Abstract
We estimated the prevalence of astrovirus, sapovirus, and norovirus among patients enrolled in research protocols and receiving medical care at the Clinical Center of the National Institutes of Health, Bethesda, MD, a clinical research hospital with a large immunocompromised patient population. We identified patients whose fecal specimens were submitted to the Clinical Center for testing on the Biofire FilmArray Gastrointestinal Panel from September 15, 2015 through November 30, 2016. Among 442 patients with fecal specimens submitted for multiplex testing, 11% had norovirus identified, 2% had astrovirus, and 2% had sapovirus. Like norovirus, astrovirus was detected in multiple sequential samples from a single patient, consistent with chronic infection or the occurrence of multiple reinfections. Coinfection with non-viral gastrointestinal pathogens was detected in 31% of patients with positive results for norovirus, astrovirus, or sapovirus. Norovirus remains common in this immunocompromised patient population, and both sapovirus and astrovirus are present.
Keywords: Norovirus, Sapovirus, Astrovirus, Immunocompromised host
1. Introduction
Norovirus is now considered the primary viral causative agent of acute diarrhea (Lopman et al., 2016; Verstraeten et al., 2016, 2017). Astrovirus, a member of the family Astroviridae, and sapovirus, a member of the family Caliciviridae, are also known viral causes of acute gastroenteritis (AGE); however, the prevalence of these agents in immunodeficient populations has not been well described. Two large studies of pediatric and adult outpatients in managed-care organizations in multiple regions of the United States found norovirus in 4–6% of fecal samples submitted for routine clinical testing, and sapovirus and astrovirus in 1–2% of these (Grytdal et al., 2016; Hall et al., 2011). The estimated community incidence was 65–152.2 per 1000 person-years for norovirus, 9–22.5 per 1000 person-years for sapovirus and 8.5–18 per 1000 person-years for astrovirus (Grytdal et al., 2016; Hall et al., 2011).
While infections with norovirus, astrovirus, and sapovirus are self-limiting in immunocompetent patients, chronic excretion of all of these viruses has been reported among immunodeficient patients (Osborne et al., 2015; Roos-Weil et al., 2011; Wunderli et al., 2011). Estimates of the prevalence of sapovirus and astrovirus for immunodeficient patients are limited, and available only for pediatric populations. One study at a tertiary care children’s hospital found that for immunocompromised patients, 15% were positive for norovirus and 5% for astrovirus (no sapovirus was detected), similar to the general patient population of the same hospital (Osborne et al., 2015). A study of predominantly immunocompromised pediatric patients at a tertiary care hospital in the UK found similar rates of 10% for norovirus, 1% for astrovirus, and 6% for sapovirus (Brown et al., 2016).
The implementation of the BioFire FilmArray Gastrointestinal (GI) Panel for detection of GI pathogens at the National Institutes of Health Clinical Center has allowed for detection of these viruses in all fecal samples sent for testing as part of routine clinical care. The multiplex FilmArray panel provides simultaneous detection of 22 different enteric pathogens directly from fecal specimens (Spina et al., 2015). To assess the impact of this new testing method on the detection of less prevalent GI pathogens, we analyzed data from patients enrolled in research protocols and receiving medical care at the Clinical Center. The objective of this study was to estimate the prevalence of astrovirus, sapovirus, and norovirus in this immunocompromised population among patients whose fecal samples were submitted for diagnostic testing, as well as to describe chronic excretion and coinfections.
2. Materials and methods
We identified all patients for whom fecal specimens were submitted to the Clinical Center for testing on the FilmArray between September 15, 2015 and November 30, 2016, and who had norovirus, sapovirus, astrovirus, adenovirus, or rotavirus identified. The NIH Clinical Center is a clinical research hospital, where all patients are enrolled in IRB-approved protocols and many are immunocompromised, through either primary or acquired immunodeficiency conditions, or secondary to immunosuppressive therapy for conditions such as hematologic malignancies and solid tumors (Bok et al., 2016). This retrospective analysis was conducted as part of a quality improvement study for the hospital following the implementation of the FilmArray panel and was determined to be exempt from IRB review by the NIH Office of Human Subjects Research Protections.
