Abstract
Since the earliest investigations of olefin metathesis catalysis, light has been the choice for controlling the catalyst activity on demand. From the perspective of energy efficiency, temporal and spatial control, and selectivity, photochemistry is not only an attractive alternative to traditional thermal manufacturing techniques but also arguably a superior manifold for advanced applications like additive manufacturing (AM). In the last three decades, pioneering work in the field of ring-opening metathesis polymerization (ROMP) has broadened the scope of material properties achievable through AM, particularly using light as both an activating and deactivating stimulus. In this Perspective, we explore trends in photocontrolled ROMP systems with an emphasis on approaches to photoinduced activation and deactivation of metathesis catalysts. Recent work has yielded a myriad of commercial and synthetically accessible photosensitive catalyst systems, although comparatively little attention has been paid to achieving precise control over polymer morphology using light. Metal-free, photophysical, and living ROMP systems have also been relatively underexplored. To take fuller advantage of both the thermomechanical properties of ROMP polymers and the operational simplicity of photocontrol, clear directions for the field are to improve the reversibility of activation and deactivation strategies as well as to further develop photocontrolled approaches to tuning cross-link density and polymer tacticity.
Keywords: olefin metathesis, photocatalysis, ruthenium, ring-opening metathesis, photocontrol
Introduction
Plastics manufacturing plays a vital role in our everyday lives. As consumer demand for manufactured products continues to grow exponentially, the consumption of electrical and thermal energy and the production of CO2 have become unsustainable. In fact, CO2 emissions directly associated with energy generated for plastics manufacturing are set to eclipse those of coal power by the year 2030.1 It is increasingly clear that more energy- and atom-efficient manufacturing practices are needed to combat the global challenge of climate change. Additive manufacturing (AM), where material is deposited to create a 3D form, presents a viable alternative to traditional manufacturing, minimizing energy consumption and enabling distributed manufacturing to be realized at competitive cost. While AM comes in many flavors from the perspective of materials and production methods, photocured polymer resin systems require minimal energy input for curing and have progressed in development to commercial relevance. Photopolymer AM is now used in the manufacturing of a wide variety of consumer products including dental products, hearing aids, footwear, jewelry, and sporting goods. However, the polyacrylate resins at the forefront of commercial applications are ultimately limited in terms of their mechanical performance and thermochemical stability.2 As the demand for AM products expands to other industries, new materials are needed to address these challenges, driving the need for new polymerization chemistries.
Ring-opening metathesis polymerization (ROMP) expands the range of accessible properties that can be achieved by AM polymers. During ROMP, cyclic olefin monomers are polymerized to yield polyalkenamers, and advanced Ru-based catalysts now enable fine control over their molecular weight, stereochemistry, and tacticity, among other parameters. ROMP can be carried out under air in the presence of various impurities and is thus ideally suited for AM. Further, polynorbornenes produced by ROMP, most notably polydicyclopentadiene (pDCPD), exhibit excellent mechanical properties, are chemically resistant, and can used in applications with operating temperatures up to 400 °C.3,4 Research over the past decades has shown that thermally latent ROMP precatalysts can be initiated with light, opening new possibilities in photomediated AM.5,6 However, ROMP-based AM has only recently garnered commercial attention from companies such as Polyspectra7−11 and Promerus,12−15 whose resins have novel long-term stability and tunable materials properties (Figure 1). The precedent from their work has motivated further research and development on catalyst design and Ru carbene photochemistry.
Figure 1.
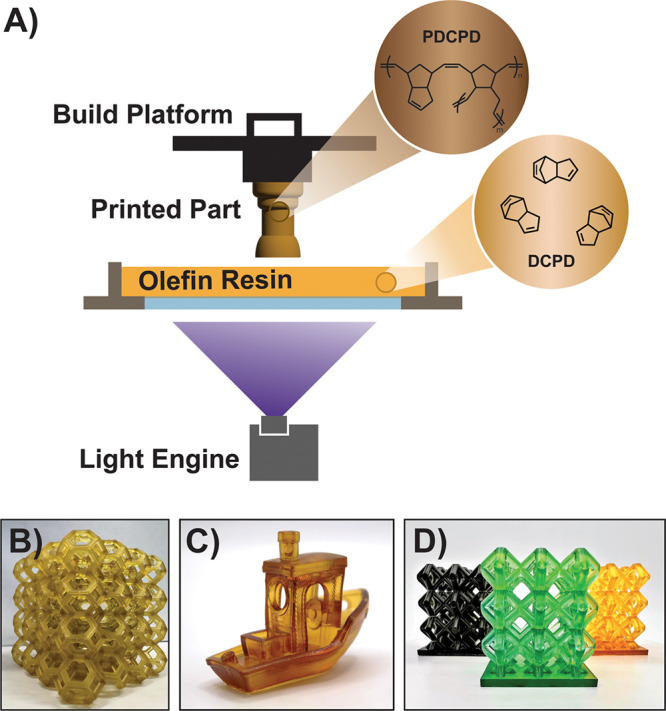
Photoactivation of olefin metathesis reactions enables control over the shape of a printed part during AM. A) Schematic representation of a bottom-up approach to AM with olefin resins, particularly DCPD. (B-D) Images of DPCD-based printed parts utilizing resins from (B) Promerus, (C) Leguizamon et al.,6 and (D) polySpectra. Images are provided by means of (B) Promerus and (D) polySpectra.
While radical photopolymerization has been studied extensively, photoactivation of olefin metathesis reactions is relatively underexplored. Compared to bulk activation methods like heat or redox chemistry, the energy efficiency and spatiotemporal control achieved through light irradiation is unparalleled. Fast and selective activation, and in some cases deactivation, can be achieved by judicious tuning of formulation and process parameters, including chromophores, initiating species, activating wavelengths, and light intensities. This means that polymerization can, in many cases, be turned on and off with tremendous ease–a desirable feature for fast, high-precision AM of materials with pixel-sized resolution. The spatial control afforded by light gives precise control over the shape of a printed part or the extent of cure in a given area of the polymer resin.
Photoactivation of ROMP was first reported nearly three decades ago,16 and significant progress has been made on understanding how olefin metathesis catalysts can be initiated with light. In fact, we now understand that most Ru-based carbene complexes can be photoinitiated, either directly or through the use of photoacids or photosensitizers, demonstrating a vast potential design space for this chemistry in AM.17−20 More work is needed to understand the photochemical mechanisms of Ru catalyst activation, to unravel how light can be leveraged to influence polymer stereochemistry and network structure, and to develop even more efficient catalyst systems that are latent, robust to environmental impurities such as water and oxygen, and can be rapidly initiated using low intensity visible light.19,20
This mini-review details some of the most significant reports of the last two decades as they pertain to photocontrolled ROMP. For the sake of focus and brevity, relevant mechanistic details are described only briefly; instead, ease of use, versatility, and fundamental insights gained from these studies are emphasized. While most research in this field has been dedicated to devising strategies for the selective activation of metathesis catalysts, unique strategies for both photoinitiation and photopatterning are also discussed. Photodeactivation strategies are also addressed. To date, only a handful of examples have demonstrated photodeactivation of olefin metathesis catalysts, the majority of which employ irreversible chemistries, limiting their potential for large-scale, economical, and energy and atom efficient photoROMP printing. Heterogeneous and photophysical strategies for photocontrolled polymerization are also conspicuously scarce but could provide versatility to both enhance resin latency and modulate cured resin properties. In the progression of photoROMP toward rapid manufacturing of complex, highly resolved architectures, new strategies for reversibly controlling catalyst activity must be developed, along with an improved understanding of the relationship between polymerization medium and catalyst activity.
