Abstract
There are scarce data regarding the rate of the occurrence of primary open-angle glaucoma (POAG) and visible lamina cribrosa pores (LCPs) in the eyes of individuals with African ancestry; the potential impact of these features on disease burden remains unknown. We recruited subjects with POAG to the Primary Open-Angle African American Glaucoma Genetics (POAAGG) study. Through regression models, we evaluated the association between the presence of LCPs and various phenotypic features. In a multivariable analysis of 1187 glaucomatous eyes, LCPs were found to be more likely to be present in eyes with cup-to-disc ratios (CDR) of ≥0.9 (adjusted risk ratio (aRR) 1.11, 95%CI: 1.04–1.19, p = 0.005), eyes with cylindrical-shaped (aRR 1.22, 95%CI: 1.11–1.33) and bean pot (aRR 1.24, 95%CI: 1.13–1.36) cups versus conical cups (p < 0.0001), moderate cup depth (aRR 1.24, 95%CI: 1.06–1.46) and deep cups (aRR 1.27, 95%CI: 1.07–1.50) compared to shallow cups (p = 0.01), and the nasalization of central retinal vessels (aRR 1.33, 95%CI: 1.23–1.44), p < 0.0001). Eyes with LCPs were more likely to have a higher degree of African ancestry (q0), determined by means of SNP analysis (aRR 0.96, 95%CI: 0.93–0.99, p = 0.005 for per 0.1 increase in q0). Our large cohort of POAG cases of people with African ancestry showed that LCPs may be an important risk factor in identifying severe disease, potentially warranting closer monitoring by physicians.
Keywords: lamina cribrosa pores, primary open-angle glaucoma, African ancestry, stereo optic disc images
1. Introduction
Glaucoma describes a group of eye diseases that progressively damage the optic nerve, subsequently causing the loss of visual fields. Glaucoma is the leading cause of irreversible blindness worldwide [1]. Primary open-angle glaucoma (POAG), the most common form of glaucoma, disproportionately affects African-ancestry individuals. These individuals are 4 to 5 times more likely to have POAG and up to 15 times more likely to experience vision loss from the disease when compared with European Americans [2,3,4]. Prior studies have investigated genetic and other risk factors that contribute to the disproportionate burden of POAG in African-ancestry populations [1,5,6]. One study suggested that structural differences in the optic nerve head (ONH) contribute to the increased prevalence and severity of POAG in these individuals [7]. It has also been demonstrated that African Americans with ocular hypertension have significantly larger optic discs, optic cups, and cup-to-disc ratios (CDR) when compared with other ancestral groups [8].
The lamina cribrosa (LC) is the porous collagen structure, located at the ONH, that provides entry and exit points for the blood vessels of the retina [9]. The LC is fixed between two pressurized compartments (ocular and craniospinal) and experiences a translaminar pressure difference (TPD) that makes it the primary site of retinal ganglion cell injury in TPD-related pathologies such as POAG; however, the extent to which these findings are linked to a higher risk of glaucoma, or its progression, is lacking [10,11,12]. Structural changes to the LC in glaucomatous eyes have been reported, including more frequent slit-shaped lamina cribrosa (LCPs), posterior displacement of the LC, and thinner LC [13,14]. The total number of LCPs can simultaneously increase as neural rim damage occurs in both POAG and normal-tension glaucoma [15]. LCPs in glaucomatous eyes have a more tortuous pathway when compared to healthy control eyes [16]. Additionally, LCPs are visible in 71% of POAG patients compared to 29.3% of controls [17]. Although several studies have characterized the changes to the LC in patients with glaucoma, few have investigated the associations between LCPs and demographic and ocular characteristics in African-ancestry populations [18,19,20]. Existing data show that African-ancestry individuals have a larger total LC area and a greater number of LCPs; however, there is no evidence linking these findings to a higher risk of glaucoma [5,16]. These studies have been limited by small sample sizes.
In this study, we investigated the prevalence of and factors associated with the presence of visible optic disc LCPs in a large cohort of African-ancestry individuals. Our aim was to discover the factors linked to visible LCPs in order to aid in identifying eyes with severe disease that potentially warrant closer monitoring by physicians.
2. Materials and Methods
2.1. Subject Recruitment and Clinical Assessment
The POAAGG study was an NIH-funded study that sought to elucidate the genetic architecture of POAG in an African-ancestry cohort. All subjects were ≥35 years and self-identified as Black (African ancestry, Afro-Caribbean, or African American). University of Pennsylvania (UPenn)-certified clinical research coordinators identified subjects during clinical visits to the Scheie Eye Institute, Perelman Center for Advanced Medicine, Philadelphia VA Medical Center, and Mercy Fitzgerald Hospital, as well as two neighboring ophthalmology clinics in Philadelphia, Pennsylvania (Windell Murphy, MD; Temple University) [21]. Additionally, a multimedia marketing campaign was employed with trusted African-ancestry community leaders to disseminate information and build trust in the community [22]. Exclusion criteria have been described previously [23].
At enrollment, subjects received an ophthalmic examination and onsite interviews. Subject data captured from the interview and examination were stored in the Research Electronic Data Capture (REDCap) database [24]. Demographic and lifestyle information were collected during the onsite interview. Family-related and past medical history were also obtained during the interview and extracted from patient medical records from the UPenn EPIC and MERGE systems.
Each subject was classified as a glaucoma case, suspect, or control by a fellowship-trained glaucoma specialist. In brief, cases were defined as having an open iridocorneal angle and characteristic optic nerve defects with corresponding visual field loss [23]. All subjects signed an informed consent form and provided a DNA sample. The University of Pennsylvania Institutional Review Board approved the study and the informed consent process, and the research adhered to the tenets of the Declaration of Helsinki.
2.2. Grading of Color Stereo Images of the ONH
Images were captured using the Topcon TRC 50EX retinal camera (Topcon Corp. of America, Oakland, NJ, USA) during the examination. Thirty-degree color stereo disc photographs of POAAGG subjects were uploaded to a secure server at the Scheie Image Reading Center at UPenn. The images were taken between 13 January 2004 and 25 June 2019, and were uploaded to the Scheie Image Reading Center server between 22 January 2016 and 20 April 2021. Grading occurred at the Reading Center between 6 June 2016 and 10 May 2021.
The images were analyzed by two non-physician POAAGG-certified graders to check for quantitative and qualitative phenotypic features. The grading process and its reliability have been described previously [25,26]. In brief, three non-physician graders trained by two glaucoma specialists evaluated images using a stereo viewer (Screen-Vu stereoscope, Eyesupply USA, Inc. Tampa, FL, USA). Differences in grader evaluations were reviewed and adjudicated by the Director of the Reading Center (ED), an ophthalmologist [26]. The qualitative grading process has been described in detail previously [26]. Briefly, two graders completed a standardized grading form for each stereo image pair (stereo images provide a 3D visual to qualitatively assess ocular features including cup depth) in order to determine image quality; disc shape; the shape of the cup; the presence of a tilted disc; disc hemorrhages and their location; arteriolar narrowing; both beta- and alpha-peripapillary atrophy; the narrowing of venules; the bayoneting of vessels; the pallor of the neural rim; the depth of the cup; rim plane position; the baring of the LC (grader determines if there are 0, ≤3, or >3 honeycomb-appearing visual pores); circumlinear vessels; sloping toward the outer rim of the disc; cilioretinal vessels; and gray crescents. The Reading Center Director also adjudicated disagreements between graders in terms of qualitative grading.
