Abstract
Under European Union legislation (Article 32, Regulation (EC) No 396/2005), the European Food Safety Authority provides an annual report assessing the pesticide residue levels in foods on the European market. In 2022, 96.3% of the overall 110,829 samples analysed fell below the maximum residue level (MRL), 3.7% exceeded this level, of which 2.2% were non‐compliant, i.e. results in a given sample exceeded the MRL after taking into account the measurement uncertainty. For the EU‐coordinated multiannual control programme subset, 11,727 samples were analysed of which 0.9% were non‐compliant. To assess acute and chronic risk to consumer health, dietary exposure to pesticide residues was estimated and compared with available health‐based guidance values (HBGV). Continuation of the probabilistic assessment methodology was consolidated to all pesticides listed in the 2022 EU Regulation providing the probability of a consumer being exposed to an exceedance of the HBGV. Overall, the assessed risk to EU consumer's health is low. Recommendations to risk managers are given to increase the effectiveness of European control systems and to ensure a high level of consumer protection throughout the EU.
Keywords: acute, chronic, dietary exposure, European Union, food safety, maximum residue levels, national monitoring programme, pesticide residues, probability, risk assessment
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2024.EN-8751/full
SUMMARY
The 2022 EU report on pesticide residues in food provides an overview of the official control activities on pesticide residues carried out in the EU Member States, 1 Iceland and Norway. It summarises the results of both the EU‐coordinated control programme (EU MACP) and the national control programmes (MANCP).
The analysis of the results from all reporting countries is presented in a data visualisation format, 2 to provide stakeholders with a comprehensive, easily digestible analysis of the European situation related to the findings. The conclusions and recommendations derived from the results remain within this report, giving risk managers a tool for designing future monitoring programmes and taking appropriate decisions on which pesticides and food products should be targeted.
The report also includes the outcome of the deterministic risk assessment, both acute and chronic to single substances and the consolidation of the methodology introduced last year on probabilistic exposure assessment to single substances, where probabilities of exceedance of the health‐based guidance values (HBGV) of pesticides have been calculated in different subpopulation of European consumers for the 193 pesticides (corresponding to 199 active substances) listed in the EU MACP Regulation. The purpose of these calculations is to provide readers with a deeper insight into the risks of dietary exposure to pesticides and to evidence the differences between the two methodologies (i.e. deterministic and probabilistic). The probabilistic methodology was found to provide a better estimation of real consumption events.
EU‐coordinated multiannual control programme (EU MACP)
The EU MACP randomly sampled covers the most consumed food products by European citizens as indicated in the EU MACP Regulation (EU) No 2021/601. 3 The control of these products is distributed across a 3‐year cycle, so that every 3 years the same products are analysed. A snapshot of the situation in 2022 of the pesticide residues present in those food products is provided and compared with 2019 and 2016.
In 2022, the 12 food products selected in the EU MACP were apples, strawberries, peaches (including nectarines and similar hybrids), wine (red or white), lettuces, head cabbages, tomatoes, spinaches, oat grain, barley grain, cow's milk and swine fat. A total of 11,727 samples were analysed. 4 Overall, 11,535 samples (98.4%) were found to be within the legal limits. MRLs 5 were exceeded in 192 samples (1.6%), of which 100 samples (0.9%) were found to be non‐compliant when taking into account measurement uncertainty. On average, 66.7% of the samples analysed were domestic, 22% were from other reporting country, 7.7% from third countries and 3.6% were of unknown origin. Similar rates were observed in 2021, except for the domestic samples that increased from 53.3% in 2021 to 66.7% in 2022 and for the import control from third countries that decreased from 19.6% in 2021 to 7.7% in 2022.
National programmes (EU MACP + MANCP)
The 2022 programmes (both EU MACP and MANCP) amounted a total of 110,829 samples. Of the total number of samples analysed, 106,681 samples (96.3%) fell within the legal limits. In total, MRLs were exceeded in 4148 samples (3.7%). When taking into account measurement uncertainty, 2383 samples (2.2%) triggered legal sanctions or enforcement actions. Overall results remain steady in comparison with previous year.
Dietary estimated exposure assessment
Acute and chronic exposure and health risk analysis to consumers was performed using both deterministic and probabilistic exposure modelling. The two approaches address two different assessment questions and are therefore difficult to compare. Whereas the first one calculates risks for ‘hypothetical’ consumers under conservative assumptions, the second one aims at quantifying the probability of true consumers being exposed to an exceedance of the HBGV.
The deterministic acute risk assessment was done using the Pesticide Residues Intake Model (PRIMo rev. 3.1), to the 193 individual pesticides listed in the 2022 EU MACP Regulation. Out of 38,045 samples analysed in this assessment, the estimated exposure exceeded the HBGV for 33 different pesticides in 650 samples (1.7%). In the chronic deterministic assessment, no consumer intake concern was identified.
The probabilistic acute risk assessment revealed that, for 111 active substances of the 163 assessed, the probability of an individual‐day exceeding the HBGV is estimated to be zero for the 40 commodities and 30 surveys covering 30 European subpopulation groups and 17 EU MSs assessed. In the chronic probabilistic assessment, only one active substance in two populations resulted in a consumer exceeding the HBGV, respectively.
The two calculations (i.e. deterministic and probabilistic) used in this report cannot be compared. The one providing a more realistic estimation of what consumers are exposed to, is the probabilistic calculation, as it reflects real consumption events. However, deterministic calculation in the EU is intended to decide whether a lot can be placed on the EU market.
Overall, in the samples analysed in the framework of the 2022 monitoring programmes, the estimated dietary exposure to pesticide residues for which HBGVs are available is very low for most of the EU subpopulation groups assessed. Thus, the assessed risk to EU consumer's health is low. In the cases where the estimated dietary exposure for a specific pesticide/product combination was calculated to exceed the HBGV, and for those pesticides for which no HBGV could be established, the competent authorities took appropriate and proportionate corrective measures to address potential risks to consumers such as withdrawing the product from the market or recalling it before even being placed on it.
1. BACKGROUND
1.1. Legal basis
Pesticide residues, 6 resulting from the use of ‘plant protection products’ (PPP) 7 on crops or food products that are used for food, can potentially pose a risk to public health. For this reason, a comprehensive legislative framework was established in the European Union (EU), which defines rules for the approval of active substances, their uses in plant protection products 8 and their permissible residues in food. To ensure a high level of consumer protection, legal limits or so‐called ‘maximum residue levels’ (MRLs),5 providing a wide margin of safety being based on the most critical good agricultural practice (GAP) for an intended crop and apply to a residue definition for enforcement i.e. to ensure GAP compliant uses of plant protection products (PPP). These MRLs are established in Regulation (EC) No 396/2005. 9 EU‐harmonised MRLs are set covering 381 food products/food groups. The MRLs apply to the pesticide residue, compounds and/or degradation products found after using a PPP. The description of what the MRL covers is known as ‘residue definition for enforcement’ or ‘RD’. However, there may be other residue definitions for risk assessment, which include all relevant metabolites with toxicological relevance. These risk assessment residue definitions are not used in the remit of this report. Furthermore, a default MRL of 0.01 mg/kg is applicable to the pesticides which are not explicitly mentioned in the MRL legislation or to those substances non‐renewed, where the MRL can be set to the lowest quantifiable level which sometimes can be lower than 0.01 mg/kg. The exceedance of an MRL when taking into account analytical measurement uncertainty constitutes a non‐compliant sample. Regulation (EC) No 396/2005 imposes the obligation on Member States to carry out controls to ensure that food placed on the market is compliant with the legal limits. This regulation establishes both EU and national control programmes:
EU‐coordinated control programme: This programme defines the food products and pesticides that should be monitored by all Member States*1 as well as the number of samples per MSs that are to be taken in respect of their population size (EFSA, 2015a) to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues. The EU‐coordinated programme (EU MACP) relevant for the calendar year 2022 was set up in Regulation (EU) No 2021/6013 hereafter referred to as ‘2022 EU MACP Regulation’ or ‘2022 monitoring programme’.
National control programmes: Member States*1 usually define the scope of national control programmes, focussing on certain products, which are expected to contain residues in concentrations exceeding the legal limits, or on products that are more likely to pose risks for consumer safety (Article 30 of Regulation (EC) No 396/2005). This article was superseded on 14 December 2022 by Regulation (EU) No 2017/625, Article 155 10 ). From 15 December 2022, the national control programmes were to be established by Member States*1 in accordance with Regulation (EU) No 2021/1355, 11 hereafter referred to as ‘MANCP’.
Temporary increase of official controls and emergency measures control programmes: in accordance with Regulation (EU) No 2019/1793 12 and its annual revisions 13 , 14 certain food products listed in its annexes are subject to requiring a temporary increase of official controls or emergency measures. These official controls are done at border control posts (BCPs) or at control points (CPs) at their entry into the Union, for a given hazard (e.g. pesticide residues, not approved food additives, mycotoxins, pentachlorophenol, dioxins and microbiological contamination) and for non‐animal origin food and feed coming from a given third country. The outcome of these controls is to be reported through the information management system for official controls (IMSOC). 15 This system does not use the same terminology as the one in EFSA. Until the two systems are aligned with each other, the Commission requested Member States*1 to submit the results of those controls also to EFSA. 16 The analysis of these controls based on the data submitted is presented in Section 4.2.3. However, during 2022 revisions, composite food was included in the annexes to be monitored. This type of food is outside the scope of this report. However, an analysis has been included in the dedicated section, based on the subset collected. These increased import controls are not considered on the exposure/risk assessments (Section 5).
According to Article 32 of Regulation (EC) No 396/2005, EFSA is responsible for preparing an Annual Report on pesticide residues, analysing the data in view of the MRL compliance of food available in the EU and the exposure of European consumers to pesticide residues. In addition, based on these findings, EFSA derives recommendations for future monitoring programmes.
Specific MRLs for food intended for infants and young children were set in Article 10 of Commission Directive 2006/141/EC, 17 Article 7 of Commission Directive 2006/125/EC 18 and Article 4 of Commission Delegated Regulation (EU) 2016/127, 19 taking into account the residue definitions as set out in Regulation (EC) No 396/2005. Following the precautionary principle, the legal limit for these types of food products was set at a low level (limit of quantification); in general, a default MRL of 0.01 mg/kg is applicable unless lower legal limits for the residue levels are defined in the above‐mentioned legislations.
Some of the active substances for which legal limits are set under Regulation (EC) No 396/2005 are also covered by Commission Regulation (EU) No 37/2010 on pharmacologically active substances. 20 For these so‐called dual use substances, Member States1 perform controls in accordance with Council Directive 96/23/EC 21 for veterinary medicinal products (VMPR). Results of the controls for dual use substances are reported within this report if MS Competent Authority has flagged as so in the remit of the 2023 ChemMon data collection (EFSA, 2023a). Otherwise, results are reported in another EFSA output on VMPR residues (EFSA, 2024b).
Regulation (EU) 2018/848 21 on organic production and labelling of organic products defines the restrictions in place for the use of plant protection products, whereas under Regulation (EU) No 2021/1165 23 certain products and substances for use in organic production are authorised. However, the MRLs set in Regulation (EC) No 396/2005 apply equally to organic food and to conventional food.
1.2. Terms of Reference
In accordance with Article 32 of Regulation (EC) No 396/2005, EFSA shall prepare an annual report on pesticide residues concerning the official control activities for food carried out in 2022.
The annual report shall include at a minimum the following information:
an analysis of the results of the controls on pesticide residues provided by EU Member States,*1
a statement of the possible reasons why the MRLs were exceeded, together with any appropriate observations regarding risk management options,
an analysis of chronic and acute risks to the health of consumers from pesticide residues,
an assessment of consumer exposure to pesticide residues based on the information provided by Member States*1 and any other relevant information available, including reports submitted under Directive 96/23/EC. 24
In addition, the report may include a recommendation on the pesticides, products or combinations of them that should be included in future monitoring programmes.
2. INTRODUCTION
This report provides a detailed insight into the control activities at European level and the results from the official control activities performed by the EU Member States,*1 including Iceland and Norway as members of the European Free Trade Association (EFTA) and of the European Economic Area (EEA). 25 This report is intended to provide information to the different stakeholders with an interest and responsibilities in the food chain, in particular food supply chain operators. Its aim is to present a comprehensive overview of residue findings in food placed on the EU market, including possible non‐compliances with legal limits, and to assess the potential exposure of consumers to pesticide residues and possible health risks. Furthermore, it gives recommendations on various possible risk management options where appropriate. The report's findings are systematically used by the Commission and the Member States*1 to establish priorities for controls of food on the market, including the most relevant substance/commodity combinations to be included in the EU MACP regulation or in the national control programmes of Member States.*1 At the same time, the report aims to address questions such as:
How frequently were pesticide residues found in food?
Which food products frequently contained pesticide residues?
Compared with previous years, are there any notable changes?
In which products were breaches of the legal limits identified by the Member States?*1 and what could be the reasons for these breaches?
What actions were taken by the national competent authorities responsible for food control to ensure that pesticide residues in food non‐compliant with the European food standards are not placed on the EU market?
Do the residues in food pose a risk to consumer health?
EFSA developed a data visualisation tool to help end‐users gain insights from the vast amount of data underpinning this report. The 2022 control programme results are presented in Appendix D – Annex I.2 An overall summary evaluation can still be found in Sections 3 and 4 of this report, but figures, maps and tables are in Annex I. The results of the dietary exposure assessments to individual pesticides are described in Section 5, complementary graphs on the acute risk assessment to the EU MACP food products are presented in Appendix B, whereas results of PRIMo rev. 3.1 tool deterministic risk assessments to single substance are presented in Appendix D – Annex II. Appendix C describes the methodologies applied in the probabilistic assessment and in Appendix D – Annex IV–VII, results of the acute and chronic probabilistic assessments are presented. Furthermore, Appendix D – Annex III to Annex VII with complementary data to this report are published in Zenodo. 26 Information on the content of these annexes can be checked in Appendix D.
The websites of the national competent authorities can be seen in Appendix A of this report.
The raw data provided by reporting countries and anonymised by EFSA can also be downloaded from the Open Science platform Zenodo 27 by typing: ‘Member‐State‐Name results from the monitoring of pesticide residues in food’. This report is made by a subset of those, applying different filters.
In addition, EFSA compiled a technical report (EFSA, 2024c) containing the descriptive information of the pesticide monitoring activity by year and submitted by the reporting countries. Here, further details at national level are provided.
3. EU‐MULTIANNUAL COORDINATED CONTROL PROGRAMME (EU MACP)
In compliance with Appendix D – Annex II of Regulation (EU) No 2021/6013, reporting countries sampled and analysed a given number of pesticide/food product combinations.
The EU MACP covers the most consumed food products in Europe. The listed products are distributed across a 3‐year cycle, so that every 3 years, the same products are analysed. In 2022, the food products included were apples, strawberries, peaches (including nectarines and similar hybrids), wine (red or white), lettuces, head cabbages, tomatoes, spinaches, oat grain, barley grain, cow's milk and swine fat. The Regulation allows in case not enough samples of cereals are retrievable, for flour of those cereals to be sampled.
A total of 11,727 samples were reported under the 2022 EU MACP. In 6023 of those samples (51.4%), no quantifiable residues were reported (residues were below the LOQ). The number of samples with pesticide residues within legally permitted levels (at or above the LOQ but below or at the MRL) was 5512 (47.0%). MRLs were exceeded in 1.6% (192) of samples, of which 0.9% (100) were found to be non‐compliant after taking into account measurement uncertainty.
The overall MRL exceedance rate decreased from 2.0% in 2019 to 1.6% in 2022. In 2016, the rate was 1.5% however neither spinaches, nor the two types of cereals (barley nor oats) were to be sampled.
Among individual food commodities, MRL exceedance rates decreased along the 3 years from 2016 to 2019 and 2022 in apples, peaches, strawberries, wine, spinaches (no samples were to be taken in 2016) and swine fat. Notably, cow's milk remained without MRL exceedances over the 3 years. On the other hand, MRL exceedance rates rose from 2016 to 2019 and to 2022 in head cabbages; in tomatoes and lettuces, the rates were higher in 2022 than in 2019, but lower than in 2016. An increase on MRL exceedance was observed in barley and oat grain from 2019 to 2022 (from 0.2% to 2.8% in barley cereals and from 0.2% to 1.5% in oat cereals, respectively). For none of the pesticides found an emergency authorisation was granted in 2022. 28 The MRL exceedances in barley grain were mainly related to prochloraz, where lots were reported to originate from the EU. This substance is not approved in the EU since 31 December 2021. Being cereals a long shelf‐life product, these exceedances might not be such, as it could have been placed on the market in accordance with its approval status the year before but sampled in 2022. Fosetyl (RD) too led to exceedances in barley, an approved substance in the EU but not authorised for use in barley. Regarding oat grains, all MRL exceedances related to six substances reported in samples grown in the EU. Of these, not approved substances reported were linuron, iprodione and chlorpyrifos; whereas approved substances without an authorisation granted on cereals were dimethomorph, fludioxonil and dodine.
The minimum number of 683 samples set in the EU MACP Regulation required to estimate a minimum of 1% MRL exceedances with a margin of error of 0.75% was reached for all commodities except for barley and oat grains (495 samples and 553 samples, respectively), and cow's milk (486 samples). The reason provided by some EU MS on the scarcity of availability of cereal grain in the EU was the Russian war of aggression against Ukraine. No given reason was provided for samples on milk.
The countries sampling the most were Germany (18.3%), Romania (11.4%) and Italy (10.3%). However, the Regulation set a minimum number based on the population size of each country. Therefore, those countries sampling the most in respect of the legal requirement were Romania (5 times more) and Denmark (3 times more). Instead, those sampling the least in respect to the minimum number given on the Regulation were Sweden (49.4%), Greece (57.8%) and Spain (65%).
On average, out of the 11,727 samples collected, 66.7% were domestic samples (an increase compared to 53.3% in 2021), 22% were from other reporting countries, 7.7% from third countries (a reduction compared to 19.6% in 2021) and 3.6% were of unknown origin.
Reporting countries do not have a common approach to take the same rate of domestic, EU or third country samples. However, it is aimed to reflect the market share present in their country. Thus, countries where more than 80% of samples were domestic were Lithuania (100%), Spain (97%), Italy (94.6%) and Greece (93.3%). Those countries that sampled the most from third countries were Northern Ireland (42.2%), Ireland (20.2%) and Iceland (20%). Belgium (20.9%), Germany (11.7%) and Iceland (10.6%) had more than 10% of samples of unknown origin. The highest non‐compliant rate by reporting country were Estonia (4.7%), Malta (4.1%) and Cyprus (2.7%).
Out of 100 non‐compliant samples reported under the EU MACP, 68% were of EU origin whereas, in 27% the place of origin was outside the European market (the remaining 5% were reported to be of unknown origin). From those food products harvested in the EU territory, 50% of the results were coming from non‐approved substances whereas for the food products of origin outside the EU, the percentage of non‐approved substances was 75%. Overall, EFSA recommends widening the analytical scope on import control samples.
For non‐compliant results coming from samples of EU origin, 18 pesticides were reported as non‐approved at EU level. The most highly reported combinations (more than 2 samples) were spinaches/dithiocarbamates (RD) 29 (6 samples), tomatoes/chlorfenapyr (RD) (4 samples), lettuces/thiophanate‐methyl (RD) (3 samples) and barley/prochloraz (RD) (3 samples). Non‐compliant results on 18 approved substances were reported most frequently for: head cabbages/fluazifop (RD) (5 samples).
Among samples reported under the EU MACP grown outside the internal EU market, 12 non‐EU approved active substances were found to be non‐compliant. The combinations most frequently reported were tomatoes/chlorfenapyr (RD) (2 samples, both coming from Morocco), tomatoes/chlorothalonil (RD) (7 samples, all coming from Türkiye), tomatoes/chlorpyrifos‐methyl (RD) (2 samples, both coming from Türkiye), apples/diflubenzuron (RD) (2 samples, both coming from Moldova) and apples/propargite (RD) (2 samples, both coming from Ukraine). Regarding approved active substances, there were four pesticides leading to non‐compliant results. The combinations reported were barley/fosetyl (RD) (2 samples, both coming from United Kingdom), tomatoes/buprofezin (RD) (1 sample from Türkiye), tomatoes/chlormequat‐chloride (RD) (1 sample from Türkiye) and head cabbages/pyridaben (RD) (1 sample from Türkiye).
EFSA recommends reporting countries, to keep monitoring these combinations in their scope of analysis of their analytical methods.
Samples from organic production systems were to be taken too in proportion to the market share of each commodity within each reporting country with a minimum of one sample per commodity listed. In total, 971 organic samples were analysed. EFSA recommends MS to fulfil the requirement on sampling by taking at least one sample per given commodity, if organic farms for the relevant products are available at country level.
In addition, 10 samples of processed cereal‐based baby food were to be sampled by each reporting country. The total number of samples reported under baby food categories amounted to 538 samples. 30 EFSA recommends MS to fulfil the requirement on sampling of at least 10 samples per given type of commodities. A comprehensive analysis of these results is reported in Section 4.3.3 where the data for all baby food samples are pooled together. This category of samples has not been included in Appendix D – Annex I.2
Annex I of Regulation (EU) No 2021/6013 also provides the list of pesticides to be analysed in each EU MACP sample taken by the EU official laboratories. In total, 193 pesticides were listed, of which 167 pesticides were to be analysed in plant origin commodities, 9 pesticides in animal origin commodities and 17 both in plant and animal commodities.
Member States*1 were to analyse these 193 pesticides listed in the EU MACP. Therefore, a target number of required analysis was calculated considering the minimum number of samples (683 samples per commodity) to be reported by each country and comparing it against the total number of reported results. Thirty‐one pesticides did not reach this minimum number of results: spinetoram (RD), mepiquat chloride (RD), heptachlor (RD), glufosinate equivalents (RD), pencycuron (RD), chlordane (RD), parathion (RD), dithianon (RD), fosetyl (RD), DDT (RD), 2‐phenylphenol (RD), cyantraniliprole (RD), methoxychlor (RD), spirotetramat (RD), haloxyfop (RD), hexachlorobenzene (RD), chlormequat‐chloride (RD), 2,4‐D (RD), bromide ion (RD), fluazifop (RD), sulfoxaflor (RD), lindane (RD), alpha‐hexachlorocyclohexane (RD), beta‐hexachlorocyclohexane (RD), glyphosate (RD), pyridalyl (RD), prochloraz (RD), formetanate (hydrochloride) (RD), fenbutatin oxide (RD), dithiocarbamates (RD) and cyflufenamid (RD). Most of these substances require a single residue method (SRM) to be quantified. Thus, EFSA recommends again to encourage MS taking the necessary measures to be able to enforce properly these substances. 31
Of the 11,727 samples, 5704 had quantified results (48.6%). Of those, more than one pesticide was quantified in 3760 samples (32.1%). The food products with a higher rate of multiple residues (greater than 10%) were apples (18.6%), strawberries (17.5%), peaches (16.9%), tomatoes (14.2%) and lettuces (12.4%).
The highest number of multiple residues was found in a sample of tomatoes where 16 different pesticides were quantified followed by strawberries where 15 different pesticides were quantified and red wine where 14 different pesticides were quantified. The tomato and the wine samples were grown in the EU whereas the strawberry sample had an unknown origin.
Detailed analyses are presented in Appendix D – Annex I.2
4. OVERALL MONITORING PROGRAMMES (EU MACP AND MANCP)
The MANCP are risk‐based sampling programmes in accordance with Article 30 of Regulation (EC) No. 396/2005 (superseded by Regulation (EU) No 2021/135511). The focus is on products likely to contain pesticide residues or for which MRL infringements were identified in previous monitoring programmes. These programmes are not designed to provide statistically representative results for residues expected in food placed on the European market.
The reporting countries define the priorities for their national control programmes considering several factors such as the importance of food products in trade or in the national diets, products with historically high residue prevalence or non‐compliance rates in previous years, the use pattern of pesticides and national laboratory capacities. The results of national control programmes cannot be used to compare countries directly as there are specific needs in each country and their dietary habits and access to local products may differ among them. The number of samples and/or the number of pesticides analysed by any reporting country is determined by the capacities of their national control laboratories and available budget resources.
The data analysis of this section is also presented in Appendix D – Annex I.2 The data are displayed into three different sections: geospatial visualisation based on overall number of samples by reporting countries, findings at residue level and analysis at food product level. Non‐compliant findings are considered by risk managers to take decisions on designing the risk based national monitoring programmes in future years. The findings are also a valuable source of information for food business operators and can be used to enhance the efficiency and safety of self‐control systems. The section on reasons for MRL exceedance remains in this report (Section 4.4). More information on the national control programmes can be found in a separate EFSA technical report that summarises the national results (EFSA, 2024c).
In 2022, 110,829 samples were reported belonging to official control programmes (both EU MACP and MANCP pooled together) from reporting countries. Of the total number of samples analysed, in 65,374 of those samples (59.0%), no quantifiable residues were reported (residues were below the LOQ). The number of samples with pesticide residues within legally permitted levels (at or above the LOQ but below or at the MRL) was 41,307 (37.3%). In total, MRLs were exceeded in 4148 samples (3.7%). When taking into account measurement uncertainty, 2383 samples (2.2%) triggered legal sanctions or enforcement actions. Overall results remain steady compared to previous years.
4.1. Geospatial findings
In 2022, the EU Member States,*1 Iceland and Norway, analysed a total of 110,829 samples for pesticide residues on/in food products covered by Regulation (EC) No 396/2005. This marks an increase on the total number of samples taken over the last 10 years.
Additionally, 12 countries reported 2021 feed samples and 9 countries reported 2266 fish samples. No MRLs are established in/on feed nor fish under Regulation (EC) No 396/2005. However, after EFSA's work (EFSA, 2023e), the monitoring of chlorates in fish is advisable. A short summary of the pesticides found in fish has been included in Appendix D – Annex I.2
Of the total number of 110,829 samples analysed, 52.3% were from the reporting countries (i.e. domestic samples), 12.8% from a different reporting country, while 30.9% had been imported to the EU from third countries. The remaining 4.0% were reported as being of unknown origin.
The countries with the highest sampling rates of imported products from third countries were Bulgaria (97.1%) and Croatia (61.9%). Lithuania, Spain and Italy focussed mainly on domestic sampling (more than 80% of the samples analysed). Further, Germany, Iceland, Belgium and Czechia reported the highest rate of samples of unknown origin (14.8%, 8.1%, 7.5% and 7.1%, respectively).
Of the 72,161 samples (65.1%) grown in one of the reporting countries, 47.264 samples (65.5%) were found not to contain any residue above the LOQ, while 23.568 samples (32.7%) contained residues at or above the LOQ but below or equal to the MRL. A 1.8% (1.328 samples) of the samples exceeded the MRL and of these, 1.0% (710 samples) were non‐compliant with the MRL. The remaining 34,193 samples (30.9%) were imported from third countries, of which 15.947 samples (46.6%) were reported as without quantifiable residues, while 15.708 samples (45.9%) contained quantifiable residues within the legal limits. The samples exceeding the MRL were 2.538 (7.4%). Of these, 1537 samples (4.5%) resulted in non‐compliant samples after taking into account measurement uncertainty. The non‐compliance rate in samples coming from third countries (4.5%) was four times higher than the ones from the reporting countries (1.0%).
The remaining 4475 samples (4.0%) were reported as origin unknown of which 6.3% exceeded the MRL and 3.0% led to non‐compliant samples. EFSA recommends reporting countries to report the country of origin of samples leading to non‐compliant results, to allow EFSA drawing more solid conclusions. 32
4.2. Results by pesticide residues
In 2022, a total of 110,829 samples were analysed. Of these, 65,374 samples (59.0%) did not contain quantifiable residues (results below the LOQ for each pesticide analysed) while 37.3% of the samples analysed contained quantified residues not exceeding the legal limits (41,307 samples). Thus, in total, 96.3% of the samples fell within the legal limit (106.681 samples). This tendency seems to be constant for the last years (96.1% in 2021; 94.9% in 2020). The same applies to the MRL exceedance rate (3.9% in 2021; 3.7% in 2022) and the non‐compliant rate (2.5% in 2021; 2.2% in 2022).
More than 22.5 million analytical determinations (individual results per residues) were submitted to EFSA (see Appendix D – Annex III – Table 3.3). The number of determinations for which residue levels were quantified at or above the LOQ amounted for 113,855 (i.e. 0.5% of the total determinations) in relation to the overall number of 110,829 samples.
The reporting countries analysed in total 754 different pesticides. An analytical scope higher than 600 pesticides at country level was noted for Malta (754 pesticides), Germany (721 pesticides), Luxembourg (670 pesticides), Spain (617 pesticides), Austria (616 pesticides) and Belgium (614 pesticides). On average, 261 different pesticides were analysed per sample.
The pesticides quantified in more than 100 samples and where a quantification rate higher than 10% was reported were copper compounds (RD) (82.7%), bromide ion (RD) (17.9%), fosetyl (RD) 33 (17.3%), hydrogen cyanide (RD) (15.0%) and chlorate (RD) (10.5%). The pesticides where the MRL exceedance rate was higher than 1% were copper compounds (RD) (5.1%), ethylene oxide (RD) (2.3%) and chlordecone (RD) (1.0%).
Copper compounds (RD) 34 was reported to have been analysed in 4493 samples. Of which, in 260 samples, the MRL was exceeded (5.8%) and in 132 samples (2.9%) led to non‐compliant results. Most of the samples were of buckwheat and other pseudo‐cereals (128 samples) some with unknown origin or mainly coming from outside the EU (i.e. Paraguay, Bolivia and Uganda); 53 samples were of baby food, 41 samples were of sheep liver coming from Germany, 14 samples of honey and other apicultural products coming from Denmark, 12 samples of bovine liver and 6 samples of wild terrestrial vertebrate animals coming from Denmark. Copper findings tend to be linked to different sources rather than uniquely from a pesticide use. It is a naturally occurring substance, but it can also be present in the diet as food additive or in feed given to livestock. An update of maximum residue levels (MRLs) for copper compounds 35 in light of EFSA's scientific opinion on the re‐evaluation of the HBGV (EFSA Scientific Committee, 2023) having considered all sources of exposure, is currently ongoing. Furthermore, Regulation (EU) No 2023/731 36 requests copper to be analysed as part of 2024 EU MACP.
Ethylene oxide (RD). This substance is not approved at EU level. Its MRL is set at the LOQ level. However, out of 2026 samples where ethylene oxide was analysed, in 47 samples, the MRL was exceeded (2.3%). Of those, six samples were of curcuma coming from India, five samples were of chilli peppers from India and Uganda, five samples of peppercorn from India, Vietnam and Lebanon and four samples of dried beans from India. Based on 2022 37 Rapid Alert System for Food and Feed (RASFF) 38 notification report, a decrease in the number of notifications was observed compared to 2021. Due to the still significant number of residues reported, EFSA recommends keep monitoring ethylene oxide in line with the latest revision of the increase of official controls Regulation (EU) 2023/1110 39 (prior to publication of this report) set the frequency of controls at borders to 20% for certain given food products. 40
Chlordecone (RD) 41 was reported to have been analysed in 10,701 samples. 42 Of which, in 110 samples, 43 the MRL was exceeded (1.0%). 44 Of those, 86 45 samples were reported in fat (74 in bovine fat, 6 in swine fat and 6 more in sheep fat) coming from France. In cassava roots coming from the French oversea territories (Guadeloupe and Martinique, and Dominica), the MRL was also exceeded in 18 samples. EFSA derived temporary MRLs for chlordecone in certain products of animal origin with the health‐based guidance values derived by the French authorities to whom the presence of this pesticide is known (EFSA, 2020a). In accordance with the policy for persistent organic pollutants, existing MRLs should be regularly reviewed, taking into account results from pesticide monitoring programmes, since contamination of food is expected to gradually decrease over time.
Details on the samples exceeding the MRL can be consulted in Appendix D – Annex III – Table 3.2.
4.2.1. Multiple pesticide residues
Multiple residues in one single sample may result from the application of different types of pesticides (e.g. application of herbicides, fungicides or insecticides against different pests or diseases) or the use of different active substances aiming at avoiding the development of resistant pests or diseases and/or uptake of persistent residues from soil from treatments used in previous seasons or spray/dust drift to fields adjacent to treated fields. In addition to multiple residues resulting from the agricultural practice, multiple residues may also occur as a result of mixing or blending products with different treatment histories at different stages in the supply chain, including contamination during food processing. According to the present EU legislation, the presence of multiple residues within a sample remains compliant, as long as each individual residue level does not exceed the individual MRL set for each active substance.
Of the 110,829 samples analysed, 45,455 samples (41.0%) contained one or several pesticides in quantifiable concentrations. Multiple residues were reported in 25,499 samples (23.0%); in an individual sample of chilli peppers under the form of paprika powder of unknown origin, up to 43 different pesticides were reported.
The highest frequency of multiple residues in unprocessed products was reported for sweet peppers, table grapes, strawberries, apples, peaches, tomatoes, oranges, lemons, pears, lettuce and mandarins.
The highest frequency of multiple residues in processed food samples was reported in table grapes as raisins, wine grapes as red wine, cumin seed as dried herb, grape leaves and similar species as salted vegetables, paprika powder and polished rice.
4.2.2. Results on glyphosate
Glyphosate is approved for use in the EU until 15 December 2033. 46 Therefore, related to the 2022 data in this report, glyphosate could be used as an active substance in Plant Protection Products (PPPs), subject to each PPP being authorised by national authorities following an evaluation of its safety. In this section, all data received relevant to the parent or to any of the metabolites/degradation products is presented.
EFSA considers an analysis on the occurrence data received on glyphosate of interest. In 2022, glyphosate was reported by 25 countries analysing 15,307 samples of different food products, of which 444 were samples from feed.
Regarding food samples, in 14,563 of the samples (98.0%) glyphosate was not quantified. In 258 samples (1.7%), glyphosate was quantified at levels above the LOQ but below the MRL and in 42 samples (0.3%) the residue levels exceeded the MRL, mainly in buckwheat and other pseudo‐cereals. The exceedance rate was slightly higher than in 2021 (0.15%). Of these, after taking into account measurement uncertainty, 22 samples (0.15%) were non‐compliant. Glyphosate residues were analysed in 599 baby food samples and were all below the LOQ, except for one sample that exceeded the MRL.
Glyphosate metabolites were analysed in different food samples: AMPA (9322 samples), AMPA‐N‐acetyl (825 samples) and N‐acetyl glyphosate (7145 samples) and trimethyl‐sulfonium cation (5874 samples). AMPA was quantified in nine samples (0.097%), mainly in soyabeans. No quantified sample was reported for AMPA‐N‐acetyl nor N‐acetyl glyphosate, only relevant in genetically modified organism crops.
Trimethyl‐sulfonium cation resulting from the use of glyphosate was quantified in 46 samples (0.78%), mainly in cultivated fungi and tea.
In crops or parts of crops exclusively used for animal feed production, where MRLs are not set, the following substances were quantified: glyphosate (40.8%), AMPA (10%) and trimethyl‐sulfonium cation (4.5%); no results were reported as above LOQ on N‐acetyl glyphosate and no AMPA‐N‐acetyl result was reported. According to the MRL review (EFSA, 2019d), there are uses authorised on grass and other feed items with very high application rates. With the new restrictions of approval, these uses will not be authorised any longer in the EU and concentrations found in feed items are likely to decrease.
4.2.3. Results on temporary increase on import controls
According to the provisions of Regulation (EU) 2019/179312 on temporary increase on import controls, certain foods were subject to an increased frequency of official controls for certain pesticides at border control posts (BCPs) into the EU territory. The data presented in this section are a subset of the one sent by reporting countries to TRACES 47 through the Integrated Management System for Official Controls (IMSOC) 48 platform. Some of these controls may enter the Rapid Alert System for Food and Feed 49 of the European Commission. More information can be found in 2022 RASFF report 50 (European Union, 2023).
The total number of samples reported to EFSA covered by Annex I of Regulation 396/2005 were 561 samples. Of those, 52 samples (9.3%) were considered non‐compliant with EU legislation on pesticide residues.
The 2022 revisions of this Regulation included composite food products. 51 Of these composite food, 169 samples 52 were reported to EFSA where of those, two samples (1.2%) lead to non‐compliant (e.g. ethylene oxide in ‘Asian‐style noodles other than glass noodles’ and in ‘food supplements and similar preparations from India’).
The results presented in this section are based on the data reported directly to EFSA for the sampling year 2022. Other data might have been reported directly to DG SANTE. 53 Therefore, this section may not give the overall picture of the situation.
A description of the required controls regarding hazard analysis, type of food products and countries of origin, relevant for the calendar year 2022 can be found in Appendix D – Annex III – Table 3.4.
4.3. Results by food products
4.3.1. Results by processed versus unprocessed food products
Of the 110,829 total samples reported in 2022, 9117 samples (8.2%) were of processed food, excluding 1783 baby food samples (Section 4.3.3). The compliance of these samples is checked against the maximum residue levels in the respective raw agricultural commodity after applying a processing factor derived for the given processed technique as per Article 20 of Regulation (EC) No 396/2005. 54 In 340 samples (3.7%), residues exceeding the corresponding MRL were found. Of these, 212 samples (2.3%) were non‐compliant taking into account the measurement uncertainty. Both rates are lower than in 2021, where the MRL exceedance rate was 4.5% and the non‐compliant rate was 3.1%.