The Origins of PhotoROMP
The first commercial process using ROMP was developed in 1976 for the production of poly(norbornene) under the trade name Norsorex.4 Though only a niche product today, its useful properties and low feedstock pricing set the stage for future ROMP polymer systems, including cyclooctadiene, tetracyclododecene, and dicyclopentadiene (DCPD), among others. While early studies on ROMP involved the use of heterogeneous transition metal catalyst systems–the active species of which remain difficult to characterize–a tremendous amount of (Nobel Prize-winning) work has since been dedicated to creating well-defined homogeneous catalysts with tunable reactivity, i.e. metal carbene complexes.21 Though it was not further explored at the time, some early reports of these complexes and their precursors indicated photosensitivity, for example, in accelerating Hα-abstraction to form the alkylidenes.22 To the best of our knowledge, the first photoROMP system was reported in 1995 by Mühlebach and co-workers for the synthesis of poly(norbornene) derivatives.23 This work inspired the development of a wealth of simple and straightforward methodologies for photoinduced ROMP, though early systems suffered from poor catalyst latency, stability, synthetic accessibility, and functional group tolerance.24
Catalyst Photoactivation
Realization of photoROMP requires catalyst systems that are active in response to light yet are otherwise (thermally) latent. Ideally, the rate of their spontaneous background polymerization at room temperature must be nominal to maximize the processing window of the prepolymer mixture, and the conversions between inactive and active catalyst states occur rapidly to enable spatiotemporal control. The plurality of developments in these systems, and most of the works described herein, utilize ruthenium vinylidene species as the catalysts (either as the initiating species or formed in situ), though photoinitiated ROMP also occurs with a variety of metals including tungsten,16 molybdenum,25 and rhenium.26
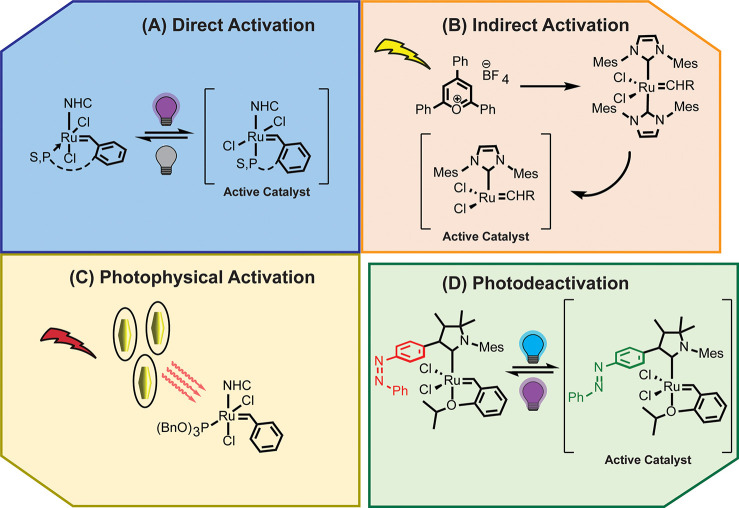
Recent designs of Ru-based photoROMP catalysts can be grouped into three categories (Figure 2): those that initiate polymerization via 1) direct photoactivation (e.g., photoinduced ligand dissociation or isomerization), 2) indirect photoactivation (e.g., ligand dissociation mediated by photoacid generators), and 3) photophysical activation.
Figure 2.
Notable examples of the three categories of photoROMP using Ru-based catalysts and of photodeactivation of Ru-catalysts. Direct activation is well illustrated by Lemcoff and co-workers’ library of S-chelated species that are thermally latent but undergo cis-to-trans isomerization upon direct irradiation with UV light.32,33 Alternatively, indirect activation through such species as photoredox catalysts can enable the use of lower irradiation intensities and longer wavelengths of light.52 The addition of carbon nanotubes, or as shown, plasmonic gold nanoparticles, enables NIR activation of thermally latent Ru-catalyst via photophysical means.63 Though generally irreversible, clever methods for reversible photodeactivation exist including the use of an azobenzene-functionalized NHC as introduced by the Hong group.68
Direct Activation
For the scope of this review, “direct activation” is broadly defined as a strategy in which a catalyst is converted from an inactive state to an active state through photoexcitation of the metal carbene complex.
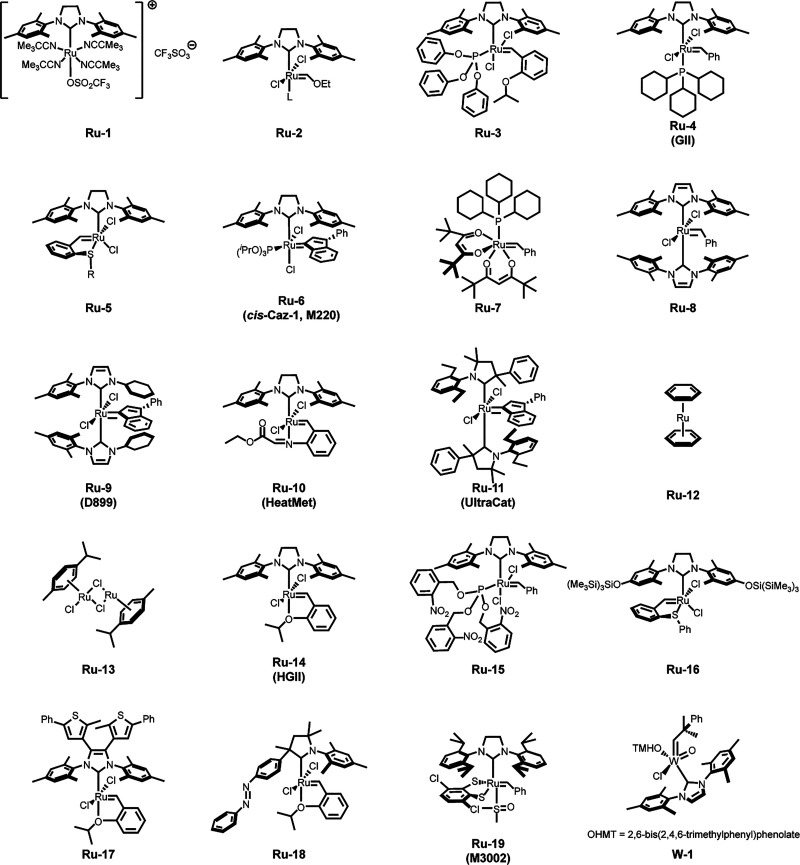
Photodissociation of labile ligands remains the most common strategy for designing direct-activation systems. The archetype for direct activation systems was established by the Buchmeiser group using triflate salts of ruthenium N-hetercyclic carbene (NHC) precatalysts (Ru-1, Figure 3).27,28 Through a combination of quantum chemical calculations and irradiation experiments, it was revealed that these otherwise latent species were capable of initiating polymerization through photoinduced dissociation of two tert-butyl isocyanide ligands. Weitekamp and co-workers demonstrated that latent Fischer-type carbenes (Ru-2) can be directly photoactivated to pattern 2D and 3D structures with ROMP, as well as the ability to incorporate a wide variety of functional groups into the final polymer network.11,29 Recently, the Lemcoff group reported a photodissociation approach to activating phosphite-ligated Hoveyda-Grubbs (HG2) catalysts (e.g., Ru-3), extending the potlife of HG2-olefin monomer systems up to 9 h.30
Figure 3.
Catalysts are referenced throughout this Perspective. Common names, when available, are included.
