Key Points
Question
What is the strength of association between surrogate markers used as primary end points in clinical trials to support Food and Drug Administration (FDA) approval of drugs treating nononcologic chronic diseases and clinical outcomes?
Findings
No meta-analyses of clinical trials examining the strength of association between treatment effects measured using surrogate markers and clinical outcomes were identified for 22 (59%) of 37 surrogate markers for 32 chronic diseases, whereas at least 1 was identified for 15 (41%), although few reported high-strength evidence of treatment effect associations.
Meaning
Most surrogate markers used as primary end points in clinical trials to support FDA approval of drugs treating nononcologic chronic diseases lack high-strength evidence of associations with clinical outcomes from published meta-analyses.
Abstract
Importance
Surrogate markers are increasingly used as primary end points in clinical trials supporting drug approvals.
Objective
To systematically summarize the evidence from meta-analyses, systematic reviews and meta-analyses, and pooled analyses (hereafter, meta-analyses) of clinical trials examining the strength of association between treatment effects measured using surrogate markers and clinical outcomes in nononcologic chronic diseases.
Data sources
The Food and Drug Administration (FDA) Adult Surrogate Endpoint Table and MEDLINE from inception to March 19, 2023.
Study Selection
Three reviewers selected meta-analyses of clinical trials; meta-analyses of observational studies were excluded.
Data Extraction and Synthesis
Two reviewers extracted correlation coefficients, coefficients of determination, slopes, effect estimates, or results from meta-regression analyses between surrogate markers and clinical outcomes.
Main Outcomes and Measures
Correlation coefficient or coefficient of determination, when reported, was classified as high strength (r ≥ 0.85 or R2 ≥ 0.72); primary findings were otherwise summarized.
Results
Thirty-seven surrogate markers listed in FDA’s table and used as primary end points in clinical trials across 32 unique nononcologic chronic diseases were included. For 22 (59%) surrogate markers (21 chronic diseases), no eligible meta-analysis was identified. For 15 (41%) surrogate markers (14 chronic diseases), at least 1 meta-analysis was identified, 54 in total (median per surrogate marker, 2.5; IQR, 1.3-6.0); among these, median number of trials and patients meta-analyzed was 18.5 (IQR, 12.0-43.0) and 90 056 (IQR, 20 109-170 014), respectively. The 54 meta-analyses reported 109 unique surrogate marker–clinical outcome pairs: 59 (54%) reported at least 1 r or R2, 10 (17%) of which reported at least 1 classified as high strength, whereas 50 (46%) reported slopes, effect estimates, or results of meta-regression analyses only, 26 (52%) of which reported at least 1 statistically significant result.
Conclusions and Relevance
Most surrogate markers used as primary end points in clinical trials to support FDA approval of drugs treating nononcologic chronic diseases lacked high-strength evidence of associations with clinical outcomes from published meta-analyses.
This study examines the strength of association between surrogate markers used as primary end points in clinical trials to support FDA approval of drugs treating nononcologic chronic diseases and clinical outcomes.
Introduction
Clinical trials increasingly use surrogate markers, such as imaging findings or laboratory measurements, as primary end points.1,2,3 Surrogate markers, which are expected to predict target outcomes of interest (eg, clinical outcomes that directly measure how people feel, function, or survive),4 offer the advantage of reducing the duration, size, and total cost of trials.5,6 In 2018, the Food and Drug Administration (FDA) publicly released an Adult Surrogate Endpoint Table of more than 100 surrogate markers that may be used as primary end points in clinical trials that form the basis of traditional or accelerated approval of new drugs or biologics. Although this table was designed to serve as a reference for drug developers, it does not provide justification for surrogate selection in terms of strength of evidence of associations between the treatment effects measured using the surrogate marker and those measured using disease-relevant clinical outcomes.
In oncology, surrogate markers, such as progression-free survival and objective response rate, are weakly associated with clinical outcomes such as overall survival and quality of life.7,8,9,10 However, to our knowledge, no studies have examined such associations for surrogate markers used as primary end points in clinical trials to support FDA approval of drugs treating nononcologic chronic diseases, which compose one-third of disease indications listed in FDA’s table. Accordingly, our objective was to systematically review the literature to identify and summarize all meta-analyses of clinical trials examining the strength of association between treatment effects measured using surrogate markers listed in FDA’s Adult Surrogate Endpoint Table and those measured using any clinical outcomes for nononcologic chronic diseases. We focused on published meta-analyses as opposed to independently meta-analyzing clinical trial data because FDA has maintained that the use of surrogate markers in traditional approval requires, at a minimum, evidence from meta-analyses of clinical trials demonstrating an association between surrogate markers and clinical outcomes to establish surrogacy.11
Methods
This review, prospectively registered on OSF.io, is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Institutional review board approval and participant informed consent were not required for this review because it used only previously published research.
Surrogate Marker Selection
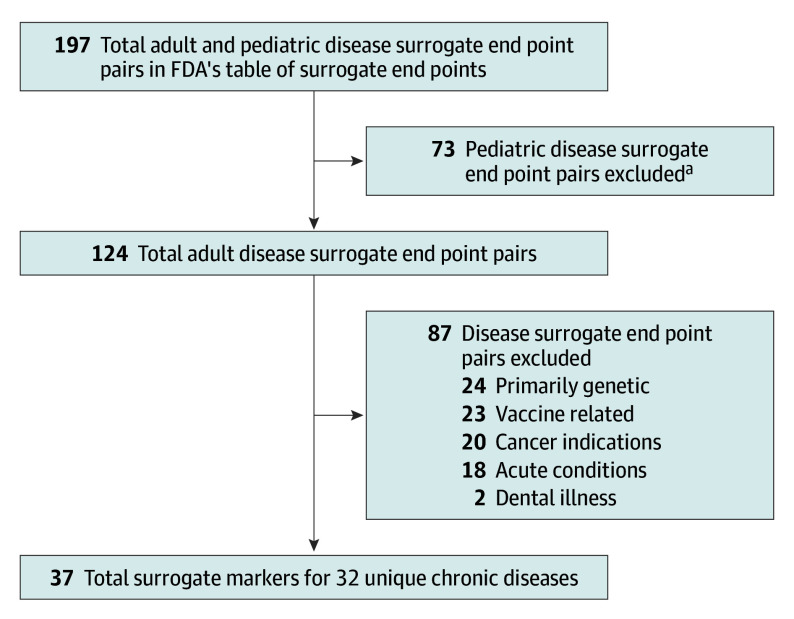
On August 2, 2022, we reviewed FDA’s Adult Surrogate Endpoint Table12 to identify all surrogate markers that may be used as primary end points in clinical trials to support FDA approval of drugs treating chronic diseases (Figure 1; eMethods in Supplement 1). We did not consider FDA’s Pediatric Surrogate Endpoint Table and excluded any surrogate markers for diseases listed in FDA’s Adult Surrogate Endpoint Table that were acute illnesses (eg, skin infection), primarily genetic (eg, Fabry disease, cystic fibrosis), oncology related,9,10 or vaccine related.
