Abstract
Background:
Erythromelalgia, which has primary and secondary presentations, causes heat, pain, and redness in the skin. The condition seems to have an autonomic basis, with vasomotor dysfunction causing dilatation of some blood vessels and constriction of others. No consistently effective treatments have been reported. Anticonvulsant, antidepressant, antihistamine, anti-inflammatory, antihypertensive, analgesic, nutritional, and topical approaches have been tried as were lidocaine infusions, nerve blocks, and thoracic and lumbar sympathectomies. Interosseous membrane stimulation appears to affect the local autonomic milieu in the extremity being treated. This approach was used on a patient with erythromelalgia.
Case:
A 36-year-old woman with erythromelalgia was treated with interosseous membrane stimulation. Eight treatments were given over a 1-year timeframe at 1–3-month intervals.
Results:
This patient repeatedly experienced much relief from her burning paresthesias, swelling, diaphoresis, and ruddy discoloration of her extremities for 6–8 hours following each treatment. The intensity of her discomfort subsided gradually over time.
Conclusions:
Interosseous membrane stimulation is a safe, simple, and effective treatment for erythromelalgia, which is notoriously refractory to treatment. This patient's response to treatment might have been a result of localized derangement of her autonomic nervous system. It is possible that manipulation of the autonomic milieu of an extremity is a significant factor in the mechanism of action of interosseous membrane stimulation.
Keywords: erythromelalgia, autonomic vascular control, interosseous membrane, complex regional pain syndrome
INTRODUCTION
Erythromelalgia is a rare condition that presents with heat, pain, and redness in the skin. Primary and secondary presentations have been described. The condition appears to have an autonomic basis with vasomotor dysfunction resulting in dilatation of some blood vessels and constriction of others. No consistently effective treatments have been reported. Anticonvulsant, antidepressant, antihistamine, anti-inflammatory, antihypertensive, analgesic, nutritional, and topical treatments have been tried. Lidocaine infusions, nerve blocks, and thoracic and lumbar sympathectomies have been performed. Interosseous membrane stimulation appears to affect the local autonomic milieu in the extremity being treated. This treatment was tried on a patient with erythromelalgia.1–3
In the April 2023 article “Interosseous Membrane Stimulation: A Treatment for Painful Peripheral Neuropathy,” it was hypothesized that stimulation of the interosseous membrane, in turn, stimulates autonomic nerve endings embedded in the membrane, resulting in a modulation of blood flow in the treated extremity.4 The current authors had the opportunity to treat a young woman who had erythromelalgia by applying interosseous membrane stimulation. It appears that her response supported the above hypothesis.
CASE
A 36-year-old woman presented in December of 2021 with a 2-month history of painful paresthesia in her hands during the day and at night. She noticed a ruddy/red discoloration of her hands that also became warm, moist, and swollen. Rarely, the same complaints involved her feet, notably, on 1 occasion, when she wore sandals and her feet were exposed to the sun. She would wake at night several days per week with both hands feeling prickly, tingly, and weak. She reported that she had to hold a coffee cup in both of her hands because she was afraid of dropping it. Her symptoms fluctuated in intensity and were incapacitating for several hours up to several days at a time. Occasionally during a flare-up, when her hands were the most uncomfortable, she felt “jittery” or “trembly,” with an associated generalized tremor, tachycardia, and an internal tremulous feeling.
This patient had extensive evaluations. After an initial referral by her internist to neurology, other specialists were consulted in dermatology, rheumatology, and infectious diseases and allergies. Laboratory evaluations and their results included: a negative COVID nucleic-acid test; a normal complete blood count; Chem 7; Ca++; Mg++; thyroid-stimulating hormone; T4 [thyroxine]; a gastrointestinal panel; an ANA [antinuclear antibodies] panel; negative RA [rheumatoid arthritis]; negative HIV [human immunodeficiency virus] and syphilis panels; below-normal range C-reactive protein; normal sedimentation rate; negative cryoglobulins; negative lupus anticoagulant; negative ANCA [antineutrophil cytoplasmic antibodies; normal SPEP [serum protein electrophoresis]; nonreactive hepatitis-B antigen, and a negative hepatitis-C screen. An initial Lyme screen indicated what was believed to be a false-positive result, and confirmatory Western Blot testing was negative x 2.
