Abstract
Acute gastroenteritis caused by noroviruses often has a duration of 2–3 days and is characteristically self-limiting. In contrast, chronic infection caused by noroviruses in immunocompromised individuals can last from weeks to years, making clinical management difficult. The mechanisms by which noroviruses establish persistent infection, and the role of immunocompromised hosts as a reservoir for noroviruses in the general human population, are not known. However, study of this patient cohort may lead to new insights into norovirus biology and approaches to treatment.
Keywords: Acquired immunodeficiency, chronic infection, immunocompromised, immunosuppressed, norovirus
Introduction
Noroviruses belong to the genus Norovirus, a large and diverse genus in the positive-strand RNA virus family Caliciviridae. The association of noroviruses with acute gastroenteritis is well established. The disease burden in the USA alone is an estimated 19–21 million episodes of gastroenteritis annually, with c. 400 000 emergency department visits, 56 000–71 000 hospitalizations, and as many as 570–800 deaths [1,2]. Noroviruses have been reported as the leading cause of severe diarrhoea in infants and young children requiring medical intervention in the USA, now that rotavirus vaccines have been successfully deployed [3,4]. Vaccines for noroviruses are not yet available, but recombinant virus-like particle vaccines have shown promise in clinical trials [5].
Noroviruses can establish a persistent infection in immunocompromised hosts, resulting in prolonged virus shedding and gastrointestinal disease that, over time, can become increasingly debilitating and life-threatening [6-8]. In a review of 123 deaths attributed to noroviruses, a serious underlying condition was reported for 17 individuals at the time of death, with ten (58%) of the deaths occurring in patients who were immunocompromised by chemotherapy or transplantation [9]. There is presently no virus-specific drug available to treat norovirus infection, although the urgent need for such drugs in immunocompromised patients has gained recent attention [8]. This review will focus on the current understanding of chronic norovirus infection that characteristically occurs in immunocompromised individuals, and the prospects for prevention and treatment.
Immunocompromised Patient Groups at Risk
Children
In early studies of the genetic diversity of noroviruses, the Toronto virus (formerly called ‘minireovirus’ and now classified as a reference GII.3 norovirus strain) was identified as a genetically distinct ‘Norwalk-like virus’ in sick children receiving care at the Children’s Hospital in Toronto, Canada [10]. Four of the 11 paediatric patients studied at the Children’s Hospital shedding norovirus were designated as immunosuppressed, with underlying conditions of leukaemia, post-liver transplant status, or severe combined immunodeficiency. Since then, an increasing number of case study descriptions have linked norovirus infection to conditions in children that are characteristically associated with immunosuppression, such as inherited immune disorders [11-13], small-bowel transplantation [14], kidney transplantation [15], haematopoietic stem cell transplantation (HSCT) [16,17], and cancer or cancer treatment [17-19]. Noroviruses have also been detected in children experiencing complications that may arise from immunosuppression. Haemophagocytic lymphohistiocytosis was reported recently in association with chronic norovirus infection of duration 40 days following bone marrow transplantation for treatment of relapsed myelogenous leukaemia in a 24-month-old child [20]. In a study of 27 paediatric patients with pneumatosis intestinalis, 17 (63%) were immunocompromised, with noroviruses being the predominant pathogens detected (23.5% prevalence) following screening for bacterial and viral agents [21]. The first study to systematically examine the prevalence of noroviruses in children with inherited immune deficiencies reported that noroviruses were the most commonly detected pathogens in the 62 children studied, and that shedding was prolonged, with 57.1% of faecal samples still being positive after a median of 9.5 months of follow-up [13]. It is of interest that the investigators reported evidence of norovirus viraemia in 25% of these paediatric cases. A retrospective study of diarrhoea in 55 haematopoietic stem cell transplant recipients aged <21 years showed that 49 developed diarrhoea and eight (6.3%) were positive for the presence of norovirus in stool [22]. The overall cumulative incidence of norovirus infection in this cohort was 12.9%, and the median time for norovirus clearance was 145 days (range: 13–263 days). A survey of two paediatric hospitals in the metropolitan Atlanta area that examined the prevalence of noroviruses [23] reported the detection of noroviruses in 15 of 92 (16.3%) of the patient stools tested. It was noteworthy that 11 of 15 (73.3%) of the norovirus-positive stools were obtained from immunocompromised patients (n = 47), indicating an overall norovirus prevalence of 23.4% in this group.