The processed food products with a non‐compliance rate higher than 10% and more than 10 samples reported were grape leaves and similar species (40.8%) mainly coming from salted and canned processing, dried cumin seed (20.0%), dried parsley (19.1%), dried wild fungi (14.3%) and basil and edible flowers (10.5%).
On the contrary, 99,929 samples (90.2%) were reported as unprocessed food products. 55 Of these, 3735 samples (3.7%) had residues exceeding the MRL, of which 2156 samples (2.2%) were non‐compliant after taking into account measurement uncertainty. Those unprocessed food products for which more than 100 samples were reported and the non‐compliance rate was higher than 10% were basil and edible flowers (31.3%), passion‐fruits/maracujas (15.6%), buckwheat and other pseudo‐cereals (14.9%), chilli peppers (13.7%), okra (lady's fingers) (12.9%), sheep liver (12.6%), cassava roots/manioc (12. 4%) and granate apples/pomegranates (10.3%).
4.3.2. Results on organic products
No specific MRLs are established for organic products. The MRLs set in Regulation (EC) No 396/2005 apply equally to organic food and to conventional food. However, Regulation (EU) No 2021/116523 authorises certain products and substances for use in organic production. In 2022, the quantification and MRL exceedance rates were lower in organic food compared to conventionally produced food (i.e. non‐organic) for all food product categories except for animal products (the quantification rate) and cereals (the exceedance rate). However, this was due to copper, a substance authorised in organic farming, having other uses such as feed supplement and fertilisers.
In 2022, 6717 samples labelled as organic (excluding baby food) were reported, corresponding to 6.1% of the total samples, a slight decrease compared to 2021 (7.4%). Of those, 971 samples were reported under the EU MACP.
Overall, 5305 samples flagged as organic did not contain quantifiable residues (79% of the analysed samples vs. 82.8% in 2021); 1252 samples contained quantified residues below or at the MRL level (18.6% vs. 15.4% in 2021) and 160 samples were reported with residue levels above their corresponding MRLs (2.4% vs. 1.8% in 2021), of which 1.4% (92 samples) were non‐compliant.
The pesticides with higher quantification rate (i.e. at levels above the LOQ but below the MRL) were copper compounds (RD) 56 (688 samples, 78.3%, mainly in cereals), bromide ion (RD) (101 samples, 14%, mainly in tomatoes, lettuces and oat) and chlorates (RD) (86 samples, 8.6%, mainly in buckwheat and other pseudo‐cereals). The pesticides exceeding the MRL the most was copper compounds (RD) (105 samples, 12%).
Most of the quantified substances often present in samples flagged as organic, are either because they are authorised for use (e.g. copper compounds), they occur naturally (e.g. bromide ion, copper), they occur as degradation product of a sanitisation process (e.g. chlorate) or are persistent contaminants of already banned substances (e.g. hexachlorobenzene [RD]). The highest MRL exceedance rate (i.e. above 10%) was 11.9% (105 samples) reported in copper compound mainly in ‘buckwheat and other pseudo‐cereals’ samples.
The occurrence of other pesticides not authorised in organic farming can – as for conventional products – be the result of authorisation in organic farming (copper), spray drift, environmental contaminations or contaminations during handling, packaging, storage or processing of organic products. This occurrence could also be linked to the incorrect labelling of conventionally produced food as organic food. Therefore, EFSA recommends reporting countries to elucidate possible reasons for occasionally quantified findings not permitted in products labelled as organic. EFSA also recommends widening the analytical scope on organic samples as much as possible.
4.3.3. Results on baby food
Reporting countries analysed 1783 samples of foods for infants and young children as defined in Regulation (EU) No 2016/12719 and Directive 2006/141/EC,17 herein referred to as baby food. The types of samples were 1142 samples of baby foods other than processed cereal‐based foods, 259 samples of processed cereal‐based foods for infants and young children, 147 samples of infant formulae, 118 samples of food for infants and young children and 117 samples of follow‐on formulae.
From the overall number of baby food samples analysed, 739 samples (41.5%) were flagged as organic samples. Of the total, 538 baby food samples (30.2%) were flagged as EU MACP.
In 1441 samples (80.8%), no residues were quantified (a rate lower than in 2021 – 87.5%). Quantified samples with residues at or above the LOQ but below the MRL were found in 269 samples (15.1%).
The MRLs in baby food are established at the default MRL of 0.01 mg/kg, except for a given number of substances which are set much lower17 (EFSA, 2018c). In 2022, in average, 898 different pesticides were analysed.
The substance most widely found in quantified concentrations was copper compounds in 61.8% of the samples. Copper compound is a naturally occurring substance but can also be present in the diet being a food additive or intake by livestock through feeding stuff. Moreover, when reporting pesticide residues in infant food, in accordance with Article 2 (3) of Regulation (EU) No 2021/601, 57 the results shall be reported based on the reconstituted product. When reconstituting a sample mainly water is added to the process. The adding of water can be a significant contributor of copper. 58 The presence of copper in baby food can also be explained by the authorisation as a microelement in the formulae's manufactured from cows' milk proteins or protein hydrolysates (EFSA, 2014a).
In 75 samples (4.2% of samples), the MRL was exceeded. Of these, when measurement uncertainty was taken into account, 15 samples led to non‐compliant results (0.8% of samples), practically the same as in 2021 (0.6%) but lower than in 2020 (1.7%). The pesticides most frequently found to exceed the MRL were copper compounds (RD) (13.6% of samples) and chlorates (RD) (1.6% of samples). While copper compounds are naturally occurring substances, chlorate findings are explained as occurring after sanitisation practice in the food change; thus, its presence is not due to a pesticide use.
4.3.4. Results on animal products
A total of 23,377 samples of animal products were reported. The results showed that 21,593 samples were free of quantifiable residues (92.4% vs. 85.9% in 2021) while 1540 samples (6.6%) contained pesticides in quantifiable concentrations at or below the MRL, a significant decrease compared to 2021 (12.8%). MRL exceedances were identified in 244 samples (1.0% vs. 1.3% in 2021), of which 134 (0.6%) were deemed non‐compliant when measurement uncertainty was taken into account.
Regarding the animal commodities with hight MRL exceedance (above 10 samples) were bovine fat (76 samples), honey and other apicultural products (54 samples), sheep liver (44 samples), cow's milk (21 samples), swine fat (15 samples) and bovine liver (12 samples).
The pesticides with a higher quantification rate were copper compounds (RD) (71.4%), chlordecone (RD) (16.7%) and bromide ion (RD) (14.8%).
Regarding MRL exceedance frequency: chlordecone (RD) 59 exceeded the most in 86 fat samples, of which 74 were of bovine fat, six samples of sheep fat and six samples of swine fat. Copper compounds (RD) followed exceeding MRL in 76 samples with the following distribution: 41 sheep liver samples, 14 honey and other apicultural products samples, 12 bovine liver samples, 6 wild terrestrial vertebrate animal's samples and 3 poultry muscle samples. Copper compound findings in animal commodities tend to be linked not only to pesticide uses but to the use as feed supplement taken up by livestock. Benzalkonium chloride (BAC) (RD) (13 cow's milk samples and 1 swine fat sample) and didecyldimethylammonium chloride (DDAC) (RD) (7 cow's milk samples, 1 swine liver samples, 2 swine fat samples, 1 bovine muscle sample and 1 muscle sample of other farmed terrestrial animals) were found mostly in products derived from milk such as cream due to its generation when sanitising agent products are used under industrial practice.
In honey, 1272 samples were reported. In 1078 samples (84.7%), no quantifiable levels of residues were reported (residues were below the LOQ). The number of samples with pesticide residues within the legally permitted levels (at or above the LOQ but below or at the MRL) was 148 (11.6%). MRLs were exceeded in 46 samples (3.6% vs. 2.1% in 2021), of which 28 samples (2.2% vs. 1.6% in 2021) were found to be non‐compliant taking the measurement uncertainty into account. In total, 32 different pesticides were reported in honey samples. The most frequent quantified pesticides were acetamiprid (59 samples, leading to 10 samples exceeding the MRL), thiacloprid (32 samples, without MRL exceedance) and amitraz (30 samples, without MRL exceedances). A decrease of thiacloprid findings is noted due to the stop at EU level of its use. EFSA recommends that reporting countries keep analysing animal products for these substances.
Despite no MRLs are applicable to fish under Regulation (EC) No 396/2005, 2266 fish samples were reported, covered by an analytical scope of 277 pesticides. In 57 samples (2.5%), pesticide residues at levels quantified at or above the limit of quantification were reported. In total, nine different pesticides were reported. The most frequent pesticides quantified in more than 10 samples were copper compounds (RD) (12 results in tuna samples), DDT (RD) (14 results of which 6 in fish meat samples, 2 in herring samples and 2 in smelt samples) and mercury (RD) (10 results in tuna samples). These findings cannot be directly linked to recent pesticide uses.
4.4. Reasons for MRL exceedances/non‐compliances
The legal limits (MRLs) are established based on supervised residue trials that reflect the residue levels expected under field conditions or for animal products, animal feeding studies based on appropriate dietary requirements of different food‐producing animals. The MRL value is estimated using statistical methods and is usually established to cover at least the upper confidence interval of the 95th percentile of the expected residue distribution. Therefore, a percentage of approximately 1% of MRL exceedances are expected even if good agricultural practices (GAP) are fully respected. A sample is non‐compliant when at least one pesticide is quantified at a level that after taking into account measurement uncertainty, the lower tail of the distribution is above the MRL value (European Commission, 2021). When a non‐compliant sample is identified, a call for action at Member State level in line with Article 50 of Regulation (EC) No 178/2002 60 is required. Generally, Member States*1 reply with appropriate measures to non‐compliances (e.g. administrative fines, RASFF notifications 61 and follow up actions, etc.).
In 2022, out of 110,829 samples reported, 4148 samples contained pesticide residues exceeding their respective MRLs (3.7%), very similar to 2021 (3.9%). When taking into account measurement uncertainty, 2383 samples resulted into non‐compliance (2.2%), similar to 2021 (2.5%).
Several possible reasons for MRL exceedances are summarised below:
-
•
For samples coming from third countries:
-
–
The use of non‐approved pesticides for which no import tolerance 62 is granted (either because not requested or because having done so, the request was unsuccessful) (e.g. chlorpyrifos in cumin seed and rice from India, in dried beans from Madagascar, in oranges from Egypt, in table grapes from Iran and grapefruits from Türkiye; imidacloprid in rice from India and Pakistan, in grape leaves from Egypt; tricyclazole and thiamethoxam in rice and cumin seed from India).
-
–
GAP not respected or registered use follows different treatment pattern: use of approved pesticide deviating from the application rates, preharvest intervals, number or method of applications (e.g. acetamiprid in teas from China, in grape leaves from Egypt, in cumin seed from India and in granate apples/pomegranates from Türkiye; lambda‐cyhalothrin in teas from China, grape leaves from Türkiye and Egypt, in passion fruits/maracujas from Colombia and buprofezin in lemons and sweet peppers/bell peppers from Türkiye).Contamination from previous pesticide use: uptake of residues from the soil (e.g. persistent pesticides used in the past such as chlordecone in French overseas territories in tannias and dasheen taros).
-
–
Processing techniques used in third countries mainly with the view of reducing microbiological contamination (i.e. Salmonella sp. in sesame seeds), found to lead to not approved residues (e.g. ethylene oxide in guar gum and curry powder from India).
-
–
Misuses of non‐approved pesticides (e.g. chlorpyrifos‐methyl in grapefruit from Türkiye, propiconazole in mandarin from South Africa and imidacloprid in dragon fruit from Thailand).
-
•
For samples originating from the internal market (reporting countries):
-
–
Use of approved pesticides but not in the crop for which the GAP is authorised (e.g. fluazifop‐P in head cabbage and lettuces, imazalil in potatoes, pirimiphos‐methyl in tomatoes, MCPA and MCPB in barley grain and fosetyl in barley grain).
-
–
GAP not respected in accordance with application rates, preharvest intervals, number or method of applications of the pesticide product (e.g. deltamethrin in spinaches, lambda‐cyhalothrin in celeries).
-
–
Cross contamination: spray drift or other accidental contamination (e.g. acetamiprid and prosulfocarb in kales, chlorpropham in potatoes when stored in facilities with a history of this pesticide having been used)
-
–
Environmental contamination resulting from inappropriate application (e.g. prosulfocarb in parsley, nicotine in bananas)
-
–
Use of non‐EU approved pesticides (e.g. acrinathrin in spinach, chlorpyrifos in Chinese cabbage and in barley grain, chlorpyrifos‐methyl in grapefruits and chlorfenapyr in tomatoes) that have not been subject to emergency authorisations 63 granted during 2022.
-
–
Natural presence of the substance in the crop (e.g. copper compounds in buckwheat and other pseudo‐cereals).
-
–
Presence of biocide residues used as pesticides in the past and continuing to be monitored under the pesticide legislation (e.g. chlorate in different food commodities).
-
–
Environmental contamination of persistent organic pollutants (POP) included in the Stockholm Convention of prohibited substances (UNEP, 2001). These substances are no longer used as pesticides but are very persistent in the environment and found to contaminate and concentrate in the food chain (e.g. dieldrin in carrots or in squash).
More details on the pesticide/crop combinations exceeding the legal limits are compiled in Appendix D – Annex III – Table 3.2.
5. DIETARY EXPOSURE AND ANALYSIS OF HEALTH RISKS
Regulation (EC) No 396/2005, Article 32, requests EFSA to conduct an analysis on the health risks to European consumers and publish this within its annual report on pesticide residues. This analysis is based on the results from the official controls provided by reporting countries, which are combined with data on food consumption to obtain estimates of exposure to those pesticide residues.
The analyses of the risk to consumer health are done by acute (short‐term) and chronic (long‐term) exposure assessments. EFSA relates the estimated amount of a residue intake with its corresponding health‐based guidance value (HBGV). These values set the estimated residue intake in food without an appreciable risk to consumers.9
For acute risk assessment, the short‐term dietary exposure from a pesticide residue is compared to the substance's acute reference dose (ARfD, in mg of residue/kg bw).
For chronic risk assessment, the long‐term dietary exposure from a pesticide residue is compared to the substance's acceptable daily intake (ADI, in mg of residue/kg bw per day).
The HBGV (i.e. ARfD and ADI) derived at EU level in the framework of assessments under Regulation (EC) No 1107/2009 or Regulation (EC) No 396/2005 were selected, when available. In case it was agreed that an ARfD/ADI was not needed, no risk assessment is performed.
Active substances for which EFSA's most recent assessment could not conclude on the establishment of a HBGV were treated according to one of the following two cases:
The assessment of the genotoxic potential in vivo of the substance could not be completed (e.g. insufficient data): In such case, a tentative risk assessment was conducted using HBGV based on the current knowledge (e.g. dimethoate (EFSA, 2018g)).
The substance was concluded to be an in vivo mutagen: In such cases, it was considered not possible to set any HBGV, and thus, the maximum residue levels (MRLs) were established at the limit of quantification to protect consumers, but no assessment was conducted within the remit of this report (e.g. omethoate; EFSA, 2017b).
For substances that were never reviewed by EFSA, HBGV established by other bodies (e.g. EPA, JMPR) were used. In some cases, the use of ADI (in acute assessments) or tolerable daily intake (TDI, in mg of residue/kg bw per day) (in chronic assessments) values were used as a (conservative) surrogate of the HBGV. These assessments were considered tentative.
In this report, the way in which the estimated exposure based on the residue level in the food analysed is related to the amount eaten reported in a given consumption survey undergoes two different approaches: the deterministic and the probabilistic. In the deterministic is considered only one point estimate, normally the highest whereas in the probabilistic there is a correlation between residue concentration and consumption, done by Monte‐Carlo simulation, i.e. samples are selected at random multiple times, capturing a more realistic situation.
Probabilistic calculations cannot be seen as a refinement of deterministic calculations. Such a refinement of the deterministic calculations by probabilistic modelling would require specific conditions, as described in the guidance of the EFSA PPR Panel (2012) with respect to the ‘high residue event’ case. The probabilistic methodology introduces an estimation of the exposure in relation to the underlying selected population. It calculates the overall risk in this population, based on the actual consumption of the most consumed food commodities by real consumers and based on all the occurrence data present in those food commodities.
From the above, the reader needs to understand that the deterministic and probabilistic calculations provide different types of information.
5.1. Acute deterministic risk assessment
The deterministic exposure assessment to single substances of the acute health risk to consumers has been performed using the International Estimation of Acute Intake (IESTI) (FAO, 2017) equation implemented in the Pesticide Residues Intake Model (PRIMo) (EFSA, 2018a) based on its revision 3.1 (EFSA, 2019c). The IESTI equation (i.e. depending on the type of food commodity cases: 1, 2a, 2b and 3) has been adjusted by substituting the highest residue (HR) from field residue trials with the highest concentration found in occurrence data by pesticide/food commodity combination. All the other parameters have remained the same as those applied in PRIMo rev. 3.1.
In Appendix D – Annex II, the outcome of the deterministic exposure assessments is included. The ARfD values used are reported in Appendix D – Annex III – Table 3.5, indicating if a tentative assessment was conducted.
5.1.1. Methodology for the estimation of acute deterministic exposure
In the case of acute deterministic exposure assessment, the risk refers to a consumer that would consume a large portion of one single commodity containing a high residue concentration of one pesticide (i.e. in this report, for each pesticide/commodity combination, the lots containing the highest recorded concentration of residues). For each pesticide, separate calculations are performed for each of the most consumed commodities. Due to the in‐built assumptions of the deterministic model, the likelihood of such an extreme exposure event is not considered. For example, a single high residue found in/on a specific pesticide/commodity combination will always generate the same estimation of the exposure, irrespective if it is based on a single finding (above the LOQ) or in multiple quantified residues in different samples with higher chances of being consumed. In parallel, the large portion for the respective food commodity is derived from the 97.5th percentile of the portion size for consumers only and does not consider the frequency of consumption.
For the first time, the 36 commodities listed in the 3‐year cycle of the EU MACP Regulation are covered (i.e. apples, aubergines/eggplants, bananas, broccoli, carrots, cauliflowers, cultivated fungi, grapefruits, head cabbages, kiwi fruits (green, red, yellow), lettuces, melons, onions, oranges, peaches, pears, potatoes, spinaches, strawberries, sweet peppers/bell peppers, table grapes, tomatoes, dry beans, barley, oat, rice, rye, wheat, olive oil, wine, eggs (chicken), liver (bovine), fat (bovine), fat (poultry), fat (swine) and milk (cattle)) together with the 193 pesticides listed in the same regulation.
The results reported in the framework of the 2022 EU MACP were pooled with the results reported under the national programmes of the same year, excluding all samples reported as suspect sampling.
A total of 38,045 samples were subject to this assessment of which, 25,780 samples (67.8%) were taken under the framework of the national programmes. The assessment was based on the following considerations:
The exposure calculations were carried out separately for each pesticide/commodity combination as it is considered unlikely that a consumer would eat two or more different food products in large portions within a short period of time and that all these food products would contain residues of the same pesticide at the highest level observed during the reporting year.
For each single pesticide/commodity combination, the highest measured residue concentration reported to EFSA for the year 2022 was used as input value for the calculation of the exposure according to the IESTI methodology implemented in EFSA PRIMo rev. 3.1 (EFSA, 2018a, 2019c). This also applies to products that are usually bulk/blended and processed before they are placed on the market (e.g. cereal grains, dry beans, olive oil or wine). According to the IESTI methodology, the exposure should be calculated based on median residue concentration, to reflect the fact that residues in the consumed product reaching the consumer are levelled out. However, taking the highest residue for these blended commodities should account for the heterogeneity found in the market and the fact that the samples analysed in the EU MACP are mostly taken close to the consumers (e.g. retailer or wholesale level). This is a realistic approach followed by EFSA also in previous reports (EFSA, 2018e, 2021a). When considering cereal grains to retrieve the highest residue concentration, results from raw grains and whole grain flour 64 were pooled, accounting for one of the 36 commodities. This is a conservative element that is likely to lead to an overestimation of the dietary exposure.
The variability factor is estimated to be five or seven times higher in the first unit of a food product consumed than the residue concentration measured on the overall sample. This aims to cover the non‐uniform residue distribution among the individual units in the same sample. For food commodities with a unit weight of more than 250 g (i.e. aubergines (egg plants), broccoli, cauliflower, grapefruit, head cabbage, lettuce, melons and table grapes), a variability factor of 5 was applied. For mid‐sized products with a unit size anywhere from 25 to 250 g (i.e. apples, bananas, carrots, cultivated fungi, kiwi, onions, orange, peaches, pears, potatoes, tomatoes and sweet peppers), a variability factor of 7 was applied; no variability factor was used for commodities with unit weights less than 25 g, processed, blended or animal products (i.e. virgin olive oil, wheat, bovine fat and chicken eggs). 65 This approach does not differ from the one used in PRIMo rev. 3.1.
Consumption data used for the deterministic exposure assessment were those used in PRIMo model revision 3.1 reported for the respective unprocessed product (EFSA, 2018a, 2019c). The 97.5th percentile of the consumption distribution was taken for the 11 Member States (Belgium, Czechia, Germany, Denmark, Finland, France, Ireland, Italy, Lithuania, Poland and Spain) derived from national food surveys for different population age groups. In cases where the number of respondents reporting consumption of a certain commodity were low, alternative percentiles were selected.
Considering that some food items may undergo treatment before consumption (e.g. peeling, cooking, etc.), processing factors (PFs) were used in the estimation of the exposure for specific pesticide/crop combinations when available. The source to retrieve processing factor was the EU processing factor database (Zincke et al., 2022) and other more recent EFSA outputs. In the absence of PFs, it is assumed that the residues on the raw commodity are entirely transferred to the product as consumed. Appendix D – Annex II – Table 3.6 contains a list of the processing factors for pesticide/crop combinations used in the context of this acute risk assessment, different than 1.
Monitoring data reported to EFSA are based on different sampling approaches. For the EU MACP, random sampling it taken (coded under EFSA terminology as selective sampling using ST10A code) aiming at representing the market. Samples taken under the various national programmes (MANCP) that are carried out following risk‐based sampling (Art. 30 of Regulation (EC) No 396/2005 and Regulation (EU) No 2021/135511) are still considered when assessing consumer exposure (coded under EFSA terminology as objective sampling using ST20A code). Under the increased control plans (Regulation (EU) No 2019/179312), suspect samples do not reflect food placed on the market as they are border inspections rejected from entering the EU territory if not compliant with the MRL (coded under EFSA terminology as ST30A code) (EFSA, 2023a). Samples obtained through suspect sampling (ST30A), were considered not representative for this assessment and as such excluded. Sensitivity analysis undergone by EFSA testing the impact of pooling selective sampling (ST10A) and objective sampling (ST20A), showed that the exposure calculation did not significantly affect the outcome (EFSA, 2022a). Thus, both types of sampling plans were pooled for the acute exposure assessment.
Fat‐soluble pesticide residues reported in bovine, swine or poultry meat 66 samples were recalculated to the fat fraction assuming default fat contents of 20%, 20% or 10%, respectively (if fat percentage was not reported) (FAO, 2017). This approach was implemented only in the cases of samples with quantified residues (results ≥ LOQ).
Pesticide/commodity combinations for which no sample had quantified residues were not considered in the acute exposure assessment. These samples are considered safe to the consumer.
The exposure estimation to pesticides was based on the residue levels expressed according to the definition established for enforcement (which is in accordance with the EU MRL legislation) and were not converted into the residue definition defined for risk assessment. No proper database exists with factors that convert one into the other. This approach may lead to an underestimation of the estimated exposure assessment as the risk assessment residue definitions often contain metabolites that are not included in the enforcement one..
Results from samples analysed with analytical methods for which the LOQ was greater than the corresponding MRL were disregarded.
For bromopropylate (RD), alpha‐hexachlorocyclohexane (RD), beta‐hexachlorocyclohexane (RD), chlordane (RD), dieldrin (RD), heptachlor (RD), hexachlorobenzene (RD), hexaconazole (RD), methoxychlor (RD) and permethrin (RD), the acute risk assessment was performed with the available ADI/pTDI reference value. ARfD values are not currently available for these pesticides. The use of the ADI/TDI instead of the ARfD is a conservative element to consider in the risk assessment because for most pesticides, the ADI/TDI is set at a lower level than the ARfD.
For the legal residue definition of fenvalerate containing esfenvalerate (a compound with a different toxicological profile), the acute risk assessment was based on the ARfD of the authorised active substance esfenvalerate (EFSA, 2014b).
For the legal residue definition of ‘cyfluthrin (cyfluthrin including other mixtures of constituent isomers (sum of isomers))’, based on EFSA opinion (EFSA, 2020b), the cyfluthrin toxicological profile was taken and HBGV derived for cyfluthrin used in this assessment.
For the legal residue definition of lambda‐cyhalothrin (including gamma‐cyhalothrin) (sum of R,S and S,R isomers), the acute risk assessment was based on the two isomers. Thus, two different scenarios were built, one using the acute toxicological profile of lambda isomer and another using the acute toxicological profile of gamma isomer being both approved active substance (EFSA, 2017a).
For cypermethrin residue definition (cypermethrin including other mixtures of constituent isomers (sum of isomers)), the toxicological profile selected to perform the risk assessment was the one of cypermethrin (mixture of all) being the only approved combination in the EU (EFSA, 2018f).
Most of the active substances belonging to the group dithiocarbamates were not approved in the EU in 2022 (except metiram and ziram). However, in view of having a thorough coverage of all possible uses and misuses, all six active substances (mancozeb, maneb, metiram, propineb, thiram and ziram) were considered in the risk assessment. Furthermore, no analytical method is available to differentiate residues from each of the active substances.
5.1.2. Results
The results of the acute deterministic risk assessment are summarised in Figure 1. The numbers in the cells should be read and interpreted based on the following information:
Numbers in the cells express the exposure as a percentage of the ARfD (or ADI/TDI, if ARfD not available) for the highest residues reported by pesticide/crop combination. For the rest of the residues quantified below the highest, the % of ARfD was calculated and results are presented in Appendix B.
When processing factors (PF) have been used, the % of ARfD resulted has been marked with a ‘PF’.
- When no numbers are reported in the cells, either:
- no residues were quantified in any sample for that specific pesticide/food combination (i.e. residue concentration < LOQ),
- the acute risk assessment is not relevant as no ARfD needed to be derived therefore, not calculated (e.g. 2‐phenylphenol) or
- an evaluation concluding on the absence of acute hazard for the substance is not available (i.e. bromide ion, 67 isocarbophos and omethoate).
FIGURE 1.
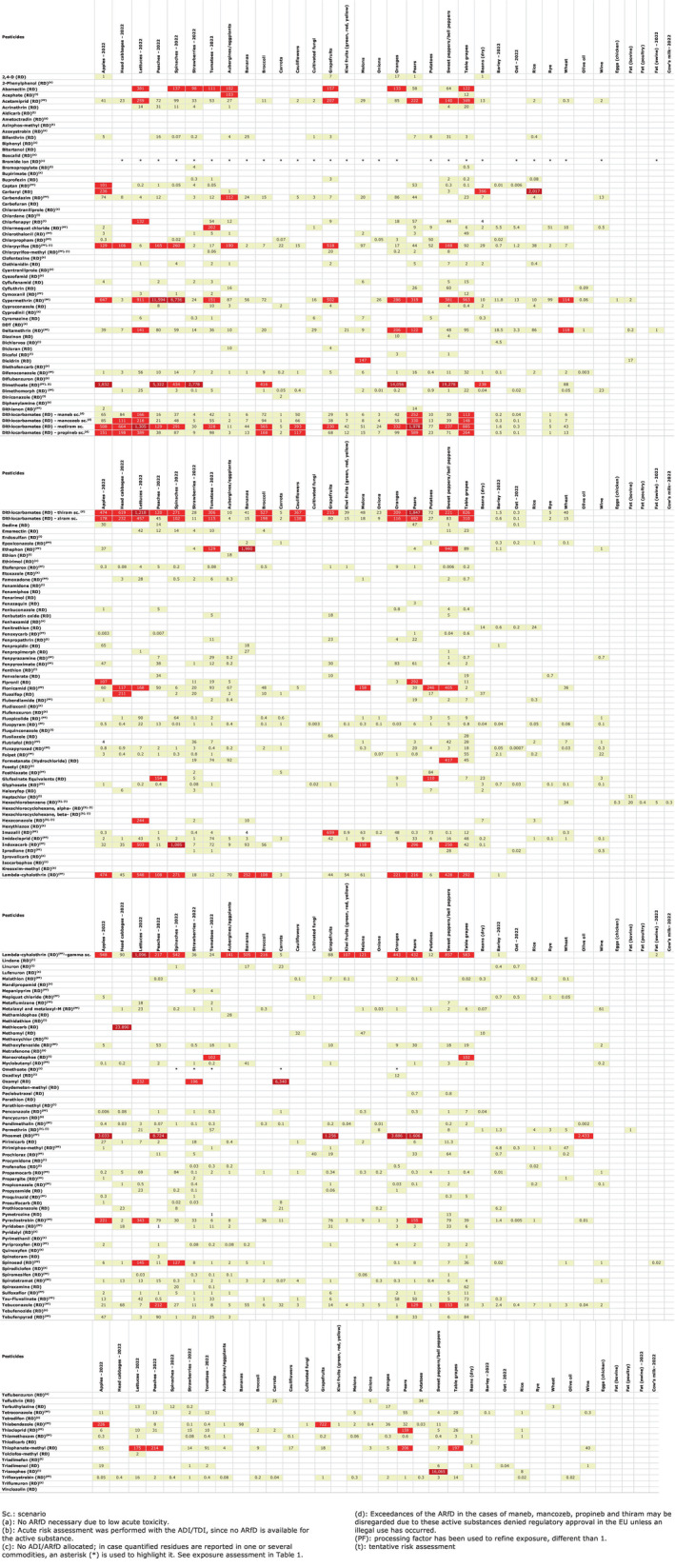
Results of acute dietary risk assessment for the highest residues reported by pesticide/commodity combination (values are expressed as a percentage of the acute health‐based guidance value or ARfD).
The colour of the plot cells should be interpreted as follows:
Yellow cells indicate that the estimated exposure was lower than the pesticide's ARfD.
Red cells indicate a potential risk to consumer health estimated on the basis of the conservative assumptions used in the exposure calculation (see Section 5.1.1 reference to the methodology bullet point) because the exposure is higher than the pesticide's ARfD; light red cells correspond to acute exposure estimates ranging from above 100% to 1000% of the ARfD, and dark red cells correspond to acute exposure estimates above 1000% of the ARfD.
Although this year acute exposure assessment has been done with all 36 commodities present in the last 3‐year cycle instead of the 12 commodities assessed in the past years, and samples of the MANCP have been pooled with the EU MACP, the overall picture remains the same. Those pesticides showing an exceedance of the ARfD in the 12 commodities of the 2022 EU MACP also show exceedances when the other 24 commodities are included. Thus, enlarging the scope of the commodities has allowed analysing the situation of the market in a wider and more reliable way.
Among the 193 pesticides present in the 2022 EU MACP, the acute risk assessment results in 38,045 samples were as follows (Figure 1):
No health‐based guidance values (ARfD) were allocated for three pesticides: bromide ion,67 isocarbophos 68 and omethoate. 69 Given their relevance, an estimated acute exposure based on the commodities where results were quantified is presented in Table 1, while they are marked with footnote c) in Figure 1.
The setting of an ARfD was not necessary for 36 pesticides. Therefore, acute adverse effects to the consumer would not be expected for the following substances: 2‐phenylphenol (RD), ametoctradin (RD), azoxystrobin (RD), biphenyl (RD), boscalid (RD), bupirimate (RD), chlorantraniliprole (RD), clofentezine (RD), cyantraniliprole (RD), cyazofamid (RD), cyprodinil (RD), DDT (RD), diethofencarb (RD), diflubenzuron (RD), diphenylamine (RD), ethirimol (RD), etoxazole (RD), fenhexamid (RD), fludioxonil (RD), flufenoxuron (RD), fosetyl (RD), hexythiazox (RD), iprovalicarb (RD), kresoxim‐methyl (RD), lufenuron (RD), mandipropamid (RD), metrafenone (RD), pencycuron (RD), pyridalyl (RD), pyrimethanil (RD), quinoxyfen (RD), spirodiclofen (RD), tebufenozide (RD), teflubenzuron (RD), tetradifon (RD), triflumuron (RD). These pesticides are marked with footnote a) in Figure 1.
There were no quantified results for 23 pesticides, in any of the tested samples of the commodities listed under the 3‐year cycle of the EU MACP. These pesticides were aldicarb (RD), azinphos‐methyl (RD), bitertanol (RD), carbofuran (RD), chlordane (RD), dicofol (RD), endosulfan (RD), fenamidone (RD), fenamiphos (RD), fenarimol (RD), fenthion (RD), fluquinconazole (RD), alpha‐hexachlorocyclohexane (RD), beta‐hexachlorocyclohexane (RD), lindane (RD), methidathion (RD), methoxychlor (RD), oxydemeton‐methyl (RD), parathion (RD), parathion‐methyl (RD), procymidone (RD), triadimefon (RD) and vinclozolin (RD). Acute dietary exposure to any of these pesticides would not be expected to pose a concern to consumer health as no residues were reported above the LOQ.
Quantified levels for 97 pesticides resulted in exposures below the health‐based acute reference values in all tested samples of the commodities listed under the 3‐year cycle of the EU MACP. This means that acute dietary exposure to these pesticides would be expected not to be of consumer health concern. The specific pesticides were 2,4‐D (RD), acephate (RD), acrinathrin (RD), bifenthrin (RD), bromopropylate (RD), buprofezin (RD), chlorothalonil (RD), chlorpropham (RD), chlorpyrifos‐methyl (RD), clothianidin (RD), cyflufenamid (RD), cyfluthrin (RD), cymoxanil (RD), cyproconazole (RD), cyromazine (RD), diazinon (RD), dichlorvos (RD), dicloran (RD), difenoconazole (RD), dimethomorph (RD), diniconazole (RD), dithianon (RD), dodine (RD), emamectin (RD), epoxiconazole (RD), ethion (RD), etofenprox (RD), famoxadone (RD), fenazaquin (RD), fenbuconazole (RD), fenbutatin oxide (RD), fenitrothion (RD), fenoxycarb (RD), fenpropathrin (RD), fenpropidin (RD), fenpropimorph (RD), fenpyrazamine (RD), fenpyroximate (RD), fenvalerate (RD), flubendiamide (RD), fluopicolide (RD), fluopyram (RD), flusilazole (RD), flutriafol (RD), fluxapyroxad (RD), folpet (RD), fosthiazate (RD), glyphosate (RD), haloxyfop (RD), heptachlor (RD), hexachlorobenzene (RD), imidacloprid (RD), iprodione (RD), linuron (RD), malathion (RD), mepanipyrim (RD), mepiquat chloride (RD), metaflumizone (RD), metalaxyl and metalaxyl‐M (RD), methamidophos (RD), methomyl (RD), methoxyfenozide (RD), myclobutanil (RD), oxadixyl (RD), paclobutrazol (RD), penconazole (RD), pendimethalin (RD), permethrin (RD), pirimicarb (RD), pirimiphos‐methyl (RD), prochloraz (RD), profenofos (RD), propamocarb (RD), propargite (RD), propiconazole (RD), propyzamide (RD), proquinazid (RD), prosulfocarb (RD), prothioconazole (RD), pymetrozine (RD), pyridaben (RD), pyriproxyfen (RD), spinetoram (RD), spiromesifen (RD), spirotetramat (RD), spiroxamine (RD), sulfoxaflor (RD), tau‐fluvalinate (RD), tebufenpyrad (RD), tefluthrin (RD), terbuthylazine (RD), tetraconazole (RD), thiamethoxam (RD), thiodicarb (RD), tolclofos‐methyl (RD), triadimenol (RD), trifloxystrobin (RD).
There were 33 pesticides quantified in 650 samples (716 results) out of 38,045 samples (1.7%) at levels exceeding their respective health‐based acute reference values: phosmet (RD) (172 samples), imazalil (RD) (133 samples), lambda‐cyhalothrin (RD) (106 samples), cypermethrin (RD) (58 samples), acetamiprid (RD) (51 samples), thiabendazole (RD) (43 samples), ethephon (RD) (29 samples), indoxacarb (RD) (14 samples), pyraclostrobin (RD) (12 samples), flonicamid (RD) (12 samples), dimethoate (RD) (11 samples), chlorpyrifos (RD) (11 samples), abamectin (RD) (10 samples), tebuconazole (RD) (9 samples), thiophanate‐methyl (RD) (8 samples), deltamethrin (RD) (6 samples), oxamyl (RD) (4 samples), carbaryl (RD) (4 samples), spinosad (RD) (3 samples), glufosinate equivalents (RD) (2 samples), monocrotophos (RD) (2 samples), thiacloprid (RD) (2 samples), captan (RD) (2 samples), fipronil (RD) (2 samples), fluazifop (RD) (2 samples), hexaconazole (RD) (1 sample), carbendazim (RD) (1 sample), methiocarb (RD) (1 sample), chlormequat chloride (RD) (1 sample), chlorfenapyr (RD) (1 sample), dieldrin (RD) (1 sample), triazophos (RD) (1 sample), formetanate (hydrochloride) (RD) (1 sample).