Figure 1. Surrogate Marker Selection Flowchart.
FDA indicates Food and Drug Administration.
aThe FDA has a separate Pediatric Surrogate Endpoint Table.
Data Sources and Selection
For each surrogate marker, we conducted independent systematic reviews for English-language meta-analyses of clinical trials through searches of MEDLINE (Ovid ALL) from inception to March 19, 2023, using broad study design (eg, meta-analyses), correlation, surrogate marker, and chronic disease–specific terminology (eMethods in Supplement 1). Reference lists of eligible studies were reviewed manually.
Study Selection
Three reviewers (J.D.W., S.Y., and L.R.G.) independently screened titles and abstracts. Full-text versions of potentially eligible articles were subsequently reviewed to identify summary or individual patient-level data meta-analyses, systematic reviews and meta-analyses, and pooled analyses (hereafter, meta-analyses) of clinical trials. Meta-analyses were eligible if they (1) reported summary measures of associations, including correlation coefficients, coefficients of determination, slopes, effect estimates, results from meta-regression analyses, or any other metrics of association or prediction (eg, surrogate threshold effect13) for the association between treatment effects measured using the surrogate marker and any clinical outcome (ie, surrogate marker–clinical outcome pairs)12; and (2) included at least 1 component study in which the disease, patient population, and medication(s) were the same as described in FDA’s table. We excluded pooled analyses containing only 2 component studies, meta-analyses containing observational studies, earlier versions of updated meta-analyses, and meta-analyses evaluating only individual patient-level correlations (ie, they did not examine changes attributable to treatment) (eMethods in Supplement 1).
Data Extraction
For each eligible meta-analysis, 3 reviewers (J.D.W., S.Y., and H.D.) recorded study characteristics and identified all correlation coefficients or coefficients of determination (eMethods in Supplement 1). If none were reported, we abstracted slopes and effect estimates from regression-based analyses or other metrics of association or prediction. Last, we recorded the authors’ subjective interpretation of the association between each surrogate marker–clinical outcome pair.
Data Synthesis and Analysis
For each surrogate marker–clinical outcome pair, the number of correlation coefficients, coefficients of determination, slopes, effect estimates, or results from meta-regression analyses that were statistically significant (P < .05) was identified. Correlation coefficients or coefficients of determination were then classified as providing high-strength evidence according to criteria proposed by the Institute for Quality and Efficiency in Health Care (r ≥ 0.85 or R2 ≥ 0.72).14 For meta-analyses reporting inconsistent evidence across multiple associations for the same surrogate marker–clinical outcome pair (eg, high- and low-strength correlations across different subgroups or follow-up durations), the evidence was classified as mixed. Descriptive statistics were calculated to characterize the eligible meta-analyses and evidence examining the strength of association between surrogate markers and clinical outcomes with RStudio (Posit) and R (R Foundation for Statistical Computing).
Results
Study Characteristics
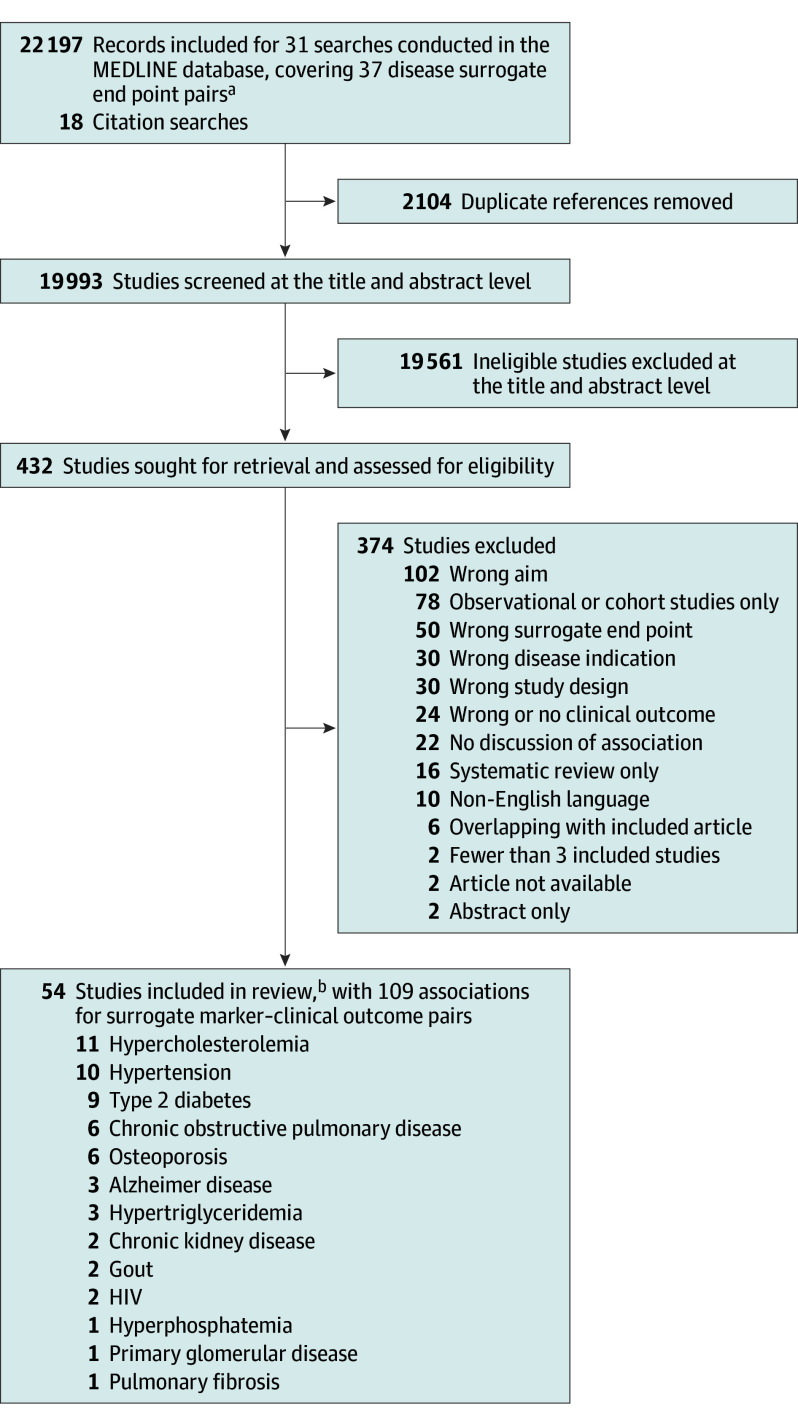
There were 37 surrogate markers listed in FDA’s Adult Surrogate Endpoint Table that may be used as primary end points for clinical trials of 32 unique nononcologic chronic diseases (7 for accelerated approval and 30 for traditional approval) (Table; eMethods in Supplement 1). For these 37 surrogate markers, 31 independent systematic reviews were conducted because the table contained similar diseases (eg, Cushing disease and Cushing syndrome) and some surrogate markers were listed multiple times. Overall, 19 993 records were identified for title and abstract screening (median, 216 records; range, 3-5541) (Figure 2; eFigures 1-31 in Supplement 1).
