Abstract
This study evaluated the immediate and 1-year postoperative outcomes of 14 patients with ruptured Valsalva aneurysmal sinus (RSVA) using symmetric ventricular septal defect (VSD) occluder for transcatheter closure (TCC). The sites of rupture were from the non-coronary sinus to the right atrium (RA) in 10 cases (71.4%), the right coronary sinus (RCS) to the RA in 3 cases (21.4%) and the RCS to the right ventricle in 1 case (7.2%). The defects (5-11 mm) were closed with a symmetrical VSD device. During the follow-up (12 months), the enlarged heart of the patients had significantly shrunk and the NYHA improved after closure. In 1 case, a moderate residual shunt was present and the patient suffered from hemolysis at 2 h after the operation, and 1 patient was transferred to surgery for aortic regurgitation 1 year after the initial treatment of RSVA. In conclusion, the TCC of RSVA with the China made symmetrical VSD occluder is safe and effective.
Keywords: rupture of sinus of Valsalva aneurysm, transcatheter closure, symmetrical closure devices
Introduction
Sinus of Valsalva aneurysm is a rare congenital cardiovascular malformation, accounting for 0.1-3.5% of congenital heart disease cases (1). Previous studies have revealed that 40-70% of patients develop a ruptured sinus of Valsalva aneurysm (RSVA), which frequently results in a large left-to-right shunt from the aorta to the cardiac level and may cause heart failure or sudden mortality (2). According to a previous study, RSVA was more likely to involve the right coronary sinus (RCS) (70%) and the non-coronary sinus (NCS; 29%) and less than 1% involved the left coronary sinus (3). Surgery used to be the primary treatment for RSVA. However, surgical procedures may result in a high incidence of aortic regurgitation and residual leakage due to invasive injury to the sinus of Valsalva (4). Therefore, Cullen et al (5) first conducted transcatheter closure (TCC) of RSVA in 1994, and with the improvement of interventional techniques and the advances in interventional devices, there have been an increasing number of reports on the TCC of RSVA globally (6). Currently, as far as we know, there is not a specialized occluder for RSVA and the majority of interventional therapies use the Amplatzer ventricular septal defect (VSD) or patent ductus arteriosus (PDA) occluders (7-9). Domestic symmetrical VSD devices are widely used due to their light weight, complete occlusion and low incidence of severe atrioventricular block. However, it is rarely reported for the treatment of RSVA (10). Since 2012, symmetrical VSD closure has been used to treat RSVA through catheterization at Xijing Hospital (Xi'an, China), and determination of immediate results and 12-month follow-ups after treatment were conducted to evaluate its safety and effectiveness.
Patients and methods
Research subjects
This retrospective study was approved by the ethics committee of Xijing Hospital, Air Force Medical University (Xi'an, China; approval no. KY20172078-C-1) and was undertaken in compliance with the tenets of the Declaration of Helsinki and its later amendments for research on human subjects. All participants provided their written informed consent to participate in the present study. A review of the patients' medical records was performed and patients aged 18 years or older and had an RSVA confirmed using thoracic echocardiography were included in the present study. The exclusion criteria were as follows: RSVA outlet >8 mm or with aortic regurgitation, hemodynamic instability, severe pulmonary hypertension due to RSVA, associated with active infective endocarditis, severe liver and kidney dysfunction and coagulation dysfunction. Percutaneous intervention was performed after obtaining written informed consent for the procedure. The final dataset included 14 consecutive patients treated at Xijing Hospital (Xi'an, China) between January 2012 and March 2022.