TABLE 1.
Estimated acute exposure without comparison to ARfD/ADI values.
| Pesticide | Food product | Acute estimated exposure (in mg/kg bw per day) |
|---|---|---|
| Bromide ion (RD) | Grapefruit | 3.0 × 10−3 |
| Oranges | 0.019 | |
| Peaches | 3.0 × 10−3 | |
| Table grapes | 3.0 × 10−3 | |
| Wine | 1.0 × 10−3 | |
| Strawberries | 3.0 × 10−3 | |
| Kiwis | 6.0 × 10−3 | |
| Bananas | 0.016 | |
| Potatoes | 0.108 | |
| Carrots | 0.039 | |
| Onions | 4.0 × 10−3 | |
| Tomatoes (requested in 2022 EU MACP) | 0.105 | |
| Sweet/bell peppers | 0.025 | |
| Aubergines (egg plants) | 0.022 | |
| Melons | 0.229 | |
| Broccoli | 0.014 | |
| Cauliflowers | 0.010 | |
| Head cabbage | 0.022 | |
| Lettuce (requested in 2022 EU MACP) | 0.109 | |
| Spinach | 0.097 | |
| Cultivated fungi | 1.0 × 10−4 | |
| Beans (dry) | 2.0 × 10−3 | |
| Oats | 2.0 × 10−4 | |
| Rice | 0.069 | |
| Rye | 1.0 × 10−3 | |
| Wheat | 0.012 | |
| Fat (swine) | 2.0 × 10−4 | |
| Omethoate (RD) | Oranges | 5.0 × 10−4 |
| Strawberries | 3.0 × 10−4 | |
| Carrots | 3.0 × 10−5 | |
| Tomatoes | 1.0 × 10−4 | |
| Spinach | 4.0 × 10−5 |
The ARfD exceedances were distributed among the 3‐year cycle of the EU MACP commodities as: grapefruits (193 samples), pears (91 samples), peaches (74 samples), oranges (67 samples), apples (59 samples), lettuces (49 samples), table grapes (41 samples), sweet peppers/bell peppers (37 samples), spinaches (34 samples), bananas (32 samples), tomatoes (7 samples), head cabbages (5 samples), melons (5 samples), strawberries (4 samples), wheat (3 samples), aubergines/eggplants (3 samples), potatoes (3 samples), broccoli (3 samples), beans (dry) (2 samples), carrots (1 sample), kiwi fruits (green, red, yellow) (1 sample), rice (1 sample) and olives for oil production (1 sample). The available acute health‐based guidance values were not exceeded in any sample of cauliflower, cultivated fungi, onion, wine, barley, oat nor rye cereals and the six animal commodities (bovine, poultry and swine fat, bovine liver, chicken eggs nor cow's milk). No results above the LOQ were reported for bovine liver.
Dithiocarbamates (RD)
Additionally to those 33 pesticides, dithiocarbamates (RD) also exceeded the ARfD for one or more of the six active substances the residue definition includes. During 2022, metiram and ziram were still approved for used in the EU, not so the rest, with existing import tolerances for mancozeb. Overall, 381 samples were quantified on dithiocarbamates expressed as CS2. When conducting all six scenarios, exceedances of the % of ARfD could not be excluded in 315 samples.
Of the 381 samples with residues of CS2 quantified above the LOQ, six samples of spinaches lead to non‐compliant results; all samples had administrative consequences or follow‐up investigations. In the rest of the samples, the MRL was not exceeded. In 2023, EFSA finalised the comprehensive MRL review of dithiocarbamates (EFSA, 2023d) where uses (EU and third countries) were only reported for metiram, ziram and mancozeb. Existing CXLs (most of them derived in the 90s and not based on a recent evaluation thus subject to uncertainties related to possible outdated data not complying fully with the current scientific standards) and background levels of CS2 were also considered, allowing to derive MRLs that accommodate better the current situation. Lacking specific methods for the individual determination of each precursor, EFSA is not able to identify from which of the active substances the observed ARfD exceedances come from. Since under the comprehensive review, MRLs were derived not only based on the results of residue trials but considering as well CS2 naturally occurring in certain crops and not related to the use as plant protection products, EFSA suggests the change of the residue definition for enforcement as ‘dithiocarbamates (dithiocarbamates determined and expressed as CS2, including maneb, mancozeb, metiram, propineb, thiram and ziram)’. The implementation of the MRLs proposed in this review is currently discussed by risk managers. In the meantime, Member States*1 are recommended to develop specific analytical methods for each precursor to refine the risk assessment and to decide whether administrative follow‐up actions are required. Moreover, to be vigilant to CS2 levels in commodities where no use was notified under the 2023 MRL review. Furthermore, we recommend keeping reporting to EFSA background levels from organic sampling of all possible commodities (including minor uses) to build a comprehensive CS2 database.
A more detailed analysis on the estimated exposure by pesticide exceeding the ARfD in more than 50 samples is presented in the following paragraphs. Out of those 33, in 7 pesticides (dimethoate (RD) in 7 samples, ethephon (RD) in 3 samples, oxamyl (RD) in 1 sample, methiocarb (RD) in 1 sample, triazophos (RD) in 1 sample, carbaryl (RD) in 1 sample and indoxacarb (RD) in 1 sample), exceedances of the ARfD were high (i.e. above 1000%). However, those exceedances correspond to 15 samples out of 38,045 (0.04%) where EU Competent Authorities are expected to take the necessary measures to withdraw the food products from the market.
Phosmet (RD) (172 samples)
The estimated exposure based on phosmet residue levels exceeded the ARfD in peaches (50 samples), apples (40 samples), oranges (39 samples), pears (38 samples), grapefruits (4 samples) and olive oil (1 sample). All residues remained in levels above the LOQ but below or equal to the MRL, so no action was taken except for two samples where warning measures were established.
The approval of phosmet expired in February 2022, with a grace period granted until November 2022 to allow Member States,*1 third countries and food business operators to adapt themselves to the requirements which result from the modification of the MRLs in Regulation (EU) 2023/1029. 70 All MRLs were lowered to the LOQ including those based on CODEX, and the substance was moved to Annex V of Regulation (EC) 396/2005 as for some commodities, the LOQ needed to be lowered to a lower level than the default 0.01* mg/kg, i.e. to 0.005* mg/kg.
Imazalil (RD) (133 samples)
The estimated exposure on imazalil residues exceeded the ARfD in 133 samples of grapefruits. Only one was flagged as non‐compliant and a warning to the food operator was given. In the rest (132), residues remained in levels above the LOQ but below or equal to the MRL. No peeling factor was derived, although peel–pulp ratios were considered at Codex level, resulting in an HR of 0.84 mg/kg for the edible portion when the MRL was stablished. By extrapolating an existing peeling factor of 0.05 (Zincke et al., 2022) derived in oranges and mandarins, refinement of the exposure could be done leading to non‐exceedances situation of the ARfD in any of the samples.
Lambda‐cyhalothrin (RD) (106 samples)
Both active substances: gamma‐cyhalothrin and lambda‐cyhalothrin were approved for uses in the EU in 2022. The residue definition that covers both active substances is lambda‐cyhalothrin (including gamma‐cyhalothrin) (sum of R,S and S,R isomers). Both active substances have been considered in two different scenarios (see Annex II), where in each one, each HBGV was used. In lambda‐cyhalothrin scenario, the less potent of the two, the estimated exposure in 106 samples led to an exceedance of the acute estimates in pears (22 samples), oranges (18 samples), spinaches (17 samples), bananas (14 samples), lettuce (8 samples), sweet peppers/bell peppers (7 samples), table grapes (7 samples), peaches (5 samples), broccoli (2 samples), apples (2 samples), melons (2 samples), kiwi fruits (green, red, yellow) (1 sample) and aubergines/eggplants (1 sample). Authorised uses on these commodities were notified only for lambda‐cyhalothrin (EFSA, 2015c), and not for gamma‐ in the framework of the MRL review 71 process for each active substance.
These results may be explained due to the different versions of PRIMo used in the exposure calculations, version 2 (EFSA, 2015c) versus version 3.1 in this assessment, and the divergences resulting from the use of the highest residue of supervised trials in the IESTI equation and the MRL in place. In the frame of the most recent MRL application for lambda‐cyhalothrin on avocado (EFSA, 2023f), the initial input values (i.e. the highest residue values derived in residue trials) assessed by EFSA in 2015c were introduced in PRIMo rev. 3.1 using lambda isomer's HBGV. Exceedances of the estimated exposure were calculated for pears, peaches, apples and boiled broccoli. Therefore, options for further refinement of the exposure assessment, e.g. in the context of confirmatory data assessment, in the process of the renewal in the approval, or under a specific mandate shall be explored for these four commodities. For the rest of the commodities, the exceedances shall not be considered of concern as the chances of coinciding the high residue measured, the consumption of a large portion (P97.5 of the most critical diet, considering consumers only) and the inequal distribution across the composite sample 72 are very low.
The IESTI methodology is currently under review 73 in the EU and efforts are ongoing to also review it at international level.
Cypermethrin (RD) (58 samples)
Cypermethrin (RD) residues exceeded the ARfD in apples (8 samples), table grapes (8 samples), spinaches (7 samples), peaches (7 samples), grapefruits (5 samples), oranges (5 samples), pears (5 samples), sweet peppers/bell peppers (4 samples), lettuces (4 samples), tomatoes (3 samples) and wheat (2 samples).
Cypermethrin as sum of isomers was approved in 2022. 74 It is a mixture of eight isomers (four diastereomeric pairs of enantiomers of alpha, beta, theta and zeta) where there are three chiral centres, resulting in eight stereoisomers and in up to four chromatographic peaks/signals when using a non‐chiral column where alpha and beta isomers can be distinguished from the rest; not so zeta from the mixture of cypermethrins (EFSA, 2023b). Alpha and beta isomers are not approved for use in the EU, but as according to the MRL review, import tolerances are in place for zeta‐cypermethrin and CODEX MRLs for cypermethrins (including zeta and alpha isomers) were implemented in the EU legislation (FAO, 2009; EFSA, 2010, 2016a).
In the EFSA comprehensive MRL review (EFSA, 2023b) on the authorised uses of cypermethrins measures for reduction of consumer exposure assessment are provided for risk managers consideration such as the lowering of the MRLs in place for oranges, table grapes, grapefruits, broccoli, tomatoes, aubergine/eggplants, melons and wheat. For apples, pears, peaches, sweet pepper/bell peppers, lettuces and spinaches, no safe uses were identified thus, in the Article 12 review of existing MRLs, EFSA recommended to lower the MRLs to the LOQ value. The new MRL values derived by EFSA in the last MRL review are currently under discussion by risk managers.
Acetamiprid (RD) (51 samples)
Acetamiprid (RD) residues exceeded the ARfD in table grapes (23 samples), pears (11 samples), lettuce (10 samples), grapefruits (6 samples) and sweet peppers/bell peppers (1 sample). Only one sample was reported as non‐compliant.
The MRL review of acetamiprid (EFSA, 2011) was carried out using PRIMo rev. 2 and the acute HBGV of 0.1 mg/kg bw derived in 2004. In 2016, in the framework of the renewal of the approval, the HBGV were lowered to 0.025 mg/kg bw (EFSA, 2016b). In 2018, EFSA performed a review of the existing MRLs in view of the lower HBGV (EFSA, 2018b). New MRL proposals have been derived in a recent MRL application, using PRIMo 3.1 (EFSA, 2021c). A comprehensive review of new toxicological data and the existing MRL is ongoing. 75
Thiabendazole (RD) (43 samples)
Thiabendazole (RD) residues exceeded the ARfD in grapefruits (41 samples), pears (1 sample) and apples (1 sample). The MRL in grapefruits in place is 7 mg/kg, implementing a Codex MRL derived in 2006 (FAO, 2006). The highest residue of the residue trials was 5.2 mg/kg. No PF was available in the database when these calculations were performed. However, if the most recent PF of 0.047 (EFSA, 2021b) was to be considered, all 41 samples would not have resulted in an exceedance of the ARfD. EFSA recommends keep updating the EU PF database in accordance with Article 41 to Regulation (EC) No 396/2005.
In the frame of the EU MACP, no HBGV could be derived for three pesticides. Thus, the estimated acute exposure is presented in Table 1 based on the commodities where results were quantified:
Bromide ion (RD) has to be analysed in sweet peppers/bell peppers. It was quantified in 823 samples. The acute risk assessment will be conducted by EFSA after having finalised the scientific opinion on the risks for human health related to the presence of bromide ion in food67 and if the outcome deems necessary the setting of an ARfD. An estimation of the acute exposure using the food consumption data from EFSA PRIMo rev. 3.1 is presented in Table 1.
Isocarbophos (RD) was not reported in any of the sample.
Omethoate (RD) was quantified in strawberries (1 samples from China and another one from Iran), in oranges (1 sample from Egypt), in carrots (1 sample from Poland), in spinach (1 sample from Romania) and in tomatoes (1 sample from Romania). Two of the six samples were destroyed. For the other two, information was not provided to EFSA. The presence of omethoate in food is likely to come from the use of dimethoate, being its metabolite. However, dimethoate was not approved in the EU from mid‐2020. MRLs in 2022 were set at the lowest quantifiable level (LOQ). 76 The estimated exposure using the food consumption data in EFSA PRIMo rev. 3.1 is presented in Table 1.
Further details on the acute deterministic dietary risk assessment results for the pesticide residues found in the 36 food products covered by the 2022 EU MACP are presented in Appendix B – Figures B.1, B.2, B.3, B.4, B.5, B.6, B.7, B.8, B.9, B.10, B.11, B.12, B.13, B.14, B.15, B.16, B.17, B.18, B.19, B.20, B.21, B.22, B.23, B.24, B.25, B.26, B.27, B.28, B.29, B.30, B.31, B.32, B.33, B.34, B.35. In these charts, the results for samples containing residues at or above the LOQ are presented individually, expressing the percentage of the ARfD. There could be some cases where the ARfD was exceeded due to recent lowering of the ARfD value, while the samples were still within the MRL. In other cases, the exceedance of the ARfD is due to the gap between the highest residue found under residue trials, used by the IESTI equation, and the MRL. The different dithiocarbamate scenarios have not been addressed.
Moreover, this year contribution of the 36 commodities mostly consumed in the EU and listed in the 3‐year cycle of the EU MACP compared to the 12 included in previous years, did not show a significant increase in the number of samples exceeding the acute HBGV as the percentages remain in the same range (1.7% in 2022 vs. ~ 1% in the previous 3 years) (see Figure 1). This finding justifies the approach of including the basket of EU products most widely consumed (i.e. 36) to get a better overview of the EU market.
In all cases, the exposure estimates were performed (i) according to the residue definition for enforcement (which does not account for some of the metabolites which the residue definition for risk assessment does), (ii) for extreme consumers, where large portions were considered and the variability factors are high (i.e. the highest residue in one individual unit due to a lack of uniformity of the sample, could be 7 or 5 times higher), (iii) no peeling or processing was considered unless specific processing/peeling factors were available (even if some PFs were applied to refine the exposure considering consumer practices such as peeling, cooking, frying and baking, this was not done consistently for all pesticides due to the lack of appropriate factors), (iv) ADI/TDI values were used in some cases, and in others, recently derived ARfD values not known before 2022 possibly overestimated the assessment. Overall, the results of the estimated acute exposure assessment reflect the outcome of a deterministic methodology with a given purpose aiming at deciding if a sample is to be withdrawn from the market or not and whether is to be reported in the RASFF notification system.
5.2. Acute probabilistic risk assessment
The acute probabilistic exposure assessment aims at estimating the probabilities of exceedance of the ARfD of the pesticides in different subpopulation of European consumers reported. Probabilistic methodology expands the scope of the acute exposure assessment by introducing the likelihood of exposure events. Results of the probabilistic assessment refer to an exposure distribution, providing information both on the magnitude of exposure and on the probability of individuals being exposed at such a level.
The probabilistic exposure assessment is executed to calculate the probabilities of exceedance of the ARfD, in different national subpopulation of European consumers. This year, the scope was expanded to all pesticides listed in the 2022 EU MACP Regulation (not only to those exceeding the percentage of ARfD in the acute deterministic risk assessment (Section 5.1), as done in 2021 (EFSA, 2023c).
The 2022 EU MACP program listed 193 pesticides, of which 163 have an ARfD. An individual acute probabilistic exposure assessment was therefore conducted for each of these 163 active substances.
The probability of exceeding the ARfD is to be understood as a characterisation of the overall risk for each population groups under assessment for the given survey considered and based on the actual consumption of 40 food commodities (see Appendix C.1.1) i.e. by real consumers. The occurrence data considered on those 40 food commodities were the one reported over 3 years (i.e. years 2020, 2021 and 2022). As these calculations are not conducted for consumers consuming only a certain commodity and do not focus on one single sample of the respective commodity, they cannot be considered as refinements of the deterministic calculations reported in Section 5.1.
5.2.1. Data and methodology
The acute probabilistic exposure to pesticide residues was assessed in accordance with the guidance on probabilistic modelling of dietary exposure to pesticide residues (EFSA PPR Panel, 2012). Hence, acute exposure estimates were obtained using a two‐dimensional method where variability of exposure within the population is modelled by means of an inner loop execution, and confidence intervals around the acute exposure estimates are modelled through an outer loop execution (see Figure 2).
FIGURE 2.
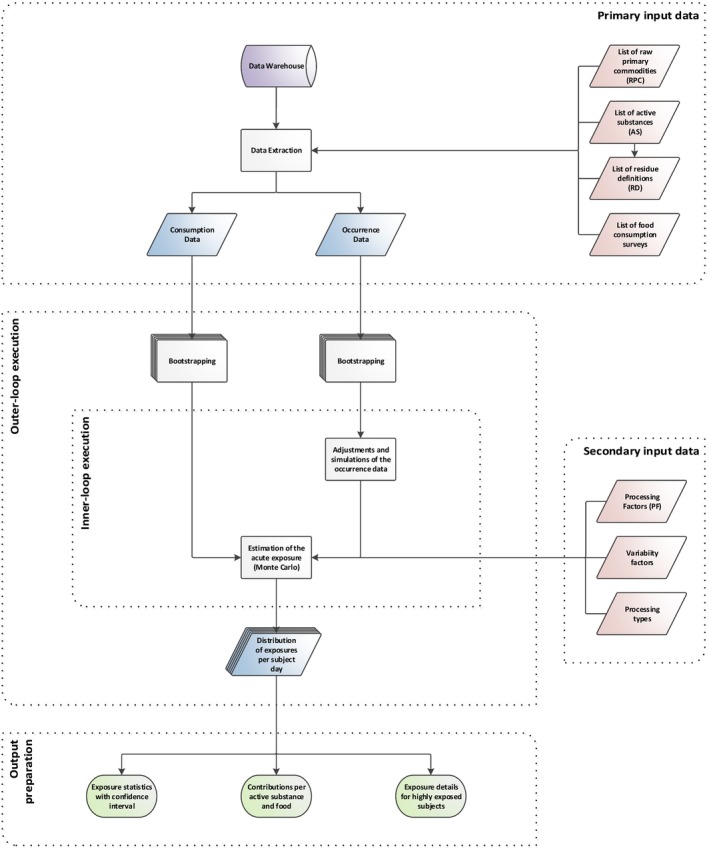
General process for probabilistic estimation of acute exposure to pesticide residues.
The primary input data required for probabilistic modelling of exposure are the occurrence data (i.e. the amounts of pesticide residues that were present in foods during the years 2020, 2021 and 2022) and food consumption data (i.e. the types and amounts of those foods consumed in a person's diet). These data are extracted from the EFSA Data Warehouse for the relevant food commodities, active substances (restricted to those covered by the 2022 EU MACP programme), associated residue definitions and dietary surveys. See Appendix C – for a full description of the data used.
Within the inner loop execution, occurrence data are subject to several simulations and imputations. These adjustments are intended to account for inaccuracies and missing information in the occurrence data set (e.g. unspecific measurements, measurements below the analytical limit of quantification (LOQ), etc.). The consumption data and adjusted occurrence data are then used to estimate acute dietary exposures using an empirical Monte Carlo simulation (i.e. with 100,000 iterations). This results in a distribution that represents the variability of acute exposures within the population. The different simulations performed during the inner loop execution require the use of additional data, referred to as secondary input data (e.g. processing factors [PFs]).
To quantify the confidence around the acute exposure distributions, the model uses an outer loop execution where the inner loop execution is repeated several times. Prior to each execution, the original consumption and occurrence data sets are modified by means of bootstrapping, a random resampling technique for quantifying uncertainty. By repeating the inner loop execution multiple times (i.e. 100), the model produces multiple distributions of exposure. The differences between those distributions reflects the impact of the sampling variability, i.e. the uncertainty depending on the sample size.
During the output preparation, summary statistics (i.e. percentiles of exposure) are generated for the multiple distributions, resulting in multiple estimates for each percentile of exposure. From these multiple estimates, confidence intervals around each percentile are produced. Subsequently, to identify risk drivers, details on the highly exposed consumers are extracted (i.e. consumers with exposure exceeding the 99th percentile) and average contributions per food commodity are calculated.
The primary and secondary input data for the probabilistic exposure assessment were selected and prepared according to the following principles.
The pesticides selected were those listed in 2022 EU MACP programme.
The assessment was also restricted to the 35 raw primary commodities (RPCs) of plant origin that were ever considered in the EU MACP and foods specifically intended for infants and young children. In addition, courgettes were also included because, according to EFSA's design assessment of the pesticide monitoring programme (EFSA, 2015a), courgettes are consumed in higher amounts than other commodities previously included in the EU MACP (e.g. spinach and broccoli). Processed foods associated with aforementioned RPCs were also included in the assessment. The full list of commodities can be seen in Appendix C.1.1.
Regarding the occurrence data, all samples analysed for any of the aforementioned substances (or associated residue definitions) were extracted from the EFSA Data Warehouse. Extracted samples referred to the monitoring years 2020, 2021 and 2022 were collected either under the EU MACP, the MANCP (national control programmes) or a combination of those (SSD codes for programme type K005A, K009A and K018A). Samples associated with increased control programmes on imported food (SSD code K019A) were excluded as they were not considered to be representative of the market. Furthermore, only samples obtained through selective or objective sampling were retained (SSD codes sample strategy ST10A and ST20A). Samples obtained through suspect sampling (ST30A) were not considered to be representative of the market and therefore excluded.
The occurrence data were not converted into the residue definition for risk assessment.
The extracted occurrence data do not only refer to the raw primary commodities. When sufficient data were available for the associated processed foods (e.g. apple juice), monitoring data for the processed foods were also extracted.
Consumption data used for the probabilistic exposure assessment were extracted from the RPC Consumption Database (EFSA, 2019a). To cover as many population groups as possible without compromising the reliability of intake estimates at the higher percentiles of the exposure distribution, only the dietary surveys with more than 300 survey participants per relevant age class were retained. This resulted in the selection of 30 population groups, covering three different age classes (i.e. adults, other children and toddlers) and 17 different countries. The limit of 300 survey participants for performing probabilistic exposure assessment has been derived based on experience gained so far. However, EFSA intends to initiate a research project that will derive more accurate criteria. Full list of surveys used can be seen in Appendix C.1.5.
For relevant active substances, RPCs and processing techniques, available processing factors were extracted from the European database on PFs, which is the most recent and the most comprehensive compilation of PFs currently available at EU level (Zinckeet al., 2022). Only the PFs indicated as reliable or indicative were extracted. PFs indicated as unreliable were excluded from the assessment.
As part of the inner loop execution , the primary and secondary input data are processed as follows to obtain the acute exposure distributions.
When measurements refer to an unspecific residue definition (i.e. a residue definition that may be associated with multiple active substances), they were all assigned to the active substance under consideration. EFSA acknowledges that this approach may generate an important bias for substance/commodity combinations that are not authorised. EFSA will therefore explore possibilities to allow for better consideration of the authorisation status in future assessments.
Measurements below the LOQ (i.e. left‐censored data) are imputed with ½ LOQ for a number of samples equal to the number of samples with positive results (i.e. above the LOQ) for the same substance/commodity combination, provided that the use of the substance is authorised for that commodity. All other results below the LOQ were imputed with a zero (i.e. assuming a no‐residue situation). As for the handling of unspecific residue definitions, EFSA acknowledges that this approach may generate an important bias; still this assumption is better representative of the real state at the market compared to the imputation considered in the past report (i.e. setting ½ LOQ to all substances reported a result below the LOQ when at least one positive result was reported).
Acute dietary exposure (i.e. within a single day) is modelled by means of an empirical Monte Carlo simulation. This means that individual days are selected at random from the consumption data set (i.e. all the records related to the consumptions of one individual during a specific day) and, for each food commodity consumed within that day, random samples of the occurrence data set are assigned. Based on the measured concentrations, the individuals' consumptions and the individuals' body weights, the acute exposures resulting from each food commodity within each individual day are calculated. The exposures resulting from residues of the substance in different foods consumed within the same day are summed up to obtain total acute exposure within each day. This process is repeated 100,000 times for the 30 population groups. This results in an empirical exposure distribution per population group, each consisting of 100,000 exposure estimates.
As for the deterministic exposure assessment, the probabilistic exposure assessment also accounts for unit‐to‐unit variability for all food commodities which may contain non‐uniform residue distributions (see Section 5.1.1). In this case, however, the unit‐to‐unit variability is modelled using a beta‐distribution instead of a fixed variability factor.
The acute exposure modelling also accounts, where possible, for the effect of processing prior to consumption, either by using available monitoring data in the processed food or by incorporation of a specific processing factor. When information on the effect of processing was not available, it was assumed that all residues present in the RPC will reach the end consumer without any loss of residues, which is generally expected to overestimate the actual exposure.
All acute exposure estimates are expressed as percentage of ARfD. Hence, a calculated value greater than 100% suggests that the estimated exposure exceeds the ARfD for that active substance. This also allows to calculate within each subpopulation the percentage of individual‐days that have an exposure exceeding the ARfD.
The outer loop execution allows to estimate the 95% confidence intervals around the calculated percentage of individual‐days exceeding the ARfD. This is evidenced by means of lower bound (LB, i.e. 2.5th percentile), middle bound (MB, i.e. 50th percentile) and upper bound (UB, i.e. 97.5th percentile) estimates.
All extractions, simulations, imputations and calculations were programmed with SAS® Studio 3.81 (Enterprise Edition). A more detailed description of the input data and methodologies applied is provided in Appendix C.
5.2.2. Results
The probabilistic risk assessment was executed for the 163 active substances having an ARfD deriving from the 193 pesticide of the 2022 EU MACP program (see section Annex III – Table 3.1). When multiple active substances are associated with a common residue definition (e.g. dithiocarbamates), each active substance was considered separately in contrast with the deterministic assessment, where some active substances were disregarded on the ground of the approval status at EU level. The methodology followed is described in Section 5.2.1, and was carried out to those 163 active substances with a set ARfD.
The results of the acute probabilistic risk assessment are summarised in Table 2. It is reported as the middle bound (MB) (50th percentile) of the confidence interval for the percentage of individual‐days exceeding the ARfD. For each population class (adults, toddlers and other children), the minimum and the maximum value among different countries is presented in the table. Out of the 163 active substances, 52 substances resulted in a positive percentage (i.e. not zero) of individual‐days exceeding the ARfD (at the MB), in at least one survey.
TABLE 2.
Summary of the acute probabilistic risk assessment results.
| Active substance | Middle bound of the percentage of individual‐days exceeding the ARfD h | |||||
|---|---|---|---|---|---|---|
| Adults | Other children | Toddlers | ||||
| Min a | Max b | Min c | Max d | Min e | Max f | |
| Abamectin | 0.0000 | 0.0000 | 0.0000 | 0.0010 | 0.0002 | 0.0014 |
| Acetamiprid | 0.0000 | 0.0009 | 0.0006 | 0.0029 | 0.0009 | 0.0063 |
| Carbaryl | 0.0000 | 0.0000 | 0.0000 | 0.0014 | 0.0000 | 0.0027 |
| Carbendazim | 0.0000 | 0.0028 | 0.0000 | 0.0024 | 0.0000 | 0.0041 |
| Carbofuran | 0.0011 | 0.0177 | 0.0015 | 0.0120 | 0.0010 | 0.0114 |
| Carbosulfan | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0002 |
| Chlormequat chloride | 0.0000 | 0.0004 | 0.0000 | 0.0010 | 0.0007 | 0.0014 |
| Chlorothalonil | 0.0000 | 0.0008 | 0.0000 | 0.0004 | 0.0000 | 0.0000 |
| Chlorpropham | 0.0003 | 0.0037 | 0.0015 | 0.0087 | 0.0022 | 0.0155 |
| Cyhalothrin, gamma‐ | 0.0009 | 0.0083 | 0.0086 | 0.0362 | 0.0263 | 0.0439 |
| Cyhalothrin, lambda‐ | 0.0000 | 0.0027 | 0.0015 | 0.0070 | 0.0062 | 0.0119 |
| Cypermethrin, sum | 0.0106 | 0.0644 | 0.0187 | 0.0511 | 0.0265 | 0.0779 |
| Cypermethrin, alpha‐ | 0.1155 | 0.3675 | 0.1580 | 0.2738 | 0.2211 | 0.3124 |
| Cypermethrin, beta‐ | 0.0808 | 0.2849 | 0.1193 | 0.2136 | 0.1713 | 0.2385 |
| Cypermethrin, zeta‐ | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0009 |
| Deltamethrin, cis‐ | 0.0000 | 0.0000 | 0.0000 | 0.0009 | 0.0000 | 0.0026 |
| Esfenvalerate | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0004 |
| Ethephon | 0.0019 | 0.0088 | 0.0051 | 0.0291 | 0.0193 | 0.0806 |
| Flonicamid | 0.0000 | 0.0008 | 0.0000 | 0.0033 | 0.0006 | 0.0028 |
| Fluazifop‐P | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0002 |
| Formetanate hydrochloride | 0.0000 | 0.0007 | 0.0000 | 0.0018 | 0.0002 | 0.0022 |
| Fosthiazate | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0005 |
| Glufosinate‐ammonium | 0.0000 | 0.0000 | 0.0000 | 0.0014 | 0.0003 | 0.0016 |
| Imazalil | 0.0000 | 0.0198 | 0.0000 | 0.0093 | 0.0024 | 0.0345 |
| Indoxacarb | 0.0000 | 0.0036 | 0.0006 | 0.0050 | 0.0012 | 0.0051 |
| Mancozeb | 0.0000 | 0.0000 | 0.0010 | 0.0077 | 0.0044 | 0.0105 |
| Maneb | 0.0000 | 0.0000 | 0.0000 | 0.0030 | 0.0009 | 0.0049 |
| Metiram | 0.0147 | 0.0693 | 0.0978 | 0.2010 | 0.1574 | 0.2668 |
| Thiram | 0.0127 | 0.0579 | 0.0837 | 0.1736 | 0.1363 | 0.2312 |
| Ziram | 0.0010 | 0.0058 | 0.0109 | 0.0365 | 0.0259 | 0.0480 |
| Methiocarb | 0.0002 | 0.0047 | 0.0001 | 0.0007 | 0.0000 | 0.0011 |
| Methomyl | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0001 |
| Oxamyl | 0.0020 | 0.0265 | 0.0014 | 0.0291 | 0.0020 | 0.0393 |
| Phosmet | 0.0064 | 0.0972 | 0.0206 | 0.1142 | 0.0382 | 0.1927 |
| Prochloraz | 0.0000 | 0.0009 | 0.0000 | 0.0306 | 0.0018 | 0.0037 |
| Propiconazole | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0007 |
| Propineb | 0.0000 | 0.0038 | 0.0070 | 0.0264 | 0.0169 | 0.0368 |
| Pyraclostrobin | 0.0000 | 0.0000 | 0.0000 | 0.0008 | 0.0000 | 0.0000 |
| Tebuconazole | 0.0000 | 0.0019 | 0.0000 | 0.0018 | 0.0000 | 0.0037 |
| Thiabendazole | 0.0000 | 0.0049 | 0.0000 | 0.0249 | 0.0039 | 0.0126 |
| Thiophanate‐methyl | 0.0000 | 0.0009 | 0.0000 | 0.0022 | 0.0005 | 0.0016 |
| Acephate g | 0.0000 | 0.0000 | 0.0000 | 0.0005 | 0.0000 | 0.0001 |
| Chlorpyrifos g | 0.0000 | 0.0018 | 0.0009 | 0.0058 | 0.0029 | 0.0081 |
| Dichlorvos g | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0004 |
| Dimethoate g | 0.0085 | 0.0692 | 0.0309 | 0.1431 | 0.0567 | 0.1457 |
| Diniconazole g | 0.0000 | 0.0016 | 0.0000 | 0.0023 | 0.0000 | 0.0011 |
| Diniconazole‐M g | 0.0000 | 0.0017 | 0.0000 | 0.0024 | 0.0000 | 0.0008 |
| Furathiocarb g | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0002 |
| Heptachlor g | 0.0000 | 0.0010 | 0.0000 | 0.0005 | 0.0000 | 0.0005 |
| Hexachlorocyclohexane (HCH), beta‐isomer g | 0.0000 | 0.0007 | 0.0001 | 0.0007 | 0.0001 | 0.0010 |
| Permethrin g | 0.0000 | 0.0033 | 0.0000 | 0.0025 | 0.0000 | 0.0033 |
| Triazophos g | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0002 |
Lowest estimated probability of exceeding the ARfD among the 15 adult populations.
Highest estimated probability of exceeding the ARfD among the 15 adult populations.
Lowest estimated probability of exceeding the ARfD among the 10 child populations.
Highest estimated probability of exceeding the ARfD among the 10 child populations.
Lowest estimated probability of exceeding the ARfD among the 5 toddler populations.
Highest estimated probability of exceeding the ARfD among the 5 toddler populations.
Active substance with a tentative ARfD.
Even if the estimated probability is 0.0000% by the model, it does not mean the true probability on the real population is 0. Therefore, the probability should be considered close to zero.
For example, for abamectin, it is shown that among the five surveys on toddlers, the middle‐bound value for the percentage of individual‐days exceeding the ARfD varies from a minimum of 0.0002% (i.e. 2 individual‐day out of 1000,000) in one country to a maximum of 0.0014% (i.e. 14 individual‐day out of 1000,000) in another country. The actual countries can be retrieved from Appendix D – Annex IV – Table 4.4, where the minimum is reached in the Netherlands, while the maximum is reached in Denmark. This means that, in Danish toddlers, the percentage of individual‐days exceeding the ARfD is estimated at 0.0014%. For the other toddler populations, the estimate is lower declining to 0.0002% in the Netherlands.
Contrary to the deterministic calculation where EFSA identified the number of samples that might pose a risk for consumers, the probabilistic risk assessment provides more insight on how those samples may potentially affect the acute risk in different subpopulations within the EU, i.e. the probability of the ARfD being exceeded in a population group.
In Appendix D – Annex IV and Annex V, detailed information on the probabilistic acute risk assessment is reported. The annexes contain information on the set ARfD values and whether a primary or tentative value was selected; infographics by active substance, population group and countries are presented too.
Important to note that the assessment is affected by different types of uncertainties. On the one hand, there are some considerations that might lead to an underestimation of the probabilities, such as the restriction of the consumption data to 36 highly consumed RPCs 77 only instead of whole diets; and the use of the residue definition for enforcement and not for risk assessment (where additional metabolites may have contributed e.g. phosmet, methiocarb) in the calculations. These sources of uncertainties are common to deterministic and probabilistic calculations.
On the other hand, the probabilities here presented are in general overestimated mainly due to the uncertainty introduced with the bootstrapping selection of consumers and occurrence data, and the lack of processing factors for processed food consumption.
For the 2021 report, the concentration for analytical results below LOQ were imputed to be equal to ½ LOQ when at least one quantified result was reported for the same substance/commodity combination. This year, the analytical results below LOQ are imputed to be equal to ½ LOQ only for a number of results equal to the total number of positive results (i.e. above the LOQ) for the same substance/commodity combination, provided that the use of the substance is authorised for that commodity. This different approach tends to represent more realistically the state of the market.
To illustrate those uncertainties, the 10 pesticides with the higher estimated probability for an individual‐days to exceed the ARfD are discussed below. A summary of the most contributing RPCs by substance is also discussed below, but for the detail by country the reader is referred to the Appendix D – Annex IV and Annex V.
Cyhalothrin, gamma‐
For gamma‐cyhalothrin, the exposure estimation is mainly driven by bananas and mandarin juice, followed by unprocessed beans (with pods), spinaches and lettuce. However, in the ongoing MRL review71 of gamma‐cyhalothrin, no uses on these commodities were notified to EFSA. Thus, this scenario would only apply in cases of occurring misuses.
Cypermethrins (sum of isomers), alpha‐cypermethrin and beta‐cypermethrin
For cypermethrins (sum of isomers), the exposure estimation is mainly driven by barley malt and wheat (semolina, or flour semi‐refined) for which no processing factor is available; therefore, an overestimation is expected. Should a specific processing factor be available, a more accurate and lower exposure estimate would be obtained. There is also a clear contribution from unprocessed foods such as spinaches and table grapes in a small number of population groups.
