Abstract
Background
Depression is a known risk factor for cardiovascular disease (CVD), but the potential sex differences in this association remain unclear.
Objectives
The aim of this study was to investigate the association between depression and subsequent CVD events, and to explore potential sex differences.
Methods
The authors conducted a retrospective analysis using the JMDC Claims Database between 2005 and 2022. The study population included 4,125,720 individuals aged 18 to 75 years without a history of cardiovascular disease or renal failure and missing data at baseline. Participants were followed up for a mean of 1,288 days to assess the association between depression and subsequent CVD events, such as myocardial infarction, angina pectoris, stroke, heart failure, and atrial fibrillation.
Results
Our analysis revealed a significant association between depression and subsequent composite CVD events in both men and women, with a stronger association observed in women. The HR for the composite endpoint was 1.64 (95% CI: 1.59-1.70) in women and 1.39 (95% CI: 1.35-1.42) in men after multivariable adjustment (P for interaction <0.001). Furthermore, the individual components of the composite endpoint were also associated with depression in both men and women, each of which was also observed to be more strongly associated in women.
Conclusions
Our study provides evidence of a significant association between depression and subsequent CVD events in both men and women, with a more pronounced association observed in women. These findings highlight the importance of addressing depression and tailoring prevention and management strategies according to sex-specific factors.
Key Words: cardiovascular disease, depression, epidemiology, sex difference
Central Illustration
Depression and cardiovascular disease (CVD) are 2 prevalent health conditions that have been shown to be closely linked.1 Recent studies have demonstrated that depression is associated with an increased risk of subsequent cardiovascular events, including myocardial infarction (MI), angina, stroke, and cardiovascular mortality.2, 3, 4, 5
Sex differences in CVD events have also been highlighted, and we have recently demonstrated a possible sex difference in the relationship between hypertension and the development of atrial fibrillation (AF).6 However, there is still controversy over the evidence on sex differences in the impact of depression on CVD. Some studies have suggested that the association between depression and CVD is stronger in women than in men.2,5 Women with depression have been found to be at a higher risk of developing CVD, and the adverse effects of depression on cardiovascular health are more pronounced in women than in men. In some studies, the association has been found to be stronger in men than in women,3 whereas in others, there has been no significant sex difference.4 Moreover, the potential mechanisms underlying the sex difference in the association between depression and CVD are still not well understood and are the subject of ongoing research.
Identification of sex-specific factors in the adverse effects of depression on subsequent cardiovascular outcomes may help in the development of targeted prevention and treatment strategies that address the specific cardiovascular risks faced by depressed patients.
The purpose of this study is to investigate sex differences in the association between depression and subsequent CVD events including MI, angina, stroke, heart failure (HF), and AF, and to present evidence to address this association. A better understanding of the sex differences in the association between depression and CVD will allow health care providers to optimize care for both men and women with depression, ultimately leading to improved cardiovascular health outcomes for these populations.
Methods
The JMDC Claims Database is available for anyone who would purchase it from JMDC Inc, a health care venture corporation in Japan.
Study population
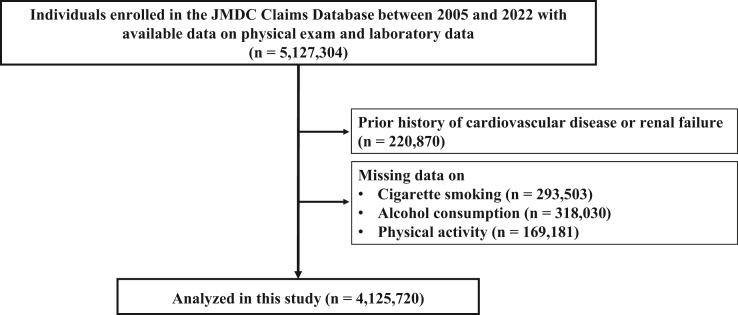
In this observational cohort study, we used the JMDC Claims Database, consisting of a combined database of health checkup and administrative claims database (both outpatient and inpatient settings) in Japan.7, 8, 9 The JMDC Claims Database covered individuals who were mainly employees and their family members in Japan between January 2005 and May 2022. Japan has a universal health insurance system, and the JMDC Claims Database consists of administrative claims records reimbursed by insurance (eg, medical diagnoses, pharmacological prescriptions) from more than 60 insurers, and medical diagnoses are registered in the form of International Classification of Diseases-10th Revision (ICD-10) coding. We identified 5,127,304 individuals with available health checkup data on physical examination and laboratory data at health checkup. We excluded individuals with a history of MI, angina pectoris, stroke, HF, AF, and renal replacement therapy, as well as those with missing data on cigarette smoking, alcohol consumption, and physical activity. Finally, we obtained 4,125,720 participants in our study (Figure 1).
Figure 1.
