This cohort study evaluates the characteristics, treatment patterns, and outcomes of patients with unresectable stage III non–small cell lung cancer who did or did not receive durvalumab.
Key Points
Question
Is consolidation durvalumab after chemoradiotherapy (CRT) in routine US clinical practice associated with better outcomes in patients with unresectable stage III non–small cell lung cancer (NSCLC)?
Findings
In this cohort study, durvalumab after CRT was associated with lower risk of progression and/or death, with the findings consistent with the phase 3 PACIFIC trial.
Meaning
This study suggests support for continued use of consolidation durvalumab after CRT as the standard of care for patients with unresectable stage III NSCLC.
Abstract
Importance
The PACIFIC trial established consolidation durvalumab as the standard of care following chemoradiotherapy (CRT) for patients with unresectable stage III non–small cell lung cancer (NSCLC). Understanding its benefit in routine US clinical practice is critical.
Objective
To report characteristics, treatment patterns, and outcomes of patients who did or did not receive durvalumab.
Design, Setting, and Participants
Two prespecified cohorts were curated in this retrospective cohort study (SPOTLIGHT). Deidentified patient-level data from a US database (Flatiron Health) were analyzed. Patients had unresectable stage III NSCLC, were diagnosed on or after January 1, 2011, had 2 or more visits on or afterward, and received CRT. Data were analyzed from May 2021 to October 2023.
Exposures
Patients started durvalumab after CRT (durvalumab cohort) or ended CRT without durvalumab (nondurvalumab cohort) by June 30, 2019, to allow 15 or more months of follow-up from CRT end.
Main Outcomes and Measures
End points included progression-free survival (PFS), overall survival (OS), time to first subsequent therapy or death (TFST), and time to distant metastasis or death (TTDM).
Results
The durvalumab cohort included 332 patients (median [IQR] age, 67.5 [60.8-74.0] years; 187 were male [56.3%], 27 were Black [8.7%], 33 were other races [10.7%], and 249 were White [80.6%]) and the nondurvalumab cohort included 137 patients (median (IQR) age, 70.0 [64.0-75.0] years; 89 [65.0%] were male, 11 [8.9%] were Black, 19 [15.4%] were other races, and 93 [75.6%] were White). Most patients had a smoking history (durvalumab, 316 patients [95.2%] and nondurvalumab, 132 patients [96.4%]) and Eastern Cooperative Oncology Group performance status 0 through 1 (durvalumab, 251 patients [90.9%] and nondurvalumab, 88 patients [81.5%]). Median (IQR) CRT duration was 1.6 (1.4-1.8) months for the durvalumab cohort and 1.5 (1.4-1.8) months for the nondurvalumab cohort. Median time to durvalumab discontinuation was 9.5 months (95% CI, 7.8-10.6 months). Median TFST and TTDM were not reached (NR) in the durvalumab cohort and 8.3 months (95% CI, 4.8-11.8 months) and 11.3 months (95% CI, 6.4-14.5 months), respectively, in the nondurvalumab cohort. Median PFS and OS were 17.5 months (95% CI, 13.6-24.8 months) and NR in the durvalumab cohort and 7.6 months (95% CI, 5.2-9.8 months) and 19.4 months (95% CI, 11.7-24.0 months) in the nondurvalumab cohort. In Cox regression analyses of patients who completed concurrent CRT without progression, durvalumab was associated with a lower risk of progression or death (hazard ratio [HR], 0.36; 95% CI, 0.26-0.51) and lower risk of death (HR, 0.27; 95% CI, 0.16-0.43), adjusted for prior platinum agent and patient characteristics.
Conclusions and Relevance
In this cohort study, findings were consistent with PACIFIC, and durvalumab was associated with a lower risk of progression and/or death. Further investigation is warranted to explain why patients did not receive durvalumab after its approval.
Introduction
Approximately 30% of patients with non–small cell lung cancer (NSCLC) present with stage III disease, which is unresectable in most cases.1 Historically, the standard of care for unresectable stage III NSCLC (stage III UR-NSCLC) was platinum-based chemotherapy administered concurrently with radiotherapy (cCRT) with curative intent. However, most patients recurred after cCRT with median progression-free survival (PFS) of 8 to 9 months and 5-year overall survival (OS) rates of approximately 13% to 20%. Additionally, survival was not improved with induction or consolidation chemotherapy or other systemic therapies.2,3,4,5,6,7,8,9,10 The placebo-controlled phase 3 Global Study to Assess the Effects of MEDI4736 Following Concurrent Chemoradiation in Patients With Stage III Unresectable Non–Small Cell Lung Cancer (PACIFIC) trial established up to 12 months of consolidation durvalumab as the new standard of care for patients with stage III UR-NSCLC and no progression following cCRT.11,12
Durvalumab is a human immunoglobulin G1 monoclonal antibody that selectively binds to programmed cell death ligand-1 (PD-L1), blocking its interaction with programmed cell death-1 (PD-1) and CD80, and thereby enhancing T-cell activation and promoting antitumor response.13 In PACIFIC, durvalumab significantly improved the primary end points of PFS and OS with manageable safety,11,12 findings reinforced by updated analyses at 5 years’ follow-up,14 when median OS was 47.5 months with durvalumab vs 29.1 months with placebo, and the estimated 5-year OS rate was 42.9% vs 33.4%, respectively; median PFS was 16.9 vs 5.6 months. These findings have led to global regulatory approvals of durvalumab and broad acceptance of the PACIFIC regimen in clinical practice.