Moreover, the concentration of cypermethrin (sum of isomers), alpha‐cypermethrin and beta‐cypermethrin is derived from the results on the residue definition ‘cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers))’, taking the full amount for each active substance in each scenario. This leads to a very conservative estimation as no uses, nor import tolerances are approved in Europe for alpha and beta isomers, and they represent a maximum of 25% (each) in the mixture.
The new MRL values derived by EFSA in the last MRL review are currently under discussion by risk managers and, since for several commodities MRLs are recommended to be lowered, a reduction of the exposure may be expected in the coming years.
Dimethoate
For dimethoate, the exposure estimation is mainly driven by mandarins, peaches, oranges followed by cucumbers and olive oil. No peeling factor for oranges is available leading to a potential overestimation for this specific commodity. Compared to the 2021 report in which the estimation was highly impacted by a very conservative assumption on the imputation on the LOQ, the assumption done this year drastically refines the exposure estimation giving more realistic results. In order to refine exposure, the availability of PFs (despite its non‐approval status at EU) and/or lower LOQs (already set at 0.01 mg/kg) would be desirable (EFSA, 2024a).
Ethephon
For ethephon, the exposure estimation is mainly driven by bananas followed by unprocessed tomatoes, sweet/bell peppers and table grapes. No peeling factor is available for bananas. The main contributors are all unprocessed foods; therefore, the assessment is not impacted by the lack of refinement in the processing. Considering that ethephon is a systemic compound (EFSA, 2009) used as plant growth regulator, even if a peeling factor for bananas would be available, the refinement would not impact the results significantly. Therefore, no further improvements are expected for this estimation.
Imazalil
For imazalil, the exposure estimation is mainly driven by processed oranges, grapefruits and mandarins followed by potatoes. Occurrence data for orange juice and grapefruit juice were used for the estimation. Therefore, for these processed commodities, no further refinement is possible. However, when the juice concentrate contributed the most, no processing factor was available. In order to refine exposure, PF or occurrence data reported on concentrated juice, is needed.
Dithiocarbamates (maneb, mancozeb, metiram, propineb, thiram and ziram, expressed as CS 2 )
The exposure estimation is the highest of all precursors for metiram, mainly driven by unprocessed pears and head cabbages followed by broccoli, apples, cauliflowers and mandarins.
However, the concentration of maneb, mancozeb, metiram, propineb, thiram and ziram is derived from the results on the residue definition ‘dithiocarbamates (dithiocarbamates expressed as CS2, including maneb, mancozeb, metiram, propineb, thiram and ziram)’, taking the full amount for each active substance. This leads to a very conservative estimation and the true percentage of consumers exceeding the ARfD is expected to be considerably lower, considering also that among the active substances included in the dithiocarbamates residue definition, in 2022 ziram was approved and metiram approval period was extended until 31 January 2024.
Oxamyl
For oxamyl, the exposure estimation is mainly driven by cucumbers, beans (with pods) and carrots. Compared to the 2021 report in which the estimation was highly impacted by a very conservative assumption on the imputation on the LOQ, the assumption done this year drastically reduced the exposure estimation giving more realistic results. Regulation (EU) 2024/331 78 (not yet applicable in 2022) lowers the LOQs even further than 0.01 mg/kg. This will refine the exposure estimation of oxamyl (EFSA, 2023g, 2024a).
Phosmet
For phosmet, the exposure estimation is mainly driven by olive oil and apples followed by peaches, oranges and mandarins. Regarding these commodities, there is little room for refinements as apples and peaches may be consumed unpeeled. However, no processing factor is available for oranges and mandarins and since the active substance is not systemic, this may lead to an overestimation in the calculations.
The grace period for its approval expired in November 2022. As per Regulation (EU) 2023/1029, 79 all MRLs were lowered to the LOQ, including those based on CODEX, and the substance was moved to Annex V of Regulation (EC) 395/2005 as for some commodities, the LOQ needed to be lower than the default 0.01* mg/kg, i.e. to 0.005* mg/kg. The latter is expected to lower the exposure to phosmet.
Summary
Overall, within this probabilistic risk assessment, the 163 active substances with a valid ARfD (i.e. resulting from 193 pesticides) have been analysed. For most of the substances, the probability for a consumer intake to exceed the ARfD is extremely low. For 111 of them, the middle bound (median value) of the confidence interval of the percentage of individual‐days potentially exceeding the ARfD is estimated to be 0%, keeping in mind that the upper bound (97.5th percentile) of the confidence intervals might be higher. The probabilistic approach is modelling sampling uncertainty to give a plausible confidence interval for the estimates analysed. The detail on the confidence interval is reported in Appendix D – Annexes IV and V.
Furthermore, the assessment still needs to account for additional uncertainties that may either overestimate or underestimate the probabilistic exposure estimates reported above. Further efforts will be made in the future to improve estimates for such active substances. For thiram, alpha‐cypermethrin and beta‐cypermethrin, the methodology also needs to be improved to take better account of their non‐approval status in the EU, most likely leading to an overestimation of the exposure estimates. Similarly, for cypermethrins, phosmet, gamma‐cyhalothrin and dimethoate, exposure estimates are expected to be overestimated due to the lack of processing factors.
5.3. Chronic deterministic risk assessment
In the chronic deterministic assessments, the risk of one ‘virtual’ consumer is calculated, assuming an average consumption of all commodities consumed by the population the consumer belongs to.
The chronic risk assessment estimates the dietary exposure to pesticides from food over a long period. Its calculation is based on a deterministic approach developed by JMPR (FAO, 2017). It consists of multiplying the average measured pesticide concentration by the average commodity's daily consumption per capita and summing up the results for all commodities within a given dietary habit.
In Appendix D – Annex II, the outcome of the deterministic exposure assessments is included. The ADI/TDI values for all the active substances mentioned in this report are found in Appendix D – Annex III – Table 3.5.
5.3.1. Methodology for the estimation of chronic exposure
Opposite to the deterministic acute assessment where only the 36 commodities covered by the 3‐year cycle of the 2022 EU MACP are included in the calculations, the chronic assessment deals with samples submitted in 2022 by the reporting countries under EU MACP and MANCP, covering the unprocessed products listed in Annex I (part A) of Regulation (EC) No 396/2005 for which consumption data are available in PRIMo rev. 3.1. In total, 64,932 samples were considered.
EFSA calculated three scenarios for chronic deterministic risk assessment: the lower bound, the middle bound and the upper bound.
The lower bound scenario: It assumes samples with non‐quantified residues (i.e. samples with residue levels reported as being < LOQ) do not contain any residue. This scenario is the less conservative one as it disregards the contribution of residues potentially present in small amounts below the LOQ. It may result in an underestimation of the chronic deterministic exposure.
The middle‐bound scenario: It assumes samples with non‐quantified residues (i.e. samples with residue levels reported as being < LOQ) contain residues at a level of ½ LOQ, 80 only if the active substance precursor leading to residues, was approved at EU level during 2022. For the non‐approved substances, the imputed value was zero instead of half of the LOQ value.
The upper bound scenario: It assumes samples with non‐quantified residues (i.e. samples with residue levels reported as being < LOQ) contain residues at a level of LOQ,80 only if the active substance precursor leading to residues, was approved at EU level during 2022. For the non‐approved substances, the imputed value was zero instead of half of the LOQ value.
The mean residue concentration from the analytical results for any given pesticide/crop combination was used. Pesticide/commodity combinations for which no sample had quantified residues were not considered in the chronic exposure assessment. These are assumed to represent a no residue/no exposure situation.
These scenarios are used by EFSA to frame the boundaries of a more realistic exposure estimate to pesticide residues. They are similar to the ones established in the probabilistic Section Appendix C.1.4, 81 The aim of the different scenarios is to better address the uncertainties linked to the presence of residues at levels below the LOQ.
The following was considered in these scenarios' calculations:
Only results for unprocessed products with available consumption data were used for this exposure calculation. Thus, no PF were use.
Only data on the 193 pesticides listed in 2022 EU MACP and for which the analysis covered their full RD for enforcement were used.
Results from samples analysed with analytical methods for which the LOQ was greater than the corresponding MRL were disregarded.
Only samples obtained through selective or objective sampling were retained (SSD codes ST10A and ST20A). Samples obtained through suspect sampling (ST30A) were considered not representative for this assessment and as such excluded (EFSA, 2023a).
Consumption data used for the deterministic exposure assessment were those used in PRIMo revision 3.1 (EFSA, 2018a). The mean consumption was taken for the 13 Member States (Germany, Denmark, Spain, Finland, France, Ireland, Italy, Lithuania, the Netherlands, Poland, Portugal, Romania and Sweden) derived from national food surveys and the relevant GEMS/Food Cluster diets relevant for the EU Member States (i.e. Cluster diet G06, G07, G08, G10, G11 and G15).
The estimation of chronic exposure is based on the residue definition for enforcement and was not converted into the one defined for risk assessment. Thus, possible underestimation of the exposure assessment can be expected.
For the legal residue definition of fenvalerate containing esfenvalerate, a compound with a different toxicological profile, the chronic risk assessment was based on the ADI of the authorised active substance esfenvalerate (EFSA, 2014b).
For the legal residue definition of ‘cyfluthrin (cyfluthrin including other mixtures of constituent isomers (sum of isomers))’, based on EFSA opinion (EFSA, 2020b), the cyfluthrin toxicological profile was taken and HBGV derived for cyfluthrin used in this assessment.
For the legal residue definition of lambda‐cyhalothrin (including gamma‐cyhalothrin) (sum of R,S and S,R isomers), the chronic risk assessment was based on the chronic toxicological profile of gamma isomer being the most potent of the two approved active substance (EFSA, 2017a).
Related to cypermethrin residue definition (cypermethrin including other mixtures of constituent isomers (sum of isomers)), the chronic profile selected to perform the risk assessment was the one of cypermethrin (EFSA, 2018f).
Most of the dithiocarbamate active substances were not approved in the EU in 2022, except metiram and ziram, and import tolerances on mancozeb. The monitoring data used reflects the common moiety method reporting total CS2. Thus, to have a thorough coverage of all possible uses and misuses, and since the different precursors have different ADI values, all six active substances (mancozeb, maneb, metiram, propineb, thiram and ziram) were considered in the deterministic chronic assessment.
For heptachlor, hexachlorobenzene, alpha‐hexachlorocyclohexane and beta‐hexachlorocyclohexane, the estimated chronic exposure assessment was performed with pTDI reference value. These values have never been formally established at EU level and the toxicological dossiers of these substances are very old. Their findings are due to persistence in the environment. Therefore, these assessments are to be considered tentative.
5.3.2. Results
The results of the chronic deterministic exposure assessment expressed as the highest percentage of the ADI from all populations considered for each pesticide (lower bound, middle‐bound and upper bound scenarios) are reported in Table 3.
TABLE 3.
Results of the chronic dietary exposure assessment.
| Pesticide | Chronic exposure (in % of ADI) | ||
|---|---|---|---|
| Lower‐bound | Middle‐bound | Upper‐bound | |
| 2,4‐D (RD) | 0.26 | 0.72 | 1.2 |
| 2‐Phenylphenol (RD) | 0.24 | 0.28 | 0.32 |
| Abamectin (RD) | 0.06 | 6.1 | 12.1 |
| Acephate (RD)* | 0.16 | 0.16 | 0.16 |
| Acetamiprid (RD) | 0.87 | 1.3 | 1.8 |
| Acrinathrin (RD) | 0.003 | 0.003 | 0.003 |
| Aldicarb (RD)* | 0.00002 | 0.00002 | 0.00002 |
| Ametoctradin (RD) | 0.0007 | 0.0009 | 0.001 |
| Azoxystrobin (RD) | 0.29 | 0.38 | 0.47 |
| Bifenthrin (RD) | 0.21 | 0.21 | 0.21 |
| Biphenyl (RD)* | 0.002 | 0.002 | 0.002 |
| Boscalid (RD) | 1.1 | 1.6 | 2 |
| Bromopropylate (RD)* | 0.001 | 0.001 | 0.001 |
| Bupirimate (RD) | 0.02 | 0.18 | 0.33 |
| Buprofezin (RD) | 0.01 | 0.36 | 0.7 |
| Captan (RD) | 1 | 1.1 | 1.2 |
| Carbaryl (RD) | 0.45 | 0.45 | 0.45 |
| Carbendazim (RD) | 0.23 | 0.23 | 0.23 |
| Carbofuran (RD) | 2.9 | 2.9 | 2.9 |
| Chlorantraniliprole (RD) | 0.004 | 0.01 | 0.02 |
| Chlorfenapyr (RD)* | 0.08 | 0.08 | 0.08 |
| Chlormequat‐chloride (RD) | 12.8 | 12.9 | 13 |
| Chlorothalonil (RD) | 0.04 | 0.04 | 0.04 |
| Chlorpropham (RD) | 0.11 | 0.11 | 0.11 |
| Chlorpyrifos (RD)* | 1.1 | 1.1 | 1.1 |
| Chlorpyrifos‐methyl (RD)* | 0.03 | 0.03 | 0.03 |
| Clofentezine (RD) | 0.01 | 0.62 | 1.2 |
| Clothianidin (RD) | 0.003 | 0.003 | 0.003 |
| Cyantraniliprole (RD) | 0.1 | 1.1 | 2.2 |
| Cyazofamid (RD) | 0.009 | 0.025 | 0.04 |
| Cyflufenamid (RD) | 0.02 | 0.19 | 0.38 |
| Cyfluthrin (RD) | 0.003 | 0.003 | 0.003 |
| Cymoxanil (RD) | 0.008 | 0.2 | 0.4 |
| Cypermethrin (RD) | 1.3 | 6.4 | 11.5 |
| Cyproconazole (RD) | 0.02 | 0.02 | 0.02 |
| Cyprodinil (RD) | 0.84 | 1.2 | 1.5 |
| Cyromazine (RD) | 0.01 | 0.01 | 0.01 |
| DDT (RD)* | 0.003 | 0.003 | 0.003 |
| Deltamethrin (RD) | 0.92 | 3.2 | 5.4 |
| Diazinon (RD) | 0.64 | 0.64 | 0.64 |
| Dichlorvos (RD)* | 0.03 | 0.03 | 0.03 |
| Dicloran (RD) | 0.003 | 0.003 | 0.003 |
| Dicofol (RD)* | 0.01 | 0.01 | 0.01 |
| Dieldrin (RD)* | 0.47 | 0.47 | 0.47 |
| Diethofencarb (RD) | 0.00001 | 0.00001 | 0.00001 |
| Difenoconazole (RD) | 0.85 | 2.9 | 4.9 |
| Diflubenzuron (RD) | 0.004 | 0.004 | 0.004 |
| Dimethoate (RD)* | 1.3 | 1.3 | 1.3 |
| Dimethomorph (RD) | 0.12 | 0.27 | 0.45 |
| Diniconazole (RD)* | 0.28 | 0.28 | 0.28 |
| Diphenylamine (RD) | 0.0002 | 0.0002 | 0.0002 |
| Dithianon (RD) | 1.2 | 1.9 | 2.7 |
| Dithiocarbamates (RD)–mancozeb sc. | 5.8 | 5.8 | 5.8 |
| Dithiocarbamates (RD)–maneb sc. | 2.6 | 2.6 | 2.6 |
| Dithiocarbamates (RD)–metiram sc. | 5.3 | 5.3 | 5.3 |
| Dithiocarbamates (RD)–propineb sc. | 5.7 | 5.7 | 5.7 |
| Dithiocarbamates (RD)–thiram sc. | 7.4 | 7.4 | 7.4 |
| Dithiocarbamates (RD)–ziram sc. | 24.7 | 57.3 | 89.8 |
| Dodine (RD) | 0.07 | 0.15 | 0.24 |
| Emamectin (RD) | 0.16 | 5.7 | 11.3 |
| Endosulfan (RD)* | 0.0005 | 0.0005 | 0.0005 |
| Epoxiconazole (RD) | 0.99 | 0.99 | 0.99 |
| Ethephon (RD) | 4.6 | 5.9 | 7.1 |
| Ethion (RD)* | 0.005 | 0.005 | 0.005 |
| Ethirimol (RD) | 0.02 | 0.02 | 0.02 |
| Etofenprox (RD) | 0.2 | 0.6 | 1 |
| Etoxazole (RD) | 0.004 | 0.13 | 0.26 |
| Famoxadone (RD) | 0.08 | 0.08 | 0.08 |
| Fenamidone (RD)* | 0.0003 | 0.0003 | 0.0003 |
| Fenamiphos (RD) | 0.004 | 0.004 | 0.004 |
| Fenazaquin (RD) | 0.006 | 0.44 | 0.88 |
| Fenbuconazole (RD) | 0.02 | 0.02 | 0.02 |
| Fenbutatin Oxide (RD) | 0.006 | 0.006 | 0.006 |
| Fenhexamid (RD) | 0.11 | 0.16 | 0.2 |
| Fenitrothion (RD) | 0.01 | 0.01 | 0.01 |
| Fenoxycarb (RD) | 0.009 | 0.009 | 0.009 |
| Fenpropathrin (RD)* | 0.003 | 0.003 | 0.003 |
| Fenpropidin (RD) | 0.06 | 0.53 | 1 |
| Fenpropimorph (RD) | 0.37 | 0.37 | 0.37 |
| Fenpyrazamine (RD) | 0.009 | 0.03 | 0.05 |
| Fenpyroximate (RD) | 0.09 | 1.1 | 2.1 |
| Fenvalerate (RD) | 0.005 | 0.3 | 0.58 |
| Fipronil (RD) | 1.4 | 1.4 | 1.4 |
| Flonicamid (RD) | 0.38 | 1.3 | 2.3 |
| Fluazifop (RD) | 0.47 | 0.94 | 1.6 |
| Flubendiamide (RD) | 0.01 | 0.58 | 1.2 |
| Fludioxonil (RD) | 0.34 | 0.38 | 0.41 |
| Flufenoxuron (RD) | 0.03 | 0.03 | 0.03 |
| Fluopicolide (RD) | 0.07 | 0.13 | 0.18 |
| Fluopyram (RD) | 1.4 | 2.8 | 4.1 |
| Flusilazole (RD) | 0.003 | 0.003 | 0.003 |
| Flutriafol (RD) | 0.05 | 0.05 | 0.05 |
| Fluxapyroxad (RD) | 0.35 | 1 | 1.7 |
| Folpet (RD) | 0.61 | 0.7 | 0.78 |
| Formetanate (Hydrochloride) (RD) | 0.02 | 0.92 | 1.8 |
| Fosetyl (RD) | 4.2 | 4.3 | 4.5 |
| Fosthiazate (RD) | 0.02 | 0.72 | 1.4 |
| Glufosinate Equivalents (RD) | 0.23 | 0.23 | 0.23 |
| Glyphosate (RD) | 1.1 | 1.1 | 1.2 |
| Haloxyfop (RD) | 0.5 | 0.5 | 0.5 |
| Heptachlor (RD)* | 0.04 | 0.04 | 0.04 |
| Hexachlorobenzene (RD)* | 0.03 | 0.03 | 0.03 |
| Hexachlorocyclohexane, beta‐ (RD)* | 0.0002 | 0.0002 | 0.0002 |
| Hexaconazole (RD)* | 0.03 | 0.03 | 0.03 |
| Hexythiazox (RD) | 0.04 | 0.36 | 0.68 |
| Imazalil (RD) | 16.9 | 17.4 | 17.9 |
| Imidacloprid (RD) | 0.02 | 0.02 | 0.02 |
| Indoxacarb (RD) | 0.25 | 0.25 | 0.25 |
| Iprodione (RD) | 0.004 | 0.004 | 0.004 |
| Iprovalicarb (RD) | 0.07 | 0.21 | 0.38 |
| Kresoxim‐Methyl (RD) | 0.0005 | 0.01 | 0.02 |
| Lambda‐cyhalothrin (RD) | 2.1 | 10 | 17.8 |
| Lambda‐cyhalothrin (RD)–gamma sc. | 4.4 | 20.8 | 37.1 |
| Linuron (RD) | 0.05 | 0.05 | 0.05 |
| Lufenuron (RD) | 0.004 | 0.004 | 0.004 |
| Malathion (RD) | 0.06 | 0.3 | 0.58 |
| Mandipropamid (RD) | 0.04 | 0.07 | 0.09 |
| Mepanipyrim (RD) | 0.03 | 0.17 | 0.34 |
| Mepiquat Chloride (RD) | 0.18 | 0.23 | 0.29 |
| Metaflumizone (RD) | 0.05 | 0.3 | 0.55 |
| Metalaxyl and metalaxyl‐M (RD) | 0.02 | 0.15 | 0.29 |
| Methamidophos (RD) | 0.12 | 0.12 | 0.12 |
| Methidathion (RD)* | 0.008 | 0.008 | 0.008 |
| Methiocarb (RD) | 1.5 | 1.5 | 1.5 |
| Methomyl (RD) | 0.05 | 0.05 | 0.05 |
| Methoxyfenozide (RD) | 0.02 | 0.14 | 0.25 |
| Metrafenone (RD) | 0.008 | 0.02 | 0.04 |
| Monocrotophos (RD)* | 0.08 | 0.08 | 0.08 |
| Myclobutanil (RD) | 0.38 | 0.38 | 0.38 |
| Oxadixyl (RD)* | 0.0003 | 0.0003 | 0.0003 |
| Oxamyl (RD) | 0.35 | 11.6 | 22.9 |
| Paclobutrazol (RD) | 0.0001 | 0.09 | 0.19 |
| Parathion‐methyl (RD)* | 0.0003 | 0.0003 | 0.0003 |
| Penconazole (RD) | 0.03 | 0.35 | 0.67 |
| Pencycuron (RD) | 0.0001 | 0.0001 | 0.0001 |
| Pendimethalin (RD) | 0.002 | 0.002 | 0.002 |
| Permethrin (RD)* | 0.05 | 0.05 | 0.05 |
| Phosmet (RD) | 3.6 | 3.6 | 3.6 |
| Pirimicarb (RD) | 0.09 | 0.33 | 0.57 |
| Pirimiphos‐Methyl (RD) | 6.2 | 8.5 | 11.8 |
| Prochloraz (RD) | 0.43 | 0.43 | 0.43 |
| Procymidone (RD)* | 0.07 | 0.07 | 0.07 |
| Profenofos (RD)* | 0.007 | 0.007 | 0.007 |
| Propamocarb (RD) | 0.12 | 0.16 | 0.2 |
| Propargite (RD) | 0.01 | 0.01 | 0.01 |
| Propiconazole (RD) | 0.06 | 0.06 | 0.06 |
| Propyzamide (RD) | 0.0015 | 0.05 | 0.1 |
| Proquinazid (RD) | 0.17 | 0.79 | 1.4 |
| Prosulfocarb (RD) | 0.04 | 1.6 | 3.1 |
| Prothioconazole (RD) | 0.008 | 0.16 | 0.32 |
| Pymetrozine (RD) | 0.0002 | 0.0002 | 0.0002 |
| Pyraclostrobin (RD) | 0.46 | 0.84 | 1.2 |
| Pyridaben (RD) | 0.07 | 0.55 | 1.1 |
| Pyridalyl (RD) | 0.02 | 0.08 | 0.15 |
| Pyrimethanil (RD) | 1.5 | 1.6 | 1.7 |
| Pyriproxyfen (RD) | 0.3 | 0.53 | 0.77 |
| Quinoxyfen (RD) | 0.00009 | 0.00009 | 0.00009 |
| Spinosad (RD) | 0.18 | 0.82 | 1.5 |
| Spirodiclofen (RD) | 0.09 | 0.09 | 0.09 |
| Spiromesifen (RD) | 0.03 | 0.13 | 0.22 |
| Spirotetramat (RD) | 0.19 | 0.67 | 1.1 |
| Spiroxamine (RD) | 0.06 | 0.15 | 0.25 |
| Sulfoxaflor (RD) | 0.04 | 0.29 | 0.56 |
| Tau‐Fluvalinate (RD) | 0.27 | 2.5 | 4.8 |
| Tebuconazole (RD) | 0.41 | 1.1 | 1.8 |
| Tebufenozide (RD) | 0.05 | 0.56 | 1.1 |
| Tebufenpyrad (RD) | 0.04 | 1 | 2 |
| Teflubenzuron (RD) | 0.004 | 0.004 | 0.004 |
| Tefluthrin (RD) | 0.002 | 0.54 | 1.1 |
| Terbuthylazine (RD) | 0.004 | 1.1 | 2.2 |
| Tetraconazole (RD) | 0.12 | 2.4 | 4.7 |
| Tetradifon (RD)* | 0.00009 | 0.00009 | 0.00009 |
| Thiabendazole (RD) | 1.7 | 1.8 | 1.9 |
| Thiacloprid (RD) | 0.08 | 0.08 | 0.08 |
| Thiamethoxam (RD) | 0.17 | 0.17 | 0.17 |
| Thiodicarb (RD) | 0.0004 | 0.0004 | 0.0004 |
| Thiophanate‐methyl (RD) | 0.27 | 0.27 | 0.27 |
| Tolclofos‐Methyl (RD) | 0.00003 | 0.004 | 0.008 |
| Triadimefon (RD)* | 0.0001 | 0.0001 | 0.0001 |
| Triadimenol (RD) | 0.002 | 0.002 | 0.002 |
| Triazophos (RD)* | 0.05 | 0.05 | 0.05 |
| Trifloxystrobin (RD) | 0.11 | 0.24 | 0.37 |
| Triflumuron (RD) | 0.11 | 0.11 | 0.11 |
| Vinclozolin (RD) | 0.00001 | 0.00001 | 0.00001 |
| Azinphos‐methyl (RD)* | n.r. | ||
| Bitertanol (RD) | n.r. | ||
| Chlordane (RD)* | n.r. | ||
| Fenarimol (RD) | n.r. | ||
| Fenthion (RD)* | n.r. | ||
| Fluquinconazole (RD)* | n.r. | ||
| alpha‐ Hexachlorocyclohexane (RD)* | n.r. | ||
| Lindane (RD)* | n.r. | ||
| Methoxychlor (RD) | n.r. | ||
| Oxydemeton‐Methyl (RD) | n.r. | ||
| Parathion (RD) | n.r. | ||
| Spinoteram (RD) | n.r. | ||
| Bromide ion (RD)** | No ADI | ||
| Isocarbophos (RD)** | No ADI | ||
| Omethoate (RD)** | No ADI | ||
Abbreviations: n.r., No quantified residues in any of the samples analysed; sc., scenario.
Tentative risk assessment.
Active substance for which no ADI was established.
No chronic consumer intake concerns were identified for any of the European diets incorporated in PRIMo rev. 3.1 when the risk assessment was based on the different scenarios. The top highest chronic risk estimates corresponded to the upper bound scenario on ziram with 89.8% of the ADI (NL, toddler with major contributor apples, followed by pears) and gamma‐cyhalothrin with 37.1% of the ADI (NL, toddler with major contributor apple followed by bananas and pears).
In the upper bound scenario, the estimated chronic exposure for 174 pesticides (accounting for 4 dithiocarbamate scenarios) was less than 10% of the ADI of which for 127 of them, the result was lower or equal to 1% of the ADI.
For azinphos‐methyl (RD), bitertanol (RD), chlordane (RD), fenarimol (RD), fenthion (RD), fluquinconazole (RD), alpha‐hexachlorocyclohexane (RD), lindane (RD), methoxychlor (RD), oxydemeton‐methyl (RD), parathion (RD), spinetoram (RD) covered by the 2022 EU MACP, quantifiable residues were not reported for any of the food samples tested, and therefore, they were excluded from the calculation.
As there are no ADI value for bromide ion, isocarbophos and omethoate, no chronic risk assessment was undertaken. The active substance bromide ion was quantified in 89 different food commodities. Until EFSA has finalised its scientific opinion on the risks for human health related to the presence of bromide ion in food and risks on animal health and transfer from feed to food of animal origin related to the presence of bromine ion in feed,67 EFSA will not perform a full chronic risk assessment of this substance. The active substance omethoate was quantified in 25 different food commodities. There was no sample with quantified results on isocarbophos; thus, the exposure was not calculated. The exposure estimates for the substances quantified using the food consumption in EFSA PRIMo rev. 3.1 are reported in Table 4.
TABLE 4.
Results of chronic exposure assessment for omethoate and bromide ion.
| Pesticide | Chronic exposure (in mg/kg bw per day) | ||
|---|---|---|---|
| Lower bound approach | Middle‐bound approach | Upper bound approach | |
| Bromide ion67 | 2.5 × 10−3 | 7.5 × 10−3 | 0.0126 |
| Omethoate 82 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 |
The new approach to handle left censored data (i.e. middle and upper bound) by imputing half the LOQ or the full LOQ value depending on whether the active substance was approved or not, made the results reported as LOQ to contributing less. This is a refinement that made the results more realistic.
Taking into consideration all food items for which consumption data are provided in PRIMo rev. 3.1, the highest contributors to the overall EU pesticide dietary exposure remain those food items covered by the 3‐year cycle of the EU‐coordinated programme. This can be seen in Appendix D – Annex II, on spreadsheet ‘Report’ on the graph presenting the % of ADI by commodity, on the category gathered under ‘other products’. The % of ADI for these other products categories is smaller than the sum of the % ADI of all the 36 commodities in the EU MACP.
5.4. Chronic probabilistic risk assessment
In the chronic probabilistic modelling, the risk of hundreds of real consumers is calculated, based on their own individual dietary pattern. This is more realistic, and also allows capturing extreme levels of exposure within consumer populations.
EFSA calculated the probabilities of exceedance of the ADI of pesticides in different subpopulation of European consumers. The purpose of these calculations is to provide readers with a deeper insight into the risk of dietary exposure to pesticides by extending the list of pesticides to the 193 included in 2022 EU MACP Regulation in respect to last year's (EFSA, 2023c) where only the 29 pesticides found to exceed the acute HBGV in the deterministic risk assessment where considered. Samples included were those of 2020, 2021 and 2022. Further details can be found in Appendix C.
Chronic risks depend on the average chronic exposure, and not on single exposure events, as this is the case for acute effects. Hence, chronic exposure assessments, regardless of being deterministic or probabilistic, rely on the assumption that all commodities contain an average residue concentration, calculated from the available monitoring data.
The main difference between chronic deterministic and probabilistic exposure assessment is in the handling of consumption data. In deterministic assessments, the risk of one ‘virtual’ consumer is calculated, assuming an average consumption of all commodities consumed by the population the consumer belongs to. In contrast, in probabilistic modelling, the risk of hundreds of real consumers is calculated, based on their own individual dietary pattern. This allows capturing the distribution of the exposure, including the high end of the distribution for extreme levels of the exposure within consumer populations subgroups.
5.4.1. Data and methodology
As for the acute probabilistic exposure assessment (see Section 5.2), chronic estimates were also obtained using a two‐dimensional method where variability of exposure within the population is modelled by means of an inner loop execution, and confidence intervals around the chronic exposure estimates are modelled through an outer loop execution. The main differences compared to the acute exposure assessments are described below (see also Figure 3).
FIGURE 3.
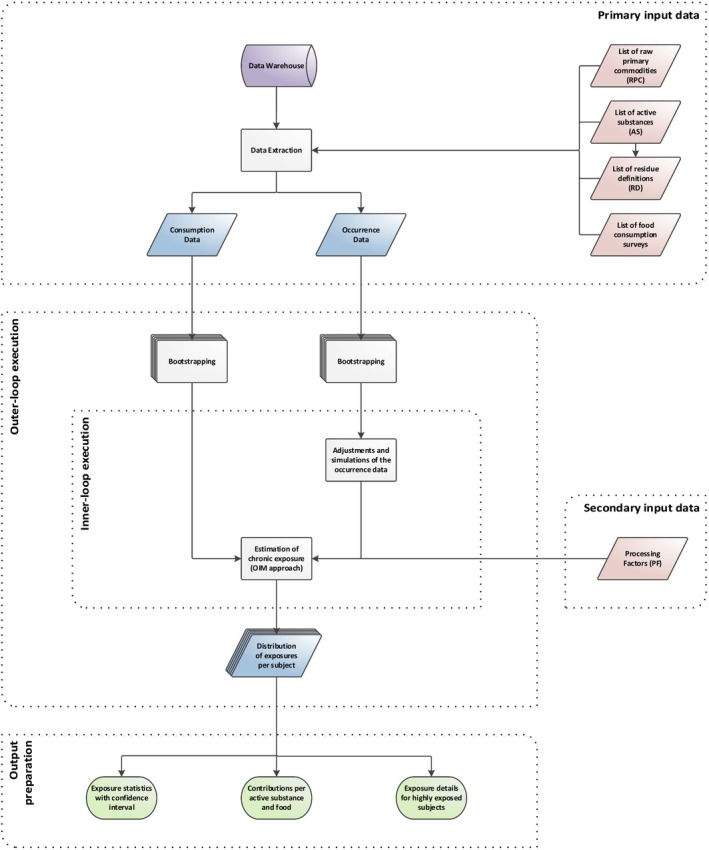
General process for probabilistic estimation of chronic exposure to pesticide residues.
Whereas acute exposure (within the inner loop execution) is modelled through a Monte Carlo simulation, the chronic exposure is modelled through the Observed Individual Means (OIM) approach (EFSA, 2012). This method uses the mean consumption over the survey days of each individual to estimate the individuals' long‐term consumption for each food commodity. Using the individuals' body weight and the mean occurrence values calculated for each food commodity, the individuals' chronic exposures resulting from each food commodity are calculated and added to obtain the individuals' total chronic exposure. Exposure estimates are then expressed as percentage of ADI. Hence, a calculated exposure greater than 100% suggests that the estimated exposure exceeds the ADI for that active substance, and a health risk cannot be excluded. This also allows to calculate within each subpopulation the percentage of consumers that have an exposure exceeding the ADI.
Further details on the input data and methodologies applied for probabilistic exposure assessment are provided in Appendix C.
5.4.2. Results
The 2022 EU MACP listed 193 pesticides. These pesticides (i.e. residue definition) could be derived from different active substances on which HBGV are derived. The chronic probabilistic exposure assessment was conducted to 199 active substances for which a HBGV was set. The methodology followed is described in Section 5.4.1.
Among all substances analysed, the only one having a percentage of consumers exceeding the ADI at the MB in at least one population group was imazalil with 0.1546% for adults in Finland and 0.010% for adults in Germany. For all the other substances, the model is not showing any consumer exposure exceeding the ADI at the MB.
Pirimiphos‐methyl shows some population groups with a percentage of consumers exceeding the ADI above zero percent at the upper bound of the CI with 0.584% for toddlers in Bulgaria and 0.019% for adults in Germany.
To better describe the magnitude of the exposure to those two active substances, Table 5 provides the exposure curves obtained from this analysis described by means of their 50th, 95th, 99th, 99.9th percentiles (both for middle bound and upper bound scenarios) in the table below extracted from Annex VI – Table B.1.4. Only the two substances and the population groups abovementioned are reported. A value of 100% corresponds to an exposure equal to the ADI. The percentage of consumers exceeding ADI is also reported.
TABLE 5.
MB and UB exposure curves and percentage of individuals exceeding the ADI for imazalil and pirimiphos‐methyl.
| Substance | Population class | Country | 50th Pctl (MB) (%) | 50th Pctl (UB) (%) | 95th Pctl (MB) (%) | 95th Pctl (UB) (%) | 99th Pctl (MB) (%) | 99th Pctl (UB) (%) | 99.9th Pctl (MB) (%) | 99.9th Pctl (UB) (%) | Percentage of consumers exceeding ADI (MB) (%) | Percentage of consumers exceeding ADI (UB) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imazalil | Adults | FI | 0.1258 | 0.1589 | 1.2902 | 1.5132 | 5.2998 | 7.8074 | 78.5190 | 217.2474 | 0.155 | 0.348 |
| Imazalil | Adults | DE | 0.0136 | 0.0363 | 0.7461 | 0.8556 | 10.9884 | 13.3161 | 46.1980 | 55.8815 | 0.010 | 0.029 |
| Pirimiphos‐methyl | Adults | DE | 0.6559 | 0.8613 | 2.3594 | 4.5115 | 3.8858 | 7.7976 | 7.1927 | 14.7851 | 0.000 | 0.019 |
| Pirimiphos‐methyl | Toddlers | BG | 2.8313 | 3.5769 | 5.8289 | 6.9596 | 7.7444 | 12.1743 | 42.6831 | 108.7358 | 0.000 | 0.584 |
For the remaining active substances (i.e. 199–2 = 197), the percentage of consumers exceeding the ADI is estimated to be zero for every population group analysed.
However, as is the case in the acute exposure calculations (Section 5.2.2), these estimates are subject to multiple uncertainties that may either underestimate or overestimate the exposure.
Imazalil
The exposure estimation for imazalil is driven by processed oranges, grapefruits and mandarins in the form of concentrated juices, for which no processing factor was applied. A refinement of the exposure calculation (i.e. extrapolating processing factors from orange concentrated juice) should be explored to address this significant bias in the calculations. Therefore, EFSA recommends not only deriving PF on juices but to do so on concentrated juices.