Table. Surrogate Markers for Nononcologic Chronic Disease Treatments in the FDA’s Adult Surrogate Endpoint Table.
| Disease or use | Patient population | Surrogate end point |
|---|---|---|
| Surrogate markers appropriate for accelerated approval with at least 1 meta-analysis of clinical trialsa | ||
| Alzheimer disease | Patients with mild cognitive impairment or mild dementia stage of Alzheimer disease | Reduction in amyloid-β plaques |
| Primary glomerular diseases associated with significant proteinuria | Patients with primary glomerular disease associated with significant proteinuria | Proteinuria (urinary protein to creatinine ratio) |
| Surrogate markers appropriate for accelerated approval without any meta-analyses of clinical trialsa | ||
| HDV | Patients with HDV infection with or without cirrhosis | ≥2 Log reduction in HDV RNA plus normalization of ALT HDV below the LLOQb |
| MAC lung disease | Patients with MAC lung disease | Sputum culture conversion to negative result by 6 mo |
| NASH | Patients without cirrhosis with NASH liver fibrosis | Histopathologic findings of (1) resolution of steatohepatitis with no worsening of fibrosis, or (2) improvement of fibrosis with no worsening of steatohepatitis, or (3) bothc |
| Primary biliary cholangitis | Patients with primary biliary cholangitis | Serum alkaline phosphatase and bilirubin |
| Pulmonary tuberculosis | Patients with active pulmonary tuberculosis | Sputum culture conversion to negative result |
| Surrogate markers appropriate for traditional approval with at least 1 meta-analysis of clinical trials | ||
| Chronic kidney disease | Patients with chronic kidney disease secondary to multiple etiologies | Estimated glomerular filtration rate |
| COPD | Patients with COPD | FEV1 |
| Gout | Patients with gout | Serum uric acid level |
| HIV-1 | Patients with HIV-1 | Undetectable plasma HIV RNA |
| Hypercholesterolemia | Patients with heterozygous familial and nonfamilial hypercholesterolemia | Serum low-density lipoprotein cholesterol level |
| Hyperphosphatemia | Patients with hyperphosphatemia and receiving dialysis | Serum phosphate level |
| Hypertension | Patients with hypertension | Systolic blood pressure |
| Diastolic blood pressure | ||
| Hypertriglyceridemia | Patients with severe hypertriglyceridemia | Serum triglyceride level |
| Osteoporosis | Patients with glucocorticoid-induced osteoporosis | Bone mineral density |
| Men with osteoporosis | ||
| Pulmonary fibrosis | Patients with pulmonary fibrosis | FVC |
| Secondary hyperparathyroidism associated with chronic kidney disease | Patients with secondary hyperparathyroidism associated with chronic kidney disease | Serum iPTH level |
| Type 2 diabetes mellitus | Patients with type 2 diabetes mellitus | Serum hemoglobin A1c level |
| Surrogate markers appropriate for traditional approval without any meta-analysis of clinical trialsa | ||
| Asthma | Patients with asthma | FEV1 |
| Chronic kidney disease | Patients with chronic kidney disease secondary to multiple etiologies | Serum creatinine level |
| Cushing disease | Patients with Cushing disease for whom pituitary surgery is not an option or has not been curative | Urine free cortisol level |
| Cushing syndrome | Patients with endogenous Cushing syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or are not candidates for surgery | |
| Exocrine pancreatic insufficiency | Patients with exocrine pancreatic insufficiency due to cystic fibrosis | Fecal coefficient of fat absorption |
| HBV | Patients with HBV infection with or without cirrhosis | Undetectable plasma HBV DNA for indefinite treatment |
| HBsAg loss for finite treatment | ||
| HCV | Patients with HCV infection with or without cirrhosis | Sustained viral response (HCV RNA) |
| HIV-1 | Patients at high risk of sexually acquired HIV-1 | Serum HIV antibody |
| >0.5 Log reduction in plasma HIV RNA | ||
| Hypothyroidism | Patients with hypothyroidism | Serum TSH level |
| Lupus nephritis | Patients with active lupus nephritis | CRR, defined as (1) a response in the urine proteinuria level (protein to creatine ratio); and (2) preservation or improvement of kidney function (estimated glomerular filtration rate) |
| Opioid use disorder | Patients with opioid use disorder | Urine toxicology test result for opioids |
| Osteoporosis | Postmenopausal women with osteoporosis | New morphometric vertebral fractures |
| Paget disease | Patients with Paget disease | Serum alkaline phosphatase level |
| Primary hyperthyroidism | Patients with hypercalcemia due to primary hyperparathyroidism | Serum calcium level |
| Systemic sclerosis–interstitial lung disease | Patients with systemic sclerosis–interstitial lung disease | FVC |
| Tobacco dependence | Cigarette smokers | Exhaled carbon monoxide level |
Abbreviations: ALT, alanine transaminase; COPD, chronic obstructive pulmonary disease; CRR, complete renal response; FDA, Food and Drug Administration; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; iPTH, intact parathyroid hormone; LLOQ, lower limit of quantification; MAC, Mycobacterium avium complex; NASH, nonalcoholic steatohepatitis; TSH, thyroid-stimulating hormone.
Measuring the strength of association between the relevant surrogate marker and clinical outcomes. Surrogate markers appropriate for accelerated approval and traditional approval are mutually exclusive.
The agency anticipates that this surrogate end point could be appropriate for use as a primary efficacy clinical trial end point for drug or biologic approval, although it has not yet been used to support an approved new drug application or biologics license application.
Surrogate end point is part of a composite of biomarker surrogate end points.
Figure 2. Study Flowchart.
aeFigures 1 through 31 in Supplement 1 provide flowcharts for the individual chronic diseases.
bFour meta-analyses were applicable to 2 disease indications.