For all patients testing positive for these viruses, data on primary diagnosis, underlying immunodeficiency, immunosuppressive medication in the 30 days prior to testing, coinfecting pathogens detected by the multiplex panel, and selected clinical features were abstracted from medical records. Medications considered immunosuppressive included corticosteroids, anti-T-cell agents, TNF inhibitors, and other immunosuppressive monoclonal antibodies (S1 Table). Clinical features were compared between viruses and statistical significance was assessed at P < 0.05 using Fisher’s exact test. Chronic excretion was defined as more than one positive test for a given virus greater than 30 days apart. As this diagnostic method does not allow for genotyping of strains, these sequential positive tests may represent chronic excretion of a single strain or reinfection with the same virus.
3. Results
Over the study period, 932 fecal samples from 442 patients were tested on the FilmArray. Of these 442 patients, 48 (11%) tested positive for norovirus, 11 (2%) tested positive for sapovirus, and 7 (2%) tested positive for astrovirus (Table 1). No patients tested positive for either adenovirus or rotavirus. One of these patients had a sample that tested positive for both sapovirus and norovirus, and one tested positive for astrovirus and sapovirus in separate samples, for a total of 64 study patients. The median age of the virus-positive study patients was 33 (range 3–79), with 1 (2%) patient aged <5 years and 10 (16%) between 5 and 18 years (Table 2). At the time of their first positive result, 33 (54%) were inpatients. Of the 63 patients with available symptom data, most patients presented with diarrhea (58, 92%), while approximately half presented with nausea/vomiting (32, 51%) and abdominal pain (34, 54%). No significant differences in symptoms were seen by virus.
Table 1.
Prevalence and chronic excretion of norovirus, astrovirus, and sapovirus among 442 patients tested at the National Institutes of Health Clinical Center between September 15, 2015 and November 30, 2016.
| Norovirus | Astrovirus | Sapovirus | |
|---|---|---|---|
|
| |||
| Prevalence (of 442 patients tested) – n, (%) | 48 (11) | 7 (2) | 11 (2) |
| Patients with chronic excretion – n(%) | 16 (33) | 1 (14) | 0 (0) |
| Days of chronic excretion – median (range) | 189 (72–372) | 132 | NA |
NA = not applicable.
Table 2.
Demographics and comorbidities.
| All patients, n = 64* | Norovirus, n = 48 | Astrovirus, n = 7 | Sapovirus, n = 11 | |
|---|---|---|---|---|
|
| ||||
| Age – median (IQR) | 33 (23–46) | 34 (22–46) | 29 (23–55) | 28 (23–40) |
| Inpatient – no. (%) | 33 (52) | 24 (50) | 3 (43) | 6 (56) |
| Diagnosed with a PID – no. (%) | 35 (55) | 30 (63) | 2 (29) | 4 (36) |
| Hematopoietic stem cell transplant – no. (%) | 27 (42) | 17 (35) | 6 (86) | 6 (55) |
| HIV/AIDS – no. (%) | 3 (5) | 1 (2) | 1 (33) | 1 (9) |
| Hematologic malignancy – no. (%) | 20 (31) | 14 (30) | 4 (57) | 3 (27) |
| Immunosuppressive medication – no. (%) | 39 (61) | 26 (54) | 6 (86) | 9 (82) |
| Immunocompromised† – no. (%) | 58 (91) | 43 (74) | 7 (100) | 10 (91) |
PID = Primary immunodeficiency; HIV/AIDS = Human immunodeficiency virus/acquired immunodeficiency syndrome.
All patients positive for norovirus, astrovirus, or sapovirus; includes one patient with a sample positive for both norovirus and sapovirus, and one patient who tested positive for astrovirus and sapovirus at separate points during the study period.
Due to PID, HIV/AIDS, immunosuppressive medication, or hematologic malignancy.