For the following surveys, at least one consumer exceeding the ADI was identified:
-
–
For adults in Finland, the exceedance is linked to a high consumption of orange juice concentrate for two consumers consuming 200 and 622.5 g/day
-
–
For adults in Germany, the exceedance is linked to a high consumption of orange juice concentrate for one consumer consuming 420 g/day.
Pirimiphos‐methyl
The exposure estimation for pirimiphos‐methyl is linked to wheat germ 83 for which a processing factor was not applied.
For the following surveys, at least one consumer exceeding the ADI was identified:
-
–
For adults in Germany, the exceedance is linked to a high consumption of wheat germ for one consumer 180 g/day
-
–
For toddlers in Bulgaria, the exceedance is linked to a high consumption of wheat germ for one consumer 30 g/day
Even if the MB of percentage of consumers exceeding the ADI for this substance is at zero percent, the result for this substance is presented because it contains the only two other consumers that are exceeding the ADI, at the upper bound, among all the other substances analysed.
Summary
Overall, the chronic probabilistic risk assessment shows that, only for two active substances (out of the 199 analysed), the ADI is exceeded for one or more consumers. The estimate is linked to a high (or extreme) consumption event of a specific processed food (for which a processing factor is not available) by few (1 to 2) consumers per survey.
Moreover, it is confirmed that based on the surveys used and their size, the estimated probability to exceed the ADI is 0% for 197 out of 199 active substances. For the remaining two substances, the estimated probability for consumers to exceed the ADI ranges from 0 to 0.15% (i.e. 15 individuals out of 10,000). However, these estimates are subject to a high consumption of orange juice concentrate of an extreme consumer in a survey, and thus, it is not representative of all consumers in the EU. Compared to the deterministic approach that considers the average consumption of specific food among the population of the surveys, the probabilistic approach is analysing the average consumption of any single consumer. Therefore, it is more sensitive to extreme consumers and gives a clearer view on the probability that certain consumers within the population might be at risk. The probabilistic risk assessment is performed on occurrence data from 2020 to 2022, while the deterministic is performed on the 2022 data only. Furthermore, the source of consumption data is different, being RPC Consumption Database (EFSA, 2019a) for the probabilistic approach different from the surveys included in PRIMo rev. 3.1 for the deterministic assessments.
6. CONCLUSIONS AND RECOMMENDATIONS
The 2022 EU report on pesticide residues in food, prepared by EFSA in accordance with Article 32 of Regulation (EC) No 396/2005, provides an overview of the official control activities on pesticide residues carried out in the EU Member States,*1 Iceland and Norway. Visuals of the results are presented in Appendix D – Annex I2 allowing stakeholders to scroll through.
A total of 110,829 samples were analysed, representing a 26.1% increase compared with 2021 (87,863 samples). Of the total, 96.3% of the samples fell within the legal limit, being a similar figure over the last years (96.1% in 2021; 94.9% in 2020); of these, 65,374 samples (59.0%) did not contain quantifiable residues (results below the LOQ for each pesticide analysed), while 37.3% contained quantified residues not exceeding the legal limits (41,307 samples). The MRL exceedance rate slightly decreased from 3.9% in 2021 to 3.7% in 2022 (4148 samples). When taking into account measurement uncertainty, 2.2% (2383 samples) of all samples triggered legal sanctions or enforcement actions, a slight decrease in comparison with 2021 (2.5%).
Of the total 110,829 samples, 72,161 (65.1%) were reported as having origin in one of the reporting countries. Of these, 47,264 samples (65.5%) did not show quantifiable results (i.e. below the LOQ), while 23,567 samples (32.7%) contained residues at or above the LOQ but below or equal to the MRL. 1328 samples (1.8%) exceeded the MRL, and of these, 710 samples (1.0%) were non‐compliant with the MRL (after taking into account measurement uncertainty). The remaining 34,193 samples (30.9%) were imported from third countries, of which 15.947 samples (46.6%) were reported as without quantifiable residues, while in 15.708 samples (45.9%) contained quantifiable residues within the legal limits. The MRL exceedance rate (7.4%) and non‐compliance rate (4.5%) were four times higher than in those food products grown in one of the reporting countries. The remaining 4475 samples (4.0%) were reported as origin unknown of which, 282 samples (6.3%) exceeded the MRL.
The random sampling of the 12 most consumed commodities by European citizens, listed in the 2022 EU MACP (Regulation (EU) No 2021/601) (i.e. apples, strawberries, peaches (including nectarines and similar hybrids), wine (red or white), lettuces, head cabbages, tomatoes, spinaches, oat grain, barley grain, cow's milk and swine fat), provides a snapshot of the level of pesticide residues in those food products. These were compared with the same food products as sampled in 2019 and 2016 EU monitoring programmes.
A total of 11,727 samples were reported under the EU MACP and analysed for 193 pesticide residues. In 6023 of those samples (51.4%), no quantifiable residues were reported (residues were below the LOQ). The number of samples with pesticide residues within legally permitted levels (at or above the LOQ but below or at the MRL) was 5512 (47.0%). MRLs were exceeded in 1.6% (192) of samples, of which 0.9% (100) were found to be non‐compliant after taking into account measurement uncertainty.
The overall MRL exceedance rate decreased from 1.9% in 2019 to 1.6% in 2022. Among individual food commodities, MRL exceedance rates decreased along the 3 years from 2016 to 2019 and 2022 in apples, peaches, strawberries, wine, spinaches (no samples were to be taken in 2016) and swine fat. Notably, cow's milk remained without MRL exceedances over the period. On the other hand, MRL exceedance rates rose from 2016 to 2019 and to 2022 in head cabbages; in tomatoes and lettuces, the rates were higher in 2022 than in 2019, but lower than in 2016. An increase in MRL exceedance was observed in barley and oat grain from 2019 to 2022.
On average, out of the total EU MACP samples, 66.7% were domestic samples (an increase compared to 53.3% in 2021), 22% were from other reporting countries, 7.7% from third countries (a decrease compared to 19.6% in 2021) and 3.6% were of unknown origin.
By food products, quantification and MRL exceedance rates were lower in organic food compared to conventionally produced food (i.e. non‐organic) for all food product categories except for animal products (the quantification rate) and cereals (the exceedance rate). This finding was mainly due to copper, a substance authorised in organic farming, having other uses such as feed supplement and fertilisers. Similarly, copper authorised as a microelement, and chlorates resulted after sanitisation practices apply in the food chain, were responsible for most part of the exceedances in baby food.
The results from the monitoring programmes are a valuable source of information for estimating the dietary exposure of EU consumers. An analysis of the acute and chronic estimated intake of residues to consumers was performed using the adjusted deterministic Pesticide Residues Intake Model (PRIMo rev. 3.1), based on available dietary surveys of the EU MS. The pilot methodology previously introduced in 2021 report was updated for acute and chronic probabilistic assessments by applying it to the 193 pesticides listed in the 2022 EU MACP Regulation for which HBGV were derived. The results provide the percentage of estimated intake exposure to pesticide residues exceeding the HBGV in different subpopulation of European consumers. Probabilistic exposure assessment cannot be considered as a refinement of the deterministic exposure assessment.
Deterministic acute exposure assessment was carried out with the 193 pesticides listed in the EU MACP. For the first time this year, the commodities selected were those of the 3‐year cycle, i.e. 36 commodities instead of the usual 12, in total, 38,045 samples. The estimated intake exposure exceeded the health‐based guidance value (ARfD) in 1.7% of the samples. The pesticides more often found to exceed (in more than 50 samples) were phosmet (RD) (172 samples), imazalil (RD) (133 samples), lambda‐cyhalothrin (RD) (106 samples), cypermethrin (RD) (58 samples) and acetamiprid (RD) (51 samples). By commodities, the ones with higher exceedance of the ARfD (in more than 50 samples) were grapefruits (193 samples), pears (91 samples), peaches (74 samples), oranges (67 samples) and apples (59 samples). Insights and potential refinements of the exposure calculations have been discussed.
Deterministic chronic exposure assessment was conducted on 64,932 samples. The assessment included the pesticides of the 2022 EU MACP and those unprocessed commodities for which consumption data are available in PRIMo rev 3.1. No exceedance of the estimated intake exposure of the ADI was observed for any pesticide.
The probabilistic acute risk assessment revealed that, for 111 active substances of the 163 assessed, the probability of an individual‐day exceeding the ARfD is estimated to be zero for the 40 commodities and 30 surveys covering 30 European subpopulation groups and 17 EU MSs under assessment. The highest estimated probability of exceeding the ARfD was calculated in alpha‐cypermethrin, metiram and phosmet. For all three active substances, actions are under discussion by risk managers.
Probabilistic chronic risk assessment was conducted to the 193 pesticide residues covered by the 2022 EU MACP (corresponding to 199 active substances with a derived ADI as) and only imazalil resulted in a percentage of consumers exceeding the ADI (at the middle‐bound exposure) in two populations. For all the other substances, the model is not showing any consumer exposure exceeding the HBGV at the middle bound.
The two calculations (i.e. deterministic and probabilistic) used in this report cannot be compared. The one providing a more realistic estimation of what consumers are exposed to, is the probabilistic calculation, as it reflects real consumption events. However, deterministic calculation in the EU is intended to decide whether a lot can be placed on the EU market.
Overall, in the samples analysed in the framework of the 2022 monitoring programmes, the estimated dietary exposure to pesticide residues for which HBGVs are available, is very low for most of the EU subpopulation groups assessed. Thus, the assessed risk to EU consumer's health is low. For the specific pesticide/product combinations where exceedance of the health‐based guidance value was calculated, further refinements are still possible.
Based on the 2022 pesticide monitoring findings, EFSA recommends the following:
EFSA reiterates its previous recommendation to Member States*1 to take the necessary measures to fulfil the minimum number of samples set in Annex II of the EU MACP Regulations regarding the 12 food commodities and the specific provisions on baby food and organic (as applicable).
-
In samples of EU origin, and randomly taken, the following active substance (approved and non‐approved)/crop combinations were leading to non‐compliance results (in more than two samples):
-
○spinaches: dithiocarbamates (RD),
-
○tomatoes: chlorfenapyr (RD),
-
○lettuces: thiophanate‐methyl (RD),
-
○barley: prochloraz (RD),
-
○head cabbages/fluazifop (RD).
EFSA recommends reporting countries to keep analysing these pesticide/crop combinations and clarify the reasons for these findings.
-
○
EU non‐approved substances were responsible for 75% of the non‐compliant samples, randomly taken, from third countries. More precisely, chlorfenapyr (RD), chlorothalonil (RD) and chlorpyrifos‐methyl (RD) were found in tomatoes, and diflubenzuron (RD) and propargite (RD) in apples at levels leading to non‐compliant results. Among the approved active substances, fosetyl (RD) in barley, buprofezin (RD) and chlormequat‐chloride (RD) in tomatoes and pyridaben (RD) in head cabbage were found at levels leading to non‐compliant results. EFSA recommends reporting countries to keep monitoring these combinations while widening the scope of analysis on imported samples.
MRL exceedance rate in spinaches decreased notably from 6.7% in 2019 to 3.4% in 2022. However, spinaches still represent the commodity with the highest exceedance rate in the EU MACP, and thus, it is recommended to keep monitoring this commodity. MRL exceedances rose from 2019 to 2022 in barley (0.2% vs. 2.8%) and oat grain (0.2% vs. 1.5%), with most of samples from EU origin. Member States*1 are recommended to elucidate the reasons for these findings.
Thirty‐one pesticides: spinetoram (RD), mepiquat chloride (RD), heptachlor (RD), glufosinate equivalents (RD), pencycuron (RD), chlordane (RD), parathion (RD), dithianon (RD), fosetyl (RD), DDT (RD), 2‐phenylphenol (RD), cyantraniliprole (RD), methoxychlor (RD), spirotetramat (RD), haloxyfop (RD), hexachlorobenzene (RD), chlormequat‐chloride (RD), 2,4‐D (RD), bromide ion (RD), fluazifop (RD), sulfoxaflor (RD), lindane (RD), alpha‐hexachlorocyclohexane, (RD), beta‐hexachlorocyclohexane, (RD), glyphosate (RD), pyridalyl (RD), prochloraz (RD), formetanate(hydrochloride) (RD), fenbutatin oxide (RD), dithiocarbamates (RD), cyflufenamid (RD), most of them requiring the use of single residue methods, did not reach the target number of analysis. EFSA reiterates its recommendation to reporting countries to take necessary measures to be able to enforce properly these substances.
When considering the results of the overall monitoring programmes (EU MACP and MANCP), samples imported from third countries showed a fourfold higher non‐compliance rate (4.5%) compared with food produced within the EU (1%). Member States' National authorities are recommended to keep monitoring pesticide residues in samples imported from third countries with a wide analytical scope. Furthermore, 4% of the samples were still reported with origin unknown, out of which 3% were non‐compliant. Although an improvement in reporting the origin of the sample is observed in comparison with 2021 (5.7%), EFSA reiterates the past recommendation to report this piece of information, particularly of those samples leading to non‐compliance results, together with the actions taken, to draw more solid conclusions.
- The active substances with the highest MRL exceedance rate for the overall monitoring programmes were naturally occurring substance with uses other than as a pesticide (e.g. copper), ethylene oxide (RD) that triggered specific incident with strict measures and an increase frequency on import controls and chlordecone as a banned persistent organic pollutants used in the past as pesticides in France overseas territories, known problem to this country. In the case of copper compounds, an increase from 1% to 5.1% was observed when comparing results of 2021 with 2022. EFSA is currently reassessing the MRLs for this active substance considering the new HBGV derived in 2022. For ethylene oxide, a decrease from 6.6% (2021) to 2.3% (2022) was noticed, while for chlordecone a small increase from < 1% in 2021 versus 1% in 2022 was reported. Particularly, National Competent Authorities should consider the following pesticide/sample combinations in their monitoring programmes:
- copper compounds (RD) in buckwheat and other pseudo‐cereals (from third countries) and in animal matrices (sheep and bovine liver and honey, of EU origin) and baby food,
- ethylene oxide (RD) in turmeric/curcuma, chilli peppers, peppercorn, and dried beans, all from third countries, mostly India,
- chlordecone (RD) in bovine fat and cassava roots from French overseas territories).
Regarding animal commodities, besides copper and chlordecone, BAC (RD) and DDAC (RD) used for disinfection, led to MRL exceedances in bovine milk, bovine muscle, swine liver, swine fat and muscle of other farmed terrestrial animals. MRL exceedance (3.6% in 2022 vs. 2.1% in 2021) and non‐compliance rate (2.1% in 2022 vs. 1.6% in 2021) slightly increased over the last year in honey. Moreover, honey still presents the highest number of quantified pesticides (32) among the animal products. Of which, acetamiprid was the most frequent quantified (with 10 samples exceeding the MRL), followed by thiacloprid, for which a decrease in its quantification rate was observed due to the decision of non‐approval (no MRL exceedances) and amitraz (with no MRL exceedances reported). Reporting countries are recommended to keep monitoring honey and other apicultural products in their national programmes, with a wide analytical scope and investigate the reasons for the presence of these substances.
The number of samples with multiple pesticide residues (23.0%) decreased compared with the previous year (26.4%). Unprocessed sweet peppers, table grapes, strawberries, apples, peaches, tomatoes, oranges, lemons, pears, lettuce and mandarins were the commodities having the highest multiple residues quantified. The highest frequency of multiple residues in processed food samples was found in raisins, red wine, cumin seed as dried herb, grape leaves and similar species as salted vegetables, paprika powder and polished rice. EFSA recommends reporting countries to continue monitoring these foodstuffs under their programmes.
The MRL exceedance (3.7%) and non‐compliance (2.3%) rates observed in processed food in 2022 were lower than those reported in 2021 (4.5% for MRL exceedance and 3.1% for non‐compliance). Those processed food products exhibiting the higher non‐compliance rate were grape leaves and similar species (40.9%) mainly coming from salted and canned processing, dried cumin seed (20%), dried parsley (19.1%), dried wild fungi (14.3%) and basil and edible flowers (10.5%) mainly coming from mint. Unprocessed food products showed MRL exceedance (3.7%) and non‐compliance (2.2%) in the same range as those processed, with basil and edible flowers (31.3%), passion‐fruits/maracujas (15.6%), buckwheat and other pseudo‐cereals (14.9%), chilli peppers (13.7%), okra (lady's fingers) (12.9%), sheep liver (12.6%), cassava roots/manioc (12.4%) and granate apples/pomegranates (10.3%) being those with the highest non‐compliance rate. It is recommended to continue monitoring these processed and unprocessed food items in the various national control programmes throughout the EU.
In organic farming, MRL exceedance and non‐compliance rates kept the same range in 2022 compared with to 2021 (exceedances: 2.4% in 2022 vs. 1.8% in 2021; non‐compliance rate: 1.4% in 2022 vs. 1% in 2021); however, non‐authorised substances in organic farming were still reported sporadically in samples coming from third countries: chlorpyrifos (RD) (mostly in dry beans, rice and cumin seed from India) and propiconazole (mostly in rice and cumin seed from India and table olives from EU) and labelled as organic. Reporting countries are recommended to investigate the reasons for these findings and to widen the analytical scope in organic samples as much as possible.
Actions taken by reporting countries for some non‐compliant samples leading to acute exceedances of the intake estimate exposure were not provided to EFSA. Considering that this is an important piece of information for traceability on non‐compliant samples and analysis of potential health risks, reporting countries' competent authorities should make sure that this information is provided when reporting the sample results to EFSA.
Given the outcome of the exposure calculations where exceedances of the acute HBGV were driven by food products that are usually consumed peeled or processed, i.e. imazalil in citrus fruits; thiabendazole in grapefruits; cypermethrin in barley and wheat; and lambda‐cyhalothrin in oranges and spinaches, authorisation holders of these active substances are recommended to generate processing (and peeling) factors to further refine the risk assessment. Furthermore, in the case of juices, it is recommended to derived PF also for concentrated juices. Overall, if the properties of the pesticide indicate that it might concentrate in a given processed fraction, authorisation holders are requested to ensure that requirements of exaggerated rates in tests according to OECD (OECD, 2008) are followed when generating processing factors.
Reporting countries may consider strengthening the monitoring of pesticide residues in processed food commodities.
EFSA recommends keeping investing efforts to develop analytical methods specific for each of the dithiocarbamate precursors for the refinement of the risk assessment. Meanwhile, EFSA proposes the determination of the dithiocarbamates by degrading the precursors to CS2 and be vigilant to CS2 levels in commodities where no use was notified under the 2023 MRL comprehensive review. Moreover, reporting countries are recommended to keep reporting to EFSA background levels of all possible commodities from organic production (including minor uses) to build a robust database on CS2 background levels (e.g. naturally occurring).
REPORTING COUNTRY CODES
- AT
Austria
- BE
Belgium
- BG
Bulgaria
- CY
Cyprus
- CZ
Czechia
- DE
Germany
- DK
Denmark
- EE
Estonia
- EL
Greece
- ES
Spain
- FI
Finland
- FR
France
- HR
Croatia
- HU
Hungary
- IE
Ireland
- IS
Iceland
- IT
Italy
- LT
Lithuania
- LU
Luxembourg
- LV
Latvia
- MT
Malta
- NI
Northern Ireland
- NL
The Netherlands
- NO
Norway
- PL
Poland
- PT
Portugal
- RO
Romania
- SE
Sweden
- SI
Slovenia
- SK
Slovak Republic
ABBREVIATIONS
- ADI
Acceptable Daily Intake
- ARfD
Acute Reference Dose
- BAC
Benzalkonium Chloride
- BCP
Border Control Posts
- CAG
Cumulative Assessment Group
- CP
Control Point
- CS2
Carbon disulfide
- DDAC
Didecyldimethylammonium chloride
- DWH
EFSA's scientific Data Warehouse
- EEA
European Economic Area
- EFTA
European Free Trade Association
- EU MACP
EU‐coordinated multiannual control programme
- EUPT
European Proficiency Test
- EURL
European Union Reference Laboratory
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HBGV
Health‐based guidance value
- HCH
Hexachlorocyclohexane
- HRM
Highest Residue Measured
- LOD
Limit of Detection
- LOQ
Limit of Quantification
- MANCP
Multiannual National Control Programme
- MRL
Maximum Residue Level
- POP
Persistent Organic Pollutants
- pTDI
Provisional Tolerable Daily Intake
- PRIMo
Pesticide Residue Intake Model
- RD
Residue Definition
- SSD
Standard Sample Description
- VMPR
Veterinary medicinal product residues
- WHO
World Health Organization
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00338
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Supporting information
Outcome of the Member State, IS and NO consultation
ACKNOWLEDGEMENTS
EFSA wishes to thank Anneli Widenfalk from the Swedish National Food Agency for her valuable proposals and her independent scientific review of this report. EFSA wishes to thank too Valentina Bocca, Guoda Bubnyte, Elisa Corsini, Violetta Costanzo Elisa Fasanelli, Rubén Fuertes, Luna Greco, Martin Josheski, Sara Levorato, Luc Mohimont, Andrea Nironi, Hermine Reich, Giuseppe Antonio Triacchini and Irene Zanetti for their support provided in this scientific output as well as a special recognition to Hypertech for their work. Lastly, EFSA wishes to acknowledge all European Competent Authorities and Member State bodies that provided data to generate this scientific output.
APPENDIX A. Authorities responsible for reporting pesticide residues by country
A.1.
| Country | National competent authority | Web address for published national monitoring reports |
|---|---|---|
| Austria | Federal Ministry of Social Affairs, Health, Care and Consumer Protection | https://www.verbrauchergesundheit.gv.at/lebensmittel/lebensmittelkontrolle/monitoring/pestizid.html |
| Austrian Agency for Health and Food Safety | https://www.ages.at/themen/rueckstaende‐kontaminanten/pflanzenschutzmittel‐rueckstaende/pestizidmonitoringberichte/ | |
| Belgium | Federal Agency for the Safety of the food Chain (FASFC) | https://www.favv‐afsca.fgov.be/publicationsthematiques/pesticide‐residue‐monitoring‐food‐plant‐origin.asp |
| Bulgaria | Risk Assessment Centre on Food Chain | https://www.babh.government.bg/en/ |
| Croatia | Ministry of Agriculture | https://www.mps.hr/ |
| Cyprus | Ministry of Health, Pesticides Residues Laboratory of the State General Laboratory | https://www.moh.gov.cy/sgl |
| Ministry of Health, Department of Medical and Public Health Services (MPHS) | ||
| Czech Republic | Czech Agriculture and Food Inspection Authority | https://www.szpi.gov.cz |
| State Veterinary Administration | https://www.svscr.cz | |
| Denmark | Danish Veterinary and Food Administration | https://foedevarestyrelsen.dk/publikationer |
| National Food Institute, Technical University of Denmark | https://www.food.dtu.dk/publikationer/kemikaliepaavirkninger/pesticider‐i‐kosten | |
| Estonia | Veterinary and Food Board | https://www.vet.agri.ee |
| Finland | Finnish Food Authority, Finnish Customs and National Supervisory Authority for Welfare and Health | https://www.ruokavirasto.fi/en/companies/food‐sector/production/common‐requirements‐for‐composition/residues‐of‐plant‐protection‐products/control‐of‐plant‐protection‐product‐residues‐in‐food/ |
| France | Ministère de l'économie et des finances / Direction générale de la concurrence, de la consommation et de la répression des fraudes (DGCCRF) | https://www.economie.gouv.fr/dgccrf/securite/produits‐alimentaires |
| Ministère de l'Agriculture et de l'Alimentation, Direction générale de l'alimentation (DGAL) | https://agriculture.gouv.fr/plans‐de‐surveillance‐et‐de‐controle | |
| Germany | Federal Office of Consumer Protection and Food Safety (BVL) | www.bvl.bund.de/berichtpsm |
| Greece | Ministry of Rural Development and Food | https://www.minagric.gr/index.php/en/citizen‐menu/foodsafety‐menu |
| https://www.minagric.gr/index.php/el/for‐farmer‐2/crop‐production/fytoprostasiamenu/ypoleimatafyto | ||
| Hungary | National Food Chain Safety Office | https://www.nebih.gov.hu |
| Iceland | MAST – The Icelandic Food and Veterinary Authority | www.mast.is |
| Ireland | Department of Agriculture Food and the Marine | www.pcs.agriculture.gov.ie |
| Italy | Ministero della Salute – Direzione Generale per l'Igiene e la Sicurezza degli Alimenti e la Nutrizione – Ufficio 7 | https://www.salute.gov.it/portale/fitosanitari/dettaglioContenutiFitosanitari.jsp?lingua=italiano&id=1105&area=fitosanitari&menu=vegetali |
| Latvia | Ministry of Agriculture | www.zm.gov.lv |
| Food and Veterinary Service of Latvia | ||
| Lithuania | National Food and Veterinary Service (SFVS) | www.nmvrvi.lt |
| Luxembourg | Ministry of Health, Directorate for public health, Division of Food Safety (Secualim) | www.securite‐alimentaire.public.lu |
| Ministry of Health, Administration of Veterinary Services (ASV) | ||
| Malta | Malta Competition and Consumer Affairs Authority | www.mccaa.org.mt |
| Netherlands | Netherlands Food and Consumer Product Safety Authority (NVWA) | www.nvwa.nl |
| Norway | Norwegian Food Safety Authority | www.mattilsynet.no |
| www.mattilsynet.no/mat_og_vann/uonskede_stofferimaten/rester_av_plantevernmidler_i_mat/#overvakings_og_kartleggingsprogrammer | ||
| Poland | The State Sanitary Inspection | www.gis.gov.pl |
| Portugal | Direção‐Geral de Alimentação e Veterinária (DGAV) | www.dgv.min‐agricultura.pt/portal/page/portal/DGV/genericos?generico=4217393&cboui=4217393t |
| Romania | National Sanitary Veterinary and Food Safety Authority | www.ansvsa.ro |
| Ministry of Agriculture and Rural Development | www.madr.ro | |
| Ministry of Health | ||
| Slovakia | State Veterinary and Food Administration of the Slovakian Republic | www.svps.sk/ |
| Public Health Authority of the Slovakian Republic | ||
| Slovenia | Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection | www.uvhvvr.gov.si/si/delovna_podrocja/ostanki_pesticidov |
| Spain | Spanish Agency for Food Safety and Nutrition (AESAN) | www.aecosan.msssi.gob.es/AECOSAN/web/seguridad_alimentaria/subseccion/programa_control_residuos.htm |
| Sweden | Swedish Food Agency | www.livsmedelsverket.se |
| Northern Ireland 1 | Department of Agriculture, Environment and Rural Affairs (DAERA). Sanitary and Phytosanitary Policy and Logistics Division | www.daera‐ni.gov.uk/ |
APPENDIX B. Detailed results on deterministic risk assessment
B.1.
Results of acute risk assessment for food products in focus of the 2022 EU MACP expressed as estimated exposure in percentage of the ARfD are presented in this appendix.
In the following figures, 84 the acute exposure calculated for each sample with residues above the LOQ is presented individually, expressing the result as a percentage of the ARfD. The blue dots refer to results reported under the EU‐coordinated programme, whereas the orange dots refer to findings in samples that were analysed in the framework of the national control programmes. The figures in brackets next to the name of the pesticides represent the number of samples with residues below the LOQ, number of samples with quantified residues below or at the MRL and the number of samples with residues above the MRL. 85
Apples, aubergines/eggplants, bananas, broccoli, carrots, cauliflowers, cultivated fungi, grapefruits, head cabbages, kiwi fruits (green, red, yellow), lettuces, melons, onions, oranges, peaches, pears, potatoes, spinaches, strawberries, sweet peppers/bell peppers, table grapes, tomatoes, dry beans, barley, oat, rice, rye, wheat, olive oil, wine, eggs (chicken), liver (bovine), fat (bovine), fat (poultry), fat (swine) and milk (cattle).
FIGURE B.1.
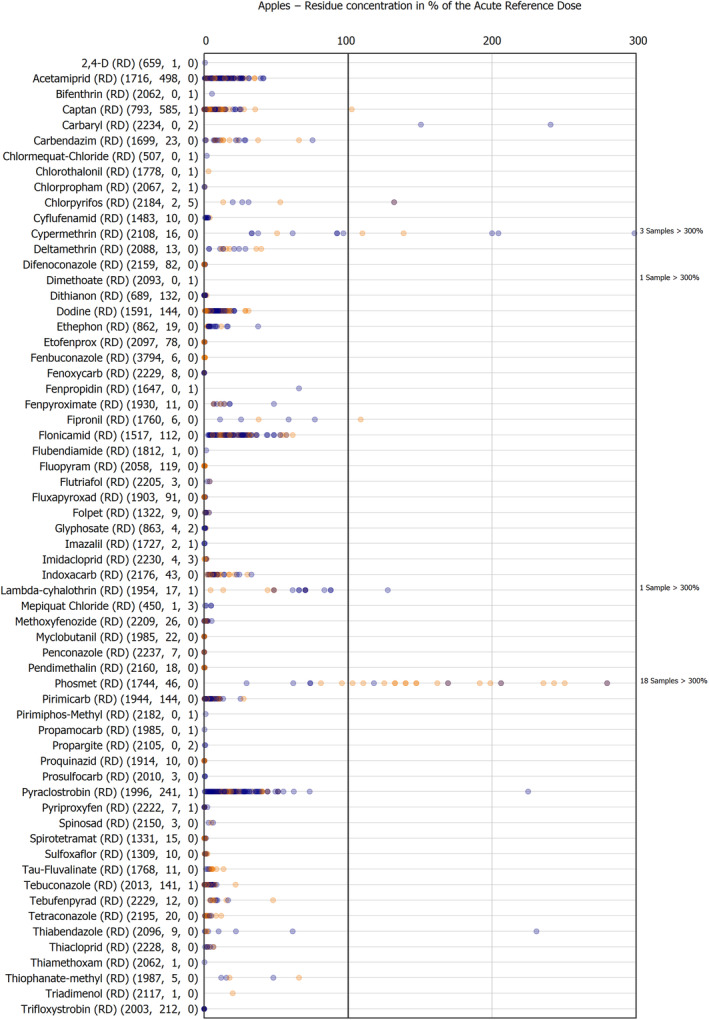
Acute dietary exposure assessment – apples.
FIGURE B.2.
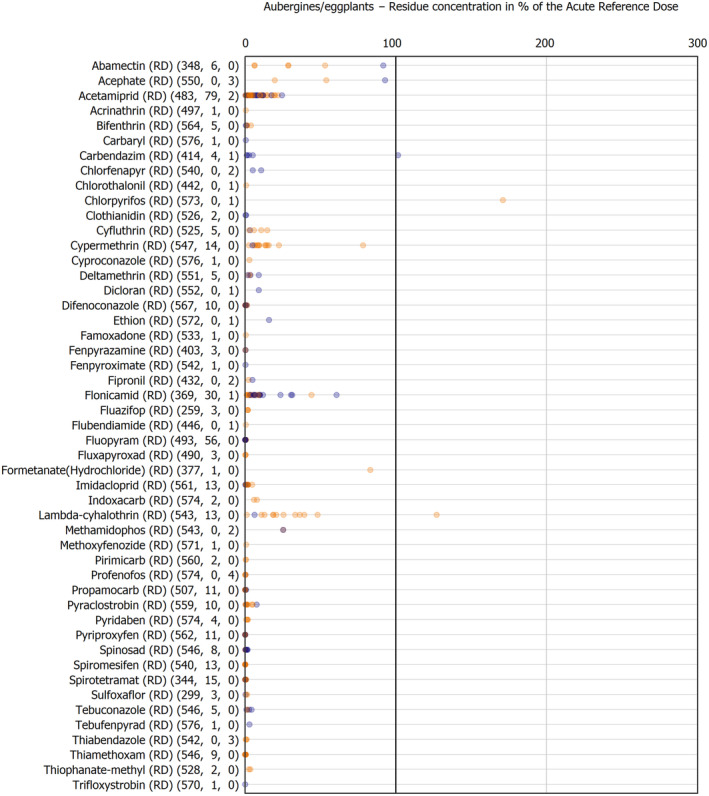
Acute dietary exposure assessment – aubergines/eggplants.
FIGURE B.3.
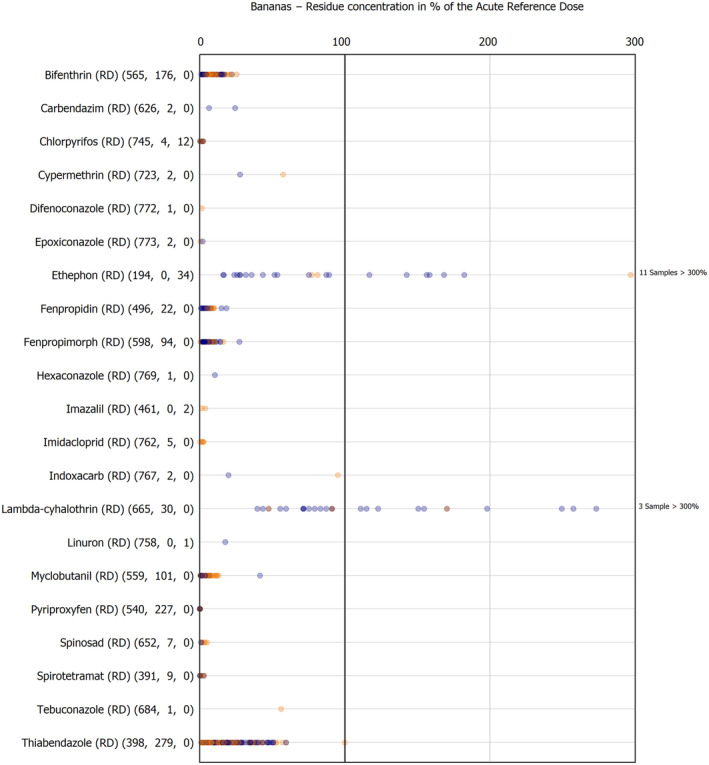
Acute dietary exposure assessment – bananas.
FIGURE B.4.
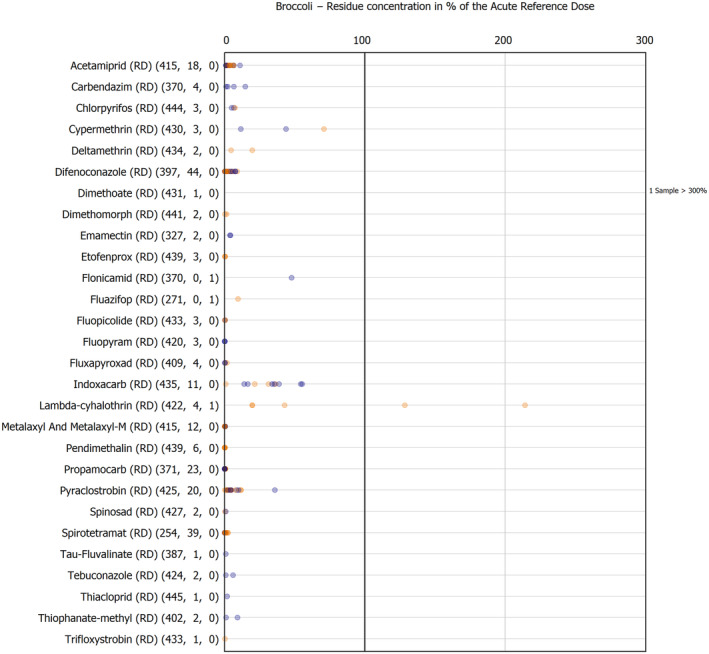
Acute dietary exposure assessment – broccoli.
FIGURE B.5.
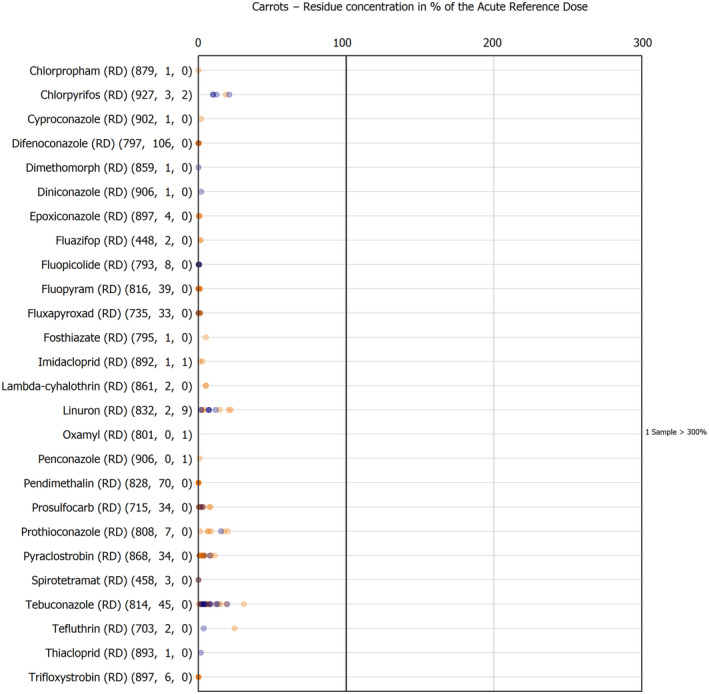
Acute dietary exposure assessment – carrots.
FIGURE B.6.