Another approach to designing latent catalyst systems relies on photoisomerization. Lemcoff and co-workers have developed a library of catalysts bearing pincer-type benzylidene ligands with coordinating sulfur atoms (e.g., Ru-5). These S-chelated species are thermally latent, and thus active only at elevated temperatures, but undergo cis-to-trans isomerization upon irradiation with UV light. Subsequent dissociation of the sulfur–ruthenium bond yields a metathesis-active species.31 The electronic characteristics of both the NHC and the pincer ligand can be tuned to affect the efficiency of activation as well as the activating wavelength.32 The Lemcoff group was also the first to recognize the photoreactivity of the commercially available, phosphite-coordinated cis-Caz-1 (Ru-6).33 Inspired by this discovery, they explored the potential of a P-chelated congener of their S-chelated series. Unfortunately, this catalyst exhibited poor reactivity, presumably due to the strong coordination of the phosphite group. However, this finding would later inform the design of a photodeactivated catalyst system based on photolabile nitrobenzyl-phosphite ligands (discussed below).34 The promise of these systems, both employing apical ligands with selective and tunable lability, naturally sparked a wealth of creative new strategies for photoactivation.30,35−37 Subsequent catalyst design inspired by Lemcoff’s original S-phenyl and S-naphthyl catalysts have yielded a swath of photoactive Ru carbenes with improved activity as well as remarkable regioselectivity.38 Studies on the electronic character of the chelating group, as well as the halide ligands, have also provided remarkable insight into the factors affecting mechanism and energetics of photoisomerization and initiation.32,39
Leveraging the stability of less reactive isomers can also enable latency, even in readily available catalysts. In a recent report, Stawiasz et al. demonstrated that the phosphite chelate of the commercially available Grubbs’ second generation catalyst (Ru-4) undergoes UV-induced photoisomerization and subsequent ligand dissociation to generate a species active for frontal polymerization of DCPD.40 Photoisomerization, particularly of adducts of commercially available catalysts, holds tremendous promise as an operationally simple method to engender reactivity on demand.
Indirect Activation
Converse to direct activation, “indirect activation” is defined here as a strategy in which a catalyst is converted from an inactive state to an active state through reaction with a photogenerated species. In these systems, a molecule other than the metal carbene complex is photoexcited and then undergoes a secondary chemical reaction with a metal complex to generate a species that is metathesis-active.
The earliest strategies for indirect photoactivation naturally evolved from photochemical synthetic pathways toward metal carbene complexes. Though rarely explored since, the in situ generation of carbenes through the reaction of precatalysts and photogenerated ligands offers the unique advantage of total catalyst latency at the expense of slower activation kinetics. For example, initiation of ruthenium sandwich complexes (e.g., Ru-12) by ligand photodissociation was utilized for the polymerization of poly(norbornene) derivatives by Mühlebach et al. as early as 1995.23 In 1992, Demonceau et al. showed that a ruthenium-cymene complex promoted ROMP of strained cyclic olefins upon activation with ethyl diazoacetate, presumably through the in situ formation of the metathetically activate vinylidene.41,42 This observation of carbene transfer directly inspired recent work on related complexes by Chemtob and co-workers, who used a photogenerated NHC to promote the formation of a ruthenium vinylidene species from a sandwich complex precatalyst (Ru-13).43 This study also revealed a limitation of this activation strategy: during polymerization, uncontrolled, nonmetathetic cross-linking (between 11 and 34%) was observed, presumably as a result of the highly enthalpic polymerization. This gave rise to inconsistent material properties between samples. Though certainly a limitation of the system, this study highlights the importance of the interplay between thermal and photo contributions to reactivity.
The first reported indirect activation strategies for photoROMP using preformed catalysts closely followed Buchmeiser and co-workers’ reports of UV-activated Ru(II) complexes. In 1998, the Grubbs group reported the acid activation of ROMP catalysts via ligand sequestration.44 Nearly a decade later, they demonstrated the use of a photoacid generator to convert acetylacetonate-functionalized Grubbs-type precatalysts (e.g., Ru-7) to their metathesis-active forms.17 The strategy of photoacid generation has since been expanded upon;18 though its robustness is limited, owing to inherent sensitivity of various monomers and Ru vinylidines to acidic and basic functionalities.45 Nonetheless, this approach inspired a multitude of related chemistries employing precatalyst species with chemo-,46 photo-,47 and redox-labile ligands.48,49
Bis-NHC species are particularly well-suited for photoactivation, as the dissociation of NHCs is thermodynamically disfavored. This renders such catalysts latent even at elevated temperatures but still capable of generating the active species with an external energy input or transmetallating agent.50,51 The sensitivity of NHC complexes is also easily synthetically tuned, allowing greater specificity in the choice of activating stimulus (i.e., wavelength, activation mechanism, etc.). For example, the Rovis group accomplished polymerization of DCPD with visible light by combining a thermally latent bis-NHC complex (Ru-8) and a photoredox catalyst.52 Photoinduced electron transfer from a pyrylium photooxidant to the catalyst led to the dissociation of one NHC group, forming the metathesis-active 14 electron species. In addition to the benefits of visible light over UV-initiation from the perspective of energy input and user safety, this system exhibited excellent spatial control and microscale resolution. The Buchmeiser group demonstrated a similar system employing NHC-coordinated W-vinylidene species (e.g., W-1) in 2D photolithography.53 Pushing toward even lower-energy radiation sources—a near-IR-initiated ROMP system using an osmium photooxidant was also investigated.54 Though this system required acetone as a cosolvent and therefore exhibited limited feasibility for applications in photolithography, the use of NIR radiation as the activating stimulus enabled through-barrier initiation, a feature impossible with visible light methods. It also exhibited remarkable reversibility over several on-and-off irradiation cycles.
Changing the ligand sphere affects not only photoactivation energy and kinetics but also regioselectivity. Compared to their dichloro congeners, the less well-studied diiodo ruthenium vinylidenes exhibit remarkable selectivity for terminal olefins and reticence toward ring opening of even highly strained internal alkenes.55,56 However, the two can be interconverted by anionic ligand exchange, enabling the facile synthesis of catalyst systems with impressive latency or activity toward ring-closed species. Leveraging this selectivity, the Lemcoff group demonstrated that DCPD could be stored at ambient temperatures in the presence of a diiodo S-chelated ruthenium benzylidene for over 24 h without a reaction. However, in the presence of a chloride photocage molecule, 8-(chloromethyl)-4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a diaza-s-indacene (Cl-BODIPY), the monomer was fully polymerized within 30 min upon irradiation.57 This study not only expands the avenues available for tailoring catalyst reactivity on demand but also sets the stage for photoswitchable polymerization and depolymerization chemistries.
Indirect activation strategies enable reactive prepolymer resins to exist stably over long processing windows, a feat difficult to achieve with thermally isomerizable precatalysts. This is particularly useful for frontal polymerization, which requires enthalpic polymerizations driven by highly active catalysts to sustain a reaction front. Since thermal initiation occurs even at room temperature according to Arrhenius kinetics, storage stability for frontal-ROMP systems employing thermally latent catalysts is inherently limited under even mild processing conditions.58 Gradual consumption of the monomer may result in gelation or stalled fronts due to lower exotherms, and the use of extremely latent catalyst necessarily increases the exothermicity required for front propagation. The contradictory requirements for catalyst reactivity and latency render the engineering of frontal polymerization systems a unique challenge.
By contrast, the use of light as an activating stimulus enables the use of highly latent catalysts. Stawiasz et al. demonstrated that long-lived prepolymer solutions of bis-NHC indenylidene precatalyst D899 (Ru-9) in dicyclopentadiene (DCPD) could be prepared and stored for up to two months without significant spontaneous background polymerization.59 Subsequent frontal polymerization of the resin was accomplished through a cascade reaction involving UV photoreduction of CuII by an acryidinium photocatalyst, followed by transmetalation of one of the Ru-coordinated NHC ligands.