Flowchart
We identified 5,127,304 individuals with available health checkup data on physical examination and laboratory data at health checkup in the JMDC Claims Database. We excluded individuals with a history of myocardial infarction, angina pectoris, stroke, heart failure, atrial fibrillation, and renal replacement therapy, as well as those with missing data on cigarette smoking, alcohol consumption, and physical activity. Finally, we obtained 4,125,720 participants in our study.
Ethics
This study was performed in accordance with the ethical guidelines of the University of Tokyo (approval by the Ethical Committee [Clinical Research Review Board] of the University of Tokyo: 2018-10862) and the principles of the Declaration of Helsinki. The requirement for informed consent was waived because all data in the JMDC Claims Database were anonymized and deidentified.
Definition of depression
We defined individuals with a history of depression as those diagnosed with depression (ICD-10 codes: F32.0-32.9, F33.0-33.3, F33.8, F33.9, F34.1, and F41.2) before undergoing the initial health check-up.
Variables and measurement
We collected the following data using standardized protocols at the initial health check-up of each participant: body mass index (BMI), blood pressure, and fasting laboratory values. Information regarding cigarette smoking (current or noncurrent) was self-reported. We defined obesity as a BMI of ≥25 kg/m2.10 Hypertension was defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or usage of blood pressure-lowering medications. Diabetes mellitus was defined as fasting glucose level of ≥126 mg/dL or usage of glucose-lowering medications. Dyslipidemia was defined as low-density lipoprotein cholesterol level of ≥140 mg/dL, high-density lipoprotein cholesterol level of <40 mg/dL, triglyceride level of ≥150 mg/dL, or usage of lipid-lowering medications. Physical inactivity was defined as not exercising for 30 minutes at least twice a week or not walking for 1 hour each day, as we previously described.11
Outcomes
Information on the outcomes was collected between January 2005 and May 2022. The primary outcome was a composite endpoint including MI (ICD-10 codes: I210, I211, I212, I213, I214, and I219), angina pectoris (ICD-10 codes: I200, I201, I208, and I209), stroke (ICD-10 codes: I630, I631, I632, I633, I634, I635, I636, I638, I639, I600, I601, I602, I603, I604, I605, I606, I607, I608, I609, I610, I611, I613, I614, I615, I616, I619, I629, and G459), HF (ICD-10 codes: I500, I501, I509, and I110), and AF (ICD-10 code: I480-I484, and I489). The secondary primary outcomes included MI, angina pectoris, stroke, HF, and AF.
Statistical analysis
We analyzed the study population stratified by sex. The data are expressed as median (Q1, Q3) for continuous variables or number (percentage) for categorical variables. The statistical significance of differences in clinical characteristics between participants with and without depression was assessed using Wilcoxon rank-sum test and chi-square test for continuous variables and for categorical variables, respectively. We performed analyses using Cox proportional hazards regression to examine the association between depression and incident CVD. We calculated HRs in an unadjusted model (model 1); an age-adjusted model (model 2); and after adjustment for age, BMI, hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, alcohol consumption, and physical inactivity using the forced entry method (model 3). To examine whether depression had a differential relationship with incident CVD by sex, multiplicative interaction terms for sex were calculated. We performed a stratified subgroup analysis by age (≥50 years vs <50 years) or obesity defined as BMI of ≥25 kg/m2. We performed 4 sensitivity analyses to validate our primary results. First, we set an induction period of 1 year, and we analyzed 3,326,132 participants. Second, we defined depression as having a diagnosis of depression and use of antidepressants (Anatomical Therapeutic Chemical Classification System code N06A). In this case scenario, participants having a diagnosis of depression without use of antidepressants (n = 105,245) were excluded from the analysis. Third, we imputed missing data on cigarette smoking, alcohol consumption, and physical inactivity, as previously described.12,13 Briefly, we performed the analysis using the multiple imputation by the chained equation method with 20 iterations described by Aloisio et al,14 and obtained the HRs with SEs based on Rubin’s rules.15 Fourth, because death could be regarded as a competing risk with CVD events, we also conducted a competing risks analysis using the Fine-Gray subdistribution hazard modeling.16,17 Statistical significance was set at a P value <0.05. All statistical analyses were conducted using STATA version 17 (StataCorp LLC).
Results
Clinical characteristics
The clinical characteristics of the study participants is summarized in Table 1. The median age was 44 years (Q1, Q3: 36-52 years), and 2,370,986 participants were men. The median age was 44 years (Q1, Q3: 36-52 years) in both men and women. Depression was observed in 99,739 participants (4.2%) in men, whereas 78,358 (4.5%) in women. In both men and women, the prevalence of obesity, hypertension, diabetes mellitus, dyslipidemia, and physical inactivity was more common in individuals having depression.
Table 1.