Adults
Chronic norovirus infection has been documented in adults following HSCT [24-27], kidney transplantation [28-31], heart transplantation [32], human immunodeficiency virus infection [33], and cancer or cancer treatment [18,34]. Elderly populations are at increased risk for a serious outcome from infectious diseases [35], including norovirus illness [36-38], with declining immune function thought to be a contributory factor.
Nosocomial Norovirus Infections
Risk factors in nosocomial outbreaks
Noroviruses constitute the leading cause of severe viral gastroenteritis prompting admission to a hospital emergency department [39]. A number of studies have also documented the important role of noroviruses in nosocomial infections. In a survey of 289 hospitals in the USA that had initiated outbreak investigations in the previous 12 months, noroviruses were the most frequently detected nosocomial pathogens, accounting for 53 of 291 (18.2%) of the investigated confirmed outbreaks and resulting in the highest rate of hospital unit closures (65%) [40]. An analysis of risk factors for norovirus disease in one university hospital found that immunocompromised patients were at increased risk for a severe clinical outcome following norovirus infection [38]. Prolonged shedding by immunocompromised individuals has been suggested as a source of virus strains for nosocomial infections [41]. A nosocomial outbreak of norovirus in a bone marrow transplant unit was attributed, in part, to the longer shedding periods and hospital stays of these patients [26]. An immunocompromised patient suffering from an acute norovirus infection was identified as an index case for the introduction of norovirus into a hospital transplantation care unit where the patient was admitted for treatment of complications resulting from graft-versus-host disease following HSCT [27].
Genetic diversity and evolution of noroviruses in nosocomial outbreaks
Persistent or chronic viral infections are known to be caused by many positive-strand RNA viruses, and they are well documented in members of the family Caliciviridae. Feline calicivirus (FCV), a member of the genus Vesivirus and an agent of upper respiratory illness in cats, can establish a chronic infection in cats, even in the presence of prior vaccination and an intact immune system [42]. The upper respiratory tract was proposed as a major site of FCV persistence [43]. Moreover, evidence was shown for the evolution of antigenic variation in FCV strains over time, suggesting the presence of selective pressure driven by an adaptive immune response [44]. Murine norovirus (MNV), which is more closely related to human noroviruses, can establish asymptomatic and persistent infection in mice, with both virus and mouse host differences being linked to chronic infection [45,46]. It has been proposed that the colon may be an important site of persistent MNV replication in mice [47,48], and it is of interest that lesions in the colon have been proposed as sites of virus replication in preterm babies with severe norovirus infection [49]. One study identified the presence of MNV antigen in the mesenteric lymph nodes of certain immunodeficient mice, suggesting lymphoid tissue as another site of persistent infection [50]. Possible adaptive changes during persistent MNV infection have been linked to the N-terminal open reading frame 1 protein (NS1/2) [48], the RNA-dependent RNA polymerase (NS7) [47], and the minor structural protein VP2 [47], but the mechanisms of norovirus persistence will require additional investigation.
Norovirus infection in immunocompromised patients can begin with an episode of acute gastroenteritis, but the inability to clear the virus can lead to chronic infection. There is presently no evidence for naturally occurring norovirus strains that have evolved to preferentially establish persistent infection in human hosts. The predominant norovirus genotypes reported in immunocompromised individuals belong to geno-group II, which is reflective of the predominance of this genotype as the cause of acute gastroenteritis in the general population. Table 1 summarizes representative studies, many in hospitals or transplant units, reporting the genotypes of strains associated with chronic infections in immunocompromised hosts.
TABLE 1.