Recently, Leguizamon and co-workers demonstrated that the use of a tertiary amine and photosensitizer accelerated ROMP of DCPD in an additive manufacturing system with a wide range of commercial Ru catalysts (e.g., Ru-6,-10, and -11).6,60,61 It was presumed that a radical or energy transfer mechanism and possibly subsequent catalyst isomerization and ligand dissociation enabled the formation of metathetically active species. The accelerating effect was observed for photosensitizer and catalyst mixtures and could be enhanced via the addition of a tertiary amine. Though the mechanism of acceleration has yet to be fully elucidated, this evidence suggests that photosensitization is an effective strategy for the indirect activation of virtually any latent metathesis catalyst. This represents a promising approach to robust and versatile ROMP systems that merits greater attention. Precatalyst activation and addressing the associated challenges therein are emerging areas of research that will certainly receive more attention in the future.
Photophysical Activation
To date, only a small number of photophysical methods of catalyst release or activation have been explored and thus present tremendous potential for development. Here, we define photophysical methods as those involving photoexcitation and subsequent processes that involve primarily physical (e.g., heating) rather than chemical changes. This includes thermal activation of metathesis catalysts by plasmonic heating or photomediated catalyst release from microcapsules, for example. Fréchet and co-workers demonstrated an early archetype for encapsulation of ROMP catalysts using light-rupturable microcapsules.62 Incorporation of carbon nanotubes in the microcapsules enabled them to absorb at NIR frequencies, resulting in fast, photothermally driven release of the Grubbs second-generation catalyst in DCPD (Ru-4). More recently, the Lemcoff and Weismann groups used plasmonic gold nanoparticles, absorbing at visible and NIR frequencies, to initiate thermally latent catalysts for photoROMP of DCPD as well as ring-closing metathesis.63 This strategy allowed tuning of the nanoparticle size and geometry to vary the activation wavelength, as well as the potential for multistage photocuring (a desirable feature for some fabrication techniques). Recent work on encapsulated metathesis catalysts in neat DCPD extended resin pot lives to over 12 months, also enabling the use of highly active initiators such as HG2 (Ru-14) for frontal polymerization.64 Though catalyst release was achieved only through thermal activation, research into photoinduced capsule degradation mechanisms is ongoing. Direct chemical activation of metathesis catalysts is an attractive area of study, and future work on photoactivation should not discount the benefits of heterogeneous manufacturing systems utilizing photophysical methods.
Deactivating Catalysts
It is important to note that the activation strategies discussed to this point, with few exceptions, rely on controlling the mechanism of initiation. Often, this occurs through irreversible ligand dissociation or in situ carbene generation. We note that in such systems photogeneration of the active species results in propagation that continues unabated. Only a handful of examples to date have demonstrated systems capable of reversible activation. Exploration of ways to couple deactivation processes with light or other stimuli offers opportunities not only for photolithography but also can be leveraged to control runaway reactions–a serious consideration in industrial-scale ROMP. Pioneering work by the Fogg group on catalyst deactivation through metallacyclobutane deprotonation sets the stage for the most prominent deactivation strategy yet explored.65 Combining photolabile nitrobenzyl-derived photobase generators (well explored systems for thiol-Michael addition polymerization)66 with Grubbs-type catalysts (Ru-15), the Lemcoff group created a photoactivated ROMP system with an orthogonal UV–C activated “kill switch”.34 Though this system was amenable to orthogonal photoactivation and decomposition at distinct wavelengths, partial decomposition of the catalyst at the activation wavelength rendered the system unsuitable for photolithography. Foster and co-workers addressed this issue by separating catalyst and inhibitor into two antagonistic systems.5 Using the thermally latent Heatmet catalyst (Ru-10), which bears an N-chelating pincer vinylidene ligand and a photosensitizer, the activation wavelength was pushed further into the visible region. This enabled selective and orthogonal activation of the initiator and the inhibiting photobase at distinct wavelengths, making the system amenable to continuous AM. Another strategy for chromatic orthogonality in catalyst activation and deactivation was devised by Lemcoff and co-workers using latent S-chelated ruthenium catalysts with protected supersilyl-functionalized NHC ligands (Ru-16).47 The catalyst was active upon UVA irradiation but destroyed by UVC-photogenerated supersilyl groups. Crucially, in both examples, activation and deactivation occurred through irreversible processes. Though this is advantageous for AM processes, it also requires high catalyst loadings to overcome decomposition and limits the recyclability of the resins, which are poisoned by photogenerated amines or silyl groups.
Reversible strategies for direct and indirect activation and deactivation are rare in the field of photoROMP, owing to the challenges enumerated above in addition to kinetic complications. An early example from Teator et al. saw the use of a dithienylethene-functionalized NHC (Ru-17) to toggle the reactivity of a metathesis catalyst between ROMP and ring-closing metathesis.67 Though the catalyst remained active in this system and both direct photoactivation and deactivation processes were sluggish, this nonetheless demonstrated potential as a way to extend the lifetime of an additive manufacturing ROMP resin. The Hong group, taking clever advantage of well-established photoisomerization chemistry, devised a metathesis catalyst bearing an azobenzene-functionalized NHC (e.g., Ru-18).68 The photoisomerization of this group from the cis to trans configuration resulted in steric congestion of the metal center, slowing olefin insertion. This resulted in an excellent temporal control. Unfortunately, the thermally favored form of the catalyst was also the metathesis-active species. This meant that 1) control over ROMP necessitated continuous irradiation to maintain a latent state, which is not ideal from a manufacturing perspective, and 2) heat transfer from the exothermic polymerization process resulted in some catalyst deactivation. Just as in photoexcitation and photodegradation strategies, catalyst activation and degradation must be decoupled in an ideal photoROMP system. Future photoROMP studies would benefit tremendously from modeling to capture the full kinetic complexity of the catalyst equilibria and heat transfer associated with polymerization.
Using Light to Control Polymer Properties
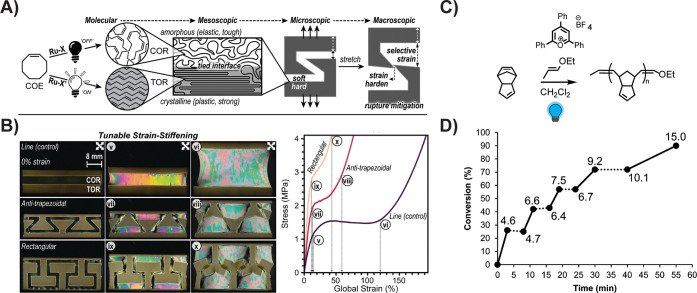
Polymer stereochemistry dictates the packing patterns of individual polymer chains, giving rise to different microstructures that, in turn, dictate the mechanical properties of the bulk material. Olefin metathesis reactions operate under kinetic or equilibrium control, depending on the susceptibility of backbone olefins toward secondary metathesis reactions. ROMP of norbornene monomers produces a statistical product distribution of stereo- and regioisomers. In nonstereoselective olefin metathesis systems where secondary metathesis may occur, the ratio of E- and Z-olefins, which is typically about 9:1, E:Z, reflects the thermodynamic energy difference between the two isomers. Thus, most metathesis systems yield a mixture of cis- and trans olefins, leading to largely amorphous materials. Stereoselective metathesis catalysis have therefore received considerable attention in the past decade, both for small molecule and polymer synthesis.69 Page and co-workers envisioned photoactivation of stereoselective catalysts as an inexpensive, operationally simple way to generate polymers with spatially resolved stereochemistry and therefore integrated stiff and elastic domains (Figure 4). Screening of a library of photoactive and stereoselective catalysts (e.g., Ru-19) afforded a visible light-sensitive system for the polymerization of cis-cyclooctene, which could be isomerized to the trans isomer in illuminated regions with the use of a nonstereoselective catalyst (e.g., Ru-8).70 Selective irradiation yielded materials with microscopically precise and mechanically robust “hard” and “soft” domains within the poly(cyclooctene) rubber. Unlike traditional approaches to making multimaterials, e.g., varying local cross-link density, controlling tacticity decreases the risk of brittle failure and interfacial stress between domains.71−75 Future work in this area should seek to expand the monomer scope, allowing access to a broader range of materials, and explore the utility of tacticity gradients. Furthermore, this highlights the need for photobased approaches to control cross-link density, as both microstructure and chemical cross-linking affect thermoset strength and toughness.