2.3. Re-Grading for LCPs
In order to make the data more robust and prevent the misdiagnosis of LCPs, we re-graded images with LCPs where one or both original graders reported >3 pores or where both agreed that there were ≤3 (n = 2703). Between 31 March 2022 and 8 August 2022, a third, independent grader used an improved grading protocol with clear examples of LCPs and simplified the grading response to a binary value, with “Yes” for >3 pores and “No” for ≤3 pores. We used the presence of three pores as a threshold. This is because, typically, if there are at least three pores present, there will be multiple beyond that point (Figure 1). We created a spreadsheet containing the data regarding 3142 eyes, including 2703 eyes from regrading and 439 eyes where the original graders agreed the classification was “None”. Eyes marked “None” were excluded from the regrading due to higher degrees of intergrader reliability, as seen in previous papers [25]. We removed 199 images after being determined as ungradable, bringing the final total to 2943 eyes from 1551 patients for our analysis.
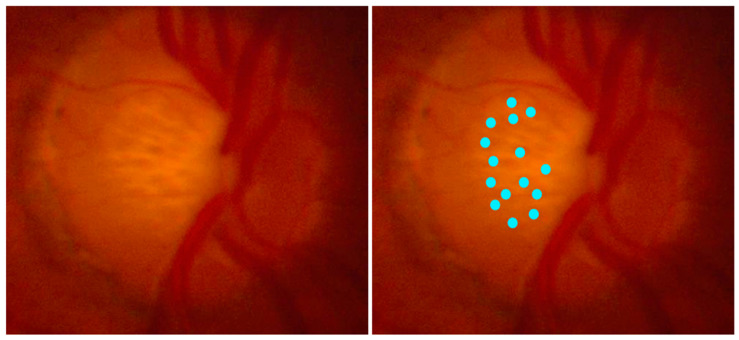
Figure 1.
Fundoscopic image of optic nerve head with multiple LCPs (left). Blue dots highlighting the location of LCPs at the ONH (right).
2.4. Specimen Collection and Ancestry Analysis
Peripheral blood or saliva samples were collected from all study subjects to perform genomic analysis [1,27]. Genotyping was conducted using the Multi-Ethnic Genotyping Array (MEGA)V2 (EX) consortium chip on the Infinium iSelect platform produced by Illumina FastTrack Services (Illumina, San Diego, CA, USA). Directly genotyped variants and samples were subjected to rigorous quality control, as detailed elsewhere [27].
After quality control, 1,108,459 SNPs were analyzed to determine continental ancestry using the 1000 Genomes Project version 5a dataset, as detailed in a previous publication [1]. This dataset contains five continental populations: African, Admixed American, East Asian, European, and South Asian. In brief, principal components were extracted from the genetic relationship matrix, using PLINK (version 1.90), in order to analyze continental ancestry. FastSTRUCTURE software (version 1.0) detected two-way admixture proportions among autosomal genotypes in POAAGG using two ancestral populations as a model [28]. This generated ancestral components q0 and q1, representing African and European ancestral components, respectively. The variables are continuous and sum to 1.0, with lower values of q0 denoting higher degrees of African ancestry and lower degrees of European ancestry [1]. In a previous publication, we found that the cases examined had a significantly lower mean value of q0 compared to controls, indicating a greater degree of African ancestry [1].
2.5. Genetic Analysis
A previous genome-wide association study (GWAS) identified potential causal loci for POAG in 11,275 individuals of African ancestry (6003 cases, 5272 controls). This mega-analysis included subjects from the African Descent and Glaucoma Evaluation Study (n = 1999) [29], the Genetics of Glaucoma in People of African Descent (GGLAD) consortium (n = 2952) [30], and the POAAGG study (n = 6324). A total of 46 risk loci were associated with POAG at a level of genome-wide significance. Replication analyses, trait colocalization analyses, and functional studies implicated three likely causal loci: rs1666698 located in DBF4P2, rs34957764 in ROCK1P1, and rs11824032 in ARHGEF12. For each SNP, we classified individuals based on the allele frequency (0, 1, 2) of risk gene. A total of 1966 eyes were evaluated in a univariable logistic regression analysis to determine the association between these SNPs and LCPs. Inter-eye correlation was accounted for using a GEE model.
2.6. Statistical Analysis
We used univariable Poisson regression models to evaluate the association between LCPs and demographic, ocular, clinical, and genetic characteristics among POAG cases. Cases were specifically chosen to understand LCPs as markers of a disease’s severity and its relationship with other glaucomatous features. Multivariable analyses were performed using a backward stepwise variable selection utilizing variables with p < 0.20, which were drawn from univariate analyses. Two separate multivariate analyses were reported (with and without consideration of clinical phenotype data) as there was a lack of detailed clinical phenotypic data for some POAG patients. All models accounted for inter-eye correlation by using generalized estimation equations (GEE). We reported risks ratios (RR), their 95%CI, and p-values for risk factors, drawing from univariable Poisson regression models and adjusted risk ratios (aRRs) from multivariable analyses. All statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
Demographic characteristics are described in Table 1. A total of 2117 eyes (71.9%) had LCPs. Individuals with a moderate BMI range of 25–30 had higher risk of LCPs, as opposed to values on both extremes (BMI < 25 and BMI ≥ 30) (p = 0.048). Higher degrees of African ancestry, as shown by lower values of the q0 variable, were associated with LCPs (RR = 0.97, 95%CI: 0.95–0.99, p = 0.006). Hypertension conferred a lower risk of LCPs (RR = 0.92, 95%CI: 0.87–0.98, p = 0.01).
Table 1.
