Abstract
Heart failure (HF) represents a complex clinical syndrome affecting multiple organs and systems of the body, which is a global public health concern because of its high prevalence, mortality, and medical cost. Asia, with its vast population, diverse ethnicities, and complex health care systems, faces challenges in the prevention and management of HF. However, unlike in Western nations, data on HF epidemiology is still limited in Asia. In this review, we will summarize available information regarding the burden of HF in Asia from the aspects of occurrence, etiology and risk factors, outcome, and management of HF, to provide insights for reducing the burden of HF and improving the prognosis of patients with HF.
Key Words: Asia, epidemiology, global burden of diseases, heart failure
Central Illustration
Highlights
-
•
In Asia, comprehensive data regarding the epidemiology and burden of HF remain limited.
-
•
This review summarizes the occurrence, causes, outcomes, and management of HF in Asia, from both the GBD data and registry studies.
-
•
HF remains a serious public health problem in Asia with significant regional variation; thus, strengthening the prevention and standardized management of HF in Asia is urgently needed.
Heart failure (HF) represents a clinical syndrome encompassing diverse etiologies. The latest universal definition of HF proposed it as a condition with symptoms and/or signs caused by a structural and/or functional cardiac abnormality, and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.1 By integrating objective quantification alongside symptomatic manifestations, this universal definition provided a more precise and comprehensive elucidation of this complex clinical syndrome than the previous definitions. Although there have been recent revisions in the definition of HF, the significant heterogeneity in HF definition still existed in prior epidemiological investigations. Previous studies have employed a diverse array of criteria to define HF, such as the Framingham criteria,2 the definition proposed by the European Society of Cardiology,3 among others. Consequently, in interpreting the epidemiological data derived from these studies, consideration should be afforded to the specific definition of HF that was applied in each respective study.
Besides, HF syndrome embodies a progressive continuum of disease with certain stages. Therefore, it becomes imperative to determine the staging of HF to identify individuals at higher risk of developing HF. Compared with the stages of HF originally described in 2013,4 the updated guidelines revised stages of HF as follows: “at risk for HF” (Stage A), “pre-HF” (Stage B), “symptomatic HF” (Stage C), and “advanced HF” (Stage D). Notably, the definition of Stage B now incorporates elevated concentrations of natriuretic peptides and/or troponins, thus accounting for the evolving role of biomarkers and more accurately characterizing patients with structural and subclinical cardiac disease who are potential candidates for targeted preventive measures.1,5,6
HF has traditionally been categorized according to left ventricular ejection fraction (LVEF) by the clinical practice guidelines. This categorization includes heart failure with reduced ejection fraction (HFrEF), heart failure with mildly reduced or midrange ejection fraction (HFmrEF), and heart failure with preserved ejection fraction (HFpEF), classified based on specified LVEF thresholds of ≤40%, 41% to 49%, and ≥50%, respectively.5,7 In this review, we will also discuss the epidemiology of HF in Asia based on these latest classifications of HF.
HF continues to represent a global public health concern, with a potential trend for increased prevalence in the foreseeable future. Worldwide, the prevalent HF cases amounted to 56.19 million individuals, and several nations and territories demonstrated an upward trajectory from 1990 to 2019, especially in limited-income countries.8 The continent of Asia, marked by its vast population; rich diversity of ethnicities, cultures, and socioeconomic strata; and multifaceted health care systems, confronts a multitude of challenges in the prevention and management of HF. In light of these circumstances, this comprehensive review analyzed the open database of GBD (Global Burden of Diseases, Injuries, and Risk Factors Study) 2019 and searched the relevant publications of HF registries in Asia to describe the epidemiological landscape and disease burden of HF in Asia.
In this review, we first provided updated data on the occurrence of HF, including the prevalence and incidence according to the regions, nations and territories, and also the HF classifications. Then, we described the etiologies, comorbidities and risk factors of HF in Asia. At last, the outcome and the advancement in HF management were demonstrated, which aimed to provide the latest insights for the prevention and management of HF in Asia.
Prevalence of HF
Prevalence of HF around the world and Asia from GBD data
The age-standardized prevalence of HF varies substantially across different regions. From the 4 world regions of GBD 2019, there were 31.89 million (95% uncertainty interval [UI]: 25.94-39.25 million) prevalent cases of HF in Asia (Asia and Oceania), with an age-standardized rate (ASR) of 722.45 per 100,000 population (95% UI: 591.97-891.64 per 100,000). The ASR of HF in Asia and Oceania were lower than that in America (810.42 per 100,000 population) and Africa (709.89 per 100,000 population), and higher than that in Europe (606.61 per 100,000 population).
Prevalence of HF across Asian regions and countries from GBD data
We further analyzed the HF burden across 5 Asian regions according to the 21 GBD regions: East Asia, South Asia, Southeast Asia, Central Asia, and high-income regions of Asia-Pacific. Based on this regional stratification, our analysis revealed that in the year 2019, the ASR of HF prevalence per 100,000 population was highest in East Asia (1,014.06) and lowest in South Asia (389.97). Furthermore, we noted intermediate values in other regions, with the ASR per 100,000 population of 455.28 in High-income Asia Pacific, 544.18 in Central Asia, and 755.95 in Southeast Asia.
Years lived with disability (YLDs) were calculated by multiplying the estimated prevalence of HF by the associated disability weight. The standardization of YLDs with respect to age and gender was carried out utilizing the GBD reference population. Comprehensive methodological details can be accessed in prior publications.9, 10, 11 In the GBD 2019, HF resulted in 5.05 million (95% UI: 3.28-7.26 million) YLDs across the world, with an ASR per 100,000 population of 63.92 (95% UI: 41.49-91.95 million). Among Asian regions, East Asia (90.93 per 100,000 population) had the highest ASR of YLDs, and South Asia exhibited the lowest ASR of YLDs (34.41 per 100,000 population).
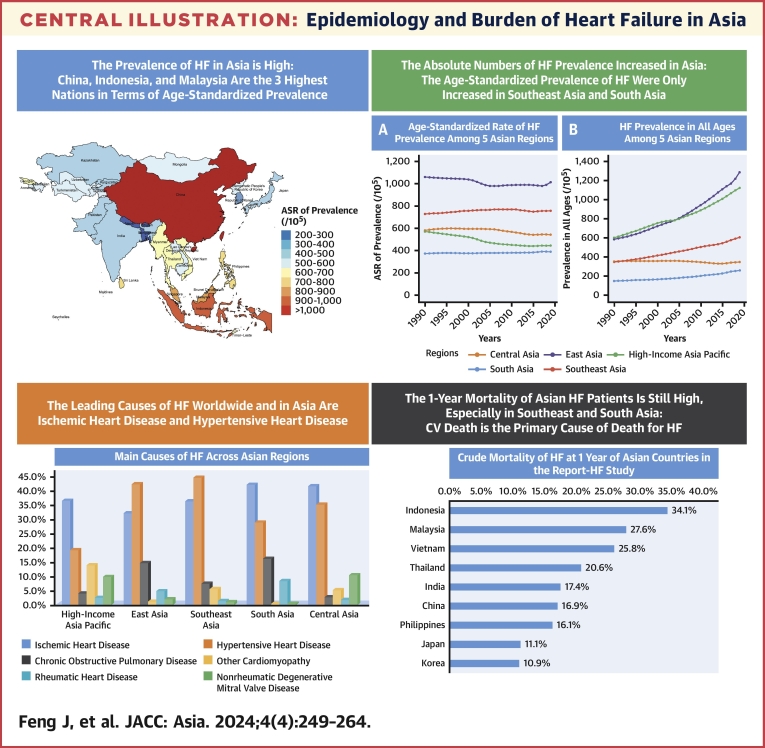
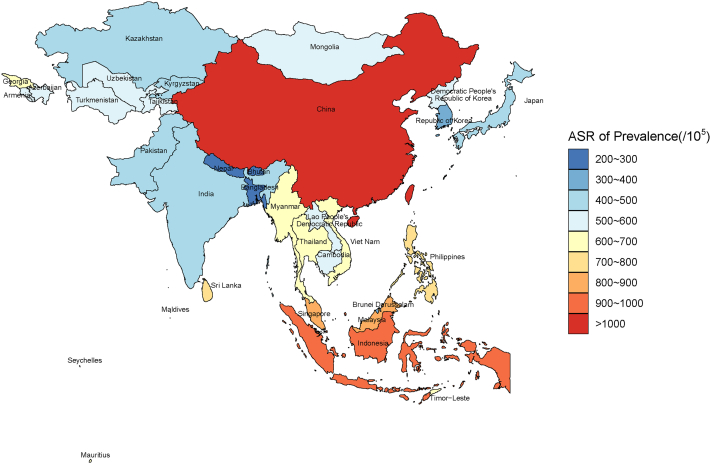
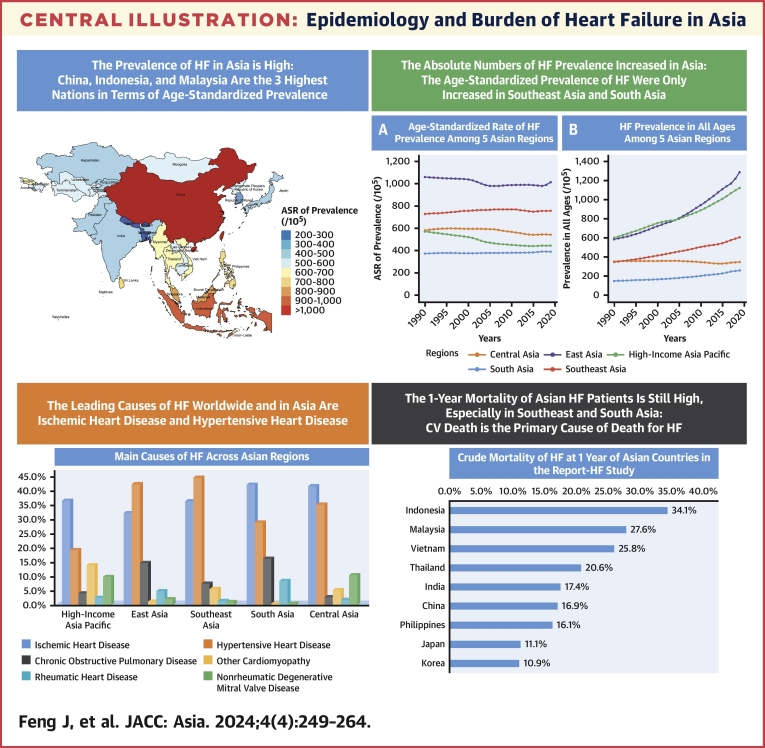
From the GBD data, the country and territorial ASR of HF prevalence ranged from 211.86 to 1,032.84 cases per 100,000 population. China (1,032.84), Indonesia (900.90), and Malaysia (809.47) are the 3 highest nations in terms of ASR for prevalence of HF in 2019. Conversely, Nepal (211.86 per 100,000 population), Bhutan (255.54 per 100,000 population), and Bangladesh (275.00 per 100,000 population) reported the lowest rates (Table 1, Figure 1). The ASR of YLDs among Asian nations and territories in 2019 demonstrated a similar pattern to the ASR of prevalence (Supplemental Table 1, Supplemental Figure 1).