For 22 of the 37 surrogate markers (59%) that may be used as primary end points for 21 chronic diseases, no eligible meta-analysis of clinical trials quantifying the strength of association between the treatment effects measured using the relevant surrogate marker and any clinical outcome was identified (Table; eFigures 1-17 in Supplement 1). Of these surrogate markers, 17 (77%) were classified by FDA as being appropriate for traditional approval, including forced expiratory volume in the first second of expiration (FEV1) for asthma and urine toxicology for opioid use disorder, and 5 (23%) as being appropriate for accelerated approval.
For 15 of the 37 surrogate markers (41%) that may be used as primary end points for 14 chronic diseases (Table), at least 1 meta-analysis of clinical trials was identified, totaling 54 unique meta-analyses (median, 2.5; IQR, 1.3-6.0). Of these surrogate markers, 13 (87%) were classified by FDA as being appropriate for traditional approval and 2 (13%) for accelerated approval. The median number of trials and patients meta-analyzed in these 54 meta-analyses was 18.5 (IQR, 12.0-43.0) and 90 056 (IQR, 20 109-170 014), respectively.
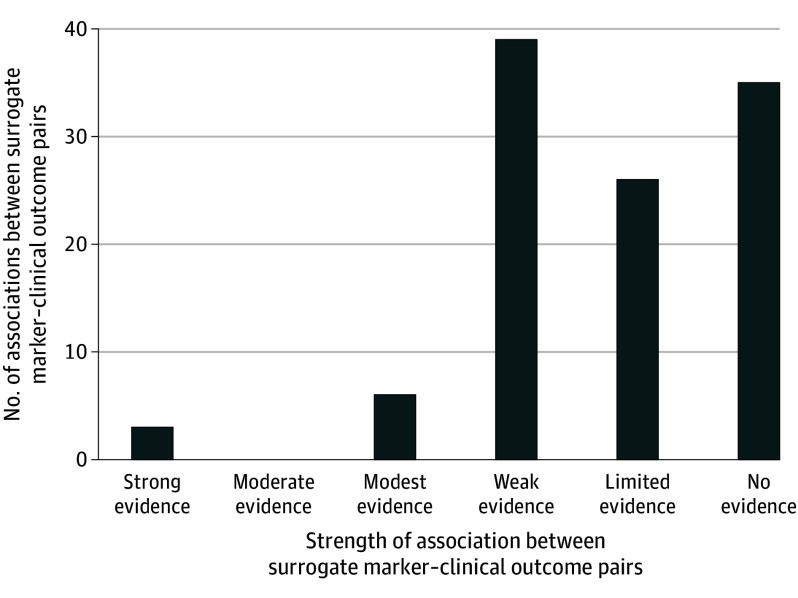
The 54 meta-analyses, 14 (26%) of which had any industry funding, reported 200 total associations for 109 unique surrogate marker–clinical outcome pairs (Figure 3; eTable 1A and B in Supplement 1). At least 1 correlation coefficient or coefficient of determination was reported for 59 (54%) surrogate marker–clinical outcome pairs, 10 (17%) of which reported at least 1 that provided high-strength evidence (ie, r ≥ 0.85 or R2 ≥ 0.72). The remaining 50 pairs (46%) reported only slopes, effect estimates, results from meta-regression analyses, or another metric of association or prediction, 26 (52%) of which reported at least 1 statistically significant result.
Figure 3. Strength of the Associations Between 109 Surrogate Marker–Clinical Outcome Pairs.
Strong evidence: correlation coefficients or coefficients of determination values reported for all associations examined, and all associations classified as statistically significant and high strength according to criteria proposed by the Institute for Quality and Efficiency in Health Care (r ≥ 0.85 or R2 ≥ 0.72).14 Moderate evidence: r or R2 values reported for all associations examined, and 1 or more (but not all) classified as statistically significant and high strength. Modest evidence: r or R2 values reported for some associations examined, and 1 or more (but not all) classified as statistically significant and high strength. Any other r, R2, slopes, effect estimates, or results from meta-regression analyses classified as statistically significant. Weak evidence: no r or R2 values classified as both statistically significant and high strength, but all r, R2, slopes, effect estimates, or results from meta-regression analyses classified as statistically significant. Limited evidence: no r or R2 values classified as both statistically significant and high strength; some r, R2, slopes, effect estimates, or results from meta-regression analyses classified as statistically significant and some not. No evidence: no r or R2 values classified as statistically significant and high strength, and all r, R2, slopes, effect estimates, or results from meta-regression analyses classified as nonstatistically significant.
Surrogate Markers That May Be Used for Accelerated Approval
Alzheimer Disease Surrogate Marker: Amyloid-β Plaque Deposition
Of 792 records screened, 3 meta-analyses offered inconsistent evidence on the strength of association between reduction in amyloid-β plaque deposition and several cognitive rating scales for Alzheimer disease (eTable 2 and eFigure 18 in Supplement 1). One reported low-strength correlation coefficients between reductions in amyloid-β deposition and changes in the Clinical Dementia Rating Scale sum of boxes (Pearson r = 0.51; P = .09 [n = 9 randomized clinical trials {RCTs}]) and Alzheimer’s Disease Assessment Scale–Cognitive subscale (Pearson r = 0.68; P = .02 [n = 9 RCTs]),15 whereas another reported that a decrease in amyloid-β level detected by positron emission tomography of 0.1 standardized uptake value ratio unit was not associated with a reduction in the Clinical Dementia Rating Scale sum of boxes score (0.051 point; 95% CI, −0.027 to 0.13 [n = 10 RCTs]) or the Mini Mental State Examination score (0.087 point; 95% CI, −0.042 to 0.22 [n = 14 RCTs]).16 However, a third reported that a 0.1-unit decrease in amyloid-β level detected by positron emission tomography was associated with statistically significant decreased reductions in the Clinical Dementia Rating Scale sum of boxes score (0.09 point; 95% CI, 0.03-0.15 [n = 15 RCTs]), Mini Mental State Examination score (0.13 point; 95% CI, 0.017-0.24 [n = 16 RCTs]), and Alzheimer’s Disease Assessment Scale–Cognitive score (0.33 point; 95% CI, 0.12-0.55 [n = 15 RCTs]).17
Primary Glomerular Disease Surrogate Marker: Proteinuria
Of 19 records screened, 1 meta-analysis offered high-strength evidence of the association between proteinuria and the composite end point of doubling of serum creatinine level, end-stage kidney disease, or death (R2 = 0.91; 95% bayesian credible interval, 0.47-1.00 [n = 11 RCTs]) (eTable 3 and eFigure 19 in Supplement 1).18
Surrogate Markers That May Be Used for Traditional Approval
Chronic Kidney Disease Surrogate Marker: Estimated Glomerular Filtration Rate
Of 731 records screened, 2 meta-analyses offered consistent evidence on the strength of association between reductions in estimated glomerular filtration rate and a range of clinical outcomes (eTable 4 and eFigure 20 in Supplement 1). One reported a high-strength coefficient of determination between estimated glomerular filtration rate and the composite end point of doubling of serum creatine level, estimated glomerular filtration rate less than 15 mL/min/m2, and treated end-stage kidney disease (R2 = 0.97; 95% bayesian credible interval, 0.78-1.00 [n = 47 RCTs]),19 whereas another reported that 30% and 40% reductions in estimated glomerular filtration rate were associated with statistically significant improvements in treated end-stage kidney disease.20
Chronic Obstructive Pulmonary Disease Surrogate Marker: FEV1
