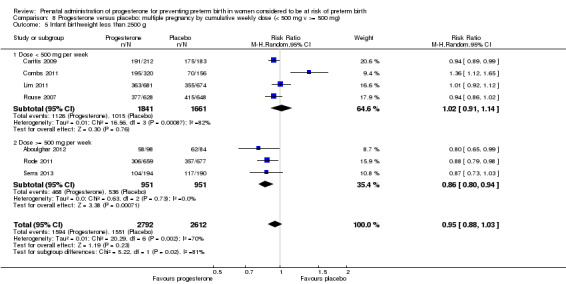
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 5 Infant birthweight less than 2500 g.
8.6. Analysis.
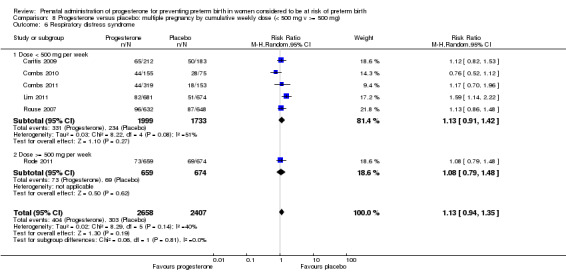
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 6 Respiratory distress syndrome.
8.7. Analysis.
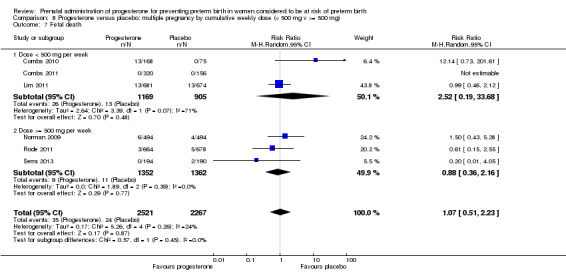
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 7 Fetal death.
8.8. Analysis.
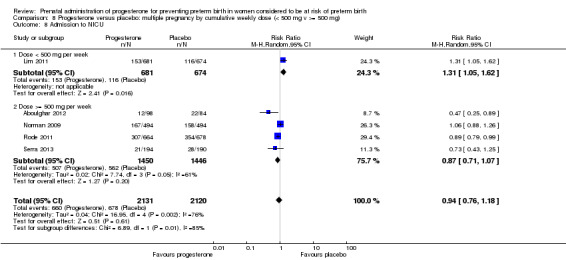
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 8 Admission to NICU.
Progesterone versus placebo/no treatment for women following presentation with threatened preterm labour
Five randomised controlled trials involving a total of 384 women and infants were included in the meta‐analysis.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcomes perinatal death Analysis 9.1, and preterm birth less than 34 weeks' gestation Analysis 9.2, there were no statistically significant differences identified when compared with placebo. Major neurodevelopmental handicap in childhood was not reported.
9.1. Analysis.
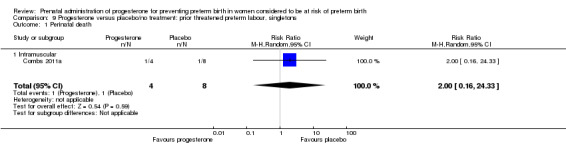
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 1 Perinatal death.
9.2. Analysis.
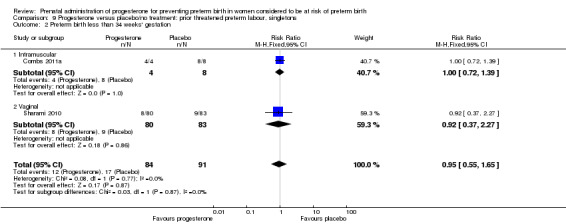
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 2 Preterm birth less than 34 weeks' gestation.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, there was a statistically significant reduction in the risk of:
infant birthweight less than 2500 g (one study; 70 infants; RR 0.52, 95% CI 0.28 to 0.98), Analysis 9.11.
9.11. Analysis.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 11 Infant birthweight less than 2500 g.
There were no statistically significant differences for any of the other outcomes analysed: preterm birth less than 37 weeks' gestation Analysis 9.10, respiratory distress syndrome Analysis 9.12, intraventricular haemorrhage grade III or IV Analysis 9.13, periventricular leucomalacia Analysis 9.14, needed for mechanical ventilation Analysis 9.15, necrotising enterocolitis Analysis 9.16, neonatal sepsis Analysis 9.17, fetal death Analysis 9.18, neonatal death Analysis 9.19, or neonatal length of hospital stay Analysis 9.20.
9.10. Analysis.
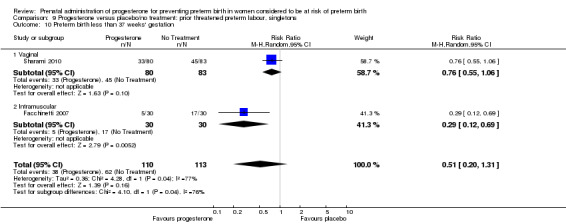
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 10 Preterm birth less than 37 weeks' gestation.
9.12. Analysis.
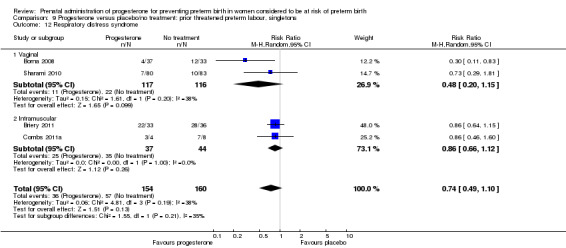
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 12 Respiratory distress syndrome.
9.13. Analysis.
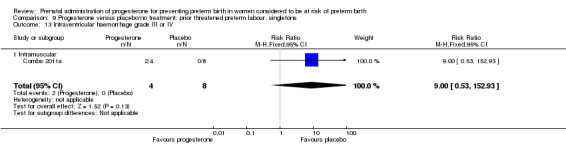
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 13 Intraventricular haemorrhage grade III or IV.
9.14. Analysis.
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 14 Periventricular leucomalacia.
9.15. Analysis.
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 15 Use of assisted ventilation.
9.16. Analysis.
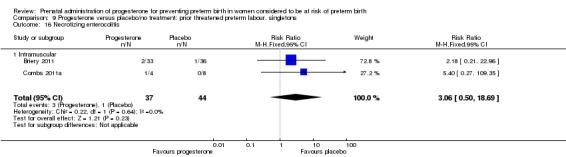
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 16 Necrotizing enterocolitis.
9.17. Analysis.
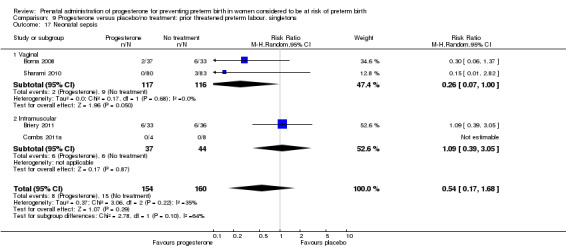
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 17 Neonatal sepsis.
9.18. Analysis.
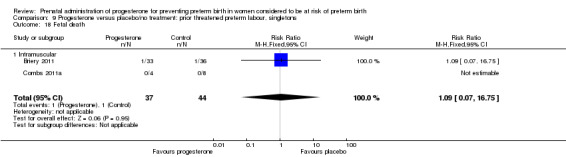
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 18 Fetal death.
9.19. Analysis.
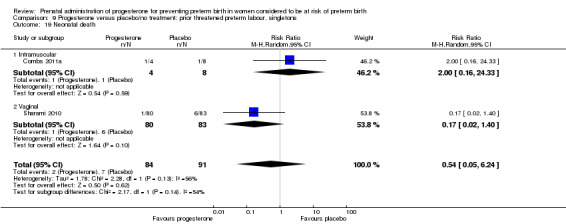
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 19 Neonatal death.
9.20. Analysis.
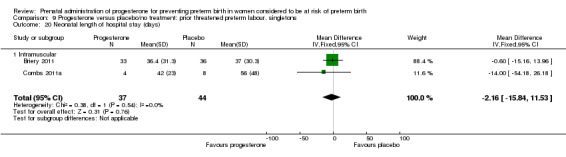
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 20 Neonatal length of hospital stay (days).
Secondary maternal outcomes
For women administered progesterone during pregnancy, when compared with placebo, there were no statistically significant differences for any of the outcomes analysed: pregnancy prolongation Analysis 9.3; Analysis 9.4; Analysis 9.5; Analysis 9.6, spontaneous vaginal birth Analysis 9.7, caesarean section Analysis 9.8, or use of tocolysis Analysis 9.9.
9.3. Analysis.
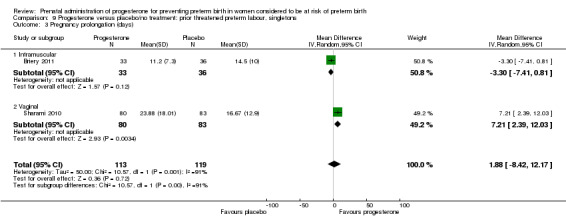
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 3 Pregnancy prolongation (days).
9.4. Analysis.
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 4 Pregnancy prolongation ‐ less than 1 week.
9.5. Analysis.
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 5 Pregnancy prolongation ‐ 1.0 to 1.9 weeks.
9.6. Analysis.
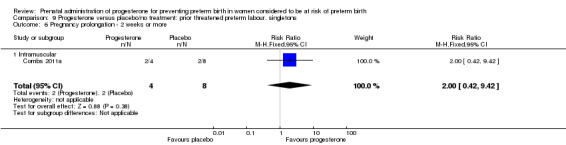
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 6 Pregnancy prolongation ‐ 2 weeks or more.
9.7. Analysis.
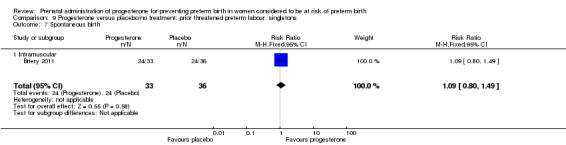
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 7 Spontaneous birth.
9.8. Analysis.
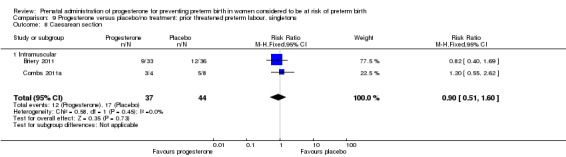
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 8 Caesarean section.
9.9. Analysis.
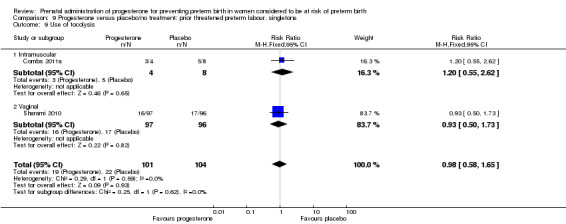
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 9 Use of tocolysis.
Secondary childhood outcomes
There were no secondary childhood outcomes reported.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses where possible for all outcomes and found no differential effect on some of the outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal), Analysis 9.2; Analysis 9.9; Analysis 9.12; Analysis 9.17; Analysis 9.19. However, for pregnancy prolongation and preterm birth less than 37 weeks' gestation, the subgroup analyses indicated a differential effect between the different routes of administration (test for subgroup differences: P = 0.001, I² = 90.5%, Analysis 9.3; P = 0.04, I² = 75.6%, Analysis 9.10), although it must be noted that in both analyses the subgroups contained only one trial.
It was not possible to assess the effect of gestational age at commencing therapy.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 mg versus greater than 500 mg) and found no differential effect for three outcomes examined: Analysis 10.3; Analysis 10.4; Analysis 10.5. However, for the two outcomes, pregnancy prolongation and preterm birth less than 37 weeks' gestation, the subgroup analyses indicated a differential effect between the different drug doses (test for subgroup differences: P = 0.001, I² = 90.5%, Analysis 10.1; P = 0.04, I² = 75.6% , Analysis 10.2).
10.3. Analysis.
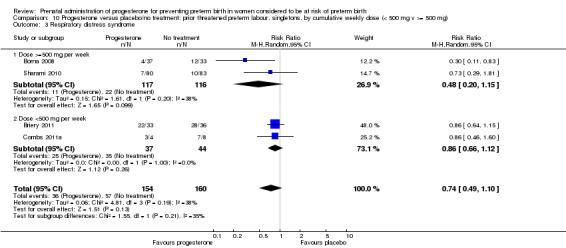
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 3 Respiratory distress syndrome.
10.4. Analysis.
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 4 Neonatal sepsis.
10.5. Analysis.
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 5 Neonatal death.
10.1. Analysis.
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 1 Pregnancy prolongation (days).
10.2. Analysis.
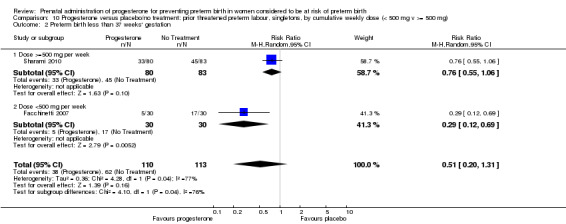
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 2 Preterm birth less than 37 weeks' gestation.
Progesterone versus placebo for women with 'other' risk factors for preterm birth
Three randomised controlled trials involving a total of 482 women and infants were included in the meta‐analysis. Data from a fourth study (Moghtadaei 2008), could not be included in the meta‐analysis because the number of women randomised to each group was not reported in the brief abstract report of the study.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcomes perinatal death Analysis 11.1 and preterm birth less than 34 weeks' gestation Analysis 11.2, there were no statistically significant differences identified when compared with placebo. The outcome major neurodevelopmental handicap in childhood were not reported.
11.1. Analysis.
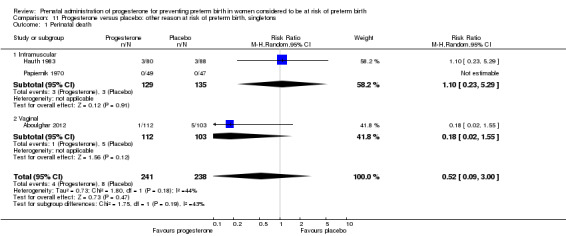
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 1 Perinatal death.
11.2. Analysis.
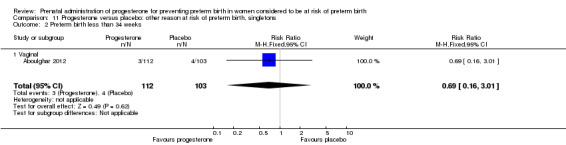
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 2 Preterm birth less than 34 weeks.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, there was a statistically significant reduction in the risk of:
infant birthweight less than 2500 g (three studies; 482 infants; RR 0.48, 95% CI 0.25 to 0.91) Analysis 11.4.
11.4. Analysis.
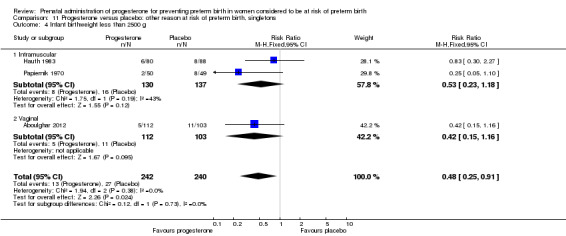
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 4 Infant birthweight less than 2500 g.
In addition, for women administered progesterone who were considered to be at risk of preterm birth for 'other' reasons, when compared with placebo, there were no statistically significant differences for the outcomes preterm birth less than 37 weeks' gestation Analysis 11.3., perinatal death Analysis 11.1, intrauterine fetal death Analysis 11.5 or neonatal death Analysis 11.6.
11.3. Analysis.
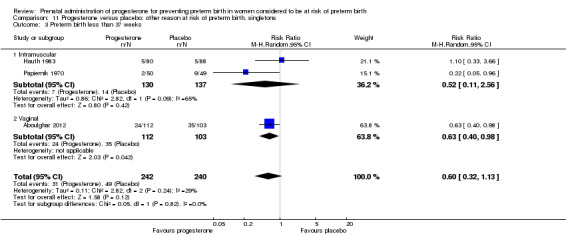
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 3 Preterm birth less than 37 weeks.
11.5. Analysis.
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 5 Intrauterine fetal death.
11.6. Analysis.
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 6 Neonatal death.
Secondary childhood outcomes
There were no secondary childhood outcomes reported.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analysis for perinatal death Analysis 12.1, preterm birth less than 37 weeks Analysis 12.2, and infant birthweight less than 2500 g, Analysis 12.3, and no differential effect was observed related for gestational age at commencing therapy, (test for subgroup differences: P = 0.10, I² = 63.9%, Analysis 12.2; P = 0.19, I² = 42.2%, Analysis 12.3).
12.1. Analysis.
Comparison 12 Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 1 Perinatal death.
12.2. Analysis.
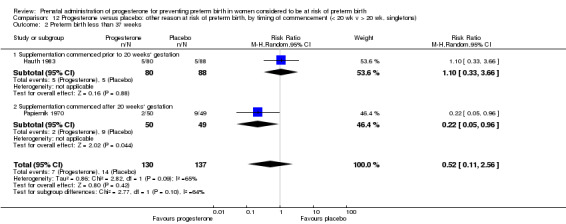
Comparison 12 Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 2 Preterm birth less than 37 weeks.
12.3. Analysis.
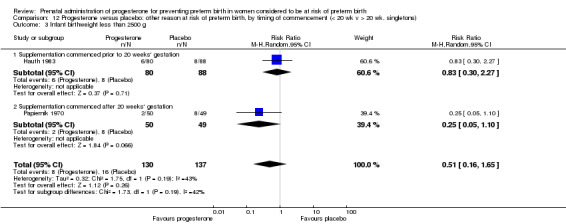
Comparison 12 Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 3 Infant birthweight less than 2500 g.
Discussion
The randomised trials identified assessed the use of progesterone in women considered to be at increased risk of preterm birth by virtue of history of spontaneous preterm birth, ultrasonographic evaluation of cervical length, presentation in threatened preterm labour, multiple pregnancy, or other reasons (including 'high preterm risk score' and active military duty).
Summary of main results
Progesterone for women with a past history of spontaneous preterm birth
For women with a past history of spontaneous preterm birth, there was a statistically significant reduction in the risk of the primary outcomes, perinatal death and in the risk of preterm birth less than 34 weeks' gestation. The reduction in risk of perinatal death is confined to the subgroup of women receiving intramuscular progesterone. There was significant heterogeneity identified for the outcomes preterm birth before 34 and 37 weeks’ gestation, with evidence of funnel plot asymmetry, raising questions about potential bias and therefore caution in interpretation. For the secondary infant and maternal outcomes, the use of progesterone was associated with a reduction in the risk of infant birthweight less than 2500 g, use of assisted ventilation, necrotising enterocolitis, neonatal death, admission to neonatal intensive care unit, preterm birth less than 37 weeks' gestation and a significant increase in prolongation in pregnancy prolongation. There were no significant differences identified for other secondary infant and maternal health outcomes with the use of progesterone. Information related to childhood health and well being is limited, with only two trials reporting two‐year follow‐up results to date (Northen 2007; O'Brien 2007), in which there were no documented differences in growth or developmental outcomes between those infants exposed in utero to progesterone and those to placebo. There was no differential effect on outcomes in terms of time of commencing therapy and dose of progesterone from the available evidence.
Further information is required about the optimal route of administration of progesterone, with the largest study to date using vaginal progesterone gel suggesting no benefit in this group of women (O'Brien 2007).
Progesterone for women with a short cervix identified on ultrasound
In the trials to date assessing the role of progesterone in women with a short cervix identified on ultrasound (Fonseca 2007; Grobman 2012; Hassan 2011; Rozenberg 2012), there were no statistically significant differences identified for the primary outcome perinatal death. Women administered progesterone were significantly less likely to have preterm birth less than 34 weeks' gestation. For the secondary infant outcomes, the use of progesterone was associated with a reduction in risk of preterm birth at less than 28 weeks' gestation. There was also a significant increase in the risk urticaria in women receiving progesterone. Further information is required about the risk of other infant health outcomes, and maternal health outcomes in this group of women. Reporting of childhood outcomes is lacking, with no trials reporting this information to date. The relative efficacy of progesterone compared with cerclage for women with a short cervix remains uncertain, as does the use of progesterone as an adjunct therapy following cerclage placement (Conde‐Agudelo 2013).
Progesterone for women with a multiple pregnancy
The role of progesterone in women with a multiple pregnancy is less clear, with no identified differences in the primary outcomes perinatal death, and preterm birth less than 34 weeks' gestation. There were also no differences identified for the other secondary infant and maternal health outcomes. Information relating to long‐term childhood health outcomes is unavailable to date. There are several ongoing randomised trials assessing the role of intramuscular (Nassar 2007) and vaginal (Wood 2007) progesterone in women with a multiple pregnancy which will contribute information about the role of progesterone in this group of women.
For multiple pregnancies, we had planned to analyse neonatal data as 'clusters' to account for dependency between twins and triplets. We anticipated that the degree of dependence between twins would vary at the outcome level i.e. in the case of preterm birth the dependency (ICC) is likely to be high, whereas in some outcomes such as perinatal death or morbidity, the ICC may be much lower. However, there were insufficient data presented in the trial reports to allow us to carry out necessary adjustment for cluster design effect ourselves and, although in several trials results had already been adjusted, we were not able to present these data in our data and analyses tables because they were not reported in a consistent way. In future updates, we will contact trial authors for additional information to allow us to carry out necessary adjustments in the analysis of the neonatal data.
Progesterone for women following presentation with threatened preterm labour
The role of progesterone for women following presentation with threatened preterm labour remains uncertain. The identified randomised trials indicate a reduction in only the risk of infant birthweight less than 2500 g. However, the outcomes have been reported in only five small trials (332 women), with only four contributing data to meta‐analysis (211 women) and are underpowered to detect differences in both maternal and infant health outcomes. There is an ongoing randomised trial assessing the role of vaginal progesterone in women presenting with symptoms or signs of threatened preterm labour which will contribute information about the role of progesterone in this group of women (Martinez 2007).
Progesterone versus placebo for women with 'other' risk factors for preterm birth
The role of progesterone in women considered to be at risk of preterm birth for 'other reasons' is uncertain, with the three randomised trials that contributed data to the meta‐analysis to date indicating no benefit in terms of perinatal death or preterm birth less than 37 weeks' gestation. However, the combined sample size of these trials is significantly underpowered to detect all but large differences in these outcomes.
There is evidence that progesterone for women with a history of previous preterm birth is associated with a reduction in preterm birth before 34 and 37 weeks gestation. As indicated earlier, there was considerable heterogeneity identified, in addition to evidence of funnel plot asymmetry, raising concern about potential bias. The observed reduction in perinatal mortality appears confined to the use of intramuscular progesterone.
However, information relating to longer‐term infant and childhood outcomes is currently insufficient, with only two randomised trials reporting to date. Ongoing follow‐up of children exposed to progesterone in utero remains a priority. Maternal outcomes following antenatal progesterone therapy were poorly reported in the available literature, including treatment side‐effects, preferences of mode of administration and satisfaction of care. Further information is required on these important issues (Greene 2003; Iams 2003). In addition, there remains uncertainty as to the optimal dose of progesterone to be administered, the optimal route of administration, and the optimal gestational age at which to commence therapy. The American College of Obstetricians and Gynecologists have issued a committee opinion relating to the use of progesterone for the prevention of preterm birth, indicating the need for further information about the optimal mode of administration (ACOG 2003), including studies directly comparing the relative efficacy of vaginal and intramuscular preparations.