It is critical to understand whether use of durvalumab after CRT in routine practice demonstrates comparable benefit with that in clinical trials. The effectiveness of the PACIFIC regimen was recently demonstrated in an observational study of patients outside the US (PACIFIC-R),15 which sheds further light on how patients are treated on an everyday basis.
Here, we report results from SPOTLIGHT, a retrospective cohort study designed to describe the characteristics, treatment patterns, and outcomes of US patients with stage III UR-NSCLC treated with CRT who did or did not receive durvalumab after CRT. We also report findings from exploratory analyses that compared outcomes in the subset of patients with no progression following cCRT (to mirror patient eligibility in PACIFIC).
Methods
Study Design and Data Source
This retrospective cohort study used deidentified, patient-level data extracted from the US Flatiron Health database (description in eMethods in Supplement 1) for a sample of patients with stage III UR-NSCLC who had received CRT (eFigure 1 in Supplement 1). The database has been independently certified as statistically deidentified and, therefore, did not require institutional review board approval or informed consent. The study included patients diagnosed with stage III UR-NSCLC on or after January 1, 2011. Two prespecified cohorts were curated: the durvalumab cohort comprised patients treated with CRT followed by durvalumab, and the nondurvalumab cohort comprised patients treated with CRT followed by either regimens that did not contain durvalumab or no subsequent treatment. Patients in the durvalumab cohort must have started durvalumab after CRT between February 1, 2018, and June 30, 2019, to allow 15 or more months potential follow-up from initiation of durvalumab to the data cutoff date of September 30, 2020. For the nondurvalumab cohort, the end of CRT had to be before or on June 30, 2019, to allow the same potential minimum duration of follow-up from the end of CRT (eFigure 2 in Supplement 1). The study was run in accordance with ethical principles consistent with the Declaration of Helsinki, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Good Clinical Practice, Good Pharmacoepidemiology Practice, and applicable legislation on noninterventional and observational studies. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients
Patients meeting inclusion criteria were aged 18 years or older with stage III (A, B, C, or unknown) NSCLC at initial diagnosis (defined in International Classification of Diseases, Ninth Revision [162.x] or Tenth Revision [C34.x or C39.9 codes]), who had received CRT and had 2 or more documented clinical visits in the Flatiron network on or after January 1, 2011 (eFigure 1 in Supplement 1). Patients were excluded if they had a 90-day or more gap between diagnosis and their first structured activity (a recording of vital information, a medication administration, a noncanceled drug order, or a laboratory test), had undergone complete resection before starting CRT, or were enrolled in a clinical trial before end of CRT. Additionally, patients in the durvalumab cohort who had surgery before initiation of durvalumab were excluded, and patients in the nondurvalumab cohort who had surgery before initiation of CRT or after initiation of CRT but before the next treatment were excluded.
End Points
The primary study objectives were to describe the demographic and clinical characteristics (with self-reported race and ethnicity recorded as described in eMethods in Supplement 1), treatment patterns, and outcomes of patients with stage III UR-NSCLC who started CRT. Race was assessed in this study because one of the primary objectives of the study was to describe the demographics and clinical characteristics of patients. The primary end points were time to first subsequent therapy or death (TFST), defined as the time to the earlier of either the start of first subsequent anticancer therapy, including surgery, or death; and time to death or distant metastasis (TTDM), defined as the time to the earlier of the first date of distant metastasis or death. Key secondary end points included time from end of CRT to start of durvalumab, number of durvalumab infusions, and the rate of prespecified adverse events (AEs) of interest: pneumonitis, esophagitis, and pain (general or local) during CRT as well as durvalumab treatment.
Exploratory end points comprised PFS and OS. PFS was defined as the time until progression or death from any cause, while OS was defined as the time to death from any cause. A patient was considered to have progressed if there was a record of progression or metastasis; in the absence of such records, a patient was considered to have progressed if he or she started subsequent anticancer therapies and discontinued durvalumab for reasons other than toxic effects. For OS analyses, patients who died after data cutoff were censored at the data cutoff date, while those lacking a record of death were censored at the earlier of a confirmed activity (eg, in-person hospital visit or treatment receipt) or the data cutoff date.