Clinical Characteristics
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Depression (−) (n = 2,271,247) |
Depression (+) (n = 99,739) |
P Value | Depression (−) (n = 1,676,376) |
Depression (+) (n = 78,358) |
P Value | |
| Age, y | 44 (36, 52) | 44 (38, 51) | <0.001 | 44 (36, 52) | 44 (36, 51) | 0.28 |
| Body mass index, kg/m2 | 23.2 (21.2, 25.6) | 23.5 (21.4, 26.1) | <0.001 | 21 (19.2, 23.4) | 21.1 (19.2, 23.8) | <0.001 |
| Obesity | 697,363 (30.7) | 34,300 (34.4) | <0.001 | 268,756 (16.0) | 14,691 (18.7) | <0.001 |
| Systolic blood pressure, mm Hg | 121 (112, 130) | 120 (111, 129) | <0.001 | 112 (102, 124) | 111 (102, 123) | <0.001 |
| Diastolic blood pressure, mm Hg | 75 (68, 83) | 76 (68, 83) | <0.001 | 69 (62, 77) | 69 (62, 77) | <0.001 |
| Hypertension | 500,541 (22.0) | 24,181 (24.2) | <0.001 | 211,269 (12.6) | 11,629 (14.8) | <0.001 |
| Diabetes mellitus | 129,122 (5.7) | 6,310 (6.3) | <0.001 | 34,442 (2.1) | 1,981 (2.5) | <0.001 |
| Dyslipidemia | 1,060,129 (46.7) | 53,032 (53.2) | <0.001 | 473,625 (28.3) | 26,597 (33.9) | <0.001 |
| Cigarette smoking | 809,137 (35.6) | 32,842 (32.9) | <0.001 | 189,557 (11.3) | 10,893 (13.9) | <0.001 |
| Alcohol consumption | 673,643 (29.7) | 24,837 (24.9) | <0.001 | 205,983 (12.3) | 8,307 (10.6) | <0.001 |
| Physical inactivity | 1,153,587 (50.8) | 54,777 (54.9) | <0.001 | 875,698 (52.2) | 43,256 (55.2) | <0.001 |
| Fast plasma glucose, mg/dL | 93 (87, 101) | 93 (87, 101) | <0.001 | 89 (84, 95) | 89 (84, 95) | <0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 121 (101, 143) | 124 (103, 146) | <0.001 | 113 (94, 136) | 116 (96, 138) | <0.001 |
| High-density lipoprotein cholesterol, mg/dL | 56 (48, 66) | 56 (47, 66) | <0.001 | 70 (60, 81) | 69 (59, 81) | <0.001 |
| Triglycerides, mg/dL | 95 (66, 142) | 103 (70, 155) | <0.001 | 64 (48, 90) | 70 (51, 101) | <0.001 |
Values are median (Q1, Q3) or n (%).
Depression and CVD between men and women
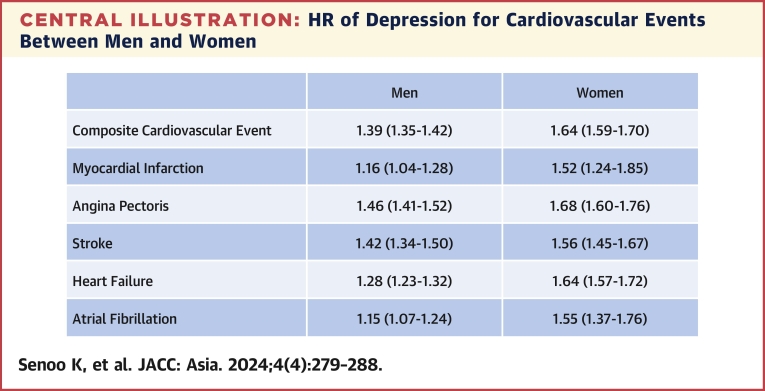
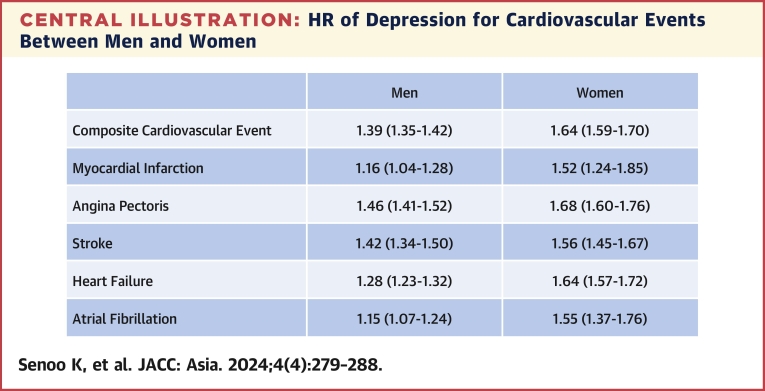
During a mean follow-up period of 1,288 ± 1,001 days (minimum 1 day, maximum 5,534 days), 119,084 CVD diagnoses were recorded in men, and the incidence of CVD was 140.1 (95% CI: 139.4-140.9) per 10,000 person-years. In women, 61,797 CVD events were recorded, and the incidence of CVD was 111.0 (95% CI: 110.1-111.8) per 10,000 person-years. Compared with participants without depression, the HR of depression for CVD was 1.39 (95% CI: 1.35-1.42) in men and 1.64 (95% CI: 1.59-1.70) in women. The P value for interaction was <0.001 in model 3, suggesting that the association of depression with incident CVD was modified by sex (Table 2). The HRs of depression for MI, angina pectoris, stroke, HF, and AF in men were 1.16 (95% CI: 1.04-1.28), 1.46 (95% CI: 1.41-1.52), 1.42 (95% CI: 1.34-1.50), 1.28 (95% CI: 1.23-1.32), and 1.15 (95% CI: 1.07-1.24), respectively. The HRs of depression for MI, angina pectoris, stroke, HF, and AF in women were 1.52 (95% CI: 1.24-1.85), 1.68 (95% CI: 1.60-1.76), 1.56 (95% CI: 1.45-1.67), 1.64 (95% CI: 1.57-1.72), and 1.55 (95% CI: 1.37-1.76), respectively. P values for interaction were all significant (Central Illustration).