Operation methods and procedures
All patients underwent clinical examination, including a chest X-ray, electrocardiogram and transthoracic echocardiography (TTE) with color Doppler interrogation. The diameter of the inner and outer orifices and blood flow velocity of RSVA were measured by transthoracic ultrasound at the aortic terminal and rupture site. Huayi symmetrical VSD device (Beijing Huayishengjie Technology Co., Ltd; http://www.starwaymedical.com) and Memory VSD device (Shanghai Shape Memory Co., Ltd.; http://www.shsma.com) were used because these two types of occluders are widely used in the treatment of ventricular septal deficiency in China. All patients received 4 mg/kg aspirin one day prior to the operation. The procedure was conducted under local anesthesia. The femoral vein and artery were accessed. Intravenous heparin (100 IU/kg) and cefazolin were administered. RSVA was measured at its aortic and rupture site with both TTE and angiography. The following steps were then performed: i) A femoral artery-ascending aorta - aortic sinus aneurysm rupture-femoral vein track was established, and a 9-10 F sheath tube was placed along the medial femoral vein of the track; ii) VSD occluders 4-6 mm larger than the rupture site diameter of the RSVA, were generally selected, and the occluders were placed in the RSVA under the guidance of echocardiography; and iii) echocardiography and aortography were performed again to observe the closure effect and function of the aortic valve. After the sealing effect was confirmed, the occluder was released. A total of eight Memory and six Huayi muscular VSD occluders were applied. Representative examples for Memory devices are provided in Fig. 1.
Figure 1.
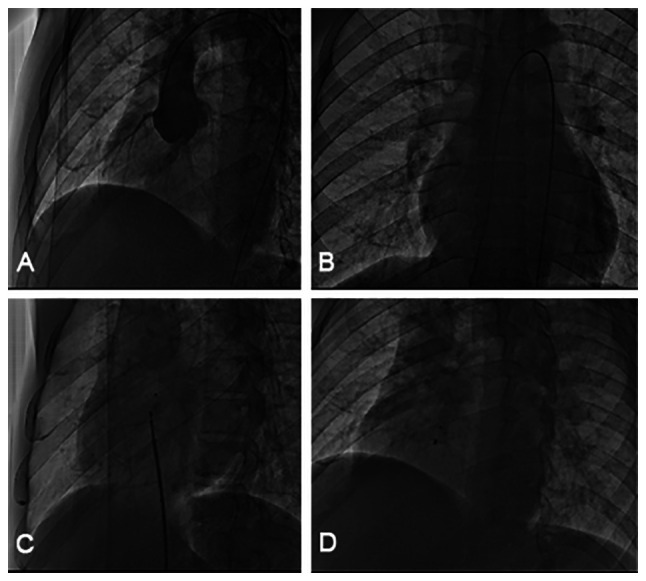
Operation of the transcatheter closure of the RSVA. (A) Aortic root angiography revealed no coronary sinus aneurysm rupturing into the right atrium. (B) A stable arteriovenous wire loop was established. (C) The RSVA was blocked using a Memory ventricular septal defect occluder delivered via the arteriovenous track. (D) Complete occlusion after device placement. RSVA, ruptured sinus of Valsalva aneurysm.
Follow-up procedure
After TCC, aspirin 4 mg/kg was used to prevent platelet aggregation for 6 months. Electrocardiogram and TTE were performed on the first day after closure. Echocardiography was then performed regularly 1, 6 and 12 months after the procedure (Fig. 2).
Figure 2.
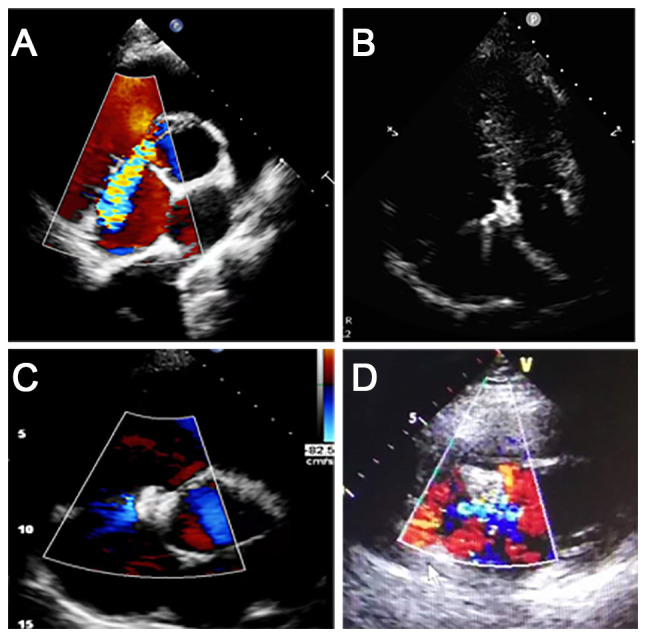
Transthoracic ultrasound follow-up after transcatheter closure. (A) Preoperative chest echocardiography revealed that the ruptured sinus of Valsalva aneurysm had broken into the non-coronary sinus. (B) TTE revealed a complete closure of the breach 1 month after the operation. (C) TTE revealed a small residual shunt 6 months after the operation. (D) TTE revealed a small residual shunt 12 months after the operation. TTE, transthoracic echocardiography.
