Abstract
Keywords: Cardiac resynchronization therapy, LBBB, Electrical optimization, QRS narrowing, Device programming, Triple fusion
Cardiac resynchronization therapy (CRT) aims to restore electrical resynchronization. Success should be reflected by electrical measure(s).1 QRS optimization on an individualized basis is standard practice with conduction system pacing (CSP) but largely ignored with CRT (devices are left at nominal settings).2 A one-size-fits all solution may underlie disappointing results with device-based CRT algorithms (e.g. LV-fusion3). Therefore, we systematically evaluated QRS optimization using paced AV interval programming with CRT, contrasting biventricular (BiV: LV&RV) vs. LV-only fusion modes.
QRS duration (QRSd) narrowing usually is used to report resynchronization with CRT. An additional element is preservation of the rapid rS inscription in V1/V2 (‘rapid intrinsic’) generated by normal intrinsic right bundle branch (iRBB) conduction.4,5 Intrinsic right bundle branch conduction is essential to ‘LV fusion’ with LV-only pacing or ‘triple fusion’ with BiV pacing.3,6,7 Intrinsic right bundle branch contribution increases at longer paced atrioventricular delay (pAVD) relative to intrinsic AVD.
We tested effect of pAVD adjustment to achieve best electrical resynchronization in heart failure patients with Strauss-type LBBB (n = 40, age 68 ± 8 years; 22 (55%) male; 13 (35%) ischaemic cardiomyopathy; LVEF 25 ± 8%). Native intervals were PR 206 ± 35 ms; rS 69 ± 12 ms and QRSd 171 ± 15 ms. The qLV/QRS was 73 ± 9%, indicating optimized LV lead position. RV-only pacing without fusion prolonged QRSd (210 ± 23 ms) and rS (104 ± 17 ms) intervals.
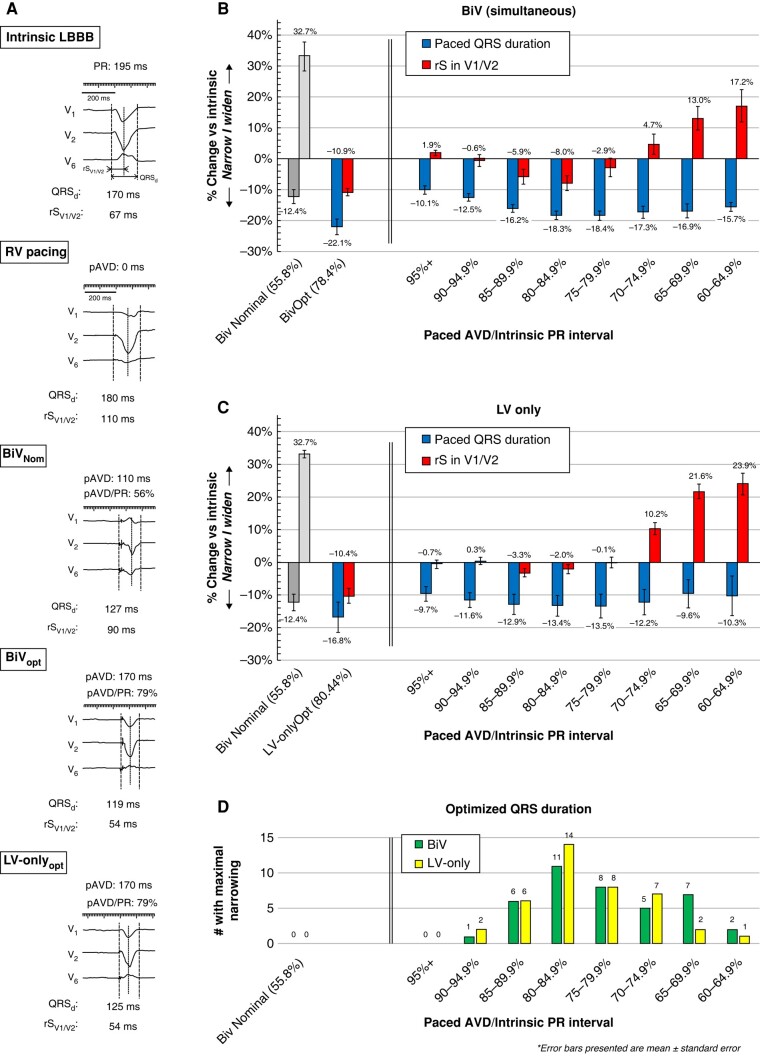
Paced QRSd and rS (r wave onset to S wave nadir in V1/V2) were measured using simultaneous 12-lead recordings during nominal BiV (BiVNom; pAVD 140/110 ms) and then during each programmed setting. In each patient, pAVD was adjusted (in increments of ∼5%) from 60% to 95% of the PR interval, firstly during BiV (simultaneous LV&RV) and then LV-only pacing. Optimal AVDs (BiVOpt and LV-onlyOpt) were determined by the narrowest QRSd and then rS measured (representative ECG presented in Figure 1A).