Table 1.
Age-Adjusted Rates of Prevalence for HF Among 5 Asian Regions From GBD Data
| Location | ASRs of Prevalence, % (95% UI) |
ASRs of Prevalence per 100,000 Population and 95% UI |
||
|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | |
| High-income Asia Pacific | 0.61 (0.50-0.75) | 0.49 (0.42-0.56) | 571.60 (471.91-698.96) | 445.28 (384.66-514.55) |
| Singapore | 0.53 (0.43-0.65) | 0.36 (0.29-0.44) | 490.72 (398.39-602.78) | 328.71 (268.09-402.06) |
| Japan | 0.65 (0.54-0.79) | 0.53 (0.46-0.60) | 603.27 (499.89-735.11) | 481.00 (418.37-550.97) |
| Brunei Darussalam | 0.47 (0.38-0.59) | 0.41 (0.34-0.51) | 443.21 (357.85-551.32) | 382.15 (310.24-472.02) |
| Republic of Korea | 0.41 (0.33-0.51) | 0.37 (0.29-0.46) | 385.12 (309.65-483.11) | 339.30 (270.67-419.87) |
| East Asia | 1.10 (0.90-1.37) | 1.09 (0.89-1.34) | 1,060.30 (863.37-1,310.49) | 1,014.06 (830.18-1,252.95) |
| China | 1.12 (0.92-1.39) | 1.11 (0.90-1.36) | 1,079.37 (880.67-1,335.51) | 1,032.84 (846.57-1,277.46) |
| Democratic People's Republic of Korea | 0.58 (0.46-0.75) | 0.58 (0.46-0.75) | 560.87 (444.58-723.28) | 549.64 (432.50-713.60) |
| Southeast Asia | 0.74 (0.59-0.94) | 0.79 (0.63-0.98) | 729.15 (581.66-919.89) | 755.95 (604.89-946.23) |
| Sri Lanka | 0.78 (0.60-1.00) | 0.78 (0.60-1.00) | 755.33 (581.66-973.48) | 737.17 (571.00-950.23) |
| Malaysia | 0.78 (0.59-1.01) | 0.86 (0.66-1.11) | 758.17 (574.29-977.43) | 809.47 (621.56-1045.58) |
| Thailand | 0.67 (0.52-0.88) | 0.68 (0.53-0.86) | 651.54 (502.92-853.63) | 646.03 (503.57-823.55) |
| Maldives | 0.78 (0.59-1.01) | 0.73 (0.56-0.94) | 762.29 (576.77-991.40) | 702.02 (536.08-904.68) |
| Timor-Leste | 0.58 (0.45-0.75) | 0.65 (0.50-0.84) | 576.74 (445.54-740.31) | 630.98 (486.42-818.38) |
| Seychelles | 0.76 (0.59-0.99) | 0.76 (0.58-1.01) | 730.55 (567.06-950.33) | 720.03 (547.32-955.05) |
| Mauritius | 0.78 (0.59-1.02) | 0.71 (0.56-0.93) | 757.00 (580.03-993.06) | 687.12 (536.54-893.83) |
| Viet Nam | 0.65 (0.50-0.85) | 0.70 (0.56-0.88) | 638.25 (490.27-840.14) | 665.42 (536.62-839.93) |
| Myanmar | 0.64 (0.49-0.82) | 0.62 (0.47-0.80) | 629.35 (485.63-811.73) | 601.42 (460.08-780.42) |
| Cambodia | 0.57 (0.44-0.75) | 0.59 (0.45-0.76) | 560.61 (430.86-734.26) | 568.26 (434.83-741.38) |
| Philippines | 0.75 (0.60-0.95) | 0.82 (0.66-1.03) | 731.70 (585.36-924.78) | 787.31 (629.80-990.66) |
| Indonesia | 0.85 (0.68-1.07) | 0.94 (0.75-1.19) | 835.45 (666.27-1,050.39) | 900.90 (717.73-1,138.87) |
| Lao People's Democratic Republic | 0.56 (0.43-0.73) | 0.58 (0.45-0.76) | 549.15 (422.46-713.94) | 560.53 (434.91-724.73) |
| South Asia | 0.38 (0.31-0.48) | 0.40 (0.32-0.50) | 374.17 (302.18-470.48) | 389.97 (314.38-487.19) |
| Bhutan | 0.25 (0.20-0.33) | 0.26 (0.21-0.34) | 251.56 (195.69-327.52) | 255.54 (199.01-330.03) |
| Bangladesh | 0.26 (0.20-0.34) | 0.28 (0.22-0.37) | 259.89 (200.46-337.34) | 275.00 (213.50-355.43) |
| India | 0.40 (0.32-0.50) | 0.42 (0.34-0.52) | 390.14 (315.11-487.72) | 406.20 (328.33-505.76) |
| Nepal | 0.23 (0.18-0.30) | 0.22 (0.17-0.29) | 226.08 (174.66-294.04) | 211.86 (164.68-276.13) |
| Pakistan | 0.40 (0.32-0.51) | 0.42 (0.34-0.53) | 396.82 (319.37-496.95) | 405.12 (327.72-504.86) |
| Central Asia | 0.61 (0.47-0.78) | 0.58 (0.45-0.74) | 582.38 (453.44-748.24) | 544.18 (425.82-697.68) |
| Kazakhstan | 0.60 (0.46-0.78) | 0.53 (0.41-0.68) | 573.91 (437.55-739.35) | 492.32 (380.35-638.56) |
| Armenia | 0.67 (0.52-0.86) | 0.61 (0.46-0.78) | 637.70 (495.41-814.90) | 567.62 (433.75-725.29) |
| Uzbekistan | 0.54 (0.42-0.70) | 0.56 (0.44-0.72) | 523.83 (404.22-671.85) | 532.43 (416.09-680.17) |
| Tajikistan | 0.62 (0.48-0.81) | 0.59 (0.45-0.76) | 599.10 (460.10-773.06) | 555.67 (424.52-724.45) |
| Turkmenistan | 0.57 (0.44-0.74) | 0.59 (0.45-0.76) | 548.14 (418.98-705.24) | 550.35 (425.56-711.45) |
| Kyrgyzstan | 0.57 (0.44-0.74) | 0.51 (0.40-0.66) | 546.45 (423.03-701.31) | 484.20 (373.74-626.12) |
| Mongolia | 0.56 (0.43-0.71) | 0.57 (0.44-0.74) | 538.33 (419.08-688.21) | 538.57 (412.28-699.69) |
| Azerbaijan | 0.65 (0.50-0.85) | 0.59 (0.46-0.77) | 623.55 (477.79-813.92) | 557.22 (431.43-722.75) |
| Georgia | 0.72 (0.55-0.93) | 0.71 (0.55-0.92) | 680.10 (520.86-887.86) | 670.44 (518.82-859.31) |
ASR = age-standardized rate; GBD = Global Burden of Disease; HF = heart failure; UI = uncertainty interval.