Overall, 58 (91%) of 64 patients were immunocompromised due to a primary immunodeficiency (PID), human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), a hematologic malignancy, or immunosuppressive medication, including 43 (74%) of 48 norovirus-positive patients, 7 (100%) of 7 astrovirus-positive patients, and 10 (91%) of 11 sapovirus-positive patients (Table 2). Of the 64 study patients, 35 (55%) patients had a PID; the most common PIDs were Common Variable Immune Deficiency (10/34, 29%) and X-linked Severe Combined Immune Deficiency (6/34, 18%). Hematologic malignancies were diagnosed in 20 (31%) patients, 39 (61%) had received immunosuppressive medication, and 3 (5%) patients were diagnosed with HIV/AIDS.
Among norovirus patients, 16 (33%) of 48 were identified with chronic excretion, with a median duration of 189 days (range 72–372; Table 1). Of these 16 patients, 15 were known to be immunocompromised due to one or more causes: 12 patients were diagnosed with PIDs, 4 had hematologic malignancies, and 3 had received immunosuppressive therapy. Of 7 astrovirus-positive patients, one had evidence of chronic excretion (132 days between positive samples). This patient was diagnosed with myelodysplastic syndrome and was receiving immunosuppressive therapy following a stem cell transplant. No sapovirus-positive patient had evidence of chronic excretion.
Coinfection with a non-viral pathogen was detected in at least one fecal sample from 20 (31%) of 64 patients, including 16 (33%) of 48 norovirus-positive patients, 2 (29%) of 7 astrovirus-positive patients, and 2 (18%) of 11 sapovirus-positive patients (Fig. 1). The two most commonly detected coinfecting pathogens were C. difficile and enteropathogenic E. coli (EPEC). Of 11 pediatric patients, 6 (55%) had coinfections, compared with 14 (26%) of 53 adult patients. However, this difference was not statistically significant.
Fig. 1.
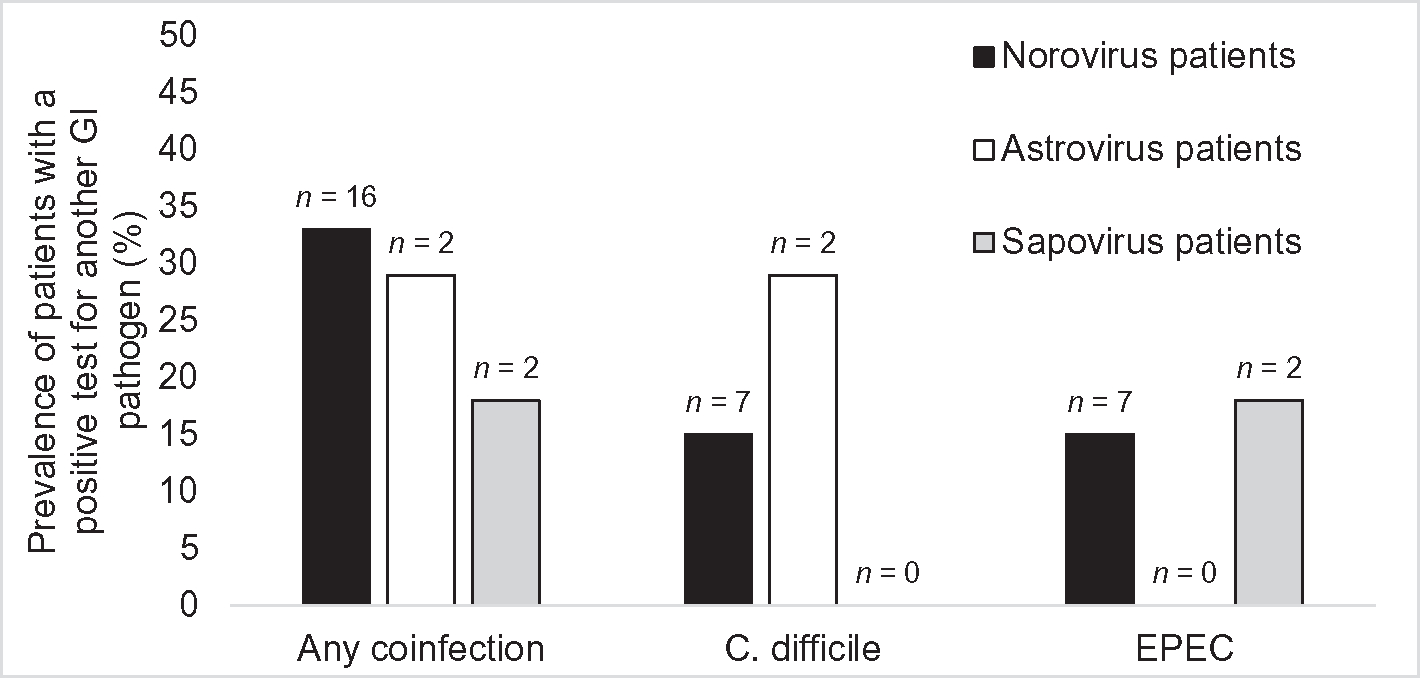
Prevalence of patients coinfected with another GI pathogen, among those with norovirus, astrovirus, or sapovirus. EPEC = Enteropathogenic E. coli; n = no. of coinfected patients.