Norovirus genotypes detected in immunocompromised patients
| Setting (country) | Underlying condition or treatment | Genotypes | Reference |
|---|---|---|---|
| Children’s hospital (Canada) | Cancer, liver transplantation, SCIDs | GII.3 | [10] |
| Hospital (UK) | Bone marrow transplantation | GII.4 | [52] |
| Hospital (UK) | HSCT | GII.3, GII.4, GII.7 | [24] |
| Hospital (Switzerland) | HSCT, lung transplantation | GII.4 | [25] |
| Hospital (The Netherlands) | Cancer, leukopenia, HSCT | GII.3, GII.4 | [17] |
| Hospital (Germany) | Cancer | GII.3, GII.4 | [19] |
| Individual patient (Sweden) | Heart transplantation | GII.3 | [32] |
| Hospital (USA) | Intestinal transplantation | GII.4 | [14] |
HSCT, haematopoietic stem cell transplantation; SCID, severe combined immunodeficiency.
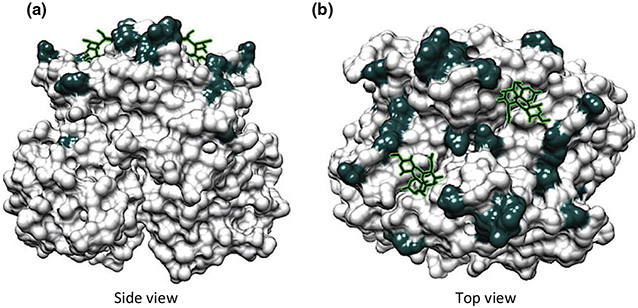
A growing number of outbreak investigations in hospitals have employed molecular techniques to elucidate the source and spread of noroviruses in nosocomial outbreaks [17,51-53]. An investigation of three separate norovirus transmission events resulting in gastroenteritis outbreaks in a hospital linked the source to three immunocompromised individuals with probable chronic shedding [41]. Sequence analysis of noroviruses shed over time in chronically ill patients may allow an in-depth understanding of virus adaptation and spread in the presence of little or no host immune pressure [54,55]. There is evidence that noroviruses in chronically infected immunocompromised patients accrue amino acid substitutions on the outer surface of the viral capsid over time that may change their antigenic profile [32,55] (Fig. 1). An important area of future investigation will be to determine whether these mutations are meaningful for treatment and clinical management.
FIG. 1.
Modelling of amino acid changes on the surface of a GII.4 norovirus capsid following chronic infection over a period of almost 1 year in an immunocompromised patient (adapted from reference [55]). (a) Side view of VP1 dimer. (b) Top view of VP1 dimer. Amino acids that differ over time are indicated in dark grey, and histo-blood group antigen-binding sites are shown as stick models. It is noteworthy that the majority of mutations are predicted to occur on the exposed surface of the viral capsid. A number of mutations correspond to antigenic changes as described in the cited article.
The infectivity and transmissibility of noroviruses shed by chronically infected individuals in comparison with those of noroviruses shed by individuals with acute infection has not been fully established. One model proposes that norovirus transmission is most efficient in the early phase of an acute norovirus infection [56], when virus titres in stools have been shown to be highest [57]. A detailed analysis of three nosocomial outbreaks in different facilities found that norovirus gastroenteritis cases were most often linked to transmission events involving symptomatic patients or healthcare workers, with asymptomatic individuals being less likely to transmit virus [58]. In healthy adult volunteers challenged with Norwalk virus, the peak of virus shedding (median concentration reported as 95 × 109 genomic copies per gram of faeces), occurred at a median of day 4 after inoculation, whether infection was symptomatic or asymptomatic, and the median duration of shedding was 28 days, well after symptoms had resolved [59]. The prevalence of asymptomatic infection in immunocompetent hosts has been estimated to be as high as 12% [60], indicating that it will be challenging to determine whether chronic shedders serve as a reservoir for the general population without intense epidemiological surveillance and viral genome sequencing. Immunocompromised children with norovirus infection were reported to shed median viral concentrations of 3.6 × 108 cDNA copies per gram of stool collected between 2 days and 14 days post-onset [19]. In another study that examined norovirus viral loads in highly immunosuppressed patients and children, the median viral load was reported to be 2.9 × 107 genomic copies per milliliter of stool [61]. Further study of viral load and evolution in immunocompromised individuals will be needed to correlate virus titre with symptoms, severity of disease, and efficiency of transmission [62].