Figure 1.
Age-Standardized Prevalence Rate of Heart Failure in Asia From GBD 2019
From the GBD (Global Burden of Disease) data, the national and territorial age-standardized rate (ASR) of heart failure prevalence ranged from 211.86 to 1,032.84 cases per 100,000 population in Asia. China (1,032.84), Indonesia (900.90), and Malaysia (809.47) are the 3 highest nations in terms of ASR for prevalence of HF in 2019. Conversely, Nepal (211.86), Bhutan (255.54), and Bangladesh (275.00) reported the lowest rates.
Trend of HF prevalence change in Asia from GBD data
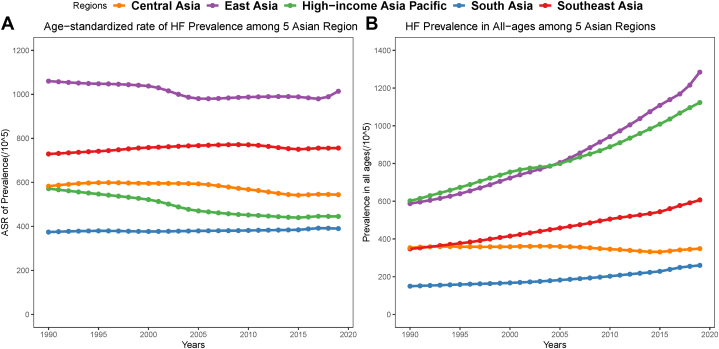
In terms of the trend of HF prevalence change in Asia, among 5 GBD regions in Asia, 3 showed a decrease in the ASR of HF from 1990 to 2019, with the largest decreases being in high-income Asia Pacific (22.10%), followed by Central Asia (6.56%) and East Asia (4.36%). In contrast, only 2 GBD regions in Asia showed an increased trend: Southeast Asia (3.68%) and South Asia (4.22%). The age-standardized and unstandardized prevalence of HF per 100,000 population in 5 Asian regions from 1990 to 2019 is presented in Figure 2. Nevertheless, the absolute numbers of HF prevalent cases among all Asian regions have increased from 1990 to 2019 (from 33% increase in Central Asia to 186% increase in East Asia).
Figure 2.
Prevalence of HF in Asia From GBD 1990 to 2019
(A) ASR of HF prevalence among 5 Asian GBD (Global Burden of Disease) data regions from 1990 to 2019. 3 showed a decrease in the ASR of HF from 1990 to 2019, with the largest decreases being in high-income Asia Pacific (22.10%), followed by Central Asia (6.56%) and East Asia (4.36%). Only 2 GBD regions in Asia showed an increased trend, with the Southeast Asia (3.68%) and South Asia (4.22%). (B) Unstandardized prevalence of HF in 5 Asian GBD regions from 1990 to 2019. The absolute numbers of HF prevalent cases among all Asian regions have increased from 1990 to 2019 (from 33% increase in Central Asia to 186% increase in East Asia). Abbreviations as in Figure 1.
From 1990 to 2019, the percentage change in the ASR of prevalence differed among Asian countries and territories. A total of 14 countries and territories showed increases, whereas others demonstrated decreases during the measurement period. Over the period of 1990 to 2019, Timor-Leste (9.40%), Indonesia (7.83%), and Philippines (7.60%) showed the largest increases, whereas Singapore (33.01%), Japan (20.27%), and Kazakhstan (14.22%) demonstrated the largest decreases. The trend of age-standardized and unstandardized prevalence of HF per 100,000 population in these 6 countries from 1990 to 2019 is presented in Supplemental Figure 2.
Prevalence of HF from registry studies
Furthermore, data about the prevalence of HF at the country and territorial level in Asia can also be obtained from some population-based registry studies, although such data resources are relatively limited across Asian countries.
For instance, In China, a study conducted in 2000 found that the prevalence of chronic HF among the Chinese population, age 35 to 74 years, was 0.9%, with gender-specific estimates of 0.7% for men and 1.0% for women.12 A decade later, in the CHS (China Hypertension Survey) enrolling 22,158 participants from 2012 to 2015, the prevalence of HF increased to 1.3% among the Chinese adult population age 35 years and older, an estimated 890 million patients.13,14 Research conducted in Taiwan, China, revealed a rise in HF prevalence from 0.63% in 2001 to 1.40% in 2016. This marked a 2.22-fold increase over 16 years, projecting an estimated prevalence of 4.45% by 2050.15
Japan is one of the most aged countries in the world; however, there have been no population-based studies that precisely examined the prevalence of HF in Japan as far as we know. One report estimated the number of Japanese outpatients with left ventricular dysfunction was 979,000 in 2005 (0.8% of the total population). It is foreseen that this number will gradually rise with the ageing population, reaching 1.3 million by the year 2030.16,17
In South Korea (Republic of Korea), a prevalence-based, cost-of-illness study was conducted using the 2014 Health Insurance Review and Assessment Service-National Patients Sample data. The study identified a total of 475,019 adults age 19 years and above with HF in 2014, resulting in an estimated prevalence rate of 12.4 individuals per 1,000 adults.18
Prevalence of HF according to LVEF from registry studies
LVEF plays an important role in categorizing HF patients because of its notable implications for prognosis and the varying responses to treatment strategies. Furthermore, the inclusion criteria for many clinical trials heavily rely on LVEF values.5,7,19 Therefore, it is necessary to understand the phenotypic characteristics of Asian HF patients based on their LVEF status.
In China, in the population-based CHS study, the weighted prevalences of HFrEF, HFmrEF, and HFpEF were found to be 0.7%, 0.3%, and 0.3%, respectively. In Chinese patients with HF, 40% had HFrEF, 23% had HFmrEF, and 37% had HFpEF. Additionally, 32.8%, 1.1%, and 1.6% of individuals were categorized as having “mild,” “moderate,” and “severe” left ventricular diastolic dysfunction, respectively,13 based on the recommendations for the evaluation of left ventricular diastolic function by echocardiography.20 The China-HF (China-Heart Failure) registry study included 13,687 patients with HF from 2012 to 2015 (Stage I) and included 34,938 patients from 2017 to 2020 (Stage II), the proportions of HFrEF, HFmrEF, and HFpEF were 36.5%, 16.6%, and 46.9% in China-HF Stage I, and 40.2%, 21.8%, and 38.0% in China-HF Stage II.21,22
In Japan, several observational studies have consistently reported that approximately one-half of hospitalized patients with decompensated HF had HFpEF.23, 24, 25, 26 The Chronic Heart Failure Analysis and Registry in the Tohoku District-2 Study analyzed 3,480 consecutive HF patients with available echocardiography data. This study revealed a distribution of 21.0% HFrEF, 17.1% HFmrEF, and 61.9% HFpEF.24 This increase in patients with HF may be attributed largely to the increase in HFpEF incidence. A series of epidemiological studies carried out in the Tohoku district (CHART [Chronic Heart Failure Analysis and Registry in the Tohoku District] 1 and 2) unveiled a noteworthy trend. The proportion of patients with HFpEF exhibited a rise from 50.6% in the years 2000 to 2005 to 68.7% in the period of 2006 to 2010.27 It is worth noting that the proportion of patients with HFpEF in Japan surpasses that observed in China as well as in Western countries.26
In the context of Southeast Asia, findings from the INTER-CHF (International-Congestive Heart Failure) study indicate that 39% of patients in Malaysia and the Philippines exhibited a reduced LVEF (<40%).28 On the other hand, in India, Indonesia, and Thailand, data gathered from the International REPORT-HF (Registry to Assess Medical Practice with Longitudinal Observation for Treatment of Heart Failure) demonstrated the proportions of HFrEF, HFmrEF, and HFpEF were 59%, 18%, and 23%.29 In South Asia, the NHFR (National Heart Failure Registry of India) presented that the most common classification was HFrEF (65%), followed by HFmrEF (22%) and HFpEF (13%).