Overall completeness and applicability of evidence
The majority of studies to date have reported short‐term maternal and infant outcomes, with only two studies reporting longer‐term childhood outcomes (Northen 2007; O'Brien 2007).
As outlined above, there are a number of ongoing studies which will contribute to the evidence relating to the use of progesterone for women considered at risk of preterm birth. The trials by Crowther (Crowther 2007), Norman (Norman 2012), and Perlitz (Perlitz 2007) will contribute data from women with a prior preterm birth; van Os (van Os 2011) will contribute data from women with a short cervix identified by ultrasound; Nassar (Nassar 2007), and Wood (Wood 2007) will contribute data from women with a multiple pregnancy; and Martinez (Martinez 2007) will contribute data from women who present with symptoms or signs of threatened preterm labour.
Quality of the evidence
Overall, the trials included in this review were considered to be of good to fair quality. Of the 36 included trials, adequate randomisation methods were described in 23 (Borna 2008; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Facchinetti 2007; Glover 2011; Grobman 2012; Hassan 2011; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012; Senat 2012; Serra 2013), allocation concealment was assessed to be at low risk of bias in 23 (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hassan 2011; Johnson 1975; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013), and blinding was achieved with the use of a placebo agent in 25 (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hartikainen 1980; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Rouse 2007; Serra 2013; Sharami 2010).Twenty‐one of the 36 included studies were assessed as being at low risk of bias for other potential sources of bias (Borna 2008; Briery 2011; Caritis 2009; Combs 2010; Combs 2011; da Fonseca 2003; Facchinetti 2007; Fonseca 2007; Glover 2011; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Saghafi 2011a; Sharami 2010).
We assessed whether studies that included multiple pregnancies accounted appropriately for non‐independence of babies from the same pregnancy in the analysis. There were 14 studies that included a multiple pregnancy (Aboulghar 2012; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Elsheikhah 2010; Fonseca 2007; Hartikainen 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013) and in seven studies adjustment appears to have been made in the analysis (Caritis 2009; Combs 2010; Combs 2011; Fonseca 2007; Lim 2011; Norman 2009; Rode 2011). In the remaining seven studies (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010; Hartikainen 1980; Rouse 2007;Senat 2012; Serra 2013), it is not clear that any adjustment was made. There was not enough information in the trial reports for us to carry out the necessary adjustments ourselves in the analysis of neonatal outcomes. This information will be sought for future updates.
Potential biases in the review process
The possibility of introducing bias was present at every stage of the reviewing process. We attempted to minimise bias in a number of ways: two review authors assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias is not an exact science and includes many personal judgements.
Agreements and disagreements with other studies or reviews
Despite the differences in methodology and included studies, our findings of a reduction in preterm birth before 34 weeks' and 28 weeks' gestation for women with a short cervix identified on ultrasound examination, and a reduction in infant respiratory distress syndrome are consistent with those reported in an individual patient data meta‐analysis by Romero and colleagues (Romero 2012). Similarly, the findings of a proposed individual patient data meta‐analysis for women with a multiple pregnancy are awaited to evaluate if there are specific subgroups of women with a multiple pregnancy who may receive benefit from progesterone therapy (Schuit 2012).
Authors' conclusions
Implications for practice.
Summary of the available information
Progesterone for women with a past history of spontaneous preterm birth
The use of progesterone in this group of women is associated with a reduction in the risk of perinatal death, preterm birth before 34 weeks' and 37 weeks' gestation, infant birthweight less than 2500 g, use of assisted ventilation, necrotising enterocolitis, neonatal death, admission to neonatal intensive care unit, and prolongation of pregnancy. There is limited information about longer‐term childhood health. In addition, further information is required as to the optimal route of administration of progesterone, the optimal dose to be administered, and the best time to commence therapy.
Progesterone for women with a short cervix identified on ultrasound
The use of progesterone in this group of women is associated with a reduction in the risk of preterm birth less than 34 and 28 weeks' gestation, and a significant increase in the risk urticaria in women receiving progesterone. Further information is required about other maternal, infant and childhood health outcomes. Of particular note, there are no comparative data reported on longer‐term childhood health from trials conducted to date. In addition, further information is required as to the optimal route of administration of progesterone, the optimal dose to be administered, and the best time to commence therapy.
Progesterone for women with a multiple pregnancy
The use of progesterone in this group of women is not associated with any statistically significant differences in perinatal death, preterm birth and other maternal, infant, and childhood health outcomes.
Progesterone for women following presentation with threatened preterm labour
The use of progesterone in this group of women was associated with a statistically significant reduction in the risk of infant birthweight less than 2500 g. However, for all other outcomes, the role of progesterone for women presenting following symptoms or signs of threatened preterm labour is uncertain.
Progesterone for women with 'other' risk factors for preterm birth
The use of progesterone in this group of women was associated with a statistically significant reduction in the risk of infant birthweight less than 2500 g. However, for all other outcomes, the role of progesterone in women considered to be at risk of preterm birth for 'other reasons' is uncertain.
Implications for research.
Further well‐designed randomised controlled trials are required to assess the optimal timing, mode of administration and dose of administration of progesterone when given to women considered to be at increased risk of early birth, by virtue of previous history of spontaneous preterm birth, short cervix identified by transvaginal ultrasound, following arrest of symptoms or signs of threatened preterm labour, or on the basis of 'other' risk factors. Assessment of longer‐term infant and childhood outcomes remains a priority.
There are several randomised trials that are currently addressing the use of progesterone for preterm birth which will contribute data in the future ‐ seeCharacteristics of ongoing studies for details.
What's new
| Date | Event | Description |
|---|---|---|
| 27 July 2015 | Amended | Corrected spelling of included study Fonseca 2007 (was previously listed as Fonesca 2007). Text has been added to Published notes about this review being split into two reviews following two new protocols. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 13 August 2013 | Amended | Robert Cincotta added to the byline. Contribution to this update added. |
| 5 August 2013 | Amended | Contact details amended for Caroline Crowther. |
| 12 June 2013 | New citation required and conclusions have changed | This updated review is now comprised of 36 included studies (involving 8523 women and 12,515 infants). There are now 11 ongoing studies. The results and conclusions have changed: Progesterone versus placebo for women with a history of preterm birth: now significant findings for perinatal mortality; significant findings for additional infant and maternal secondary outcomes. Progesterone versus placebo for women with a short cervix: significant findings now identified for secondary infant outcome (preterm birth less than 28 weeks' gestation) and secondary maternal outcome (adverse drug reaction (urticaria); now no significant findings for neonatal sepsis. Progesterone versus placebo for women with a multiple pregnancy: now no significant findings for any of the reported outcomes. Progesterone versus no treatment/placebo for women following presentation with threatened preterm labour: now no significant findings for two secondary infant outcomes (preterm birth less than 37 weeks' gestation; respiratory distress syndrome (RDS)). Progesterone versus placebo for women with 'other' risk factors for preterm birth: now significant findings for one secondary infant outcome (infant birthweight less than 2500 g). |
| 14 January 2013 | New search has been performed | Search updated. Data from 25 new trials incorporated into this update. |
| 27 January 2012 | Amended | Fifty‐two reports for 44 trials added to Studies awaiting classification. |
| 31 December 2008 | New search has been performed | Search updated. A search in October 2007 identified 17 new trials. We included five (Borna 2008; Facchinetti 2007; Fonseca 2007; O'Brien 2007; Rouse 2007); added a follow‐up report to Meis 2003; and excluded one (Walch 2005). Ten trials are ongoing (Bruinse 2007; Maurel 2007; Grobman 2007; Martinez 2007; Nassar 2007; Perlitz 2007; Rode 2007; Rozenberg 2007; Serra 2007; Wood 2007). A further updated search in December 2008 identified one more report of Borna 2008; five more reports of O'Brien 2007; six more reports of Rouse 2007; one more report of Crowther 2007; one more report of Bruinse 2007; three ongoing studies (Creasy 2008; Starkey 2008; Swaby 2007); and one study which is awaiting classification (Moghtadaei 2008a). The review's conclusions have not changed. |
| 5 November 2008 | Amended | Converted to new review format. |
| 31 March 2005 | New search has been performed | Search updated and new studies found and included or excluded. |
Notes
This review examines prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Women considered in this review includes women considered at high risk because of multiple pregnancy as well as women with singleton pregnancies considered at high risk for various clinical reasons (history of preterm birth, short cervix, threatened preterm labour and other risk factors). This review included 36 trials with several trials recruiting only women with multiple pregnancies. The results of this review may be easier to interpret and more clinically relevant if the results for women with multiple and singleton pregnancy are assessed and reported separately.
Consequently, this review will no longer be updated in it's current form but will be split into two separate reviews:
Prenatal administration of progesterone for preventing preterm birth in women with a multiple pregnancy
Prenatal administration of progesterone for preventing preterm birth in women with a singleton pregnancy
Each of these reviews will be prepared following publication of a new protocol.
Acknowledgements
We thank Lynn Hampson for searching the trials register.
We would like to thank Gabriella Behzadi for the translation of the paper by Akbari 2009 et al.
We would like to thank Therese Dowswell, Research Associate in the Cochrane Pregnancy and Childbirth Group, for her support and advice with the update in 2013.
As part of the pre‐publication editorial process, this review has been commented on by six peers (an editor and five referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods used for previous version of this review
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (December 2008).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane LIbrary 2008, Issue 1). See below for search terms.
We did not apply any language restrictions.
Searching other resources
We also manually cross referenced key publications.
We did not apply any language restrictions.
We searched the International Clinical Trials Register (using the terms pregnancy, progesterone and ante/prenatal) to identify ongoing registered clinical trials.
Search terms for CENTRAL
Search terms included free text terms pregnancy, preterm birth, progesterone, progestogen, intramuscular, vaginal, oral, perinatal morbidity, perinatal mortality, and randomis(z)ed controlled trial. Please contact review author for exact strategy.
Appendix 2. Methods for the previous version of this review
We used the standard methods of The Cochrane Collaboration (Higgins 2008). Review authors independently assessed trials for inclusion in the review and extracted the data. Any differences were resolved by discussion with all co‐authors.
Assessment of risk of bias in included studies
The methodology used to assess risk of bias of studies included in the previous version of this review is given in Appendix 2.
For this update, the following methods were used.
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g., random number table; computer random number generator);
inadequate (any non random process, e.g.,, odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g., telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies will be judged at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. Blinding will be assessed separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
adequate;
inadequate:
unclear;
where 'adequate' is less than 20% losses to follow‐up.
(5) Selective reporting bias
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2008). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses.
Data management and analysis
We conducted data management and analysis using RevMan software (RevMan 2008). V Flenady, R Cincotta and J Dodd independently extracted data. Results are reported as mean differences for continuous variables, and risk ratios for categorical outcomes, both with 95% confidence intervals.
We conducted the meta‐analysis using the fixed‐effect model, and assessed heterogeneity by visual inspection of the outcomes tables and by using two statistics (H and I2 test) of heterogeneity (Higgins 2002). Where we discovered statistical heterogeneity, this was explored.
Results are presented by reason women were considered to be at risk of preterm birth, including:
past history of spontaneous preterm birth (including preterm premature rupture of membranes);
multiple pregnancy;
ultrasound identified short cervical length;
fetal fibronectin testing;
presentation with symptoms or signs of threatened preterm labour;
other reason for risk of preterm birth.
Planned subgroup analyses included an assessment of the effect of: (1) time of treatment commencing (before 20 weeks' gestation versus after 20 weeks' gestation); (2) route of administration (intramuscular, intravaginal, oral, intravenous); and (3) different dosage regimens (divided arbitrarily into a cumulative dose of less than 500 mg per week and a dose of greater than or equal to 500 mg per week). To evaluate the effect of subgroup comparisons, we considered confidence intervals (where non‐overlap was taken to indicate a statistically significant difference).
Data and analyses
Comparison 1. Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 6 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.33, 0.75] |
| 1.1 Intramuscular | 3 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.73] |
| 1.2 Vaginal | 2 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.29] |
| 1.3 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.59] |
| 2 Preterm birth less than 34 weeks | 5 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.69] |
| 2.1 Intramuscular | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Vaginal | 4 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.44] |
| 2.3 Oral | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.90] |
| 3 Preterm birth less than 37 weeks | 10 | 1750 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.74] |
| 3.1 Intramuscular | 4 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.52, 0.75] |
| 3.2 Vaginal | 5 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.92] |
| 3.3 Oral | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.11] |
| 4 Threatened preterm labour | 2 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.47, 1.62] |
| 4.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.87] |
| 4.2 Vaginal | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.11] |
| 5 Spontaneous vaginal delivery | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.18] |
| 5.1 Vaginal | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.18] |
| 6 Caesarean section | 3 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.20] |
| 6.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 6.2 Vaginal | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.30] |
| 7 Antenatal corticosteroids | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 7.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 7.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.26] |
| 8 Antenatal tocolysis | 4 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.35] |
| 8.1 Intramuscular | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 8.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.74] |
| 8.3 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.35] |
| 9 Infant birthweight less than 2500 g | 4 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.79] |
| 9.1 Intramuscular | 3 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.81] |
| 9.2 Vaginal | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.74] |
| 10 Respiratory distress syndrome | 3 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.16] |
| 10.1 Intramuscular | 1 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.04] |
| 10.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.43] |
| 10.3 Oral | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.30] |
| 11 Use of assisted ventilation | 3 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.90] |
| 11.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.01] |
| 11.2 Oral | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.92] |
| 11.3 Vaginal | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.07, 0.81] |
| 12 Intraventricular haemorrhage ‐ all grades | 3 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.20, 2.46] |
| 12.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.82] |
| 12.2 Vaginal | 2 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.46, 3.77] |
| 13 Intraventricular haemorrhage ‐ grade III or IV | 2 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.21, 11.75] |
| 13.1 Intramuscular | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.12, 52.09] |
| 13.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.55] |
| 14 Periventricular leucomalacia | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.52] |
| 14.1 Vaginal | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.52] |
| 15 Retinopathy of prematurity | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.69] |
| 15.1 Intramuscular | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.69] |
| 16 Necrotising enterocolitis | 3 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.89] |
| 16.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.03] |
| 16.2 Vaginal | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.92] |
| 17 Neonatal sepsis | 3 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.08, 2.23] |
| 17.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.35, 3.59] |
| 17.2 Vaginal | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 1.01] |
| 18 Patent ductus arteriosus | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.18] |
| 18.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.18] |
| 19 Intrauterine fetal death | 4 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.26, 1.69] |
| 19.1 Intramuscular | 3 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.14, 1.69] |
| 19.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.33, 4.51] |
| 20 Neonatal death | 6 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.76] |
| 20.1 Intramuscular | 3 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.87] |
| 20.2 Vaginal | 2 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.18] |
| 20.3 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.59] |
| 21 Developmental delay | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 2.04] |
| 21.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 2.04] |
| 22 Intellectual impairment | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.05, 31.34] |
| 22.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.05, 31.34] |
| 23 Motor Impairment | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.76] |
| 23.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.76] |
| 24 Visual Impairment | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.16, 4.57] |
| 24.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.16, 4.57] |
| 25 Hearing Impairment | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.24] |
| 25.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.24] |
| 26 Cerebral palsy | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.48] |
| 26.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.48] |
| 27 Learning difficulties | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.92] |
| 27.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.92] |
| 28 Height less than 5th centile | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.49] |
| 28.1 Intramuscular | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.49] |
| 29 Weight less than 5th centile | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.05] |
| 29.1 Intramuscular | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.05] |
| 30 Adverse drug reaction | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.15] |
| 30.1 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.15] |
| 31 Pregnancy prolongation (weeks) | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | 4.47 [2.15, 6.79] |
| 31.1 Oral | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | 4.47 [2.15, 6.79] |
| 32 Apgar score < 7 | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.25] |
| 32.1 Intramuscular | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.25] |
| 33 Admission to neonatal intensive care unit | 3 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.14, 0.40] |
| 33.1 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.49] |
| 33.2 Vaginal | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.49] |
| 34 Neonatal length of hospital stay (days) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.67, 5.67] |
| 34.1 Oral | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.67, 5.67] |
| 35 Infant weight at 6 months follow‐up (g) | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 29.0 [‐209.62, 267.62] |
| 35.1 Vaginal | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 29.0 [‐209.62, 267.62] |
| 36 Infant weight at 12 months follow‐up (g) | 1 | 379 | Mean Difference (IV, Fixed, 95% CI) | ‐88.0 [‐381.48, 205.48] |
| 36.1 Vaginal | 1 | 379 | Mean Difference (IV, Fixed, 95% CI) | ‐88.0 [‐381.48, 205.48] |
| 37 Infant weight at 24 months follow‐up (g) | 1 | 287 | Mean Difference (IV, Fixed, 95% CI) | ‐40.0 [‐482.41, 402.41] |
| 37.1 Vaginal | 1 | 287 | Mean Difference (IV, Fixed, 95% CI) | ‐40.0 [‐482.41, 402.41] |
| 38 Infant length at 6 months follow‐up (cm) | 1 | 430 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.59, 0.79] |
| 38.1 Vaginal | 1 | 430 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.59, 0.79] |
| 39 Infant length at 12 months follow‐up (cm) | 1 | 376 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 39.1 Vaginal | 1 | 376 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 40 Infant length at 24 months follow‐up (cm) | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.23, 0.83] |
| 40.1 Vaginal | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.23, 0.83] |
| 41 Infant head circumference at 6 months follow‐up (cm) | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.23, 0.43] |
| 41.1 Vaginal | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.23, 0.43] |
| 42 Infant head circumference at 12 months follow‐up (cm) | 1 | 372 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 42.1 Vaginal | 1 | 372 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 43 Infant head circumference at 24 months follow‐up (cm) | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.21, 0.61] |
| 43.1 Vaginal | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.21, 0.61] |
1.37. Analysis.
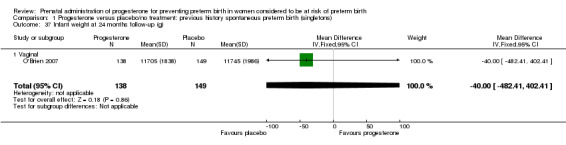
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 37 Infant weight at 24 months follow‐up (g).
Comparison 2. Progesterone versus placebo/no treatment: previous history spontaneous preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 37 weeks | 6 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.89] |
| 1.1 Therapy commences before 20 weeks | 4 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.04] |
| 1.2 Therapy commences after 20 weeks | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.78] |
Comparison 3. Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 5 | 1403 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.35, 0.97] |
| 1.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.18] |
| 1.2 Dose >= 500 mg per week | 3 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.21] |
| 2 Preterm birth less than 37 weeks | 9 | 1700 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.74] |
| 2.1 Dose < 500 mg per week | 3 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.44, 0.80] |
| 2.2 Dose >= 500 mg per week | 6 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.85] |
| 3 Threatened preterm labour | 2 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.47, 1.62] |
| 3.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.87] |
| 3.2 Dose >= 500 mg per week | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.11] |
| 4 Caesarean section | 3 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 4.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 4.2 Dose >= 500 mg per week | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 5 Antenatal corticosteroids | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 5.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 5.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.26] |
| 6 Need for tocolysis | 3 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.52] |
| 6.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 6.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.74] |
| 7 Respiratory distress syndrome | 4 | 1359 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.89] |
| 7.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.05] |
| 7.2 Dose >= 500 mg per week | 3 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.23] |
| 8 Intraventricular haemorrhage ‐ all grades | 3 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.20, 2.46] |
| 8.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.82] |
| 8.2 Dose >= 500 mg per week | 2 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.46, 3.77] |
| 9 Intraventricular haemorrhage ‐ grade III or IV | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.21, 11.73] |
| 9.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.12, 51.92] |
| 9.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.55] |
| 10 Necrotising enterocolitis | 3 | 1170 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.25] |
| 10.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.03] |
| 10.2 Dose >= 500 mg per week | 2 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.15, 1.95] |
| 11 Intrauterine fetal death | 3 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.32, 2.91] |
| 11.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 6.51] |
| 11.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.33, 4.51] |
| 12 Neonatal death | 5 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.28, 0.81] |
| 12.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.03] |
| 12.2 Dose >= 500 mg per week | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.26, 0.99] |
3.8. Analysis.
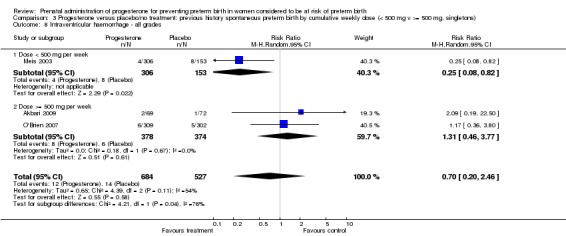
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 8 Intraventricular haemorrhage ‐ all grades.