Treatment and Control of Norovirus Infection in Immunocompromised Hosts
Treatment
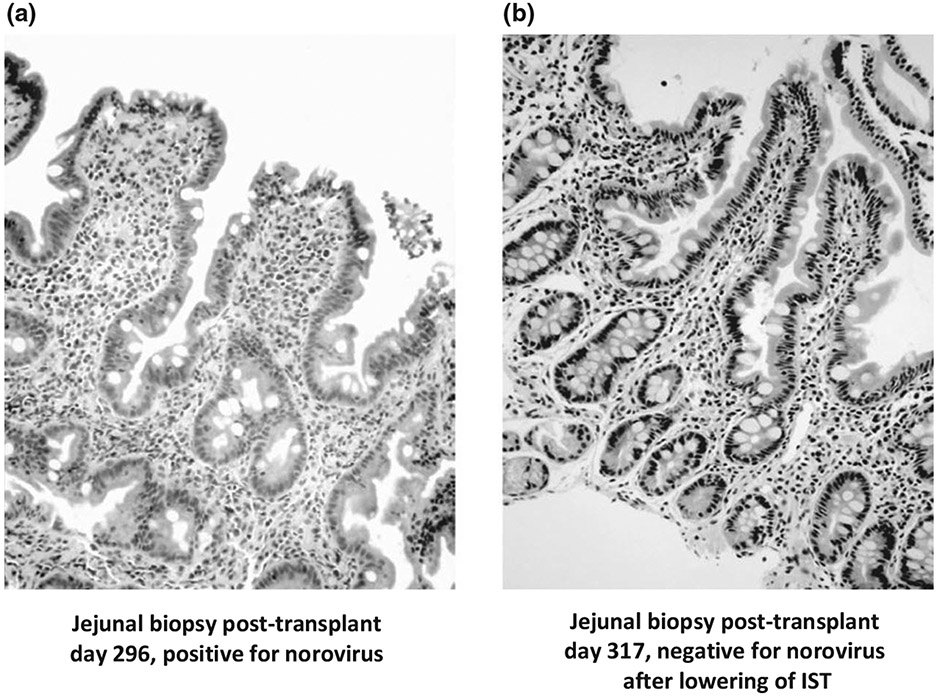
There is currently no specific antiviral drug for the treatment of norovirus gastroenteritis. Supportive fluid replacement therapy can be used to prevent dehydration, and nutritional supplementation may be required [6]. Nausea and diarrhoea, which are hallmarks of norovirus disease, are common in immunocompromised patients, making laboratory confirmation of norovirus infection an important diagnostic tool in patient management. For example, diarrhoea occurred in 79% of adult allogeneic stem cell transplant recipients in one study, but the aetiology could not be linked to any known aetiological infectious agent [63]. Conditioning therapy prior to transplantation and complications following transplantation, such as graft-versus-host disease, may cause diarrhoea [6,27]. A number of treatment strategies have been reported to improve chronic norovirus infection in immunocompromised individuals, including adjustment of immunosuppressive therapy (IST) drug types and dose levels [14,24,25,29,30], drugs approved for other pathogens [34], and passive γ-globulin therapy [31,33,64]. The recovery of normal intestinal morphology following reduction of IST in a norovirus-infected child who had undergone a small-bowel transplant is shown in Fig. 2. In mice, it was possible to clear persistent MNV infection in immunocompromised animals [59] with neutralizing antibodies, showing the potential benefit of passive immunotherapy [65]. However, there are also reports of treatment failure with breast milk, γ-globulin and IST adjustment in one immunocompromised patient [32].
FIG. 2.