Searches for occult malignancy with mammography and computed tomography of her chest, abdomen, and pelvis were negative. Because of her intermittent trembling and tachycardia, 24-hour urine collections for catecholamines and metanephrines were checked and were normal. Looking for evidence of mastocytosis and mast-cell degranulation, a baseline tryptase level was obtained and was elevated at 12.1 with the range being <11.0 mcg/L. Her symptoms, in retrospect, were not consistent with either cutaneous or systemic mastocytosis. The baseline tryptase study was not repeated. She did not have a change in symptoms after several weeks of antihistamine therapy.
This patient declined consideration of therapeutic trials of prescription medications for treating her condition. Based upon her presentation and lack of evidence for an alternative explanation, she was diagnosed with erythromelalgia, that caused her episodes of burning pain and redness in the hands, and sometimes the feet, arms, legs, ears, and face.
Symptoms of erythromelalgia can begin at any age and typically occur in spells lasting from a few minutes to several days. The onset is usually associated with an itching sensation, worsening to pain, with tender, warm-to-the-touch red skin and swelling, and sweating of the affected areas. The skin often retains a purple discoloration between spells.5 No medication, therapeutic method, or procedure has been an effective treatment consistently for individuals diagnosed with erythromelalgia.6
This patient was treated with interosseous membrane stimulation with a good response lasting for several hours up to 12 hours at a time. Treatment was carried out with a SEIRIN® 60-mm, L-type, No. 8 needle. The interosseous membrane was needled approximately midway between TH-8 (TE-8) and TH-9 (TE-9), in the depression palpable between the abductor pollicis longus and extensor carpi radialis brevis muscles. The needles were advanced ∼1 cun or about half the width of her forearm. The needles were “strummed” for ∼10 minutes until she reported a resolution of the hot prickly sensation in her hands. If she did not to begin to feel a response in by 1 minute, the needle was either advanced or repositioned to another location in the interosseous membrane, until she reported that the discomfort from her hot, prickly, and painful paresthesias was replaced with a “cool, pleasant sensation.”
Timing of her treatments was driven by her ability to get to the clinic, which was challenging, as she worked full-time and lived more than a mile from the clinic. She received the treatment 8 times in 1–3-month intervals between October 21, 2022, and September 8, 2023. Over that time she felt that her discomfort gradually decreased in intensity.
On October 23,2023, after learning that she was pregnant, this patient sent a message to the neurology nurse:
I just thought I'd send y'all a quick update on how my hands have been. Almost immediately after I found out I was pregnant my hands began to act normal. They swell slightly only first thing in the morning or if I'm hot/doing physical activity. In other words, they moderately swell like a normal person again. Otherwise, there is no swelling, numbness/tingling, redness, heat, etc. I'm able to wear both my engagement and wedding rings again and they freely spin on my finger like they used to.
Using a cellphone camera as well as a digital thermometer, the current authors documented the resolution of hyperemia in this patient's hands (Table 1). This was associated with an initial increase and then decrease in skin temperature following treatment (Table 2 shows measurements in the left hand). This rather-dramatic response, resulting in resolution of the manifestations of her erythromelalgia, seemed to establish that at least 1 mechanism of action of interosseous membrane stimulation treatment is through mediation of the autonomic milieu in the extremity being treated.
Table 1.
Examination Findings
| Date | Subjective | Objective |
|---|---|---|
| April 10, 2023 | Pleasant/cool feeling in hands post-treatment Decrease in tightness and tingling |
Generalized trembling prior to treatment resolved post-treatment |
| June 23, 2023 | Feeling of warmth spread to knuckles during treatment Cool feeling post-treatment |
Shaking fingers prior to treatment resolved post treatment Decreased ruddy color of hands 2 hrs post-treatment |
| August 11, 2023 | Pleasant & cool feeling Pain decreased from 3/10 to 0/10 |
Mild tremulousness resolved with treatment |
hrs, hours.
Table 2.
Left Hand Temperatures (in Fahrenheit) Pre- and Post-Treatment
| |
Left hand temperature pretreatment |
Left hand temperature immediately post-treatment |
Left hand temperature 30 min + post-treatment |
|||
|---|---|---|---|---|---|---|
| Date | Dorsum | Palm | Dorsum | Palm | Dorsum | Palm |
| April 10, 2023 | 87.2 | 84.4 | 91.6 | 91.3 | 73.1 | 73.4 |
| June 23, 2023 | 85.1 | 89.8 | 91.9 | 86.7 | 87.2 | |
| August 11, 2023 | 92.7 | 94.5 | 81.7 | |||
min, minutes.