Of 569 records screened, 6 meta-analyses offered inconsistent evidence on the strength of association between improvements in FEV1 and several objective and subjective clinical outcomes across different subgroups and follow-up durations (ie, exacerbation rate, rescue medication use, St George’s Respiratory Questionnaire, Transition Dyspnea Index, and risk for hospitalization) (eTable 5 and eFigure 21 in Supplement 1) and only reported 2 or more high-strength coefficients of determination between change in trough FEV1 and first occurrence of a moderate to severe exacerbation across all trials (R2 = 0.85) and among those evaluating only bronchodilators (R2 = 0.88).21
Gout Surrogate Marker: Serum Uric Acid
Of 188 records screened, 2 meta-analyses offered inconsistent evidence on the strength of association between serum uric acid and various clinical outcomes (eTable 6 and eFigure 22 in Supplement 1). One reported a low-strength association between achieving serum urate level less than 6 mg/dL and the risk of patients’ experiencing gout flair (R2 = 0.0779 [n = 10 RCTs]),22 whereas another reported statistically significant slopes, suggesting that reductions in serum urate concentration were associated with worse pain and patient-reported outcomes across several scales.23
HIV Surrogate Marker: Plasma HIV RNA
Of 506 records screened, 2 meta-analyses offered inconsistent evidence on the strength of association between HIV-1 RNA viral load and progression to AIDS or death (eTable 7 and eFigure 23 in Supplement 1). One reported that increased levels of HIV-1 RNA were associated with higher rates of progression to AIDS,24 whereas another reported 1 high-strength coefficient of determination between viral load less than 200 copies/mL and progression to AIDS or death at 48 weeks (R2 = 0.86; P = .02 [n = 5 RCTs]) and 8 low-strength correlations for different viral load levels and follow-up durations.25
Hypercholesterolemia Surrogate Marker: Low-Density Lipoprotein Cholesterol
Of 3463 records screened, 11 meta-analyses reported inconsistent evidence on the strength of association between reduction in low-density lipoprotein cholesterol and various clinical outcomes (eTable 8 and eFigure 24 in Supplement 1). Although all meta-analyses claimed that reductions in low-density lipoprotein cholesterol were associated with statistically significant improvements in at least 1 clinical outcome, only 1 meta-analysis reported high-strength coefficients of determination between low-density lipoprotein cholesterol and major vascular events (R2 = 0.85), major coronary events (R2 = 0.85), and vascular mortality (R2 = 0.75 [n = 25 RCTs]) among trials investigating statin therapy.26
Hyperphosphatemia Surrogate Marker: Serum Phosphorus
Of 556 records screened, 1 meta-analysis reported low-strength correlations between changes in serum phosphorus level and improvements in all-cause mortality (r = 0.23; 95% credible interval, −0.48 to 0.69 [n = 12 RCTs]) or cardiovascular mortality (r = 0.54; 95% credible interval, −0.98 to 1.0 [n = 4 RCTs]) (eTable 9 and eFigure 25 in Supplement 1).27
Hypertension Surrogate Marker: Diastolic and Systolic Blood Pressure
Of 5541 records screened, 10 meta-analyses offered inconsistent evidence on the strength of association between reductions in systolic or diastolic blood pressure and improvements in cardiovascular outcomes and all-cause mortality (eTable 10 and eFigure 26 in Supplement 1). The most recent meta-analysis reported that reductions of 5 mm Hg in systolic blood pressure were associated with decreased incidence of major adverse cardiovascular events (hazard ratio = 0.89 [95% CI, 0.86-0.92] and hazard ratio = 0.91 [95% CI, 0.89-0.94] among participants with and without cardiovascular disease, respectively [n = 48 RCTs]).28
Hypertriglyceridemia Surrogate Marker: Serum Triglyceride
Of 1869 records screened, 3 meta-analyses offered inconsistent evidence on the strength of association between serum triglyceride level and several clinical outcomes (eTable 11 and eFigure 27 in Supplement 1). One reported that each increase of 10 mg/dL in absolute triglyceride level was associated with a nonstatistically significant lower risk of vascular events (log relative risk, 0.4; 95% CI, −3.8 to 4.8 [n = 64 RCTs]),29 whereas another reported that changes in triglyceride levels were predictive of cardiovascular events (log rate ratio slope, 0.488; P = .005 [n = 40 RCTs]) but the observed slope was no longer statistically significant when limited to RCTs of primary prevention (slope, 0.373; P = .11 [n = 25 RCTs]).30 A third meta-analysis reported that each reduction of 1 mmol/L (88.5 mg/dL) in triglyceride level was associated with a lower risk of major vascular events (relative risk, 0.84; 95% CI, 0.75-0.94 [n = 44 RCTs]).31
Osteoporosis Surrogate Marker: Bone Mineral Density
Of 1356 records screened, 6 meta-analyses offered inconsistent evidence on the strength of association between increases in bone mineral density (ie, hip, femoral neck, and spine bone mineral density) and reductions in the risk of several fracture types (ie, hip, vertebral, nonvertebral, and spine fracture) (eTable 12 and eFigure 28 in Supplement 1). The 2 most recent meta-analyses reported 18 coefficients of determination, of which 3 were considered high strength (hip bone mineral density and vertebral fractures, R2 = 0.73 [95% CI, 0.41-0.83], P < .001 [n = 14 RCTs]; femoral neck bone mineral density and nonvertebral fractures, R2 = 0.65 [95% CI, 0.33-0.77], P < .001 [n = 17 RCTs]; and spine bone mineral density and vertebral fractures, R2 = 0.63 [95% CI 0.41-0.73], P < .001 [n = 30 RCTs]).32,33
Pulmonary Fibrosis Surrogate Marker: Forced Vital Capacity
Of 85 records screened, 1 evaluated the strength of association between forced vital capacity and the end points of mortality (hazard ratio, 1.20; 95% CI, 1.12-1.28 per 2.5% relative decline in forced vital capacity during 3 months) and disease progression (hazard ratio, 1.46; 95% CI, 1.36-1.57 per 2.5% relative decline in forced vital capacity during 3 months [n = 6 RCTs]) among treated individuals (eTable 13 and eFigure 29 in Supplement 1).34
Secondary Hyperparathyroidism Surrogate Marker: Serum Parathyroid Hormone