Intestinal biopsy of a paediatric small-intestine transplant patient. (a) During acute norovirus infection at day 296 post-transplant. (b) After resolution of symptoms at day 317 post-transplant following reduction of immunosuppressive therapy (IST), which allowed virus clearance. Note the severe blunting of intestinal villi during acute infection, and the regeneration of normal intestinal morphology after virus clearance. Adapted from reference [14].
Infection control
Noroviruses are highly infectious and stable in the environment. A review of transmission routes in 54 nosocomial outbreaks identified person-to-person spread (18.5%) and foodborne sources (3.7%) as the major known routes, but a clear route of transmission could not be identified in the majority (77.8%) of the outbreaks [66]. Infection control has remained the first line of defence in reducing norovirus exposure and spread [67], and is especially important in the immunocompromised population [7]. Hospitals and long-term-care facilities caring for immunocompromised patients should adhere strictly to rigorous infection control practices, to limit exposure of patients, staff and visitors to noroviruses [68]. Immunocompromised individuals should follow food safety guidelines that minimize exposure to contaminated food and water, and avoid contact with individuals who might be ill. The US CDC has published online guidelines for the prevention of norovirus infection, including management in healthcare settings www.cdc.gov/norovirus/index.html and resources on effective disinfectants. Infection control strategies with the most positive outcomes have been reviewed in the nosocomial setting [66], and include restricting movement of patients and staff, enhanced environmental cleaning, and attention to hand-washing. The practice of ‘presenteesim’ (a term for working while sick) should be avoided by healthcare workers managing immunocompromised patients: this practice has been linked to norovirus outbreaks in settings such as a bone marrow transplantation unit [26].
Vaccines
Norovirus vaccine development and evaluation is underway [69], but it will probably be several more years before licensed vaccines are available [70]. Immune correlates are not fully understood, but it is presumed that the adaptive immune system, including antibodies, CD4 lymphocytes, and CD8 lymphocytes, is critical in protection [46,71]. This is supported by data from a norovirus vaccine study that showed a correlation between the presence of serum antibodies with a histo-blood group antigen blocking titre of at least 200 and a reduced frequency of viral illness and infection upon virus challenge [69]. A norovirus vaccine may be of limited use in the general immunocompromised population with debilitated T-cell and B-cell function, with the exception of patients who would be eligible for incorporation of norovirus vaccination (when available) into the panel of recommended vaccines administered prior to or following organ or stem cell transplantation and IST [72]. Effective norovirus vaccines that lower the overall disease prevalence in the general population or in healthcare workers could indirectly benefit immunodeficient individuals by lowering the risk of exposure and subsequent infection.
Antiviral drugs
Noroviruses associated with human disease do not grow in cell culture, so drug design and screening have relied primarily on recombinant DNA-based research tools (such as recombinant norovirus replicative enzymes) or cell culture-adapted calicivirus models, such as MNV or FCV. A number of compounds, inhibitors and therapeutic antibodies are under investigation in the basic research setting [8,73]. Promising candidate drugs for the noroviruses will require safety and efficacy testing in adult volunteer challenge studies, and a proposed design of clinical drug trials in immunocompromised patients has recently been described [8].
Future Perspectives
Noroviruses are increasingly being recognized to constitute a major risk factor for debilitating diarrhoea and chronic gastroenteritis in immunocompromised patients. It is not known whether these individuals play a major role in the epidemiology of the virus, or are responsible for only sporadic and occasional transmission. There is a need for effective treatment in this special at-risk population to clear chronic norovirus infection and restore normal intestinal function. Such treatment may also offer benefits to patient groups who are at risk from acute life-threatening diarrhoea, including infants, young children, and the elderly.
Acknowledgements
I would like to thank R. Prevots, S. Sosnovtsev and G. I. Parra of NIAID, NIH for critical reading and contributions to this manuscript. K. Green is supported by the Intramural Research Program of the NIAID, NIH, USA.
Footnotes
Transparency Declaration
The author has no conflict of interest to declare in relation to this article.