Comparison 4. Progesterone versus placebo: ultrasound identified short cervix, singletons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 3 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.29] |
| 1.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.17] |
| 1.2 Intramuscular | 1 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.46, 2.72] |
| 2 Preterm birth less than 34 weeks | 2 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.90] |
| 2.1 Vaginal | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.87] |
| 2.2 Intramuscular | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.43, 1.46] |
| 3 Preterm labour | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 3.1 Intramuscular | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 4 Prelabour spontaneous rupture of membranes | 2 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.68, 2.62] |
| 4.1 Intramuscular | 2 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.68, 2.62] |
| 5 Side effects (any) | 2 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 5.1 Intramuscular | 2 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 6 Side effects (injection site) | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.17] |
| 6.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.17] |
| 7 Side effects (urticaria) | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [1.11, 22.78] |
| 7.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [1.11, 22.78] |
| 8 Side effects (nausea) | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.83] |
| 8.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.83] |
| 9 Pregnancy prolongation (days) | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.29, 6.29] |
| 9.1 Intramuscular | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.29, 6.29] |
| 10 Caesarean section | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 10.1 Intramuscular | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 11 Antenatal tocolysis | 2 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.11] |
| 11.1 Intramuscular | 2 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.11] |
| 12 Preterm birth less than 37 weeks | 3 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 12.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 12.2 Intramuscular | 2 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.28] |
| 13 Preterm birth less than 28 weeks | 2 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.93] |
| 13.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 0.97] |
| 13.2 Intramuscular | 1 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.30] |
| 14 Infant birthweight less than 2500 g | 3 | 1379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 14.1 Vaginal | 2 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 14.2 Intramuscular | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.30] |
| 15 Respiratory distress syndrome | 4 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 1.00] |
| 15.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.29, 0.85] |
| 15.2 Intramuscular | 2 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.58] |
| 16 Apgar score < 7 | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 16.1 Intramuscular | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 17 Need for assisted ventilation | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.16] |
| 17.1 Vaginal | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.16] |
| 18 Intraventricular haemorrhage ‐ all grades | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 18.1 Vaginal | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 19 Intraventricular haemorrhage ‐ grades III or IV | 2 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.17, 5.60] |
| 19.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.73] |
| 19.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.18, 22.08] |
| 20 Periventricular leucomalacia | 3 | 1282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.38, 8.24] |
| 20.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Intramuscular | 2 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.38, 8.24] |
| 21 Retinopathy of prematurity | 2 | 916 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.23, 4.42] |
| 21.1 Vaginal | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.25, 104.70] |
| 21.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.21] |
| 22 Necrotising enterocolitis | 3 | 1374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.78] |
| 22.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.30, 3.11] |
| 22.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 23 Neonatal sepsis | 3 | 1374 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.20] |
| 23.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.15, 2.25] |
| 23.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.97] |
| 24 Intrauterine fetal death | 3 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 24.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.28, 2.42] |
| 24.2 Intramuscular | 1 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.04 [0.45, 35.92] |
| 25 Neonatal death | 4 | 1571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.13] |
| 25.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.15, 1.15] |
| 25.2 Intramuscular | 2 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.16] |
| 26 Admission to neonatal intensive care unit | 2 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 26.1 Intramuscular | 2 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
Comparison 5. Progesterone versus placebo: ultrasound identified short cervix, singletons by cumulative weekly dose (<500 mg v >=500 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Periventricular leucomalacia | 2 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.13, 16.87] |
| 1.1 Dose < 500 mg per week | 1 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.45, 35.81] |
| 1.2 Dose >= 500 mg per week | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.73] |
| 2 Admission to neonatal intensive care unit | 2 | 834 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.72, 1.65] |
| 2.1 Dose < 500 mg per week | 1 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.27] |
| 2.2 Dose >= 500 mg per week | 1 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.83, 2.55] |
Comparison 6. Progesterone versus placebo: multiple pregnancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 7 | 4136 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 1.1 Intramuscular | 4 | 2228 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.30, 3.71] |
| 1.2 Vaginal | 3 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.41] |
| 2 Preterm birth less than 34 weeks | 6 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.27] |
| 2.1 Vaginal | 5 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 2.2 Intramuscular | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.73, 2.59] |
| 3 Preterm PROM | 3 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.74, 1.70] |
| 3.1 Intramuscular | 2 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.72, 1.71] |
| 3.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.74] |
| 4 Adverse drug reaction | 2 | 1162 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.19] |
| 4.1 Intramuscular | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.54, 1.01] |
| 4.2 Vaginal | 1 | 494 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.08] |
| 5 Caesarean section | 8 | 3136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.02] |
| 5.1 Intramuscular | 5 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 5.2 Vaginal | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.84, 0.98] |
| 6 Spontaneous birth | 2 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.38] |
| 6.1 Intramuscular | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.75, 1.04] |
| 6.2 Vaginal | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.21, 2.49] |
| 7 Assisted birth | 2 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.57, 1.76] |
| 7.1 Intramuscular | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 7.2 Vaginal | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.44, 1.24] |
| 8 Satisfaction with therapy | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.35, 0.35] |
| 8.1 Vaginal | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.35, 0.35] |
| 9 Antenatal tocolysis | 7 | 2642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 9.1 Intramuscular | 5 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 9.2 Vaginal | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 10 Antenatal corticosteroids | 2 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.26] |
| 10.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.17] |
| 10.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.81, 3.49] |
| 11 Preterm birth less than 37 weeks | 8 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.14] |
| 11.1 Intramuscular | 4 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.22] |
| 11.2 Vaginal | 4 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.85, 1.13] |
| 12 Preterm birth less than 28 weeks | 5 | 1855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.75, 1.95] |
| 12.1 Intramuscular | 3 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.68, 2.07] |
| 12.2 Vaginal | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.51, 3.19] |
| 13 Infant birthweight less than 2500 g | 7 | 5404 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 13.1 Intramuscular | 4 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.14] |
| 13.2 Vaginal | 3 | 1902 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.94] |
| 14 Apgar score < 7 at 5 minutes | 4 | 3451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.21] |
| 14.1 Intramuscular | 2 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 14.2 Vaginal | 2 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.28, 1.23] |
| 15 Respiratory distress syndrome | 6 | 5065 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 15.1 Intramuscular | 5 | 3732 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.42] |
| 15.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.79, 1.48] |
| 16 Use of assisted ventilation | 4 | 3392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.16] |
| 16.1 Intramuscular | 2 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 16.2 Vaginal | 2 | 1717 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.36] |
| 17 Intraventricular haemorrhage ‐ grades III or IV | 4 | 2368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.92] |
| 17.1 Intramuscular | 4 | 2368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.92] |
| 18 Intraventricular haemorrhage ‐ all grades | 2 | 2688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.75, 4.21] |
| 18.1 Intramuscular | 1 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.77] |
| 18.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.62, 4.66] |
| 19 Periventricular leucomalacia | 3 | 1091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.05, 3.02] |
| 19.1 Intramuscular | 3 | 1091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.05, 3.02] |
| 20 Retinopathy of prematurity | 5 | 3668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.91] |
| 20.1 Intramuscular | 4 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.20, 2.06] |
| 20.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.26, 4.07] |
| 21 Chronic lung disease | 2 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.13, 27.80] |
| 21.1 Intramuscular | 2 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.13, 27.80] |
| 22 Necrotising enterocolitis | 6 | 5059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.50, 1.75] |
| 22.1 Intramuscular | 5 | 3726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.52, 1.88] |
| 22.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.63] |
| 23 Neonatal sepsis | 6 | 5065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.62] |
| 23.1 Intramuscular | 5 | 3732 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.72] |
| 23.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.61, 2.13] |
| 24 Fetal death | 6 | 4788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.71, 2.09] |
| 24.1 Intramuscular | 3 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.77, 3.12] |
| 24.2 Vaginal | 3 | 2714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.35, 1.95] |
| 25 Neonatal death | 7 | 5170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.48, 2.10] |
| 25.1 Intramuscular | 4 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.31, 2.10] |
| 25.2 Vaginal | 3 | 2714 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.36, 4.95] |
| 26 Admission to NICU | 5 | 4251 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.18] |
| 26.1 Vaginal | 4 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 26.2 Intramuscular | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| 27 Perinatal death | 7 | 4133 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.61, 2.08] |
| 27.1 Intramuscular | 4 | 2228 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.30, 3.71] |
| 27.2 Vaginal | 3 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.67, 2.35] |
| 28 Preterm birth less than 34 weeks | 6 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.24] |
| 28.1 Vaginal | 5 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| 28.2 Intramuscular | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.73, 2.59] |
| 29 Preterm PROM | 3 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.60] |
| 29.1 Intramuscular | 2 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.72, 1.71] |
| 29.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.12] |
| 30 Caesarean section | 8 | 3136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.01] |
| 30.1 Intramuscular | 5 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 30.2 Vaginal | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.98] |
| 31 Antenatal tocolysis | 7 | 2642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 31.1 Intramuscular | 5 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 31.2 Vaginal | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.05] |
| 32 Antenatal corticosteroids | 2 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 32.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.17] |
| 32.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.76, 3.31] |
| 33 Preterm birth less than 37 weeks | 8 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.13] |
| 33.1 Intramuscular | 4 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.22] |
| 33.2 Vaginal | 4 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 34 Preterm birth less than 28 weeks | 5 | 1855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.80, 2.04] |
| 34.1 Intramuscular | 3 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.68, 2.07] |
| 34.2 Vaginal | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.63, 3.69] |
| 35 Infant birthweight less than 2500 g | 7 | 5401 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.89, 1.04] |
| 35.1 Intramuscular | 4 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.14] |
| 35.2 Vaginal | 3 | 1899 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.96] |
| 36 Apgar score < 7 at 5 minutes | 4 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.27] |
| 36.1 Intramuscular | 2 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 36.2 Vaginal | 2 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.41, 1.58] |
| 37 Use of assisted ventilation | 4 | 3389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 37.1 Intramuscular | 2 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 37.2 Vaginal | 2 | 1714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.59] |
| 38 Fetal death | 6 | 4785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.79, 2.29] |
| 38.1 Intramuscular | 3 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.77, 3.12] |
| 38.2 Vaginal | 3 | 2711 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.49, 2.48] |
| 39 Neonatal death | 7 | 5167 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.62, 2.21] |
| 39.1 Intramuscular | 4 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.31, 2.10] |
| 39.2 Vaginal | 3 | 2711 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.84, 3.72] |
| 40 Admission to NICU | 5 | 4248 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.17] |
| 40.1 Vaginal | 4 | 2893 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.07] |
| 40.2 Intramuscular | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| 41 Sensitivity analysis for perinatal death (assuming total non‐independence) | 7 | 2068 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.43, 1.65] |
| 41.1 Intramuscular | 4 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.29, 3.36] |
| 41.2 Vaginal | 3 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.28, 2.07] |
| 42 Sensitivity analysis for perinatal death (assuming 1% non‐independence) | 7 | 4091 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.44, 1.90] |
| 42.1 Intramuscular | 4 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.29, 3.58] |
| 42.2 Vaginal | 3 | 1888 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.41] |
Comparison 7. Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 37 weeks | 4 | 1467 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 1.1 Supplementation commenced prior to 20 weeks' gestation | 2 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 1.2 Supplementation commenced after 20 weeks' gestation | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.38, 2.54] |
| 2 Neonatal death | 3 | 2738 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.93] |
| 2.1 Supplementation commenced prior to 20 weeks' gestation | 2 | 1750 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.29, 2.91] |
| 2.2 Supplementation commenced after 20 weeks' gestation | 1 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.47, 3.81] |
| 3 Admission to NICU | 2 | 2343 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.95, 1.43] |
| 3.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.88, 1.26] |
| 3.2 Supplementation commenced after 20 weeks' gestation | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
Comparison 8. Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 7 | 4136 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 1.1 Dose < 500 mg per week | 4 | 2228 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.30, 3.71] |
| 1.2 Dose >= 500 mg per week | 3 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.41] |
| 2 Preterm birth less than 34 weeks | 6 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.27] |
| 2.1 Dose < 500 mg per week | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.73, 2.59] |
| 2.2 Dose > 500 mg per week | 5 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 3 Antenatal tocolysis | 7 | 2642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 3.1 Dose < 500 mg per week | 5 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 3.2 Dose >= 500 mg per week | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 4 Preterm birth less than 37 weeks | 8 | 3489 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.06] |
| 4.1 Dose < 500 mg per week | 6 | 2380 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.88, 1.15] |
| 4.2 Dose >= 500 mg per week | 4 | 1109 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 5 Infant birthweight less than 2500 g | 7 | 5404 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 5.1 Dose < 500 mg per week | 4 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.14] |
| 5.2 Dose >= 500 mg per week | 3 | 1902 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.94] |
| 6 Respiratory distress syndrome | 6 | 5065 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 6.1 Dose < 500 mg per week | 5 | 3732 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.42] |
| 6.2 Dose >= 500 mg per week | 1 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.79, 1.48] |
| 7 Fetal death | 6 | 4788 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.51, 2.23] |
| 7.1 Dose < 500 mg per week | 3 | 2074 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.19, 33.68] |
| 7.2 Dose >= 500 mg per week | 3 | 2714 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.36, 2.16] |
| 8 Admission to NICU | 5 | 4251 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.18] |
| 8.1 Dose < 500 mg per week | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| 8.2 Dose >= 500 mg per week | 4 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
Comparison 9. Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 1.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 2 Preterm birth less than 34 weeks' gestation | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.65] |
| 2.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.72, 1.39] |
| 2.2 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.37, 2.27] |
| 3 Pregnancy prolongation (days) | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐8.42, 12.17] |
| 3.1 Intramuscular | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐7.41, 0.81] |
| 3.2 Vaginal | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 7.21 [2.39, 12.03] |
| 4 Pregnancy prolongation ‐ less than 1 week | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.07, 2.37] |
| 4.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.07, 2.37] |
| 5 Pregnancy prolongation ‐ 1.0 to 1.9 weeks | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.16, 24.33] |
| 5.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.16, 24.33] |
| 6 Pregnancy prolongation ‐ 2 weeks or more | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.42, 9.42] |
| 6.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.42, 9.42] |
| 7 Spontaneous birth | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.49] |
| 7.1 Intramuscular | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.49] |
| 8 Caesarean section | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.51, 1.60] |
| 8.1 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.51, 1.60] |
| 9 Use of tocolysis | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.58, 1.65] |
| 9.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.62] |
| 9.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.73] |
| 10 Preterm birth less than 37 weeks' gestation | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.31] |
| 10.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.06] |
| 10.2 Intramuscular | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.69] |
| 11 Infant birthweight less than 2500 g | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.98] |
| 11.1 Vaginal | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.98] |
| 12 Respiratory distress syndrome | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.49, 1.10] |
| 12.1 Vaginal | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.15] |
| 12.2 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 13 Intraventricular haemorrhage grade III or IV | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.53, 152.93] |
| 13.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.53, 152.93] |
| 14 Periventricular leucomalacia | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Use of assisted ventilation | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.37] |
| 15.1 Vaginal | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.37] |
| 16 Necrotizing enterocolitis | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.50, 18.69] |
| 16.1 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.50, 18.69] |
| 17 Neonatal sepsis | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.17, 1.68] |
| 17.1 Vaginal | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.00] |
| 17.2 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.05] |
| 18 Fetal death | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.75] |
| 18.1 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.75] |
| 19 Neonatal death | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.24] |
| 19.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 19.2 Vaginal | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.40] |
| 20 Neonatal length of hospital stay (days) | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐15.84, 11.53] |
| 20.1 Intramuscular | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐15.84, 11.53] |
| 21 Apgar score less than seven at five minutes | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 21.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 22 Prelabour spontaneous rupture of membranes | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.45] |
| 22.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.45] |
| 23 Preterm birth less than 28 weeks' gestation | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 23.1 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 24 Apgar score less than seven at five minutes | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 24.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 25 Admission to neonatal intensive care unit | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.27, 9.07] |
| 25.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.27, 9.07] |
9.21. Analysis.
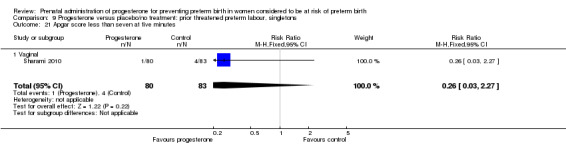
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 21 Apgar score less than seven at five minutes.
9.22. Analysis.
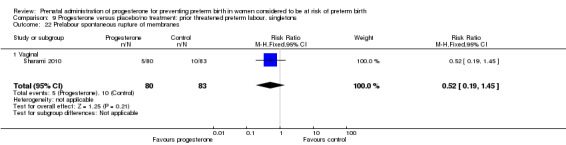
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 22 Prelabour spontaneous rupture of membranes.
9.23. Analysis.
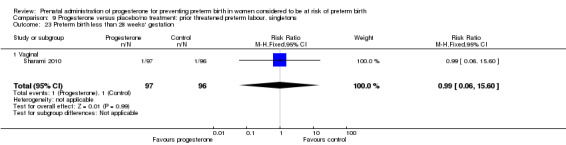
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 23 Preterm birth less than 28 weeks' gestation.
9.24. Analysis.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 24 Apgar score less than seven at five minutes.
9.25. Analysis.
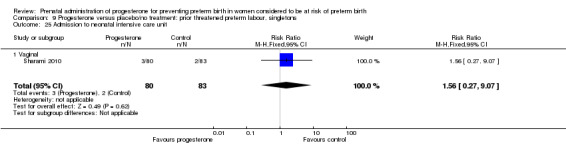
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 25 Admission to neonatal intensive care unit.
Comparison 10. Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy prolongation (days) | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐8.42, 12.17] |
| 1.1 Dose < 500 mg per week | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐7.41, 0.81] |
| 1.2 Dose >= 500 mg per week | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 7.21 [2.39, 12.03] |
| 2 Preterm birth less than 37 weeks' gestation | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.31] |
| 2.1 Dose >=500 mg per week | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.06] |
| 2.2 Dose <500 mg per week | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.69] |
| 3 Respiratory distress syndrome | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.49, 1.10] |
| 3.1 Dose >=500 mg per week | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.15] |
| 3.2 Dose <500 mg per week | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 4 Neonatal sepsis | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.17, 1.68] |
| 4.1 Dose >=500 mg per week | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.00] |
| 4.2 Dose <500 mg per week | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.05] |
| 5 Neonatal death | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.24] |
| 5.1 Dose <500 mg per week | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 5.2 Dose >=500 mg per week | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.40] |
Comparison 11. Progesterone versus placebo: other reason at risk of preterm birth, singletons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 3 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.09, 3.00] |
| 1.1 Intramuscular | 2 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.23, 5.29] |
| 1.2 Vaginal | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.55] |
| 2 Preterm birth less than 34 weeks | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 3.01] |
| 2.1 Vaginal | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 3.01] |
| 3 Preterm birth less than 37 weeks | 3 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.32, 1.13] |
| 3.1 Intramuscular | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.56] |
| 3.2 Vaginal | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 0.98] |
| 4 Infant birthweight less than 2500 g | 3 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.91] |
| 4.1 Intramuscular | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.18] |
| 4.2 Vaginal | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.16] |
| 5 Intrauterine fetal death | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.45] |
| 5.1 Intramuscular | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.45] |
| 6 Neonatal death | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [0.27, 112.73] |
| 6.1 Intramuscular | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [0.27, 112.73] |
| 7 Admission to neonatal intensive care unit | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.11] |
| 7.1 Vaginal | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.11] |
11.7. Analysis.
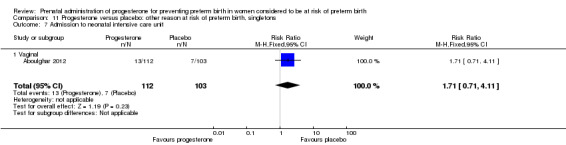
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 7 Admission to neonatal intensive care unit.
Comparison 12. Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.23, 5.29] |
| 1.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.23, 5.29] |
| 1.2 Supplementation commenced after 20 weeks' gestation | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Preterm birth less than 37 weeks | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.56] |
| 2.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.33, 3.66] |
| 2.2 Supplementation commenced after 20 weeks' gestation | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 3 Infant birthweight less than 2500 g | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.65] |
| 3.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.30, 2.27] |
| 3.2 Supplementation commenced after 20 weeks' gestation | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aboulghar 2012.
| Methods | Single‐centre prospective placebo‐controlled randomised clinical trial. IVF Center, Cairo, Egypt. |
|
| Participants | 313 women at high risk of preterm birth with pregnancies conceived by IVF or ICSI. Inclusion criteria: Healthy pregnant women who conceived after IVF/ICSI between 18‐24 weeks of gestation, with a first pregnancy, singleton or dichorionic twins, normal uterine and cervical anatomy, and normal fetal anatomy. Exclusion criteria: Previous pregnancy, serious fetal anomalies for which termination may be considered such as major heart anomaly or major CNS anomaly. All women received progesterone injections as luteal phase support which they continued if pregnant until the day of the first ultrasound |
|
| Interventions | Vaginal progesterone 200 mg twice daily from randomisation until delivery or 37 weeks’ gestation. Total number randomised: n = 161 women (161 analysed, 210 babies). Placebo vaginal suppositories from randomisation until 37 weeks’ gestation. Total number randomised: n = 152 women (145 women analysed, 187 babies). |
|
| Outcomes | Primary outcomes were: preterm birth of singleton and twin pregnancies before 37 completed weeks and before 34 completed weeks. Secondary outcomes: neonatal morbidity and mortality (live‐born children who died < 28 days after delivery) and take‐home baby rate (live‐birth rate per patient). Birthweight > 2500 g; 1500‐2500 g; < 1500 g; NICU admissions. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States, “Dark, sealed envelopes containing the intervention taken from a table of numbers” – not clear how the table of number generated – does not state “random number table”. |
| Allocation concealment (selection bias) | Low risk | Refers to “dark, sealed, sequentially numbered envelopes” and the envelopes were picked by a nurse not involved in the study. The envelopes had been created by a third party not involved in the allocation process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow diagram clearly displays participant flow in the study. 410 women recruited, 313 randomised; none lost to follow‐up in progesterone group and 6 lost to follow‐up in placebo group and 1 excluded because of termination of pregnancy after diagnosis of trisomy 21. States “Intention–to‐treat principle was followed during data analysis.” |
| Selective reporting (reporting bias) | Low risk | All expected outcome results reported. |
| Other bias | Unclear risk | None apparent, although baseline characteristics table not presented. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States “single blinding” and that “the patient was informed about the allocated arm” so presumably the clinician/personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear, but probably low risk. Placebo‐controlled trial. |
Akbari 2009.
| Methods | Randomised. Single centre, Lorestan. |
|
| Participants | 150 women randomised: 75 to each group. Inclusion criteria: 1. Single child pregnancy with the exact age of conception based on LMP was determined and was verified by sonogram before reaching 20 weeks. If the LMP was not available the exact age of pregnancy was based on 2 sonograms that were verified on at least 2 separate weeks; 2. Women with a history of 1 or 2 previous early childbirths before reaching 37 weeks of pregnancy or women with a history of prophylactic cervical cerclage or uterine anomalies (unicornuate uterus, bicornuate uterus, septate uterus, arcuate uterus, uterus didelphys); and 3. Older than 18 years, younger than 35 years. Exclusion criteria: 1. Rupture of membranes PROM; 2. Large known fetal anomalies; 3 Cervix dilatation larger than 4 cm; 4. Contraindications for tocolysis including fetal distress, chorioamnionitis, pre‐eclampsia, and haemodynamic instability; 5. Allergies to progesterone (dizziness, mygan, visual disturbances, depression, and increased blood sugar during previous consumption of this drug were considered allergic reactions to the hormone.); 6. Not following up with patients; 7. Multiple pregnancies; 8.The existence of an illness in the mother that necessitated medication, such as high blood pressure, cancer, tension, thromboembolic disease, Kennedy’s disease, illnesses that are treated for asthma with oral beta‐adrenergic; 9. Age younger than 18 or older than 35; 10. Existence of IUGR fetuses; 11. Unwarranted vaginal bleeding. |
|
| Interventions | Experimental intervention: 100 mg of prophylactic vaginal progesterone daily between 24th and 34th week of gestation ‐ Cyclogest. Control/Comparison intervention: the other group received no treatment and were monitored. |
|
| Outcomes | Mean gestational age at time of delivery. Preterm delivery before 37th week gestation. Preterm delivery before 34th week gestation. Respiratory distress syndrome. Low birthweight. Birthweight. Need for oxygen. Infant Apgar score. Need for mechanical ventilator. Hospitalisation in NICU. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear “150 women that had passed the entrance criterion to the study were divided randomly into two groups of 75.” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 individuals from the control group and 4 from the group receiving progesterone were excluded from the study – reasons for exclusion not clear – but in table of results – 6 people appear to be missing from denominator for the progesterone group (report 69) and not 4 as described? Results presented for 69 women in progesterone group and 72 in control. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported. |
| Other bias | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Borna 2008.
| Methods | Method of randomisation: random number table. Allocation concealment: unclear. Blinded outcome assessment: no. Completeness of follow‐up: outcome data available for 70 women. | |
| Participants | 70 women presenting between 24 and 34 weeks' gestation with symptoms and signs of threatened preterm labour, where acute symptoms were arrested following use of tocolytic medication. | |
| Interventions | Daily intravaginal pessary (400 mg) versus no treatment. | |
| Outcomes | Interval from randomisation to birth. | |
| Notes | Trial conducted in Tehran, Iran. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data available. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in the abstract appear to have been reported upon (latency period until delivery; respiratory distress syndrome; low birthweight; birthweight; recurrent preterm birth; admission to intensive care unit; neonatal sepsis). |
| Other bias | Low risk | Baseline characteristics were similar (see table 1, page 61). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of care givers and women. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
Briery 2011.