Univariable analysis of the association of demographic features and visible pores in the Lamina Cribrosa among POAAGG glaucoma cases (n = 2943 eyes).
| n | Visible Pores in the Lamina Cribrosa (n = 2117 Eyes) | RR (95%CI) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | ≤60 | 682 | 511 (74.9%) | Ref | 0.08 |
| (60, 70] | 836 | 618 (73.9%) | 0.99 (0.92, 1.06) | ||
| (70, 80] | 880 | 606 (68.9%) | 0.92 (0.85, 0.99) | ||
| ≥80 | 545 | 382 (70.1%) | 0.94 (0.86, 1.02) | ||
| Sex | Male | 1213 | 890 (73.4%) | Ref | 0.22 |
| Female | 1730 | 1227 (70.9%) | 0.97 (0.92, 1.02) | ||
| Body mass index | <25 | 689 | 502 (72.9%) | 0.97 (0.91, 1.04) | 0.048 |
| 25–30 | 979 | 732 (74.8%) | Ref | ||
| ≥30 | 1275 | 883 (69.3%) | 0.93 (0.87, 0.99) | ||
| Diabetes | No | 1794 | 1312 (73.1%) | Ref | 0.14 |
| Yes | 1143 | 801 (70.1%) | 0.96 (0.91, 1.01) | ||
| Missing | 6 | 4 | |||
| q0 (per 0.1 increase in q0) | 1807 | 1278 (70.7%) | 0.97 (0.95,0.99) | 0.006 | |
| Hypertension | No | 641 | 490 (76.4%) | Ref | 0.01 |
| Yes | 2296 | 1623 (70.7%) | 0.92 (0.87, 0.98) | ||
| Missing | 6 | 4 | |||
| Family history of glaucoma | No | 1191 | 847 (71.1%) | Ref | 0.63 |
| Yes | 1599 | 1153 (72.1%) | 1.01 (0.96, 1.07) | ||
| Missing | 153 | 117 | |||
| Alcohol use | No | 1556 | 1114 (71.6%) | Ref | 0.79 |
| Yes | 1346 | 971 (72.1%) | 1.01 (0.95, 1.06) | ||
| Missing | 41 | 32 | |||
| Tobacco use | No | 1339 | 982 (73.3%) | Ref | 0.19 |
| Yes | 1604 | 1135 (70.8%) | 0.96 (0.91, 1.02) | ||
| Previous glaucoma surgery | No | 2067 | 1497 (72.4%) | Ref | 0.43 |
| Yes | 868 | 614 (70.7%) | 0.98 (0.92, 1.04) | ||
| Missing | 8 | 6 | |||
The associations between qualitative ocular phenotypes and LCPs are shown in Table 2. LCPs were less likely to be present in eyes with an oval disc shape (RR = 0.92, 95%CI: 0.87–0.96, p = 0.003) compared to those with a round shape. Eyes with LCPs were also more likely to display the nasalization of the central vessels (RR = 1.39, 95%CI: 1.32–1.45, p < 0.0001), large CDR ≥ 0.9 (RR = 1.29, 95%CI: 1.20–1.38, p < 0.0001) or the bayoneting of vessels (RR = 1.24, 95%CI: 1.19–1.30, p < 0.0001), with moderate (RR = 1.45, 95%CI: 1.28–1.65, p < 0.0001) or deep cup depth (RR = 1.77, 95%CI: 1.56–2.01, p < 0.0001) compared to shallow depth, and bean pot/partial bean pot-shaped cups (RR = 1.59, 95%CI: 1.49–1.69, p < 0.0001) or cylinder-shaped cups (RR = 1.33, 95%CI: 1.25–1.42, p < 0.0001) compared to cone-shaped cups.
Table 2.
Univariable analysis of the associations between disc features determined by central reading center and visible pores in the Lamina Cribrosa among POAAGG glaucoma cases (n = 2943 eyes).
| n | Visible Pores in the Lamina Cribrosa (n = 2117 Eyes) | RR (95%CI) | p-Value | ||
|---|---|---|---|---|---|
| Disc shape | Round | 1247 | 940 (75.4%) | Ref | 0.003 |
| Oval | 1592 | 1102 (69.2%) | 0.92 (0.87, 0.96) | ||
| Other | 10 | 6 (60.0%) | 0.80 (0.45, 1.42) | ||
| Missing | 94 | 69 | |||
| Cup disc ratio | <0.9 | 1003 | 683 (68.1%) | Ref | <0.0001 |
| ≥0.9 | 268 | 235 (87.7%) | 1.29 (1.20, 1.38) | ||
| Missing | 1672 | 1199 | |||
| Shape of cup | Conical | 1107 | 645 (58.3%) | Ref | <0.0001 |
| Cylindrical | 1241 | 965 (77.8%) | 1.33 (1.25, 1.42) | ||
| Bean pot/partial bean pot | 395 | 365 (92.4%) | 1.59 (1.49, 1.69) | ||
| Other | 14 | 8 (57.1%) | 0.98 (0.62, 1.55) | ||
| Missing | 186 | 134 | |||
| Cup depth | Shallow | 317 | 154 (48.6%) | Ref | <0.0001 |
| Moderate | 1737 | 1225 (70.5%) | 1.45 (1.28, 1.65) | ||
| Deep | 704 | 605 (85.9%) | 1.77 (1.56, 2.01) | ||
| Missing | 185 | 133 | |||
| Tilted disc | No | 2547 | 1832 (71.9%) | Ref | 0.80 |
| Yes | 275 | 200 (72.7%) | 1.01 (0.93, 1.10) | ||
| Missing | 121 | 85 | |||
| Disc hemorrhage | No | 2802 | 2017 (72.0%) | Ref | 0.29 |
| Yes | 48 | 31 (64.6%) | 0.90 (0.73, 1.11) | ||
| Missing | 93 | 69 | |||
| Arteriole narrowing | No | 2802 | 2010 (71.7%) | Ref | 0.22 |
| Yes | 48 | 38 (79.2%) | 1.10 (0.95, 1.28) | ||
| Missing | 93 | 69 | |||
| Beta parapapillary atrophy | No | 856 | 627 (73.2%) | Ref | 0.36 |
| Yes | 2087 | 1490 (71.4%) | 0.97 (0.92, 1.03) | ||
| Venule narrowing | No | 2806 | 2016 (71.8%) | Ref | 0.90 |
| Yes | 44 | 32 (72.7%) | 1.01 (0.84, 1.21) | ||
| Missing | 93 | 69 | |||
| Bayonetting | No | 2462 | 1703 (69.2%) | Ref | <0.0001 |
| Yes | 481 | 414 (86.1%) | 1.24 (1.19, 1.30) | ||
| Nasalization of central vessels | No | 1751 | 1095 (62.5%) | Ref | <0.0001 |
| Yes | 1095 | 949 (86.7%) | 1.39 (1.32, 1.45) | ||
| Missing | 97 | 73 | |||
| Pallor of the neural rim | No | 2730 | 1958 (71.7%) | Ref | 0.37 |
| Yes | 119 | 90 (75.6%) | 1.05 (0.94, 1.18) | ||
| Missing | 94 | 69 | |||
The associations of the available clinical phenotypes that were obtained within a ±90-day range of the image date are shown in Table 3. Eyes with LCPs had a significantly larger mean CDR (0.76 versus 0.62, p < 0.0001) and worse visual field mean deviation (−8.72 dB versus −6.35 dB, p = 0.007) than eyes without LCPs.
Table 3.