Figures 1–3 are matched with temperature measurements with a digital infrared thermometer at 3 timepoints: (1) before treatment; (2) immediately post-treatment, and (3) 4 hours post-treatment. As can be seen in these images, the patient's ruddy discoloration of her skin was reduced over that timeframe. She had an associated resolution of her discomfort.
FIG. 1.

Hand pretreatment (August 11, 2023, at 1:13 pm).
FIG. 2.

Hand immediately post-treatment (August 11, 2023, at 1:25 pm).
FIG. 3.

Hand 30 minutes post-treatment (August 11, 2023, at 1:56 pm).
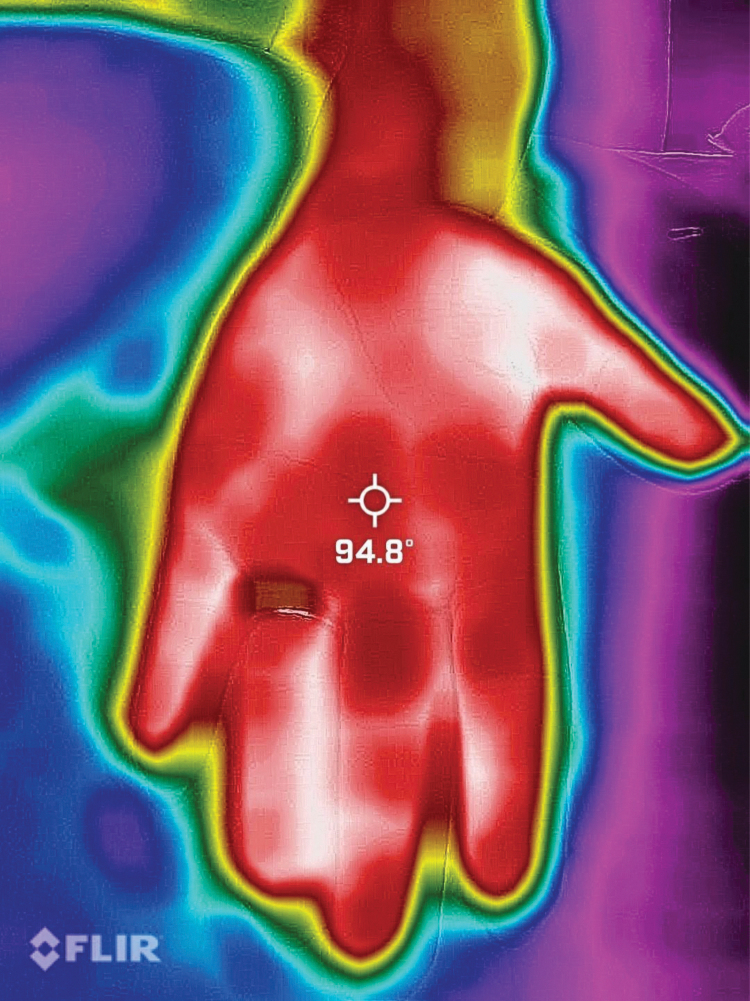
Figures 4–9 show the dramatic visualization of these changes documented with a thermography application (forward-looking infrared [FLIR]) attached to a smartphone.
FIG. 4.
Palm pretreatment temperature (in Fahrenheit) on FLIR [forward-looking infrared] application (August 11, 2023, at 1:13 pm).
FIG. 5.
Fingers pretreatment temperature (in Fahrenheit) on FLIR [forward-looking infrared] application (August 11, 2023, at 1:14 pm).
FIG. 6.
Palm immediately post-treatment temperature (in Fahrenheit) on FLIR [forward-looking infrared] application (August 11, 2023, at 1:25 pm).
FIG. 7.
Fingers immediately post-treatment temperature (in Fahrenheit) on FLIR [forward-looking infrared] application (August 11, 2023, at 1:25 pm).
FIG. 8.
Palm 30 minutes post-treatment temperature (in Fahrenheit) on FLIR [forward-looking infrared] application (August 11, 2023, at 1:56 pm).
FIG. 9.
Fingers 30 minutes post-treatment temperature (in Fahrenheit) on FLIR [forward-looking infrared] application (August 11, 2023, at 1:56 pm).