4. Discussion
The prevalence of norovirus, astrovirus, and sapovirus found in this primarily immunocompromised patient population is similar to previous reports in adult populations. The prevalence of norovirus is slightly higher, at 11% compared to 4–6%, while the prevalence of astrovirus and sapovirus is 2% each, within the range of 1–2% in other studies (Grytdal et al., 2016; Hall et al., 2011). These estimates are also similar to the two published reports in pediatric immunocompromised patient populations, which found a prevalence of 10–15% for norovirus, and a prevalence of ≤6% for astrovirus and sapovirus (Brown et al., 2016; Osborne et al., 2015). These results indicate that sapovirus and astrovirus, although less common than norovirus, are important to the etiology of AGE in immunocompromised patients.
The high rate of coinfection (31%) found in this study is consistent with previous laboratory-based studies using the FilmArray, which have found rates of multiple pathogen detection of approximately 30% (Spina et al., 2015; Stockmann et al., 2016). The high prevalence of C. difficile coinfections was also consistent with past studies of norovirus epidemiology at this research hospital, which found that C. difficile was the most common coinfection among patients with norovirus, at 9% (Bok et al., 2016). This prevalence of coinfection is particularly relevant following recent epidemiologic studies in low and middle income settings, suggesting that synergy between coinfecting GI pathogens can increase the risk of diarrhea(Bhavnani et al., 2012), or increase the severity of AGE (Shrivastava et al., 2017; Zhang et al., 2016). Moreover, the increased detection of GI coinfections due to the expanded implementation of multiplex assays poses questions for interpretation of the results and clinical management of AGE patients, but a paucity of information is available on this topic (Liesman and Binnicker, 2016). Given the high rates of coinfection, further research is needed to address the role of coinfection in pathogenesis and clinical outcomes among immunodeficient and other populations.
Chronic excretion of norovirus or astrovirus was frequent in this primarily immunocompromised patient population, although such excretion was more common for norovirus. Because chronic excretion is often debilitating and sometimes fatal (Saif et al., 2011; Woodward et al., 2015; Wunderli et al., 2011), the frequent occurrence and prolonged duration of viral shedding observed in our study has important implications for proper clinical management of immunocompromised patients. The limitations of this study include the lack of viral genotype information, the relatively small sample size and the short time period of observation. As this is a retrospective study of data from clinical diagnostics, samples were not saved and therefore not available for genotyping. Thus, among patients with multiple positive samples, our methods could not distinguish between chronic infections and reinfections with different strains of the same pathogen. However, a previous study of norovirus among immunodeficient patients at this center, which did include genotyping, found that all patients with sequential norovirus-positive samples had chronic excretion of a single virus strain, rather than reinfection (Bok et al., 2016). Therefore, most of these sequential positive samples likely represent chronic infection rather than reinfection. The short time frame (14 months) of the study period likely led to an underestimation of both the prevalence and duration of chronic excretion, as some patients may have been excreting the virus either before or after the period captured. In addition, given the severity of disease in the Clinical Center patient population, these results may not be generalizable to immunodeficient populations at other centers. Nevertheless, the implementation of the multiplex assay allowed for the increased detection of previously underrecognized viral pathogens in this immunodeficient population, and highlighted new questions raised by this diagnostic technique, including the clinical interpretation of coinfections. Expanded implementation of the FilmArray GI panel will allow systematic screening to determine the true prevalence of astrovirus, sapovirus and other rarely assayed pathogens.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diagmicrobio.2018.05.017.