Statistical Analysis
All analyses were performed with SAS version 9.4 or higher (SAS Institute), and R version 4.1.0 (R Project for Statistical Computing). Descriptive analyses were conducted to evaluate time-to-event end points (time to durvalumab discontinuation, TFST, TTDM, PFS, and OS) per Kaplan-Meier method. Medians with 2-sided 95% CI and landmark rates were reported. The index date was durvalumab initiation for the durvalumab cohort and CRT initiation for the nondurvalumab cohort.
As a comparative analysis and to mirror patient eligibility in PACIFIC, exploratory analyses compared PFS and OS in patients who completed (without progressing during) cCRT (described in eMethods in Supplement 1). Cox regression analyses were used to evaluate the association of durvalumab with PFS and OS, separately, adjusting for prior platinum agent and available patient characteristics (age, sex, race, region, histological examination, disease stage, smoking history, and Eastern Cooperative Oncology Group [ECOG] performance status) at the end of CRT.
Sensitivity analyses were performed using treatment time-distribution matching,16 a different method to address immortal time bias (described in eMethods in Supplement 1). Data were analyzed from May 2021 to October 2023.
Results
The overall analysis set included a total of 469 patients with stage III UR-NSCLC, including 332 patients who received consolidation durvalumab (durvalumab cohort) and 137 patients who did not receive durvalumab (nondurvalumab cohort) (eFigure 1 in Supplement 1). Patients were most frequently from the south (221 patients [50.0%]) and the majority were treated at community practices (447 patients [95.3%]) (eTable 1 and eTable 2 in Supplement 1).
Durvalumab Cohort
Patients
In the durvalumab cohort (332 patients), the median (IQR) age was 67.5 (60.8-74.0) years. A total of 187 (56.3%) patients were male, 27 (8.7%) were Black, 33 (10.7%) were other races, 249 (80.6%) were White, and 316 (95.2%) had a history of smoking; 251 (90.9%) had a known ECOG performance status of 0 to 1, and either stage IIIA (194 patients [58.4%]) or IIIB (128 patients [38.6%]) disease at initial diagnosis (eTable 1 in Supplement 1). In addition, 36 evaluable patients (65.5%) had positive (ie, ≥1%) PD-L1 tumor cell expression and 56 patients (91.8%) had negative (ie, wild-type) EGFR variant status; the numbers of patients with missing data for each were high (277 and 271 patients, respectively).
Treatment Patterns
Median (IQR) duration of CRT was 1.6 (1.4-1.8) months (eTable 3 in Supplement 1). Most patients (309 of 332 patients [93.1%]) received complete cCRT; the majority (285 patients [85.8%]) received carboplatin-based chemotherapy, most frequently carboplatin-paclitaxel (243 patients [73.2%]). The main reasons for stopping CRT were treatment completion (289 patients [87.0%]) and toxic effects (43 patients [13.0%]); no patients discontinued CRT due to progression.
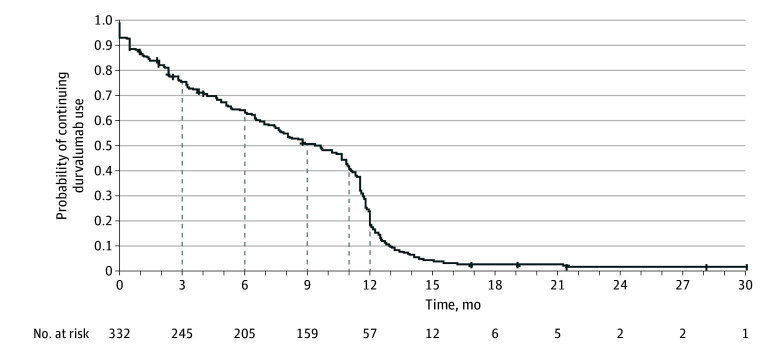
Median (IQR) time from end of CRT to start of durvalumab was 42 (29-65) days with 167 patients (50.3%) starting durvalumab within the suggested period of 42 days after the end of CRT. Patients received a median (range) of 16.5 (1-60) durvalumab infusions, administered every 2 weeks. At data cutoff, 314 patients (94.6%) had discontinued durvalumab, primarily due to completing treatment based on physician assessment (133 patients [40.1%]), progression (72 patients [21.7%]), or treatment AEs (63 patients [19.0%]) (eTable 4 in Supplement 1). Discontinuation within 3 months of starting durvalumab was most commonly due to toxic effects (34 patients [42.5%]), and between 3 and 6 months was most commonly due to disease progression (18 patients [48.6%]). Median time to durvalumab discontinuation was 9.5 months (95% CI, 7.8-10.6 months); 40.5% of patients received treatment for 11 months or more (Figure 1; eTable 3 in Supplement 1). After stopping durvalumab, 103 of 332 patients (31.0%) received subsequent anticancer therapy (eTable 5 in Supplement 1).