Incidence of HF in Asia
Population-based studies on the incidence of HF within the Asian region are relatively limited. In China, a recent population-based study utilized records from 50 million individuals ≥25 years of age from the national urban employee basic medical insurance in 6 provinces during the year 2017. Incident cases were defined as individuals diagnosed with HF in 2017, with a preceding 4-year disease-free period (2013-2016). The incidence of HF was determined by applying age standardization to the 2010 Chinese census population. The age-standardized incidence was 275 per 100,000 person-years, accounting for 3.0 million newly diagnosed HF patients aged 25 years and above. Notably, the incidence increased with age, with rates of 158, 892, and 1,655 per 100,000 person-years for individuals age 25-64, 65-79, and ≥80 years, respectively.30 In a study of Taiwan, China, the incidence of HF in year 2016 was 2.19 per 1,000 person-years. Over the period from 2001 to 2016, there was a slight overall temporal trend indicating a decrease in incidence (from 2.44 to 2.19 per 1,000 person-years, respectively).15
In India, the annual incidence of HF attributed to conditions such as coronary heart disease, hypertension, obesity, diabetes, and rheumatic heart disease was conservatively estimated to be 491,600 to 1.8 million cases.31
In Japan, a study that estimated the annual incidence of HF using data from the United States suggested that 10 of 1,000 individuals aged 65 years or older develop new-onset heart failure each year.32 This estimation speculated that more than 0.3 million Japanese individuals developed new-onset HF among the 31.9 million people aged 65 years or older in 2013. Furthermore, it is anticipated that more than 0.37 million new cases of HF will arise in 2025.33
Etiology, Comorbidities, and Risk Factors
Causes of HF from GBD data
According to the 2019 GBD data, the leading causes of HF worldwide are ischemic heart disease and hypertensive heart disease. The proportion of HF attributed to ischemic heart disease and hypertensive heart disease are 37.32% and 32.84%, respectively. Ischemic heart disease is more likely to affect higher-income areas.34 In addition to heart diseases, chronic obstructive pulmonary disease (COPD) is a main cause of HF (Table 2). During the period from 1990 to 2019, the ASR of HF prevalence attributable to hypertensive heart disease experienced an increase, rising from 219.54 to 233.77 per 100,000 population. In contrast, the ASR of HF from other causes decreased.8
Table 2.
Top 10 Causes of HF Among Asian Regions From GBD 2019
| Global | High-Income Asia Pacific | East Asia | Southeast Asia | South Asia | Central Asia | |
|---|---|---|---|---|---|---|
| Ischemic heart disease | 37.32 | 36.19 | 31.89 | 36.06 | 41.82 | 41.37 |
| Hypertensive heart disease | 32.84 | 19.11 | 42.02 | 44.28 | 28.66 | 34.95 |
| Chronic obstructive pulmonary disease | 9.22 | 3.98 | 14.54 | 7.27 | 16.06 | 2.60 |
| Other cardiomyopathy | 6.67 | 13.79 | 1.01 | 5.41 | 0.31 | 5.05 |
| Rheumatic heart disease | 3.70 | 2.38 | 4.70 | 1.27 | 8.28 | 1.67 |
| Nonrheumatic degenerative mitral valve disease | 2.95 | 9.75 | 1.85 | 0.93 | 0.38 | 10.30 |
| Nonrheumatic calcific aortic valve disease | 1.41 | 4.70 | 0.36 | 0.07 | 0.06 | 1.13 |
| Alcoholic cardiomyopathy | 1.19 | 1.25 | 0.19 | 0.25 | 0.18 | 0.60 |
| Congenital heart anomalies | 1.02 | 1.96 | 1.12 | 1.13 | 1.49 | 0.79 |
| Myocarditis | 0.73 | 2.17 | 1.01 | 0.66 | 0.24 | 0.45 |
Values are %.
GBD = Global Burden of Disease; HF = heart failure.
Among the 5 Asian regions analyzed within the GBD data, ischemic heart disease emerged as the most prevalent cause of HF in high-income Asia Pacific, South Asia, and Central Asia. However, in East Asia and Southeast Asia, ischemic heart disease ranked as the second cause of HF, and hypertensive heart disease is the primary cause of HF. COPD is the third cause of HF in East Asia, Southeast Asia, and South Asia, whereas cardiomyopathy is the third cause of HF in high-income Asia Pacific and nonrheumatic degenerative mitral valve disease is the third cause of HF in Central Asia.
Causes of HF and comorbidities from registry studies
Data from some global or national-level HF registry studies have also provided information on the etiology and comorbidities of HF in Asian patients. The INTER-CHF study enrolled 5,823 patients with HF and evaluated 1-year mortality, 2,660 patients were in Asia, including China, India, and Southeast Asia (Malaysia and the Philippines). Ischemic heart disease was identified as the primary cause of HF in Southeast Asia, accounting for 56% of cases, and it contributed to 46% of cases in India and 45% in China. Hypertensive heart disease emerged as the second leading cause of HF in India and Southeast Asia, representing 14% and 15% of cases, respectively. Notably, idiopathic dilated cardiomyopathy ranked as the second main cause of HF in China, accounting for 15% of cases. Valvular heart disease was responsible for 12% of HF cases in Southeast Asia, 12% in India, and 11% in China.28
In the REPORT-HF study, 18,102 patients hospitalized for HF were enrolled from 44 countries on 6 continents. These countries were categorized based on a modified WHO regional classification. In Southeast Asia (India, Indonesia, and Thailand), the etiology of HF was as follows: ischemic heart disease (37%), hypertensive heart disease (24%), cardiomyopathy (23%), and valvular heart disease (7%). Regarding comorbidities, the most prevalent conditions included anemia (54%), coronary artery disease (CAD) (51%), and hypertension (47%). Additionally, type 2 diabetes, chronic kidney disease, and atrial fibrillation accounted for 42%, 10%, and 8% of the comorbidities, respectively.29
The ASIAN-HF (Asian Sudden Cardiac Death in Heart Failure) registry is a prospective study that enrolled individuals with symptomatic HF from 3 Asian regions: South Asia, Southeast Asia, and Northeast Asia, including 11 countries and territories. Overall, 6,480 patients with HF aged over 18 years were recruited. In the ASIAN-HF registry, an ischemic etiology was more common in Southeast Asia (62%) vs South Asia (35%) vs Northeast Asia (30%), and in HFrEF vs HFpEF. In terms of comorbidities, the prevalence of hypertension was highest in HF patients in Southeast (67%) and Northeast (55%) Asia, whereas it was lower in South Asia (38%). Atrial fibrillation had a prevalence of 31.5% among HF patients in Northeast Asia, 4.6% in South Asia, and 19.8% in Southeast Asia. The prevalence of diabetes in HF patients was 51.3% in Southeast Asia, 35.9% in South Asia, and 33.6% in Northeast Asia.35
Another publication of ASIAN-HF prospectively studied the specific clinical and echocardiographic characteristics in 1,204 patients with HFpEF. The mean age of the study population was 68 ± 12 years (37% were age <65 years). In total, 70% of patients had ≥2 comorbidities. These data show that Asian patients with HFpEF are relatively young and have a high burden of comorbidities.36 Besides, a study of ASIAN-HF reported the ethnic differences of comorbidities among 5,276 stable patients with HFrEF. Compared with Chinese ethnicity, Malays and Indians had higher odds of CAD as a comorbidity, whereas Koreans and Japanese had lower odds. This highlights the heterogeneity among Asian patients with stable HFrEF, and the influence of ethnicity on patient characteristics.37
Regarding other national-level registries, in the China-HF stage II registry, common etiologies included CAD (48.3%), valvular heart disease (18.7%), and dilated cardiomyopathy (16.3%). Common comorbidities included hypertension (56.3%), diabetes mellitus (31.5%), and atrial fibrillation (17.6%).22 It is worth noting that the prevalence of hypertension, diabetes, and atrial fibrillation in Chinese patients with HF was notably lower compared with estimates from the GWTG-HF (Get With The Guidelines-Heart Failure) study conducted in the United States.38 Data from the China Cardiovascular Association Database-HF Center Registry included 41,708 hospitalized HFpEF patients with 1 year of follow-up from 31 provinces of China, highlighting the common precipitating factors for index hospitalization for HF. Among these factors, ischemia (26.6%), infection (14.4%), and arrhythmia (10.5%) were the 3 most frequently observed. A significant proportion (67.4%) of these patients had 3 or more comorbidities. The most prevalent comorbidities included hypertension (65.2%), coronary heart disease (60.3%), and atrial fibrillation (41.2%).39
In the National Heart Failure Registry of India, ischemic heart disease was the predominant etiology for HF (72%), followed by dilated cardiomyopathy (18%). Rheumatic valvular heart disease was present in 5.9% of the study population. Valve diseases other than rheumatic valvular heart disease contributed to 2.1% of the cases. Isolated right HF was noted in 62 (0.6%) participants. Hypertension and diabetes were the most frequent comorbid conditions (48.9% and 42.3%, respectively). Atrial arrhythmia was prevalent in 9.5% of the study population. Chronic kidney disease, stroke, and COPD were reported in 8.5%, 3.0%, and 6.9% of the study population, respectively.40 Additionally, the etiology and comorbidities of HF patients in Japan and South Korea are presented in Table 3.
Table 3.