References
- 1.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting—United States, 2001–2009. J Infect Dis 2013; 207: 1058–1065. [DOI] [PubMed] [Google Scholar]
- 2.Hall AJ, Lopman BA, Payne DC et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19: 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne DC, Vinje J, Szilagyi PG et al. Norovirus and medically attended gastroenteritis in US children. N Engl J Med 2013; 368: 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo HL, Neill FH, Estes MK et al. Noroviruses: the most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J Pediatric Infect Dis Soc 2013; 2: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atmar RL, Estes MK. Norovirus vaccine development: next steps. Expert Rev Vaccines 2012; 11: 1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med 2012; 367: 2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atmar J, Mullen E. Norovirus in immunocompromised patients. Oncol Nurs Forum 2013; 40: 434–436. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman SS, Green KY, Korba BE. Treatment of norovirus infections: moving antivirals from the bench to the bedside. Antiviral Res 2014; 105C: 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control 2013; 41: 654–657. [DOI] [PubMed] [Google Scholar]
- 10.Lew JF, Petric M, Kapikian AZ, Jiang X, Estes MK, Green KY. Identification of minireovirus as a Norwalk-like virus in pediatric patients with gastroenteritis. J Virol 1994; 68: 3391–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xerry J, Gallimore CI, Cubitt D, Gray JJ. Tracking environmental norovirus contamination in a pediatric primary immunodeficiency unit. J Clin Microbiol 2010; 48: 2552–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallimore CI, Lewis D, Taylor C, Cant A, Gennery A, Gray JJ. Chronic excretion of a norovirus in a child with cartilage hair hypoplasia (CHH). J Clin Virol 2004; 30: 196–204. [DOI] [PubMed] [Google Scholar]
- 13.Frange P, Touzot F, Debre M et al. Prevalence and clinical impact of norovirus fecal shedding in children with inherited immune deficiencies. J Infect Dis 2012; 206: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman SS, Chatterjee NK, Fushino ME, et al. Calicivirus enteritis in an intestinal transplant recipient. Am J Transp 2003; 3: 764–768. [DOI] [PubMed] [Google Scholar]
- 15.Chehade H, Girardin E, Delich V, Pascual MA, Venetz JP, Cachat F. Acute norovirus-induced agranulocytosis in a pediatric kidney transplant recipient. Transpl Infect Dis 2012; 14: E27–E29. [DOI] [PubMed] [Google Scholar]
- 16.Saif MA, Bonney DK, Bigger B et al. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant 2011; 15: 505–509. [DOI] [PubMed] [Google Scholar]
- 17.Siebenga JJ, Beersma MF, Vennema H, van Biezen P, Hartwig NJ, Koopmans M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J Infect Dis 2008; 198: 994–1001. [DOI] [PubMed] [Google Scholar]
- 18.Simon A, Schildgen O, Maria Eis-Hubinger A et al. Norovirus outbreak in a pediatric oncology unit. Scand J Gastroenterol 2006; 41: 693–699. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig A, Adams O, Laws HJ, Schroten H, Tenenbaum T. Quantitative detection of norovirus excretion in pediatric patients with cancer and prolonged gastroenteritis and shedding of norovirus. J Med Virol 2008; 80: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 20.Salvador C, Meister B, Larcher H, Crazzolara R, Kropshofer G. Hemophagocytic lymphohistiocytosis after allogeneic bone marrow transplantation during chronic norovirus infection. Hematol Oncol 2014; 32: 102–106. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Kim YJ, Lee JH et al. Norovirus: a possible cause of pneumatosis intestinalis. J Pediatr Gastroenterol Nutr 2011; 52: 314–318. [DOI] [PubMed] [Google Scholar]
- 22.Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 2012; 18: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 23.Munir N, Liu P, Gastanaduy P, Montes J, Shane A, Moe C. Norovirus infection in immunocompromised children and children with hospital-acquired acute gastroenteritis. J Med Virol 2014; 86: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 24.Roddie C, Paul JP, Benjamin R et al. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis 2009; 49: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 25.Boillat Blanco N, Kuonen R, Bellini C et al. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl Infect Dis 2011; 13: 213–215. [DOI] [PubMed] [Google Scholar]