Figure 4.
Stereochemical and selective photoactivation of olefin monomers. (A) Page and co-workers developed a method for stereoselective ROMP of cyclooctene to yield patterned crystallinity. (B) Images of samples with patterned crystallinity and their corresponding stress–strain curves in vertical tension. (C) Metal-free-ROMP for the formation of linear PDCPD. Conversion of norbornene over time undergoing metal-free-ROMP. Periods of exposure to blue light and periods of no irradiation are indicated by solid and dotted lines, respectively. Data point labels indicate Mn values (kDa) as determined by GPC analysis. (A and B) are reprinted from ref [70]. Reprinted with permission from AAAS (2023). (C–D) are reproduced from ref [78]. Copyright (2015) American Chemical Society.
A related but underexplored approach to the photocontrol of tacticity takes advantage of the inherent isomerizability of the olefin moiety. Michaudel and co-workers have undertaken extensive research on stereoretentive ROMP, particularly of cis-phenylenevinylenes (PVs). The polymer, which was synthesized in its all-cis form, was rapidly photosiomerized to the all-trans configuration upon irradiation with 365 nm light.76 Owing to the inherent insolubility of these highly aromatic species, this technique is limited to solution-phase polymerization. Nonetheless, it is easy to envision the extension of this principle to bulk manufacturing: rather than controlling the activity of the catalyst, choosing a photolabile monomer might allow yet another avenue to tacticity control employing only a single catalyst.
Metal-Free ROMP
Applications of ROMP in biological or electronic applications are yet relatively unexplored, owing in part to the high cost of typical transition metal carbene catalysts as well as the dubious biological compatibility of residual Ru species. Metal-free alternatives to traditional ROMP naturally offer a route to avoid expensive and inefficient procedures required to remove catalyst from the final polymer product and to avoid the use of expensive transition metals altogether. In 2001, Chiba et al. reported the first example of electrocatalytic olefin cross-metathesis.77 The mechanism was determined to proceed via electrocyclization and subsequent cycloreversion, similar to traditional catalytic metathesis, following one-electron oxidation of a vinyl ether. Years later, taking direct inspiration from these studies, the Boydston group envisioned a metal-free ROMP (MF-ROMP) system for norbornenes utilizing photoredox mediators to generate the initiating radical cation (Figure 4).78 Conveniently, the Mn growth of polymer chains was determined to correlate with monomer conversions during on–off cycles, indicating that the polymerization possessed both “living” characteristics and the advantage of temporal control afforded by light stimulus. However, this approach was found to be limited in monomer scope, as intermolecular reactions between propagating radical cations and neighboring olefins drastically limited conversion of monomers possessing multiple alkene groups. Furthermore, this approach offered no means to control polymer tacticity.79
Recent work has addressed many of these initial shortcomings. Judicious choice of initiator structure and counterion was determined to drastically improve tacticity control of poly(norbornene).80 Whereas linear pDCPD has historically been difficult to produce with transition metal catalysts owing to their high reactivity, its synthesis was recently achieved in a metal-free system, enabling access to a manifold of unexplored ROMP polymer structures.81 This could enable the synthesis of elusive macromonomers for viscosity and shrinkage control in ROMP-based AM systems. Incorporation of vinyl ether initiators into the polymer backbone in MF-ROMP systems has also enabled the synthesis of acid-degradable block copolymers, indicating the intrinsic utility of this unique system.82 While transition-metal-based catalytic systems offer undeniably superior reactivity, MF-ROMP excels from the perspective of greenness, initiation latency, cost, and potential bio- and electronic compatibility. MF-ROMP should no longer be considered a niche system but rather a competitive alternative in the broader scope of photoROMP systems.
Conclusions and Outlook
From the perspective of balancing thermomechanical properties and energy efficiency, ROMP polymers represent a versatile and robust alternative to polyacrylates, particularly in the realm of AM. Existing strategies for low-energy activation and through-barrier polymerization hold great promise for the facile initiation and generation of functional materials. However, several challenges are yet unmet. Whereas photoactivation strategies have been largely focused on controlling the kinetics of initiation, realization of an ideal photocontrol system will require a polymerization catalyst that can be both activated and deactivated reversibly. This may require developing systems in which a catalyst antagonist can interact with a propagating species rather than just an initiator. Strategies for reversible chain growth that are unimolecular in the propagating catalyst, akin to those developed for controlled polymerization strategies (ATRP, RAFT, etc.), must also be considered, although they may be complicated by the associated enthalpy of polymerization as well as the kinetics of chain transfer. Work from the Kilbinger group is expanding the scope of “living” ROMP using vinyl ether and 1,3-diene moieties as chain transfer agents.83−86 Incorporation of a photosensitization mechanism into these systems represents the next challenge on the horizon. Catalysts that can be reversibly and selectively photoactivated or deactivated without sacrificing activity or being compromised by concomitant thermal deactivation must also be devised. Multimolecular strategies for reversible deactivation, for example, by the presence of radical species, are extremely promising.
As with any photopolymerization strategy, finding ways to further tune the initiating wavelength, whether through catalyst design or novel photosensitization strategies, is of primary interest. Broadening the gap between activating wavelengths could enable combinations of photoinitiated polymerization chemistries, enabling greater control over both polymerization kinetics and material properties. The Page group demonstrated a photoredox strategy for the synthesis of interpenetrating polyurethane and acrylate networks.87 It is easy to envision extending this principle to ROMP. For example, a system in which a photobase generator and ROMP initiator are both present and activated orthogonally.
Physical methods of releasing either catalyst or polymerization inhibitor must also be considered as a way to address the issues enumerated above.62,64 One unique advantage of such systems over chemically derived photocontrol methods is that the enthalpy of polymerization, which has been shown to affect the degree of cross-linking in pDCPD systems, is directed by swelling or melting of the incorporated nanoparticles. This could allow for more precise control over the degree of cross-linking in the photoROMP thermosets. Furthermore, the versatility of photophysical systems allows selective photorelease of the initiator or inhibitor (or both, orthogonally) to match the desired properties of the system. The incorporated nanoparticles also represent a unique chemical interface for both the catalyst as well as the resultant polymer and thus present an opportunity to easily generate materials with tunable mechanical properties. Better understanding of the role of the polymerization medium in both heat transfer and catalyst solvation must be accomplished for 2D and 3D photolithographic methods. It may not require total reinvention of the wheel, either: ten years ago, Grubbs and co-workers described a photolithographic system utilizing readily prepared ruthenium vinylidene ether complexes saturated with secondary olefins.11 The answers to the questions of reversible photocontrolled ROMP may be right in front of us.
Acknowledgments
The authors thank Leah Appelhans for their review of this manuscript. This paper describes objective technical results and analysis. This work was supported by the Laboratory Directed Research and Development program at Sandia National Laboratories, a multimission laboratory managed and operated by the National Technology and Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International, Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA-0003525. This work was also sponsored by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory managed by UT-Battelle LLC for the U.S. DOE.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy (DOE) or the U.S. Government.
The authors declare the following competing financial interest(s): The authors declare the existence of intellectual property based on some of the technologies described. RAW has an ownership interest in polySpectra.
References
- Vallette J.The new coal: Plastics and climate change; 2021. https://www.beyondplastics.org/plastics-and-climate (accessed 2024-04-08).