Univariable analysis of the association of clinical measures and visible pores in the Lamina Cribrosa among POAAGG glaucoma cases (n = 2943 eyes).
| Visible Lamina Cribrosa Pores (n = 2117 Eyes) |
No Visible Lamina Cribrosa Pores (n = 826 Eyes) |
p-Value | |
|---|---|---|---|
| Highest IOP (mmHg) | |||
| N | 1149 | 441 | 0.46 |
| Mean (SD) | 19.15 (6.56) | 19.47 (6.76) | |
| Range | (2.00, 56.00) | (7.00, 52.00) | |
| Central corneal thickness (µm) | |||
| N | 869 | 332 | 0.28 |
| Mean (SD) | 533.43 (38.34) | 536.71 (40.06) | |
| Range | (420.00, 690.00) | (433.00, 659.00) | |
| Cup disc ratio | |||
| N | 918 | 353 | <0.0001 |
| Mean (SD) | 0.76 (0.15) | 0.62 (0.20) | |
| Range | (0.10, 1.00) | (0.10, 1.00) | |
| Visual acuity (logMAR) | |||
| N | 872 | 328 | 0.56 |
| Mean (SD) | 0.31 (0.66) | 0.29 (0.62) | |
| Range | (−0.12, 5.00) | (−0.12, 6.00) | |
| Nerve Fiber layer thickness (µm) | |||
| N | 410 | 159 | 0.16 |
| Mean (SD) | 73.38 (14.69) | 75.38 (12.38) | |
| Range | (38.00, 120.00) | (46.00, 100.00) | |
| Visual field MD | |||
| N | 489 | 200 | 0.007 |
| Mean (SD) | −8.72 (9.59) | −6.35 (8.71) | |
| Range | (−33.15, 22.08) | (−32.00, 21.01) | |
The results of our genetic variant analysis are included in Supplemental Table S1. The univariable analysis of genetic variants and LCPs in glaucoma cases did not unveil significant associations between the studied SNPs and LCPs.
We performed multivariate analyses, considering variables with p < 0.20 in the univariate analysis, in a backward variable selection model. Table 4 shows the results when all variables from the univariate analysis were included, resulting in a total of 654 eyes, of which 457 had LCPs (69.9%). CDRs ≥ 0.9 (aRR = 1.21, 95%CI: 1.11–1.33, p < 0.0001), th nasalization of central vessels (aRR = 1.34, 95%CI: 1.20–1.48, p < 0.0001), and lower q0 values (aRR = 0.96, 95%CI: 0.93–0.99, p = 0.005) were all significantly associated with LCPs. Cylindrical-shaped cups (aRR = 1.41, 95%CI: 1.24–1.61) and bean pot/partial bean pot cups (aRR = 1.36, 95%CI: 1.19–1.55) were more likely to have LCPs when compared to cone-shaped cups (p < 0.0001).
Table 4.
Multivariable analysis of the associations between features and visible pores in the Lamina Cribrosa among POAAGG glaucoma cases with complete data that included q0 (n = 654 eyes).
| n | Visible Pores in Lamina Cribrosa Present (n = 457 Eyes) | aRR (95%CI) | p-Value * | ||
|---|---|---|---|---|---|
| Cup Disc Ratio | <0.9 | 526 | 342 (65.0%) | Ref | <0.0001 |
| ≥0.9 | 128 | 115 (89.8%) | 1.21 (1.11, 1.33) | ||
| Shape of cup | Conical | 277 | 149 (53.8%) | Ref | <0.0001 |
| Cylindrical | 273 | 214 (78.4%) | 1.41 (1.24, 1.61) | ||
| Bean pot/partial bean pot | 100 | 92 (92.0%) | 1.36 (1.19, 1.55) | ||
| Other | 4 | 2 (50.0%) | 1.07 (0.38, 3.01) | ||
| Nasalization of central vessels | No | 387 | 225 (58.1%) | Ref | <0.0001 |
| Yes | 267 | 232 (86.9%) | 1.34 (1.20, 1.48) | ||
| q0 (per 0.1 increase in q0) | 645 | 457 (69.9%) | 0.96 (0.93, 0.99) | 0.005 | |
The analysis shown in Table 5 excluded q0, yielding 1187 total eyes, of which 856 (72.1%) had LCPs. The results were similar to those obtained when ancestry was included, which are shown in Table 5. LCPs were more likely to be present in eyes with a cylindrical cup shape (aRR = 1.22, 95%CI: 1.11–1.33) and bean pot/partial bean pot-shaped cups (aRR = 1.24, 95%CI: 1.13–1.36) when compared to the cone-shaped cups (p < 0.0001), as well as during the nasalization of the central retinal vessels (aRR = 1.33, 95%CI: 1.23–1.44, p < 0.0001), CDRs ≥ 0.9 (aRR = 1.11, 95%CI: 1.04–1.19, p = 0.005), and lastly with moderate (aRR = 1.24, 95%CI: 1.06–1.46) or deep cup depths (aRR = 1.27, 95%CI: 1.07–1.50) compared to shallow depths (p = 0.01).
Table 5.
Multivariable analysis of the associations between disc features and visible pores in the Lamina Cribrosa among POAAGG glaucoma cases with complete data that did not include q0 (n = 1187 eyes).
| n | Visible Pores in Lamina Cribrosa (n = 856 Eyes) | aRR (95%CI) | p-Value * | ||
|---|---|---|---|---|---|
| Cup Disc Ratio | <0.9 | 945 | 644 (68.1%) | Ref | 0.005 |
| ≥0.9 | 242 | 212 (87.6%) | 1.11 (1.04, 1.19) | ||
| Shape of cup | Conical | 525 | 317 (60.4%) | Ref | <0.0001 |
| Cylindrical | 480 | 372 (77.5%) | 1.22 (1.11, 1.33) | ||
| Bean Pot/Partial Bean Pot | 174 | 162 (93.1%) | 1.24 (1.13, 1.36) | ||
| Other | 8 | 5 (62.5%) | 1.06 (0.65, 1.72) | ||
| Cup depth | Shallow | 167 | 86 (51.5%) | Ref | 0.01 |
| Moderate | 735 | 520 (70.7%) | 1.24 (1.06, 1.46) | ||
| Deep | 285 | 250 (87.7%) | 1.27 (1.07, 1.50) | ||
| Nasalization of central vessels | No | 673 | 405 (60.2%) | Ref | <0.0001 |
| Yes | 514 | 451 (87.7%) | 1.33 (1.23, 1.44) |
4. Discussion
In this study, we investigated the prevalence and risk factors associated with the presence of visible LCPs in a large African-ancestry cohort. We showed that LCPs were associated with extremely large CDR, deep optic disc cups, cylindrical and bean pot-shaped cups, the nasalization of central retinal vessels, and higher degrees of African ancestry. Our univariate analysis initially suggested an association between LCPs and moderate BMI, but this was not supported in multivariate analysis. The impact of BMI on LC morphology remains unclear; a recent study found no difference in LC depth or thickness between obese and non-obese individuals [31].