Statistical analysis
Statistical analysis was conducted using SPSS (version 19.0; IBM Corp.) software. Repeated-measures ANOVA followed by the Bonferroni test was used for the two sets of heart size data. An independent-samples unpaired t-test was used to compare operation time between the two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
General characteristics of patients
A total of 14 patients were included in the present study, comprising 10 males and 4 females, with an age range of 24-66 years (mean, 38±13 years). Among them, 10 patients (71.4%) had an NCS rupture into the right atrium (RA), 3 patients (21.4%) had an RCS rupture into the RA and 1 patient (7.2%) had an RCS rupture into the right ventricle (RV). Furthermore, 1 patient had atrial fibrillation, 1 patient had patent foramen ovale (PFO) and VSD, and 1 patient had VSD. The mean diameter of the defect at the aortic end was 8.56±1.63 mm (median, 9.00 mm) and the mean diameter at the rupture site measured 5.06±1.18 mm (median, 5.00 mm). The mean procedure time was 61±17 min. All patients completed a 12-month follow-up. The patient characteristics were summarized in Table I.
Table I.
Clinical, echocardiographic and baseline variables, procedural variables, and immediate and mid-term outcomes.
| Case no. | Age, years/sex | NYHA class | Associated diseases | Defect location | Defect size by ECHO, inner/outer opening, mm | Closure device size, mm | Device supplier | Operation time, min | Residual shunt at discharge/follow-up | AR at discharge/ follow-up | NYHA class at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24/M | II | None | NCS to RA | 9/5 | VSD16 | Memory | 70 | Middle/none | Small/small | I |
| 2 | 50/M | II | VSD | NCS to RA | 8/4 | VSD12 | Memory | 55 | None/none | Small/small | I |
| 3 | 28/F | III | None | NCS to RA | 10/7 | VSD16 | Huayi | 60 | None/none | None/small | I |
| 4 | 27/F | III | None | NCS to RA | 8.7/5.7 | VSD14 | Memory | 90 | None/none | None/none | I |
| 5 | 43/M | IV | AF | NCS to RA | 9/4.5 | VSD16 | Huayi | 85 | None/none | None/small | II |
| 6 | 40/M | II | HTN | NCS to RA | 7/4 | VSD12 | Memory | 40 | None/none | Small/none | I |
| 7 | 35/M | III | None | NCS to RA | 10/4.4 | VSD16 | Memory | 30 | None/none | None/none | I |
| 8 | 48/M | II | None | NCS to RA | 10/4.6 | VSD16 | Memory | 50 | None/none | Middle/small | I |
| 9 | 66/F | II | PFO, VSD | NCS to RV | 5/3 | VSD14 | Memory | 75 | None/none | None/small to middle | I |
| 10 | 48/F | III | None | RCS to RA | 7.2 | VSD10 | Memory | 65 | None/none | None/small | I |
| 11 | 51/M | II | None | RCS to RA | 10/7 | VSD16 | Huayi | 45 | None/none | None/small | I |
| 12 | 23/M | II | None | RCS to RA | 7/5.6 | VSD16 | Huayi | 80 | None/none | None/none | I |
| 13 | 23/M | II | None | NCS to RA | 8/5 | VSD14 | Huayi | 60 | None/none | None/none | I |
| 14 | 31/M | II | PFO | NCS to RA | 11/6 | VSD16 | Huayi | 45 | None/none | None/none | I |
M, male; F, female; NYHA, New York Heart Association; ECHO, echocardiography; AR, aortic regurgitation; NCS, non-coronary sinus; RCS, right coronary sinus; RA, right atrium; RV, right ventricle; VSD, ventricular septal defect; AF, atrial fibrillation; HTN, hypertension; PFO, patent foramen ovale; Huayi, Beijing Huayishengjie Technology Co., Ltd.; Memory, Shanghai Shape Memory Co., Ltd.