- Ligon-Auer S. C.; Schwentenwein M.; Gorsche C.; Stampfl J.; Liska R. Toughening of photo-curable polymer networks: a review. Polym. Chem. 2016, 7 (2), 257–286. 10.1039/C5PY01631B. [DOI] [Google Scholar]
- Mol J. C. Industrial applications of olefin metathesis. J. Mol. Catal. A: Chem. 2004, 213 (1), 39–45. 10.1016/j.molcata.2003.10.049. [DOI] [Google Scholar]
- Nickel A.; Edgecombe B. Industrial Applications of ROMP. Polymer Science: A Comprehensive Reference 2012, 4, 749–759. 10.1016/B978-0-444-53349-4.00106-0. [DOI] [Google Scholar]
- Foster J. C.; Cook A. W.; Monk N. T.; Jones B. H.; Appelhans L. N.; Redline E. M.; Leguizamon S. C. Continuous Additive Manufacturing using Olefin Metathesis. Advanced Science 2022, 9 (14), 2200770. 10.1002/advs.202200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguizamon S. C.; Monk N. T.; Hochrein M. T.; Zapien E. M.; Yoon A.; Foster J. C.; Appelhans L. N. Photoinitiated Olefin Metathesis and Stereolithographic Printing of Polydicyclopentadiene. Macromolecules 2022, 55 (18), 8273–8282. 10.1021/acs.macromol.2c01244. [DOI] [Google Scholar]
- Balasubramanian A.; Weitekamp R. A.. Olefin metathesis photopolymers. US11725077B2: 2021.
- Weitekamp R.; Grubbs R. H.; Atwater H. A.; Fakonas J.. Direct photopatterning of robust and diverse materials. US 10799613B2: 2020.
- Weitekamp R.Photoactive Catalyst Compositions. US 20180067393A1: 2020.
- Weitekamp R.; Grubbs R. H.. Photoinitiated olefin methathesis polymerization. US 9207532B2: 2015.
- Weitekamp R. A.; Atwater H. A.; Grubbs R. H. Photolithographic Olefin Metathesis Polymerization. J. Am. Chem. Soc. 2013, 135 (45), 16817–16820. 10.1021/ja4093083. [DOI] [PubMed] [Google Scholar]
- Burtovyy O.; Zhang L.; Langsdorf L.; Niemiec A.; Gastaldo D.; Skilskyj D.; Skowerski K.; Rhodes L. In Latent, UV-activated ROMP Catalyst System; ISOM23, 2019.
- Burtovyy O.; Zhang W.; Langsdorf L.; Rhodes L. F.. High impact strength 3d printing materials derived from polycycloolefin monomers and crosslinkers. WO 2020132665A1: 2022.
- Burtovyy O.Stable mass polymerizable polycycloolefin compositions as 3D printing materials and a method of fabrication thereof. WO 2021142402A1: 2022.
- Burtovyy O.; Barchok M. L.; Zhang W.; Rhodes L. F.. Polycycloolefin monomers and catalyst activated by compound capable of generating photoacid as 3d printing materials. WO 2020006345A1: 2022.
- van der Schaaf P. A.; Hafner A.; Mühlebach A. Photoinduced Ring Opening Metathesis Polymerization (PROMP) with Photochemically Generated Schrock Type Catalysts. Angewandte Chemie International Edition in English 1996, 35 (16), 1845–1847. 10.1002/anie.199618451. [DOI] [Google Scholar]
- Keitz B. K.; Grubbs R. H. A Tandem Approach to Photoactivated Olefin Metathesis: Combining a Photoacid Generator with an Acid Activated Catalyst. J. Am. Chem. Soc. 2009, 131 (6), 2038–2039. 10.1021/ja807187u. [DOI] [PubMed] [Google Scholar]
- Joo W.; Chen C. H.; Moerdyk J. P.; Deschner R. P.; Bielawski C. W.; Willson C. G. Photoinitiated ring-opening metathesis polymerization. J. Polym. Sci., Part A: Polym. Chem. 2019, 57 (17), 1791–1795. 10.1002/pola.29449. [DOI] [Google Scholar]
- Doerr A. M.; Burroughs J. M.; Gitter S. R.; Yang X.; Boydston A. J.; Long B. K. Advances in Polymerizations Modulated by External Stimuli. ACS Catal. 2020, 10 (24), 14457–14515. 10.1021/acscatal.0c03802. [DOI] [Google Scholar]
- Zhou Y.-N.; Li J.-J.; Wu Y.-Y.; Luo Z.-H. Role of External Field in Polymerization: Mechanism and Kinetics. Chem. Rev. 2020, 120 (5), 2950–3048. 10.1021/acs.chemrev.9b00744. [DOI] [PubMed] [Google Scholar]
- Casey C. P. 2005 Nobel Prize in Chemistry. Development of the Olefin Metathesis Method in Organic Synthesis. J. Chem. Educ. 2006, 83 (2), 192. 10.1021/ed083p192. [DOI] [Google Scholar]
- Schaverien C. J.; Dewan J. C.; Schrock R. R. Multiple metal-carbon bonds. 13. Preparation and Characterization of Monocyclopentadienyl Mononeopentylidene Complexs of Niobium and Tantalum Inlcuding the First Details of an Alpha-Abstraction Process. J. Am. Chem. Soc. 1986, 108 (10), 2771–2773. 10.1021/ja00270a056. [DOI] [Google Scholar]
- Karlen T.; Ludi A.; Mühlebach A.; Bernhard P.; Pharisa C. Photoinduced ring opening metathesis polymerization (PROMP) of strained bicyclic olefins with ruthenium complexes of the type [(η6 arene1) Ru (η6 arene2)] 2+ and [Ru (Nc R) 6] 2+. J. Polym. Sci., Part A: Polym. Chem. 1995, 33 (10), 1665–1674. 10.1002/pola.1995.080331013. [DOI] [Google Scholar]
- Hafner A.; van der Schaaf P. A.; Mühlebach A.; Bernhard P.; Schaedeli U.; Karlen T.; Ludi A. Thermal-and photoinduced ring-opening metathesis polymerization (ROMP)/(PROMP): an efficient tool in polymer chemistry. Prog. Org. Coat. 1997, 32 (1–4), 89–96. 10.1016/S0300-9440(97)00064-7. [DOI] [Google Scholar]
- Subbotina I.; Shelimov B.; Kazanskij V. Olefin metathesis over silica-supported molybdena catalysts activated by UV-irradiation in the presence of alkanes. Kinetika i Kataliz 1997, 38, 742–748. [Google Scholar]
- Tarasov A. L.; Shelimov B. N.; Kazansky V. B.; Mol J. C. Olefin metathesis on supported rhenium catalysts activated by γ-irradiation. J. Mol. Catal. A: Chem. 1997, 115 (1), 219–228. 10.1016/S1381-1169(96)00104-5. [DOI] [Google Scholar]
- Wang D.; Wurst K.; Buchmeiser M. R. Cationic versus Neutral RuII––N Heterocyclic Carbene Complexes as Latent Precatalysts for the UV Induced Ring Opening Metathesis Polymerization. Chem.—Eur. J. 2010, 16 (43), 12928–12934. 10.1002/chem.201001999. [DOI] [PubMed] [Google Scholar]
- Wang D.; Wurst K.; Knolle W.; Decker U.; Prager L.; Naumov S.; Buchmeiser M. R. Cationic RuII Complexes with N Heterocyclic Carbene Ligands for UV Induced Ring Opening Metathesis Polymerization. Angew. Chem., Int. Ed. 2008, 47 (17), 3267–3270. 10.1002/anie.200705220. [DOI] [PubMed] [Google Scholar]