Table 2.
Association of Depression With Risk of Developing Cardiovascular Disease Between Men and Women
| Men |
Women |
P Value for Interaction | |||||
|---|---|---|---|---|---|---|---|
| Depression (−) (n = 2,271,247) |
Depression (+) (n = 99,739) |
P Value | Depression (−) (n = 1,676,376) |
Depression (+) (n = 78,358) |
P Value | ||
| Composite | |||||||
| Events | 112,638 | 6,446 | 57,639 | 4,158 | |||
| Incidence | 138.1 (137.3-138.9) | 188.3 (183.8-193.0) | 107.9 (107.0-108.8) | 182.3 (176.8-187.9) | |||
| Unadjusted | 1.00 (Reference) | 1.36 (1.33-1.40) | <0.001 | 1.00 (Reference) | 1.69 (1.64-1.74) | <0.001 | |
| Age-adjusted | 1.00 (Reference) | 1.43 (1.40-1.47) | <0.001 | 1.00 (Reference) | 1.69 (1.64-1.74) | <0.001 | |
| Multivariable | 1.00 (Reference) | 1.39 (1.35-1.42) | <0.001 | 1.00 (Reference) | 1.64 (1.59-1.70) | <0.001 | <0.001 |
| Myocardial infarction | |||||||
| Events | 7,279 | 359 | 1,506 | 105 | |||
| Incidence | 8.6 (8.4-8.8) | 10.0 (9.0-11.1) | 2.7 (2.6-2.9) | 4.4 (3.6-5.3) | |||
| Unadjusted | 1.00 (Reference) | 1.16 (1.04-1.29) | 0.007 | 1.00 (Reference) | 1.60 (1.32-1.96) | <0.001 | |
| Age-adjusted | 1.00 (Reference) | 1.23 (1.11-1.37) | <0.001 | 1.00 (Reference) | 1.60 (1.31-1.95) | <0.001 | |
| Multivariable | 1.00 (Reference) | 1.16 (1.04-1.28) | 0.008 | 1.00 (Reference) | 1.52 (1.24-1.85) | <0.001 | 0.0173 |
| Angina pectoris | |||||||
| Events | 49,141 | 3,026 | 24,121 | 1,803 | |||
| Incidence | 59.1 (58.5-59.6) | 86.1 (83.1-89.2) | 44.5 (43.9-45.0) | 77.0 (73.6-80.7) | |||
| Unadjusted | 1.00 (Reference) | 1.46 (1.40-1.51) | <0.001 | 1.00 (Reference) | 1.73 (1.65-1.82) | <0.001 | |
| Age-adjusted | 1.00 (Reference) | 1.51 (1.46-1.57) | <0.001 | 1.00 (Reference) | 1.73 (1.65-1.81) | <0.001 | |
| Multivariable | 1.00 (Reference) | 1.46 (1.41-1.52) | <0.001 | 1.00 (Reference) | 1.68 (1.60-1.76) | <0.001 | <0.001 |
| Stroke | |||||||
| Events | 23,988 | 1,367 | 11,950 | 829 | |||
| Incidence | 28.6 (28.2-28.9) | 38.3 (36.3-40.4) | 21.9 (21.5-22.3) | 35.0 (32.7-37.5) | |||
| Unadjusted | 1.00 (Reference) | 1.34 (1.27-1.42) | <0.001 | 1.00 (Reference) | 1.60 (1.49-1.72) | <0.001 | |
| Age-adjusted | 1.00 (Reference) | 1.45 (1.37-1.53) | <0.001 | 1.00 (Reference) | 1.60 (1.49-1.72) | <0.001 | |
| Multivariable | 1.00 (Reference) | 1.42 (1.34-1.50) | <0.001 | 1.00 (Reference) | 1.56 (1.45-1.67) | <0.001 | 0.0350 |
| Heart failure | |||||||
| Events | 55,231 | 2,952 | 27,085 | 1,982 | |||
| Incidence | 66.3 (65.8-66.9) | 83.6 (80.6-86.6) | 49.9 (49.3-50.5) | 84.6 (81.0-88.4) | |||
| Unadjusted | 1.00 (Reference) | 1.26 (1.21-1.31) | <0.001 | 1.00 (Reference) | 1.70 (1.62-1.78) | <0.001 | |
| Age-adjusted | 1.00 (Reference) | 1.32 (1.28-1.37) | <0.001 | 1.00 (Reference) | 1.69 (1.62-1.77) | <0.001 | |