Summary of National-Level Registry Studies in China, India, Japan, and Korea
| China-HF Stage I21,22 2012-2015 | China-HF Stage II22 2017-2020 | National Heart Failure Registry of India40 2019-2020 | CHART-227 2006-2010 | JCARE-CARD48 2004-2006 | KorAHF50 2011-2012 | |
|---|---|---|---|---|---|---|
| Regions | China | China | India | Japan | Japan | Korea |
| Sample size | 13,687 | 34,938 | 10,851 | 4,735 | 2,675 | 2,066 |
| Age, y | 65 ± 15 | 67 ± 14 | 60 ± 14 | 70 ± 12 | 71 ± 13 | 69 ± 14 |
| Male | 59.1 | 60.8 | 69.0 | 68.4 | 59.7 | 55.0 |
| HF classification | ||||||
| HFrEF | 36.5 | 40.2 | 65.0 | 58.0 | 56.0 | |
| HFmrEF | 16.6 | 21.8 | 22.0 | 16.0 | ||
| HFpEF | 46.9 | 38.0 | 13.0 | 68.7 | 26.0 | |
| Etiology | ||||||
| Ischemic heart disease | 49.6 | 48.3 | 71.9 | 47.1 | 32.0 | 38.0 |
| Hypertensive heart disease | 9.9 | 24.6 | 6.0 | |||
| Valvular heart disease | 15.5 | 18.7 | 2.1 | 23.8 | ||
| Dilated cardiomyopathy | 16.0 | 16.3 | 18.0 | 24.0 | ||
| Comorbidities | ||||||
| Hypertension | 50.9 | 56.3 | 48.9 | 74.3 | 52.6 | 59.0 |
| Diabetes mellitus | 21.0 | 31.5 | 42.3 | 23.3 | 29.8 | 36.0 |
| CKD | 40.0 | 41.9 | 8.5 | 47.3 | 11.7 | |
| COPD | 6.9 | 6.5 | ||||
| Atrial fibrillation | 24.4 | 17.6 | 9.5 | 31.0 | 35.0 | 27.0 |
| Anemia | 21.1 | 22.0 | 20.7 | |||
| Medications | ||||||
| ACEI or ARB or ARNI | 68.2 | 78.7 | 61.9 | 76.4 | 79.1 | 65.0 |
| Beta-blocker | 61.5 | 82.2 | 75.9 | 49.0 | 57.5 | 44.0 |
| MRA | 75.0 | 87.8 | 65.5 | 42.2 | 40.0 | |
Values are n, mean ± SD, or %.
ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blockers; ARNI = angiotensin receptor-neprilysin inhibitor; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
Risk factors of HF
Lifestyles and cardiovascular health metrics
The adverse impact of a combination of unhealthy lifestyle factors on the risk of HF has been predominantly reported in Western populations. The CKB (China Kadoorie Biobank) cohort study provided some new perspectives in Asian populations. This study developed a healthy lifestyle score (HLS) based on parameters including smoking, drinking, physical activity, diet, body mass index, and waist circumference, and compared it with a more comprehensive set of metrics that included cardiovascular disease (CVD) risk markers (blood pressure, blood glucose, and blood lipids) in addition to the HLS. This expanded set of factors, referred to as “ideal cardiovascular health metrics” (ICVHMs), was evaluated in 487,197 participants. Over a median follow-up period of 10 years, a total of 4,208 incident cases of HF were recorded. The findings showed significant associations between both the HLS and ICVHMs and the risk of HF. Notably, ICVHMs exhibited a more robust predictive capability for HF risk in comparison to lifestyle factors alone. Consequently, it was established that the combination of lifestyle factors and cardiometabolic status substantially enhanced the prediction of HF risk.41
Another study from CKB investigated the genetic association with incident HF as well as the modification effect of ICVHMs on such genetic association. The results indicated that genetic risk and adherence to ICVHMs were independently correlated with the risk of developing HF. This observation underscored that maintaining a favorable cardiovascular (CV) health status was linked to a reduced risk of HF, regardless of the genetic predisposition.42
Air pollution
Air pollution and its link to CVD represent a growing concern. A study aimed to estimate the short-term effects of fine particulate matter (PM2.5) on CVD admissions in Beijing, China. The study involved a data set comprising 460,938 electronic hospitalization summary reports for CVD cases from 2013 to 2017. The analysis explored the association between PM2.5 exposure and hospitalizations for both overall CVD and specific causes of CVD, such as HF, while adjusting for seasonal variations, days of the week, public holidays, and weather conditions. The findings revealed a significant increase in total CVD admissions with every 10 μg/m3 rise in PM2.5 concentration from the previous day to the current day. This association was particularly pronounced among older adults (age ≥65 years) and for specific conditions such as coronary heart disease and atrial fibrillation. However, the observed elevated risk did not reach statistical significance for HF hospitalizations.43
In another meta-analysis that included 100 studies covering 20 countries worldwide, 81 of which investigated short-term exposure and 19 long-term exposure to various air pollutants (including PM2.5, PM10, NO2, SO2, CO, and O3), the relationship between these pollutants and HF hospitalization, incidence, or mortality was explored. The results showed a significant association between PM2.5, PM10, NO2, SO2, and CO and an elevated risk of HF, irrespective of the duration of exposure. Importantly, the adverse associations between most pollutants and HF were more pronounced in low- and middle-income countries compared with high-income countries.44 The study emphasizes the ongoing need for sustained public and environmental policies and actions aimed at mitigating air pollution, because they play a crucial role in reducing the burden of HF.
Ambient temperature
Epidemiological studies have provided compelling evidence of relationships between ambient temperature and CVD. A national time-series analysis in 184 cities across China included data on daily hospital admissions for CVD. Temperature variability was assessed by computing the SD of daily minimum and maximum temperatures over exposure days. Throughout the study duration, there were 8.0 million hospital admissions for CVD. At the national average level, a 1 °C increase in temperature variability at 0 to 1 days was associated with a 0.44% rise in hospital admissions for CVD and 0.48% increase in hospitalization for HF. The estimates decreased but remained significant when controlling for ambient fine particulate matter.45 This finding suggested that short-term temperature variability exposure may elevate the risk of HF, offering new insights into the health effects of climate change.
Outcome
Global or regional-level registry studies
Contemporary data on outcomes in HF across Asia remain relatively limited. In the INTER-CHF study, 1-year mortality rate was 23% in India, 15% in Southeast Asia (Malaysia and the Philippines), and 7% in China. Notably, regional differences persisted after multivariable adjustments.28 In REPORT-HF study, among Asian countries, the crude 1-year mortality was highest in Indonesia (34.1%) and lowest in Korea (10.9%).29 The characteristics and 1-year mortality of HF patients in Asian countries in REPORT-HF study is shown in Table 4.
Table 4.