Deployment success and complications
All 14 patients were successfully treated with the domestic symmetrical VSD closure. All of the patients were implanted once, and after the procedure, there were no instances of device displacement, the device falling off, device thrombosis or ineffective endocarditis. Furthermore, the dyspnea of the patients was reduced, edema disappeared and the grade of the NYHA class was improved.
Hemolysis and hemoglobinuria occurred in 1 patient (case 1) 5 h after intervention and a moderate residual shunt was revealed using thoracic echocardiography. Therefore, dexamethasone 10 mg was administered along with rehydration and alkaline urination, and the patient recovered on day 10 after conservative treatment. In the present study, 1 patient (case 5) was transferred to surgery for aortic regurgitation 1 year after the initial treatment of RSVA. A total of 5 patients developed procedure-related aortic regurgitation.
Heart cavity after closure
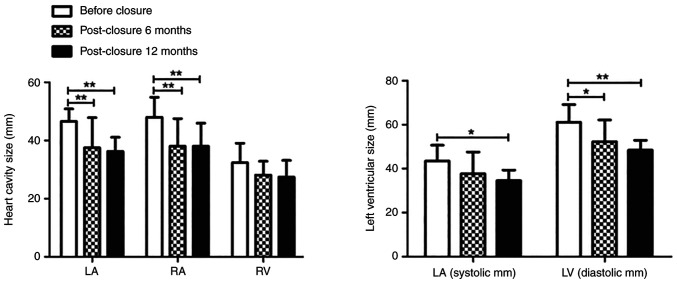
The results illustrated that the left atrium, RA, LV (systolic) and LV (diastolic) were significantly reduced 6 and 12 months after the operation compared with that before the TCC (F=5.66, P=0.02; F=17.80, P<0.001; F=6.72, P=0.01; F=8.95, P<0.001; respectively). There was no statistically significant difference in the RV change 6 and 12 months after the operation compared with that before the TCC (Table II and Fig. 3).
Table II.
Heart cavity after closure.
| Heart cavity | Before closure | Post-closure 6 months | Post-closure 12 months | F-value | P-value |
|---|---|---|---|---|---|
| LA, mm | 46.57±4.31 | 37.53±10.36 | 36.28±4.86 | 5.66 | 0.02 |
| RA, mm | 48.00±6.88 | 38.00±9.55 | 38.00±7.98 | 17.80 | <0.001 |
| RV, mm | 32.36±6.73 | 28.15±4.72 | 27.43±5.74 | 1.44 | 0.27 |
| Systolic LV, mm | 43.50±7.23 | 37.70±9.86 | 34.57±4.79 | 6.72 | 0.01 |
| Diastolic LV, mm | 61.07±8.12 | 52.31±9.84 | 48.43±4.50 | 8.95 | <0.001 |
LA, left atrium; RA, right atrium; RV, right ventricle; LV, left ventricle.
Figure 3.
Size of cardiac cavities 6 and 12 months after occlusion. The size of the LA (mm), RA (mm) and LV (mm) reduced significantly 6 and 12 months after occlusion. The RV (mm) did not significantly change. *P<0.05; **P<0.01. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
Effects of the two types of devices
In the present study, two domestic symmetrical VSD devices were used and there were no significant differences in the operation time (F=0.31; P=0.76) (Table III).
Table III.