- 26.Doshi M, Woodwell S, Kelleher K, Mangan K, Axelrod P. An outbreak of norovirus infection in a bone marrow transplant unit. Am J Infect Control 2013; 41:820–823. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz S, Vergoulidou M, Schreier E et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 2011; 117: 5850–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westhoff TH, Vergoulidou M, Loddenkemper C et al. Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant 2009; 24: 1051–1053. [DOI] [PubMed] [Google Scholar]
- 29.Schorn R, Hohne M, Meerbach A et al. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin Infect Dis 2010; 51: 307–314. [DOI] [PubMed] [Google Scholar]
- 30.Roos-Weil D, Ambert-Balay K, Lanternier F et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation 2011; 92: 61–69. [DOI] [PubMed] [Google Scholar]
- 31.Chagla Z, Quirt J, Woodward K, Neary J, Rutherford C. Chronic norovirus infection in a transplant patient successfully treated with enterally administered immune globulin. J Clin Virol 2013; 58: 306–308. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson M, Hedlund KO, Thorhagen M et al. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J Virol 2003; 77: 13117–13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wingfield T, Gallimore CI, Xerry J et al. Chronic norovirus infection in an HIV-positive patient with persistent diarrhoea: a novel cause. J Clin Virol 2010; 49: 219–222. [DOI] [PubMed] [Google Scholar]
- 34.Siddiq DM, Koo HL, Adachi JA, Viola GM. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect 2011; 63: 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crossley K, Peterson PK. Infections in the elderly—new developments. Curr Clin Top Infect Dis 1998; 18: 75–100. [PubMed] [Google Scholar]
- 36.van Asten L, Siebenga J, van den Wijngaard C et al. Unspecified gastroenteritis illness and deaths in the elderly associated with norovirus epidemics. Epidemiology 2011; 22: 336–343. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi TK, DeSalvo T, Lee L et al. Hospitalizations and mortality associated with norovirus outbreaks in nursing homes, 2009–2010. JAMA 2012; 308: 1668–1675. [DOI] [PubMed] [Google Scholar]
- 38.Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect 2006; 12: 69–74. [DOI] [PubMed] [Google Scholar]
- 39.Bresee JS, Marcus R, Venezia RA et al. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis 2012; 205: 1374–1381. [DOI] [PubMed] [Google Scholar]
- 40.Rhinehart E, Walker S, Murphy D, O’Reilly K, Leeman P. Frequency of outbreak investigations in US hospitals: results of a national survey of infection preventionists. Am J Infect Control 2012; 40: 2–8. [DOI] [PubMed] [Google Scholar]
- 41.Sukhrie FH, Siebenga JJ, Beersma MF, Koopmans M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J Clin Microbiol 2010; 48: 4303–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen NC, Hawkins KF. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination. Vet Microbiol 1995; 47: 141–156. [DOI] [PubMed] [Google Scholar]
- 43.Dick CP, Johnson RP, Yamashiro S. Sites of persistence of feline calicivirus. Res Vet Sci 1989; 47: 367–373. [PubMed] [Google Scholar]
- 44.Johnson RP. Antigenic change in feline calicivirus during persistent infection. Can J Vet Res 1992; 56: 326–330. [PMC free article] [PubMed] [Google Scholar]
- 45.Wobus CE, Thackray LB, Virgin HW. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 2006; 80: 5104–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomov VT, Osborne LC, Dolfi DV et al. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J Virol 2013; 87: 7015–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arias A, Bailey D, Chaudhry Y, Goodfellow I. Development of a reverse-genetics system for murine norovirus 3: long-term persistence occurs in the caecum and colon. J Gen Virol 2012; 93 (Pt 7): 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nice TJ, Strong DW, McCune BT, Pohl CS, Virgin HW. A single-amino-acid change in murine norovirus NS1/2 is sufficient for colonic tropism and persistence. J Virol 2013; 87: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelizzo G, Nakib G, Goruppi I et al. Isolated colon ischemia with norovirus infection in preterm babies: a case series. J Med Case Rep 2013; 7: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward JM, Wobus CE, Thackray LB et al. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol Pathol 2006; 34: 708–715. [DOI] [PubMed] [Google Scholar]