Figure 1. Time to Durvalumab Treatment Discontinuation in the Durvalumab Cohort.
Four patients continued to receive durvalumab treatment, and 14 were lost to follow-up at the time of data cutoff. Two patients discontinued durvalumab after completing 26 infusions and restarted durvalumab 4 months later; the discontinuation date was the date that each completed their 26th infusion. One patient discontinued durvalumab due to disease progression and restarted durvalumab 3 months later after a second progression; the discontinuation date for this patient was the date of the last durvalumab administration after the first progression.
Effectiveness
Median TFST and median TTDM were not reached (NR) (eFigure 3 in Supplement 1). Median PFS was 17.5 months (95% CI, 13.6-24.8 months), with a 24-month PFS rate of 44.4% (95% CI, 38.4%-50.3%) (eFigure 4 in Supplement 1). Median OS was NR; the rate of OS at 24 months was 71.5% (95% CI, 65.6%-76.6%) (eFigure 4 in Supplement 1).
Safety
In the durvalumab cohort, 275 patients (82.8%) reported AEs of interest during CRT, including pain (218 patients [65.7%]), esophagitis (144 patients [43.4%]), and pneumonitis (21 patients [6.3%]), which were not mutually exclusive (ie, patients may have had more than 1 AE of interest) (eTable 6 in Supplement 1). After initiation of durvalumab, 270 (81.3%) developed at least 1 AE of interest, including pain (252 patients [75.9%]), pneumonitis (79 patients [23.8%]), and esophagitis (10 patients [3.0%]), which were not mutually exclusive (eTable 7 in Supplement 1). Overall, 30 patients (9.0%) discontinued a durvalumab-based regimen due to an AE of interest, most commonly attributed to pneumonitis (23 patients [6.9%]), although this could be due to a combination of AEs of interest (eTable 7 in Supplement 1).
Nondurvalumab Cohort
Patients
The median (IQR) age of the 137 patients in the nondurvalumab cohort was 70.0 (64.0-75.0) years. A total of 89 patients (65.0%) were male, 11 (8.9%) were Black, 19 (15.4%) were other races, and 93 (75.6%) were White; most had a history of smoking (132 patients [96.4%]), known ECOG performance status 0 to 1 (88 patients [81.5%]), and either stage IIIA (89 patients [65.0%]) or IIIB (45 patients [32.8%]) disease (eTable 2 in Supplement 1); in addition, 14 evaluable patients (56.0%) had positive (ie, ≥1%) PD-L1 tumor cell expression, and 27 patients (84.4%) had a negative (ie, wild-type) EGFR variant status; the numbers of patients with missing data for each were high (112 and 105 patients, respectively).
Treatment Patterns
In the nondurvalumab cohort, median (IQR) duration of CRT was 1.5 (1.4-1.8) months, with 98 patients (71.5%) receiving complete cCRT (eTable 8 in Supplement 1). Overall, the majority of patients received carboplatin-based chemotherapy (117 patients [85.4%]), most commonly carboplatin-paclitaxel (96 patients [70.1%]). The main reasons for stopping CRT were treatment completion (86 patients [62.8%]) and treatment toxic effects (26 patients [19.0%]); 5 patients (3.6%) discontinued CRT due to progression.
Effectiveness
Median TFST was 8.3 months (95% CI, 4.8-11.8 months), while median TTDM was 11.3 months (95% CI, 6.4-14.5 months) (eFigure 5 in Supplement 1). Median PFS was 7.6 months (95% CI, 5.2-9.8 months), with a 24-month PFS rate of 8.8% (95% CI, 3.8%-16.5%) (eFigure 6 in Supplement 1). Median OS was 19.4 months (95% CI, 11.7-24.0 months), with a 24-month OS rate of 41.0% (95% CI, 30.7%-51.1%) (eFigure 6 in Supplement 1).
Safety
In the nondurvalumab cohort, 103 patients (75.2%) reported AEs of interest during CRT, including pain (89 patients [65.0%]), esophagitis (47 patients [34.3%]), and pneumonitis (8 patients [5.8%]). These AEs were not mutually exclusive (ie, patients may have had more than 1 AE of interest) (eTable 6 in Supplement 1).
Exploratory Analyses
Main Analyses
A total of 299 of 332 patients from the durvalumab cohort and 77 of 137 patients from the nondurvalumab cohort met the conditions for inclusion in the exploratory analyses. Patient characteristics were largely similar between the 2 groups analyzed (Table). However, the durvalumab group vs nondurvalumab group had a lower proportion of patients who were aged 65 years or older (184 patients [61.5%] vs 56 patients [72.7%]), Black or African American (23 patients [8.1%] vs 8 patients [11.4%]), from the South (134 patients [47.3%] vs 42 patients [59.2%]) or had ECOG performance status higher than 1 (26 patients [10.8%] vs 8 patients [17.4%]), and a higher proportion of patients with stage IIIB/IIIC disease at initial diagnosis (126 patients [42.1%] vs 21 patients [27.3%]).