Of 53 records screened, 1 offered low-strength evidence on the strength of association between serum parathyroid hormone level and all-cause mortality (r = 0.12; 95% credible interval, −0.61 to 0.73 [n = 12 RCTs]) and cardiovascular mortality (r = −0.03; 95% credible interval, −0.91 to 0.91 [n = 6 RCTs]) and continuous serum parathyroid hormone level and all-cause mortality (r = −0.69; 95% credible interval, −0.88 to −0.18 [n = 17 RCTs]) and cardiovascular mortality (r = −0.28; 95% credible interval, −0.98 to 0.96 [n = 5 RCTs]) (eTable 14 and eFigure 30 in Supplement 1).27
Type 2 Diabetes Mellitus Surrogate Marker: Hemoglobin A1c
Of 1766 records screened, 9 meta-analyses offered inconsistent evidence on the strength of association between reduction in hemoglobin A1c level and improvements in all-cause mortality, myocardial infarction, stroke, heart failure, kidney injury, cardiovascular death, hospitalization for heart failure, coronary heart disease, treatment-related discontinuations, neuropathy, and peripheral vascular events (eTable 15 and eFigure 31 in Supplement 1). One offered high-strength evidence for the outcome of fatal stroke (R2 = 1.00 [n = 18 RCTs]),35 whereas 2 offered high-strength evidence for the outcome of major adverse cardiovascular events.35,36
Discussion
This systematic review of the evidence examining surrogate markers listed in FDA’s Adult Surrogate Endpoint Table that may be used as clinical trial end points to support FDA approval of drugs treating nononcologic chronic diseases found that more than half had no published meta-analysis examining the strength of their association with any clinical outcome. Nearly 80% of these surrogate markers without a published meta-analysis were classified by FDA as being appropriate for traditional approval, including FEV1 for asthma and serum creatinine for chronic kidney disease. For surrogate markers for which at least 1 eligible meta-analysis of clinical trials was identified, most lacked high-strength evidence of treatment effect associations with any clinical outcome. Only 3 surrogate markers, FEV1 for chronic obstructive pulmonary disease for the outcome of time to first occurrence of a moderate to severe exacerbation, hemoglobin A1c for type 2 diabetes mellitus for the outcome of fatal stroke, and proteinuria for primary glomerular disease treatment, were found to have consistent evidence from published meta-analyses demonstrating a high-strength treatment effect association with a clinical outcome.
There is a lack of consensus on the minimum strength of association between the treatment effects measured using surrogate markers and those measured using target clinical outcomes necessary to establish surrogacy. Although correlation coefficients and coefficients of determination from meta-analyses of clinical trials are often used to evaluate the strength of association between surrogate markers and clinical outcomes,8,9,14 these values are purely statistical, rely on arbitrary cutoffs, and fail to capture information about the study design, target outcome, and generalizability of the evidence. Although more comprehensive methods to establish surrogacy have been proposed (eg, the Biomarker-Surrogacy Evaluation Schema),13,37 enhanced clinical trial reporting4,38 and greater availability of shared individual patient-level data will facilitate more precise analyses and estimates.
To further enhance transparency, FDA’s Adult Surrogate Endpoint Table could explicitly report the target outcomes intended to be predicted by each surrogate marker for each disease. The FDA has maintained that the use of surrogate markers in traditional approval requires, at minimum, evidence from meta-analyses of clinical trials.11 The agency could publicly report and routinely update an accompanying summary of evidence supporting marker surrogacy for clinical benefit, or clarify when no studies are available. Although individual studies may be the only source of evidence for surrogate markers classified by FDA as being appropriate for accelerated approval, FDA could reconsider whether to allow surrogate markers with low-strength evidence from multiple meta-analyses to be used as primary end points in clinical trials supporting traditional drug approval. Additional studies explicitly designed to establish surrogacy may be necessary for surrogate markers with no or inconsistent evidence.39
Limitations
This study has several limitations. First, our findings may not apply to surrogate markers used in clinical trials of drugs treating chronic diseases but not listed in FDA’s Adult Surrogate Endpoint Table. Second, unpublished studies submitted to FDA and other regulators may not have been identified. Third, by our relying on the published literature indexed in MEDLINE, some published studies indexed in other databases may not have been identified. However, the search terms were broad and MEDLINE (Ovid) indexes information from more than 5600 journals. Fourth, individual studies and meta-analyses using observational cohorts were excluded because these studies are not considered robust sources of evidence for evaluating drug treatment effects or establishing surrogacy.40 Fifth, identified meta-analyses could be susceptible to publication bias because studies showing significant associations between surrogate markers and clinical outcomes may be more likely to be published than those showing no association. Sixth, exploratory subgroup analyses were not recorded, although they could reveal variations for specific subgroups or settings of use.
Conclusions
More than half of the surrogate markers listed in FDA’s Adult Surrogate Endpoint Table that may be used as primary end points in clinical trials supporting FDA approval of drugs treating nononcologic chronic diseases were not found to have a published meta-analysis evaluating associations between treatment effects measured using surrogate markers and clinical outcomes. For the surrogate markers for which at least 1 meta-analysis was identified, most lacked high-strength evidence of treatment effect associations with clinical outcomes. These findings highlight the importance of making publicly available a summary of the evidence supporting surrogate end points that may be used to support FDA approval of drugs treating chronic disease, which can aid drug sponsors in choosing appropriate surrogate end points for trials and guide physicians and their patients to better interpret the clinical benefits of drugs approved according to listed surrogate markers.