We noted that several morphological changes to the optic cup were associated with the presence of visible LCPs. These LCP-associated cup features could be surrogates for the thinning of LC, the posterior displacement of LC, laminar deformation, and the curvature of LC, which have been described by Swept Source Optical Coherence Tomography [29]. Thinner LC is a risk factor that can cause normal-tension glaucoma suspects to convert to glaucoma cases [30]. LC thinning has also been associated with large CDRs and decreased vessel density in glaucoma suspects [32,33,34]. LC parameters such as cup depth, LC depth, prelaminar tissue thickness, and LC curvature index were associated with higher IOPs and thinner nerve fiber layers [35], as well as with a faster rate of RNFL loss [36]. Depth variations in the cup floor also differed between groups of normal subjects and glaucoma patients. This surface variability at the floor of the optic cup may represent a measurement of LC fragility that has been implicated, but not previously estimated, in glaucomatous eyes [37]. Glaucomatous eyes appear to undergo multiple changes in the LC, which may predispose patients to or be the result of other phenotypic changes.
Cone-shaped cups, used as a reference in this study, have a gradually sloping wall and may undergo the least deformation of the LC. Cylinder-shaped cups and bean pot-shaped cups possibly have more severe deformation of the LC. Although this has not been proven, longitudinal studies of glaucomatous eyes with imaging moralities such as SD-OCT should inform us in the future as to whether conical cups progress to cylinder-shaped cups and then to bean pot cups—and whether the LCPs start appearing at this point. The depth of the cups was independently associated with the presence of visible LCPs after adjusting for the shape of cups. OCT emerged as a comprehensive modality capable of more fully assessing LCPs. One such study showed an increased ratio of beam thickness to pore diameter and enhanced pore diameter variability in glaucomatous eyes compared to healthy controls [38]. To our knowledge, no study has compared the visibility of LCPs in OCT to traditional methods; however, OCT’s capability to assess microstructural features provides more robust data [32].
Visual field loss has been associated with elongated LCPs in glaucomatous patients; pores become more elongated and less circular with increasing field loss [39]. Small, round pores were associated with mild field loss, oval pores with moderate field loss, and striate or slit-shaped pores with advanced field loss [14]. We did not characterize the LCP shapes in this study, but did find a significant association between the presence of visible LCPs and worse visual fields in the univariate analysis.
We were unable to find an association between the three SNPs previously implicated in a large GWAS of POAG. Thus, our results suggest that these specific SNPs may not be determinants for the visibility of LCPs in African-ancestry patients. To our knowledge, no other study has explored genetic associations with visible LCPs. Future studies with larger sample sizes or additional variants may be warranted in order to explore potential associations more comprehensively.
A strong association was found between visible LCPs and the nasalization of central vessel trunk (CRVT) that had not been reported previously. The nasal displacement of CRVT has been reported as helping to predict the progression of central visual fields [40,41]. CRV has a nasally angled pathway through the LC [42], but it appears that the more nasally located CRVT in glaucoma is present in the prelaminar area and not in the LC deformation area [43].
We found that lower q0 values, which corresponded with higher degrees of African ancestry, were associated with the presence of visible LCPs. The optic disc features in blacks and whites with normal IOPs were different, with larger cup volumes and CDRs present in blacks [44]. In a large cohort of subjects with ocular hypertension, African Americans had significantly larger optic discs, optic cups, neuroretinal rims, and CDRs than other ancestral groups [8]. A number of studies on glaucoma subjects have shown that blacks display greater progression of glaucoma-related damage, a greater incidence of hemorrhage in and around the disc, earlier onset of POAG, greater prevalence and higher risk of IOPs, higher risk of reoperation after MIGS procedures, lower utilization of eye care services, and greater rates of blindness when compared to whites [45,46,47,48,49,50,51,52,53].
The relationship between morphological changes in the LC between African-descent (AD) and European-descent (ED) subjects was not consistent. While anterior lamina cribrosa surface depth (ALCSD) was found to be deeper in AD than ED glaucoma patients in a cross-sectional study [54], a different result was observed in a longitudinal study among glaucoma patients, where the migration of ALCSD was observed to be more posterior in ED compared to AD subjects [55,56]. Other studies showed that, in healthy individuals, African-descent groups had a greater acute posterior bowing of the LC and thinner LC [57,58]. Experiments on healthy eyes received from eye banks showed that, even though blacks had larger total LC area and a greater number of laminar pores than whites, the connective tissue proportion and pore size distribution in the laminae cribrosae of AD individuals were almost identical to those of ED individuals [59]. Ethnic differences in optic disc features in healthy eyes are not limited to blacks and whites, but also includes Chinese and Indian individuals, with Chinese people showing a deeper LC [60].
Our study has multiple limitations to note. There is variability in the treatment status and treatment type among cases, which could affect associations between LCPs and variables such as IOP. Next, the grading of cup features is subjective, and therefore less reliable when compared to objective processes. There were other features, such as axial eye length or other refractive measures, that we were unable to assess in this study; these features may have an effect on LCPs visibility and subsequent grader evaluation. We used double grading by experienced graders and the adjudication of discrepancies by an ophthalmologist to provide a robust evaluation. Additionally, it should be noted that the LC morphology continued to change, particularly in eyes with glaucoma, as deformation and remodeling of the neural and connective tissues of the optic nerve head took place [61]. Therefore, cross-sectional studies such as this are limited in our interpretation. Similarly, as our data were cross-sectional, we were unable to determine causality in terms of our significant associations. We lacked other ethnic groups to draw comparisons from, which may limit the generalizability of our study. We chose to focus exclusively on individuals of African ancestry with POAG in order to address the large disparities in terms of the prevalence of the condition and vision loss faced by this understudied population. Lastly, we opted to use data within a 90-day range of the subjects’ first imaging date. Many of the early images of eyes do not have corresponding phenotypic data in this range and as such, numerous data are in the “missing category”.
In conclusion, in our large cohort of glaucoma cases among individuals of African ancestry, we show that LCPs are associated with greater degrees of optic disc cupping and higher proportions of African ancestry. These results should inform those who manage patients with glaucoma, encouraging them to carefully follow patients who manifest LCPs in order to allow for timely treatment to prevent progression of the disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vision8020024/s1, Supplemental Table S1. Univariable analysis of the association of genetic variants and visible pores in the Lamina Cribrosa among POAAGG glaucoma cases (n = 1966 eyes).