| Multivariable | 1.00 (Reference) | 1.28 (1.23-1.32) | <0.001 | 1.00 (Reference) | 1.64 (1.57-1.72) | <0.001 | <0.001 |
| Atrial fibrillation | |||||||
| Events | 15,972 | 721 | 3,872 | 265 | |||
| Incidence | 19.0 (18.7-19.3) | 20.1 (18.7-21.6) | 7.1 (6.8-7.3) | 11.1 (9.9-12.5) | |||
| Unadjusted | 1.00 (Reference) | 1.06 (0.98-1.14) | 0.136 | 1.00 (Reference) | 1.58 (1.39-1.79) | <0.001 | |
| Age-adjusted | 1.00 (Reference) | 1.16 (1.07-1.24) | <0.001 | 1.00 (Reference) | 1.57 (1.39-1.78) | <0.001 | |
| Multivariable | 1.00 (Reference) | 1.15 (1.07-1.24) | <0.001 | 1.00 (Reference) | 1.55 (1.37-1.76) | <0.001 | <0.001 |
Multivariable analysis = adjusted for age, body mass index, hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, alcohol consumption, and physical inactivity.
Central Illustration.
HR of Depression for Cardiovascular Events Between Men and Women
The association of depression with incident cardiovascular event is pronounced in women.
Subgroup analysis
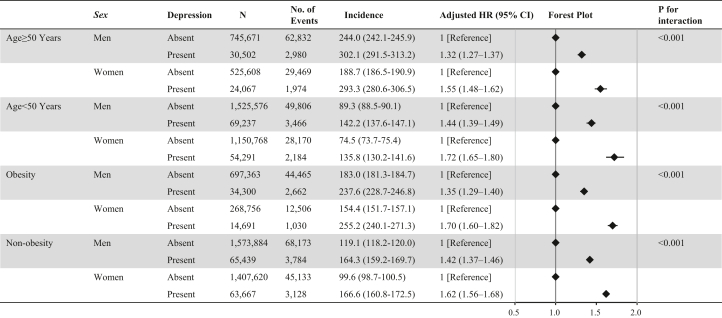
Depression was associated with a higher risk of developing CVD in men and women, not only in people aged ≥50 years, but also in those aged <50 years. The P value for the interaction evaluating the relationship between depression and incident CVD between men and women was statistically significant in both people aged ≥50 and <50 years. The association of depression with incident CVD was greater in women than in men among both people with and without obesity (Figure 2).
Figure 2.
Subgroup Analyses
We examined the association of depression with the risk of developing cardiovascular disease between men and women stratified by age and obesity. Models were adjusted for age, body mass index, hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, alcohol consumption, and physical inactivity.
Sensitivity analyses
First, we included individuals with a follow-up period longer than 1 year to exclude people having latent CVD at baseline, and the main results were unchanged in this population (Supplemental Table 1). Second, in the scenario in which depression was defined as having a diagnosis of depression and use of antidepressants, the main results did not change (Supplemental Table 2). Third, after multiple imputations for missing data, our primary results were consistent (Supplemental Table 3). Fourth, our primary results were consistent with those of a competing risks model (Supplemental Table 4).
Discussion
Our study provides evidence of a significant association between depression and subsequent CVD events in both men and women, with a more pronounced association observed in women. Furthermore, the individual components of the composite endpoint were also associated with depression in both men and women, each of which was also observed to be more strongly associated in women.