Characteristics and 1-Year Mortality of Asian Countries in the REPORT-HF Study
| Country | Regions (as the Study Design) | Income Classification | No. of Patients Discharged Alive | Age, y Median (IQR) |
Women, % | Crude Mortality Rate at 1 y |
|---|---|---|---|---|---|---|
| Indonesia | Southeast Asia | Lower middle income | 337 | 56 (49-63) | 38.6 | 34.1 |
| Malaysia | Western Pacific | Upper middle income | 134 | 54 (46-63) | 20.9 | 27.6 |
| Vietnam | Western Pacific | Lower middle income | 182 | 66 (57-78) | 46.7 | 25.8 |
| Thailand | Southeast Asia | Upper middle income | 472 | 65 (57-75.5) | 48.7 | 20.6 |
| India | Southeast Asia | Lower middle income | 1,483 | 62 (53-70) | 32.0 | 17.4 |
| China | Western Pacific | Upper middle income | 1,436 | 67 (57-77) | 36.1 | 16.9 |
| Philippines | Western Pacific | Lower middle income | 508 | 61 (50.5-71) | 41.7 | 16.1 |
| Japan | Western Pacific | High income | 108 | 79 (70-85) | 40.7 | 11.1 |
| Korea | Western Pacific | High income | 558 | 73 (62-79) | 50.5 | 10.9 |
Data from the REPORT-HF study by Tromp et al.29
In the ASIAN-HF registry, crude 1-year all-cause mortality was 9.6% in patients with symptomatic HF. Asian patients with HFrEF had a worse 1-year mortality than those with HFpEF (10.6% vs 5.4%, respectively). When considering regional disparities, Southeast Asian patients had the highest 1-year all-cause mortality at 13.0%, followed by South Asian patients at 7.5% and Northeast Asian patients at 7.4%.35 Additionally, another study of the ASIAN-HF registry revealed that higher socioeconomic indicators at both the country and patient levels were associated with an improved quality of life and a reduced risk of all-cause mortality or HF-related hospitalization.46 Regarding the causes of death in patients with HF, the ASIAN-HF registry found that the primary cause of death was CV in 60.9% of cases, followed by unknown/presumed CV causes at 25.9%, and non-CV causes at 13.2%. The risk of CV-related death was slightly higher in HFrEF patients (54.0%) compared with HFpEF patients (53.3%), whereas non-CV causes of death were more frequent in HFpEF (23.3%) than in HFrEF (12.0%) at 1 year.35
National or territorial-level registry studies
Regarding the data on outcomes in national-level registries, in Japan, the ATTEND (acute decompensated heart failure syndromes) registry enrolled 4,842 patients hospitalized for HF, which demonstrated that the length of stay was extremely long (mean 30 days), with 6.4% in-hospital mortality.47 In the JCARE-CARD (Japanese Cardiac Registry of Heart Failure in Cardiology) enrolling 2,675 patients hospitalized for worsening HF in 164 hospitals, unadjusted in-hospital mortality was higher in patients with HFpEF vs HFrEF (6.5% vs 3.9%; P = 0.03), and postdischarge mortality with an average of 2.4 years of follow-up (22.7% vs 17.8%; P = 0.058). After multivariate adjustments, no significant differences were observed in the mortality rate. Moreover, patients with preserved EF had similar rehospitalization rates (36.2% vs 33.4%; P = 0.515) compared with patients with reduced EF.48 Another study, the JASPER (Japanese Heart Failure Syndrome With Preserved Ejection Fraction) Registry, included 535 consecutive hospitalized HFpEF patients from 15 hospitals. The in-hospital mortality rate was 1.3% and the 2-years rate of death or HF hospitalization was 40.8%, of which one-half of deaths had a cardiac cause.49
In Korea, the KorAHF (Korean Acute Heart Failure Registry) was established to assess the clinical profiles, treatment approaches, hospital trajectories, and outcomes of individuals admitted for acute heart failure (AHF) syndrome.50,51 Among 3,466 participants, of whom 1,653 were patients with LVEF, <40% were selected for the analysis. The in-hospital mortality was determined to be 6.6%. The 1,527 patients who survived and were discharged were followed up for a median of 1 year. The 60-day mortality was 3.8%, and the 1-year mortality reached 9.2%. Additionally, the rates of mortality or rehospitalization were 4.6% at 60 days and 14.1% at the end of 1 year.51
In China, the crude in-hospital mortality was 4.1% in China-HF Stage I (2012-2015) and significantly higher in patients with HFrEF vs HFpEF (4.0% vs 2.4%, respectively).21 Subsequently, the in-hospital mortality displayed a decline to 2.8% in China-HF Stage II (2017-2020),22 which was close to the in-hospital mortality in the GWTG-HF registry in the United States.38 The China PEACE 5p-HF enrolled adult patients hospitalized for HF from 52 hospitals in China. Among 4,898 patients, the rate of cardiovascular death was 3.9% in 30 days after discharge and 13.4% in 1 year after discharge. The rate of HF-related hospitalization was 5.2% within 30 days after discharge and 24.2% within 1-year postdischarge.52 In the China Cardiovascular Association Registry, which included 41,708 hospitalized HFpEF patients with 1-year follow-up in China in this study between 2017 and 2021, the 1-year rate of CV death was 3.1% and hospitalization for HF was 13.6%.39 Another study in Taiwan, China, included 633,098 hospitalized patients with HF and investigated the association between income level and outcomes. The overall in-hospital mortality was 4.1%. Patients in the low-income group faced nearly double the risk of in-hospital mortality and postdischarge events compared with the high-income group, partially attributed to reduced utilization of GDMT.53 Other research in Taiwan, China, showed that the incident mortality after newly diagnosed HF was estimated to be 38.5%, 52.2%, 62.1%, 69.6%, and 75.5% at 2, 4, 6, 8, and 10 years during the follow-up.15
In India, of 5,269 patients with acute decompensated HF enrolled in the Indian College of Cardiology National Heart Failure Registry study, the in-hospital mortality was 6.98%, while the 30-day mortality reached 12.35%, and the 30-day rehospitalization rate was 7.98%.54 In another study, the National Heart Failure Registry (NHFR) of India recruited 10,851 ADHF patients. Over the course of this study, a 90-day mortality rate of 14.2% was observed, alongside an 8.4% re-admission rate.40 Moreover, in a specific study involving 1,205 patients admitted for AHF in the THFR (Trivandrum Heart Failure Registry) of India in 2013, the 90-day all-cause mortality was 2.43 per 1,000 person-days.55 A 3-year mortality of 44.8% and a 5-year mortality rate of 59% was documented.56,57 The primary causes of death were attributed to sudden cardiac events (46%) and pump failure (49%).57
Management of HF in Asia
Despite recent progress in the diagnosis and treatment of HF, notable challenges remain in achieving standardized HF management. First, there exists a significant disparity in the uniformity of HF diagnosis and treatment practices among hospitals in different geographical regions. Furthermore, many individuals with HF do not receive comprehensive postdischarge management and education. This gap in care increases the risk of rehospitalization caused by worsening HF or even mortality.
Guideline-directed medical therapy and management
In the ASIAN-HF study, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) were prescribed to 77%, beta-blockers to 79%, and mineralocorticoid receptor antagonists (MRA) to 58%, with substantial regional variation. The guideline recommended dose was achieved in only 17% of cases for ACEIs/ARBs, 13% for beta-blockers, and 29% for MRA.58 Guideline-directed medical therapies (GDMTs) at recommended doses are underutilized in patients with HFrEF. Improved uptake and up-titration of GDMTs are needed for better patient outcomes. The rates of GDMT utilization in other Asian registries are shown in Table 3.
In China, the China-HF registry was established to comprehensively evaluate the management of HF in a large cohort of patients. The results from the first stage (2012-2015) showed that for 13,687 patients in China-HF Stage I, eligible patients with HFrEF received ACEIs/ARBs at a rate of 67.5%, beta-blockers at a rate of 70.0%, and MRA at a rate of 74.1%.21 To address the deficiencies like understandardized HF management reflected by China-HF stage I, the Heart Failure Medical Union in China was established to carry out continuous quality promotion projects and establish the Medical Quality Evaluation Index System for HF in China.59 Meanwhile, the China-HF Stage II (2017-2020) study was launched. The results suggested that echocardiographic assessment of LVEF and natriuretic peptide testing were conducted in 93.7% and 93.0% of cases, respectively. In China-HF Stage II, adherence to standardized guidelines showed improvement compared with China-HF Stage I, but generally remained lower than the GWTG-HF registry. Specifically, 78.7% of eligible patients received RAS inhibitors and 82.2% were prescribed β-blockers. Notably, a higher proportion of eligible patients were discharged with MRA (87.8%) compared with the GWTG-HF registry.22 This study reflected a significant improvement in the standardized management of HF within the country. However, the substantial gaps in the quality of care for HF patients in China still persist.
In another study of China, the China PEACE 5r-HF study focused on evaluating the adherence to quality measures for HF care at the hospital level. The quality of HF care was assessed by the adherence to 4 performance measures. In total, 10,004 hospital admissions for HF at 189 hospitals were included in this study. The median rates for key performance measures were as follows: LVEF assessment during hospitalization was 66.7%, β-blocker prescription for eligible patients at discharge was 14.8%, ACEI/ARB prescription for eligible patients at discharge reached 57.1%, and scheduled follow-up appointments at discharge were made at a rate of 11.5%. The findings of this study suggest that there is wide heterogeneity in the quality of care for HF among hospitals, suggesting the need for a national strategy to improve and standardize the quality of HF care in China.60
In India, the Cardiology Society of India-Kerala Acute Heart Failure Registry described the GDMT prescribing patterns and 90-day mortality rates in patients admitted with AHF in Kerala, India. A total of 7,507 patients with AHF were included and only one-fourth (28%) of patients with HFrEF received GDMT, which is defined as the combination of ACEI/ARB/ARNI, β-blocker, and MRA. The mortality of AHF patients was independently associated with GDMT initiation. This study emphasizes that quality improvement initiatives should be implemented as soon as possible, focusing on increasing GDMT prescription and improving the survival of HF patients in India.