| Methods | Placebo‐controlled double‐blind randomised clinical trial. Single site, USA. |
|
| Participants | 69 women randomised: 33 to 17‐OH group and 36 to placebo. Inclusion criteria: women who presented with singleton, vertex gestations to university’s obstetric emergency area with a diagnosis of PPROM at 20‐30 weeks' gestation, typically dated by ultrasound, were eligible. Exclusion criteria: severe fetal or placental disease that might bias neonatal outcomes such as intrauterine growth restriction (< 5th percentile), suspected placental abruption, and confirmed placenta previa. Also excluded were patients already taking 17P, and some with signs and symptoms of chorioamnionitis, non‐reassuring fetal assessments or severe medical/obstetric diseases such as sickle cell disease with the crisis, insulin‐dependent diabetes, and severe pre‐eclampsia. |
|
| Interventions | Experimental intervention: weekly injections of 17P (250 mg) until 34 weeks or delivery, whichever came first. Control/Comparison intervention: weekly injections of placebo until 34 weeks or delivery, whichever came first. |
|
| Outcomes | Gestational age at birth; route of delivery; indications for delivery; birthweight; 5‐minute Apgar score; total NICU days; significant neonatal morbidity (sepsis, seizures); respiratory distress syndrome; patent ductus arteriosis; intraventricular haemorrhage; necrotising enterocolitis; bronchopulmonary dysplasia; death during neonatal period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Central allocation – pharmacy‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients lost – 1 person from 17P group who was previously enrolled in another study and 1 in placebo group refused the injection. “All 69 were analyzed (intention to treat).” |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes, apart from intraventricular haemorrhage, are reported in tables – although number of seizures were recorded. |
| Other bias | Low risk | No significant differences in demographic statistics between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Patients, their families, research personnel, and physicians/nurses were not aware of the study group assignment”. Also described as “double‐blind”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Caritis 2009.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. 14 centres, USA. |
|
| Participants | 134 women randomised: 71 to 17 alph‐hydroxyprogesterone caproate; 63 to placebo Inclusion criteria: pregnant women with triplets were eligible if their gestational age was at least 16 weeks and no more than 20 weeks. Exclusion criteria: serious fetal anomalies, 2 or more fetuses in 1 amniotic sac, suspected twin‐to‐twin transfusion syndrome, marked ultrasonographic growth discordance, planned non study progesterone therapy after 16 weeks, in‐place or planned cerclage, major uterine anomaly, unfractionated heparin therapy at any dose, and major chronic medical diseases. |
|
| Interventions | Experimental intervention: weekly injections of 17‐OHPC* (250 mg in 1 mL castor oil) starting at 16‐20 weeks and ending at delivery or 35 weeks' gestation. *17 Alpha‐Hydroxyprogesterone Caproate Control/Comparison intervention: weekly injections of placebo (1mL castor oil) starting at 16‐20 weeks and ending at delivery or 35 weeks' gestation. |
|
| Outcomes | Primary outcomes: composite of delivery or fetal loss before 35 completed weeks of gestation (245 days) – fetal loss included: miscarriage, termination, or stillbirth occurring any time after randomisation. Secondary outcomes: selected individual maternal and neonatal outcomes and a composite of serious neonatal outcomes. Composite serious adverse neonatal outcomes included: neonatal death, respiratory distress syndrome, culture‐proven sepsis, necrotising enterocolitis stage II or III, bronchopulmonary dysplasia, intraventricular haemorrhage grade III or IV, or periventricular leucomalacia or severe retinopathy of prematurity stage III or higher. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The simple urn method of randomization with stratification according to clinical center, was used to create a randomization sequence for each center.” |
| Allocation concealment (selection bias) | Low risk | The injections were prepared by a research pharmacy and boxes of 17‐OHPC and placebo were packaged for each centre according to randomisation sequences – so appears to be central allocation – pharmacy‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Outcome data were available for 100% of the assigned women, and for all of the 402 fetuses.” No exclusions apparent. ITT stated in statistical methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported. |
| Other bias | Low risk | The baseline characteristics of the 2 study groups were similar (Table 1). This study included women with multiple pregnancies (triplet) and there appears to be adjustment for neonatal binary outcomes ‐ used log binomial regression to calculate relative risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The participating women, their caregivers, and the research personnel were not aware of the study group assignment”. Also described as “double‐blinded”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Cetingoz 2011.
| Methods | Randomised placebo‐controlled double‐blind study. Department of Obstetrics and Gynecology, Istanbul. |
|
| Participants | 160 women randomised: 84 allocated to intervention and 76 allocated to placebo. Inclusion criteria: high‐risk pregnant women: twin pregnancies; pregnancies with at least 1 spontaneous preterm birth; uterine malformation. Exclusion criteria: not stated. |
|
| Interventions | Experimental intervention: micronized progesterone (100 mg) administered daily by vaginal suppository between 24 and 34 weeks of gestation. Control/Comparison intervention: placebo (100 mg) administered daily by vaginal suppository between 24 and 34 weeks of gestation. |
|
| Outcomes | Delivery < 37 weeks. Delivery < 34 weeks. Preterm labour admission. NICU admission. Neonatal death. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number list ‐ “Patients were allocated according to randomised number table". |
| Allocation concealment (selection bias) | Low risk | Random‐number list generated centrally by research hospital pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 170 high‐risk women were eligible – 10 women were excluded before randomisation due to abortion (n = 2), delivery between 20 and 24 weeks (n = 7) and application of cervical cerclage (n = 1). 160 women were randomised – 10 lost during follow‐up, 6 from the placebo group and 4 from intervention group. 150 women analysed (intervention group ‐ n = 80 ‐ prior preterm birth = 37; uterine malformation = 4; twin gestation = 39) and (placebo group ‐ n = 70 ‐ prior preterm birth = 34; uterine malformation = 8; twin gestation = 28). Analysis was performed according to ITT principle. |
| Selective reporting (reporting bias) | Low risk | Yes – all expected outcomes reported |
| Other bias | Unclear risk | Groups were similar in regard to age, pregravid BMI, parity, abortion, and ratio of high‐risk groups according to baseline characteristics table. There were no statistically significant differences in demographics. This study included singleton and twin pregnancies ‐ Odd ratio presented, but does not state whether any adjustments made in the analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The participating women, their care‐givers, and the research personnel were unaware of the woman’s study‐group assignments.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Combs 2010.
| Methods | Double‐blind, randomised clinical trial. Multicentre, Obstetrix Collaborative Research Network, USA. |
|
| Participants | 81 women randomised: 56 allocated to 17P and 25 to placebo. Inclusion criteria: mothers carrying trichorionic‐triamniotic triplets – confirmed at 15‐23 week detailed second‐trimester ultrasound examination – showing normal amniotic fluid volume and no major fetal anomalies. Exclusion criteria: women with symptomatic uterine contractions, rupture of fetal membranes, any contraindication to interventions intended to prolong the pregnancy, a pre‐existing medical condition that might be worsened by progesterone, or a pre‐existing medical condition carrying a high risk of preterm delivery. Women less than 18 years of age, had an allergy to 17P or the oil vehicle, had taken any progesterone‐derivative medication after 15 weeks of gestation, or had undergone placement of cervical cerclage for treatment of cervical change in the current pregnancy. |
|
| Interventions | Experimental intervention: 17 alpha‐hydroxyprogesterone caproate (17P) (250 mg in 1 mL castor oil) – weekly injections starting at 16‐22 weeks and continued until 34 weeks or delivery. Weekly repeat injections were carried out at the site or at home with partner administering after training. Injection diary for partner injections and measurement of unused medication returned by patient used to assess compliance with home administration. Control/Comparison intervention: identical appearing placebo (in 1 mL castor oil). |
|
| Outcomes | Primary outcomes: composite neonatal morbidity defined as 1 or more of: perinatal death (stillbirth, neonatal death, miscarriage); respiratory distress syndrome; use of oxygen therapy at 28 days of life; neonatal sepsis proven by blood culture; pneumonia; intraventricular haemorrhage (grade III or IV); periventricular leucomalacia; necrotising enterocolitis requiring surgery; retinopathy of prematurity; newborn asphyxia. Secondary outcomes: individual neonatal morbidities listed above; gestational age at delivery; birthweight; maternal side effects. Other outcomes reported: Mean weeks of gestation. Delivery before 28, 32 or 35 week. Reason for delivery before 32 weeks (spontaneous; indicated). Reason for delivery, all deliveries (spontaneous; indicated). Caesarean delivery. Tocolysis used. Antenatal corticosteroids. Maternal complications: Pre‐eclampsia or gestational hypertension. Gestational diabetes. Chorioamnionitis. Sepsis. Postpartum endometritis. Neonatal outcomes: Birthweight, g. Head circumference, cm. Total hospital stay, days. NICU. Intermediate care. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme. |
| Allocation concealment (selection bias) | Low risk | Random‐number generated centrally by pharmacy. “Progesterone or identical‐appearing placebo was compounded by pharmacy and shipped in advance to each study site in coded prenumbered kits. To randomise the research nurse contacted the central pharmacy by telephone or fax to obtain the code number for the kit assigned to that patient.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 248 women identified with triplets, 147 eligible for trial inclusion, of these 89 gave consent (61%) and were given trial injection. Of these, 81 (91%) returned for randomisation. No loss – 81 women randomised and outcome data available for all 81 mothers and 243 offspring. “Analysis was by the “intention‐to‐treat” principle. Accordingly, outcomes for each patient were tabulated according to the assigned group (17P vs placebo) regardless of her compliance.” |
| Selective reporting (reporting bias) | Low risk | Yes – all expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics of the participants were similar. There were no significant difference between the groups in the percentage with cervix length less than 2.5 cm 17% in the 17P group versus 35% in the placebo group or with positive fibronectin, 11% versus 9%. Mean compliance was 98.5% in the 17P group and 96.1% in the placebo group. There was no significant difference between groups in the percentage of injections given by clinic staff (27% versus 30%), by patients themselves (13% versus 15%), or by their designated representatives (61% versus 56%). Included multiple pregnancies (triplet) ‐ adjustment made in analysis ‐ used repeated measures model, where each infant was considered as a repeated measure. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Subjects, physicians, and study personnel remained blinded as to group assignment until after completion of the trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Data were abstracted by study personnel who remained blinded to each subject’s group assignment.” |
Combs 2011.
| Methods | Double‐blind, randomised clinical trial. Multicentre – 18 sites, Obstetrix Collaborative Research Network, USA. |
|
| Participants | 240 women randomised: 160 allocated to 17Pc and 80 to placebo. Inclusion criteria: women were eligible if they had a dichorionic‐diamniotic twin pregnancy at 15‐23 weeks' gestation and if they had completed a detailed ultrasound examination – showing no major fetal anomalies. Exclusion criteria: women were excluded if they were < 18 years old; had taken any progestins > 15 weeks of gestation; or had symptomatic uterine contractions, rupture of the fetal membranes, any contraindication to prolonging the pregnancy, any pre‐existing condition that might be worsened by progesterone, or a pre‐existing medical condition carrying a high risk of preterm delivery. |
|
| Interventions | Experimental intervention: 17 alpha‐hydroxyprogesterone caproate (17P) (250 mg in 1 mL castor oil) – weekly injections starting at 16‐24 weeks and continued until 34 weeks or delivery. Weekly repeat injections were carried out at the site or at home with partner administering after training. Injection diary for partner injections and measurement of unused medication returned by patient used to assess compliance with home administration. Control/Comparison intervention: identical appearing placebo (in 1 mL castor oil). |
|
| Outcomes | Primary outcomes: composite neonatal morbidity defined as 1 or more of: perinatal death (stillbirth, neonatal death, miscarriage); respiratory distress syndrome; use of oxygen therapy at 28 days of life; neonatal sepsis proven by blood culture; pneumonia; intraventricular haemorrhage (grade III or IV); periventricular leucomalacia; necrotising enterocolitis requiring surgery; retinopathy of prematurity; newborn asphyxia. Secondary outcomes: individual neonatal morbidities listed above; gestational age at delivery; birthweight; maternal side effects. Other outcomes reported: Mean weeks of gestation. Delivery before 28, 32 or 34 or 37 weeks. Reason for delivery before 37 weeks (spontaneous; indicated). Caesarean delivery. Tocolysis used. Antenatal corticosteroids. Maternal complications: Pre‐eclampsia or gestational hypertension. Gestational diabetes. Chorioamnionitis. Sepsis. Postpartum endometritis. Neonatal outcomes: Birthweight, g. Birthweight < 2500 g. Birthweight < 1500 g. Birthweight < 1000 g. Small‐for‐gestational age. All births. Births < 2500 g. Head circumference, cm. Total hospital stay, days. NICU. Intermediate care. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme. |
| Allocation concealment (selection bias) | Low risk | Random‐number generated centrally by pharmacy. “Progesterone or identical‐appearing placebo was compounded by pharmacy and shipped in advance to each study site in coded prenumbered kits. To randomise the research nurse contacted the central pharmacy by telephone or fax to obtain the code number for the kit assigned to that patient.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1450 women identified with twin pregnancy, 254 eligible and consented and were given trial injection. Of these, 240 returned for randomisation. No loss in progesterone group – 160 women allocated, 160 mothers delivered and 320 perinates with known outcome. 80 women allocated to placebo – 2 lost to follow‐up – 78 women delivered and 156 perinates with known outcome. “Outcomes for each patient were tabulated according to assigned group regardless of her compliance.” |
| Selective reporting (reporting bias) | Low risk | Yes – all expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics of the subjects were similar. Mean compliance was 96.4% in the 17P group and 98.7% in the placebo group. Included multiple pregnancies (twin) ‐ adjustment made in analysis ‐ used repeated measures model, where each infant was considered as the repeated measure. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Subjects, physicians, and study personnel remained blinded as to group assignment until after completion of the trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Combs 2011a.
| Methods | Double‐blind, placebo‐controlled randomised clinical trial. Multicentre – 18 sites, Obstetrix Collaborative Research Network, USA. |
|
| Participants | 12 women randomised: 4 allocated to 17P and 8 to placebo. The trial was terminated early because of 2 separate issues related to the supply of 17P. Inclusion criteria:women were eligible if they were at least 18 years old, had a singleton pregnancy at 23.0 to 31.9 weeks of gestation and PROM. Exclusion criteria: women were excluded with contraindications to expectant management; with known fetal abnormalities; with history of allergy to 17P or castor oil; with medical conditions that might adversely interact with 17P; with medical conditions treated with systemic steroid medications; or with a cervical cerclage present at the time of PROM. |
|
| Interventions | Experimental intervention: 17 alpha‐hydroxyprogesterone caproate (17P) (250 mg in 1 mL castor oil) – weekly intramuscular injections Control/Comparison intervention: identical appearing placebo (in 1 mL castor oil). |
|
| Outcomes | Primary outcomes: prolongation of pregnancy until favourable gestational age. Composite neonatal morbidity defined as 1 or more of: stillbirth, neonatal death, infant death before hospital discharge; respiratory distress syndrome; intracranial haemorrhage grade III or IV; necrotising enterocolitis stage 2 or 3; culture proven sepsis within 72 hours of birth; periventricular leucomalacia. Secondary outcomes: pregnancy prolongation (interval from randomisation to delivery). Individual neonatal morbidities listed above; gestational age at delivery; birthweight; maternal side effects. Other outcomes reported: Delivery at 34 weeks or more. Delivery at 32‐33.9 weeks with documented fetal lung maturity. Mean gestational age at delivery. Delivery before 32 or 34 weeks. Pulmonary maturity testing. Latency – randomisation to delivery. Less than 1 week. 1.0 to 1.9 weeks. 2.0 2.0 weeks or more. Reason for delivery before 34 weeks (spontaneous; chorioamnionitis; fetal indications). Maternal complications: Pre‐eclampsia or gestational hypertension. Gestational diabetes. Chorioamnionitis. Sepsis. Caesarean delivery. Tocolysis in first 48 hours. Antenatal corticosteroids. Neonatal outcomes: Birthweight, g. Total hospital stay, days. NICU stay, days. Newborns with congenital anomaly. Adverse events not tabulated elsewhere. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence was used by the trial statistician to generate a randomisation code. |
| Allocation concealment (selection bias) | Low risk | Random‐number generated by a statistician and held centrally at each site’s inpatient pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up – but only 12 patients were randomised – 8 to placebo and 4 to 17P Appears to have been ITT. |
| Selective reporting (reporting bias) | Low risk | Yes – all expected outcomes reported . |
| Other bias | Unclear risk | The trial was stopped early due to 2 separate issues related to the supply of 17P. Only 12 patients were randomised and because of the early termination, the trial was grossly under‐powered to make conclusions as to the efficacy or safety of 17P in women with PROM. Too few patients to assess baseline imbalance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Participants and research personnel were blinded to group assignment throughout.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
da Fonseca 2003.
| Methods | Method of randomisation: random number table. Allocation concealment: sequential sealed envelopes; allocation to either drug A or B; allocation of groups revealed after last woman birthed. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for 142 women (15 women excluded after randomisation). | |
| Participants | 157 women considered to be at 'high risk' for preterm birth due to history of previous preterm birth, cervical suture, uterine malformation. | |
| Interventions | Nightly intravaginal pessary of either 100 mg progesterone or placebo from 24 weeks until 28 weeks' gestation, or birth if earlier. | |
| Outcomes | Preterm birth before 37 weeks' gestation; preterm birth before 34 weeks' gestation. | |
| Notes | Trial conducted in Sao Paulo, Brazil. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Adequate; sequential sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 women (less than 1%) post‐randomisation exclusions. |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes (incidence of preterm delivery; frequency of uterine contractions). |
| Other bias | Low risk | "The two groups were found similar in regard to age, risk factors for preterm delivery, and obstetric history." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Caregivers and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded. |
Elsheikhah 2010.
| Methods | Described as “randomized controlled study” in abstract title. Single centre, Cairo, Egypt. |
|
| Participants | 100 women: 50 allocated to progesterone group and 50 to placebo. Inclusion criteria: women with twin pregnancy. Exclusion criteria: patients with anomalies were excluded. |
|
| Interventions | Experimental intervention: vaginal progesterone 200 mg daily for 10 weeks from the 24th to the 34th week of gestation. Control/Comparison intervention: placebo for the same duration. |
|
| Outcomes | Mean cervical length. Mean gestational age at delivery. Fetal complications (not specified). NICU admissions. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Data limited – only reported as an abstract. |
| Allocation concealment (selection bias) | Unclear risk | Data limited – only reported as an abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data limited – only reported as an abstract. |
| Selective reporting (reporting bias) | Unclear risk | Data limited – only reported as an abstract. |
| Other bias | Unclear risk | Data limited – only reported as an abstract. Multiple pregnancies ‐ no adjustment apparent from abstract report. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data limited – only reported as an abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data limited – only reported as an abstract. |
Facchinetti 2007.
| Methods | Method of randomisation: random number table. Allocation concealment: randomisation list managed by senior midwife; allocation to either progesterone or placebo. Blinding of outcome assessment: no. Completeness of follow‐up: outcome data available for 60 women. | |
| Participants | 60 women presenting between 25 and 33 + 6 weeks' gestation with symptoms and signs of threatened preterm labour, where acute symptoms were arrested following use of tocolytic medication (atosiban). | |
| Interventions | 341 mg intramuscular 17OHP administered every 4 days to 36 weeks' gestation. | |
| Outcomes | Cervical length as assessed by transvaginal ultrasound. Secondary outcomes included preterm birth < 37 weeks, and infant birthweight. | |
| Notes | Trial conducted in Modena, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Unclear; list managed by "senior midwife" with allocation to either progesterone or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for all women. |
| Selective reporting (reporting bias) | High risk | Cervical length was measured by transvaginal ultrasound scanning at discharge and at day 7 and 21. Results for cervical length at discharge were not fully reported. All other outcomes appear to have been reported upon (cervical length at 7 and 21 days; preterm delivery < 37 weeks and < 35 weeks; birthweight). |
| Other bias | Low risk | No differences were found between the 2 groups for baseline characteristics (see table, page 3). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding ‐ not possible "The study was not double blind because it was not sponsored; therefore, the preparation of true placebo (same vial, same oil without active compound) was not possible." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessor. |
Fonseca 2007.
| Methods | Method of randomisation: not stated. Allocation concealment: central randomisation process; identically appearing treatment packs. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for all 250 women randomised. | |
| Participants | 250 women undergoing transvaginal ultrasound assessment of cervical length, where the cervical length was measured to be 15 mm or less. Women with both singleton and multiple pregnancies were eligible to participate (226 singleton and 24 with twin pregnancies). | |
| Interventions | Nightly intravaginal pessary of either 200 mg micronised progesterone or placebo from 24 weeks until 33 + 6 weeks' gestation, or birth if earlier. | |
| Outcomes | Primary outcome: spontaneous preterm birth less than 34 weeks' gestation. Secondary outcomes: infant birthweight, fetal death, neonatal death, major adverse outcomes (intraventricular haemorrhage, respiratory distress syndrome, retinopathy of prematurity, necrotising enterocolitis), need for neonatal special care (NICU, ventilation, phototherapy, treatment for proven or suspected sepsis, blood transfusion). | |
| Notes | Trial conducted in 5 maternity hospitals in London (UK), Santiago (Chile), Sao Paulo (Brazil), and Greece. This data analysed in subgroup of women with short cervix ‐ multiples only made up a small proportion of total group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation generation not stated. |
| Allocation concealment (selection bias) | Low risk | Adequate; central randomisation; identical appearing treatment packs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported (spontaneous delivery before 34 completed weeks; birthweight; fetal or neonatal death; major adverse outcomes before discharge; need for neonatal special care). |
| Other bias | Low risk | "There were no significant differences in baseline characteristics between the placebo and progesterone groups" (see Table 1, page 465). Singleton and twin pregnancies ‐ adjustment made for infant outcomes, “ the analyses of infant outcomes used robust standard errors and were clustered on a maternal identifier to account for the non‐independence of twin pairs.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants, caregivers, outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants, caregivers, outcome assessors. |
Glover 2011.
| Methods | Pilot, single‐centre, randomised, double‐blind, placebo‐controlled trial. Single centre, Miami Valley Hospital, Ohio, USA. |
|
| Participants | Inclusion criteria: women < 20 weeks' gestation and had at least 1 prior spontaneous preterm birth of a live‐born singleton infant between 200/7 weeks and 366/7 weeks' gestation. Exclusion criteria: multiple gestations, the presence of major fetal anomalies, progesterone use in current pregnancy, the presence of a cervical cerclage and the presence of a placenta previa. |
|
| Interventions | 36 women randomised: 20 allocated to progesterone group and 16 allocated to placebo group. Experimental intervention: 400 mg (2 200‐mg capsules) of oral micronized progesterone MP. Administration of the tables was initiated between 160/7 and 19 6/7weeks and was continued until the completion of the 33rd week of gestation. Control/Comparison intervention: control group took 2 identical placebo capsules for the same time period. |
|
| Outcomes | Rate of recurrent spontaneous preterm birth. Serum progesterone levels. Neonatal morbidity and mortality: birthweight (g) mean (SD); male gender; 5‐minute Apgar (mean); ventilator use; neonatal length of stay (mean), days. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was done by the hospital’s research pharmacy using a standard randomization table methodology for two groups.” |
| Allocation concealment (selection bias) | Low risk | Central allocation – pharmacy controlled: “After subjects were randomized to their respective group, the research pharmacy dispensed a 1‐month supply of either progesterone or placebo tablets in identical prescription bottles, which were labelled identically as “progesterone study medication.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45 patients were eligible for randomisation – 9 women didn't complete the initial evaluation or failed to present to the pharmacy for randomisation and were excluded. Appears that 36 were randomised, but only 33 analysed. 3 more participants were excluded – 1 from the MP group as became apparent that she had not had previous spontaneous preterm birth as she had been induced for severe eclampsia; 1 from the placebo group had a spontaneous abortion at 14 weeks; and another from placebo group did not complete her prenatal care at this centre and delivered elsewhere. 3 appear to have been excluded after randomisation, see above. Analysis appears to be ITT: 2 women ended their participation in the study – but both delivered at this institution and were included in their respective group for all analyses. |
| Selective reporting (reporting bias) | Unclear risk | In methods reports that neonatal mortality will be reported – but was not reported. |
| Other bias | Low risk | Similar baseline characteristics – no statistically significant differences between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The study subjects’ physicians were aware of the study participation but were blinded to the group assignment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Grobman 2012.