Effect comparison of the two types of device.
| Device supplier | Number of times used in operation | Operation time, min | Residual shunt | Aortic regurgitation | F-value | P-value |
|---|---|---|---|---|---|---|
| Memory | 8 | 59±20 | 1 | 2 | 0.31 | 0.76 |
| Huayi | 6 | 63±17 | 0 | 3 |
Huayi, Beijing Huayishengjie Technology Co., Ltd.; Memory, Shanghai Shape Memory Co., Ltd.
Concomitant congenital heart disease
In the present study, there were 2 cases with VSD. Due to the defect being small (1.3 mm), 1 case was not treated. In the other case, the patient had PFO and VSD, and the sinus aneurysm of the aorta ruptured into the right ventricle; therefore, the VSD occluder blocked both the rupture and the VSD. Angiography revealed the disappearance of residual shunt and auscultation of cardiac murmur. Echocardiographic re-examination revealed that the position of the occluder was normaland no residual shunt was revealed in the VSD.
Discussion
RSVA is mainly caused by congenital dysplasia of elastic fibers in the middle of the aortic root and is more common in males compared with females (ratio, ~4:1) (11). Aortic sinus aneurysm mainly involves the RCS (~70%), followed by the NCS (~29%) and rarely involves the left coronary sinus (3). The occurrence of these aneurysms may be secondary due to events and conditions (including trauma, bacterial endocarditis, syphilis, cystic medial necrosis and atherosclerosis) that weaken the juncture between the media and the annulus fibrosus of the aorta (12). Most frequently, it is congenital in origin (13) due to either a congenital absence of continuity between the aortic media and the annulus fibrosis, or a developmental structural defect in the aortic annulus itself, which may gradually give way under aortic pressure to form an aneurysm. Aortic sinus aneurysm may rupture into the RA, RV, pulmonary artery, left ventricle or pericardial cavity, and usually manifest as a sudden chest pain or acute heart failure (14). The disease process of either ruptured or non-ruptured supraventricular arrhythmias (SVAs) is difficult to determine due to the rarity of these lesions. Adams et al (15) revealed that the mean survival of patients with untreated ruptured SVA was 3.9 years. Therefore, early intervention is required in these patients (16).
Since 1994, when Cullen et al first carried out transcatheter intervention to block RSVA, transcatheter closure has been widely applied for its clinical advantages and economic benefits (5,17). However, due to the low incidence of aortic sinus aneurysm, there is no specialized device to block RSVA. According to related reports, the Amplatzer occluder has been the most widely used device in the world (7-9). There are few studies on the device made in China to treat RSVA. Chen et al (2) evaluated the safety and effectiveness of a domestic small lumbar VSD closure device for the treatment of ruptured aneurysm of valsalva sinus (RSVA). From 2005 to 2010, Chen et al (2) treated 7 patients with RSVAusing a domestic small lumbar VSD device, and during the 12-month follow-up, there were no cases of hemolysis, arrhythmia, device embolism, infective endocarditis, heart failure or mortality. This demonstrates that the domestic small lumbar VSD device was safe and effective as a therapy for RSVA Xiao et al (18) retrospectively analyzed 35 patients after treatment of RSVA using a domestic small lumbar VSD occluder and PDA device. The study revealed that 1 patient developed severe obstructive aortic insufficiency and 2 cases failed. However, 32 of the patients successfully completed transcatheter closure, and there were no cases of infective endocarditis, residual shunt, thrombosis, device displacement, severe aortic regurgitation, severe arrhythmia or mortality after an average follow-up of 73.5 months (18).
The symmetrical VSD occluder is a device developed in China. It is made of superelastic nickel-titanium alloy wire and has a self-expanding double-disc structure with a connection in the middle. The middle part, which is called the waist, is also made of nickel-titanium alloy. The diameter of both sides of the plates is the same and the waist height is 2.5-4 mm. The new domestic Nitinol VSD blocker has the advantages of being easily operated and recyclable, a reliable curative effect and low cost, which has resulted in it being rapidly popularized and applied in clinical practice. Animal experiments have demonstrated that with the memory VSD occluder, endothelialization began on the 7th day after implantation, and endothelialization was completed on the 30th day after the operation, which is superior to the VSD occluder that has been marketed (19). Clinical studies have indicated that the memory VSD occluder may be used for membranous or muscular ventricular septal defects (20,21). Furthermore, a multi-center clinical study demonstrated that the incidence of complete atrioventricular block is significantly reduced (20,22). However, as far as we know, it is rarely reported for the treatment of RSVA.