Table. Patient Demographics, Treatment, and Clinical Characteristics at the End of Chemoradiotherapy (Main Exploratory Analyses).
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Durvalumab group (n = 299) | Nondurvalumab group (n = 77) | |
| Age, median (IQR), y | 67.0 (60.0-74.0) | 70.0 (64.0-74.0) |
| Age ≥65 y | 184 (61.5) | 56 (72.7) |
| Sex | ||
| Male | 167 (55.9) | 46 (59.7) |
| Female | 132 (44.1) | 31 (40.3) |
| Race | ||
| Asian | 6 (2.1) | 2 (2.9) |
| Black or African American | 23 (8.1) | 8 (11.4) |
| White | 228 (80.6) | 51 (72.9) |
| Othera | 26 (9.2) | 9 (12.9) |
| Missingb | 16 | 7 |
| Smoking history | ||
| Yes | 284 (95.0) | 73 (94.8) |
| No | 15 (5.0) | 4 (5.2) |
| ECOG performance statusc | ||
| 0-1 | 214 (89.2) | 38 (82.6) |
| >1 | 26 (10.8) | 8 (17.4) |
| Missingb | 59 | 31 |
| AJCC disease stage at initial diagnosisd | ||
| IIIA | 173 (57.9) | 56 (72.7) |
| IIIB | 116 (38.8) | 20 (26.0) |
| IIIC | 10 (3.3) | 1 (1.3) |
| Histology | ||
| Squamous | 147 (50.9) | 35 (47.9) |
| Nonsquamous | 142 (49.1) | 38 (52.1) |
| Missingb | 10 | 4 |
| PD-L1 tumor cell expressione | ||
| ≥1% | 35 (66.0) | 8 (66.7) |
| <1% | 18 (34.0) | 4 (33.3) |
| Missingb | 246 | 65 |
| EGFR variant statuse | ||
| Negative | 54 (94.7) | 14 (100) |
| Positive | 3 (5.3) | 0 |
| Missing/unknown/indeterminateb | 242 | 63 |
| Region | ||
| South | 134 (47.3) | 42 (59.2) |
| Midwest | 70 (24.7) | 11 (15.5) |
| Northeast | 44 (15.5) | 11 (15.5) |
| West | 35 (12.4) | 7 (9.9) |
| Missingb | 16 | 6 |
| Prior platinum agent | ||
| Any cisplatin | 45 (15.1) | 8 (10.4) |
| Carboplatin only | 254 (84.9) | 69 (89.6) |
Abbreviations: AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed cell death-ligand 1.
The groups included in other race are not specified in the database.
Missing values were not imputed; all reported percentages were calculated using the number of patients with available data as the denominator.
Between the chemoradiotherapy (CRT) end date and 42 days after the CRT end date, inclusive.
Based on the date range that patients were diagnosed in this study, they may have been diagnosed according to either the 7th or 8th editions of the AJCC Cancer Staging Manual.
No PD-L1 or EGFR data were available within 4 weeks before or after the end of CRT, so this is, instead, within 4 weeks before or after the date of the start of CRT. If multiple records were available, the record closest to the CRT start date is reported. If a patient had values both before and after the CRT start date of the same duration of time from that date, then the value before the date was analyzed.
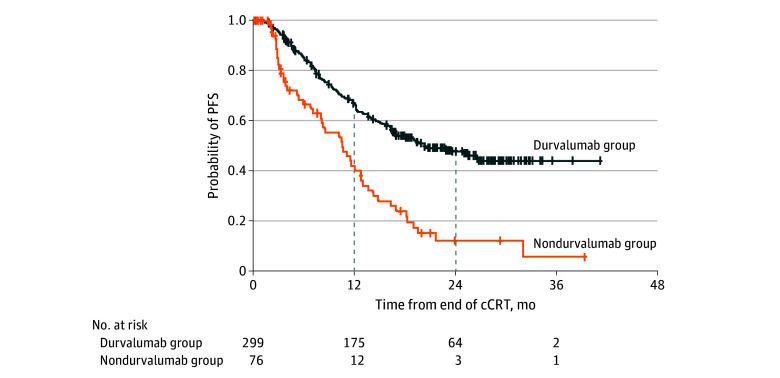
Median PFS was 20.0 months (95% CI, 16.2 months-not estimable [NE]) in the durvalumab group and 10.2 months (95% CI, 6.7-12.4 months) in the nondurvalumab group (Figure 2); 12-month PFS rates were 63.8% (95% CI, 57.9%-69.1%) and 40.2% (95% CI, 27.3%-52.8%), respectively, and 24-month PFS rates were 47.8% (95% CI, 41.5%-53.8%) and 12.3% (95% CI, 4.7%-23.8%). In the Cox regression analysis for PFS (adjusted for prior platinum agent and patient characteristics), durvalumab was associated with a lower risk of progression or death (HR, 0.36; 95% CI, 0.26-0.51).