eMethods. Meta-Analyses of Clinical Trials
eTable 1. Original Search Results
eTable 1A. Surrogate Marker-Clinical Outcome Pairs From Meta-Analyses of Clinical Trials With Correlation Coefficients, Coefficients of Determination, or Results From Meta-Regression Analyses
eTable 1B. Surrogate Marker-Clinical Outcome Pairs From Meta-Analyses of Clinical Trials With Correlation Coefficients, Coefficients of Determination, or Results From Meta-Regression Analyses Classification for Figure 2
eTable 2. Alzheimer’s Disease
eTable 3. Primary Glomerular Disease
eTable 4. Chronic Kidney Disease
eTable 5. Chronic Obstructive Pulmonary Disease
eTable 6. Gout
eTable 7. HIV
eTable 8. Hypercholesterolemia
eTable 9. Hyperphosphatemia
eTable 10. Hypertension
eTable 11. Hypertriglyceridemia
eTable 12. Osteoporosis
eTable 13. Pulmonary Fibrosis
eTable 14. Secondary Hyperparathyroidism
eTable 15. Type 2 Diabetes
eFigure 1. Asthma
eFigure 2. Cushing’s Disease / Cushing’s Syndrome
eFigure 3. Exocrine Pancreatic Insufficiency
eFigure 4. Hepatitis B
eFigure 5. Hepatitis C
eFigure 6. Hepatitis D
eFigure 7. Hypothyroidism
eFigure 8. Lupus Nephritis
eFigure 9. Mycobacterium Avium Complex (MAC) Lung Disease
eFigure 10. Non-alcoholic Steatohepatitis (NASH)
eFigure 11. Opioid Use Disorder
eFigure 12. Paget’s Disease
eFigure 13. Primary Biliary Cholangitis
eFigure 14. Primary Hyperparathyroidism
eFigure 15. Pulmonary Tuberculosis
eFigure 16. Systemic Sclerosis-Interstitial Lung Disease
eFigure 17. Tobacco Dependence
eFigure 18. Alzheimer’s Disease
eFigure 19. Primary Glomerular Diseases Associated With Significant Proteinuria
eFigure 20. Chronic Kidney Disease
eFigure 21. Chronic Obstructive Pulmonary Disease
eFigure 22. Gout
eFigure 23. Human Immunodeficiency Virus (HIV)
eFigure 24. Hypercholesterolemia
eFigure 25. Hyperphosphatemia
eFigure 26. Hypertension
eFigure 27. Hypertriglyceridemia
eFigure 28. Osteoporosis
eFigure 29. Pulmonary Fibrosis
eFigure 30. Secondary Hyperparathyroidism
eFigure 31. Type 2 Diabetes Mellitus
eReferences.
Data Sharing Statement
References
- 1.US Food and Drug Administration . Surrogate endpoint resources for drug and biologic development. Accessed January 23, 2023. https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development
- 2.Zhang AD, Puthumana J, Downing NS, Shah ND, Krumholz HM, Ross JS. Assessment of clinical trials supporting US Food and Drug Administration approval of novel therapeutic agents, 1995-2017. JAMA Netw Open. 2020;3(4):e203284. doi: 10.1001/jamanetworkopen.2020.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Use of surrogate end points in healthcare policy: a proposal for adoption of a validation framework. Nat Rev Drug Discov. 2016;15(7):516. doi: 10.1038/nrd.2016.81 [DOI] [PubMed] [Google Scholar]
- 4.Ciani O, Manyara AM, Davies P, et al. A framework for the definition and interpretation of the use of surrogate endpoints in interventional trials. EClinicalMedicine. 2023;65:102283. doi: 10.1016/j.eclinm.2023.102283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittes J, Lakatos E, Probstfield J. Surrogate endpoints in clinical trials: cardiovascular diseases. Stat Med. 1989;8(4):415-425. doi: 10.1002/sim.4780080405 [DOI] [PubMed] [Google Scholar]
- 6.Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health. 2017;20(3):487-495. doi: 10.1016/j.jval.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Walia A, Haslam A, Prasad V. FDA validation of surrogate endpoints in oncology: 2005-2022. J Cancer Policy. 2022;34:100364. doi: 10.1016/j.jcpo.2022.100364 [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992-1994. doi: 10.1001/jamainternmed.2015.5868 [DOI] [PubMed] [Google Scholar]
- 9.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389-1398. doi: 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
- 10.Gyawali B, Hey SP, Kesselheim AS. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine. 2020;21:100332. doi: 10.1016/j.eclinm.2020.100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98(1):34-46. doi: 10.1002/cpt.136 [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration . Table of surrogate endpoints that were the basis of drug approval or licensure. Accessed September 21, 2022. https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure
- 13.Burzykowski T, Buyse M. Surrogate threshold effect: an alternative measure for meta-analytic surrogate endpoint validation. Pharm Stat. 2006;5(3):173-186. doi: 10.1002/pst.207 [DOI] [PubMed] [Google Scholar]
- 14.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen . Validity of surrogate endpoints in oncology. Accessed December 12, 2023. https://www.iqwig.de/download/a10-05_executive_summary_v1-1_surrogate_endpoints_in_oncology.pdf
- 15.Avgerinos KI, Ferrucci L, Kapogiannis D. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res Rev. 2021;68:101339. doi: 10.1016/j.arr.2021.101339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackley SF, Zimmerman SC, Brenowitz WD, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ. 2021;372(156):n156. doi: 10.1136/bmj.n156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang M, Zhu L, Gabelle A, et al. Effect of reduction in brain amyloid levels on change in cognitive and functional decline in randomized clinical trials: an instrumental variable meta-analysis. Alzheimers Dement. 2023;19(4):1292-1299. doi: 10.1002/alz.12768 [DOI] [PubMed] [Google Scholar]
- 18.Inker LA, Mondal H, Greene T, et al. Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual-patient meta-analysis. Am J Kidney Dis. 2016;68(3):392-401. doi: 10.1053/j.ajkd.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 19.Inker LA, Heerspink HJL, Tighiouart H, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30(9):1735-1745. doi: 10.1681/ASN.2019010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambers Heerspink HJ, Tighiouart H, Sang Y, et al. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 randomized controlled trials. Am J Kidney Dis. 2014;64(6):860-866. doi: 10.1053/j.ajkd.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 21.Zider AD, Wang X, Buhr RG, Sirichana W, Barjaktarevic IZ, Cooper CB. Reduced COPD exacerbation risk correlates with improved FEV1: a meta-regression analysis. Chest. 2017;152(3):494-501. doi: 10.1016/j.chest.2017.04.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamp L, Morillon MB, Taylor WJ, et al. Serum urate as surrogate endpoint for flares in people with gout: a systematic review and meta-regression analysis. Semin Arthritis Rheum. 2018;48(2):293-301. doi: 10.1016/j.semarthrit.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 23.Topless R, Noorbaloochi S, Merriman TR, Singh JA. Change in serum urate level with urate-lowering therapy initiation associates in the immediate term with patient-reported outcomes in people with gout. Semin Arthritis Rheum. 2022;56:152057. doi: 10.1016/j.semarthrit.2022.152057 [DOI] [PubMed] [Google Scholar]