Several potential mechanisms may contribute to sex differences in the association between depression and CVD. One possible explanation is that women may experience more severe and persistent symptoms of depression compared with men, leading to a greater impact on cardiovascular health.18,19 Additionally, women may be more likely to experience depression during critical periods of hormonal changes, such as during pregnancy or menopause, which could contribute to a greater impact on cardiovascular health.20 Another potential mechanism underlying the sex difference in the association between depression and CVD is related to differences in cardiovascular risk factors between men and women. Studies have shown that women with depression may be more likely to have traditional cardiovascular risk factors, such as hypertension, diabetes, and obesity, compared with men with depression.21 These coexisting traditional risk factors may contribute to the development of CVD and could potentially exacerbate the adverse effects of depression on cardiovascular health in women.22 Furthermore, differences in health care utilization and treatment between men and women may contribute to the sex difference in the association between depression and CVD. Women are more likely to seek health care services and may be more likely to be diagnosed with depression than men.23,24 However, it has been shown that women with depression may be less likely to receive appropriate treatment for their condition.25 This highlights the importance of early detection and appropriate treatment of depression in women to help reduce the risk of CVD. Last, sex-specific differences in biological factors, such as genetics and hormonal profiles, may contribute to the sex difference in the association between depression and CVD. For example, studies have suggested that the female sex hormone estrogen may have a protective effect on cardiovascular health, and disruptions in estrogen levels, such as during menopause, may increase the risk of CVD in women.26, 27, 28 Thus, addressing hormonal changes in women may be an important consideration in the prevention and management of CVD in women with depression. However, the exact mechanisms underlying the sex difference in the association between depression and CVD are still not fully understood and require further research to elucidate.
This study also found that the impact of sex differences on the association between depression and individual cardiovascular outcomes was consistent. Therefore, recognizing the important role of depression in the development of each cardiovascular outcome, we emphasize the importance of a comprehensive, patient-centered approach to CVD prevention and management.29 Addressing depression and its associated risk factors in both men and women not only improves mental health, but also may improve overall cardiovascular health. In the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial, cognitive therapy intervention after MI improved depressive symptoms but did not reduce mortality.30 However, in a post hoc analysis, patients in the ENRICHD trial who received selective serotonin reuptake inhibitors had significantly lower all-cause and cardiovascular mortality rates.31 Observational data also suggest that serotonin reuptake inhibitors may be associated with a reduction in the onset of MI.32 Tricyclic antidepressant toxicity results predominantly from myocardial sodium-channel blockade. Subsequent ventricular dysrhythmias, myocardial depression, and hypotension cause CV collapse.33 Further research is needed on the association between treatment for depression and CV outcomes. Importantly, this does not preclude appropriate screening and treatment of depression in cardiac patients for the purpose of improving depression itself. Depression is the third leading cause of morbidity worldwide, and organizations such as the Institute of Medicine emphasize that screening and treatment of depression is a priority for U.S. health care in the 21st century.34 Treating depression may improve patients’ quality of life and compliance with cardiac care recommendations.
Health care providers should incorporate routine screening and treatment for depression into standard clinical practice for all patients, regardless of gender as the results of this study show that depression has an adverse effect on the development of CVD in both men and women. Both men and women should also consider addressing modifiable cardiovascular risk factors such as hypertension, diabetes, and obesity that may exacerbate the adverse effects of depression on cardiovascular health. Future research should fully understand the mechanisms linking depression and CVD and answer whether treatment of depression can improve cardiovascular mortality and morbidity. Meanwhile, the current American College of Cardiology/American Heart Association guideline35 recommends evaluation for symptoms of depression and consideration of treatment for depression. Failure to recognize depression in patients with CVD is a failure to provide the best care for the patient. The results of this study strongly support the recognition that depression is an important risk factor for the development of CVD, particularly in women, and that assessing the risk of CVD in depressed patients and treating and preventing depression may lead to the prevention of CVD in women, which is consistent with the objectives of “Go Red for Women,” a campaign led by the American Heart Association and the American College of Cardiology. The campaign aims to protect women's health by encouraging women to learn about the risk factors for CVD, encourage them to practice a healthy lifestyle, and promote early detection and treatment of CVD. In the future, we would like to ensure that all women, including those with depression, will understand their heart health and learn about the risk factors for CVD, and should be encouraged to work towards the prevention of CVD.