Figure 2. Exploratory Analysis of Progression-Free Survival (PFS).
PFS was measured from the end of chemoradiotherapy (CRT) to progression or death from any cause. One patient in the nondurvalumab group was not assessed for progression after the end of cCRT and, therefore, was excluded from the PFS analysis. cCRT indicates concurrent chemoradiotherapy.
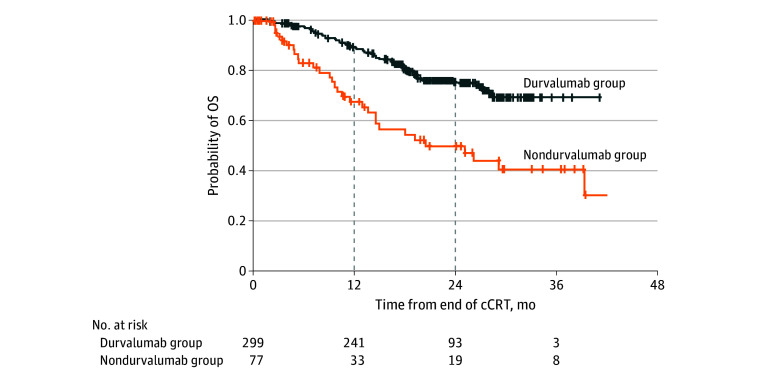
Median OS in the durvalumab group was not reached vs 24.8 months (95% CI, 13.4 months-NE) in the nondurvalumab group (Figure 3); the 12-month OS rate was 88.6% (95% CI, 84.3%-91.8%) vs 68.0% (95% CI, 53.9%-78.5%) and the 24-month OS rate was 75.4% (95% CI, 69.5%-80.3%) vs 50.3% (95% CI, 35.7%-63.2%). In the Cox regression analysis for OS (adjusted for prior platinum agent and patient characteristics), durvalumab was associated with a lower risk of death (HR, 0.27; 95% CI, 0.16-0.43). The results of sensitivity analyses for the main exploratory analyses were consistent, as summarized in eFigure 7, eFigure 8, and eFigure 9 in Supplement 1.
Figure 3. Exploratory Analysis of Overall Survival (OS).
OS was measured from the end of chemoradiotherapy (CRT) to death from any cause. cCRT indicates concurrent chemoradiotherapy.
Discussion
Findings from SPOTLIGHT were consistent with PACIFIC,11,12,14 demonstrating the effectiveness of durvalumab in everyday US clinical practice and increasing our understanding of the management of patients with stage III UR-NSCLC. In exploratory analyses of patients with no progression following cCRT, which mirrored patient eligibility in PACIFIC, those who received durvalumab had better outcomes vs patients who did not, despite relatively similar patient demographic and clinical characteristics; however, it should be acknowledged that data pertaining to ECOG performance status, PD-L1 tumor cell expression, and molecular testing (ie, the presence of an EGFR variant), all of which can impact immunotherapy decisions in this setting,17,18 were incomplete. Findings were also consistent with effectiveness data recently reported for the PACIFIC-R study of non-US patients, which did not include a comparative analysis of patients who did not receive durvalumab.15 With a relatively short median follow-up, OS, TFST, and TTDM data in SPOTLIGHT were immature (medians were not reached in the durvalumab group); however, the landmark rates appear consistent with those in PACIFIC.14 Finally, the reductions in risk of progression or death (HR, 0.36) and in risk of death (HR, 0.27), as associated with durvalumab treatment in SPOTLIGHT, were notable.
These US data suggest that, among patients with stage III UR-NSCLC receiving cCRT or sCRT, most received cCRT, which is consistent with the clinical evidence19 and consequential standard of care recommendations for patients fit enough to tolerate cCRT. In PACIFIC, consolidation durvalumab had to start within 42 days of completing CRT and continued for up to 12 months, with 42.7% receiving 12 months.11 In SPOTLIGHT, median time to start of durvalumab was 42 days after CRT end, and 40.5% received treatment for 11 or more months. In the PACIFIC-R study, median time to durvalumab start was 56 days from CRT end and 19.8% of patients were treated for at least 12 months.15 Results from both studies suggest that many patients in everyday practice may start durvalumab outside of the 42-day window after completing CRT. The reasons for this may be the challenges or inherent delays in arranging scans and scheduling the next line of therapy, as well as patients’ recovery from toxic effects, in everyday clinical practice vs a clinical trial.