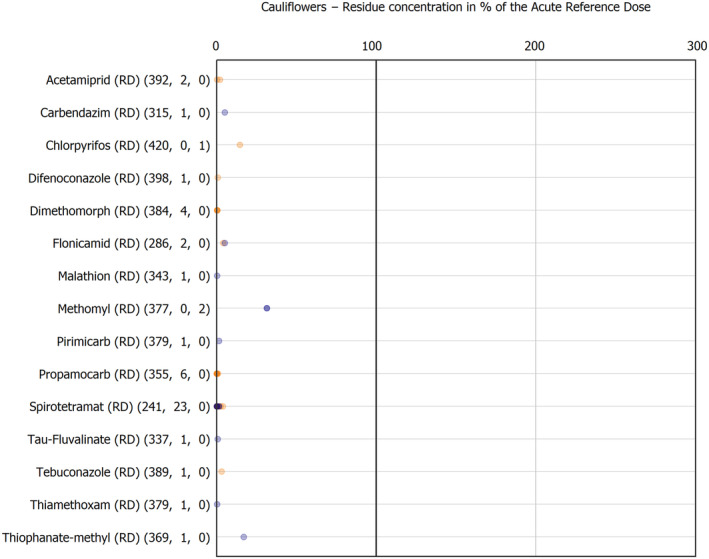
Acute dietary exposure assessment – cauliflowers.
FIGURE B.7.
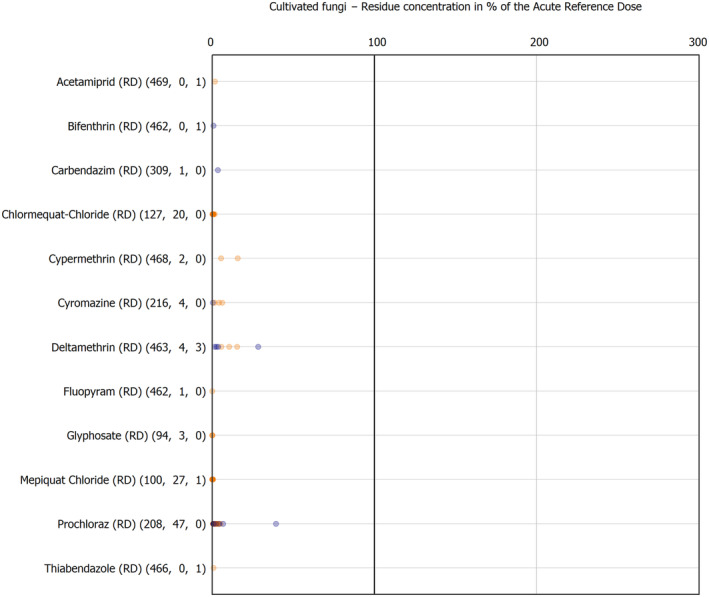
Acute dietary exposure assessment – cultivated fungi.
FIGURE B.8.
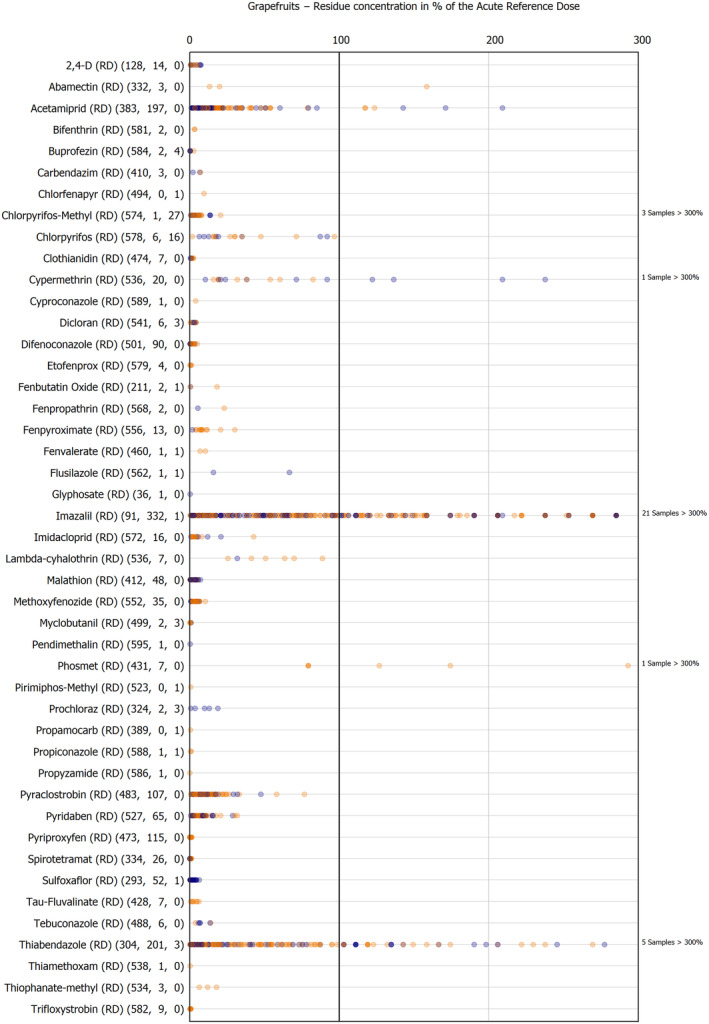
Acute dietary exposure assessment – grapefruits.
FIGURE B.9.
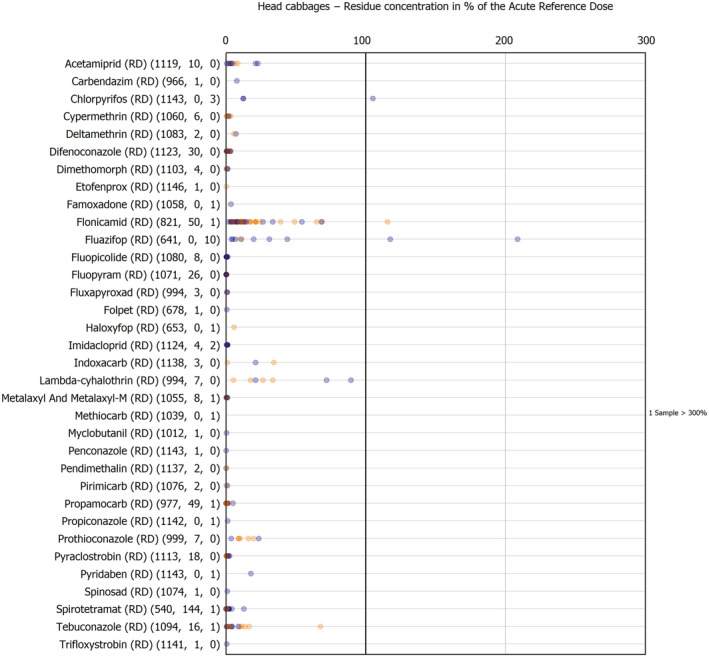
Acute dietary exposure assessment – head cabbages.
FIGURE B.10.
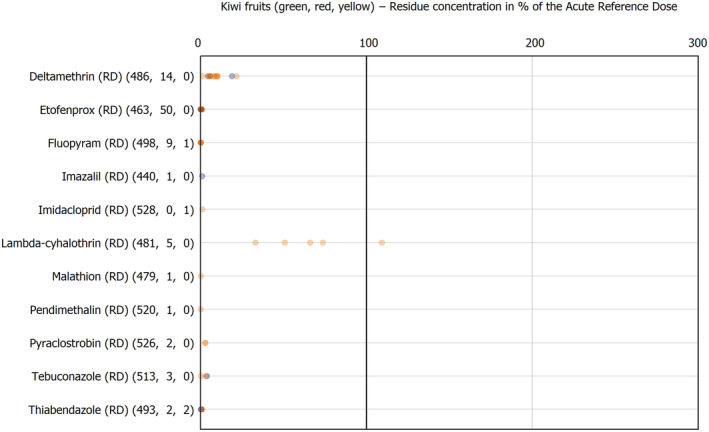
Acute dietary exposure assessment – kiwi fruits (green, red, yellow).
FIGURE B.11.
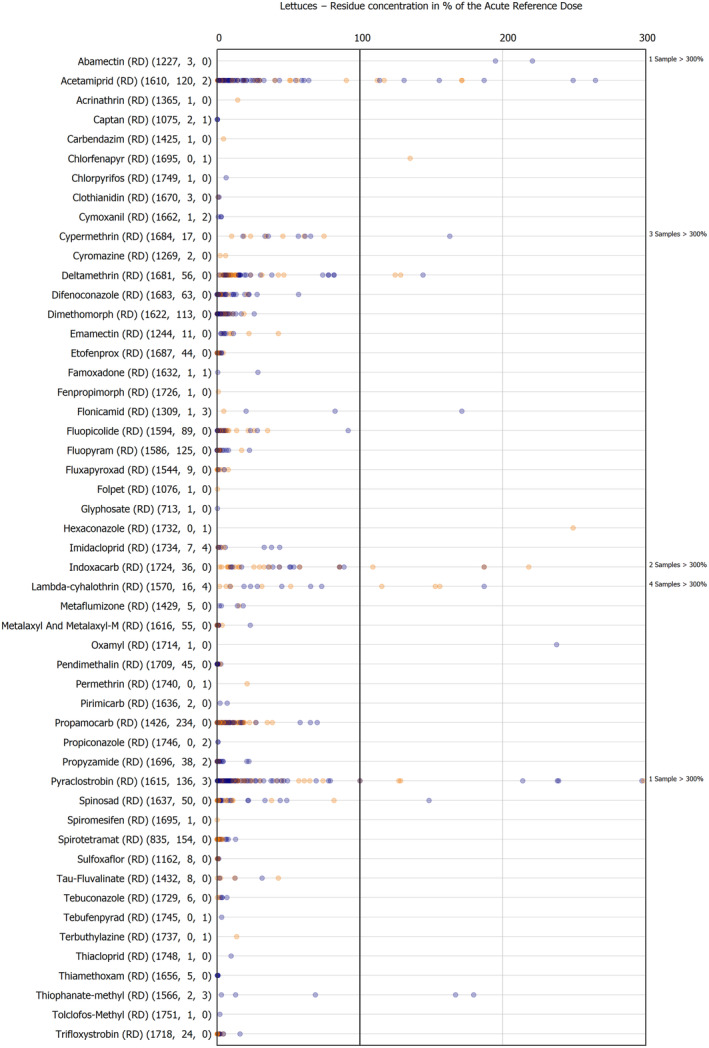
Acute dietary exposure assessment – lettuces.
FIGURE B.12.
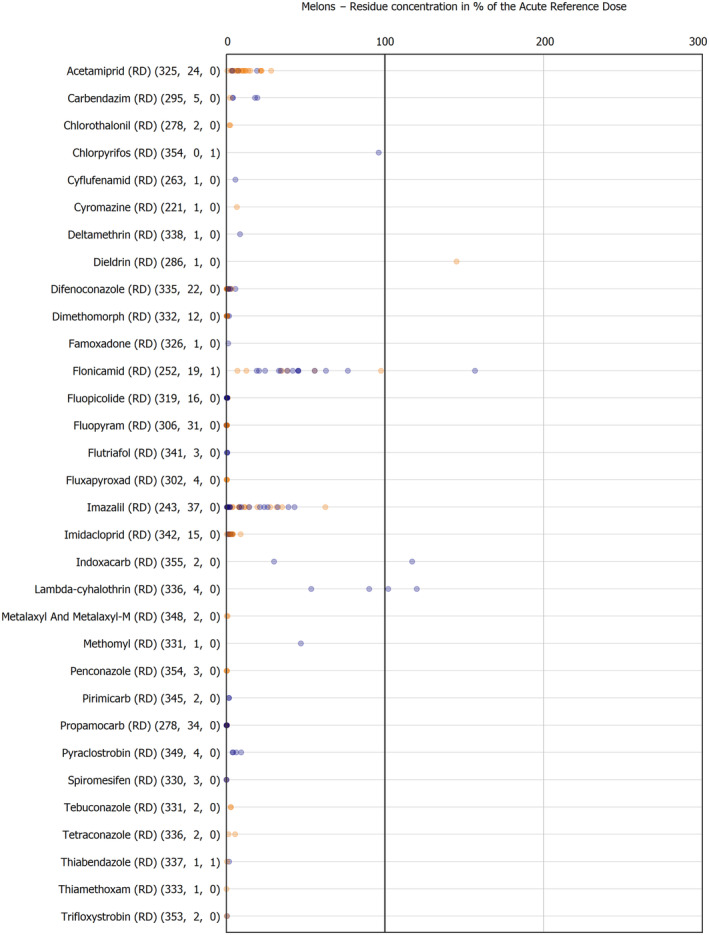
Acute dietary exposure assessment – melons.
FIGURE B.13.
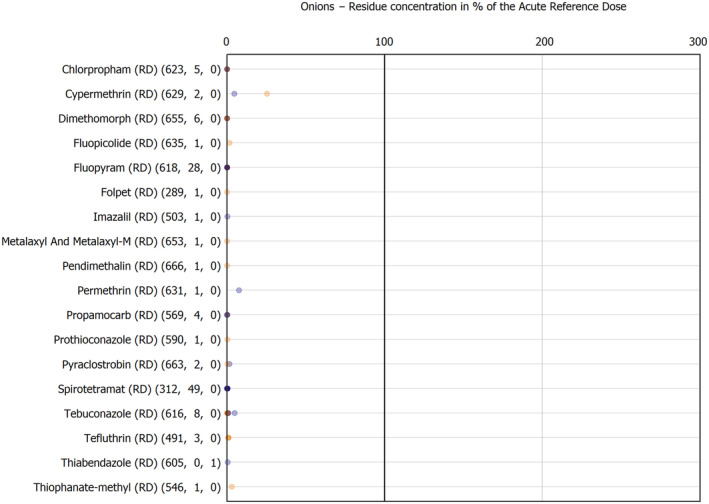
Acute dietary exposure assessment – onions.
FIGURE B.14.
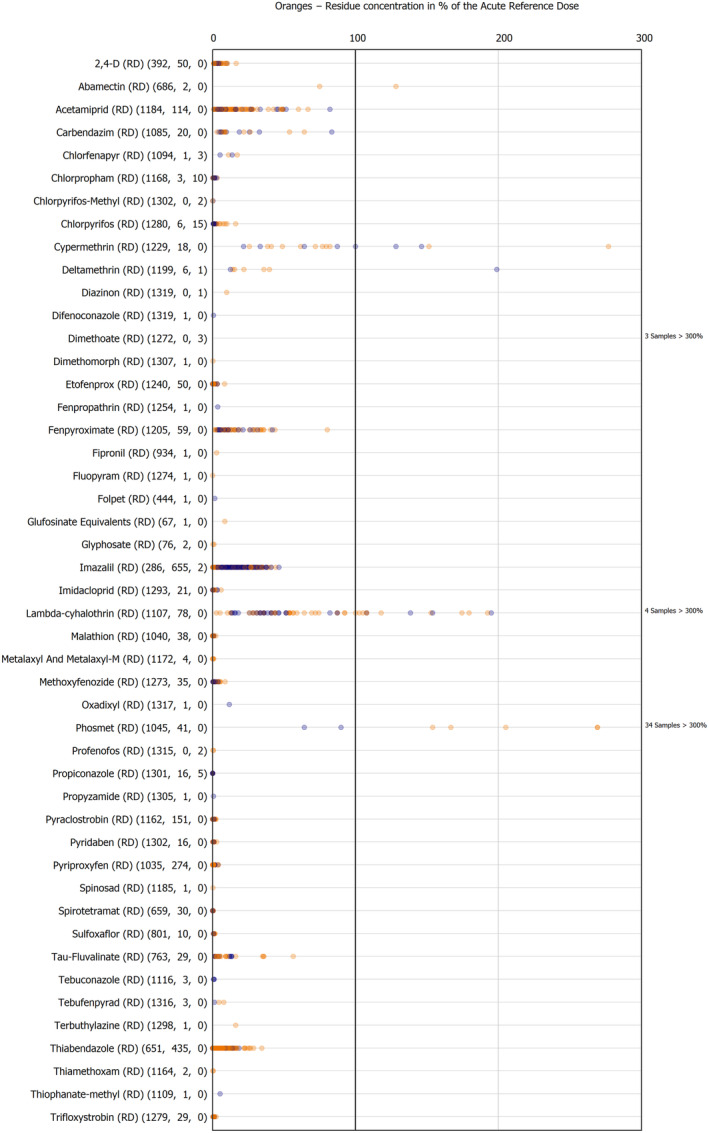
Acute dietary exposure assessment – oranges.
FIGURE B.15.
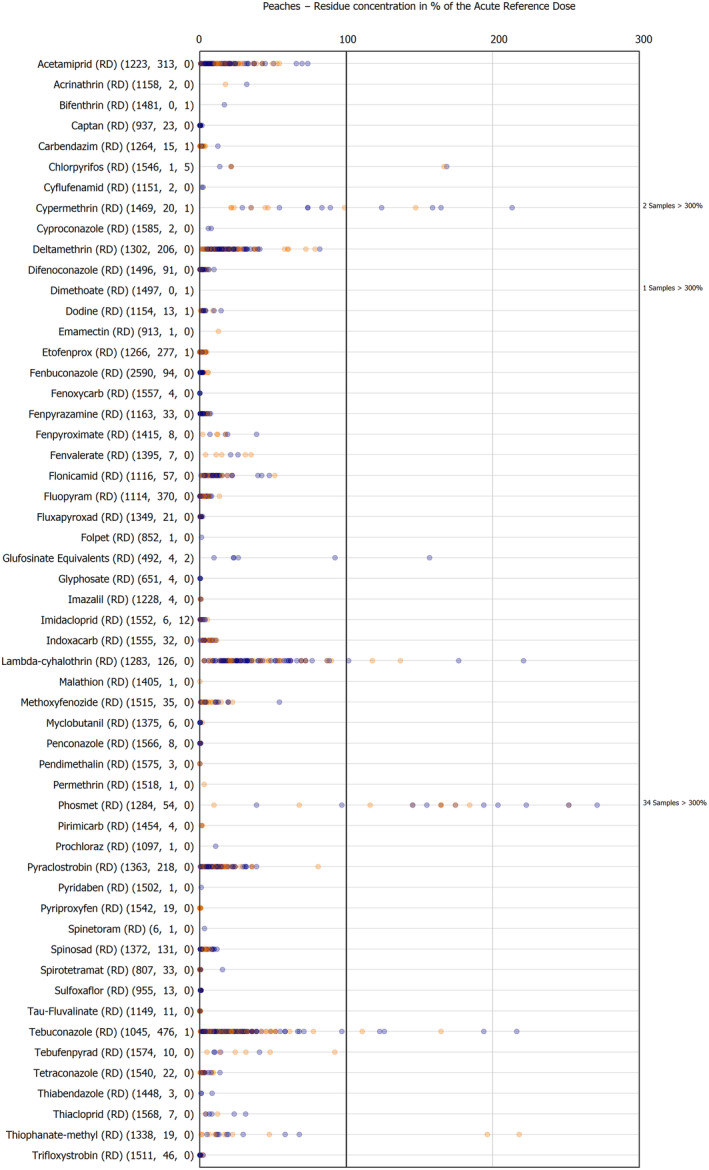
Acute dietary exposure assessment – peaches.
FIGURE B.16.
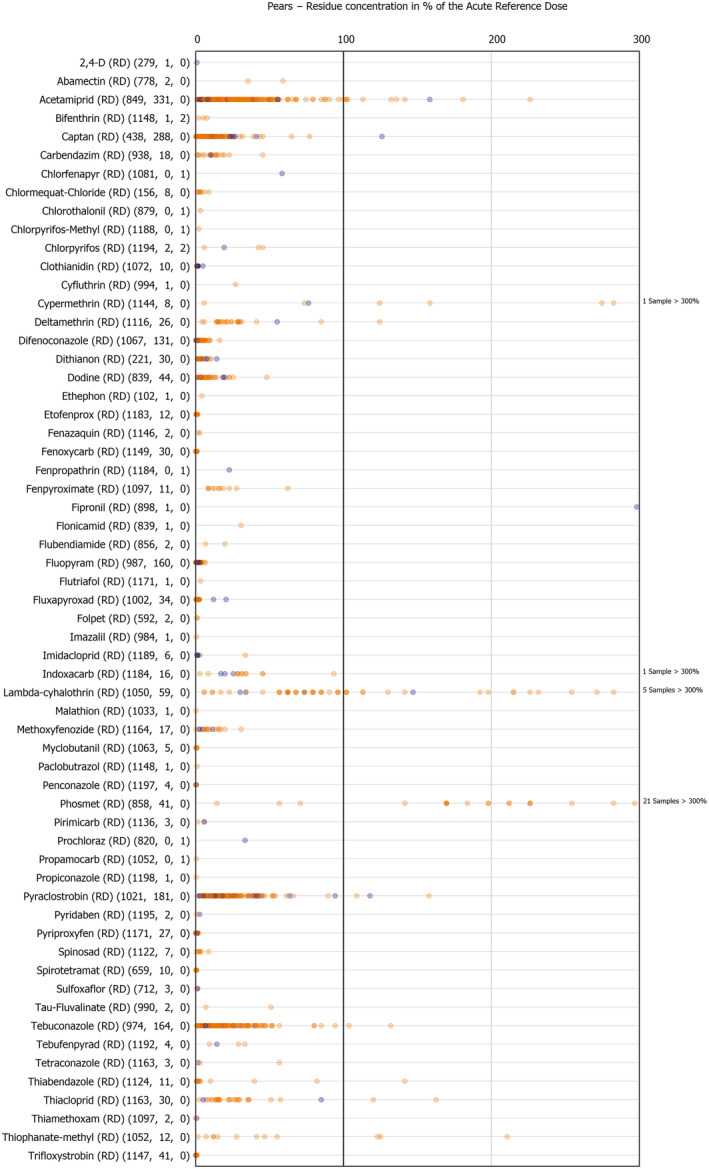
Acute dietary exposure assessment – pears.
FIGURE B.17.
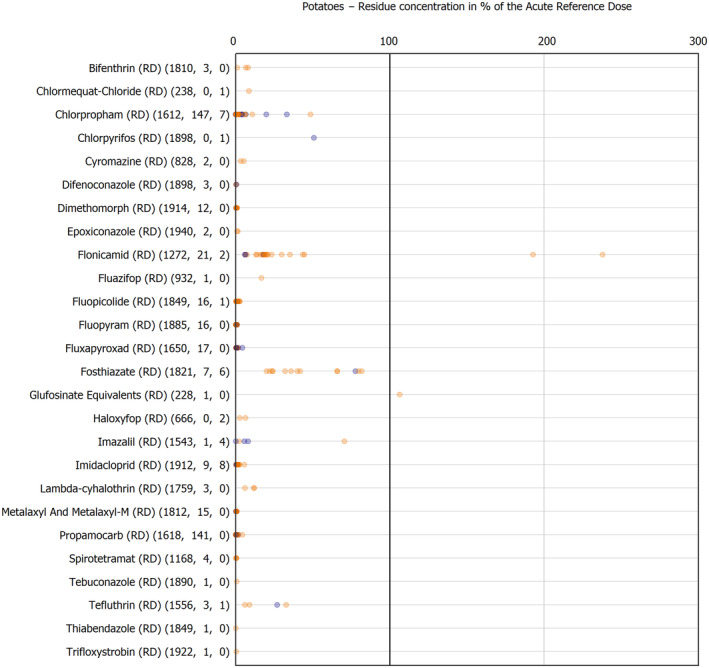
Acute dietary exposure assessment – potatoes.
FIGURE B.18.
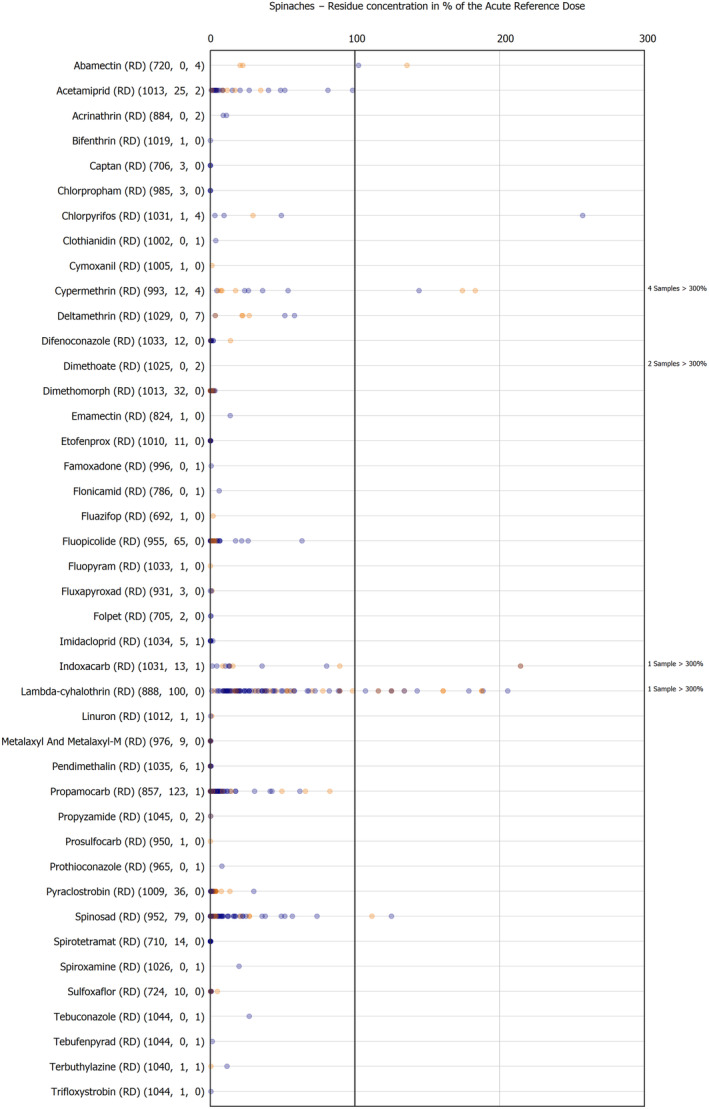
Acute dietary exposure assessment – spinaches.
FIGURE B.19.
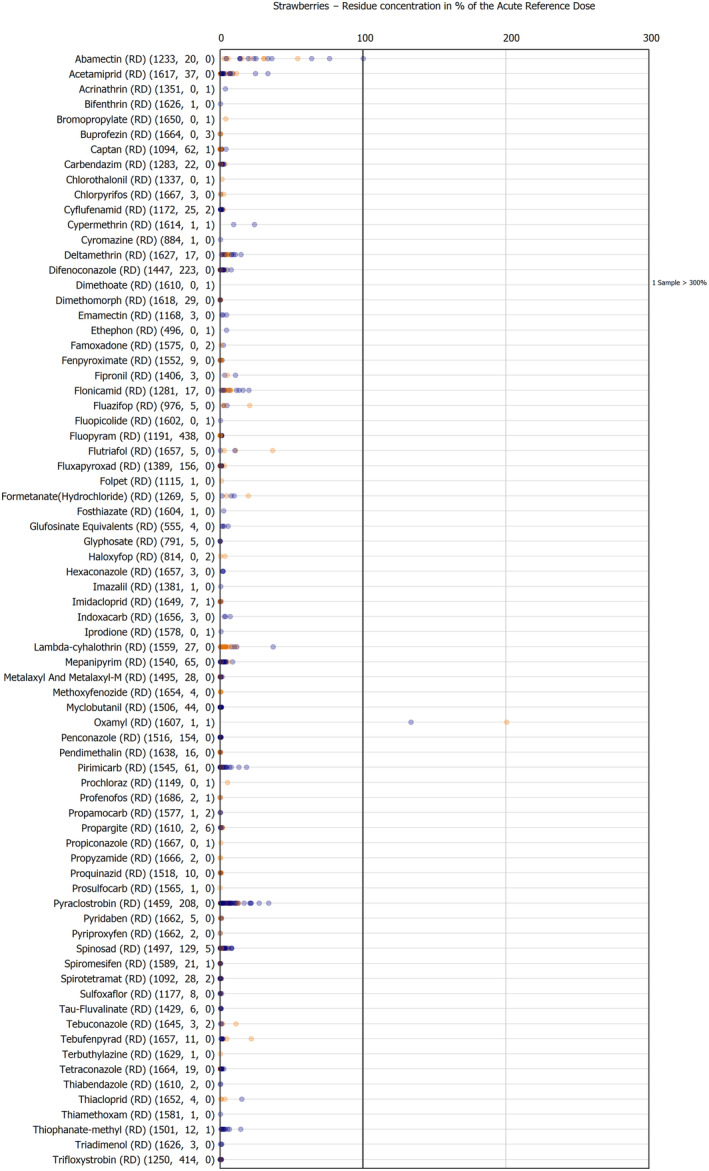
Acute dietary exposure assessment – strawberries.
FIGURE B.20.
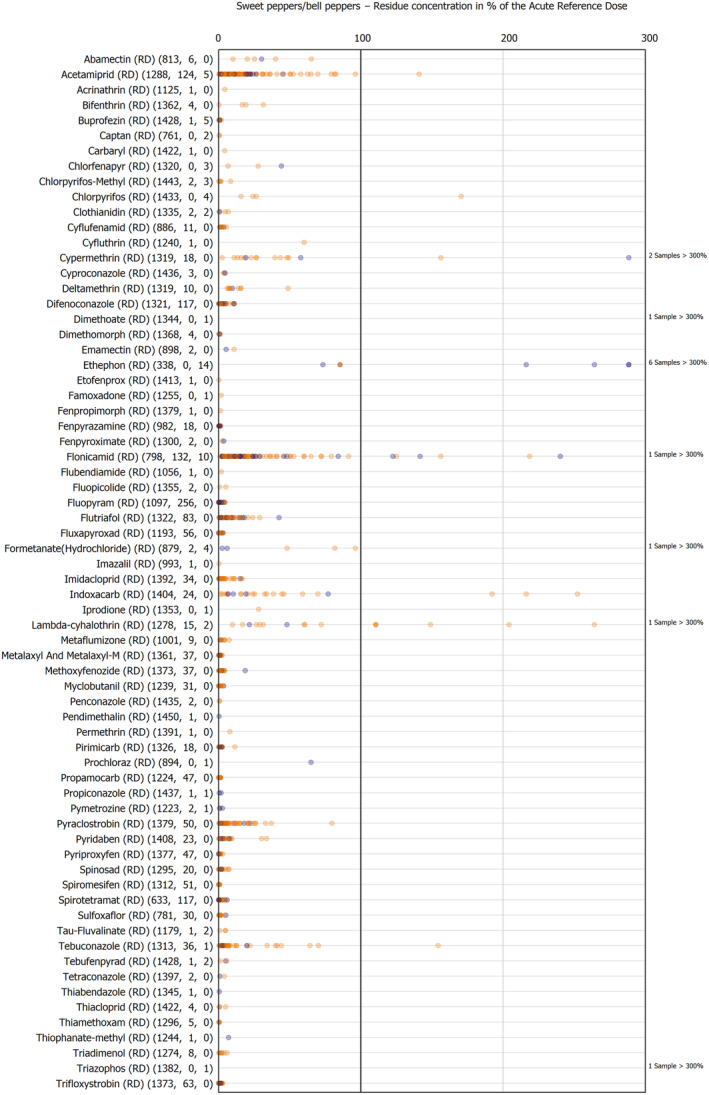
Acute dietary exposure assessment – sweet peppers/bell peppers.
FIGURE B.21.
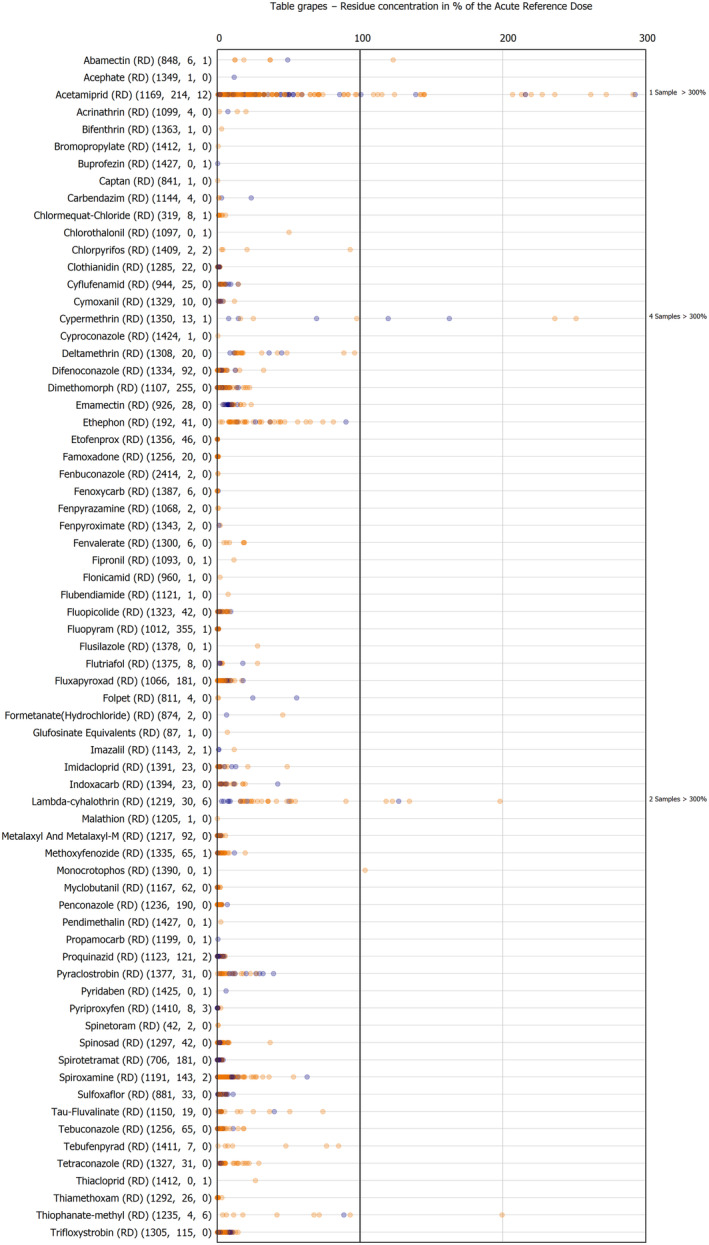
Acute dietary exposure assessment – table grapes.
FIGURE B.22.
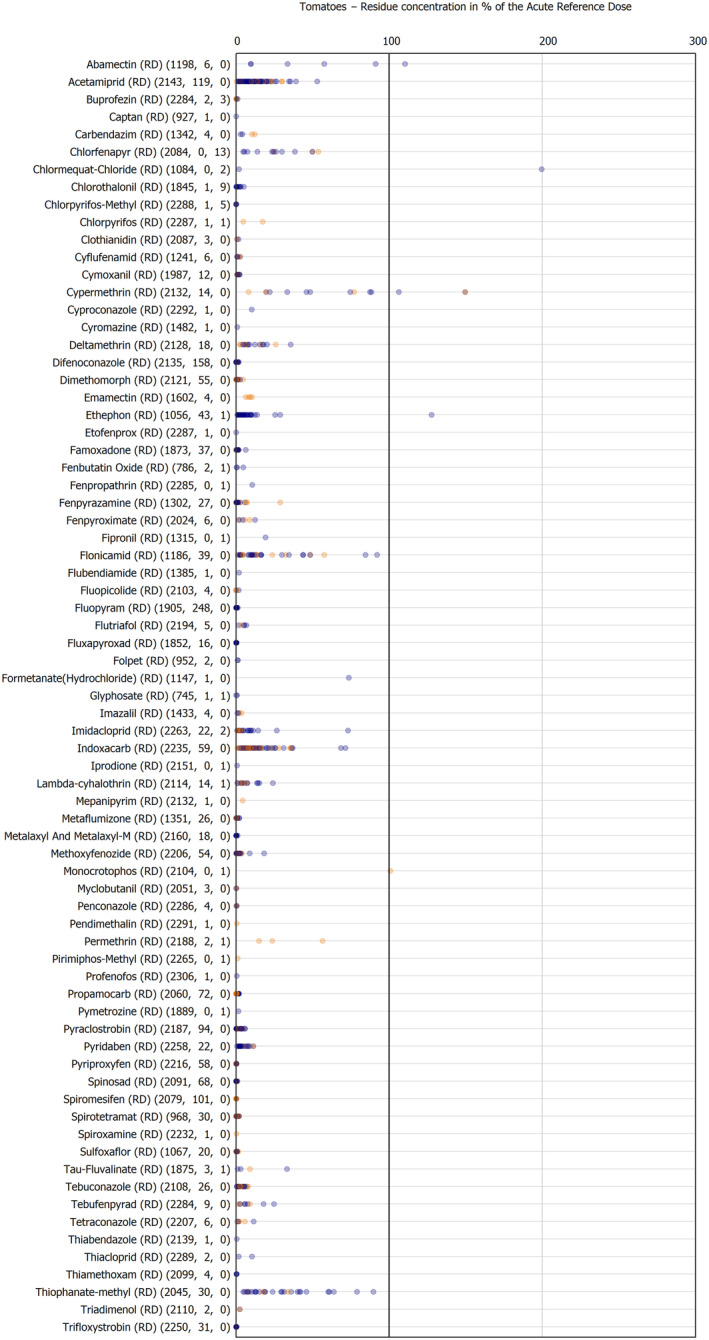
Acute dietary exposure assessment – tomatoes.