Author Contributions
Conceptualization: J.A.J., E.D., J.M.O.; Data curation: E.D., Y.C., Y.Z., E.J.S., R.L. and G.-S.Y.; Formal Analysis: Y.C., Y.Z., D.Z. and G.-S.Y.; Funding acquisition: J.M.O.; Investigation: J.A.J., E.D., R.J.S., E.M.-E., V.A., P.S.S., E.J.S. and J.M.O.; Resources: J.M.O.; Supervision: E.D., G.-S.Y. and J.M.O.; Visualization: J.A.J., E.D., R.J.S., Y.C. and D.Z.; Writing—original draft: J.A.J. and E.D.; Writing—review & editing: all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of The University of Pennsylvania (IRB protocol #812036).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Eye Institute, Bethesda, Maryland (grant #1RO1EY023557) and Vision Research Core Grant (P30 EY001583). Funds also come from the Jeffrey W. Berger Research Award, Minority Ophthalmology Mentoring Program Research Grant, F.M. Kirby Foundation, Research to Prevent Blindness, The UPenn Hospital Board of Women Visitors, and The Paul and Evanina Bell Mackall Foundation Trust. The Ophthalmology Department at the Perelman School of Medicine and the VA Hospital in Philadelphia, PA also provided support. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cole B.S., Gudiseva H.V., Pistilli M., Salowe R., McHugh C.P., Zody M.C., Chavali V.R.M., Ying G.S., Moore J.H., O’Brien J.M. The Role of Genetic Ancestry as a Risk Factor for Primary Open-Angle Glaucoma in African Americans. Investig. Ophthalmol. Vis. Sci. 2021;62:28. doi: 10.1167/iovs.62.2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broman A.T., Quigley H.A., West S.K., Katz J., Munoz B., Bandeen-Roche K., Tielsch J.M., Friedman D.S., Crowston J., Taylor H.R., et al. Estimating the Rate of Progressive Visual Field Damage in Those with Open-Angle Glaucoma, from Cross-Sectional Data. Investig. Ophthalmol. Vis. Sci. 2008;49:66. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tielsch J.M. Racial Variations in the Prevalence of Primary Open-Angle Glaucoma: The Baltimore Eye Survey. JAMA. 1991;266:369. doi: 10.1001/jama.1991.03470030069026. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz B. Causes of Blindness and Visual Impairment in a Population of Older AmericansThe Salisbury Eye Evaluation Study. Arch. Ophthalmol. 2000;118:819. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A., Tielsch J.M., Katz J., Quigley H.A., Gottsch J.D., Javitt J.C., Martone J.F., Royall R.M., Witt K.A., Ezrine S. Racial Differences in the Cause-Specific Prevalence of Blindness in East Baltimore. N. Engl. J. Med. 1991;325:1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Amero K.K., González A.M., Osman E.A., Larruga J.M., Cabrera V.M., Al-Obeidan S.A. Mitochondrial DNA Lineages of African Origin Confer Susceptibility to Primary Open-Angle Glaucoma in Saudi Patients. Mol. Vis. 2011;17:1468–1472. [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai C., Zangwill L., Gonzalez C., Irak I., Garden V., Hoffman R., Weinreb R. Ethnic Differences in Optic Nerve Head Topography. J. Glaucoma. 1995;4:248–257. doi: 10.1097/00061198-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zangwill L.M., Weinreb R.N., Berry C.C., Smith A.R., Dirkes K.A., Coleman A.L., Piltz-Seymour J.R., Liebmann J.M., Cioffi G.A., Trick G., et al. Racial Differences in Optic Disc Topography: Baseline Results from the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch. Ophthalmol. 2004;122:22–28. doi: 10.1001/archopht.122.1.22. [DOI] [PubMed] [Google Scholar]
- 9.Quigley H.A., Addicks E.M., Green W.R., Maumenee A.E. Optic Nerve Damage in Human Glaucoma. II. The Site of Injury and Susceptibility to Damage. Arch. Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 10.Morgan W.H., Yu D.Y., Alder V.A., Cringle S.J., Cooper R.L., House P.H., Constable I.J. The Correlation between Cerebrospinal Fluid Pressure and Retrolaminar Tissue Pressure. Investig. Ophthalmol. Vis. Sci. 1998;39:1419–1428. [PubMed] [Google Scholar]
- 11.Morgan W.H., Chauhan B.C., Yu D.-Y., Cringle S.J., Alder V.A., House P.H. Optic Disc Movement with Variations in Intraocular and Cerebrospinal Fluid Pressure. Investig. Ophthalmol. Vis. Sci. 2002;43:3236–3242. [PubMed] [Google Scholar]
- 12.Siaudvytyte L., Januleviciene I., Daveckaite A., Ragauskas A., Bartusis L., Kucinoviene J., Siesky B., Harris A. Literature Review and Meta-Analysis of Translaminar Pressure Difference in Open-Angle Glaucoma. Eye. 2015;29:1242–1250. doi: 10.1038/eye.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoji T., Kuroda H., Suzuki M., Ibuki H., Araie M., Yoneya S. Glaucomatous Changes in Lamina Pores Shape within the Lamina Cribrosa Using Wide Bandwidth, Femtosecond Mode-Locked Laser OCT. PLoS ONE. 2017;12:e0181675. doi: 10.1371/journal.pone.0181675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller K.M., Quigley H.A. The Clinical Appearance of the Lamina Cribrosa as a Function of the Extent of Glaucomatous Optic Nerve Damage. Ophthalmology. 1988;95:135–138. doi: 10.1016/S0161-6420(88)33219-7. [DOI] [PubMed] [Google Scholar]
- 15.Tezel G., Trinkaus K., Wax M.B. Alterations in the Morphology of Lamina Cribrosa Pores in Glaucomatous Eyes. Br. J. Ophthalmol. 2004;88:251–256. doi: 10.1136/bjo.2003.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B., Lucy K.A., Schuman J.S., Sigal I.A., Bilonick R.A., Lu C., Liu J., Grulkowski I., Nadler Z., Ishikawa H., et al. Tortuous Pore Path Through the Glaucomatous Lamina Cribrosa. Sci. Rep. 2018;8:7281. doi: 10.1038/s41598-018-25645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healey P.R., Mitchell P. Visibility of Lamina Cribrosa Pores and Open-Angle Glaucoma. Am. J. Ophthalmol. 2004;138:871–872. doi: 10.1016/j.ajo.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Tian H., Li L., Song F. Study on the Deformations of the Lamina Cribrosa during Glaucoma. Acta Biomater. 2017;55:340–348. doi: 10.1016/j.actbio.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Glidai Y., Lucy K.A., Schuman J.S., Alexopoulos P., Wang B., Wu M., Liu M., Vande Geest J.P., Kollech H.G., Lee T., et al. Microstructural Deformations within the Depth of the Lamina Cribrosa in Response to Acute In Vivo Intraocular Pressure Modulation. Investig. Ophthalmol. Vis. Sci. 2022;63:25. doi: 10.1167/iovs.63.5.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiumehr S., Park S.C., Dorairaj S., Teng C.C., Tello C., Liebmann J.M., Ritch R. In Vivo Evaluation of Focal Lamina Cribrosa Defects in Glaucoma. Arch. Ophthalmol. 2012;130:552–559. doi: 10.1001/archopthalmol.2011.1309. [DOI] [PubMed] [Google Scholar]