The profile of prespecified AEs of interest in SPOTLIGHT were broadly in line with those already known in this setting.11,20 Treatment-related pneumonitis is of particular concern as both thoracic radiation and immunotherapy pose a risk of pneumonitis to a patient population that often has underlying lung dysfunction secondary to comorbidities or lung function compromised by tumors treated with CRT.20 Of note, differentiation between immunotherapy-induced and radiation-induced pneumonitis can be difficult. However, in SPOTLIGHT, the reported incidence of pneumonitis during durvalumab treatment (23.8%) was less than that typically observed in Asian patients,21 consistent with that in PACIFIC (any-grade pneumonitis or radiation pneumonitis, 33.9%) and PACIFIC-R (any-grade pneumonitis or interstitial lung disease, 17.9%), and did not lead to greater rates of treatment discontinuation due to pneumonitis (6.9% in SPOTLIGHT, 6.3% in PACIFIC, and 9.5% in PACIFIC-R).
Limitations
The SPOTLIGHT study is limited by its retrospective, observational design. That said, we used random sampling of patients from the Flatiron database during an initial selection process to minimize the effects of selection bias. In addition, for the main exploratory analyses, we adjusted for the prior platinum agent and patient characteristics as assessed at the end of CRT (the time point from which outcomes were measured), and the treatment benefit associated with durvalumab remained evident, though there may be additional socioeconomic or patient frailty–related factors that were not collected in this study. We addressed the potential for immortal time bias with a different method in the sensitivity analyses, which also demonstrated the benefits of consolidation durvalumab. Other limitations stem from those intrinsic to the Flatiron database; data are drawn mainly from community practices within the Flatiron network and may have missing variables, values, or records of visits. In addition, the definition of progression used in the analyses may not be consistent with that used in clinical trials. Specifically, for PFS, a patient may have progressed some time before the date of recorded disease progression. In SPOTLIGHT, however, we also evaluated TFST and TTDM as supportive measures to PFS, and their landmark estimates were consistently numerically higher in patients receiving consolidation durvalumab (eFigure 3 and eFigure 5 in Supplement 1). Upcoming analyses of TFST and TTDM with more mature data will shed further light on the potential clinical benefit of treatment. Finally, a more detailed analysis of toxic effects was not available, as we limited collection of safety data to only 3 prespecified AEs of interest (albeit the most prevalent ones).
Conclusions
In summary, patients with stage III UR-NSCLC who received durvalumab consolidation after CRT had better outcomes than those who did not receive durvalumab, supporting continued use of durvalumab as the standard of care. Further investigation of durvalumab administration patterns (eg, factors leading to the decision not to administer durvalumab) is warranted to explain why patients did not receive durvalumab after its approval.
eMethods.
eFigure 1. Selection of Patients From Flatiron Database: Attrition Flow Chart
eFigure 2. Study Design
eTable 1. Patient Demographics and Clinical Characteristics at Durvalumab Initiation (Durvalumab Cohort)
eTable 2. Patient Demographics and Clinical Characteristics at CRT Start (Nondurvalumab Cohort)
eTable 3. Type and Duration of CRT and Duration of Durvalumab Treatment (Durvalumab Cohort)
eTable 4. Reasons for Discontinuation of Durvalumab by Duration of Treatment (Durvalumab Cohort)
eTable 5. First Subsequent Therapy After Discontinuation of Durvalumab by Duration of Durvalumab Treatment (Durvalumab Cohort)
eFigure 3. Time to First Subsequent Therapy or Death and Time to Distant Metastasis or Death in the Durvalumab Cohort
eFigure 4. Progression-Free Survival and Overall Survival in the Durvalumab Cohort
eTable 6. Incidence and Management of Adverse Events of Interest Occurring During CRT (Durvalumab and Nondurvalumab Cohorts)
eTable 7. Incidence and Management of Adverse Events of Interest Occurring During Durvalumab Treatment (Durvalumab Cohort)
eTable 8. Type and Duration of CRT (Nondurvalumab Cohort)
eFigure 5. Time to First Subsequent Therapy or Death and Time to Distant Metastasis or Death in the Nondurvalumab Cohort
eFigure 6. Progression-Free Survival and Overall Survival in the Nondurvalumab Cohort
eFigure 7. Time to Start of Durvalumab From End of Chemoradiotherapy (Durvalumab Group)
eFigure 8. Sensitivity Analyses of Time to First Subsequent Therapy or Death and Time to Distant Metastasis or Death
eFigure 9. Sensitivity Analyses of Progression-Free Survival and Overall Survival
eResults.