DISCUSSION
The first reported case of erythromelalgia was in 1878 by Mitchell. While the condition was originally known as Mitchell disease, Mitchell himself suggested using the term erythromelalgia.7 The Greek words that comprise this term are: erythros meaning red; melos meaning limb; and algas meaning pain.8 Primary idiopathic erythromelalgia not related to a recognized underlying disease is the most-common presentation; inherited erythromelalgia is less-common. Secondary erythromelalgia related to autoimmune conditions— polycythemia vera, Raynaud's phenomena, thrombocytopenia, and neuropathy—may occur.3 No single medication, therapeutic method, or procedure has been consistently effective for individuals who were treated for erythromelalgia.3
Treatment of this condition with interosseous membrane stimulation has not been described previously. The current authors postulate that this treatment was effective because it affected the autonomic milieu of the extremities being treated. Other acupuncture treatments also likely affect the local autonomic milieu of an extremity. For instance, MH-7 (P-7) traditionally has an indication as a Sedation point for dispersing Excess Fire and Excess Yang symptoms and Fire-type skin problems, such as redness and heat in the palms. When needling MH-7, the underlying flexor retinaculum as well as the distal portion of the interosseous membrane are both potentially stimulated. The connective tissues of the forearm are innervated, containing free nerve endings (Pacini and Ruffini corpuscles from branches of the median, ulnar, and radial nerves). Perhaps needling MH-7 also induced an autonomic response sufficient to reset the autonomic milieu in the extremity of the current patient.9
The current first author (M.F.) has also successfully treated manifestations and discomfort related to complex regional pain syndrome of the hand and foot, another condition believed to be secondary to autonomic dysregulation in distal extremities. In this first author's clinic, discomfort experienced by patients with painful neuropathy, and arthritic pain in the fingers of a patient with psoriatic arthritis, have shown the same excellent response to treatment. An identical thermographic response to treatment of these conditions was documented, suggesting that the response to interosseous membrane stimulation in those conditions is also autonomically mediated.
The autonomic nervous system (ANS) regulates certain involuntary functions, including the diameter of the opened blood vessels. In the article by Freedman and Bierwirth, during interosseous membrane stimulation treatment, patients described some variation of a wave of spreading “warmth,” associated with a “pleasant feeling,” or a “floating feeling” that replaced their discomfort.4 The sensation of warmth suggests an increase of blood flow in the affected limb.4 Autonomic innervation of tendons, ligaments, and joint capsules was described in 2001 by Ackermann et al.10 Sensory nerve endings have been found throughout the interosseous membrane of the forearm, a structure similar to the interosseous membrane of the leg. The interosseous membrane has large sensory fibers with encapsulated endings specialized for detecting vibration and pressure.11
The pathophysiology of erythromelalgia is unknown. As suggested online by the National Organization for Rare Disorders,
[i]n erythromelalgia, additional evidence indicates that ring-shaped muscle regions (sphincters) of certain blood vessels that control blood flow from small arteries (arterioles) to capillaries (i.e., precapillary sphincters) may be abnormally narrowed while “arteriovenous shunts” are open. (According to researchers, blood flow through skin capillaries primarily provides necessary oxygen and nutrients to cells. Arteriovenous shunts, which are blood vessels that directly connect certain arteries and veins and thus bypass the capillary network, are thought to play a role in regulating temperature.) Constriction of some precapillary sphincters while arteriovenous shunts are open may lead to increased total blood flow but decreased transport of oxygen and nutrients to cells. This results in a simultaneous insufficient oxygen supply (hypoxia) and excess of blood (hyperemia) in affected skin. The presence of hypoxia may in turn trigger increased localized blood flow to skin regions, thus exacerbating pain, heat sensation, and redness.5
Control of blood flow to the extremities is a complex process. Spinal and supraspinal control have been identified as well as the presence of a local sympathetic venoarteriolar axon reflex.12 Peripheral circulation is controlled centrally by the nervous system and through local conditions in the areas of the blood vessels.13