Supplementary Material
Acknowledgements
This study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and the NIH Clinical Center.
Footnotes
Competing Interests
The authors have no competing interests to declare.
References
- Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JNS. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern Ecuador. Am J Epidemiol 2012;176(5):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok K, Prevots DR, Binder AM, Parra GI, Strollo S, Fahle GA, et al. Epidemiology of norovirus infection among immunocompromised patients at a tertiary care research hospital, 2010–2013. Open Forum Infect Dis 2016;3, ofw169. [Oxford University Press; ]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR, Shah D, Breuer J. Viral gastrointestinal infections and norovirus genotypes in a paediatric UK hospital, 2014–2015. J Clin Virol 2016;84:1–6. [DOI] [PubMed] [Google Scholar]
- Grytdal SP, DeBess E, Lee LE, Blythe D, Ryan P, Biggs C, et al. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among Kaiser Permanente member populations in the United States, 2012–2013. PLoS One 2016;11(4), e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Rosenthal M, Gregoricus N, Greene SA, Ferguson J, Henao OL, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis 2011;17(8):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesman RM, Binnicker MJ. The role of multiplex molecular panels for the diagnosis of gastrointestinal infections in immunocompromised patients. Curr Opin Infect Dis 2016;29(4):359–65. [DOI] [PubMed] [Google Scholar]
- Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med 2016;13(4), e1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CM, Montano AC, Robinson CC, Schultz-Cherry S, Dominguez SR. Viral gastroenteritis in children in Colorado 2006–2009. J Med Virol 2015;87(6):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos-Weil D, Ambert-Balay K, Lanternier F, Mamzer-Bruneel MF, Nochy D, Pothier P, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation 2011;92(1):61–9. [DOI] [PubMed] [Google Scholar]
- Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, et al. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant 2011;15(5):505–9. [DOI] [PubMed] [Google Scholar]
- Shrivastava AK, Kumar S, Mohakud NK, Suar M, Sahu PS. Multiple etiologies of infectious diarrhea and concurrent infections in a pediatric outpatient-based screening study in Odisha, India. Gut Pathog 2017;9(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina A, Kerr KG, Cormican M, Barbut F, Eigentler A, Zerva L, et al. Spectrum of enteropathogens detected by the FilmArray GI panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect 2015;21(8):719–28. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Pavia AT, Graham B, Vaughn M, Crisp R, Poritz MA, et al. Detection of 23 gastrointestinal pathogens among children who present with diarrhea. J Pediatr Infect Dis Soc 2016;6(3):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten T, Cattaert T, Harris J, Lopman B, Tam CC, Ferreira G. Estimating the burden of medically attended norovirus gastroenteritis: modeling linked primary care and hospitalization datasets. J Infect Dis 2017;216(8):957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten T, Jiang B, Weil JG, Lin JH. Modelling estimates of norovirus disease in patients with chronic medical conditions. PLoS One 2016;11(7), e0158822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JM, Gkrania-Klotsas E, Cordero-Ng AY, Aravinthan A, Bandoh BN, Liu H, et al. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am J Gastroenterol 2015;110(2):320–7. [DOI] [PubMed] [Google Scholar]
- Wunderli W, Meerbach A, Guengoer T, Berger C, Greiner O, Caduff R, et al. Astrovirus infection in hospitalized infants with severe combined immunodeficiency after allogeneic hematopoietic stem cell transplantation. PLoS One 2011;6(11), e27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S-X, Zhou Y-M, Xu W, Tian L-G, Chen J-X, Chen S-H, et al. Impact of co-infections with enteric pathogens on children suffering from acute diarrhea in Southwest China. Infect Dis Poverty 2016;5(64). 10.1186/s40249-016-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