| Methods | Multicentre, randomised controlled trial. Multicentre, Maternal‐Fetal Units Network, USA. |
|
| Participants | 657 women recruited between April 2007 and May 2011 in various centres in the USA 657 women randomised: 327 to OHPc group and 330 to placebo. Inclusion criteria: nulliparous women with a singleton gestation between 16 and 22 3/7 weeks with cervical length less than 30 mm. Exclusion criteria: Women that had selective fetal reduction for multiple pregnancy, had evidence of additional fetal pole/embryo at 12 weeks or more, vaginal bleeding, prolapsed membranes, major fetal anomaly, current or planned cerclage, maternal medical condition associated with preterm delivery (e.g. hypertension) prior cervical surgery, or planned preterm delivery. |
|
| Interventions | Experimental intervention: 250 mg IM weekly 17 alpha hydroxyprogesterone caproate (17‐OHPc) given by nurse until delivery or up to 36 weeks and 6 days gestation. Control/Comparison intervention: An identical appearing placebo.Weekly IM injections of placebo (castor oil) given by study nurse until delivery or up to 36 weeks and 6 days gestation. |
|
| Outcomes | Maternal outcomes: Preterm birth before 37 weeks Birth before 35 weeks Preterm rupture of membranes Delivery < 28 weeks Tocolytic therapy Caesarean delivery Side effects (any; injection site; urticaria; nausea) Perinatal outcomes: Fetal death Neonatal death Respiratory distress syndrome Necrotising enterocolitis grade II or III Intraventricular haemorrhage grade II or IV Periventricular leucomalacia Early‐onset sepsis Retinopathy of prematurity grade III or IV Birthweight < 2500 g 5‐minute Apgar < 7 NICU admission Length of NICU stay, days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data co‐ordinating centre to created the computer‐generated randomisation sequence. Simple turn method of randomisation used. |
| Allocation concealment (selection bias) | Low risk | Simple randomisation method stratified by centre. Study treatments were research pharmacy prepared. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were accounted for in the analysis. Discontinuation of treatment was greater in the placebo group 33/330 vs 18/327 but there was an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported upon. |
| Other bias | Unclear risk | Groups appeared balanced at baseline (slightly more white Hispanic in the placebo group and this group were slightly younger). The study was stopped early as the study monitoring committee decided that further recruitment after data for 591 available for interim analysis would be unlikely to show benefit (planned 1000 women , data for 657). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was stated that neither patients nor medical staff were aware of treatment group and the study was placebo controlled. It was stated that the IM injections appeared identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was stated that the trial was blinded to research staff collecting outcome data. The trial was placebo controlled. |
Hartikainen 1980.
| Methods | Method of randomisation: stated to be "placebo controlled and double blind". Allocation concealment: not stated. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for 77 women. | |
| Participants | 77 women with a twin pregnancy. | |
| Interventions | Weekly intramuscular injection of either 250 mg 17‐hydroxyprogesterone caproate or placebo from 28 weeks' gestation until 37 weeks' gestation or birth if earlier. | |
| Outcomes | Perinatal death. | |
| Notes | Trial conducted in Finland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for all participants. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported (duration of pregnancy; spontaneous delivery before 37 weeks; weights of neonates; perinatal mortality; neonatal morbidity (respiratory problems; omphalitis; pulmonary infection); maternal levels of progesterone; estradiol). |
| Other bias | Unclear risk | "The gestational age at the onset of medication, the gestational age at diagnosis of twin pregnancy and the patient's age were similar in both groups." (see table 1, page 693). "The factors commonly regarded as risk factors for premature delivery showed no differences between groups." (see table 2, page 693). Multiple pregnancies ‐ abstract only available – limited data – no adjustment apparent. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Caregivers and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors. |
Hassan 2011.
| Methods | Multicentre randomised double‐blind placebo‐controlled trial. Multicentre – 44 centres in 10 countries, USA. |
|
| Participants | 465 women randomised: 236 allocated to progesterone and 229 to placebo. Inclusion criteria: singleton gestation; gestational age between 19 + 0 and 23 + 6 weeks; transvaginal sonographic cervical length between 10 and 20 mm; without signs and symptoms of preterm labour. Exclusion criteria: planned cerclage; acute cervical dilation; allergic reaction to progesterone; current or recent progestogen treatment within 4 weeks; chronic medical conditions that would interfere with study participation or evaluation of the treatment; major fetal anomaly or known chromosomal abnormality; uterine anatomic malformation; vaginal bleeding; known or suspected clinical chorioamnionitis. |
|
| Interventions | Experimental intervention: daily micronised vaginal progesterone gel – women self‐administered the study drug once daily in the morning. Each applicator delivered 1.125 g gel containing 90 mg progesterone. Control/Comparison intervention: an identical appearing placebo ‐ each applicator delivered 1.125 g gel containing 90 mg placebo. |
|
| Outcomes | Primary outcome: preterm birth before 33 weeks. Secondary outcomes: neonatal morbidity ‐ respiratory distress syndrome; bronchopulmonary dysplasia; intraventricular haemorrhage grade III or IV; periventricular leucomalacia; proven sepsis; necrotising enterocolitis; perinatal mortality (fetal death or neonatal death) – composite scores were used to assess perinatal mortality and morbidity. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation allocation was 1:1 and was accomplished using a centralised interactive voice response system. Randomisation was stratified according to centre and risk strata (previous preterm birth between 20 and 35 weeks or no previous preterm birth) using a permuted blocks strategy with a block size of 4. |
| Allocation concealment (selection bias) | Low risk | Reported that allocation concealment accomplished in 3 ways: 1. participant study kits at each site were numbered independently from the treatment assignments in the randomisation blocks; 2. IVR system specified which kit number was to be dispensed to the subject; 3. the study drug packaging , applicators and contents were identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 733 women eligible, 268 declined, 465 randomised. 1 lost to follow‐up from progesterone group and 6 from placebo group. ITT analysis performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported upon. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Hauth 1983.
| Methods | Method of randomisation: stated to be "randomised, double blind intervention". Allocation concealment: not stated. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for all women randomised. | |
| Participants | 168 women on active military duty. | |
| Interventions | Weekly intramuscular injection of either 1000 mg 17‐hydroxyprogesterone caproate or placebo from 16 to 20 weeks until 36 weeks' gestation, or birth if earlier. | |
| Outcomes | Preterm birth before 37 weeks' gestation; birthweight less than 2.5 kg; perinatal death. | |
| Notes | Trial conducted in Lackland Airforce Base, Texas, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for all women recruited. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported (low birthweight; perinatal mortality; pregnancy‐induced hypertension; premature labour). |
| Other bias | Low risk | The groups were similar for parity, previous abortion, race, cigarette smoking, and marital status. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Caregivers and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors. |
Ibrahim 2010.
| Methods | Randomised placebo‐controlled trial. Ain Shams University Maternity Hospital, Cairo, Egypt. |
|
| Participants | 50 women randomised: 25 allocated to progesterone group and 25 to placebo group. Inclusion criteria: singleton pregnant women in their second trimester with a history of preterm labour. Exclusion criteria: women with a history medical disease during pregnancy, multiple pregnancy, abdominal or cervical cerclage, known fetal anomalies or scarred uterus. |
|
| Interventions | Experimental intervention: 17‐α‐hydoxy progesterone caproate – 1 dose of 250 mg intramuscular progesterone‐ weekly until 36 weeks. Control/Comparison intervention: standard dose of placebo IM per week until 36 weeks. |
|
| Outcomes | Mean gestation age. Birth < 37 weeks. Birth > 37 weeks. Live neonates. Need for NICU. Neonatal death. Birthweight < 2500 g. Birthweight > 2500 g. Apgar score < 7. Apgar score > 7. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported – says women were divided into 2 groups –but no details of method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | “Randomisation was done by the use of sealed envelopes which were opened by the nurse responsible for giving the injections to all participants whether Cidolut depot or placebo” – unclear whether the envelopes were opaque and sequentially numbered – so nurse may have been able to foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to be included in the results – 25 in each group. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported upon. |
| Other bias | Unclear risk | Baseline demographic characteristics not fully reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double blind – but no details described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Johnson 1975.
| Methods | Method of randomisation: stated to be "random double blind fashion". Allocation concealment: next of identical drug packages. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for 43 women (7 women excluded after randomisation). | |
| Participants | 50 women with a history of 2 previous spontaneous abortions or previous preterm birth before 36 weeks' gestation. | |
| Interventions | Weekly intramuscular injection of either 250 mg 17‐hydroxyprogesterone caproate or placebo from 'booking' until 24 weeks' gestation. | |
| Outcomes | Preterm birth before 37 weeks' gestation; birthweight less than 2.5 kg; perinatal death. | |
| Notes | Trial conducted in Baltimore, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of sequence not stated. |
| Allocation concealment (selection bias) | Low risk | Adequate; identical appearing treatment packs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 women excluded post‐randomisation (1%). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported (premature delivery; birthweight; perinatal mortality). |
| Other bias | Low risk | Groups were similar for baseline characteristics, (see table 2, page 678), although placebo group included fewer smokers and less heavy smokers than progesterone group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Caregivers and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
Lim 2011.
| Methods | Multicentre, double‐bind, placebo‐controlled randomised trial. Multicentre, 55 obstetric clinics in Netherland. |
|
| Participants | 671 women randomised: 336 allocated to progesterone and 326 allocated to placebo. Inclusion criteria: women with a multiple pregnancy and gestational age between 15 and 19 weeks. Exclusion criteria: women with a previous spontaneous preterm birth before 34 weeks, serious congenital defects or death of 1 or more fetuses, early signs of twin‐to‐twin transfusion syndrome, or primary cerclage were excluded from participation. |
|
| Interventions | Experimental intervention: 1 mL 17‐α‐hydoxyprogesterone caproate (250 mg/mL in castor oil) – starting between 16 and 20 weeks and continuing to 36 weeks. Injections were administered at the clinic, by a general practitioner or, in case the patient or a family member had a background in medical practice, at the patient’s home. Control/Comparison intervention: 1 mL placebo (castor oil) – study medication and placebo were identical in packaging, colour and consistency. |
|
| Outcomes | Primary outcomes: composite adverse neonatal outcome – severe respiratory distress syndrome; bronchopulmonary dysplasia; intraventricular haemorrhage grade II B or worse;necrotising enterocolitis; proven sepsis; death before discharge. Secondary outcomes: Side effects (soreness, itching, and swelling). Gestational age at delivery. Preterm birth before 28, 32 and 37 weeks. Length of admission to the NICU. Maternal morbidity. Hospitalisation of the mother due to (threatened) preterm labour. Costs. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An independent data manager rendered a computer‐generated list that was stratified by chorionicity, parity, and number of multiples, using random blocks of maximum block size.” |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation – “Randomization was accessible through a website” and “Allocation code was known only to ACE Pharmaceuticals” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1865 women eligible, 1194 declined participation, 671 women entered trial. 336 randomised to progesterone group and no women lost to follow‐up/326 randomised to placebo and 4 lost to follow‐up. 681 children born to 336 women in progesterone group/ 680 children born to 331 women in placebo group. Neonatal outcome available for 681 in progesterone group and 674 in placebo group. States that “all analyses were based on the intention‐to‐treat principle.” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported upon. |
| Other bias | Low risk | Baseline characteristics similar. Multiple pregnancies (twin, triplet and 1 quadruplet) ‐ adjustments made for all neonatal, delivery, pregnancy and side effect outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Participants, caregivers, and data collectors were all blinded to allocation.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, caregivers, and data collectors were all blinded to allocation.” |
Majhi 2009.
| Methods | Prospective randomised trial. Department of Obstetrics and Gynaecology, Postgraduate Instistute of Medical Education and Research (PGIMER), Chandigarh, India. |
|
| Participants | 50 women randomised: 50 allocated to progesterone and 50 allocate to no treatment. Inclusion criteria: women at high risk for preterm birth, having a singleton pregnancy and current gestation 16‐24 weeks. High risk was defined by history of at least once prior spontaneous preterm birth of a singleton infant > 20 and < 37 weeks due to spontaneous labour or preterm rupture of fetal membranes. Exclusion criteria: women with multifetal gestation, congenital malformation in the fetus, current or planned cervical cerclage or with any associated medical disorder were excluded. |
|
| Interventions | Experimental intervention: 100 mg natural micronised progesterone capsule intravaginally once daily at bedtime from 20‐24 weeks' gestation until 36 weeks. Control/Comparison intervention: no placebo – just managed according to the institute protocol. |
|
| Outcomes | Primary outcomes: Preterm birth < 37 weeks. Preterm birth ≤ 34 weeks. Secondary outcomes: Maternal hospitalisation. Vaginal delivery. LSCS. Birthweight (Mean SD). NICU. Sepsis. Hyperbilirubinaemia. Necrotising enterocolitis. Cord pH (Mean SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number tables. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes – provided centrally by Dept Biostatistics and investigators were not involved in the randomisation procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 118 women met the inclusion criteria; 100 women consented and were included – 50 assigned to each group. There was no attrition during follow‐up. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported upon. |
| Other bias | Unclear risk | Both groups were similar in all characteristics except BV, which was commoner in the study group. It was treated in both groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported – no placebo used though – so participants would have been aware of assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Meis 2003.
| Methods | Method of randomisation: computer‐generated 2:1 random number schedule. Allocation concealment: next of identical drug packages. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for 463 women. | |
| Participants | 463 women with a history of previous spontaneous preterm birth; exclusion women with multiple pregnancy, known fetal anomaly, progesterone or heparin treatment during pregnancy, current or planned cervical cerclage, hypertension, seizure disorder. | |
| Interventions | Weekly intramuscular injection of either 250 mg 17‐hydroxyprogesterone caproate or placebo from 16 to 20 weeks until 36 weeks' gestation, or birth if earlier. | |
| Outcomes | Preterm birth before 37 weeks' gestation; birthweight less than 2.5 kg; stillbirth; neonatal death; intraventricular haemorrhage; respiratory distress syndrome; bronchopulmonary dysplasia; sepsis; necrotising enterocolitis; retinopathy of prematurity; patent ductus arteriosus. | |
| Notes | Trial conducted by the Maternal‐Fetal Medicine Network, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Adequate; identical appearing treatment packs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for all 463 women recruited. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported (preterm delivery < 37, < 35, < 32 weeks; pregnancy outcomes (e.g. caesarean delivery; chorioamnionitis; fetal and neonatal outcomes (e.g. birthweight; neonatal death; ventilatory support; necrotizing enterocolitis; proven sepsis). |
| Other bias | Low risk | Groups were similar for baseline characteristics, (see table 1, page 2382), although the women in the placebo group had had more previous preterm deliveries. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded. |
Moghtadaei 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial. Single site, Iran. |
|
| Participants | 260 women randomised to treatment ‐ number randomised to placebo not reported. Inclusion criteria: women in advanced maternal age – primiparous aged 35 years or more. Exclusion criteria: not reported. |
|
| Interventions | Experimental intervention: weekly injections of 17P (250 mg) starting at 16‐20 weeks' gestation until 34 weeks. Control/Comparison intervention: matching placebo. |
|
| Outcomes | Delivery before 37 weeks. Delivery before 35 weeks. Delivery before 32 weeks. Hypertension. Diabetes. Intrauterine growth restriction. Side effects at injection site. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only abstract available – data limited. |
| Allocation concealment (selection bias) | Unclear risk | Only abstract available – data limited. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract available – data limited. |
| Selective reporting (reporting bias) | Unclear risk | Only abstract available – data limited. |
| Other bias | Unclear risk | Only abstract available – data limited. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only abstract available – data limited – although states “double blind”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only abstract available – data limited. |
Ndoni 2010.
| Methods | Prospective randomised placebo‐controlled study. Single centre, Albania. |
|
| Participants | 121 women randomised into 3 groups: IM injection prontogest (n = 52); oral progesterone (n = 43); placebo (n = 26). Inclusion criteria: 15‐22 weeks' gestation at high risk for preterm labour, hospitalised in the pathology of pregnancy clinic. Exclusion criteria: not stated. |
|
| Interventions | Experimental intervention: Group 1: Daily intramuscular injection of 17 alpha‐hydroxyprogesterone caproate (Prontogest) (n = 52). Group 2: Oral progesterone (Utrogestan) (n = 43). Control/Comparison intervention: Group 3: Identical‐looking placebo (n = 26). |
|
| Outcomes | Not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “The participants were separated in three groups..” |
| Allocation concealment (selection bias) | Unclear risk | Data limited – only reported as an abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data limited – only reported as an abstract. |
| Selective reporting (reporting bias) | Unclear risk | No outcome data was reported in the abstract. |
| Other bias | Unclear risk | Data limited – only reported as an abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data limited – only reported as an abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data limited – only reported as an abstract. |
Norman 2009.
| Methods | Double‐blind randomised placebo‐controlled trial. Multicentre, 9 UK NHS hospitals – STOPPIT study (Study Of Progesterone for the Prevention of Preterm Birth In Twins), UK. |
|
| Participants | 500 women randomised: 250 allocated to progesterone and 250 allocated to placebo. Inclusion criteria: women with twin pregnancy, with gestation and chorionicity established by scan before 20 weeks’ gestation and attending the antenatal clinic during the recruitment period. Exclusion criteria: pregnancy complicated by a recognised structural or chromosomal fetal abnormality at the time of recruitment, or if they had contraindications to progesterone, planned cervical suture, planned elective delivery before 34 weeks’ gestation, or planned intervention for twin‐to‐twin transfusion before 22 weeks’ gestation. Women with higher multiple pregnancy were also excluded. |
|
| Interventions | Experimental intervention: daily vaginal progesterone gel 90 mg starting at 24 weeks and 0 days of gestation. Each applicator of intervention contained 1.45 g of gel and delivered 1.125 g of gel containing 8% progesterone Control/Comparison intervention: placebo gel – administered in the same way as active treatment, daily from 24 weeks' gestation. Each applicator of intervention contained 1.45 g of gel and delivered 1.125 g of gel containing 8% excipients. |
|
| Outcomes | Primary: Delivery or intrauterine death before 34 weeks. Secondary: Gestation at delivery. Mode of delivery. Duration of each stage of labour. Safety outcomes: Admission to neonatal unit. Duration of neonatal unit stay. Mother died. Intrauterine death. Neonatal death. Involved or prolonged inpatient maternal hospital admission. Involved persistent/significant maternal disability or incapacity. Life threatening. Chorioamnionitis or intrauterine infection. Congenital anomaly or birth defect. Maternal symptoms. Maternal satisfaction. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “We used a randomisation schedule with permuted blocks of randomly mixed sizes to make up treatment packs (either active or placebo) for every patient, which were held in individual hospital pharmacies until use.” |
| Allocation concealment (selection bias) | Low risk | Central allocation from research network – local researcher telephoned the interactive voice response randomisation application at the UK Clinical Research Network registered trials unit to be given a participant number that corresponded to a specific treatment pack. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1483 assessed for eligibility, 983 excluded (did not meet eligibility; declined participation), 500 enrolled and randomised. 250 randomised to each group, 3 lost to follow‐up from both treatment and control groups (6 altogether) – because of withdrawal of consent or not traceable after moving out of study area. 3 therefore excluded from each analysis. Analysis was by ITT. 494 mothers and 988 babies remained for the per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported. |
| Other bias | Low risk | Baseline characteristics similar. Multiple pregnancies ‐ adjustment for some neonatal outcomes – give Odds Ratios: admission to neonatal unit; duration of neonatal unit stay. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “All study personnel and participants were masked to treatment assignment for the duration of the study.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
O'Brien 2007.
| Methods | Method of randomisation: random number table. Allocation concealment: identical appearing sequentially numbered treatment packs. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for 611 of 659 women randomised (48 (7.3%) women lost to follow‐up). | |
| Participants | 659 women with a history of prior spontaneous preterm birth. Exclusions: adverse reaction to progesterone; progesterone treatment within 4 weeks of randomisation; medical conditions; suspected genital tract malignancy; thromboembolic disease; fetal anomaly; multiple pregnancy; planned cervical cerclage. | |
| Interventions | Nightly vaginal progesterone gel (90 mg) versus placebo. | |
| Outcomes | Preterm birth less than 32 weeks; Apgar scores, infant birthweight, NICU admission. Secondary analysis of data from O'Brien 2012: infant weight, length and head circumference at 6, 12 and 24 months. |
|
| Notes | Trial conducted in 53 centres worldwide. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Adequate; identical appearing treatment packs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for 611 of 659 women randomised (7.3% women lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported (preterm birth; maternal, fetal and neonatal outcomes), detailed in table 2, page 692. |
| Other bias | Low risk | Baseline characteristics similar between groups (see table 1, page 691). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded. |
Papiernik 1970.