In the extremities, resistance vessels and capacitance, small arteries/arterioles, and small veins/venules, respectively, regulate many local conditions to maintain blood pressure and vascular flow to the body's core. Contraction and relaxation of the smooth-muscle wall of these resistance vessels—many times only a single layer in thickness—is governed by the complex integration of neurologic, metabolic, and hormonal mechanisms, to name a few. Ultimately, in the case of contraction, these pathways lead to an increase in cytoplasmic smooth-muscle cell calcium-ion concentration, phosphorylation of myosin light-chain kinase (MLCK), binding of the myosin filament to the actin filament post phosphorylation, and smooth-muscle contraction.13 In relaxation, the myosin filament is dephosphorylated via myosin light-chain phosphatase (MLCP) enabling the myosin filament to detach from the actin filament.13 The balance of phosphorylation and dephosphorylation of the myosin filament via MLCK and MLCP drives blood flow through the extremities.13
Neurologically, the sympathetic branch of the ANS is the main contributor of resistance-vessel contraction via local control of norepinephrine.13 Local peripheral vasoconstriction is controlled by the input of the sympathetic branch and release of neurotransmitters such as norepinephrine, which ultimately causes an increase in MLCK activity.14 Sympathetic withdrawal and the absence of vasoconstrictive neurotransmitters permits vasodilation14 and contributes to an increase in MLCP activity. Of note, no parasympathetic innervation has been identified in peripheral vasculature.13–16
A major contributor to vasodilation, however, is through release of nitric oxide (NO) by endothelial cells. NO is released from the endothelium in response to either a shearing stimulus or a pressure stimulus.17 Insertion of an acupuncture needle into the vascularized interosseous membrane and inducing a shear response via vibration of the needle was likely a mechanism of action causing this patient's response. Moreover, it has been demonstrated that vasodilatory and vasoconstrictive responses can be propagated from smaller vessels to larger ones, such as propagation of a neurologic signal along axons.13 This can explain the wavelike warm feeling this patient experienced as vasodilation propagated down her forearm and into her hand.
Moreover, due to the extensive vascular and neurologic integration of the interosseous membrane, it is likely that afferent sensory pathways feedback to the central nervous system.16 These signals are consolidated in the medulla's nucleus tractus solitarius (NTS) that controls efferent autonomic signals.16 It is possible that the increased peripheral forearm blood flow from vasodilation propagates a sensory afferent signal to the NTS which, in turn, inhibits sympathetic efferent signals and tilting the balance to an increased parasympathetic effect.16 This could be an explanation for the decrease in the current patient's internal feeling of tremulousness and the visible decrease of tremor in her upper extremities that was observed post treatment.
This patient's improvement after becoming pregnant was consistent with the observation that, during normal pregnancy, there is an ∼50% increase in maternal blood volume, thereby inducing systemic vasodilation. Additionally, it has been found that pregnancy induces reduced peripheral vascular resistance via increased release of NO, increased vasodilatory-receptor density, increased ß-1 sensitivity and decreased α-1 sensitivity.18,19 These physiologic changes lead to a similar response, as was seen in the proposed mechanism that is induced by the acupuncture needle.
A limitation of this study was the lack of consistency between the data sets collected at the 3 appointments was an unfortunate manifestation of attempting to do science in a purely clinical setting.
CONCLUSIONS
The current authors postulate that stimulation of the interosseous membrane during treatment may result in a discharge from autonomic fibers embedded in the interosseous membrane fibers, resulting in a local increase of blood flow. Interosseous membrane stimulation is a safe, simple, and effective treatment of erythromelalgia, a condition that is notoriously refractory to treatment. The patient's response to treatment with interosseous membrane stimulation in this case may have resulted because a localized derangement of the ANS was reset. It is possible that manipulation of the autonomic milieu of an extremity is a factor in the mechanism of action of interosseous membrane stimulation.20
AUTHOR DISCLOSURE STATEMENT
No financial conflicts of interest exist.
FUNDING INFORMATION
No funding was received for this article.
REFERENCES
- 1. Ma JE, Lee JUJ, Sartori-Valinotti JC, et al. Erythromelalgia: A review of medical management options, our approach to management. Mayo Clin Proc 2023;98(1):136–149; doi: 10.1016/j.mayocp.2022.08.005 [DOI] [PubMed] [Google Scholar]
- 2. Chinn G, Guan Z. Case report and literature review: Interventional management of erythromelalgia. Transl Perioper Pain Med 2019;6(4):91–97; doi: 10.31480/2330-4871/094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis MDP. UpToDate. Erythromelalgia. UpToDate, Wolters Kluwer: Alphen aan den Rijn, Netherlands. November 3, 2023. Available from: https://www.uptodate.com/contents/erythromelalgia [Last accessed: January 1, 2024].