- 24.Staszewski S, DeMasi R, Hill AM, Dawson D. HIV-1 RNA, CD4 cell count and the risk of progression to AIDS and death during treatment with HIV-1 reverse transcriptase inhibitors. AIDS. 1998;12(15):1991-1997. doi: 10.1097/00002030-199815000-00010 [DOI] [PubMed] [Google Scholar]
- 25.Mills EJ, Kelly S, Bradley M, Mollon P, Cooper C, Nachega J. Antiretroviral effects on HIV-1 RNA, CD4 cell count and progression to AIDS or death: a meta-regression analysis. HIV Med. 2008;9(10):849-857. doi: 10.1111/j.1468-1293.2008.00643.x [DOI] [PubMed] [Google Scholar]
- 26.Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31(2):236-244. doi: 10.1016/j.clinthera.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 27.Palmer SC, Teixeira-Pinto A, Saglimbene V, et al. Association of drug effects on serum parathyroid hormone, phosphorus, and calcium levels with mortality in CKD: a meta-analysis. Am J Kidney Dis. 2015;66(6):962-971. doi: 10.1053/j.ajkd.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 28.Blood Pressure Lowering Treatment Trialists’ Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397(10285):1625-1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15. doi: 10.1016/j.atherosclerosis.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Stauffer ME, Weisenfluh L, Morrison A. Association between triglycerides and cardiovascular events in primary populations: a meta-regression analysis and synthesis of evidence. Vasc Health Risk Manag. 2013;9:671-680. doi: 10.2147/VHRM.S52713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marston NA, Giugliano RP, Im K, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140(16):1308-1317. doi: 10.1161/CIRCULATIONAHA.119.041998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black DM, Bauer DC, Vittinghoff E, et al. ; Foundation for the National Institutes of Health Bone Quality Project . Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672-682. doi: 10.1016/S2213-8587(20)30159-5 [DOI] [PubMed] [Google Scholar]
- 33.Bouxsein ML, Eastell R, Lui LY, et al. ; FNIH Bone Quality Project . Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34(4):632-642. doi: 10.1002/jbmr.3641 [DOI] [PubMed] [Google Scholar]
- 34.Khan FA, Stewart I, Moss S, et al. Three-month FVC change: a trial endpoint for idiopathic pulmonary fibrosis based on individual participant data meta-analysis. Am J Respir Crit Care Med. 2022;205(8):936-948. doi: 10.1164/rccm.202109-2091OC [DOI] [PubMed] [Google Scholar]
- 35.Maiorino MI, Longo M, Scappaticcio L, et al. Improvement of glycemic control and reduction of major cardiovascular events in 18 cardiovascular outcome trials: an updated meta-regression. Cardiovasc Diabetol. 2021;20(1):210. doi: 10.1186/s12933-021-01401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambrosi P, Daumas A, Villani P, Giorgi R. Glycosylated hemoglobin as a surrogate for the prevention of cardiovascular events in cardiovascular outcome trials comparing new antidiabetic drugs to placebo. Cardiology. 2020;145(6):370-374. doi: 10.1159/000506004 [DOI] [PubMed] [Google Scholar]
- 37.Lassere MN. The Biomarker-Surrogacy Evaluation Schema: a review of the biomarker-surrogate literature and a proposal for a criterion-based, quantitative, multidimensional hierarchical levels of evidence schema for evaluating the status of biomarkers as surrogate endpoints. Stat Methods Med Res. 2008;17(3):303-340. doi: 10.1177/0962280207082719 [DOI] [PubMed] [Google Scholar]
- 38.Manyara AM, Davies P, Stewart D, et al. Protocol for the development of SPIRIT and CONSORT extensions for randomised controlled trials with surrogate primary endpoints: SPIRIT-SURROGATE and CONSORT-SURROGATE. BMJ Open. 2022;12(10):e064304. doi: 10.1136/bmjopen-2022-064304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyawali B, Ross JS, Kesselheim AS. Fulfilling the mandate of the US Food and Drug Administration’s accelerated approval pathway: the need for reforms. JAMA Intern Med. 2021;181(10):1275-1276. doi: 10.1001/jamainternmed.2021.4604 [DOI] [PubMed] [Google Scholar]
- 40.Buyse M, Saad ED, Burzykowski T, Regan MM, Sweeney CS. Surrogacy beyond prognosis: the importance of “trial-level” surrogacy. Oncologist. 2022;27(4):266-271. doi: 10.1093/oncolo/oyac006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Meta-Analyses of Clinical Trials
eTable 1. Original Search Results
eTable 1A. Surrogate Marker-Clinical Outcome Pairs From Meta-Analyses of Clinical Trials With Correlation Coefficients, Coefficients of Determination, or Results From Meta-Regression Analyses
eTable 1B. Surrogate Marker-Clinical Outcome Pairs From Meta-Analyses of Clinical Trials With Correlation Coefficients, Coefficients of Determination, or Results From Meta-Regression Analyses Classification for Figure 2
eTable 2. Alzheimer’s Disease
eTable 3. Primary Glomerular Disease
eTable 4. Chronic Kidney Disease
eTable 5. Chronic Obstructive Pulmonary Disease
eTable 6. Gout
eTable 7. HIV
eTable 8. Hypercholesterolemia
eTable 9. Hyperphosphatemia
eTable 10. Hypertension
eTable 11. Hypertriglyceridemia
eTable 12. Osteoporosis
eTable 13. Pulmonary Fibrosis
eTable 14. Secondary Hyperparathyroidism
eTable 15. Type 2 Diabetes
eFigure 1. Asthma
eFigure 2. Cushing’s Disease / Cushing’s Syndrome
eFigure 3. Exocrine Pancreatic Insufficiency
eFigure 4. Hepatitis B
eFigure 5. Hepatitis C
eFigure 6. Hepatitis D
eFigure 7. Hypothyroidism
eFigure 8. Lupus Nephritis
eFigure 9. Mycobacterium Avium Complex (MAC) Lung Disease
eFigure 10. Non-alcoholic Steatohepatitis (NASH)
eFigure 11. Opioid Use Disorder
eFigure 12. Paget’s Disease
eFigure 13. Primary Biliary Cholangitis
eFigure 14. Primary Hyperparathyroidism
eFigure 15. Pulmonary Tuberculosis
eFigure 16. Systemic Sclerosis-Interstitial Lung Disease
eFigure 17. Tobacco Dependence
eFigure 18. Alzheimer’s Disease
eFigure 19. Primary Glomerular Diseases Associated With Significant Proteinuria
eFigure 20. Chronic Kidney Disease
eFigure 21. Chronic Obstructive Pulmonary Disease
eFigure 22. Gout
eFigure 23. Human Immunodeficiency Virus (HIV)
eFigure 24. Hypercholesterolemia
eFigure 25. Hyperphosphatemia
eFigure 26. Hypertension
eFigure 27. Hypertriglyceridemia
eFigure 28. Osteoporosis
eFigure 29. Pulmonary Fibrosis
eFigure 30. Secondary Hyperparathyroidism
eFigure 31. Type 2 Diabetes Mellitus
eReferences.
Data Sharing Statement