Study limitations
Despite the strengths of our study, including a large sample size and high retention rate because of an electrical linkage of claims records, there are some potential limitations to consider. Our study is observational, and therefore, we cannot establish causality between depression and subsequent cardiovascular events. We used ICD-10 codes of depression, which may not accurately reflect the severity or duration of depressive symptoms. Several previous studies have reported the reliability of CVD diagnosis registered in claims databases in Japan, and the incidence of CVD in our database is comparable to other epidemiological databases.36,37 However, because of the nature of claims databases, there remains uncertainty regarding the accuracy of recorded diagnosis in our data set as well. Our study did not account for potential confounding factors such as socioeconomic status, which may influence the association between depression and subsequent CVD. However, given that our data set mainly includes employees working for relatively large companies in Japan, the difference in socioeconomic status is seemingly not so large. Our study did not include detailed information on procedural records and imaging examination. Individuals with severe depression might be excluded because of the nature of this data set, in which employees are primarily registered. Our data set does not include individuals aged >75 years, and therefore, whether our primary findings could be applicable to older individuals is unclear. The COVID-19 epidemic highlights the important interplay among CVD, COVID-19–related inflammatory conditions, and depression. Although cardiovascular impairment accounts for a significant proportion of deaths caused by COVID-19, social restrictions caused by COVID-19 have not only been associated with CVD but also emerged as a non-negligible risk factor for depression. It is known that long-term COVID complications are worse in women, including depression, decreased physical activity, and poor lifestyle habits, all of which may affect CV risk.38, 39, 40 Therefore, we should acknowledge that COVID-19 may have influenced our results as a confounder. Last, sex-specific differences in biological factors, such as genetics and hormonal profiles, may contribute to the sex difference in the association between depression and CVD.41 We conducted multiple imputations for missing variables. However, the reason for which the missing values occurred was unclear in this data set.
Conclusions
The association between depression and subsequent CVD is strong regardless of gender, hence requiring health care professionals to assess and manage depression in all individuals. Given that these associations are particularly strong in women, there is a need to better understand the potential sex-specific factors underlying the association between depression and CVD, and to develop targeted prevention and treatment strategies that address the specific cardiovascular risks faced by women with depression.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Our analysis of a nationwide epidemiological database showed a significant association between depression and subsequent composite CVD events in both men and women. The association of depression with risk of developing CVD was more pronounced in women than in men. The individual components of the composite endpoint were also associated with depression in both men and women, each of which was also observed to be more strongly associated in women.
TRANSLATIONAL OUTLOOK: The relationship of depression with subsequent CVD events is strong irrespective of sex, confirming the importance of the depression from the perspective of CVD prevention. Further, considering that these associations are pronounced in women, there is a need to better understand the potential sex-specific factors underlying the relationship of depression with incident cardiovascular disease, and to develop targeted prevention and treatment strategies that address the specific cardiovascular risks faced by women with depression.
Funding Support and Author Disclosures
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, and 21K08123). The funding sources played no role in the current study. Drs Kaneko and Fujiu have received research funding and scholarship funds from Medtronic Japan Co, Ltd, Boston Scientific Japan Co, Ltd, Biotronik Japan, Simplex Quantum Co, Ltd, and Fukuda Denshi, Central Tokyo Co, Ltd. All other authors have reported that they have no relationships relevant to the contents of this study to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Möller-Leimkühler A.M. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci. 2007;9:71–83. doi: 10.31887/DCNS.2007.9.1/ammoeller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haukkala A., Konttinen H., Uutela A., Kawachi I., Laatikainen T. Gender differences in the associations between depressive symptoms, cardiovascular diseases, and all-cause mortality. Ann Epidemiol. 2009;19:623–629. doi: 10.1016/j.annepidem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Meng R., Yu C., Liu N., et al. Association of depression with all-cause and cardiovascular disease mortality among adults in China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S.B., Jeong I.S. Sex differences in the relationship between depression and cardiovascular disease risk: a nationwide study in Korea. Osong Public Health Res Perspect. 2021;12:105–114. doi: 10.24171/j.phrp.2021.12.2.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L., Chen Y., Wang N., et al. Association between depression and risk of incident cardiovascular diseases and its sex and age modifications: a prospective cohort study in southwest China. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.765183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanazawa S., Kaneko H., Yano Y., et al. Sex differences in the association between hypertension and incident atrial fibrillation. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.026240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko H., Yano Y., Itoh H., et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation. 2021;143:2244–2253. doi: 10.1161/CIRCULATIONAHA.120.052624. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko H., Itoh H., Yotsumoto H., et al. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association blood pressure guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka S., Kaneko H., Yano Y., et al. Association between blood pressure classification using the 2017 ACC/AHA blood pressure guideline and retinal atherosclerosis. Am J Hypertens. 2021;34:1049–1056. doi: 10.1093/ajh/hpab074. [DOI] [PubMed] [Google Scholar]