Sodium-glucose cotransporter 2 inhibitors have recently proved to be therapeutic in patients with HFrEF and HFpEF. It also was recommended for patients with type 2 diabetes and either established CVD or at high cardiovascular risk to prevent hospitalizations for HF.5 However, the effectiveness of these treatments may vary between patients in Asia and those in other parts of the world. A study aimed to examine the outcomes in patients randomized in Asia in the DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure) trial. Among the 4,744 patients in the DAPA-HF trial, 1,096 (23.1%) were from Asia. The findings showed that dapagliflozin reduced the risk of the primary endpoint to a similar extent in patients from Asia as it did in other regions (P for interaction = 0.32).61 Another study conducted an individual data pooled analysis of the DAPA-HF and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trials, which evaluated the effects of dapagliflozin in HFrEF and HFmrEF/HFpEF, respectively. Among 11,007 patients, 2,322 (21.1%) were in Asia. The benefit of dapagliflozin on worsening HF or cardiovascular death was not modified by region (P interaction = 0.40). The HR of dapagliflozin vs placebo in Asia was 0.74 (95% CI: 0.61-0.91).62
A post hoc analysis of EMPEROR-Reduced trial provided an in-depth analysis of the regional differences in the effect of empagliflozin. Of 3,730 patients, 493 (13.2%) were enrolled in Asia. The ratio of total HF hospitalization to cardiovascular death was highest in Asia (5.4), followed by 4.8 in North America and 2.1 in Europe. Groups with the highest ratio (Asia) had the greatest reduction in the composite of cardiovascular death or HF hospitalization with empagliflozin, which also supported that the benefit of empagliflozin was primarily by its effect in reducing hospitalizations.63
The EMPEROR-Preserved trial provided compelling evidence that, when added to the standard of care therapy, empagliflozin effectively reduces the risk of the CV death or hospitalization for HF in patients with HFpEF. Among the 5,988 patients enrolled in the EMPEROR-Preserved trial, 686 individuals (11.5%) were from Asia. An analysis aimed to explore the influence of region and race/ethnicity on the effects of empagliflozin in EMPEROR-Preserved, which found that no interaction was observed for the treatment effect of empagliflozin for the primary endpoint by regions.64
Guideline-directed device therapy
Regarding the device therapy for HF, the application rate of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapies (CRTs) in Asian patients is still relatively low. The utilization of ICD exhibited significant disparities throughout Asia, ranging from a mere 1.5% utilization rate in Indonesia to a considerably higher 52.5% rate in Japan. This variation suggests a trend wherein regions with established government reimbursement for ICD and reduced out-of-pocket health care expenditure tend to demonstrate more ICD implantation.65
In an analysis of PARADIGM-HF (Prospective Comparison of ARNI with an ACE-Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, patients included in the PARADIGM-HF underwent evaluation to analyze ICD implantation rates and their impact on sudden cardiac death (SCD). The utilization of ICD varied across regions, reaching the highest rates in North America (56%) and the lowest in the Asia-Pacific region (1.7%). An inverse association emerged between the rate of ICD implantation and the occurrence of SCD. North America exhibited the highest ICD implantation rates (54%) and the lowest rates of SCD (1.50 events per 100 person-years), whereas the Asia-Pacific region had the lowest ICD utilization rates (1.7%) coupled with the highest SCD rates (4.89 events per 100 person-years).66
Despite a notable increase in the overall implantation rate of CRT in the total population between China HF Stages I and II, the rate of implantation in eligible patients did not exhibit a significant increase.22 In 2022, Chinese investigators conducted the first randomized controlled trial for comparing the efficacy of left bundle branch pacing (LBBP) and traditional biventricular pacing in the treatment of nonischemic cardiomyopathy patients with left bundle branch block. The findings of the trial indicate that using LBBP for CRT can significantly improve LVEF, left ventricular end-systolic volume, and NT-proBNP levels compared with biventricular pacing CRT, promoting left ventricular reverse remodeling.67 Consequently, CRT is an effective treatment strategy for HF patients with wide QRS complexes, with the approach to pacing treatment strategies evolving in recent years to incorporate options such as His bundle and left bundle branch pacing for CRT in clinical practice.
Summary and Conclusions
The prevalence of HF in Asia is notably high on a global scale, and the high YLDs emphasize the heavy disease burden. Although the age-standardized prevalence of HF has shown stability or decreasing trend in most Asian regions, increasing trends were still observed in Southeast Asia and South Asia (Central Illustration). HFpEF is becoming increasingly prevalent in Asia, particularly in countries like Japan, which is attributed to factors like population aging. Ischemic heart disease continues to be the leading cause of HF. Additionally, the impact of air pollution as a risk factor for HF should not be underestimated. In Asia, both mortality and readmission rates for HF patients remain persistently high, and CV deaths remain the primary cause of death among HF patients. Despite some progress in recent years, the standardized application of guideline-directed medical therapy is still relatively insufficient, with dosage optimization of certain medications requiring greater attention.
Central Illustration.
Epidemiology and Burden of Heart Failure in Asia
This review summarizes the occurrence, causes, outcomes, and management of heart failure (HF) in Asia, from both the GBD (Global Burden of Disease) data and registry studies. The prevalence of HF in Asia is high; China, Indonesia, and Malaysia are the 3 highest nations in terms of age-standardized prevalence. The age-standardized prevalence of HF only increased in Southeast Asia and South Asia from 1990 to 2019. The leading causes of HF worldwide and in Asia are ischemic heart disease and hypertensive heart disease. The 1-year mortality of Asian HF patients is still high. Thus, it is urgently needed to strengthen the prevention and standardized management of HF in Asia.
Future Direction
Considering the previously mentioned issues, it is necessary to focus on controlling risk factors and managing underlying diseases to enhance primary and secondary prevention of HF. Viewing HF as a comprehensive clinical syndrome that affects the entire body can improve the overall management of HF and its various comorbidities. Promoting standardized treatment should be a central focus for improving HF outcomes. Notable advancements in HF treatment, such as the use of sodium-glucose cotransporter-2 inhibitors, highlight the importance of increasing awareness and understanding of these treatments among health care professionals at all levels. Furthermore, optimizing the management of HF patients outside of the hospital setting can lead to reduced readmission rates, subsequently reducing health care costs. Last, more epidemiological data on HF in Asian countries are still needed to provide more region-specific strategies for HF prevention and management.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an introduction of the GBD project, the literature searching keywords and strategy, and a supplemental table and figures, please see the online version of this paper.
Appendix
References
- 1.Bozkurt B., Coats A.J.S., Tsutsui H., et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 2.McKee P.A., Castelli W.P., McNamara P.M., et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Yancy C., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich P., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Mohebi R., Wang D., Lau E.S., et al. Effect of 2022 ACC/AHA/HFSA criteria on stages of heart failure in a pooled community cohort. J Am Coll Cardiol. 2023;81:2231–2242. doi: 10.1016/j.jacc.2023.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 8.Yan T., Zhu S., Yin X., et al. Burden, trends, and inequalities of heart failure globally, 1990 to 2019: a secondary analysis based on the Global Burden of Disease 2019 Study. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.027852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray C.J.L., Ezzati M., Flaxman A.D., et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 10.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu D., Huang G., He J. Investigation of prevalence and distributing feature of chronic heart failure in Chinese adult population. Chin J Cardiol. 2003;31:3–6. [Google Scholar]
- 13.Hao G., Wang X., Chen Z., et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. 2019;21:1329–1337. doi: 10.1002/ejhf.1629. [DOI] [PubMed] [Google Scholar]
- 14.Metra M., Lucioli P. Corrigendum to 'Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015' [Eur J Heart Fail. 2019;21:1329-1337] Eur J Heart Fail. 2020;22:759. doi: 10.1002/ejhf.1808. [DOI] [PubMed] [Google Scholar]
- 15.Hung C.-L., Chao T.-F., Tsai C.-T., et al. Prevalence, incidence, lifetime risks, and outcomes of heart failure in Asia: a nationwide report. J Am Coll Cardiol HF. 2023;11:1454–1456. doi: 10.1016/j.jchf.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Konishi M., Ishida J., Springer J., et al. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Heart Fail. 2016;3:145–151. doi: 10.1002/ehf2.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okura Y., Ramadan M.M., Ohno Y., et al. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72:489–491. doi: 10.1253/circj.72.489. [DOI] [PubMed] [Google Scholar]