| Methods | Method of randomisation: unclear. Allocation concealment: unclear. Blinded outcome assessment: yes. Completeness of follow‐up: outcome data available for 99 women. | |
| Participants | 99 women with a "high preterm risk score". | |
| Interventions | Every 3 days intramuscular injection of either 250 mg 17‐hydroxyprogesterone caproate or placebo from 28 weeks' gestation until 32 weeks' gestation. | |
| Outcomes | Preterm birth before 37 weeks' gestation; birthweight less than 2.5 kg; perinatal death. | |
| Notes | Trial conducted in Paris, France. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for 99 women randomised. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to tell from translation. |
| Other bias | Low risk | From the translation of the paper "Each group studied was similar in age, the number of previous pregnancies, the onset of treatment and risk of preterm birth". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Caregivers and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded. |
Rai 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial. Single centre, Dept Obstetrics & Gynaecology, University College of Medical Science and Guru Teg Bahadur Hospital, Delhi. |
|
| Participants | 150 women randomised: 75 allocated to progesterone and 75 allocated to placebo. Inclusion criteria: asymptomatic women aged between 18 and 35 years who were between 18 and 24 weeks of pregnancy, with a history of at least 1 spontaneous preterm delivery (between 20 weeks and 36 weeks plus 6 days) and with a singleton live pregnancy. Exclusion criteria: women with first trimester bleeding, PROM, multiple pregnancy, fetal anomalies or active liver disease were excluded. |
|
| Interventions | Experimental intervention: 100 mg oral micronised progesterone – twice a day from recruitment (18‐24 weeks) until 36 weeks or delivery. Control/Comparison intervention: placebo ‐ twice a day from recruitment (18‐24 weeks) until 36 weeks or delivery. |
|
| Outcomes | Primary: Mean prolongation of pregnancy in weeks and days of gestation. Gestational age at delivery: < 28. 28‐31 + 6. 32‐33 + 6. 34‐36 + 6. Secondary: Use of tocolysis. Adverse drug effects. Neonatal outcomes: Neonatal age at delivery, week. Birthweight, g. NICU stay. < 24 hours. 24 hours – 1 week. > 1 week. Apgar score at 1 minute. < 6. > 6. Apgar score at 10 minute. < 6. > 6. Neonatal deaths. Respiratory distress syndrome. RDS with septicaemia. RDS with hyperbilirubinaemia. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer‐generated numbers table.” |
| Allocation concealment (selection bias) | Low risk | Central allocation suggested ‐ random number table provided by the Department of Biostatistics. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 150 assessed for eligibility, all enrolled and randomised. 75 randomised to each group – and 1 lost to follow‐up from each group – 74 analysed in each group. ITT not mentioned – but 74 from each group analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported . |
| Other bias | Low risk | Baseline characteristics similar. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The patients and the medical staff were blinded to the study medication allocation until after the last patient had delivered and the study was complete.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Rode 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial. Multicentre, 17 centres in Denmark and Austria. |
|
| Participants | 677 women were randomised: 334 allocated to progesterone and 343 allocated to placebo Inclusion criteria: women with a live, diamniotic twin pregnancy and chorionicity assessed by ultrasound before 16 weeks’ gestation were eligible for recruitment. Exclusion criteria: age < 18 years; known allergy to progesterone or peanuts (active treatment contained peanuts); history of hormone‐associated thromboembolic disorders; rupture of membranes; treatment for or signs of twin‐to‐twin transfusion syndrome; intentional fetal reduction; known major structural or chromosomal fetal abnormality; known or suspected malignancy in genitals or breasts; known liver disease; women with higher‐order multiple pregnancies; women who did not speak and understand Danish or German. |
|
| Interventions | Experimental intervention: vaginal micronized progesterone pessaries (200 mg) – self‐administered daily by participants – starting from 20‐24 weeks until 34 weeks’ gestation. Control/Comparison intervention: vaginal placebo pessaries (200 mg) – self‐administered daily by participants – starting from 20‐24 weeks until 34 weeks’ gestation. |
|
| Outcomes | Primary: Incidence of delivery before 34 + 0 weeks’ gestation. Secondary: Delivery before 22, 28, 32, 37 weeks' gestation. Delivery by caesarean section (emergency and planned). Number of liveborn infants. Miscarriage. Intrauterine death. Infant death during delivery. Neonatal death. Death after 28 days. Sudden infant death. Corticosteroids for fetal lung maturation. Tocolytic therapy. Maternal adverse outcomes (gestational diabetes; increased liver enzymes; pre‐eclampsia; thromboembolic event). Birthweight < 2500 g. Birthweight < 1500 g. Apgar score < 7 at 5 minutes. Congenital or chromosomal anomalies. Perinatal complication: Hypoglycaemia. Intraventricular haemorrhage. Jaundice. Necrotising enterocolitis. Patent ductus arteriosis. Respiratory distress syndrome. Retinopathy of prematurity. Septicemia. Admission to NICU. CPAP treatment of at least 24 hours. Respirator treatment. Neurophysiological development 6 and 18 months after estimated date of delivery (assessed via Ages and Stages Questionnaire (ASQ). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The Perinatal Epidemiology Research Unit created a randomization sequence with a 1:1 ratio.” |
| Allocation concealment (selection bias) | Low risk | Central allocation suggested ‐ used an interactive voice‐response randomisation system: “We stratified by center and chorionicity using an interactive voice‐response randomization system at the Perinatal Epidemiology Research Unit. Each local researcher telephoned the randomization system, was given a randomization number that corresponded to a specific treatment box from a given batch". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1507 assessed for eligibility, 219 excluded, 1288 informed about the trial, 526 declined to participate/did not answer, 677 randomised – 343 to each group. 2 from placebo group lost to follow‐up ‐ 343 included in analysis for progesterone group and 341 in placebo group. 9 women in the progesterone group and 4 in the placebo group never started treatment because they changed their minds with respect to participation (n = 8), they miscarried or a fetus died in utero (n = 2) or they were withdrawn from the study (n = 3). Analyses based on 343 from progesterone group and 341 from placebo group. States that all analyses were performed according to the ITT principle. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported. |
| Other bias | Low risk | Baseline characteristics were comparable for the groups but there were slightly more monochorionic gestations in the placebo group – but the difference was not statistically significant. Multiple pregnancies ‐ adjustments made for infant outcomes – present Relative Risks. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “All participants and study personnel were blinded to treatment assignment for the duration of the trial, and the randomization code was not broken before all data had been collected, including the infant follow‐up at 18 months of age.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Appears that outcome assessment was blinded – “Only the statistician and the independent Data Monitoring and Safety Committee had access to unblinded data during the study period”. |
Rouse 2007.
| Methods | Method of randomisation: the "urn" method of randomisation. Allocation concealment: next of identical appearing treatment injections. Blinded outcome assessment: yes. Completeness of follow‐up: 661 women randomised with outcome data available for 655 women. | |
| Participants | 661 women with a twin pregnancy; exclusion women with known fetal anomaly, spontaneous fetal death of a fetus after 12 weeks, presumed monoamnionic placenta, suspected twin‐twin transfusion syndrome, marked ultrasonographic growth discordance, progesterone or heparin treatment during pregnancy, current or planned cervical cerclage, hypertension, insulin‐dependent diabetes, and twin pregnancies that were the result of intentional fetal reduction. | |
| Interventions | Weekly intramuscular injection of either 250 mg 17‐hydroxyprogesterone caproate or placebo (castor oil) from 16 ‐ 20 + 3 weeks until 34 completed weeks' gestation, or birth if earlier. | |
| Outcomes | Primary outcome: composite of delivery or death prior to 35 weeks' gestation.
Secondary outcomes: randomisation to delivery interval; composite adverse outcomes (retinopathy of prematurity, respiratory distress syndrome, sepsis, necrotising enterocolitis, bronchopulmonary dysplasia, grade III or IV intraventricular haemorrhage, periventricular leucomalacia), birthweight (less than 2500 g and less than 1500 g), 5‐minute Apgar score < 7, patent ductus arteriosus, pneumonia, mechanical ventilation, seizures. Preterm birth before 37 weeks' gestation; birthweight less than 2.5 kg; stillbirth; neonatal death; intraventricular haemorrhage; respiratory distress syndrome; bronchopulmonary dysplasia; sepsis; necrotising enterocolitis; retinopathy of prematurity; patent ductus arteriosus. |
|
| Notes | Trial conducted by the Maternal‐Fetal Medicine Network, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The simple urn method of randomisation with stratification according to clinical center was used by the George Washington University Biostatistical Co‐ordinating Center to create a randomization sequence for each center,..." |
| Allocation concealment (selection bias) | Low risk | Adequate; identical appearing treatment packs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for 655 of 661women (less than 1% loss to follow‐up). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported (delivery or fetal death before 35 weeks' gestation; other obstetric and neonatal outcomes) see table 2 and 3. |
| Other bias | Unclear risk | "Baseline demographic data were similar in the two study groups" (see table 1). Multiple pregnancies ‐ no adjustment in analysis apparent. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded. |
Rozenberg 2012.
| Methods | Open‐label randomised controlled trial. Multicentre, 13 French university hospitals. |
|
| Participants | 188 women randomised. Inclusion criteria: women with singleton pregnancies admitted at 24 + 0 through 31 + 6 weeks of gestation with a cervical length < 25 mm for an episode of preterm labour that was then successfully arrested by tocolytic treatment. A course of betamethasone 12 mg, repeated after 24 hours, was given intramuscularly in all patients. Exclusion criteria: cervical dilatation > 3cm, chorioamnionitis, fetal heart rate abnormalities, placenta previa, adruptio placentae, PROM, polyhydramnios, IUGR, pregnancy‐related hypertension or pre‐eclampsia, maternal or fetal condition requiring immediate delivery, anticonvulsive treatment or participation in any other trial. |
|
| Interventions | Experimental intervention: 500 mg of intramuscular 17 alpha‐hydroxyprogesterone caproate (17 P) started after tocolysis ended and repeated twice weekly until 36 weeks or until preterm delivery. Control/Comparison intervention: no treatment with 17P. Additional management in the 2 arms was left to the discretion of the attending physician, except that progesterone was not allowed in the control group. |
|
| Outcomes | Time from randomisation to delivery, preterm delivery before 37, 34 and 32 weeks, readmissions for preterm labour, NICU, birthweight, respiratory distress, bronchopulmonary dysplasia, necrotising enterocolitis, periventricular leucomalacia, perinatal death, severe maternal or neonatal adverse effect. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised, computer‐generated randomisation process in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 188 women were randomised. Outcome data available for 184 women. States “assessed according to the intention‐to‐treat principle”. |
| Selective reporting (reporting bias) | Low risk | All expected outcome results reported. |
| Other bias | Unclear risk | A subsequent letter to the journal questioned the use of the study medication unless it had been tested for purity and many samples of the medication did not meet FDA specifications. The letter stated that this casts doubt on the findings of any study using 17Pc. The authors confirmed that the study medication was not of questionable quality. Study groups appeared similar at baseline although 12% of the intervention group and 24% of controls had had a previous preterm delivery. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding ‐ trial is described as “open label”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. High risk of bias (randomisation to the control group may have affected subsequent treatment and outcome assessment). |
Saghafi 2011a.
| Methods | Described as an interventional study. Obstetrics Clinic of Ghaem Hospital, Mashad University of Medical Sciences, Iran. |
|
| Participants | 100 women randomised: 50 allocated to progesterone and 50 allocated to placebo. Inclusion criteria: pregnant women with a previous history of preterm delivery who had no contraindication for continuing pregnancy. Exclusion criteria: women who had entered the active phase of delivery, cases of preterm PROM, pre‐eclampsia, vaginal bleeding, maternal‐fetal diseases for which continuation of the pregnancy was dangerous, symptoms of distress, and fetal anomalies. |
|
| Interventions | Experimental intervention: 250 mg of intramuscular 17 alpha‐hydroxyprogesterone caproate (17 P) weekly from 16 weeks’ gestation up to a maximum of 37 weeks’ gestation. Control/Comparison intervention: routine perinatal care. |
|
| Outcomes | Preterm delivery (< 37 weeks). Apgar score at 1 and 5 minutes (mean values given). Newborn weight (g) mean values. Vaginal delivery (n). Low birthweight neonates (%) – didn't specify what low birthweight was. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ just says "They were randomly divided into two groups...". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss. Outcome data available for all 100 women, 50 randomised to each group. |
| Selective reporting (reporting bias) | Unclear risk | Data limited – only reported as an abstract. |
| Other bias | Low risk | States that the 2 groups were similar for age, gravidity, abortions, previous preterm births ‐ see table 1. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
Senat 2012.
| Methods | Open‐label multicentre, randomised controlled trial in France – 13 French university hospitals. | |
| Participants | 165 women randomised and 161 followed up – it was not clear how many were randomised to each group. Inclusion criteria: asymptomatic women with twin pregnancy and cervical length < 25 mm between 20 and 32 weeks of gestation. Exclusion criteria: not reported in the abstract. |
|
| Interventions | Experimental intervention: 500 mg intramuscular 17 alpha‐hydroxyprogesterone caproate (17P) – twice weekly until 36 weeks or until preterm delivery. Control/Comparison intervention: no 17P. Additional management in the 2 arms was left to the discretion of the attending physician, except that progesterone was not allowed in the control group. |
|
| Outcomes | Median time to delivery; delivery before 32 and 34 weeks' gestation. | |
| Notes | Not able to report any outcome data because the number randomised to each group is not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by computer‐generated randomisation process in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | States – central randomisation. “A centralised, computer generated randomised process in a 1:1 ratio.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear – limited data reported as only an abstract. |
| Selective reporting (reporting bias) | Unclear risk | Unclear – limited data reported as only an abstract. |
| Other bias | Unclear risk | Unclear – limited data reported as only an abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as an "open label trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Described as an "open label trial". |
Serra 2013.
| Methods | 3‐arm RCT in 5 centres in Spain, individual randomisation. | |
| Participants | 294 women attending 1 of 5 university hospital centres in Spain between December 2005 and January 2008. Inclusion criteria: women were recruited at 11‐13 weeks' gestation. If they had previously been treated with vaginal progesterone it was stopped. Women were 18 years or more, dichorionic, diamniotic twin pregnancy. Exclusion criteria: singleton pregnancy, mono‐chorionic twin pregnancies, triplets or higher multiple pregnancies, elective cervical cerclage before 14 weeks' gestation, history of hepatic problems, pregnancy cholestasis, abnormal liver or kidney function, allergy to peanuts or study medication, recurrent vaginal bleeding or infection, fetal anomalies, alcohol or illicit drug use and smoking more than 10 cigarettes per day. |
|
| Interventions | Intervention: 1. 200 mg vaginal progesterone self‐inserted daily at bedtime. (98 women). 2. 400 mg vaginal progesterone self‐inserted daily at bedtime (98 women). 3. (control) placebo vaginal pessaries self‐inserted daily at bedtime (98 women). All women were provided with specially manufactured identical looking pessaries, 2 to be administered each evening. Total number randomised: n = 294. |
|
| Outcomes | Preterm birth rate < 37 weeks of gestation; early preterm birth rate < 34, 32, 28 weeks of gestation; need for tocolytic treatment; steroid treatment; rate of preterm premature rupture of membranes < 37 weeks of gestation; cervical length measurements at 20, 24, 28 weeks of gestation; perinatal mortality and morbidity; caesarean section. Local tolerance to the treatment; number of serious systemic adverse effects. Perinatal outcomes: short‐term neonatal morbidity (respiratory distress syndrome; pneumonia; early onset sepsis; seizures; graded III‐IV intraventricular haemorrhage; stage II‐II necrotising enterocolitis; and/or patent duct arteriosus). Long‐term neonatal morbidity included: broncho‐pulmonary dysplasia; periventricular leucomalacia; and/or severe retinopathy of prematurity. Birthweight < 2500 g; 5 minute Apgar score; major congenital malformation; admission to NICU; mechanical ventilation; neonatal death. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was performed by computer (SPSS Random Number Generator, using a randomisation sequence 1:1:1 ratio (blocks of nine, with no stratification).” |
| Allocation concealment (selection bias) | Low risk | Central allocation “An external monitoring centre provided a randomisation code number for each pregnant woman” “The medication was given at each visit by the hospital pharmacy department." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was stated that an ITT analysis was carried out. There was very little loss to follow‐up. 294 randomised at 20 weeks. 290 analysed – analysed as ITT. Placebo group – n = 98, n = 10 discontinued study, n = 2 lost to follow‐up, 96 analysed 200 mg progesterone group = n = 98, n = 11 discontinued study, n = 1 lost to follow‐up, 97 analysed. 400 mg progesterone group = n = 98, n = 10 discontinued study, n = 1 lost to follow‐up, 97 analysed. |
| Selective reporting (reporting bias) | Unclear risk | Most expected outcomes reported upon. However – individual outcome results for short‐term and long‐term neonatal morbidity were not reported, e.g. respiratory distress syndrome, periventricular leucomalacia. |
| Other bias | Unclear risk | Baseline characteristics similar. Management may have differed in the 5 participating centres although this was not clear. Groups appeared similar at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and staff were blinded. Medication packs were coded and contained identical appearing pessaries. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was reported that all study personnel were blind to treatment allocation for the duration of the project. |
Sharami 2010.
| Methods | Described as placebo‐controlled double‐blind RCT. Alzhara Hospital, Iran. Recruitment between 2007 and 2009. | |
| Participants | Inclusion criteria:173 randomised. Women with singleton pregnancies, gestational age 28‐36 weeks admitted with threatened preterm labour successfully treated with tocolysis with intact membranes and less than 2cm cervical dilatation. Exclusion criteria: patients with intrauterine infection, vaginal bleeding, pre‐eclampsia, urinary tract infection, intrauterine growth retardation, hypertension and heart disease, dilatation ≥ 2 cm, fetal distress and fetal abnormalities. |
|
| Interventions | Intervention: n = 86 women. 200 mg vaginal progesterone daily until 36 weeks’ gestation. Control/comparison intervention: n = 87 women. Placebo vaginal suppositories until 36 weeks’ gestation. All patients (in both groups) received 12 mg betamethasone and antibiotic prophylaxis and advised to restrict physical activity. |
|
| Outcomes | Primary: Time until delivery (latency time) PTB before 34 and 37 weeks of gestation Secondary: Selected maternal and neonatal outcomes including nausea, headache, pre‐eclampsia, PROM, chorioamnionitis, post‐partum haemorrhage for maternal complications and birth weight, Apgar score, admission to the NICU, fetal death, neonatal death, RDS, sepsis, and intraventricular haemorrhage (IVH). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just states, “Random block allocation method” – no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 270 eligible, 97 women delivered within 48 hours, a total of 173 randomised. Placebo group – n = 87, n = 4 lost to follow‐up, 83 completed follow‐up – 83 included in analysis. Progesterone group = n = 86, n = 6 lost to follow‐up, 80 completed follow‐up – 80 included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not clear. Several outcomes stated as secondary outcomes in the methods were not reported (this may have been because there were no cases but this was not stated, e.g. fetal death, intraventricular haemorrhage). |
| Other bias | Low risk | Baseline characteristics similar. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both patients and physician were blinded to the type of suppositories. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind. Not clear if outcome assessment was blind. |
CNS: central nervous system CPAP: continuous positive airways pressure ITT: intention‐to‐treat ICSI: intracytoplasmic sperm injection IUGR: intrauterine growth restriction IVF: in vitro fertilisation LSCS: lower segment Cesarian section LMP: last menstrual period NICU: neonatal intensive care unit PROM: premature rupture of membranes PPROM: preterm premature rupture of membranes PTB:preterm birth RDS: respiratory distress syndrome SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2012 | RCT embedded in a longitudinal study, but comparison of progesterone versus cerclage, so not relevant to this review. |
| Arikan 2011 | Acute treatment – being used as a tocolytic. |
| Berghella 2010 | Women were randomised to cerclage versus no cerclage – not randomised to 17P use or not – stratified at randomisation to intent to use or not use 17P. |
| Breart 1979 | Progesterone administration for prevention of miscarriage. |
| Brenner 1962 | Progesterone administration for prevention of miscarriage. |
| Chandiramani 2012 | Not clear that this is an RCT and also compares progesterone with cerclage, so not relevant to this review. |
| Corrado 2002 | Progesterone administered after amniocentesis for the prevention of miscarriage. |
| Hobel 1986 | Compares an oral progesterone agent with placebo but the only outcome reported is the rate of preterm birth as a percentage (not able to determine n = in either progesterone or placebo group). Other results reported as experimental group versus control (this allocation is not randomised but based on risk assessment at first and second antenatal visit). |
| Ionescu 2012 | Progesterone versus cerclage – so not relevant to this review. |
| Keeler 2009 | Comparison with McDonald cerclage, not placebo – topic of another review. |
| Le Vine 1964 | Quasi‐randomised trial. |
| Rust 2006 | Progesterone versus cerclage – so not relevant to this review. |
| Suvonnakote 1986 | Quasi‐randomised trial ‐ women were 'divided' into 2 groups (trial group and control group). |
| Turner 1966 | Progesterone administration for prevention of miscarriage. |
| Walch 2005 | Progesterone administration for prevention of miscarriage. |
| Yemini 1985 | Quasi‐randomised trial. |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Coomarasamy 2012.
| Trial name or title | Progesterone in recurrent miscarriages (PROMISE) study. |
| Methods | |
| Participants | 1. Women with unexplained recurrent miscarriages (3 or more consecutive first trimester miscarriages). 2. Age 18‐39 years at randomisation (likelihood of miscarriages due to chromosomal aberrations is higher in older women; such miscarriages are unlikely to be prevented by progesterone therapy). 3. Spontaneous conception (as confirmed by urinary pregnancy tests). 4. Willing and able to give informed consent. |
| Interventions | Intervention group: progesterone pessaries (400 mg twice daily) started soon as possible after a positive pregnancy test (and no later than 6 weeks' gestation) and continued to 12 weeks of gestation. Control group: placebo. Total duration of follow‐up per participant: 42 weeks. |
| Outcomes | Primary outcome: live births beyond 24 weeks. Secondary outcomes: 1. Gestation at delivery. 2. Clinical pregnancy at 6‐8 weeks. 3. On‐going pregnancy at 12 weeks (range 11‐13 weeks). 4. Miscarriage rate. 5. Survival at 28 days of neonatal life. 6. Congenital abnormalities with specific examination for genital anomalies. 7. Adverse events. |
| Starting date | 01/05/2009, anticipated end date 0105/2012. |
| Contact information | Dr Arri Coomarasamy: a.coomarasamy@bham.ac.uk |
| Notes |
Creasy 2008.
| Trial name or title | The effect of vaginal progesterone administration in the prevention of preterm birth in women with a short cervix. NCT00615550. |
| Methods | |
| Participants | Women with a singleton gestation and a short cervical length by transvaginal ultrasound defined as 10‐20 mm. |
| Interventions | Progesterone 8% vaginal gel, 1.125 g once daily, beginning at 19 0/7 to 23 6/7 weeks' gestation through 36 6/7 weeks' gestation. Placebo vaginal gel, 1.125 g once daily, beginning at 19 0/7 to 23 6/7 weeks' gestation through 36 6/7 weeks' gestation. |
| Outcomes | Reduction in the frequency of preterm birth (less than or equal to 32 6/7 weeks' gestation. |
| Starting date | March 2008. |
| Contact information | Joseph R Parella: jparella@columbialabs.com |
| Notes |
Crowther 2007.
| Trial name or title | Progesterone for the prevention of neonatal respiratory distress syndrome (The PROGRESS Study). ISRCTN20269066. |
| Methods | |
| Participants | Women with a history of previous spontaneous preterm birth. |
| Interventions | Daily vaginal progesterone vs placebo. |
| Outcomes | Neonatal lung disease. |
| Starting date | October 2005. |
| Contact information | progress@adelaide.edu.au caroline.crowther@adelaide.edu.au |
| Notes |
Martinez 2007.
| Trial name or title | Vaginal progesterone to prevent preterm delivery in women with preterm labour. NCT00536003. |
| Methods | |
| Participants | Women presenting with symptoms and signs of preterm labour, and evidence of cervical change or positive fetal fibronectin testing. |
| Interventions | Vaginal progesterone vs placebo. |
| Outcomes | Preterm birth less than 37 weeks. |
| Starting date | July 2006. |
| Contact information | Begona Martinez de Tejada: begona.mdt@bluewin.ch |
| Notes |
Nassar 2007.
| Trial name or title | Prevention of preterm delivery in twin pregnancies by 17 alpha hydroxyprogesterone caproate. NCT00141908. |
| Methods | |
| Participants | Women with a twin pregnancy. |
| Interventions | Weekly IM 17OHP vs placebo. |
| Outcomes | Preterm birth. |
| Starting date | March 2006. |
| Contact information | Anwar Nassar: an21@aub.edu.lb |
| Notes |
Norman 2012.
| Trial name or title | Trial protocol OPPTIMUM ‐ Does progesterone prophylaxis for the prevention of preterm labour improve outcome? ISRCTN14568373. |
| Methods | Double‐blind randomised placebo controlled trial. |
| Participants | Women with singleton pregnancy and at high risk of preterm labour. |
| Interventions | Prophylactice vaginal natural progesterone, 200 mg daily from 22‐34 weeks' gestation vs placebo. |
| Outcomes | Incidence of preterm delivery (before 34 weeks); neonatal outcome (composite of death and major morbidity); childhood cognitive and neurosensory outcomes at 2 years of age. |
| Starting date | Recruitment began in 2009 and is scheduled to close in Spring 2013. |
| Contact information | Jane E Norman. |
| Notes |
Perlitz 2007.