- 10.Itoh H., Kaneko H., Kiriyama H., et al. Effect of metabolically healthy obesity on the development of carotid plaque in the general population: a community-based cohort study. J Atheroscler Thromb. 2020;27:155–163. doi: 10.5551/jat.48728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko H., Itoh H., Yotsumoto H., et al. Association between the number of hospital admissions and in-hospital outcomes in patients with heart failure. Hypertens Res. 2020;43:1385–1391. doi: 10.1038/s41440-020-0505-2. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko H., Itoh H., Yotsumoto H., et al. Association of body weight gain with subsequent cardiovascular event in non-obese general population without overt cardiovascular disease. Atherosclerosis. 2020;308:39–44. doi: 10.1016/j.atherosclerosis.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Yagi M., Yasunaga H., Matsui H., et al. Impact of rehabilitation on outcomes in patients with ischemic stroke: a nationwide retrospective cohort study in Japan. Stroke. 2017;48:740–746. doi: 10.1161/STROKEAHA.116.015147. [DOI] [PubMed] [Google Scholar]
- 14.Aloisio K.M., Swanson S.A., Micali N., Field A., Horton N.J. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014;14:863–883. [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin D.B., Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 16.Morita K., Ono S., Ishimaru M., Matsui H., Naruse T., Yasunaga H. Factors affecting discharge to home of geriatric intermediate care facility residents in Japan. J Am Geriatr Soc. 2018;66:728–734. doi: 10.1111/jgs.15295. [DOI] [PubMed] [Google Scholar]
- 17.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus S.M., Young E.A., Kerber K.B., et al. Gender differences in depression: findings from the STAR∗D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Albert P.R. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40:219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromberger J.T., Kravitz H.M. Mood and menopause: findings from the Study of Women's Health Across the Nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38:609–625. doi: 10.1016/j.ogc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elovainio M., Hakulinen C., Pulkki-Råback L., et al. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health. 2017;2:e260–e266. doi: 10.1016/S2468-2667(17)30075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccarino V., Johnson B.D., Sheps D.S., et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 23.Bertakis K.D., Azari R., Helms L.J., Callahan E.J., Robbins J.A. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–152. [PubMed] [Google Scholar]
- 24.Bertakis K.D., Helms L.J., Callahan E.J., Azari R., Leigh P., Robbins J.A. Patient gender differences in the diagnosis of depression in primary care. J Womens Health Gend Based Med. 2001;10:689–698. doi: 10.1089/15246090152563579. [DOI] [PubMed] [Google Scholar]
- 25.Grote N.K., Zuckoff A., Swartz H., Bledsoe S.E., Geibel S. Engaging women who are depressed and economically disadvantaged in mental health treatment. Soc Work. 2007;52:295–308. doi: 10.1093/sw/52.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-López F.R., Larrad-Mur L., Kallen A., Chedraui P., Taylor H.S. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010;17:511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maas A.H., van der Schouw Y.T., Regitz-Zagrosek V., et al. Red alert for women's heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32:1362–1368. doi: 10.1093/eurheartj/ehr048. [DOI] [PubMed] [Google Scholar]
- 28.Regitz-Zagrosek V., Oertelt-Prigione S., Prescott E., et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 29.Rumsfeld J.S., Ho P.M. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111:250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 30.Berkman L.F., Blumenthal J., Burg M., et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 31.Taylor C.B., Youngblood M.E., Catellier D., et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 32.Sauer W.H., Berlin J.A., Kimmel S.E. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation. 2003;108:32–36. doi: 10.1161/01.CIR.0000079172.43229.CD. [DOI] [PubMed] [Google Scholar]
- 33.Blaber M.S., Khan J.N., Brebner J.A., McColm R. "Lipid rescue" for tricyclic antidepressant cardiotoxicity. J Emerg Med. 2012;43:465–467. doi: 10.1016/j.jemermed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Adams K., Corrigan J.M., editors. Priority Areas for National Action: Transforming Health Care Quality. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 35.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Saito I., Yamagishi K., Kokubo Y., et al. Association between mortality and incidence rates of coronary heart disease and stroke: the Japan Public Health Center-based prospective (JPHC) study. Int J Cardiol. 2016;222:281–286. doi: 10.1016/j.ijcard.2016.07.222. [DOI] [PubMed] [Google Scholar]
- 37.Miura K., Nakagawa H., Ohashi Y., et al. Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: a meta-analysis of 16 cohort studies. Circulation. 2009;119:1892–1898. doi: 10.1161/CIRCULATIONAHA.108.823112. [DOI] [PubMed] [Google Scholar]
- 38.Vogel B., Acevedo M., Appelman Y., et al. The Lancet Women and Cardiovascular Disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. doi: 10.1016/S0140-6736(21)00684-X. [DOI] [PubMed] [Google Scholar]
- 39.Bucciarelli V., Nasi M., Bianco F., et al. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: gender makes a difference. Trends Cardiovasc Med. 2022;32:12–17. doi: 10.1016/j.tcm.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moscucci F., Gallina S., Bucciarelli V., et al. Impact of COVID-19 on the cardiovascular health of women: a review by the Italian Society of Cardiology Working Group on 'gender cardiovascular diseases.'. J Cardiovasc Med (Hagerstown) 2023;24:e15–e23. doi: 10.2459/JCM.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bucciarelli V., Caterino A.L., Bianco F., et al. Depression and cardiovascular disease: the deep blue sea of women's heart. Trends Cardiovasc Med. 2020;30:170–176. doi: 10.1016/j.tcm.2019.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.