- 51.Gallimore CI, Cubitt DW, Richards AF, Gray JJ. Diversity of enteric viruses detected in patients with gastroenteritis in a tertiary referral paediatric hospital. J Med Virol 2004; 73: 443–449. [DOI] [PubMed] [Google Scholar]
- 52.Kundu S, Lockwood J, Depledge DP et al. Next-generation whole genome sequencing identifies the direction of norovirus transmission in linked patients. Clin Infect Dis 2013; 57: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukhrie FH, Beersma MF, Wong A et al. Using molecular epidemiology to trace transmission of nosocomial norovirus infection. J Clin Microbiol 2011; 49: 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bull RA, Eden JS, Luciani F, McElroy K, Rawlinson WD, White PA. Contribution of intra- and interhost dynamics to norovirus evolution. J Virol 2012; 86: 3219–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debbink K, Lindesmith LC, Ferris MT et al. Within host evolution results in antigenically distinct GII.4 noroviruses. J Virol 2014; 88: 7244–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zelner JL, Lopman BA, Hall AJ, Ballesteros S, Grenfell BT. Linking time-varying symptomatology and intensity of infectiousness to patterns of norovirus transmission. PLoS One 2013; 8: e68413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis 1994; 170: 34–43. [DOI] [PubMed] [Google Scholar]
- 58.Sukhrie FH, Teunis P, Vennema H et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis 2012; 54: 931–937. [DOI] [PubMed] [Google Scholar]
- 59.Atmar RL, Opekun AR, Gilger MA et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008; 14: 1553–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips G, Tam CC, Rodrigues LC, Lopman B. Prevalence and characteristics of asymptomatic norovirus infection in the community in England. Epidemiol Infect 2010; 138: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 61.Henke-Gendo C, Harste G, Juergens-Saathoff B, Mattner F, Deppe H, Heim A. New real-time PCR detects prolonged norovirus excretion in highly immunosuppressed patients and children. J Clin Microbiol 2009; 47: 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. Diagnosing norovirus-associated infectious intestinal disease using viral load. BMC Infect Dis 2009; 9: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Kraaij MG, Dekker AW, Verdonck LF et al. Infectious gastro-enteritis: an uncommon cause of diarrhoea in adult allogeneic and autologous stem cell transplant recipients. Bone Marrow Transplant 2000; 26: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Florescu DF, Hermsen ED, Kwon JY et al. Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr Transplant 2011; 15: 718–721. [DOI] [PubMed] [Google Scholar]
- 65.Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HW. Antibody is critical for the clearance of murine norovirus infection. J Virol 2008; 82: 6610–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greig JD, Lee MB. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect 2012; 140: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 67.MacCannell T, Umscheid CA, Agarwal RK, Lee I, Kuntz G, Stevenson KB. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol 2011; 32: 939–969. [DOI] [PubMed] [Google Scholar]
- 68.Dew K, Keefe V, Small K. ‘Choosing’ to work when sick: workplace presenteeism. Soc Sci Med 2005; 60: 2273–2282. [DOI] [PubMed] [Google Scholar]
- 69.Atmar RL, Bernstein DI, Harro CD et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 2011; 365: 2178–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debbink K, Lindesmith LC, Baric RS. The state of norovirus vaccines. Clin Infect Dis 2014; 58: 1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang H, Tan M, Xia M, Wang L, Jiang X. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS One 2013; 8: e63269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danziger-Isakov L, Kumar D. Vaccination in solid organ transplantation. Am J Transpl 2013; 13(suppl 4): 311–317. [DOI] [PubMed] [Google Scholar]
- 73.Rohayem J, Bergmann M, Gebhardt J et al. Antiviral strategies to control calicivirus infections. Antiviral Res 2010; 87: 162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]