| Trial name or title | Prevention of recurrent preterm delivery by a natural progesterone agent. NCT00329316. |
| Methods | |
| Participants | Women with preterm labour in a prior pregnancy. |
| Interventions | Daily vaginal progesterone gel vs placebo. |
| Outcomes | Not specified. |
| Starting date | Not yet recruiting. |
| Contact information | Yuri Perlitz: yperlitz@poria.health.gov.il |
| Notes |
Starkey 2008.
| Trial name or title | Comparing intramuscular versus vaginal progesterone for prevention of preterm birth. NCT00579553. |
| Methods | |
| Participants | Women with singleton pregnancies and history of prior spontaneous preterm birth. |
| Interventions | Weekly intramuscular injection of 17 alpha hydroxylprogesterone caproate (250 mg) or daily vaginal progesterone (100 mg). |
| Outcomes | Maternal, fetal and neonatal outcomes. |
| Starting date | October 2006. |
| Contact information | Christy Zornes: christina‐zornes@ouhsc.edu |
| Notes |
Swaby 2007.
| Trial name or title | Pilot randomised controlled trial of vaginal progesterone to prevent preterm birth in multiple pregnancy. |
| Methods | |
| Participants | Women with a multiple pregnancy. |
| Interventions | Vaginal progesterone (90 mg) or placebo gel. |
| Outcomes | Duration of pregnancy. |
| Starting date | |
| Contact information | C Swaby. University of Calgary, 1403‐29 Street, Calgary, Canada. |
| Notes |
van Os 2011.
| Trial name or title | Preventing preterm birth with progesterone: costs and effects of screening low risk women with a singleton pregnancy for short cervical length, the Triple P study. Netherlands Trial Register (NTR): NTR207. |
| Methods | Cohort study with a randomised placebo‐controlled trial embedded. Multicentre, Dutch Obstetric Consortium. |
| Participants | Inclusion criteria: women with low risk singleton pregnancies at 18‐22 weeks' gestation with a short cervix <= 30 mm. |
| Interventions | Vaginal progesterone 200 mg‐capsules of micronised progesterone ‐ vs placebo, taken between 22 and 34 weeks. |
| Outcomes | Primary outcome: composite poor neonatal outcome (death or severe morbidity): severe respiratory distress syndrome; bronchopulmonary dysplasia; periventricular leucomalacia > grade 1; intracerebral haemorrhage > grade II; necrotising enterocolitis > stage 1; proven sepsis and death before discharge from the nursery. Secondary outcomes: time to delivery; preterm birth rate before 32, 34 and 37 weeks; days of admission in neonatal intensive care unit; maternal morbidity; maternal admission days for preterm labour; costs; growth, physical condition (close examination of genital tract), neurodevelopmental outcome of child at 24 month age; cost‐effectiveness of screening for short cervical length. |
| Starting date | October 2011 ‐ not clear from paper. |
| Contact information | Paper reporting study protocol ‐ Melanie A van Os first author ‐ correspondence email: m.vanos@vumc.nl |
| Notes |
Wood 2007.
| Trial name or title | Vaginal progesterone versus placebo in multiple pregnancy. NCT00343265. |
| Methods | |
| Participants | Women with a multiple pregnancy. |
| Interventions | Daily vaginal progesterone gel vs placebo. |
| Outcomes | Primary: gestational age. |
| Starting date | June 2006. |
| Contact information | Stephen Wood: stephen.wood@calgaryhealthregion.ca |
| Notes |
IM: intramuscular vs: versus
Differences between protocol and review
In view of the increase in the number of trials published, along with the huge variation in the patient populations recruited, we have decided to categorise the studies by the reason women were considered to be at increased risk of preterm birth. We have also included longer‐term childhood health outcomes, in recognition of the need for ongoing follow‐up of children exposed antenatally to progesterone (Update 2008), and maternal quality of life (Update 2013).
Contributions of authors
For the update in 2013, J Dodd and L Jones assessed studies, extracted data and entered it into RevMan. L Jones wrote the first version of the results. J Dodd revised the review, discussion and conclusions. V Flenady, CA Crowther and R Cincotta commented on all drafts of the update (2013).
Sources of support
Internal sources
Mater Research Support Centre, Mater Health Services Brisbane, South Brisbane, Queensland, Australia.
Department of Maternal Fetal Medicine, Mater Mothers' Hospital, South Brisbane, Queensland, Australia.
The University of Adelaide, Discipline of Obstetrics and Gynaecology, Australia.
External sources
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS:10/4001/02
Declarations of interest
J Dodd, V Flenady and C Crowther are investigators in a randomised trial assessing the use of progesterone for prevention of respiratory distress syndrome (The PROGRESS Trial).
Edited (no change to conclusions)
References
References to studies included in this review
Aboulghar 2012 {published data only}
- Aboulghar MM, Aboulghar MA, Amin YM, Al‐Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reproductive BioMedicine Online 2012;25(2):133‐8. [DOI] [PubMed] [Google Scholar]
Akbari 2009 {published data only}
- Akbari S, Birjandi M, Mohtasham N. Evaluation of the effect of progesterone on prevention of preterm delivery and its complications. Scientific Journal of Kurdistan University of Medical Sciences 2009;14(3):11‐9. [Google Scholar]
Borna 2008 {published data only}
- Borna S, Sahabi N. Progesterone for maintenance tocolytic therapy after threatened preterm labour: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(1):58‐63. [DOI] [PubMed] [Google Scholar]
- Borna S, Shakoie S, Borna H. Progesterone for maintenance tocolytic therapy after threatened preterm labor. Randomized controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2008; Vol. 21, issue Suppl 1:151‐2.
Briery 2011 {published data only}
- Briery C, Veillon E, Klauser CK, Martin R, Chauhan S, Magann EF, et al. Women with prolonged premature rupture of the membrane do not benefit from weekly progesterone: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S189. [DOI] [PubMed] [Google Scholar]
- Briery CM, Veillon EW, Klauser CK, Martin RW, Chauhan SP, Magann EF, et al. Progesterone does not prevent preterm births in women with twins. Southern Medical Journal 2009;102(9):900‐4. [DOI] [PubMed] [Google Scholar]
- Briery CM, Veillon EW, Klauser CK, Martin RW, Magann EF, Chauhan SP, et al. Women with preterm premature rupture of the membranes do not benefit from weekly progesterone. American Journal of Obstetrics and Gynecology 2011;204(1):54.e1‐5. [DOI] [PubMed] [Google Scholar]
Caritis 2009 {published data only}
- Caritis SN, Rouse DJ, Peaceman AM, Sciscione A, Momirova V, Spong CY, et al. Prevention of preterm birth in triplets using 17 alpha‐hydroxyprogesterone caproate: a randomized controlled trial. Obstetrics & Gynecology 2009;113(2 Pt 1):285‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cetingoz 2011 {published data only}
- Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high‐risk pregnancies: a randomized placebo‐controlled trial. Archives of Gynecology and Obstetrics 2011;283(3):423‐9. [DOI] [PubMed] [Google Scholar]
Combs 2010 {published data only}
- Combs CA, Garite T, Maurel K, Das A, Porto M, for the OCRN. Failure of 17‐hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double‐blind, randomized clinical trial. American Journal of Obstetrics and Gynecology 2010;203(3):248.e1‐9. [DOI] [PubMed] [Google Scholar]
- Combs CA, Garite T, Porto M, Maurel K, for the OCRN. 17‐hydroxyprogesterone for triplet pregnancy does not reduce prematurity or neonatal morbidity, may increase midtrimester loss. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S168. [Google Scholar]
- Heitmann E, Lu G, Combs CA, Garite TJ, Maurel K. The impact of maternal weight upon the effectiveness of 17‐hydroxyprogesterone in preventing preterm birth among twin gestations. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S98. [Google Scholar]
- Maurel K, Combs A. 17OHP for reduction of neonatal morbidity due to preterm birth (PTB) in twin and triplet pregnancies. http://controlledtrials.com (accessed 2007).
Combs 2011 {published data only}
- Combs CA, Garite T, Maurel K, Das A, Porto M, for theOCRN. 17‐hydroxyprogesterone caproate for twin pregnancy: a double‐blind, randomized clinical trial. American Journal of Obstetrics and Gynecology 2011;204:221.e1‐8. [DOI] [PubMed] [Google Scholar]
- Combs CA, Garite TJ, Maurel K, Cebrik D. 17‐hydroxyprogesterone caproate for women with history of preterm birth in a prior pregnancy and twins in the current pregnancy. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S213. [Google Scholar]
- Combs CA, Maurel K, Garite T, for theOCRN. 17‐hydroxyprogesterone for twin pregnancy: no reduction in prematurity or neonatal morbidity. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S7. [DOI] [PubMed] [Google Scholar]
- Maurel K, Combs A. 17OHP for reduction of neonatal morbidity due to preterm birth (PTB) in twin and triplet pregnancies. http://controlledtrials.com (accessed 2007).
Combs 2011a {published data only}
- Combs CA, Garite TJ, Maurel K, Mallory K, Edwards RK, Lu G, et al. 17‐Hydroxyprogesterone caproate to prolong pregnancy after preterm rupture of the membranes: early termination of a double‐blind, randomized clinical trial. BMC Research Notes 2011;4(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
da Fonseca 2003 {published data only}
- Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo‐controlled double‐blind study. American Journal of Obstetrics & Gynecology 2003;188(2):419‐24. [DOI] [PubMed] [Google Scholar]
- Fonseca EB, Bittar RE, Carvalho MHB, Martinelli S, Zugaib M. Uterine contraction monitoring in pregnant women using vaginal natural progesterone. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):525. [Google Scholar]
Elsheikhah 2010 {published data only}
- Elsheikhah AZ, Dahab S, Negm S, Ebrashy A. Effect of prophylactic progesterone on incidence of preterm labour in spontaneous twin pregnancy, randomized controlled study. Ultrasound in Obstetrics and Gynecology 2010;36(Suppl 1):108. [Google Scholar]
Facchinetti 2007 {published data only}
- Facchinetti F, Dante G, Venturini P, Paganelli S, Volpe A. 17alpha‐hydroxy‐progesterone effects on cervical proinflammatory agents in women at risk for preterm delivery. American Journal of Perinatology 2008;25(8):503‐6. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Paganelli S, Comitinit G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17‐alpha‐hydroxyprogesterone caproate. American Journal of Obstetrics and Gynecology 2007;196:453e1‐453e4. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Paganelli S, Venturini P, Dante G. 17 alpha hydroxy‐progesterone caproate (17P) treatment reduced cervical shortening inhibiting cervical interleukin‐1 secretion. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S5. [Google Scholar]
Fonseca 2007 {published data only}
- Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. New England Journal of Medicine 2007;357:462‐9. [DOI] [PubMed] [Google Scholar]
Glover 2011 {published data only}
- Glover M, Croom CS, Sonek JD, Kovac C, McKenna D. A randomized placebo controlled trial of oral micronized progesterone to prevent recurrent preterm birth. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S172. [Google Scholar]
- Glover MM, McKenna DS, Downing CM, Smith DB, Croom CS, Sonek JD. A randomized trial of micronized progesterone for the prevention of recurrent preterm birth. American Journal of Perinatology 2011;28(5):377‐81. [DOI] [PubMed] [Google Scholar]
Grobman 2012 {published data only}
- Grobman W. RCT of progesterone to prevent preterm birth in nulliparous women with a short cervix. http://controlledtrials.com (accessed 2007).
- Grobman W. Randomized controlled trial of progesterone treatment for preterm birth prevention in nulliparous women with cervical length less than 30 mm. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S367. [Google Scholar]
- Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, et al. 17 alpha‐hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. American Journal of Obstetrics and Gynecology 2012;207(5):390.e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartikainen 1980 {published data only}
- Hartikainen‐Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 alpha‐hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstetrics & Gynecology 1980;56(6):692‐5. [PubMed] [Google Scholar]
- Hartikainen‐Sorri AL, Kauppila A, Tuimala R. Management of twin pregnancy with 17 alpha‐ hydroxyprogesterone caproate [abstract]. 9th World Congress of Gynecology and Obstetrics 1979 October 26‐31; Tokyo, Japan. 1979:298‐9.
Hassan 2011 {published data only}
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double‐blind, placebo‐controlled trial. Ultrasound in Obstetrics & Gynecology 2011;38(1):18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauth 1983 {published data only}
- Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17 alpha‐hydroxyprogesterone caproate on pregnancy outcome in an active‐duty military population. American Journal of Obstetrics and Gynecology 1983;146(2):187‐90. [DOI] [PubMed] [Google Scholar]
Ibrahim 2010 {published data only}
- Ibrahim M, Mohamed Ramy AR, Younis MA‐F. Progesterone supplementation for prevention of preterm labor: a randomized controlled trial. Middle East Fertility Society Journal 2010;15(1):39‐41. [Google Scholar]
Johnson 1975 {published data only}
- Johnson JWC, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17 alpha‐hydroxyprogesterone caproate in the prevention of premature labor. New England Journal of Medicine 1975;293(14):675‐80. [DOI] [PubMed] [Google Scholar]
- Klebanoff M, for the NICHD MFMU Network. Impact of 17alpha hydroxyprogesterone caproate administration on salivary progesterone and estriol [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S140. [Google Scholar]
Lim 2011 {published data only}
- Bruinse HW. 17 alpha hydroxyprogesterone in multiple pregnancies to prevent handicapped infants (The AMPHIA Study). http://www.controlledtrials.com (accessed 2007).
- Lim AC. The effect of 17‐alpha hydroxyprogesterone caproate on cervical length in multiple pregnancies. Reproductive Sciences 2010;17(3 Suppl 1):282A. [Google Scholar]
- Lim AC, Bloemenkamp KW, Boer K, Duvekot JJ, Erwich JJ, Hasaart TH, et al. Progesterone for the prevention of preterm birth in women with multiple pregnancies: the AMPHIA trial. BMC Pregnancy & Childbirth 2007; Vol. 7:7. [DOI] [PMC free article] [PubMed]
- Lim AC, Schuit E, Bloemenkamp K, Bernardus RE, Duvekot JJ, Erwich JJ, et al. 17alpha‐hydroxyprogesterone caproate for the prevention of adverse neonatal outcome in multiple pregnancies: A randomized controlled trial. Obstetrics and Gynecology 2011;118(3):513‐20. [DOI] [PubMed] [Google Scholar]
- Lim AC, for the ASG. Is second trimester cervical length a predictor for an effect of 17‐alpha hydroxyprogesterone caproate on neonatal morbidity in multiple pregnancies? Results from the AMPHIA trial (ISRCTN40512715). Reproductive Sciences 2010;17(3 Suppl 1):282A. [Google Scholar]
- Willekes C, Lim A, Vijgen S, Mol B, Papatsonis D, Hasaart T, et al. Mid‐pregnancy cervical length as a predictor of preterm birth in multiple pregnancies. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S54. [Google Scholar]
Majhi 2009 {published data only}
- Majhi P, Bagga R, Kalra J, Sharma M. Intravaginal use of natural micronised progesterone to prevent pre‐term birth: a randomised trial in India. Journal of Obstetrics and Gynaecology 2009;29(6):493‐8. [DOI] [PubMed] [Google Scholar]
Meis 2003 {published data only}
- Gyamfi C, Horton AL, Momirova V, Rouse DJ, Caritis SN, Peaceman AM, et al. The effect of 17‐alpha hydroxyprogesterone caproate on the risk of gestational diabetes in singleton or twin pregnancies. American Journal of Obstetrics and Gynecology 2009;201(4):392.e1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff MA, Meis PJ, Dombrowski MP, Zhao Y, Moawad AH, Northen A, et al. Salivary progesterone and estriol among pregnant women treated with 17‐alpha‐hydroxyprogesterone caproate or placebo. American Journal of Obstetrics and Gynecology 2008;199(5):506.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz G. Does gestational age at randomization affect the efficacy of alpha hydroxyprogesterone caproate (17‐OHCP) in preventing recurrent preterm delivery? [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl 1):S55. [Google Scholar]
- Manuck TA, Lai Y, Meis PJ, Dombrowski MP, Sibai B, Spong CY, et al. Progesterone receptor polymorphisms and clinical response to 17‐alpha‐hydroxyprogesterone caproate. American Journal of Obstetrics & Gynecology 2011;205(2):135.e1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis P, NICHD MFMU Network. More than one previous preterm delivery and the risk of preterm birth in women treated with 17 alpha‐hydroxyprogesterone (17P) [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6):S168. [Google Scholar]
- Meis PJ, Klebanoff M, Dombrowski MP, Sibai BM, Leindecker S, Moawad AH, et al. Does progesterone treatment influence risk factors for recurrent preterm delivery?. Obstetrics & Gynecology 2005;106(3):557‐61. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha‐hydroxyprogesterone caproate. New England Journal of Medicine 2003;348(24):2379‐85. [DOI] [PubMed] [Google Scholar]
- Meis PJ, NICHD MFMU Network. 17 alphahydroxyprogesterone caproate prevents recurrent preterm birth [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S54. [Google Scholar]
- Northen A. 4‐year follow‐up of children exposed to 17alpha hydroxyprogesterone caproate (17P) in utero. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S6. [Google Scholar]
- Northen AT, Norman GS, Anderson K, Moseley, L, Divito M, Cotroneo M, et al. Follow‐up of children exposed in utero to 17 alpha‐hydroxyprogesterone caproate compared with placebo. Obstetrics & Gynecology 2007;110(4):864‐72. [DOI] [PubMed] [Google Scholar]
- Sibai B. Plasma CRH levels at 16‐20 weeks do not predict preterm delivery in women at high‐risk for preterm delivery [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S114. [DOI] [PubMed] [Google Scholar]
- Sibai B, Meis PJ, Klebanoff M, Dombrowski MP, Weiner SJ, Moawad AH, et al. Plasma CRH measurement at 16 to 20 weeks' gestation does not predict preterm delivery in women at high‐risk for preterm delivery. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1181‐6. [DOI] [PubMed] [Google Scholar]
- Spong CY. Progesterone for prevention of recurrent preterm birth: impact of gestational age at prior delivery [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S11. [DOI] [PubMed] [Google Scholar]
- Spong CY, Meis PJ, Thom EA, Sibai B, Dombrowski MP, Moawad AH, et al. Progesterone for prevention of recurrent preterm birth: impact of gestational age at previous delivery. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1127‐31. [DOI] [PubMed] [Google Scholar]
Moghtadaei 2008 {published data only}
- Moghtadaei P, Sardari F, Latifi M. Progesterone for prevention of preterm birth and improvement in pregnancy outcomes among primiparae of advanced maternal age. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008; Vol. 93, issue Suppl 1:Fa71.
- Moghtadei P, Sardari F, Latifi M. Progesterone for prevention of preterm birth and improvement pregnancy outcomes among primiparae of advanced maternal age. Journal of Maternal‐Fetal and Neonatal Medicine 2008; Vol. 21, issue Suppl 1:122.
Ndoni 2010 {published data only}
- Ndoni E, Bimbashi A, Dokle A, Kallfa E. Treatment with different types of progesterone in prevention of preterm delivery. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):305. [Google Scholar]
Norman 2009 {published data only}
- Eddama O, Petrou S, Regier D, Norrie J, MacLennan G, MacKenzie F, et al. Study of progesterone for the prevention of preterm birth in twins (STOPPIT): findings from a trial‐based cost‐effectiveness analysis. International Journal of Technology Assessment in Health Care 2010;26(2):141‐8. [DOI] [PubMed] [Google Scholar]
- Norman J. Double blind randomised placebo controlled trial of progesterone for the prevention of preterm birth. http://controlledtrials.com (accessed 2007).
- Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double‐blind, placebo‐controlled study and meta‐analysis. Lancet 2009;373(9680):2034‐40. [DOI] [PubMed] [Google Scholar]
- Norman JE, Yuan M, Anderson L, Howie F, Harold G, Young A, et al. Effect of prolonged in vivo administration of progesterone in pregnancy on myometrial gene expression, peripheral blood leukocyte activation, and circulating steroid hormone levels. Reproductive Sciences 2011;18(5):435‐46. [DOI] [PubMed] [Google Scholar]
O'Brien 2007 {published data only}
- DeFranco EA, O'Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double‐blind, placebo‐controlled trial. Ultrasound in Obstetrics and Gynecology 2007; Vol. 30, issue 5:697‐705. [DOI] [PubMed]
- Defranco E, O'Brien J, Adair J, Lewis DF, Hall D, Phillips J, et al. Is there a racial disparity of progesterone to prevent preterm birth. American Journal of Obstetrics and Gynecology 2007; Vol. 197, issue 6 Suppl 1:S200, Abstract no: 702.
- O'Brien J, Defranco E, Adair D, Lewis DF, Hall D, Bsharat M, et al. Progesterone reduces the rate of cervical shortening in women at risk for preterm birth: secondary analysis from a randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2007; Vol. 197, issue 6 Suppl 1:S7, Abstract no: 15.
- O'Brien J, Defranco E, Hall D, Creasy G. Natural progesterone administration and the risk of medical complications of pregnancy: secondary analysis from a multinational, randomized, double blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 6 Suppl 1:S139.
- O'Brien J, Defranco E, Hall D, Phillips J, Creasy G. Do other elements of the obstetrical history provide a possible indication for progesterone supplementation? Secondary analysis from the progesterone vaginal gel trial. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 6 Suppl 1:S42.
- O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized double blind placebo controlled trial. Ultrasound in Obstetrics and Gynecology 2007;30(5):687‐96. [DOI] [PubMed] [Google Scholar]
- O'Brien JM, Defranco EA, Adair CD, Lewis DF, Hall DR, How H, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double‐blind, placebo‐controlled trial. Ultrasound in Obstetrics & Gynecology 2010;34(6):653‐9. [DOI] [PubMed] [Google Scholar]
- O'Brien JM, Steichen JJ, Phillips JA, Creasy GW. Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S223. [Google Scholar]
Papiernik 1970 {published data only}
- Papiernik‐Berkhauer E. Double blind study of an agent to prevent preterm delivery among women at increased risk [Etude en double aveugle d'un medicament prevenant la survenue prematuree de l'accouchement chez les femmes a risque eleve d'accouchement premature]. Edition Schering Serie IV 1970; Vol. 3:65‐8.
Rai 2009 {published data only}
- Rai P, Rajaram S, Goel N, Ayalur Gopalakrishnan R, Agarwal R, Mehta S. Oral micronized progesterone for prevention of preterm birth. International Journal of Gynecology & Obstetrics 2009;104(1):40‐3. [DOI] [PubMed] [Google Scholar]
Rode 2011 {published data only}
- Klein K, Rode L, Nicolaides K, Krampl‐Bettelheim E, Larsen H, Holmskov A, et al. Vaginal progesterone and the risk of preterm delivery in high‐risk twin gestations ‐ secondary analysis of a placebo‐controlled randomized trial. Ultrasound in Obstetrics & Gynecology 2011;38(S1):11. [DOI] [PubMed] [Google Scholar]
- Klein K, Rode L, Nicolaides KH, Krampl‐Bettelheim E, Tabor A, PREDICT Group. Vaginal micronized progesterone and risk of preterm delivery in high‐risk twin pregnancies: secondary analysis of a placebo‐controlled randomized trial and meta‐analysis. Ultrasound in Obstetrics & Gynecology 2011;38(3):281‐7. [DOI] [PubMed] [Google Scholar]
- Rode L. The PREDICT study. http://controlledtrials.com (accessed 2007).