Figure 1.
Electrical optimization as a function of pAVD/PR interval programming.
Figure 1B depicts summary data for QRS and rS intervals during BiV pacing. BiVNom narrowed QRSd by 12.4% (darker grey) but widened rS interval (lighter grey) vs. intrinsic LBBB. Progressive pAVD/PR lengthening abbreviated QRSd and rS. Maximum effects were seen in range 75–85%. BiVOpt reduced QRSd by 22.1% vs. intrinsic LBBB and was associated with longer pAVD than BiVNom [78 ± 7 vs. 56 ± 7% of PR interval (absolute intervals 106–276 ms)]. When compared, BiVNom produced inferior resynchronization: 12.3% wider QRS than BiVopt (149 ± 17 vs. 133 ± 15 ms) and 48% wider rS (89 ± 20 vs. 60 ± 9 ms); all P < 0.05 (maximal QRS narrowing is calculated as the mean from each individual patient whereas data presented in the figures is aggregated for each pAVD/PR interval).
LV-only pacing (Figure 1C) showed maximum QRSd shortening at pAVD/PR range 75–85% (absolute intervals 115–297 ms) and rS abbreviation at 85–90% (a broadly similar range to BiV). LV-onlyOpt reduced QRSd duration by 16.8% vs. intrinsic LBBB, marginally superior to BiVNom (142 ± 16 ms vs. 149 ± 17 ms; P < 0.05), but inferior to BiV fusion pacing (BiVOpt 133 ± 15; P < 0.05 ms). Individually, LV-onlyOpt programming was superior to BiVOpt infrequently, achieving narrowest QRS in only 4/40 patients (10%).
Notably, max QRS shortening with both BiVOpt and LV-onlyOpt was associated with minimized rS interval suggesting that iRBB contributes to electrical resynchronization in both fusion modes. pAVD/PR range 80–85% was optimal in most patients (Figure 1D) but notably in some was <65% or >90%.
Important points for CRT programming are highlighted: (1) nominal settings usually underreach maximal optimization; (2) individualized adjustment of the paced AVD is key as optimal values are widely distributed; (3) electrical resynchronization is best achieved via incorporation of rapid iRBB conduction (with either LV or BiV fusion pacing); (4) shortening pAVD truncates iRBB contribution (broadening rS) and diminishes magnitude of QRS narrowing; and (5) resynchronization is significantly enhanced by the incorporation of RV pacing to LV and iRBB (i.e. triple fusion) but requires longer pAVD/PR intervals.
The incorporation of the RV paced wavefront to improve electrical resynchronization, as demonstrated here, may be counterintuitive. Although RV pacing is deleterious when ventricular activation in HF patients is fully committed to this wavefront, its initial effects on septal activation to break down functional conduction barriers may synergize with iRBB conduction and facilitate resynchronization with LV pacing.8,9 pAVD timing is crucial to titrate this effect.10
Our findings may explain the neutral results of trials testing CRT device algorithms that apply a one-size-fits all solution.3,11 For example, the LV-only fusion pacing algorithm uses a non-programmable paced pAVD of ∼70% of the PR interval. We show optimal pAVD for this mode is widely distributed and more likely to be longer (>80% of the PR) that enables greater iRBB contribution. However, in 90% of patients, this was inferior to BiVOpt. Thus, adding RV pacing at longer pAVD (triple fusion) further enhances electrical resynchronization.7 Notably, individualized CRT programming as demonstrated here results in similar QRSd to those reported with CSP.12–14
In conclusion, individualized programming to balance iRBB, RV, and LV paced wavefronts is key to electrical resynchronization and may improve CRT outcomes. This hypothesis is being tested in the SyncAV (NCT04100148) randomized trial.