The present study reported the effect of a domestic made symmetrical occluder treatment RSVA for the first time. All of the 14 patients were successfully treated with the closure and immediate results indicated that the heart function of the patients improved, dyspnea was alleviated and motor capacity increased. At the 12-month follow-up, it was revealed that there were no cases of infective endocarditis, device thrombosis, severe aortic regurgitation, severe arrhythmia or mortality, with the exception that 1 patient underwent surgery 1 year after initial RSVA treatment. These results demonstrated that domestic symmetrical occluders were safe and effective in the treatment of RSVA. Two types of devices were used in the present study. There was no significant difference in the therapeutic effect and safety between the two types of occluder.
The RSVA is similar to PDA in morphology, but the structure of RSVA is different from PDA. PDA is muscular fibrous tissue with a strong supporting force. The muscular layer of aortic sinus aneurysms has degenerated and lacks supporting force (13), so the PDA occluder is not selected for occlusion. However, PDA occluders have also been reported to treat RSVA (23). Therefore, more clinical trials are required to determine which occluders are more effective.
Aneurysms of the sinus of Valsalva are rare, and thus, the number of treatment options are limited. At present, there are no consensus guidelines for transcatheter closure. The present study hypothesized that the successful operations were due to the size and location of the RSVA. The majority of cases that failed were due to large defects. Previous studies suggested that the defects of RSVA <10 mm are more likely to be closed using TCC, while surgery is preferred for defects >10 mm. According to the literature, the biggest defect of RSVA via TCC was 17 mm and the largest occluder was 22 mm (24). The occluder selected is generally 2-4 mm larger compared with the defect. The second factor that affected intervention was the relationship between the device and the surrounding tissue. The most common complications after occlusion were aortic regurgitation and residual shunt, and often due to these complications, a number of patients had to transfer to surgery.
The RSVA has been frequently associated with other congenital heart diseases, such as VSD or atrial septal defect (ASD). In the past, surgery was the first choice for these cases. A number of studies have attempted to plug both RSVA and VSD using a catheter. The study by Mahimarangariah et al (25) reported on a 14-year-old male patient with RSVA and VSD, in whom the RSVA and VSD was closed using TCC at the same time. Mehta et al (26) illustrated a case of RSVA with ASD. After transcatheter closure of the RSVA, echocardiography revealed that the ASD was smaller compared with the ASD before (from 22 to 18 mm) and 6 months after the ASD was occluded using interventional closure.
Interventional complications were also the focus of attention for the present study, mainly to observe the effect of the occluder on the structures of adjacent tissues such as the aortic valve, tricuspid valve and coronary arteries. In the present study, one patient had surgery for severe aortic regurgitation. Mild aortic regurgitation occurred in 5 patients, but it had no effect on heart size or function. Whether moderate aortic regurgitation requires additional surgery may depend primarily on whether the regurgitation continues to increase, whether cardiac insufficiency occurs and whether hemodynamics were affected. Kerkar et al (27) also observed a similar phenomenon with Amplatzer duct occluder for RSVA.