FIGURE B.23.
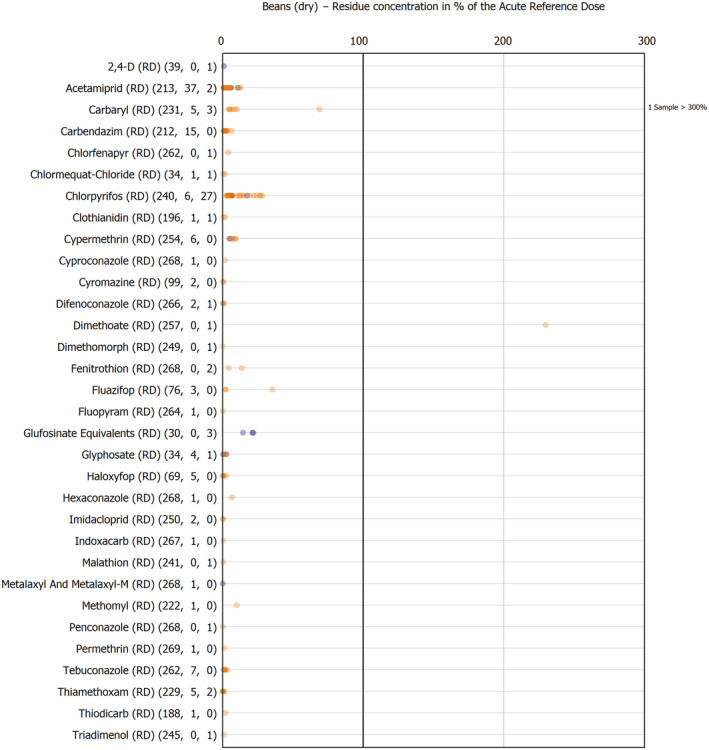
Acute dietary exposure assessment – dry beans.
FIGURE B.24.
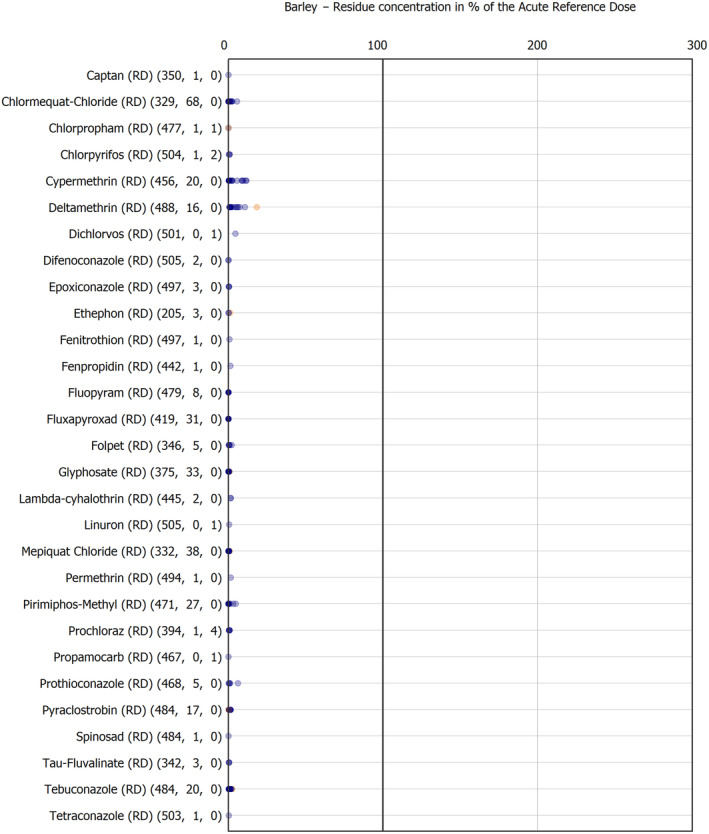
Acute dietary exposure assessment – barley cereal.
FIGURE B.25.
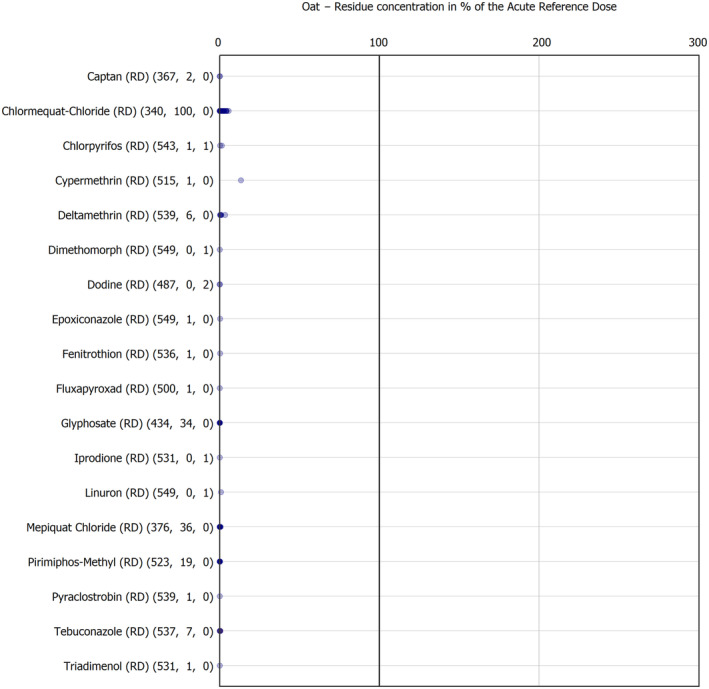
Acute dietary exposure assessment – oat cereal.
FIGURE B.26.
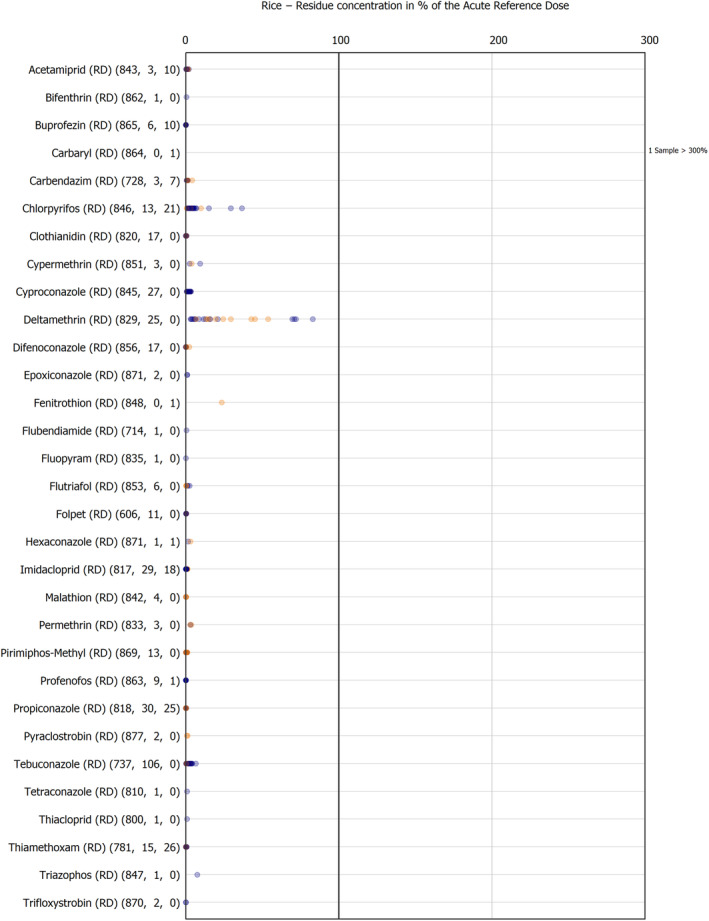
Acute dietary exposure assessment – rice.
FIGURE B.27.
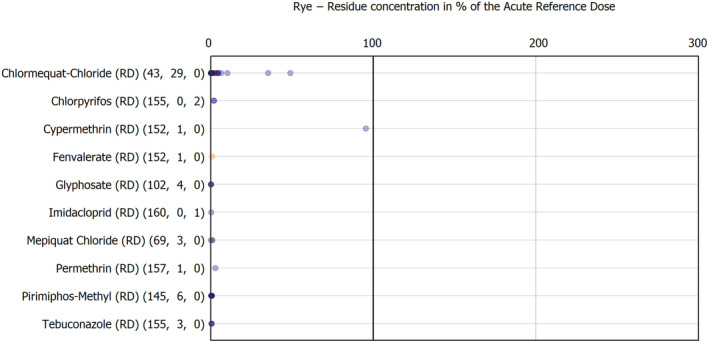
Acute dietary exposure assessment – rye cereal.
FIGURE B.28.
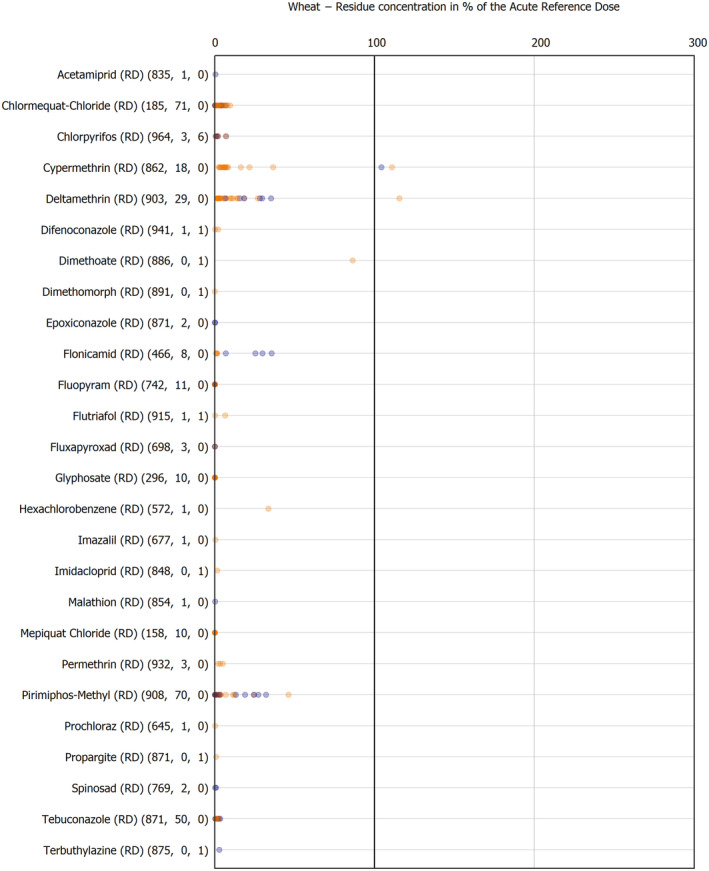
Acute dietary exposure assessment – wheat cereal.
FIGURE B.29.
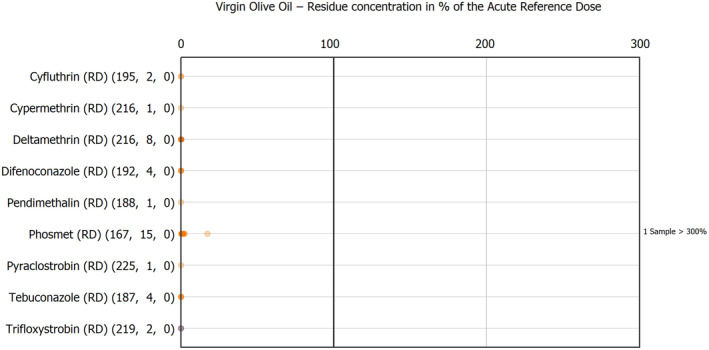
Acute dietary exposure assessment – virgin olive oil.
FIGURE B.30.
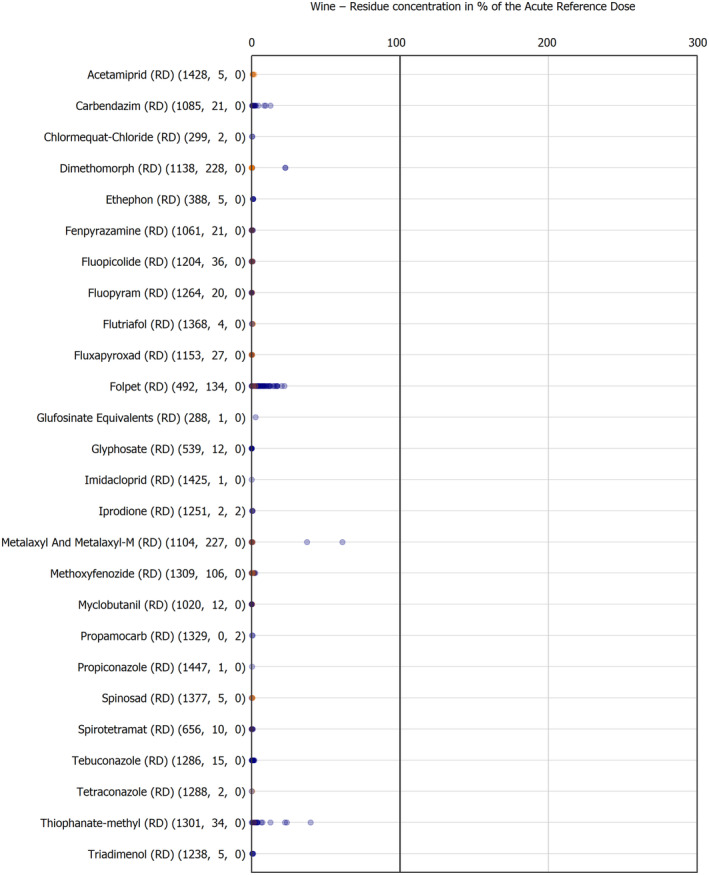
Acute dietary exposure assessment – wine.
FIGURE B.31.
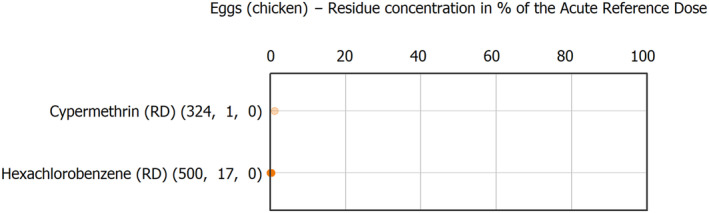
Acute dietary exposure assessment – eggs (chicken).
FIGURE B.32.
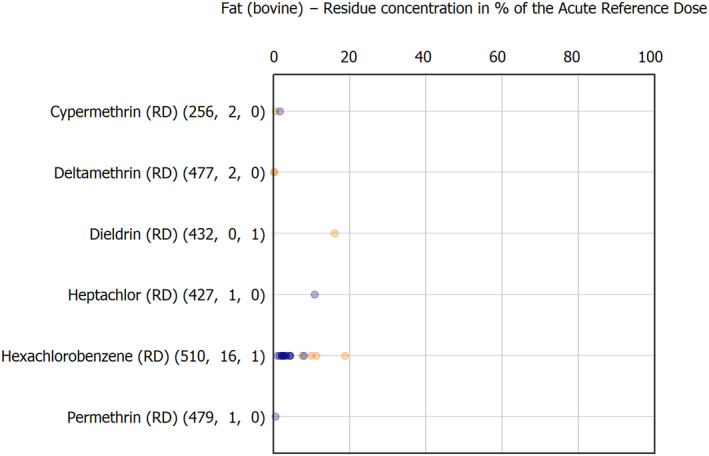
Acute dietary exposure assessment – fat (bovine).
FIGURE B.33.
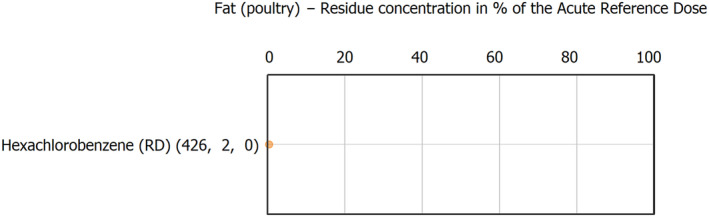
Acute dietary exposure assessment – fat (poultry).
FIGURE B.34.
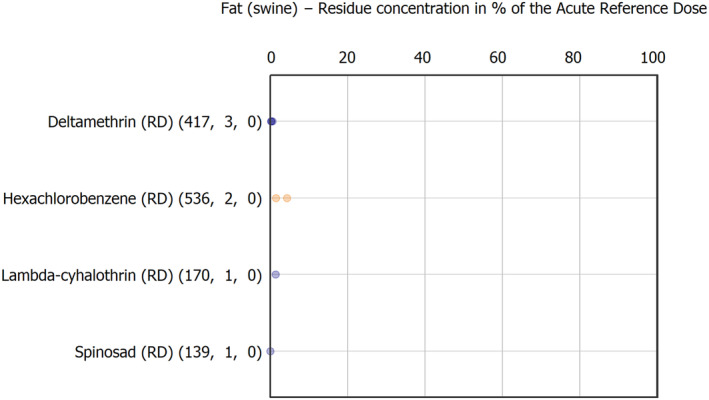
Acute dietary exposure assessment – fat (swine).
FIGURE B.35.
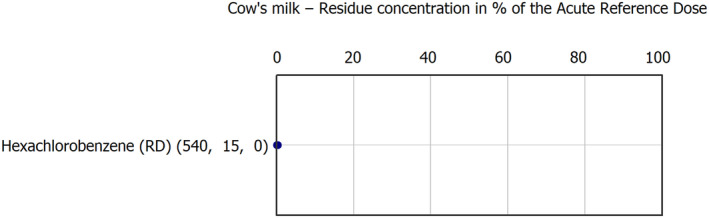
Acute dietary exposure assessment – cow's milk.
APPENDIX C. Detailed description of the input data and methodologies applied in the probabilistic assessment
C.1. Primary Input data
C.1.1. Raw primary commodities
To pilot the probabilistic risk assessment to pesticide residues, EFSA selected the 35 raw primary commodities (RPCs) of plant origin that were ever considered in the EU MACP. In addition, courgettes were also included because, according to EFSA's design assessment of the pesticide monitoring programme (EFSA, 2015a), courgettes are consumed in higher amounts than other commodities previously included in the EU MACP (e.g. spinach and broccoli). Foods specifically intended for infants and young children were integrated in the exposure assessment.
The full list of the included food commodities is provided in Table C.1.
TABLE C.1.
PRC list.
| Number of commodities | prodCode a | prodName b |
|---|---|---|
| 1 | P0110010A | Grapefruits |
| 2 | P0110020A | Oranges |
| 3 | P0110050A | Mandarins |
| 4 | P0130010A | Apples |
| 5 | P0130020A | Pears |
| 6 | P0140030A | Peaches |
| 7 | P0151010A | Table grapes |
| 8 | P0151020A | Wine grapes |
| 9 | P0152000A | Strawberries |
| 10 | P0162010A | Kiwi fruits |
| 11 | P0163020A | Bananas |
| 12 | P0211000A | Potatoes |
| 13 | P0213020A | Carrots |
| 14 | P0220020A | Onions |
| 15 | P0231010A | Tomatoes |
| 16 | P0231020A | Peppers |
| 17 | P0231030A | Aubergines (egg plants) |
| 18 | P0232010A | Cucumbers |
| 19 | P0232030A | Courgettes |
| 20 | P0233010A | Melons |
| 21 | P0241010A | Broccoli |
| 22 | P0241020A | Cauliflower |
| 23 | P0242020A | Head cabbage |
| 24 | P0251020A | Lettuce |
| 25 | P0252010A | Spinach |
| 26 | P0260010A | Beans (with pods) |
| 27 | P0260040A | Peas (without pods) |
| 28 | P0270060A | Leek |
| 29 | P0280010A | Cultivated fungi |
| 30 | P0300010A | Beans (dry) |
| 31 | P0402010A | Olives for oil production |
| 32 | P0500010A | Barley |
| 33 | P0500050A | Oats |
| 34 | P0500060A | Rice |
| 35 | P0500070A | Rye |
| 36 | P0500090A | Wheat |
| 37 | PX100001A | Baby foods other than processed cereal‐based foods |
| 38 | PX100003A | Processed cereal‐based foods for infants and young children |
| 39 | PX100004A | Infant formulae |
| 40 | PX100005A | Follow‐on formulae |
C.1.2. Active substances
The active substances under analysis are those derived by the pesticide residue definition listed in the 2022 EU MACP program. The list of active substances is presented in Appendix C.1.4.
C.1.3. Residue definitions
While the probabilistic risk assessment is executed at the level of the active substances, the occurrence data reported to EFSA refer to the residue definition for enforcement. As the residue definitions defined in Regulation (EC) No 396/2005 may change over time, single active substances may be associated with multiple residue definitions throughout the reference period 2020–2022. EFSA, therefore, collected all the residue definitions that were applicable to the selected food commodities and active substances during the reference period. The residue definitions and related active substances collected are presented in Annex III – Table 3.1 – mapping.
Depending on the metabolism and availability of analytical methods, the residue definitions may either be equal to the active substance, may include additional metabolites, or even incorporate multiple active substances. When the residue definition includes additional metabolites that are specific to the active substance (i.e. complex residue definition), the measurements of the related residue definition are assigned to all associated active substances, allowing for the estimation of exposure for each of these active substances.
C.1.4. Occurrence data
The occurrence data collected under Article 31 of Regulation (EC) No 396/2005 are the most appropriate data available to EFSA for performing a probabilistic risk assessment. These data are obtained from the official control activities carried out in the EU Member States,*1 Iceland and Norway. These data are reported to EFSA using the Standard Sample Description ver2 (SSD2) (EFSA, 2013). Although the occurrence data are collected at the level of individual measurements, the SSD2 allows identification of measurements associated to a single food sample (e.g. samples analysed for multiple pesticide residues). After validation by EFSA, the collected data are integrated in the EFSA's Scientific Data Warehouse (sDWH).
All occurrence data referring to the relevant food commodities (see Appendix C.1.1) and residue definitions (see Appendix C.1.3) were extracted from the sDWH. Only measurements validated under 2020, 2021 and 2022 EU reports on pesticide residues in food were included.
The following additional criteria were applied to the extracted data:
Only samples resulting from the EU‐coordinated control programme (EU MACP), national control programmes (MANCP) or a combination of those were selected (SSD2 programme type codes K005A, K009A and K018A). Samples associated with increased control programmes or any other type of programme were excluded as they were not considered to be representative of the market.
Only samples obtained through selective or objective sampling were retained (SSD2 sampling strategy codes ST10A and ST20A). Samples obtained through suspect sampling, or any other type of sampling were not considered to be representative of the market and therefore excluded.
When the occurrence data were primarily reported for the RPC, samples for processed commodities were excluded and the assessment was based on the RPCs. However, when a sufficient number of samples were found, the occurrence data for the processed foods were also retained. The detailed list of the processed and unprocessed products retained for the assessment is reported in Table C.2.
Only measurements reported as a numerical (i.e. quantifiable) value or as a non‐quantified value were considered useful for the assessment (SSD2 resType codes VAL and LOQ). Other result types were not considered valid and therefore excluded.
Only measurements reported for the enforcement residue definition that was applicable at the time of sampling, or for the most complete subset of that enforcement residue definition were used (SSD2 paramType codes P004A and P005A). Measurements referring to parts of the residue definition were excluded from the assessment.
When the LOQ value for a measurement could not be reported by the reporting countries (i.e. for residue definitions composed of multiple components), the median LOQ of all measurements referring to the same residue definition/commodity combination was assumed.
When the LOQ value for a measurement was found to be more than 100 times higher compared to the median LOQ of all measurements referring to the same combination of commodity and residue definition, the measurement was no longer considered valid and excluded from the assessment.
When a measurement was reported at LOQ, the concentration used for the assessment was set at ½ LOQ for a number of results equal to the number of positive results (i.e. above the LOQ) for the same substance/commodity combination, provided that the use of the substance is authorised for that commodity.
When several measurements with overlapping residue definitions were reported for the same sample, only the measurement referring to the most recent enforcement residue definition was retained for assessment.
TABLE C.2.
Complete list of products retained for the assessment.
| Prodcode a | PRODNAME b | PRODTREAT c | PRODTREAT_DESC d |
|---|---|---|---|
| P0110010A | Grapefruits | F28.A0C0S | PROCESS=Unprocessed |
| P0110020A | Oranges | F28.A07LN | PROCESS = Juicing |
| P0110020A | Oranges | F28.A0C0S | PROCESS=Unprocessed |
| P0110050A | Mandarins | F28.A0C0S | PROCESS=Unprocessed |
| P0130010A | Apples | F28.A07LN | PROCESS = Juicing |
| P0130010A | Apples | F28.A0C0S | PROCESS=Unprocessed |
| P0130020A | Pears | F28.A0C0S | PROCESS=Unprocessed |
| P0140030A | Peaches | F28.A0C0S | PROCESS=Unprocessed |
| P0151010A | Table grapes | F28.A07KG | PROCESS=Drying (dehydration) |
| P0151010A | Table grapes | F28.A0C0S | PROCESS=Unprocessed |
| P0151020A | Wine grapes | F28.A0C0S | PROCESS=Unprocessed |
| P0151020A | Wine grapes | F28.A0C00$F10.A0F2R | PROCESS=Winemaking, QUAL = White |
| P0151020A | Wine grapes | F28.A0C00$F10.A0F2S | PROCESS=Winemaking, QUAL = Red |
| P0152000A | Strawberries | F28.A0C0S | PROCESS=Unprocessed |
| P0162010A | Kiwi fruits (green, red, yellow) | F28.A0C0S | PROCESS=Unprocessed |
| P0163020A | Bananas | F28.A0C0S | PROCESS=Unprocessed |
| P0211000A | Potatoes | F28.A0C0S | PROCESS=Unprocessed |
| P0213020A | Carrots | F28.A0C0S | PROCESS=Unprocessed |
| P0220020A | Onions | F28.A0C0S | PROCESS=Unprocessed |
| P0231010A | Tomatoes | F28.A0C0S | PROCESS=Unprocessed |
| P0231020A | Sweet peppers/bell peppers | F28.A07KG$F28.A07LA | PROCESS=Drying (dehydration), PROCESS = Grinding/milling/crushing |
| P0231020A | Sweet peppers/bell peppers | F28.A0C0S | PROCESS=Unprocessed |
| P0231030A | Aubergines/eggplants | F28.A0C0S | PROCESS=Unprocessed |
| P0232010A | Cucumbers | F28.A0C0S | PROCESS=Unprocessed |
| P0232030A | Courgettes | F28.A0C0S | PROCESS=Unprocessed |
| P0233010A | Melons | F28.A0C0S | PROCESS=Unprocessed |
| P0241010A | Broccoli | F28.A0C0S | PROCESS=Unprocessed |
| P0241020A | Cauliflowers | F28.A0C0S | PROCESS=Unprocessed |
| P0242020A | Head cabbages | F28.A0C0S | PROCESS=Unprocessed |
| P0251020A | Lettuces | F28.A0C0S | PROCESS=Unprocessed |
| P0252010A | Spinaches | F28.A07KQ | PROCESS=Freezing |
| P0252010A | Spinaches | F28.A0C0S | PROCESS=Unprocessed |
| P0260010A | Beans (with pods) | F28.A0C0S | PROCESS=Unprocessed |
| P0260040A | Peas (without pods) | F28.A0C0S | PROCESS=Unprocessed |
| P0270060A | Leeks | F28.A0C0S | PROCESS=Unprocessed |
| P0280010A | Cultivated fungi | F28.A0C0S | PROCESS=Unprocessed |
| P0300010A | Beans (dry) | F28.A0C0S | PROCESS=Unprocessed |
| P0402010A | Olives for oil production | F28.A0C02$F02.A068M | PROCESS=Oil production, PART = Vegetable fats and oils (as part‐nature) |
| P0500010A | Barley | F28.A0C0S | PROCESS=Unprocessed |
| P0500050A | Oat | F28.A07LH | PROCESS=Flattening/rolling |
| P0500050A | Oat | F28.A0C0S | PROCESS=Unprocessed |
| P0500060A | Rice | F28.A0BZV | PROCESS=Polishing |
| P0500060A | Rice | F28.A0C0S | PROCESS=Unprocessed |
| P0500070A | Rye | F28.A0C0S | PROCESS=Unprocessed |
| P0500090A | Wheat | F28.A0C03$F02.A067Z$F10.A06HR | PROCESS = Grain milling, PART = Flour/meal or finely ground powder (as part‐nature), QUAL = Integral/not refined |
| P0500090A | Wheat | F28.A0C03$F02.A067Z$F10.A07XK | PROCESS = Grain milling, PART = Flour/meal or finely ground powder (as part‐nature), QUAL = White/refined |
| P0500090A | Wheat | F28.A0C0S | PROCESS=Unprocessed |
Code of the raw primary commodity as defined by EFSA's harmonised terminology for scientific research (MATRIX catalogue; EFSA, 2022b).
Name of the raw primary commodity as defined by EFSA's harmonised terminology for scientific research (MATRIX catalogue; EFSA, 2022b).
Codes of FoodEx2 facet describing the processing technique, including additional descriptors such as qualitative information, part consumed or the nature of the food (MTX catalogue; EFSA, 2015b).
Names of FoodEx2 facet describing the processing technique, including additional descriptors such as qualitative information, part consumed or the nature of the food (MTX catalogue; EFSA, 2015b).
C.1.5. Consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011). Data reported in the Comprehensive Database may either refer to raw primary commodities (RPCs), RPC derivatives (i.e. single‐component foods altered by processing) or composite foods (i.e. multicomponent). Consumption data for RPC derivatives and composite foods, however, cannot be used in exposure assessments when the occurrence data are reported for the RPCs.
To address the above issue, EFSA transformed the Comprehensive Database into a new RPC Consumption Database by means of the RPC model (EFSA, 2019a). This model converts the consumption data for composite foods or RPC derivatives into their equivalent quantities of RPCs, except foods for infants and young children. 86 The RPC model was applied to the Comprehensive Database as of 31 March 2018, when it contained results from 51 different dietary surveys carried out in 23 different Member States covering 94,523 individuals.
Furthermore, in order to cover as many populations as possible without compromising the reliability of intake estimates at the 99.9th percentile of the distribution, only the dietary surveys with more than 300 survey consumers were retained, covering 17 different countries.
-
–
Toddlers 87 : Bulgaria, Denmark, Finland, Germany, Netherlands
-
–
Other children 88 : Belgium, Bulgaria, Czechia, Finland, France, Germany, Greece, Netherlands, Spain, Sweden.
-
–
Adults 89 : Austria, Belgium, Czechia, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden.
For chronic exposure assessment, individuals who participated for only 1 day of the dietary survey were excluded because at least two survey days per individual are normally required to assess repeated exposure (EFSA, 2011).
The full data set is not reported in the remit of this report. However, the description of the variables is provided in Table C.3.
TABLE C.3.
Description of the variable contained in the food consumption database used for this assessment.
| Name | Label | Description |
|---|---|---|
| Country | Country | Country where the dietary survey took place as defined by EFSA's harmonised terminology for scientific research (COUNTRY catalogue; EFSA, 2022b) |
| Survey | Survey | Acronym of the dietary survey |
| PopClass | Population class | Participant's population class, based on age, as defined by EFSA's harmonised terminology for scientific research (AGECLS catalogue; EFSA, 2022b) |
| ORSUBID | Consumer ID | A pseudonymised consumer ID number generated by EFSA upon receipt of the data |
| Weight | Body weight | Bodyweight of the consumer (in kg) |
| ndays | Number of survey days | Number of days on which the participant's consumption was surveyed |
| Day | Survey day | Ordinal number of the day on which the participant's consumption was surveyed |
| prodCode | RPC code | Code of the raw primary commodity as defined by EFSA's harmonised terminology for scientific research (MATRIX catalogue; EFSA, 2022b) |
| prodName | RPC name | Name of the raw primary commodity as defined by EFSA's harmonised terminology for scientific research (MATRIX catalogue; EFSA, 2022b) |
| FoodEx2_Facets | Processing code | FoodEx2 facet code describing the processing technique, including additional descriptors such as qualitative information, part consumed or the nature of the food (EFSA, 2015b) |
| RPCD_amount | RPCD amount | Amount of raw primary commodity derivative (in grams) |
| RPC_amount | RPC amount | Amount of raw primary commodity (in grams) |
C.2. Secondary input data
C.2.1. Maximum residue level
Certain assumptions on the extrapolation of occurrence data (see Appendix C.1.4) require information on the maximum residue levels (MRLs). An MRL is the upper legal level of a concentration for a pesticide residue in or on food or feed set in accordance with Regulation (EC) No 396/2005. This regulation also defines a procedure for the setting and modification of MRLs. MRLs may therefore have been modified throughout the 2020–2022 reference period. In order to obtain a single list of MRLs, EFSA decided to use the MRLs as of 31 December 2022 (i.e. the end of the current reference period). Hence, it was assumed that those MRLs were applicable during the entire reference period, regardless of whether the MRL or residue definition may have changed during that period.
MRLs for the relevant food commodities (see Appendix C.1.1) and enforcement residue definitions (see Appendix C.1.3) were extracted from the EU Pesticides Database and organised in a data format that can be used directly for exposure assessment.
C.2.2. Authorised uses
In some cases, the imputations and simulations performed on the occurrence data rely on the authorisations for use of the active substance(s) (see Appendix C.1.4). While the approval status of an active substance under Regulation (EC) No 1107/2009 is regulated at EU level, the authorisations for plant protections products (PPP, i.e. formulated products containing the active substances) are delivered at national level within the EU Member States.1 A centralised database compiling these national authorisations is not yet available at EU level.
National authorisations can be reported to EFSA under Regulation (EC) No 396/2005, either for an MRL application under Article 10 or for an MRL review under Article 12. There is, however, no legal obligation to systematically report all national authorisations and the MRL review programme is still in progress. A comprehensive overview of all pesticides authorisations within the EU is therefore also not available to EFSA. Meanwhile, a tentative list of authorised uses was elaborated according to the following principles.
-
–
When the MRL for a given combination of active substance and RPC was not set at the LOQ (see Appendix C.1.2.1), the active substance was assumed to be authorised for use on that specific commodity. This assumption also accounts for uses authorised outside the EU and for which treated products may be placed on the EU market. Furthermore, this assumption concerns non‐approved substances, including persistent organic pollutants, which are assumed to be authorised on crops for which MRLs are above the LOQ.
-
–
When non‐LOQ MRLs referred to unspecific residue definitions (i.e. including or applying to multiple active substances, see also Appendix C.1.3), only the substances approved under Regulation 1107/2009 were assumed to be authorised for use on that crop. If none of the active substances was approved, it was assumed that any substance may be authorised for use outside the EU.
-
–
When non‐LOQ MRLs refer to an active substance that is phased out under Regulation 1107/2009 (e.g. carbendazim) but may still occur as a metabolite from another active substance (thiophanate‐methyl), the MRL was not considered to represent an authorised use of the active substance that was phased out.
-
–
For the group of dithiocarbamates, which comprises six substances, Regulation (EC) No 396/2005 provides specific information on the active substances that were considered for deriving the MRLs. Authorised uses for these active substances were identified accordingly.
-
–
When the MRL was set at LOQ and a review under Article 12 of Regulation (EC) No 396/2005 had not been issued, it was assumed that the use was not authorised.
-
–
For the remaining combinations of active substance and RPC (i.e. where the MRL was set at LOQ), EFSA screened the relevant reasoned opinions issued under Article 12 of Regulation (EC) No 396/2005 and the subsequent reasoned opinions issued under Article 10. Any authorised use reported in those reasoned opinions was recorded. The combinations that have been considered authorised are listed in the Annex III – Table 3.7.
C.2.3. Processing factors
Occurrence data for pesticide residues are collected at the level of the RPC (see Appendix C.1.4). Food consumption data may be collected at the level of RPC, RPC derivative or composite food, but for the purpose of this assessment, all consumption data for composite foods and RPC derivatives were converted into their equivalent quantities of RPCs (see Appendix C.1.5). Combining occurrence and consumption data at RPC level implies that all residues present in the RPC will reach the end consumer. This assumption, however, is conservative. In reality, residue concentrations will most likely change due to processing, such as peeling, washing, cooking, etc.
The effect of processing is usually addressed by means of processing factors. A processing factor accounts for the change in residue concentrations and is specific to each RPC, processing type and active substance. Processing factors are quantified by dividing the residue concentration in the processed commodity by the residue concentration in the raw commodity.
The European database on processing factors is the most recent and the most comprehensive compilation of processing factors currently available at EU level (Zincke, 2022). Processing factors for the active substances and RPCs under assessment were extracted from the database according to the following criteria:
For each active substance, RPC and processing technique only the median processing factor was extracted.
Only the processing factors indicated as reliable, or indicative were extracted. Processing factors indicated as unreliable were excluded from the assessment.
Processing techniques reported in the processing factor database were then compared to the processing techniques reported in the RPC consumption data set. The processing techniques from both databases were matched according to the following principles:
When a generic processing technique was reported in the RPC consumption database (e.g. juice) while more specific processing techniques were reported in the processing factor database (e.g. pasteurised juice and unpasteurised juice), the specific processing technique with the highest processing factor was selected.