- Rode L, Klein K, Nicolaides K, Krampl‐Bettelheim E, Vogel I, Larsen H, et al. Prevention of preterm delivery in twin gestations (PREDICT): a multicentre randomised placebo‐controlled trial on the effect of vaginal micronised progesterone. Ultrasound in Obstetrics & Gynecology 2011;38(Suppl 1):1. [DOI] [PubMed] [Google Scholar]
- Rode L, Klein K, Nicolaides KH, Krampl‐Bettelheim E, Tabor A, PREDICT G. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo‐controlled trial on the effect of vaginal micronized progesterone. Ultrasound in Obstetrics & Gynecology 2011;38(3):272‐80. [DOI] [PubMed] [Google Scholar]
Rouse 2007 {published data only}
- Caritis S, Rouse D. A randomized controlled trial of 17‐hydroxyprogesterone caproate (17‐OHPC) for the prevention of preterm birth in twins. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S2. [Google Scholar]
- Caritis SN, Simhan H. Relationship of 17‐alpha hydroxyprogesterone caproate (17‐OHPC) concentrations and gestational age at delivery in twins. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA 2008:Abstract no: 139.
- Caritis SN, Simhan HN, Zhao Y, Rouse DJ, Peaceman AM, Sciscione A, et al. Relationship between 17‐hydroxyprogesterone caproate concentrations and gestational age at delivery in twin gestation. American Journal of Obstetrics and Gynecology 2012;207(5):396.e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caritis SN, Venkat R. Impact of body mass index (BMI) on plasma concentrations of 17‐alpha hydroxyprogesterone caproate (17‐OHPC). 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA 2008:Abstract no: 138.
- Durnwald C. The impact of cervical length on risk of preterm birth in twin gestations. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 6 Suppl 1:S10.
- Durnwald CP, Momirova V, Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, et al. Second trimester cervical length and risk of preterm birth in women with twin gestations treated with 17‐ hydroxyprogesterone caproate. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(12):1360‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi C, Horton AL, Momirova V, Rouse DJ, Caritis SN, Peaceman AM, et al. The effect of 17‐alpha hydroxyprogesterone caproate on the risk of gestational diabetes in singleton or twin pregnancies. American Journal of Obstetrics and Gynecology 2009;201(4):392.e1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A, Gyamfi C. 17‐alpha hydroxyprogesterone caproate does not increase the risk of gestational diabetes in singleton and twin pregnancies. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 6 Suppl 1:S197. [DOI] [PMC free article] [PubMed]
- Manuck T, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The relationship between polymorphisms in the human progesterone receptor and clinical response to 17 alpha‐hydroxyprogesterone caproate for the prevention of recurrent spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 6 Suppl 1:S18.
- Refuerzo JS, Momirova V, Peaceman AM, Sciscione A, Rouse DJ, Caritis SN, et al. Neonatal outcomes in twin pregnancies delivered moderately preterm, late preterm, and term. American Journal of Perinatology 2010;27(7):537‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 Alpha‐hydroxyprogesterone caproate to prevent prematurity in twins. New England Journal of Medicine 2007;357:454‐61. [DOI] [PubMed] [Google Scholar]
- Simhan HN, Caritis SN. The effect of 17‐alpha hydroxyprogesterone caproate (17‐OHPC) on maternal plasma CRP levels in twin pregnancies. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA 2008:Abstract no: 140.
Rozenberg 2012 {published data only}
- Rozenberg P. Efficacy of 17 alpha‐hydroxyprogesterone caproate for the prevention of preterm delivery. http://controlledtrials.com (accessed 2007).
- Rozenberg P, Chauveaud A, Deruelle P, Capelle M, Winer N, Desbriere R, et al. Prevention of preterm delivery after successful tocolysis in preterm labor by 17 alpha‐hydroxyprogesterone caproate: a randomized controlled trial. American Journal of Obstetrics & Gynecology 2012;206(3):206.e1‐9. [DOI] [PubMed] [Google Scholar]
- Rozenberg P, Deruelle P, Chauveaud A, Capelle M, Winer N, Porcher R, et al. Prevention of preterm delivery after successful tocolysis in preterm labour by 17 alpha‐hydroxyprogesterone caproate: a randomised controlled trial. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S2‐S3. [DOI] [PubMed] [Google Scholar]
Saghafi 2011a {published data only}
- Saghafi N, Khadem N, Mohajeri T, Shakeri MT. Efficacy of 17alpha‐hydroxyprogesterone caproate in prevention of preterm delivery. Journal of Obstetrics and Gynaecology Research 2011;37(10):1342‐5. [DOI] [PubMed] [Google Scholar]
- Saghafi N, Khadem N, Mohajeri T, Shakeri MT, Amini M. Efficacy of 17alpha‐hydroxyprogesterone caproate in preterm delivery prevention. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;14(2):28‐33. [DOI] [PubMed] [Google Scholar]
Senat 2012 {published data only}
- Senat MV, Winer N, Porcher R, Rozenberg P. Prevention of preterm delivery in asymptomatic women with twin pregnancy by 17 alpha‐Hydroxyprogesterone caproate: A randomised controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):14. [Google Scholar]
Serra 2013 {published data only}
- Serra V. Natural progesterone and preterm birth in twins. http://controlledtrials.com (accessed 2007).
- Serra V, Perales A, Meseguer J, Parrilla J, Lara C, Bellver J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double‐blind multicentre trial. BJOG: an international journal of obstetrics and gynaecology 2013;120(1):50‐7. [DOI] [PubMed] [Google Scholar]
Sharami 2010 {published data only}
- Sharami SH, Zahiri Z, Shakiba M, Milani F. Maintenance therapy by vaginal progesterone after threatened idiopathic preterm labor: a randomized placebo‐controlled double‐blind trial. International Journal of Fertility and Sterility 2010;4(2):45‐50. [Google Scholar]
References to studies excluded from this review
Abbott 2012 {published data only}
- Abbott D. Relationship between cervical‐vaginal fluid elafin concentrations and subsequent cervical shortening in women at high risk of spontaneous preterm birth. Reproductive Sciences 2012;19(3Suppl):73A. [Google Scholar]
Arikan 2011 {published data only}
- Arikan I, Barut A, Harma M, Harma IM. Effect of progesterone as a tocolytic and in maintenance therapy during preterm labor. Gynecologic and Obstetric Investigation 2011;72(4):269‐73. [DOI] [PubMed] [Google Scholar]
Berghella 2010 {published data only}
- Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, et al. 17‐alpha‐hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. American Journal of Obstetrics and Gynecology 2010;202(4):351.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breart 1979 {published data only}
- Breart G, Lanfranchi M, Chavigny C, Rumeau‐Rouquette C, Sureau C. A comparative study of the efficiency of hydroxyprogesterone caproate and of chlormadinone acetate in the prevention of premature labor. International Journal of Gynecology & Obstetrics 1979;16(5):381‐4. [DOI] [PubMed] [Google Scholar]
Brenner 1962 {published data only}
- Brenner WE, Hendricks CH. Effect of medroxyprogesterone acetate upon the duration and characteristics of human gestation and labor. American Journal of Obstetrics and Gynecology 1962;83:1094‐8. [DOI] [PubMed] [Google Scholar]
Chandiramani 2012 {published data only}
- Chandiramani M. Serum progesterone concentrations in women with a previous preterm birth treated with vaginal progesterone supplementation. Reproductive Sciences 2012;19(3Suppl):189A. [Google Scholar]
Corrado 2002 {published data only}
- Corrado F, Dugo C, Cannata M, Bartolo M, Scilipoti A, Stella N. A randomised trial of progesterone prophylaxis after midtrimester amniocentesis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;100(2):196‐8. [DOI] [PubMed] [Google Scholar]
Hobel 1986 {published data only}
- Hobel CJ, Bemis RL. West Area Los Angeles prematurity prevention demonstration project. In: Papiernik E, Breart G, Spira N editor(s). Prevention of Preterm Birth. Paris: INSERM, 1986:205‐22. [Google Scholar]
- Hobel CJ, Bragonier R, Ross M, Bear M, Bemis R, Mori B. West Los Angeles premature prevention program: significant impact. Journal of Perinatal Medicine 1987;15:112. [Google Scholar]
- Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Bear M, Mori B. West Los Angeles preterm birth prevention project (LAPPP): program impact. American Journal of Obstetrics and Gynecology 1992;166:363. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Nessim S, Sandhu M, et al. The West Los Angeles preterm birth prevention project: I. program impact on high‐risk women. American Journal of Obstetrics and Gynecology 1994;170:54‐62. [DOI] [PubMed] [Google Scholar]
Ionescu 2012 {published data only}
- Ionescu AC, Gheorghiu D, Pacu I, Davitoiu B, Dimitriu M, Haradja H. Randomized trial of cerclage and progesterone to prevent spontaneous preterm brith in high‐risk women with a short cervix. Journal of Perinatal Medicine 2012;39 Suppl:Abstract no. 008. [Google Scholar]
Keeler 2009 {published data only}
- Keeler SM, Kiefer D, Rochon M, Quinones JN, Novetsky AP, Rust O. A randomized trial of cerclage vs. 17 alpha‐hydroxyprogesterone caproate for treatment of short cervix. Journal of Perinatal Medicine 2009;37(5):473‐9. [DOI] [PubMed] [Google Scholar]
Le Vine 1964 {published data only}
- Vine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgical Obstetrics and Gynecology 1964;72:30‐6. [PubMed] [Google Scholar]
Rust 2006 {published data only}
- Rust O, Larkin R, Roberts W, Quinones J, Rochon M, Reed J, et al. A randomized trial of cerclage versus 17 hydroxyprogesterone for the treatment of short cervix. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S112. [Google Scholar]
Suvonnakote 1986 {published data only}
- Suvonnakote T. Prevention of preterm labour with progesterone. Journal of the Medical Association of Thailand 1986;69(10):538‐42. [PubMed] [Google Scholar]
Turner 1966 {published data only}
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95(2):222‐7. [DOI] [PubMed] [Google Scholar]
Walch 2005 {published data only}
- Walch K, Hefler L, Nagele F. Oral dydrogesterone treatment during the first trimester of pregnancy: the prevention of miscarriage study. A double blind, prospectively randomised placebo controlled parallel group trial. Journal of Maternal‐Fetal and Neonatal Medicine 2005;18(4):265‐9. [DOI] [PubMed] [Google Scholar]
Yemini 1985 {published data only}
- Yemini M, Borenstein R, Dreazen E, Apelman Z, Mogilner B, Kessler I, et al. Prevention of premature labor by 17 alpha‐hydroxyprogesterone caproate. American Journal of Obstetrics and Gynecology 1985;151(5):574‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Coomarasamy 2012 {published data only}
- Coomarasamy A. First trimester progesterone therapy in women with a history of unexplained recurrent miscarriages: a randomised double‐blind placebo‐controlled multi‐centre trial (The PROMISE [PROgesterone in recurrent MIScarriagE] Trial). http://www.controlled‐trials.com/ISRCTN92644181/ISRCTN92644181 (accessed 11.01.2012). [DOI] [PMC free article] [PubMed]
Creasy 2008 {published data only}
- Creasy GW. The effect of vaginal progesterone administration in the prevention of preterm birth in women with a short cervix. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 2008).
Crowther 2007 {published data only}
- Armson BA, Dodd J, for the POPPI Collaborative Trial Group. POPPI: prevention of problems of preterm birth with progesterone in women at increased risk: a multicentre randomised controlled trial [abstract]. Journal of Paediatrics and Child Health 2007;43:A29. [Google Scholar]
- Ashwood P. Progesterone after previous preterm birth for the prevention of neonatal respiratory distress syndrome. WOMBAT Collaboration (www.wombatcollaboration.net/trials) (accessed 4 October 2006).
- Crowther CA, Dodd JM, McPhee AJ, Flenady V. Australasian Collaborative Trial of Vaginal Progesterone Therapy (The PROGRESS Trial). http://controlledtrials.com (accessed 2007).
- Dodd JM, Crowther CA, McPhee AJ, Flenady V, Robinson JS. Progesterone after previous preterm birth for prevention of neonatal respiratory distress syndrome (PROGRESS): a randomised controlled trial. BMC Pregnancy and Childbirth 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2007 {published data only}
- Matrinez de Tajada B. Vaginal progesterone to prevent preterm delivery in women with preterm labor. http://controlledtrials.com (accessed 2007).
Nassar 2007 {published data only}
- Nassar A. Prevention of preterm delivery in twin pregnancies by 17 alpha hydroxyprogesterone caproate. http://controlledtrials.com (accessed 2007).
- Nassar A, Awwad J, Succar J, Saassouh W, Khalife T, Hayek S, et al. Incidence of GDM in twin pregnancies of women receiving prophylactic 17‐A OH progesterone caproate. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):307‐8. [Google Scholar]
Norman 2012 {published data only}
- Norman JE, Shennan A, Bennett P, Thornton S, Robson S, Marlow N, et al. Trial protocol OPPTIMUM‐ Does progesterone prophylaxis for the prevention of preterm labour improve outcome?. BMC pregnancy and childbirth 2012;12(1):79. [PUBMED: 22866909] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlitz 2007 {published data only}
- Perlitz Y. Prevention of recurrent preterm delivery by a natural progesterone agent. http://controlledtrials.com (accessed 2007).
Starkey 2008 {published data only}
- Starkey M. Comparing IM vs. vaginal progesterone for pre‐term birth. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 April 2008).
Swaby 2007 {published data only}
- Swaby C. Pilot randomized controlled trial of vaginal progesterone to prevent preterm birth in multiple pregnancy. JOGC: Journal of Obstetrics and Gynaecology Canada 2007; Vol. 29, issue 6 Suppl 1:S47.
van Os 2011 {published data only}
- Os MA, Ven JA, Kleinrouweler CE, Pajkrt E, Miranda E, Wassenaer A, et al. Preventing preterm birth with progesterone: Costs and effects of screening low risk women with a singleton pregnancy for short cervical length, the Triple P study. BMC Pregnancy and Childbirth 2011;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2007 {published data only}
- Wood S. Vaginal progesterone versus placebo in multiple pregnancy. http://controlledtrials.com (accessed 2007).
Additional references
ACOG 2003
- American College of Obstetrics and Gynecology. Committee opinion: use of progesterone to reduce preterm birth. Obstetrics & Gynecology 2003;102:1115‐6. [DOI] [PubMed] [Google Scholar]
Adams 2000
- Adams MM, Elam‐Evans LD, Wilson HG, Gilbertz DA. Rates and factors associated with recurrence of preterm delivery. JAMA 2000;283(12):1591‐6. [DOI] [PubMed] [Google Scholar]
AIHW 2003
- Australian Institute of Health and Welfare. Australia's Mothers and Babies 2000. National Perinatal Statistics Unit, 2003. [Google Scholar]
Alfirevic 2012
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008991.pub2] [DOI] [PubMed] [Google Scholar]
Astle 2003
- Astle S, Slater DM, Thornton S. The involvement of progesterone in the onset of human labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;108(2):177‐81. [DOI] [PubMed] [Google Scholar]
Bakketeig 1979
- Bakketeig LS, Hoffman HJ, Harley EE. The tendency to repeat gestational age and birth weight in successive births. American Journal of Obstetrics and Gynecology 1979;135(8):1086‐103. [DOI] [PubMed] [Google Scholar]
Berkowitz 1993
- Berkowitz G, Papiernik E. Epidemiology of preterm birth. Epidemiologic Reviews 1993;88:233‐8. [DOI] [PubMed] [Google Scholar]
Blencowe 2012
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2162‐72. [PUBMED: 22682464] [DOI] [PubMed] [Google Scholar]
Block 1984
- Block BS, Liggins GC, Creasy RK. Preterm delivery is not predicted by serial plasma estradiol or progesterone concentration measurements. American Journal of Obstetrics and Gynecology 1984;150(6):716‐22. [DOI] [PubMed] [Google Scholar]
Bloom 2001
- Bloom SL, Yost NP, McIntire DD, Leveno KJ. Recurrence of preterm birth in singleton and twin pregnancies. Obstetrics & Gynecology 2001;98:379‐85. [DOI] [PubMed] [Google Scholar]
Carr‐Hill 1985
- Carr‐Hill RA, Hall MH. The repetition of spontaneous preterm labour. BJOG:an international journal of obstetrics and gynaecology 1985;92(9):921‐8. [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2013
- Conde‐Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O'Brien JM, Cetingoz E, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. American Journal of Obstetrics and Gynecology 2013;208(1):42.e1‐42.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Condon 2003
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proceedings of the National Academy of Sciences of the United States of America 2003;100(16):9518‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elder 1999
- Elder DE, Haga R, Evans SF, Benninger HR, French NP. Hospital admissions in the first year of life in very preterm infants. Journal of Paediatrics and Child Health 1999;35(2):145‐50. [DOI] [PubMed] [Google Scholar]
Gates 2004
- Gates S, Brocklehurst P. How should randomised trial including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynaecology 2004;111:213‐9. [DOI] [PubMed] [Google Scholar]
Goldenberg 1998
- Goldenberg RL, Rouse DJ. Prevention of premature birth. New England Journal of Medicine 1998;339:313‐20. [DOI] [PubMed] [Google Scholar]
Grazzini 1998
- Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 1998;392(6675):509‐12. [DOI] [PubMed] [Google Scholar]
Greene 2003
- Greene MF. Progesterone and preterm delivery ‐ deja vu all over again. New England Medical Journal 2003;348:2453‐5. [DOI] [PubMed] [Google Scholar]
Haas 2008
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003511.pub2] [DOI] [PubMed] [Google Scholar]
Hack 1999
- Hack M. Consideration of the use of health status, functional outcome, and quality‐of‐life to monitor neonatal intensive care practice. Pediatrics 1999;103(1 Suppl E):319‐28. [PubMed] [Google Scholar]
Haluska 1997
- Haluska GJ, Cook MJ, Novy MJ. Inhibition and augmentation of progesterone production during pregnancy: effects on parturition in rhesus monkeys. American Journal of Obstetrics and Gynecology 1997;176(3):682‐91. [DOI] [PubMed] [Google Scholar]
Haluska 2002
- Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localisation and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. Journal of the Society for Gynecological Investigation 2002;9(3):125‐36. [PubMed] [Google Scholar]
Hewitt 1988
- Hewitt BC, Newnham JP. A review of the obstetric and medical complications leading to the delivery of very low birth weight infants. Medical Journal of Australia 1988;149:234‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins J, Thompson S. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21:1559‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Iams 2003
- Iams JD. Supplemental progesterone to prevent preterm birth. American Journal Obstetrics and Gynecology 2003;188(2):303. [DOI] [PubMed] [Google Scholar]
Kaminski 1973
- Kaminski M, Goujard J, Rumeau‐Rouquette C. Prediction of low birthweight and prematurity by a multiple regression analysis with maternal characteristics known since the beginning of the pregnancy. International Journal of Epidemiology 1973;2:195‐204. [DOI] [PubMed] [Google Scholar]
Kistka 2007
- Kistka ZA, Palomar L, Lee KA. Racial disparity in the frequency of recurrence of preterm birth. American Journal of Obstetrics and Gynecology 2007;196:131.e1‐131.e6. [DOI] [PubMed] [Google Scholar]
Kramer 2000
- Kramer M, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA 2000;284:843‐9. [DOI] [PubMed] [Google Scholar]
Lopez‐Bernal 2003
- Lopez‐Bernal A. Mechanism of labour ‐ biochemical aspects. BJOG: an international journal of obstetrics and gynaecology 2003;110(Suppl 20):39‐45. [DOI] [PubMed] [Google Scholar]
Martin 2003
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. National Vital Statistics Report 2003;52(10):1‐113. [PubMed] [Google Scholar]
Mattison 2001
- Mattison DR, Damus K, Fiore E, Petrini J, Alter C. Preterm delivery: a public health perspective. Paediatric and Perinatal Epidemiology 2001;15(Suppl. 2):7‐16. [DOI] [PubMed] [Google Scholar]
McLaughlin 2002
- McLaughlin KJ, Crowther CA, Vigneswaran P, Hancock E, Willson K. Who remains undelivered more than seven days after a single course of prenatal corticosteroids and gives birth at less than 34 weeks?. Australian and New Zealand Journal of Obstetrics and Gynaecology 2002;42(4):353‐7. [DOI] [PubMed] [Google Scholar]
Mercer 1999
- Mercer BM, Goldenberg RL, Moawad AH. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcomes. American Journal of Obstetrics and Gynecology 1999;181(5 Pt 1):1216‐21. [DOI] [PubMed] [Google Scholar]
Northen 2007
- Northen AT, Norman GS, Anderson K, Moseley, L, Divito M, Cotroneo M, et al. Follow‐up of children exposed in utero to 17 alpha‐hydroxyprogesterone caproate compared with placebo. Obstetrics & Gynecology 2007;110(4):864‐72. [DOI] [PubMed] [Google Scholar]
Papiernik 1974
- Papiernik E, Kaminski M. Multifactorial study of the risk of prematurity at 32 weeks of gestation: a study of the frequency of 30 predictive characteristics. Journal of Perinatal Medicine 1974;2:30‐6. [DOI] [PubMed] [Google Scholar]
Pepe 1995
- Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Review 1995;16(5):608‐48. [DOI] [PubMed] [Google Scholar]
Petrini 2005
- Petrini J, Callaghan W, Klebanoff M. Estimated effect of 17 alpha hydroxyprogesterone caproate on preterm birth in the United States. Obstetrics & Gynecology 2005;105:267‐72. [DOI] [PubMed] [Google Scholar]
Pieber 2001
- Peiber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Molecular Human Reproduction 2001;7(9):875‐9. [DOI] [PubMed] [Google Scholar]
Rafael 2011
- Rafael TJ, Berghella V, Alfirevic Z. Cervical stitch (cerclage) for preventing preterm birth in multiple pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD009166] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robinson 2001
- Robinson JN, Regan JA, Norwitz ER. The epidemiology of preterm labour. Seminars in Perinatology 2001;25:204‐14. [DOI] [PubMed] [Google Scholar]
Romero 2012
- Romero R, Nicolaides K, Conde‐Agudelo A, Tabor A, O'Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. American Journal of Obstetrics and Gynecology 2012;206(2):124: 1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuit 2012
- Schuit E, Stock S, Groenwold RH, Maurel K, Combs CA, Garite T, et al. Progestogens to prevent preterm birth in twin pregnancies: an individual participant data meta‐analysis of randomized trials. BMC Pregnancy Childbirth 2012;15(12):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smit 1984
- Smit DA, Essed GG, deHaan J. Predictive value of uterine contractility and the serum levels of progesterone and oestrogens with regard to preterm labour. Gynecologic and Obstetric Investigation 1984;18(5):252‐63. [DOI] [PubMed] [Google Scholar]
Smith 2007
- Smith V, Devane D, Begley CM, Clarke M, Higgins S. A systematic review and quality assessment of systematic reviews of fetal fibronectin and transvaginal length for predicting preterm birth. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;133(2):134‐42. [DOI] [PubMed] [Google Scholar]
Stanley 1992
- Stanley F. Survival and cerebral palsy in low birthweight infants: implications for perinatal care. Paediatric and Perinatal Epidemiology 1992;6(2):298‐310. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cincotta 2004
- Cincotta R, Gardener G, Duncombe G, Flenady V, Dodd J. Antenatal administration of progesterone for preventing spontaneous preterm birth. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004947] [DOI] [Google Scholar]
Dodd 2006
- Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004947.pub2] [DOI] [PubMed] [Google Scholar]