Of note, the present study had limitations. The patients were included from a single center, which limits the generalizability of the results. Therefore, a well-designed multicenter study with a larger sample size is required. Although immediate and mid-term effects were demonstrated to be favorable in the present study, attention should be given to possible residual shunt as well as aortic regurgitation and the long-term effects should be observed.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was funded by the Key Research and Development Program of Shaanxi Province (grant no. 2017ZDXM-SF-049).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HL and WC confirm the authenticity of all the raw data. HL and TL contributed to the conception and design of the study. WC completed the implementation of the study and drafted the manuscript and revised it critically for important intellectual content. WC and XL completed the collection, analysis and interpretation of data. HL and TL agreed to be accountable for all aspects of the work and ensured that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics committee of Xijing Hospital (Xi'an, China) approved the study (approval no. KY20172078-C-1). All participants provided their written informed consent to participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goldberg N, Krasnow N. Sinus of Valsalva aneurysms. Clin Cardiol. 1990;13:831–836. doi: 10.1002/clc.4960131204. [DOI] [PubMed] [Google Scholar]
- 2.Chen SP, Bai Y, Zhao XX, Qin YW. Safety and efficacy of a domestic made small-waist ventricular septal defect occluder for transcatheter closure of ruptured aneurysm of the sinus of Valsalva. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40:298–301. (In Chinese) [PubMed] [Google Scholar]
- 3.Sakakibara S, Konno S. Congenital aneurysm of the sinus of Valsalva. Anatomy and classification. Am Heart J. 1962;63:405–424. doi: 10.1016/0002-8703(62)90287-9. [DOI] [PubMed] [Google Scholar]
- 4.Murashita T, Kubota T, Kamikubo Y, Shiiya N, Yasuda K. Long-term results of aortic valve regurgitation after repair of ruptured sinus of valsalva aneurysm. Ann Thorac Surg. 2002;73:1466–1471. doi: 10.1016/s0003-4975(02)03493-8. [DOI] [PubMed] [Google Scholar]
- 5.Cullen S, Somerville J, Redington A. Transcatheter closure of a ruptured aneurysm of the sinus of Valsalva. Br Heart J. 1994;71:479–480. doi: 10.1136/hrt.71.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao Y, Wang C, Li Y, Guan X, Zhang X, Wu X. Percutaneous closure versus surgical repair for ruptured sinus of valsalva aneurysm: A systematic review and meta-analysis. Front Cardiovasc Med. 2023;10(1158906) doi: 10.3389/fcvm.2023.1158906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerkar P, Suvarna T, Burkule N, Panda R. Transcatheter closure of ruptured sinus of Valsalva aneurysm using the Amplatzer duct occluder in a critically ill post-CABG patient. J Invasive Cardiol. 2007;19:E169–E171. [PubMed] [Google Scholar]
- 8.Szkutnik M, Kusa J, Glowacki J, Fiszer R, Bialkowski J. Transcatheter closure of ruptured sinus of valsalva aneurysms with an Amplatzer occluder. Rev Esp Cardiol. 2009;62:1317–1321. doi: 10.1016/s1885-5857(09)73359-6. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal G, Agarwal M, Chintala K. Transcatheter closure of ruptured sinus of Valsalva aneurysm in a pregnant woman. J Cardiol Cases. 2015;12:183–187. doi: 10.1016/j.jccase.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Xu X, Zhao X, Chen F, Bai Y, Li W, Zhang Y, Wang C, Xiang J, Wu G, et al. Percutaneous closure of ruptured sinus of Valsalva aneurysm: Results from a multicentre experience. EuroIntervention. 2014;10:505–512. doi: 10.4244/EIJV10I4A87. [DOI] [PubMed] [Google Scholar]
- 11.Henze A, Huttunen H, Bjork VO. Ruptured sinus of valsalva aneurysms. Scand J Thorac Cardiovasc Surg. 1983;17:249–253. doi: 10.3109/14017438309099360. [DOI] [PubMed] [Google Scholar]
- 12.Sarikaya S, Adademir T, Elibol A, Buyukbayrak F, Onk A, Kirali K. Surgery for ruptured sinus of Valsalva aneurysm: 25-year experience with 55 patients. Eur J Cardiothorac Surg. 2013;43:591–596. doi: 10.1093/ejcts/ezs450. [DOI] [PubMed] [Google Scholar]