- 18.Lee H., Oh S.-H., Cho H., et al. Prevalence and socio-economic burden of heart failure in an aging society of South Korea. BMC Cardiovasc Disord. 2016;16(1):215. doi: 10.1186/s12872-016-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonagh T.A., Metra M., Adamo M., et al. 2023 focused update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur Heart J. 2023;44(37):3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Appleton C.P., Gillebert T.C., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Zhang J., Butler J., et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China Heart Failure (China-HF) Registry. J Card Fail. 2017;23:868–875. doi: 10.1016/j.cardfail.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Gao C., Greene S.J., et al. Clinical performance and quality measures for heart failure management in China: the China-Heart Failure registry study. ESC Heart Fail. 2023;10:342–352. doi: 10.1002/ehf2.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiga T., Suzuki A., Haruta S., et al. Clinical characteristics of hospitalized heart failure patients with preserved, mid-range, and reduced ejection fractions in Japan. ESC Heart Failure. 2019;6 doi: 10.1002/ehf2.12418. 475-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji K., Sakata Y., Nochioka K., et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail. 2017;19:1258–1269. doi: 10.1002/ejhf.807. [DOI] [PubMed] [Google Scholar]
- 25.Yaku H., Ozasa N., Morimoto T., et al. Demographics, management, and in-hospital outcome of hospitalized acute heart failure syndrome patients in contemporary real clinical practice in Japan-observations from the prospective, multicenter Kyoto Congestive Heart Failure (KCHF) Registry. Circ J. 2018;82:2811–2819. doi: 10.1253/circj.CJ-17-1386. [DOI] [PubMed] [Google Scholar]
- 26.Obokata M., Sorimachi H., Harada T., et al. Epidemiology, pathophysiology, diagnosis, and therapy of heart failure with preserved ejection fraction in Japan. J Cardiac Fail. 2023;29:375–388. doi: 10.1016/j.cardfail.2022.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Shiba N., Nochioka K., Miura M., et al. Trend of Westernization of Etiology and Clinical Characteristics of Heart Failure Patients in Japan. Circ J. 2011;75:823–833. doi: 10.1253/circj.cj-11-0135. [DOI] [PubMed] [Google Scholar]
- 28.Dokainish H., Teo K., Zhu J., et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health. 2017;5:e665–e672. doi: 10.1016/S2214-109X(17)30196-1. [DOI] [PubMed] [Google Scholar]
- 29.Tromp J., Bamadhaj S., Cleland J.G.F., et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health. 2020;8:e411–e422. doi: 10.1016/S2214-109X(20)30004-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang H., Chai K., Du M., et al. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail. 2021;14(10) doi: 10.1161/CIRCHEARTFAILURE.121.008406. [DOI] [PubMed] [Google Scholar]
- 31.Huffman M.D., Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India. 2010;23:283–288. [PMC free article] [PubMed] [Google Scholar]
- 32.Go A.S., Mozaffarian D., Roger V.L., et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 33.Shimokawa H., Miura M., Nochioka K., et al. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 34.Bragazzi N.L., Zhong W., Shu J., et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28:1682–1690. doi: 10.1093/eurjpc/zwaa147. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald M.R., Tay W.T., Teng T.H.K., et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF Registry. J Am Heart Assoc. 2020;9(1) doi: 10.1161/JAHA.119.012199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tromp J., Teng T.-H., Tay W.T., et al. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail. 2019;21:23–36. doi: 10.1002/ejhf.1227. [DOI] [PubMed] [Google Scholar]
- 37.Lam C.S.P., Teng T.-H.K., Tay W.T., et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian Sudden Cardiac Death in Heart Failure registry. Eur Heart J. 2016;37:3141–3153. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham L.C., Fonarow G.C., Yancy C.W., et al. Regional variations in heart failure quality and outcomes: Get With The Guidelines-Heart Failure Registry. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai A., Qiu W., Zhou Y., et al. Clinical characteristics and 1-year outcomes in hospitalized patients with heart failure with preserved ejection fraction: results from the China Cardiovascular Association Database-Heart Failure Center Registry. Eur J Heart Fail. 2022;24:2048–2062. doi: 10.1002/ejhf.2654. [DOI] [PubMed] [Google Scholar]
- 40.Harikrishnan S., Bahl A., Roy A., et al. Clinical profile and 90 day outcomes of 10 851 heart failure patients across India: National Heart Failure Registry. ESC Heart Fail. 2022;9:3898–3908. doi: 10.1002/ehf2.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R., Lv J., Yu C., et al. Importance of healthy lifestyle factors and ideal cardiovascular health metrics for risk of heart failure in Chinese adults. Int J Epidemiol. 2022;51:567–578. doi: 10.1093/ije/dyab236. [DOI] [PubMed] [Google Scholar]
- 42.Yang R., Lv J., Yu C., et al. Modification effect of ideal cardiovascular health metrics on genetic association with incident heart failure in the China Kadoorie Biobank and the UK Biobank. BMC Med. 2021;19:259. doi: 10.1186/s12916-021-02122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amsalu E., Wang T., Li H., et al. Acute effects of fine particulate matter (PM2.5) on hospital admissions for cardiovascular disease in Beijing, China: a time-series study. Environ Health. 2019;18:70. doi: 10.1186/s12940-019-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia Y., Lin Z., He Z., et al. Effect of air pollution on heart failure: systematic review and meta-analysis. Environ Health Perspect. 2023;131 doi: 10.1289/EHP11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian Y., Liu H., Si Y., et al. Association between temperature variability and daily hospital admissions for cause-specific cardiovascular disease in urban China: a national time-series study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng T.K., Tay W.T., Richards A.M., et al. Socioeconomic status and outcomes in heart failure with reduced ejection fraction from Asia. Circ Cardiovasc Qual Outcomes. 2021;14(4) doi: 10.1161/CIRCOUTCOMES.120.006962. [DOI] [PubMed] [Google Scholar]
- 47.Sato N., Kajimoto K., Keida T., et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry) Circ J. 2013;77:944–951. doi: 10.1253/circj.cj-13-0187. [DOI] [PubMed] [Google Scholar]
- 48.Tsuchihashi-Makaya M., Hamaguchi S., Kinugawa S., et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction-a report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73:1893–1900. doi: 10.1253/circj.cj-09-0254. [DOI] [PubMed] [Google Scholar]
- 49.Nagai T., Yoshikawa T., Saito Y., et al. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction-a report from the Japanese Heart Failure Syndrome With Preserved Ejection Fraction (JASPER) Registry. Circ J. 2018;82:1534–1545. doi: 10.1253/circj.CJ-18-0073. [DOI] [PubMed] [Google Scholar]
- 50.Lee S.E., Cho H.J., Lee H.Y., et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 51.Youn Y.J., Yoo B.-S., Lee J.-W., et al. Treatment performance measures affect clinical outcomes in patients with acute systolic heart failure. Circ J. 2012;76:1151–1158. doi: 10.1253/circj.cj-11-1093. [DOI] [PubMed] [Google Scholar]
- 52.Hu D., Liu J., Zhang L., et al. Health status predicts short- and long-term risk of composite clinical outcomes in acute heart failure. J Am Coll Cardiol HF. 2021;9:861–873. doi: 10.1016/j.jchf.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Hung C.-L., Chao T.-F., Su C.-H., et al. Income level and outcomes in patients with heart failure with universal health coverage. Heart. 2021;107(3):208–216. doi: 10.1136/heartjnl-2020-316793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayagopal P.B., Sastry S.L., Nanjappa V., et al. Clinical characteristics and 30-day outcomes in patients with acute decompensated heart failure: Results from Indian College of Cardiology National Heart Failure Registry (ICCNHFR) Int J Cardiol. 2022;356:73–78. doi: 10.1016/j.ijcard.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Harikrishnan S., Sanjay G., Anees T., et al. Clinical presentation, management, in-hospital and 90-day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17:794–800. doi: 10.1002/ejhf.283. [DOI] [PubMed] [Google Scholar]
- 56.Sanjay G., Jeemon P., Agarwal A., et al. In-hospital and three-year outcomes of heart failure patients in South India: the Trivandrum Heart Failure Registry. J Cardiac Fail. 2018;24:842–848. doi: 10.1016/j.cardfail.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harikrishnan S., Jeemon P., Ganapathi S., et al. Five-year mortality and readmission rates in patients with heart failure in India: results from the Trivandrum Heart Failure Registry. Int J Cardiol. 2021;326:139–143. doi: 10.1016/j.ijcard.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng T.-H.K., Tromp J., Tay W.T., et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. The Lancet Glob Health. 2018;6:e1008–e1018. doi: 10.1016/S2214-109X(18)30306-1. [DOI] [PubMed] [Google Scholar]
- 59.Commission NH. Medical quality control indicators for cardiovascular disease related specialties (2021 version) Circ J. 2021;36:733–742. [Google Scholar]
- 60.Gupta A., Yu Y., Tan Q., et al. Quality of care for patients hospitalized for heart failure in China. JAMA Netw Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.18619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Docherty K.F., Anand I.S., Chiang C.-E., et al. Effects of dapagliflozin in asian patients with heart failure and reduced ejection fraction in DAPA-HF. JACC: Asia. 2022;2:139–153. doi: 10.1016/j.jacasi.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondo T., Wang X., Yang M., et al. Efficacy of dapagliflozin according to geographic location of patients with heart failure. J Am Coll Cardiol. 2023;82:1014–1026. doi: 10.1016/j.jacc.2023.05.056. [DOI] [PubMed] [Google Scholar]
- 63.Lam C.S.P., Ferreira J.P., Pfarr E., et al. Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42:4442–4451. doi: 10.1093/eurheartj/ehab360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chopra V. Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a preserved ejection fraction- results from the EMPEROR-PRESERVED Trial (abstr) J Am Coll Cardiol. 2022;79(Suppl 9):336. [Google Scholar]
- 65.Chia Y.M.F., Teng T.K., Tan E.S.J., et al. Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes. 2017;10(11) doi: 10.1161/CIRCOUTCOMES.116.003651. [DOI] [PubMed] [Google Scholar]
- 66.Rohde L.E., Chatterjee N.A., Vaduganathan M., et al. Sacubitril/Valsartan and Sudden Cardiac Death According to Implantable Cardioverter-Defibrillator Use and Heart Failure Cause: A PARADIGM-HF Analysis. J Am Coll Cardiol HF. 2020;8:844–855. doi: 10.1016/j.jchf.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Zhu H., Hou X., et al. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. J Am Coll Cardiol. 2022;80:1205–1216. doi: 10.1016/j.jacc.2022.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.