- 21.Salowe R.J., Lee R., Zenebe-Gete S., Vaughn M., Gudiseva H.V., Pistilli M., Kikut A., Becker E., Collins D.W., He J., et al. Recruitment Strategies and Lessons Learned from a Large Genetic Study of African Americans. PLoS Glob. Public Health. 2022;2:e0000416. doi: 10.1371/journal.pgph.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikut A., Vaughn M., Salowe R., Sanyal M., Merriam S., Lee R., Becker E., Lomax-Reese S., Lewis M., Ryan R., et al. Evaluation of a Multimedia Marketing Campaign to Engage African American Patients in Glaucoma Screening. Prev. Med. Rep. 2020;17:101057. doi: 10.1016/j.pmedr.2020.101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson E.S., Sankar P.S., Miller-Ellis E., Regina M., Fertig R., Salinas J., Pistilli M., Salowe R.J., Rhodes A.L., Merritt W.T., et al. The Primary Open-Angle African American Glaucoma Genetics Study: Baseline Demographics. Ophthalmology. 2015;122:711–720. doi: 10.1016/j.ophtha.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addis V., Oyeniran E., Daniel E., Salowe R., Zorger R., Lee R., Pistilli M., Maguire M., Cui Q., Miller-Ellis E., et al. Non-Physician Grader Reliability in Measuring Morphological Features of the Optic Nerve Head in Stereo Digital Images. Eye. 2019;33:838–844. doi: 10.1038/s41433-018-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel E., Addis V., Maguire M.G., McGeehan B., Chen M., Salowe R.J., Zenebe-Gete S., Meer E., Lee R., Smith E., et al. Prevalence and Factors Associated with Optic Disc Tilt in the Primary Open-Angle African American Glaucoma Genetics Study. Ophthalmol. Glaucoma. 2022;5:544–553. doi: 10.1016/j.ogla.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma S.S., Gudiseva H.V., Chavali V.R.M., Salowe R.J., Bradford Y., Guare L., Lucas A., Collins D.W., Vrathasha V., Nair R.M., et al. A Multi-Cohort Genome-Wide Association Study in African Ancestry Individuals Reveals Risk Loci for Primary Open-Angle Glaucoma. Cell. 2024;187:464–480.e10. doi: 10.1016/j.cell.2023.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raj A., Stephens M., Pritchard J.K. fastSTRUCTURE: Variational Inference of Population Structure in Large SNP Data Sets. Genetics. 2014;197:573–589. doi: 10.1534/genetics.114.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zangwill L.M., Ayyagari R., Liebmann J.M., Girkin C.A., Feldman R., Dubiner H., Dirkes K.A., Holmann M., Williams-Steppe E., Hammel N., et al. The African Descent and Glaucoma Evaluation Study (ADAGES) III: Contribution of Genotype to Glaucoma Phenotype in African Americans: Study Design and Baseline Data. Ophthalmology. 2019;126:156–170. doi: 10.1016/j.ophtha.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor K.D., Guo X., Zangwill L.M., Liebmann J.M., Girkin C.A., Feldman R.M., Dubiner H., Hai Y., Samuels B.C., Panarelli J.F., et al. Genetic Architecture of Primary Open Angle Glaucoma in Individuals of African Descent: The African Descent & Glaucoma Evaluation Study (ADAGES) III. Ophthalmology. 2019;126:38–48. doi: 10.1016/j.ophtha.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koprubasi S., Bulut E. Impact of Obesity on Peripapillary Choroidal Thickness, Macular Choroidal Thickness, and Lamina Cribrosa Morphology. Photodiagnosis Photodyn. Ther. 2023;43:103724. doi: 10.1016/j.pdpdt.2023.103724. [DOI] [PubMed] [Google Scholar]
- 32.Tan N.Y.Q., Koh V., Girard M.J.A., Cheng C.Y. Imaging of the Lamina Cribrosa and Its Role in Glaucoma: A Review. Clin. Experiment. Ophthalmol. 2018;46:177–188. doi: 10.1111/ceo.13126. [DOI] [PubMed] [Google Scholar]
- 33.Park H.-Y.L., Shin D.Y., Jeon S.J., Kim Y.-C., Jung Y., Kim E.K., Shin H.-Y., Jung K.I., Choi J.A., Lee N.Y., et al. Predicting the Development of Normal Tension Glaucoma and Related Risk Factors in Normal Tension Glaucoma Suspects. Sci. Rep. 2021;11:16697. doi: 10.1038/s41598-021-95984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omodaka K., Takahashi S., Matsumoto A., Maekawa S., Kikawa T., Himori N., Takahashi H., Maruyama K., Kunikata H., Akiba M., et al. Clinical Factors Associated with Lamina Cribrosa Thickness in Patients with Glaucoma, as Measured with Swept Source Optical Coherence Tomography. PLoS ONE. 2016;11:e0153707. doi: 10.1371/journal.pone.0153707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung K.I., Jeon S., Park C.K. Lamina Cribrosa Depth Is Associated With the Cup-to-Disc Ratio in Eyes with Large Optic Disc Cupping and Cup-to-Disc Ratio Asymmetry. J. Glaucoma. 2016;25:e536–e545. doi: 10.1097/IJG.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 36.Jeon S.-J., Park H.-Y.L., Park C.-K. Vessel Density Loss of the Deep Peripapillary Area in Glaucoma Suspects and Its Association with Features of the Lamina Cribrosa. J. Clin. Med. 2021;10:2373. doi: 10.3390/jcm10112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J., Du Y., Li J., Fan X., Lin C., Wang N. The Influence of Different Intraocular Pressure on Lamina Cribrosa Parameters in Glaucoma and the Relation Clinical Implication. Sci. Rep. 2021;11:9755. doi: 10.1038/s41598-021-87844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivers K.M., Sredar N., Patel N.B., Rajagopalan L., Queener H.M., Twa M.D., Harwerth R.S., Porter J. In Vivo Changes in Lamina Cribrosa Microarchitecture and Optic Nerve Head Structure in Early Experimental Glaucoma. PLoS ONE. 2015;10:e0134223. doi: 10.1371/journal.pone.0134223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J.-A., Kim T.-W., Weinreb R.N., Lee E.J., Girard M.J.A., Mari J.M. Lamina Cribrosa Morphology Predicts Progressive Retinal Nerve Fiber Layer Loss In Eyes with Suspected Glaucoma. Sci. Rep. 2018;8:738. doi: 10.1038/s41598-017-17843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgoyne C. The Morphological Difference between Glaucoma and Other Optic Neuropathies. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2015;35((Suppl. S1)):S8–S21. doi: 10.1097/WNO.