Conflict of interest: B.W.: employee of Abbott. N.V.: Abbott, Biotronik, Boston Scientific, EP Solutions, Implicity, Impulse Dynamics, Medtronic, Pacemate.
Contributor Information
Brian Wisnoskey, Cardiac Electrophysiology, Heart and Vascular Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Niraj Varma, Cardiac Electrophysiology, Heart and Vascular Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Data availability
Raw data will be available on reasonable request to the corresponding author.
References
- 1. Jastrzebski M, Baranchuk A, Fijorek K, Kisiel R, Kukla P, Sondej T, et al. Cardiac resynchronization therapy-induced acute shortening of QRS duration predicts long-term mortality only in patients with left bundle branch block. Europace 2019;21:281–289. [DOI] [PubMed] [Google Scholar]
- 2. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–3520. [published correction appears in Eur Heart J. 2022 May 1; 43(17):1651]. [DOI] [PubMed] [Google Scholar]
- 3. Wilkoff BL, Filippatos G, Leclercq C, Gold MR, Hersi AS, Kusano K, et al. Adaptive versus conventional cardiac resynchronisation therapy in patients with heart failure (AdaptResponse): a global, prospective, randomised controlled trial. Lancet 2023;402:1147–1157. [published correction appears in Lancet. 2023 Sep 30; 402(10408):1132]. [DOI] [PubMed] [Google Scholar]
- 4. Varma N, Jia P, Ramanathan C, Rudy Y. RV electrical activation in heart failure during right, left, and biventricular pacing. JACC Cardiovasc Imaging 2010;3:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Végh EM, Kandala J, Januszkiewicz L, et al. A new simplified electrocardiographic score predicts clinical outcome in patients treated with CRT. Europace 2018;20:492–500. [DOI] [PubMed] [Google Scholar]
- 6. ter Horst IAH, Bogaard MD, Tuinenburg AE, Mast TP, de Boer TP, Doevendans P, et al. The concept of triple wavefront fusion during biventricular pacing: using the EGM to produce the best acute hemodynamic improvement in CRT. Pacing Clin Electrophysiol 2017;40:873–882. [DOI] [PubMed] [Google Scholar]
- 7. Varma N. Fine-tuning delivery of cardiac resynchronization therapy: optimization for “triple fusion”. HeartRhythm Case Rep 2021;7:425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation 2004;109:1133–1139. [DOI] [PubMed] [Google Scholar]
- 9. Varma N, Ghanem R, Jia P. Pacing prescription for cardiac resynchronization therapy: when RV stimulation matters. J Cardiovasc Electrophysiol 2019;30:769–770. [DOI] [PubMed] [Google Scholar]
- 10. Varma N, Ghanem R, Jia P. Optimization of cardiac resynchronisation therapy: LV lead position, qLV, or paced effects?. Europace 2019;21:360. [DOI] [PubMed] [Google Scholar]
- 11. Gold MR, Ellenbogen KA, Leclercq C, Lowy J, Rials SJ, Shoda M, et al. Effects of atrioventricular optimization on left ventricular reverse remodeling with cardiac resynchronization therapy: results of the SMART-CRT trial. Circ Arrhythm Electrophysiol 2023;16:e011714. [DOI] [PubMed] [Google Scholar]
- 12. Vijayaraman P, Zalavadia D, Haseeb A, Dye C, Madan N, Skeete JR, et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm 2022;19:1263–1271. [DOI] [PubMed] [Google Scholar]
- 13. Vijayaraman P, Sharma PS, Cano Ó, Ponnusamy SS, Herweg B, Zanon F, et al. Comparison of left bundle branch area pacing and biventricular pacing in candidates for resynchronization therapy. J Am Coll Cardiol 2023;82:228–241. [DOI] [PubMed] [Google Scholar]
- 14. Jastrzębski M, Kiełbasa G, Cano O, Curila K, Heckman L, De Pooter J, et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J 2022;43:4161–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data will be available on reasonable request to the corresponding author.