- Weitekamp R. A.Multifunctional Materials: Bottom-Up and Top-Down. Ph.D. Thesis. California Institute of Technology, 2015. [Google Scholar]
- Vaisman A.; Vidavsky Y.; Baranov M.; Lehrer A.; Baraban J. H.; Lemcoff N. G. Latency for All: Enabling Latency of Hoveyda-Grubbs Second-Generation Catalysts by Adding Phosphite Ligands. J. Am. Chem. Soc. 2024, 146 (1), 73–78. 10.1021/jacs.3c10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Asuly A.; Aharoni A.; Diesendruck C. E.; Vidavsky Y.; Goldberg I.; Straub B. F.; Lemcoff N. G. Photoactivation of ruthenium olefin metathesis initiators. Organometallics 2009, 28 (16), 4652–4655. 10.1021/om9004302. [DOI] [Google Scholar]
- Alassad N.; Nechmad N. B.; Phatake R. S.; Reany O.; Lemcoff N. G. Steric and electronic effects in latent S -chelated olefin metathesis catalysts. Catalysis Science & Technology 2023, 13 (2), 321–328. 10.1039/D2CY00943A. [DOI] [Google Scholar]
- Eivgi O.; Guidone S.; Frenklah A.; Kozuch S.; Goldberg I.; Lemcoff N. G. Photoactivation of Ruthenium Phosphite Complexes for Olefin Metathesis. ACS Catal. 2018, 8 (7), 6413–6418. 10.1021/acscatal.8b01637. [DOI] [Google Scholar]
- Eivgi O.; Vaisman A.; Nechmad N. B.; Baranov M.; Lemcoff N. G. Latent Ruthenium Benzylidene Phosphite Complexes for Visible-Light-Induced Olefin Metathesis. ACS Catal. 2020, 10 (3), 2033–2038. 10.1021/acscatal.9b05079. [DOI] [Google Scholar]
- Alassad N.; Phatake R. S.; Baranov M.; Reany O.; Lemcoff N. G. Tuning the Latency by Anionic Ligand Exchange in Ruthenium Benzylidene Phosphite Complexes. Catalysts 2023, 13 (11), 1411. 10.3390/catal13111411. [DOI] [Google Scholar]
- Segalovich-Gerendash G.; Baranov M.; Lemcoff N. G.; Phatake R. S. Ruthenium Olefin Metathesis Catalysts with Six-Membered Chelating Dithioacetal Ligands: Synthesis and Reactivity. Organometallics 2023, 42 (9), 825–831. 10.1021/acs.organomet.3c00093. [DOI] [Google Scholar]
- Nechmad N. B.; Iudanov K.; Tarannam N.; Kobernik V.; Kozuch S.; Lemcoff N. G. Coordinating Additives as Activity Modulators in Diiodo Latent Olefin Metathesis Catalysts. ChemCatChem. 2023, 15 (4), e202201690. 10.1002/cctc.202201690. [DOI] [Google Scholar]
- Ginzburg Y.; Anaby A.; Vidavsky Y.; Diesendruck C. E.; Ben-Asuly A.; Goldberg I.; Lemcoff N. G. Widening the Latency Gap in Chelated Ruthenium Olefin Metathesis Catalysts. Organometallics 2011, 30 (12), 3430–3437. 10.1021/om200323c. [DOI] [Google Scholar]
- Monsigny L.; Cejas Sánchez J.; Piątkowski J.; Kajetanowicz A.; Grela K. Synthesis and Catalytic Properties of a Very Latent Selenium-Chelated Ruthenium Benzylidene Olefin Metathesis Catalyst. Organometallics 2021, 40 (21), 3608–3616. 10.1021/acs.organomet.1c00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawiasz K. J.; Paul J. E.; Schwarz K. J.; Sottos N. R.; Moore J. S. Photoexcitation of Grubbs’ Second-Generation Catalyst Initiates Frontal Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2020, 9 (11), 1563–1568. 10.1021/acsmacrolett.0c00486. [DOI] [PubMed] [Google Scholar]
- Delaude L.; Demonceau A. Retracing the evolution of monometallic ruthenium-arene catalysts for C-C bond formation. Dalton Transactions 2012, 41 (31), 9257–9268. 10.1039/c2dt30293d. [DOI] [PubMed] [Google Scholar]
- Demonceau A.; Noels A. F.; Saive E.; Hubert A. J. Ruthenium-catalysed ring-opening metathesis polymerization of cycloolefins initiated by diazoesters. J. Mol. Catal. 1992, 76 (1), 123–132. 10.1016/0304-5102(92)80151-6. [DOI] [Google Scholar]
- Trinh T. K. H.; Schrodj G.; Rigolet S.; Pinaud J.; Lacroix-Desmazes P.; Pichavant L.; Héroguez V.; Chemtob A. Combining a ligand photogenerator and a Ru precatalyst: a photoinduced approach to cross-linked ROMP polymer films. RSC Adv. 2019, 9 (48), 27789–27799. 10.1039/C9RA05831A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D. M.; Dias E. L.; Grubbs R. H.; Mohr B.. Acid activation of ruthenium metathesis catalysts and living ROMP metathesis polymerization in water. Google Patents: 2002.
- Khalimon A. Y.; Leitao E. M.; Piers W. E. Photogeneration of a Phosphonium Alkylidene Olefin Metathesis Catalyst. Organometallics 2012, 31 (15), 5634–5637. 10.1021/om3005965. [DOI] [Google Scholar]
- Liberman-Martin A. L.; Grubbs R. H. Ruthenium Olefin Metathesis Catalysts Featuring a Labile Carbodicarbene Ligand. Organometallics 2017, 36 (21), 4091–4094. 10.1021/acs.organomet.7b00615. [DOI] [Google Scholar]
- Sutar R. L.; Levin E.; Butilkov D.; Goldberg I.; Reany O.; Lemcoff N. G. A Light-Activated Olefin Metathesis Catalyst Equipped with a Chromatic Orthogonal Self-Destruct Function. Angew. Chem., Int. Ed. 2016, 55 (2), 764–767. 10.1002/anie.201508966. [DOI] [PubMed] [Google Scholar]
- Arumugam K.; Varnado Jr C. D.; Sproules S.; Lynch V. M.; Bielawski C. W. Redox-Switchable Ring-Closing Metathesis: Catalyst Design, Synthesis, and Study. Chem.—Eur. J. 2013, 19 (33), 10866–10875. 10.1002/chem.201301247. [DOI] [PubMed] [Google Scholar]
- Ahumada G.; Ryu Y.; Bielawski C. W. Potentiostatically Controlled Olefin Metathesis. Organometallics 2020, 39 (10), 1744–1750. 10.1021/acs.organomet.0c00052. [DOI] [Google Scholar]
- Dumas A.; Colombel-Rouen S.; Curbet I.; Forcher G.; Tripoteau F.; Caijo F.; Queval P.; Rouen M.; Baslé O.; Mauduit M. Highly selective macrocyclic ring-closing metathesis of terminal olefins in non-chlorinated solvents at low dilution. Catalysis Science & Technology 2019, 9 (2), 436–443. 10.1039/C8CY02115E. [DOI] [Google Scholar]
- Kamal F.; Colombel-Rouen S.; Dumas A.; Guégan J.-P.; Roisnel T.; Dorcet V.; Baslé O.; Rouen M.; Mauduit M. Activation of olefin metathesis complexes containing unsymmetrical unsaturated N-heterocyclic carbenes by copper and gold transmetalation. Chem. Commun. 2019, 55 (77), 11583–11586. 10.1039/C9CC05776E. [DOI] [PubMed] [Google Scholar]