- 13.Ott DA. Aneurysm of the sinus of valsalva. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:165–176. doi: 10.1053/j.pcsu.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Kumar GA, Parimala PS, Jayaranganath M, Jagadeesh AM. Three-dimensional transesophageal echocardiography-guided transcathetar closure of ruptured noncoronary sinus of valsalva aneurysm. Ann Card Anaesth. 2017;20(Suppl):S73–S75. doi: 10.4103/0971-9784.197807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams JE, Sawyers JL, Scott HW Jr. Surgical treatment of aneurysms of the aortic sinuses with aorticoatrial fistula; experimental and clinical study. Surgery. 1957;41:26–42. [PubMed] [Google Scholar]
- 16.Sawyers JL, Adams JE, Scott HW Jr. A method of surgical repair for ruptured aortic sinus aneurysms with aorticoatrial fistula. South Med J. 1957;50:1075–1078. doi: 10.1097/00007611-195708000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Xu X, Ding X, Liu G, Zhao Z, Zhao X, Qin Y. Comparison of immediate results and mid-term follow-up of surgical and percutaneous closure of ruptured sinus of Valsalva aneurysm. J Cardiol. 2014;63:239–243. doi: 10.1016/j.jjcc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Xiao JW, Niu MN, Wang QG, Zhang DZ, Han XM, Zhang P, Cui CS, Zhu XY. Safety and efficacy of transcatheter closure of ruptured sinus of Valsalva aneurysm. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:799–803. doi: 10.3760/cma.j.issn.0253-3758.2018.10.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Chen F, Huang X, Zhao X, Wu H, Bai Y, Qin Y. A new coated nitinol occluder for transcatheter closure of ventricular septal defects in a canine model. Biomed Res Int. 2013;2013(507919) doi: 10.1155/2013/507919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F, Li P, Liu S, Du H, Zhang B, Jin X, Zheng X, Wu H, Chen S, Han L, et al. Transcatheter closure of intracristal ventricular septal defect with mild aortic cusp prolapse using zero eccentricity ventricular septal defect occluder. Circ J. 2015;79:2162–2168. doi: 10.1253/circj.CJ-15-0301. [DOI] [PubMed] [Google Scholar]
- 21.Zhu D, Tao K, An Q, Luo S, Gan C, Lin K. Perventricular device closure of residual muscular ventricular septal defects after repair of complex congenital heart defects in pediatric patients. Tex Heart Inst J. 2013;40:534–540. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou D, Pan W, Guan L, Ge J. Transcatheter closure of perimembranous and intracristal ventricular septal defects with the SHSMA occluder. Catheter Cardiovasc Interv. 2012;79:666–674. doi: 10.1002/ccd.23344. [DOI] [PubMed] [Google Scholar]
- 23.Guan L, Zhou D, Zhang F, Pan W, Dong L, Chen H, Ge J. Percutaneous device closure of ruptured sinus of valsalva aneurysm: A preliminary experience. J Invasive Cardiol. 2013;25:492–496. [PubMed] [Google Scholar]
- 24.Sinha SK, Khanna NN, Razi M, Krishna V, Jha MJ, Mishra V, Aggarwal P, Goel A, Singh K, Thakur R, et al. Safety and feasibility of transcatheter interruption of ruptured sinus of valsalva aneurysm using the cocoon duct occluder: Immediate results and mid-term follow-up. Cardiol Res. 2017;8:154–160. doi: 10.14740/cr568w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahimarangariah J, Kikkeri HS, Rai KM, Nanjappa MC. Combined transcatheter device closure of ruptured sinus of valsalva and a post-surgical residual ventricular septal defect. Catheter Cardiovasc Interv. 2013;82:E803–E808. doi: 10.1002/ccd.24889. [DOI] [PubMed] [Google Scholar]
- 26.Mehta NK, Mishra N, Kerkar P. Percutaneous closure of ruptured sinus of valsalva aneurysm and atrial septal defect. J Invasive Cardiol. 2010;22:E82–E85. [PubMed] [Google Scholar]
- 27.Kerkar PG, Lanjewar CP, Mishra N, Nyayadhish P, Mammen I. Transcatheter closure of ruptured sinus of Valsalva aneurysm using the Amplatzer duct occluder: Immediate results and mid-term follow-up. Eur Heart J. 2010;31:2881–2887. doi: 10.1093/eurheartj/ehq323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.