Data Sharing Statement
References
- 1.Hansen RN, Zhang Y, Seal B, et al. Long-term survival trends in patients with unresectable stage III non-small cell lung cancer receiving chemotherapy and radiation therapy: a SEER cancer registry analysis. BMC Cancer. 2020;20(1):276. doi: 10.1186/s12885-020-06734-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn JS, Ahn YC, Kim JH, et al. Multinational randomized Phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33(24):2660-2666. doi: 10.1200/JCO.2014.60.0130 [DOI] [PubMed] [Google Scholar]
- 3.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flentje M, Huber RM, Engel-Riedel W, et al. GILT–A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther Onkol. 2016;192(4):216-222. doi: 10.1007/s00066-016-0941-8 [DOI] [PubMed] [Google Scholar]
- 5.Hanna N, Neubauer M, Yiannoutsos C, et al. ; Hoosier Oncology Group; US Oncology . Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26(35):5755-5760. doi: 10.1200/JCO.2008.17.7840 [DOI] [PubMed] [Google Scholar]
- 6.Senan S, Brade A, Wang LH, et al. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34(9):953-962. doi: 10.1200/JCO.2015.64.8824 [DOI] [PubMed] [Google Scholar]
- 7.Vokes EE, Herndon JE II, Kelley MJ, et al. ; Cancer and Leukemia Group B . Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: cancer and leukemia group B. J Clin Oncol. 2007;25(13):1698-1704. doi: 10.1200/JCO.2006.07.3569 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28(23):3739-3745. doi: 10.1200/JCO.2009.24.5050 [DOI] [PubMed] [Google Scholar]
- 9.Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8(1):1-20. doi: 10.5306/wjco.v8.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26(15):2450-2456. doi: 10.1200/JCO.2007.14.4824 [DOI] [PubMed] [Google Scholar]
- 11.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 12.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 13.Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052-1062. doi: 10.1158/2326-6066.CIR-14-0191 [DOI] [PubMed] [Google Scholar]
- 14.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301-1311. doi: 10.1200/JCO.21.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard N, Bar J, Garrido P, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. 2023;18(2):181-193. doi: 10.1016/j.jtho.2022.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016-1023. doi: 10.1093/aje/kwi307 [DOI] [PubMed] [Google Scholar]
- 17.Aredo JV, Mambetsariev I, Hellyer JA, et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16(6):1030-1041. doi: 10.1016/j.jtho.2021.01.1628 [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798-806. doi: 10.1016/j.annonc.2020.03.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol. 2010;28(13):2181-2190. doi: 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 20.Mielgo-Rubio X, Rojo F, Mezquita-Pérez L, et al. Deep diving in the PACIFIC: practical issues in stage III non-small cell lung cancer to avoid shipwreck. World J Clin Oncol. 2020;11(11):898-917. doi: 10.5306/wjco.v11.i11.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CK, Oh HJ, Kim YC, et al. Korean real-world data on patients with unresectable stage III NSCLC treated with durvalumab after chemoradiotherapy: PACIFIC-KR. J Thorac Oncol. 2023;18(8):1042-1054. doi: 10.1016/j.jtho.2023.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Selection of Patients From Flatiron Database: Attrition Flow Chart
eFigure 2. Study Design
eTable 1. Patient Demographics and Clinical Characteristics at Durvalumab Initiation (Durvalumab Cohort)
eTable 2. Patient Demographics and Clinical Characteristics at CRT Start (Nondurvalumab Cohort)
eTable 3. Type and Duration of CRT and Duration of Durvalumab Treatment (Durvalumab Cohort)
eTable 4. Reasons for Discontinuation of Durvalumab by Duration of Treatment (Durvalumab Cohort)
eTable 5. First Subsequent Therapy After Discontinuation of Durvalumab by Duration of Durvalumab Treatment (Durvalumab Cohort)
eFigure 3. Time to First Subsequent Therapy or Death and Time to Distant Metastasis or Death in the Durvalumab Cohort
eFigure 4. Progression-Free Survival and Overall Survival in the Durvalumab Cohort
eTable 6. Incidence and Management of Adverse Events of Interest Occurring During CRT (Durvalumab and Nondurvalumab Cohorts)
eTable 7. Incidence and Management of Adverse Events of Interest Occurring During Durvalumab Treatment (Durvalumab Cohort)
eTable 8. Type and Duration of CRT (Nondurvalumab Cohort)
eFigure 5. Time to First Subsequent Therapy or Death and Time to Distant Metastasis or Death in the Nondurvalumab Cohort
eFigure 6. Progression-Free Survival and Overall Survival in the Nondurvalumab Cohort
eFigure 7. Time to Start of Durvalumab From End of Chemoradiotherapy (Durvalumab Group)
eFigure 8. Sensitivity Analyses of Time to First Subsequent Therapy or Death and Time to Distant Metastasis or Death
eFigure 9. Sensitivity Analyses of Progression-Free Survival and Overall Survival
eResults.
Data Sharing Statement