- 4. Freedman M, Bierwirth P. Interosseous membrane stimulation: A treatment for painful peripheral neuropathy. Med Acupunct 2023;35(2):73–75; doi: 10.1089/acu.2022.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Health Service. Erythromelalgia. England. 2023. Available from: https://www.nhs.uk/conditions/erythromelalgia/ [Last accessed February 1, 2023].
- 6. NORD. Erythromelalgia. Quincy, MA. February 1, 2023. Available from: https://rarediseases.org [Last accessed: January 1, 2024].
- 7. Norton JV, Zager E, Grady JF. Erythromelalgia: Diagnosis and classification. J Foot Ankle Surg 1999;38(3):238–241; doi: 10.1016/s1067-2516(99)80060-x [DOI] [PubMed] [Google Scholar]
- 8. Jha SK, Karna B, Goodman MB. Erythromelalgia. StatPearls, StatPearls Publishing: Treasure Island, FL; 2023. [PubMed] [Google Scholar]
- 9. Feger J, Jones J. Radiopaedia. Antebrachial fascia. 2023. Available from: https://radiopaedia.org/articles/antebrachial-fascia?lang=us [Last accessed: January 1, 2024].
- 10. Ackermann PW, Li J, Finn A, et al. Autonomic innervation of tendons, ligaments and joint capsules: A morphologic and quantitative study in the rat. J Orthop Res 2001;19(3):372–378; doi: 10.1016/S0736-0266(00)90029-9 [DOI] [PubMed] [Google Scholar]
- 11. Rein S, Esplugas M, Garcia-Elias M, et al. Immunofluorescence analysis of sensory nerve endings in the interosseous membrane of the forearm. J Anat 2020;236(5):906–915; doi: 10.1111/joa.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henriksen O. Sympathetic reflex control in human peripheral tissues. Acta Physiol Scand 1991;603(suppl):33–39. PMID: . [PubMed] [Google Scholar]
- 13. Pappano AJ, Wier WG. The Peripheral Circulation and Its Control. In: Cardiovascular Physiology, 11th ed. Baltimore, MD: Elsevier; 2019. [Google Scholar]
- 14. Koep JL, Taylor CE, Coombes JS, et al. Autonomic control of cerebral blood flow: Fundamental comparisons between peripheral and cerebrovascular circulations in humans. J Physiol 2022;600(1):15–39; doi: 10.1113/JP281058 [DOI] [PubMed] [Google Scholar]
- 15. Esteves NK, Gibson OR, Khir AW, et al. Regional thermal hyperemia in the human leg: Evidence of the importance of thermosensitive mechanisms in the control of the peripheral circulation. Physiol Rep 2021;9(15):e14953; doi: 10.14814/phy2.14953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebert TJ, Stowe DF. Neural and endothelial control of the peripheral circulation—Implications for anesthesia: Part I, neural control of the peripheral vasculature. J Cardiothorac Vasc Anesth 1996;10(1):147–158; doi: 10.1016/s1053-0770(96)80190-x [DOI] [PubMed] [Google Scholar]
- 17. Vozzi F, Bianchi F, Ahluwalia A, et al. Hydrostatic pressure and shear stress affect endothelin-1 and nitric oxide release by endothelial cells in bioreactors. Biotechnol J 2014;9(1):146–54; doi: 10.1002/biot.201300016 [DOI] [PubMed] [Google Scholar]
- 18. Reyes LM, Usselman CW, Davenport MH, et al. Sympathetic nervous system regulation in human normotensive and hypertensive pregnancies. Hypertension 2018;71(5):793–803; doi: 10.1161/HYPERTENSIONAHA.117.10766 [DOI] [PubMed] [Google Scholar]
- 19. Brislane A, Davenport MH, Steinback CD. The sympathetic nervous system in healthy and hypertensive pregnancies: Physiology or pathology? Exp Physiol 2023;108(10):1238–1244; doi: 10.1113/EP089665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim S-Y, Min S, Lee H., et al. Changes in local blood flow in response to acupuncture stimulation: A systemic review. Evid Based Complement Alternat Med 2016;2016:9874207; doi: 10.1155/2016/9874207 [DOI] [PMC free article] [PubMed] [Google Scholar]