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esfandiari H., Efatizadeh A., Hassanpour K., Doozandeh A., Yaseri M., Loewen N.A. Factors Associated with Lamina Cribrosa Displacement after Trabeculectomy Measured by Optical Coherence Tomography in Advanced Primary Open-Angle Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2018;256:2391–2398. doi: 10.1007/s00417-018-4135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shon K., Hye Jo Y., Won Shin J., Kwon J., Jeong D., Kook M.S. Nasalization of Central Retinal Vessel Trunk Predicts Rapid Progression of Central Visual Field in Open-Angle Glaucoma. Sci. Rep. 2020;10:3789. doi: 10.1038/s41598-020-60355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Wang H., Pasquale L.R., Baniasadi N., Shen L.Q., Bex P.J., Elze T. Relationship Between Central Retinal Vessel Trunk Location and Visual Field Loss in Glaucoma. Am. J. Ophthalmol. 2017;176:53–60. doi: 10.1016/j.ajo.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawada Y., Araie M., Shibata H., Iwase T. Nasal Displacement of Retinal Vessels on the Optic Disc in Glaucoma Associated with a Nasally Angled Passage through Lamina Cribrosa. Sci. Rep. 2021;11:4176. doi: 10.1038/s41598-021-83720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chi T., Ritch R., Stickler D., Pitman B., Tsai C., Hsieh F.Y. Racial Differences in Optic Nerve Head Parameters. Arch. Ophthalmol. 1989;107:836–839. doi: 10.1001/archopht.1989.01070010858029. [DOI] [PubMed] [Google Scholar]
- 46.Girkin C.A., McGwin G., Xie A., Deleon-Ortega J. Differences in Optic Disc Topography between Black and White Normal Subjects. Ophthalmology. 2005;112:33–39. doi: 10.1016/j.ophtha.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Wilson R., Richardson T.M., Hertzmark E., Grant W.M. Race as a Risk Factor for Progressive Glaucomatous Damage. Ann. Ophthalmol. 1985;17:653–659. [PubMed] [Google Scholar]
- 48.Skaat A., De Moraes C.G., Bowd C., Sample P.A., Girkin C.A., Medeiros F.A., Ritch R., Weinreb R.N., Zangwill L.M., Liebmann J.M., et al. African Descent and Glaucoma Evaluation Study (ADAGES): Racial Differences in Optic Disc Hemorrhage and Beta-Zone Parapapillary Atrophy. Ophthalmology. 2016;123:1476–1483. doi: 10.1016/j.ophtha.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S.-A., Ciociola E.C., Mitchell W., Hall N., Lorch A.C., Miller J.W., Friedman D.S., Boland M.V., Elze T., Zebardast N., et al. Effectiveness of Microinvasive Glaucoma Surgery in the United States: Intelligent Research in Sight Registry Analysis 2013–2019. Ophthalmology. 2023;130:242–255. doi: 10.1016/j.ophtha.2022.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang J.H., Wang M., Frueh L., Rosner B., Wiggs J.L., Elze T., Pasquale L.R. Cohort Study of Race/Ethnicity and Incident Primary Open-Angle Glaucoma Characterized by Autonomously Determined Visual Field Loss Patterns. Transl. Vis. Sci. Technol. 2022;11:21. doi: 10.1167/tvst.11.7.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halawa O.A., Kolli A., Oh G., Mitchell W.G., Glynn R.J., Kim D.H., Friedman D.S., Zebardast N. Racial and Socioeconomic Differences in Eye Care Utilization among Medicare Beneficiaries with Glaucoma. Ophthalmology. 2022;129:397–405. doi: 10.1016/j.ophtha.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allison K., Patel D.G., Greene L. Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials. JAMA Netw. Open. 2021;4:e218348. doi: 10.1001/jamanetworkopen.2021.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnemaijer P.W.M., Lo Faro V., Sanyiwa A.J., Hassan H.G., Cook C., GIGA Study Group. Van de Laar S., Lemij H.G., Klaver C.C.W., Jansonius N.M., et al. Differences in Clinical Presentation of Primary Open-Angle Glaucoma between African and European Populations. Acta Ophthalmol. 2021;99:e1118–e1126. doi: 10.1111/aos.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhodes L.A., Huisingh C., Johnstone J., Fazio M., Smith B., Clark M., Downs J.C., Owsley C., Girard M.J.A., Mari J.M., et al. Variation of Laminar Depth in Normal Eyes with Age and Race. Investig. Ophthalmol. Vis. Sci. 2014;55:8123–8133. doi: 10.1167/iovs.14-15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gracitelli C.P.B., Zangwill L.M., Diniz-Filho A., Abe R.Y., Girkin C.A., Weinreb R.N., Liebmann J.M., Medeiros F.A. Detection of Glaucoma Progression in Individuals of African Descent Compared with Those of European Descent. JAMA Ophthalmol. 2018;136:329–335. doi: 10.1001/jamaophthalmol.2017.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta P., Zhao D., Guallar E., Ko F., Boland M.V., Friedman D.S. Prevalence of Glaucoma in the United States: The 2005–2008 National Health and Nutrition Examination Survey. Investig. Ophthalmol. Vis. Sci. 2016;57:2905–2913. doi: 10.1167/iovs.15-18469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girkin C.A., Fazio M.A., Bowd C., Medeiros F.A., Weinreb R.N., Liebmann J.M., Proudfoot J., Zangwill L.M., Belghith A. Racial Differences in the Association of Anterior Lamina Cribrosa Surface Depth and Glaucoma Severity in the African Descent and Glaucoma Evaluation Study (ADAGES) Investig. Ophthalmol. Vis. Sci. 2019;60:4496–4502. doi: 10.1167/iovs.19-26645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Girkin C.A., Belghith A., Bowd C., Medeiros F.A., Weinreb R.N., Liebmann J.M., Proudfoot J.A., Zangwill L.M., Fazio M.A. Racial Differences in the Rate of Change in Anterior Lamina Cribrosa Surface Depth in the African Descent and Glaucoma Evaluation Study. Investig. Ophthalmol. Vis. Sci. 2021;62:12. doi: 10.1167/iovs.62.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fazio M.A., Johnstone J.K., Smith B., Wang L., Girkin C.A. Displacement of the Lamina Cribrosa in Response to Acute Intraocular Pressure Elevation in Normal Individuals of African and European Descent. Investig. Ophthalmol. Vis. Sci. 2016;57:3331–3339. doi: 10.1167/iovs.15-17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Girkin C.A., Fazio M.A., Yang H., Reynaud J., Burgoyne C.F., Smith B., Wang L., Downs J.C. Variation in the Three-Dimensional Histomorphometry of the Normal Human Optic Nerve Head with Age and Race: Lamina Cribrosa and Peripapillary Scleral Thickness and Position. Investig. Ophthalmol. Vis. Sci. 2017;58:3759–3769. doi: 10.1167/iovs.17-21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan-Davies J., Taylor N., Hill A.R., Aspinall P., O’Brien C.J., Azuara-Blanco A. Three Dimensional Analysis of the Lamina Cribrosa in Glaucoma. Br. J. Ophthalmol. 2004;88:1299–1304. doi: 10.1136/bjo.2003.036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.