When a specific processing technique was reported in the RPC consumption database (e.g. mashed potato) while a more generic processing technique was reported in the processing factor database (e.g. boiled potato), the generic processing factor was applied to the specific processing techniques.
Processing factors were extrapolated between raw primary commodities with similar properties (i.e. oranges and mandarins, apples and pears, table and wine grapes, wheat and rye grain).
Processing factors for peeling were applied to the corresponding fruit with inedible peel, even when the processing technique was not specified in the RPC consumption database (i.e. oranges, mandarins, bananas and melons).
Although the European database on processing factors is the most comprehensive compilation of processing factors currently available at EU level, this compilation is limited to all processing factors that have been evaluated by EFSA until 31 December 2022. Meanwhile, additional processing factors have been assessed by EFSA in the framework of Regulation (EC) No 396/2005 and Regulation (EC) No 1107/2009. Additional processing factors evaluated by EFSA until August 2024 will be integrated in future updates of the European PF database (Kittelmann et al., 2024). The list of PFs is available in the Appendix D – Annex III – Table 3.6
C.2.4. Variability factors
The occurrence data used for the assessment are related to the average concentrations in composite laboratory samples (see Appendix C.1.4). Consumers on the other hand are exposed to individual units of the commodity. Residue concentrations may vary among the individual units, referred to as unit‐to‐unit variability. Acute exposure assessments for pesticide residues should account for variability among the single commodity units of the composite laboratory samples. To account for this variability, several parameters are required for each food commodity.
Unit weight: estimated weight for a single commodity unit.
Units per sample: estimated number of units within a composite laboratory sample.
Variability factor (VF): expected variability among the single unit concentrations, which is defined as the ratio between the 97.5th percentile and mean of the distribution of unit concentrations.
Unit weights for each commodity were retrieved from the Pesticide Residues Intake Model (EFSA, 2018a). Residue concentrations may vary among the individual units, referred to as unit‐to‐unit variability. For RPCs that have a unit weight inferior to 25 g and for processed foods that were subject to blending or bulking, the unit‐to‐unit variability is not considered relevant since the residue concentration in the composite laboratory sample is expected to reflect the residue concentration in the portion that would be consumed (FAO, 2003).
The number of units per sample was obtained from Commission Directive 2002/63/EEC, 90 establishing community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin. This directive defines a minimum weight and a minimum number of units for composite laboratory samples of each food category. Hence, the minimum number of units (as defined by Directive 2002/63/EEC) was used, unless the minimum sample weight divided by the corresponding unit weight was higher. In that case, the latter calculated value (rounded up to the next integer) was retained.
VFs were also retrieved from the Pesticide Residues Intake Model (EFSA, 2018a).
While a fixed VF is usually applied for acute deterministic calculations, for probabilistic exposure assessment, the use of a distribution of unit concentrations is considered more adequate than using a fixed VF. Therefore unit‐to‐unit variability is modelled using a beta distribution, which can be bounded between 0 and an upper limit. Indeed, if the average concentration in a composite sample is 1, the concentration in a single unit can never be higher than the number of units within the composite sample (assuming all other units have a concentration of zero). Hence, for each RPC with a unit weight exceeding 25 g, the beta distribution was parameterised with the following restrictions.
Lower bound = 0
Mean = 1
97.5th percentile = VF
Upper bound = number of units per sample
Stochastic VFs can then be drawn from the beta distribution and multiplied with the composite sample concentration to obtain a plausible estimate of the unit concentration. When the portion consumed by an individual is smaller than a single unit, the stochastic VF is directly applicable to the consumed portion. When the consumed portion is composed of multiple units however, multiple stochastic VFs will be drawn from the same beta distribution to estimate concentration in the whole portion consumed. Therefore, the concentration in the whole portion is estimated by multiplying the sample concentration with a weighted VF, which is calculated as follows.
where is the weighted VF;
is the stochastic VF drawn for unit ;
is the estimated number of units within the consumed portion (unrounded), assuming the unit weights reported in Appendix C.1.2.4;
is the number of stochastic VFs to be drawn (i.e. ceiling of ).
In Table C.4, are shown the stochastic VFs parameters for each RPCs selected for the probabilistic risk assessment. If the information is missing, it means that the unit‐to‐unit variability is not relevant.
TABLE C.4.
Variability factor parameters.
| prodCode a | prodName b | Cat_2002_63_EC c | SampWeight d | minUnits e | UnitWeight f | NrUnits g | VF h |
|
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P0110010A | Grapefruits | Large, 250 g or more | 2000 | 5 | 270.5 | 8 | 5 | 0.341154 | 2.388078 | ||
| P0110020A | Oranges | Medium, 25–250 g | 1000 | 10 | 160 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0110050A | Mandarins | Medium, 25–250 g | 1000 | 10 | 100 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0130010A | Apples | Medium, 25–250 g | 1000 | 10 | 112 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0130020A | Pears | Medium, 25–250 g | 1000 | 10 | 206.5 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0140030A | Peaches | Medium, 25–250 g | 1000 | 10 | 127.6 | 10 | 7 | 0.158404 | 1.425633 | ||
| P0151010A | Table grapes | Large, 250g or more | 2000 | 5 | 581.55 | 6 | 5 | 0.248312 | 1.241558 | ||
| P0151020A | Wine grapes | ||||||||||
| P0152000A | Strawberries | ||||||||||
| P0162010A | Kiwi fruits | Medium, 25–250 g | 1000 | 10 | 83 | 13 | 7 | 0.184385 | 2.21262 | ||
| P0163020A | Bananas | Medium, 25–250 g | 1000 | 10 | 100 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0211000A | Potatoes | Medium, 25–250 g | 1000 | 10 | 216 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0213020A | Carrots | Medium, 25–250 g | 1000 | 10 | 80 | 13 | 7 | 0.184385 | 2.21262 | ||
| P0220020A | Onions | Medium, 25–250 g | 1000 | 10 | 105.8 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0231010A | Tomatoes | Medium, 25–250 g | 1000 | 10 | 142.5 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0231020A | Peppers | Medium, 25–250 g | 1000 | 10 | 154.9 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0231030A | Aubergines (egg plants) | Large, 250 g or more | 2000 | 5 | 271 | 8 | 5 | 0.341154 | 2.388078 | ||
| P0232010A | Cucumbers | Large, 250 g or more | 2000 | 5 | 411.4 | 6 | 5 | 0.248312 | 1.241558 | ||
| P0232030A | Courgettes | Medium, 25–250 g | 1000 | 10 | 114 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0233010A | Melons | Large, 250 g or more | 2000 | 5 | 540 | 6 | 5 | 0.248312 | 1.241558 | ||
| P0241010A | Broccoli | Medium, 25–250 g | 1000 | 10 | 186 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0241020A | Cauliflower | Large, 250 g or more | 2000 | 5 | 689.9 | 6 | 5 | 0.248312 | 1.241558 | ||
| P0242020A | Head cabbage | Large, 250 g or more | 2000 | 5 | 1281.9 | 6 | 5 | 0.248312 | 1.241558 | ||
| P0251020A | Lettuce | Large, 250 g or more | 2000 | 5 | 534.7 | 6 | 5 | 0.248312 | 1.241558 | ||
| P0252010A | Spinach | ||||||||||
| P0260010A | Beans (with pods) | ||||||||||
| P0260040A | Peas (without pods) | ||||||||||
| P0270060A | Leek | Medium, 25–250 g | 1000 | 10 | 168.8 | 10 | 7 | 0.158731 | 1.428581 | ||
| P0280010A | Cultivated funghi | Medium, 25–250 g | 1000 | 10 | 25 | 40 | 7 | 0.227164 | 8.859387 | ||
| P0300010A | Beans (dry) | ||||||||||
| P0402010A | Olives for oil production | ||||||||||
| P0500010A | Barley | ||||||||||
| P0500050A | Oats | ||||||||||
| P0500060A | Rice | ||||||||||
| P0500070A | Rye | ||||||||||
| P0500090A | Wheat | ||||||||||
| PX100001A | Baby foods other than processed cereal‐based foods | ||||||||||
| PX100003A | Processed cereal‐based foods for infants and young children | ||||||||||
| PX100004A | Infant formulae | ||||||||||
| PX100005A | Follow‐on formulae |
Code of the RPC as defined by EFSA's harmonised terminology for scientific research (MATRIX catalogue; EFSA, 2019d).
Name of the RPC as defined by EFSA's harmonised terminology for scientific research (MATRIX catalogue; EFSA, 2019d).
Commodity classification defined by Table 4 of the Annex to Commission Directive 2002/63/EC.
Minimum size of each laboratory sample (expressed in g) defined by Table 4 of the Annex to Commission Directive 2002/63/EC.
Minimum size of each laboratory sample (expressed in number of units) defined by Table 4 of the Annex to Commission Directive 2002/63/EC.
Estimated weight (expressed in g) for a single commodity unit as reported in the Pesticide Residues Intake Model (EFSA, 2018a).
Estimated number of units required to obtain the minimum size of a laboratory sample, both in terms of weight and number of units.
Default VF as reported in the Pesticide Residues Intake Model (EFSA, 2018a). This factor represents the variability among the single unit concentrations, which is defined as the ratio between the 97.5th percentile and mean of the distribution of unit concentrations.
Computed parameter of the beta distribution.
Computed parameter of the beta distribution.
C.2.5. Processing types
Variability among the single commodity units of the composite laboratory samples is not relevant when the food consumed is subject to processing techniques that involve bulking and blending.
EFSA therefore extracted all processing techniques reported in the RPC consumption data (see Appendix C.1.5) and identified the processes that normally involve blending or bulking. Typically, these are processing techniques performed at industrial level (e.g. milling, oil production, etc.). Household processes, however, were assumed not to involve any bulking or blending (e.g. boiling, stewing, etc.). Although juicing may also be carried out at household level, EFSA assumed that most fruit juices are produced at industrial level.
C.2.6. Health‐Based Guidance Values
The ARfDs and ADIs established by EFSA under regulations (EC) No 1107/2009 were selected. The same assumptions taken for the deterministic assessment on tentative ADIs and ARfDs were done, see Sections 5.1 and 5.3 for details. The list of HBGVs is available in the Appendix D – Annex III – Table 3.5.
APPENDIX D. Supporting Informatiion
D.1.
Annex I – The data visualisation (EU MACP and MANCP) of 2022 ARPR: https://multimedia.efsa.europa.eu/pesticides‐report‐2022/
Annexes published in Zenodo: https://doi.org/10.5281/zenodo.10853986
Annex II – The PRIMo deterministic exposure model on the 2022 EU annual report on pesticide residue results (ARPR)
Annex III – Input and output data of the 2022 EU pesticide residues report on food
Table 3.1 – The 2022 EU coordinated multiannual programme of the Union: the residue definitions and related active substances
Table 3.1 – mapping. The residue definitions and related active substances
Table 3.2 – List of samples exceeding the MRLs, including information on the measured residue concentrations and the origin of the samples
Table 3.3 – Scope of analysis of pesticides reported
Table 3.4 – Regulation (EU) 2019/1793 on the temporary increase of official controls ‐ extract of the controls to be performed of pesticides in food
Table 3.5 – Health‐based guidance values (HBGV)
Table 3.6 – Processing factors used to refine acute exposure assessment
Table 3.7 – Authorised use
Annex IV – Acute individual Exposure Assessment, Primary of 2022 ARPR
Figure 4.1 – Histogram presenting the distribution of the hazard quotient per active substance and survey
Figure 4.2 – Box plot presenting the 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Figure 4.3 – Pie chart presenting the average contributions of RPCs to the exposures exceeding the 99th percentile
Table 4.4 – Estimated 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Table 4.5 – Distribution of the hazard quotient calculated per active substance, survey and bootstrap
Table 4.6 – Average contributions of RPCs to the exposures exceeding the 99th percentile per active substance and survey
Table 4.7 – Detailed records for consumers with exposures closer to the 99th percentile
Table 4.8 – Overview of RPCs and active substances with limited occurrence data
Annex V – Acute individual Exposure Assessment, Tentative of 2022 ARPR
Figure 5.1 – Histogram presenting the distribution of the hazard quotient per active substance and survey
Figure 5.2 – Box plot presenting the 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Figure 5.3 – Pie chart presenting the average contributions of RPCs to the exposures exceeding the 99th percentile
Table 5.4 – Estimated 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Table 5.5 – Distribution of the hazard quotient calculated per active substance, survey and bootstrap
Table 5.6 – Average contributions of RPCs to the exposures exceeding the 99th percentile per active substance and survey
Table 5.7 – Detailed records for consumers with exposures closer to the 99th percentile
Table 5.8 – Overview of RPCs and active substances with limited occurrence data
Annex VI – Chronic individual Exposure Assessment, Primary of 2022 ARPR
Figure 6.1 – Histogram presenting the distribution of the hazard quotient per active substance and survey
Figure 6.2 – Box plot presenting the 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Figure 6.3 – Pie chart presenting the average contributions of RPCs to the exposures exceeding the 99th percentile
Table 6.4 – Estimated 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Table 6.5 – Distribution of the hazard quotient calculated per active substance, survey and bootstrap
Table 6.6 – Average contributions of RPCs to the exposures exceeding the 99th percentile per active substance and survey
Table 6.7 – Detailed records for consumers with exposures closer to the 99th percentile
Table 6.8 – Overview of RPCs and active substances with limited occurrence data
Annex VII – Chronic individual Exposure Assessment, Tentative of 2022 ARPR
Figure 7.1 – Histogram presenting the distribution of the hazard quotient per active substance and survey
Figure 7.2 – Box plot presenting the 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Figure 7.3 – Pie chart presenting the average contributions of RPCs to the exposures exceeding the 99th percentile
Table 7.4 – Estimated 95% confidence intervals of the hazard quotient at different percentiles per active substance and survey
Table 7.5 – Distribution of the hazard quotient calculated per active substance, survey and bootstrap
Table 7.6 – Average contributions of RPCs to the exposures exceeding the 99th percentile per active substance and survey
Table 7.7 – Detailed records for consumers with exposures closer to the 99th percentile
Table 7.8 – Overview of RPCs and active substances with limited occurrence data
Annex A. Outcome of the Member State,1 IS and NO consultation
A.1.
Annex A is available under the Supporting Information section on the online version of the scientific output.
EFSA (European Food Safety Authority) , Carrasco Cabrera, L. , Di Piazza, G. , Dujardin, B. , Marchese, E. , & Medina Pastor, P. (2024). The 2022 European Union report on pesticide residues in food. EFSA Journal, 22(4), e8753. 10.2903/j.efsa.2024.8753
Approved: 22 March 2024
Annex A is available under the Supporting Information section .
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2024.EN-8751/full
Notes
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Windsor Framework (see Joint Declaration No 1/2023 of the Union and the United Kingdom in the Joint Committee established by the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community of 24 March 2023, OJ L 102, 17.4.2023, p. 87) in conjunction with section 24 of Annex 2 to that Framework, for the purposes of this Regulation, references to Member States include the United Kingdom in respect of Northern Ireland.
A dedicated website where EU MACP and MANCP results are presented: https://multimedia.efsa.europa.eu/pesticides‐report‐2022/
Commission Implementing Regulation (EU) 2021/601 of 13 April 2021 concerning a coordinated multiannual control programme of the Union for 2022, 2023 and 2024 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin. OJ L 127, 14.4.2021, p. 29–41.
These samples exclude those of baby food requested under the EU MACP.
The ‘maximum residue level’ (MRL) is defined as the upper legal level of concentration for a pesticide residue in or on food or feed set in accordance with Regulation (EC) No 396/2005, based on good agricultural practice and the lowest consumer exposure necessary to protect vulnerable consumers.
The term pesticide residue is used throughout this report and its annexes, to refer to measurable amounts of an active substance and/or related metabolites and/or degradation products that can be found on harvested crops or in foods of animal origin.
The term plant protection products (PPP) used throughout this report and its annexes pertains to a product containing an active substance and other substances added and/or their products to ensure, among others, plant protection against harmful organisms, influence their life processes (e.g. growth regulators), destroy or prevent growth of undesired plants or parts of them in the fields, etc.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). OJ L 95, 7.4.2017, p. 1–142.
Commission Implementing Regulation (EU) 2021/1355 of 12 August 2021 on multiannual national control programmes for pesticides residues to be established by Member States. OJ L 291, 13.8.2021, p. 120–121.
Commission Implementing Regulation (EU) 2019/1793 of 22 October 2019 on the temporary increase of official controls and emergency measures governing the entry into the Union of certain goods from certain third countries implementing Regulations (EU) 2017/625 and (EC) No 178/2002 of the European Parliament and of the Council and repealing Commission Regulations (EC) No 669/2009, (EU) No 884/2014, (EU) 2015/175, (EU) 2017/186 and (EU) 2018/1660. OJ L 277, 29.10.2019, p. 89–129.
Commission Implementing Regulation (EU) 2021/2246 of 15 December 2021 amending Implementing Regulation (EU) 2019/1793 on the temporary increase of official controls and emergency measures governing the entry into the Union of certain goods from certain third countries implementing Regulations (EU) 2017/625 and (EC) No 178/2002 of the European Parliament and of the Council. OJ L 453, 17.12.2021, p. 5–34.
Commission Implementing Regulation (EU) 2022/913 of 30 May 2022 amending Implementing Regulation (EU) 2019/1793 on the temporary increase of official controls and emergency measures governing the entry into the Union of certain goods from certain third countries implementing Regulations (EU) 2017/625 and (EC) No 178/2002 of the European Parliament and of the Council. OJ L 158, 13.6.2022, p. 1–26.
Commission Implementing Regulation (EU) 2019/1715 of 30 September 2019 laying down rules for the functioning of the information management system for official controls and its system components (the IMSOC Regulation) OJ L 261, 14.10.2019, p. 37–96.
Agenda point A.17 of the 18–19 September 2023 SCoPAFF meeting: https://food.ec.europa.eu/system/files/2023‐10/sc_phyto_20230918_ppr_sum.pdf
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1–33.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 16–35.
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding. OJ L 25, 2.2.2016, p. 1–29.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 015, 20.1.2010, p. 1.
Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. OJ L 125, 23.5.1996, p. 10.
Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. OJ L 150, 14.6.2018, p. 1–92.
Commission Implementing Regulation (EU) 2021/1165 of 15 July 2021 authorising certain products and substances for use in organic production and establishing their lists. OJ L 253, 16.7.2021, p. 13–48.
Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. OJ L 125, 23.5.1996, p. 10–32.
Iceland and Norway are considered as second countries in respect to EU MSs considered first. The rest of the countries are referred in this report as third countries.
As long as all the dithiocarbamates assessment for each single precursor have not been assessed individually, within the remit of this report ‘dithiocarbamates’ will be considered as not approved substance.
The minimum number of 10 samples of baby food category mentioned in the EU MACP was not reached by Hungary (1 samples), Portugal (5 samples), Czechia (6 samples), France (6 samples), Spain (7 samples) and no baby food samples were reported by Bulgaria, Estonia, Greece, and Lithuania.
When Member States follow a multiresidue method (MRM) and consider it sufficient to include the given number of substances to properly enforce the residue definition, the paramCode to be used is the one of the full residue definitions but flagging it as paramType P004A, when reporting data to EFSA under the ChemMon data collection (EFSA, 2023a).
EFSA will make mandatory reporting this data element in its Chemical Monitoring data collection when samples are flagged as non‐compliant.
Its findings may include residues of two approved fungicides: disodium phosphonate and phosphonic acid, the latter resulting from the use of potassium and disodium phosphonates (which can also be used as foliar feed fertiliser). A residue definition for enforcement common to all was derived ‘fosetyl‐Al (sum of fosetyl, phosphonic acid and their salts, expressed as fosetyl)’ (EFSA, 2018d).
Only six countries reported data con copper compound. As this substance will be included in the EU MACP, it is recommended to official laboratories including it on the scope of analysis.
Commission Implementing Regulation (EU) 2023/731 of 3 April 2023 concerning a coordinated multiannual control programme of the Union for 2024, 2025 and 2026 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin and repealing Implementing Regulation (EU) 2022/741. OJ L 95, 4.4.2023, p. 28–40.
Commission Implementing Regulation (EU) 2023/1110 of 6 June 2023 amending Implementing Regulation (EU) 2019/1793 on the temporary increase of official controls and emergency measures governing the entry into the Union of certain goods from certain third countries implementing Regulations (EU) 2017/625 and (EC) No 178/2002 of the European Parliament and of the Council. OJ L 147, 7.6.2023, p. 111–141.
On locust beans (carob), locust beans seeds, not decorticated, crushed or ground, mucilage and thickeners, whether or not modified, derived from locust beans or locust beans seeds, from India and guar gum from India.
Close to the finalisation of this report, a correction was made by France following an error during data transmission: The new data submitted by France to EFSA are reflected in footnotes within this section as well as within Annex III – Table 3.2 and Table 3.3. The National Summary Report (EFSA, 2024c) provides detailed and corrected information.
8612 samples with the new data provided by France.
67 samples with the new data provided by France.
The new MRL exceedance rate for chlordecone with the new data provided by France is 0.7%.
43 samples were reported in fat with the new data provided by France (37 in bovine fat, 3 in swine fat and 3 more in sheep fat).
Commission Implementing Regulation (EU) 2023/2660 of 28 November 2023 renewing the approval of the active substance glyphosate in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) No 540/2011. OJ L, 2023/2660, 29.11.2023.
Composite food products are food made of more than one product, either plant based or/and animal commodities. The MRL that applies, needs to be recalculated on a case by case depending on the ingredients and proportion contained in the sample. The type of products the regulation listed were tea, whether or not flavoured; mucilage's and thickeners, whether or not modified, derived from locust beans or locust beans seeds; tomato ketchup and other tomato sauces; xanthan gum; guar gum; mixtures of food additives containing locust bean gum or guar gum; sauces and preparations thereof; mixed condiments and mixed seasonings; mustard flours and meals and prepared mustard; food supplements containing botanicals; instant noodles containing spices/ seasonings or sauces; calcium carbonate.
These samples are not counted on the overall number of 110,829 samples.
Through IMSOC system, the real number of samples of import control are collected. More information on this can be requested through SANTE-IMPORT-CONTROLS@ec.europa.eu
Information note on Article 20 of Regulation (EC) No 396/2005 as regards processing factors and composite food and feed. SANTE/10704/2021 expected to be noted in February 2022 SCoPAFF meeting.
In the framework of this report, unprocessed food products are considered those to which an MRL is directly applicable and are listed in Annex I to Regulation (EC) No 396/2005, i.e. products such as fermented tea, dried spices, dried herbal infusions, etc.
Copper is authorised in organic production23.
Commission Implementing Regulation (EU) 2021/601 of 13 April 2021 concerning a coordinated multiannual control programme of the Union for 2022, 2023 and 2024 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin. OJ L 127, 14.4.2021, p. 29–41.
This is a known issue by French Competent Authorities on their overseas territories.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
As set in Regulation (EC) No 396/2005, an import tolerance is an MRL set for imported products to meet the needs of international trade different than the one in place in the EU, for which prevails no risk to EU consumers.
An emergency authorisation in accordance with Article 53 of Regulation (EC) 1107/20098.
According to the 2022 EU MACP control programme, samples of barley, oat, rye or wheat whole grain flour could be taken in case samples of grain were not available for monitoring purposes.
In 2017, JMPR recommended using a variability factor of 3 (which is the rounded mean of 2.8) for all commodities (FAO, 2017). At EU level, the choice of the most appropriate variability factor to be used for the acute risk assessment is still under discussion. Under CRA, for Tier II scenario a variability factor of 3.6 is applied to all commodities having a unit weight above 25 g (EFSA, 2019b).
Within the 2022 EU MACP, meat can be sampled in accordance with Table 3 of Annex to Directive 2002/63/EC.
Bromide ion assessment is put on‐hold until EFSA delivers its evaluation (M‐2022‐00105).
No samples above the LOQ were reported.
For omethoate no HBGV has been derived due to in vivo mutagenicity being demonstrated (EFSA, 2017b).
Commission Regulation (EU) 2023/1029 of 25 May 2023 amending Annexes III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for phosmet in or on certain products. OJ L 139, 26.5.2023, p. 15–27.
For many commodities, the IESTI calculations (FAO, 2017) are performed with a residue concentration 5 or even 7 times higher than the residue measured in the composite sample.
Commission Implementing Regulation (EU) 2021/2049 of 24 November 2021 renewing the approval of the active substance cypermethrin as a candidate for substitution in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 420, 25.11.2021, p. 6–13.
M‐2022‐00157.
Commission Regulation (EU) 2021/155 of 9 February 2021 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for carbon tetrachloride, chlorothalonil, chlorpropham, dimethoate, ethoprophos, fenamidone, methiocarb, omethoate, propiconazole and pymetrozine in or on certain products. OJ L 46, 10.2.2021, p. 5–33.
36 in respect of the 40 taken, not being raw primary commodities baby food and water.
Commission Regulation (EU) 2024/331 of 19 January 2024 amending Annexes II and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for oxamyl in or on certain products. OJ L, 2024/331, 22.1.2024.
Commission Regulation (EU) 2023/1029 of 25 May 2023 amending Annexes III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for phosmet in or on certain products. OJ L 139, 26.5.2023, p. 15–27.
To judge on samples reported without quantifiable residues, SANCO/12574/2015 (rev. 5) (European Commission, 2018) was applied in those cases of multicomponent residue definitions when resInfo.notSummed = Y. EFSA calculated the sum LOQ based on the individual LOQ reported and the molecular weight factor. This recalculation was used when calculating the mean middle‐bound and upper‐bound scenarios. In case no individual LOQs were reported, EFSA did not recalculate a summed LOQ and disregarded the record.
The imputation done in probabilistic of ½ LOQ for a number of results equal to the number of positive results (i.e. above the LOQ) for the same substance/commodity combination – provided that the use of the substance is authorised for that commodity – on the number of results where the value is above the LOQ, cannot be done in deterministic.
Being omethoate a non‐approved substance, for results reported below the LOQ, a zero value was inputted in the MB and UB scenarios; thus, all three resulted in the same exposure.
Wheat germ is converted to wheat by a factor of 50. Annex VI, Table 6.7 provides the amount consumed and the correspondent amount in the raw primary commodity conversion.
In the following figures, there are some cases where the ARfD was exceeded due to recent lowering in the ARfD value, while the sample residue levels were still within the MRL. In other cases, the exceedance of the ARfD is due to the IESTI equation and the gap between the highest residue level derived under residue trials and the calculation of the MRL.
Samples with residues above the MRL in the context of this report refer to samples with one or several pesticides exceeding the legal limit, as reported by the Member States including compliant and non‐compliant samples.
Consumption data for foods for infants and young children were not converted to their equivalent amounts of RPC because, in this case, chemical occurrence data are collected for the processed food commodity.
The population class ‘toddlers’ refers to participants from ≥ 12 months to < 36 months old.
The population class ‘other children’ refers to participants from ≥ 36 months to < 10 years old.
The population class ‘adults’ refers to participants from ≥ 18 years to < 65 years old.
Commission Directive 2002/63/EC of 11 July 2002 establishing Community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing Directive 79/700/EEC. OJ L 187, 16.7.2002, p. 30–43.
REFERENCES
- EFSA (European Food Safety Authority) . (2009). Review of the existing maximum residue levels (MRLs) for ethephon on request of EFSA. EFSA Journal, 7(10), 1347. 10.2903/j.efsa.2009.1347 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2010). Scientific and technical support for preparing an EU position in the 42nd session of the Codex committee on pesticide residues (CCPR). EFSA Journal, 8(11), 1560. 10.2903/j.efsa.2010.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011). Guidance of EFSA on the use of the EFSA comprehensive European food consumption database in intakes assessment. EFSA Journal, 9(3), 2097. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2012). A European tool for usual intake distribution estimation in relation to data collection by EFSA. Supporting Publications, 9(6), EN‐300. 10.2903/sp.efsa.2012.EN-300 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2013). Guidance of EFSA on the standard sample description ver. 2.0. EFSA Journal, 11(10), 3424. 10.2903/j.efsa.2013.3424 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014a). Scientific opinion on the essential composition of infant and follow‐on formulae. EFSA Journal, 12(7), 3760. 10.2903/j.efsa.2014.3760 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014b). Conclusion on the peer review of the pesticide risk assessment of the active substance esfenvalerate. EFSA Journal, 12(11), 3873. 10.2903/j.efsa.2014.3873 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015a). Pesticide monitoring program: Design assessment. EFSA Journal, 13(2), 4005. 10.2903/j.efsa.2015.4005 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015b). The food classification and description system FoodEx2 (revision 2). EFSA supporting publication, 13(2), EN‐804. 10.2903/sp.efsa.2015.en-804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015c). Reasoned opinion on the revision of the review of the existing maximum residue levels for lambda‐cyhalothrin. EFSA Journal, 13(12), 4324. 10.2903/j.efsa.2015.4324 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2016a). Scientific report of EFSA on scientific support for preparing an EU position in the 48th session of the Codex committee on pesticide residues (CCPR). EFSA Journal, 14(8), 4571. 10.2903/j.efsa.2016.4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2016b). Conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid. EFSA Journal, 14(11), 4610. 10.2903/j.efsa.2016.4610 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2017a). Reasoned opinion on the focussed review of the existing maximum residue levels for lambda‐cyhalothrin in light of the unspecific residue definition and the existing good agricultural practices for the substance gamma‐cyhalothrin. EFSA Journal, 15(7), 4930. 10.2903/j.efsa.2017.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2017b). Scientific opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal, 15(12), 5113 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018a). Guidance on use of EFSA pesticide residue intake model (EFSA PRIMo revision 3). EFSA Journal, 16(1), 5147. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018b). Focussed assessment of certain existing MRLs of concern for acetamiprid and modification of the existing MRLs for table olives, olives for oil production, barley and oats. EFSA Journal, 16(5), 5262. 10.2903/j.efsa.2018.5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018c). Scientific opinion on pesticides in foods for infants and young children. EFSA Journal, 16(6), 5286. 10.2903/j.efsa.2018.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018d). Conclusion on the peer review of the pesticide risk assessment of the active substance fosetyl. EFSA Journal, 16(7), 5307. 10.2903/j.efsa.2018.5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018e). The 2016 European Union report on pesticide residues in food. EFSA Journal, 16(7), 5348. 10.2903/j.efsa.2018.5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018f). Conclusion on the peer review of the pesticide risk assessment of the active substance cypermethrin. EFSA Journal, 16(8), 5402. 10.2903/j.efsa.2018.5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2018g). Conclusion on the peer review of the pesticide risk assessment of the active substance dimethoate. EFSA Journal, 16(10), 5454. 10.2903/j.efsa.2018.5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2019a). Technical report on the raw primary commodity (RPC) model: strengthening EFSA's capacity to assess dietary exposure at different levels of the food chain, from raw primary commodities to foods as consumed. EFSA supporting publication, 16(1), EN‐1532. 10.2903/sp.efsa.2019.EN-1532 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2019b). Cumulative dietary exposure assessment of pesticides that have acute effects on the nervous system using SAS® software. EFSA Journal, 17(9), 5764. 10.2903/j.efsa.2019.5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2019c). Pesticide Residue Intake Model–EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication, 16(3), EN‐1605. 10.2903/sp.efsa.2019.EN-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2019d). Review of the existing maximum residue levels for glyphosate according to article 12 of regulation (EC) No 396/2005 – Revised version to take into account omitted data. EFSA Journal, 17(10), 5862. 10.2903/j.efsa.2019.5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020a). Statement on the dietary exposure assessment for the temporary maximum residue levels for chlordecone in certain products of animal origin. EFSA Journal, 18(3), 6052. 10.2903/j.efsa.2020.6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020b). Conclusion on the peer review of the pesticide risk assessment of the active substance beta‐cyfluthrin. EFSA Journal, 18(4), 6058. 10.2903/j.efsa.2020.6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2021a). The 2019 European Union report on pesticide residues in food. EFSA Journal, 19(4), 6491. 10.2903/j.efsa.2021.6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2021b). Reasoned opinion on the modification of the existing maximum residue levels and setting of import tolerances for thiabendazole in various crops. EFSA Journal, 19(5), 6586. 10.2903/j.efsa.2021.6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2021c). Reasoned opinion on the modification of the existing maximum residue levels for acetamiprid in various crops. EFSA Journal, 19(9), 6830. 10.2903/j.efsa.2021.6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2022a). Scientific report on retrospective cumulative dietary risk assessment of craniofacial alterations by residues of pesticides. EFSA Journal, 20(10), 7550. 10.2903/j.efsa.2022.7550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2022b). Harmonized terminology for scientific research [data set]. Zenodo. 10.5281/zenodo.5949025 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023a). Chemical monitoring reporting guidance: 2023 data collection. EFSA Supporting publication, 20(10), 7851. 10.2903/sp.efsa.2023.EN-7851 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023b). Reasoned opinion on the review of the existing maximum residue levels for cypermethrins according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 21(3), 7800. 10.2903/j.efsa.2020.7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023c). The 2021 European Union report on pesticide residues in food. EFSA Journal, 21(4), 7939. 10.2903/j.efsa.2023.7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023d). Reasoned opinion on the review of the existing maximum residue levels for dithiocarbamates according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 21(5), 7987. 10.2903/j.efsa.2023.7987 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023e). Statement on risk assessment related to the presence of benzalkonium chloride (BAC), didecyldimethyl ammonium chloride (DDAC) and chlorates in/on fish and fish products. EFSA Journal, 21(5), 8019. 10.2903/j.efsa.2023.8019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023f). Setting of an import tolerance for lambda‐cyhalothrin in avocados. EFSA Journal, 21(12), 8464. 10.2903/j.efsa.2023.8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023g). Statement on the risk assessment of maximum residue levels (MRLs) for oxamyl in view of consumer protection. EFSA Journal, 21(3), 7823. 10.2903/j.efsa.2023.7823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2024a). Prioritisation of pesticides and target organ systems for dietary cumulative risk assessment based on the 2019–2021 monitoring cycle. EFSA Journal, 22(2), e8554. 10.2903/j.efsa.2024.8554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2024b). Report for 2022 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Supporting Publication, 21(3), EN‐8669. 10.2903/sp.efsa.2024.EN-8669 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2024c). National summary reports on pesticide residue analysis performed in 2022. EFSA Supporting Publication, 21(4), EN‐8751. 10.2903/sp.efsa.2024.EN-8751 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues) . (2012). Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA Journal, 10(10), 2839. 10.2903/j.efsa.2012.2839 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2023). Scientific opinion on the re‐evaluation of the existing health‐based guidance values for copper and exposure assessment from all sources. EFSA Journal, 21(1), 7728. 10.2903/j.efsa.2023.7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . (2018). Working document on the summing up of LOQs in case of complex residue definitions. SANCO/12574/2015. rev. 5(1). Application date: 1 January 2018. 30/11‐01/12 2015.
- European Commission . (2021). Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. SANTE/11312/2021 V2 (implemented from 01.01.2024).
- European Union . (2023). The 2022 annual report on alert and cooperation network (p. 2022). Publications Office of the European Union. 10.2875/941288T EW‐AC‐07‐22‐001‐EN‐N. ISBN: 978–92–68‐04222‐9. ISSN: 2363‐0965. [DOI] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2003). Pesticide residues in food – 2005. Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO expert group on pesticide residues. FAO Plant Production and Protection Paper 176.
- FAO (Food and Agriculture Organization of the United Nations) . (2006). Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO expert group on pesticide residues. FAO plant production and protection paper 187. https://www.fao.org/3/a0888e/a0888e.pdf
- FAO (Food and Agriculture Organization of the United Nations) . (2009). Report of the thirty‐second session of the Codex Committee on Pesticide Residues, Rome, 29 June–4 July 2009. Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission 32nd session, Rome, 29 June–4 July 2009.
- FAO (Food and Agriculture Organization of the United Nations) . (2017). Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 286 pp.
- Kittelmann, A. , Scholz, R. , Tietz, E. , Zincke, F. , & Michalski, B. (2024). Second update of the EU database of processing factors for pesticide residues. EFSA Supporting Publication, EN‐8738. 10.2903/sp.efsa.2024.EN-8738 [DOI] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) . (2008). Test No. 508: Magnitude of the Pesticide Residues in Processed Commodities, ISBN: 9789264067622. 16 October 2008. 15 pp. https://www.oecd‐ilibrary.org/environment/test‐no‐508‐magnitude‐of‐the‐pesticide‐residues‐in‐processed‐commodities_9789264067622‐en;jsessionid=3e6jrbG3N7EWn1Pa3B7bc2N2u8qVvz2z_gtjLgl3.ip‐10‐240‐5‐14
- UNEP . (2001). Stockholm Convention on Persistent Organic Pollutants, United Nation Environment Program, Stockholm Convention Secretariat . https://chm.pops.int/TheConvention/Overview/tabid/3351/
- Zincke, F. , Fischer, A. , Kittelmann, A. , Kraus, C. , Scholz, R. , & Michalski, B. (2022). First update of the EU database of processing factors for pesticide residues. EFSA supporting publication, 19(9), EN‐7453. 10.2903/sp.efsa.2022.EN-7453 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcome of the Member State, IS and NO consultation