- Theunissen C.; Ashley M. A.; Rovis T. Visible-Light-Controlled Ruthenium-Catalyzed Olefin Metathesis. J. Am. Chem. Soc. 2019, 141 (17), 6791–6796. 10.1021/jacs.8b13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso J. V.; Gebel P.; Gramm V.; Frey W.; Buchmeiser M. R. Tungsten Oxo and Tungsten Imido Alkylidene N-Heterocyclic Carbene Complexes for the Visible-Light-Induced Ring-Opening Metathesis Polymerization of Dicyclopentadiene. Macromolecules 2023, 56 (7), 2878–2888. 10.1021/acs.macromol.3c00128. [DOI] [Google Scholar]
- Cabanero D. C.; Nguyen J. A.; Cazin C. S. J.; Nolan S. P.; Rovis T. Deep Red to Near-Infrared Light-Controlled Ruthenium-Catalyzed Olefin Metathesis. ACS Catal. 2023, 13 (7), 4384–4390. 10.1021/acscatal.3c00473. [DOI] [Google Scholar]
- Ivry E.; Nechmad N. B.; Baranov M.; Goldberg I.; Lemcoff N. G. Influence of Anionic Ligand Exchange in Latent Sulfur-Chelated Ruthenium Precatalysts. Inorg. Chem. 2018, 57 (24), 15592–15599. 10.1021/acs.inorgchem.8b02917. [DOI] [PubMed] [Google Scholar]
- Nechmad N. B.; Phatake R.; Ivry E.; Poater A.; Lemcoff N. G. Unprecedented Selectivity of Ruthenium Iodide Benzylidenes in Olefin Metathesis Reactions. Angew. Chem., Int. Ed. 2020, 59 (9), 3539–3543. 10.1002/anie.201914667. [DOI] [PubMed] [Google Scholar]
- Nechmad N. B.; Kobernik V.; Tarannam N.; Phatake R.; Eivgi O.; Kozuch S.; Lemcoff N. G. Reactivity and Selectivity in Ruthenium Sulfur-Chelated Diiodo Catalysts. Angew. Chem., Int. Ed. 2021, 60 (12), 6372–6376. 10.1002/anie.202014929. [DOI] [PubMed] [Google Scholar]
- Robertson I. D.; Dean L. M.; Rudebusch G. E.; Sottos N. R.; White S. R.; Moore J. S. Alkyl Phosphite Inhibitors for Frontal Ring-Opening Metathesis Polymerization Greatly Increase Pot Life. ACS Macro Lett. 2017, 6 (6), 609–612. 10.1021/acsmacrolett.7b00270. [DOI] [PubMed] [Google Scholar]
- Stawiasz K. J.; Wendell C. I.; Suslick B. A.; Moore J. S. Photoredox-Initiated Frontal Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2022, 11 (6), 780–784. 10.1021/acsmacrolett.2c00248. [DOI] [PubMed] [Google Scholar]
- Leguizamon S. C.; Cook A. W.; Appelhans L. N. Employing Photosensitizers for Rapid Olefin Metathesis Additive Manufacturing of Poly(dicyclopentadiene). Chem. Mater. 2021, 33 (24), 9677–9689. 10.1021/acs.chemmater.1c03298. [DOI] [Google Scholar]
- Leguizamon S. C.; Lyons K.; Monk N. T.; Hochrein M. T.; Jones B. H.; Foster J. C. Additive Manufacturing of Degradable Materials via Ring-Opening Metathesis Polymerization (ROMP). ACS Appl. Mater. Interfaces 2022, 14 (45), 51301–51306. 10.1021/acsami.2c14411. [DOI] [PubMed] [Google Scholar]
- Pastine S. J.; Okawa D.; Zettl A.; Fréchet J. M. J. Chemicals On Demand with Phototriggerable Microcapsules. J. Am. Chem. Soc. 2009, 131 (38), 13586–13587. 10.1021/ja905378v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcoff N.; Nechmad N. B.; Eivgi O.; Yehezkel E.; Shelonchik O.; Phatake R. S.; Yesodi D.; Vaisman A.; Biswas A.; Lemcoff N. G.; Weizmann Y. Plasmonic visible-near infrared photothermal activation of olefin metathesis enabling photoresponsive materials. Nat. Chem. 2023, 15 (4), 475–482. 10.1038/s41557-022-01124-7. [DOI] [PubMed] [Google Scholar]
- Davydovich O.; Greenlee A. J.; Root H. D.; Jansen A. L.; Gallegos S. C.; Warner M. J.; Kent M. S.; Cardenas J. A.; Appelhans L. N.; Roach D. J.; Jones B. H.; Leguizamon S. C. Encapsulated Transition Metal Catalysts Enable Long-term Stability in Frontal Polymerization Resins. Macromolecules 2023, 56 (18), 7543–7550. 10.1021/acs.macromol.3c01146. [DOI] [Google Scholar]
- Nascimento D. L.; Reim I.; Foscato M.; Jensen V. R.; Fogg D. E. Challenging Metathesis Catalysts with Nucleophiles and Brønsted Base: Examining the Stability of State-of-the-Art Ruthenium Carbene Catalysts to Attack by Amines. ACS Catal. 2020, 10 (19), 11623–11633. 10.1021/acscatal.0c02760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xi W.; Gao G.; Wang X.; Stansbury J. W.; Bowman C. N. o-Nitrobenzyl-Based Photobase Generators: Efficient Photoinitiators for Visible-Light Induced Thiol-Michael Addition Photopolymerization. ACS Macro Lett. 2018, 7 (7), 852–857. 10.1021/acsmacrolett.8b00435. [DOI] [PubMed] [Google Scholar]
- Teator A. J.; Shao H.; Lu G.; Liu P.; Bielawski C. W. A Photoswitchable Olefin Metathesis Catalyst. Organometallics 2017, 36 (2), 490–497. 10.1021/acs.organomet.6b00913. [DOI] [Google Scholar]
- Park S.; Byun S.; Ryu H.; Hahm H.; Lee J.; Hong S. Reversibly photoswitchable catalysts for olefin metathesis reactions. ACS Catal. 2021, 11 (22), 13860–13865. 10.1021/acscatal.1c04281. [DOI] [Google Scholar]
- Dawood K. M.; Nomura K. Recent Developments in Z Selective Olefin Metathesis Reactions by Molybdenum, Tungsten, Ruthenium, and Vanadium Catalysts. Advanced Synthesis & Catalysis 2021, 363 (8), 1970–1997. 10.1002/adsc.202001117. [DOI] [Google Scholar]
- Rylski A. K.; Cater H. L.; Mason K. S.; Allen M. J.; Arrowood A. J.; Freeman B. D.; Sanoja G. E.; Page Z. A. Polymeric multimaterials by photochemical patterning of crystallinity. Science 2022, 378 (6616), 211–215. 10.1126/science.add6975. [DOI] [PubMed] [Google Scholar]
- Cox L. M.; Blevins A. K.; Drisko J. A.; Qi Y.; Ding Y.; Fiedler-Higgins C. I.; Long R.; Bowman C. N.; Killgore J. P. Tunable Mechanical Anisotropy, Crack Guiding, and Toughness Enhancement in Two-Stage Reactive Polymer Networks. Adv. Eng. Mater. 2019, 21 (8), 1900578. 10.1002/adem.201900578. [DOI] [Google Scholar]
- Bialas S.; Michalek L.; Marschner D. E.; Krappitz T.; Wegener M.; Blinco J.; Blasco E.; Frisch H.; Barner-Kowollik C. Access to Disparate Soft Matter Materials by Curing with Two Colors of Light. Adv. Mater. 2019, 31 (8), 1807288. 10.1002/adma.201807288. [DOI] [PubMed] [Google Scholar]
- Dolinski N. D.; Page Z. A.; Callaway E. B.; Eisenreich F.; Garcia R. V.; Chavez R.; Bothman D. P.; Hecht S.; Zok F. W.; Hawker C. J. Solution Mask Liquid Lithography (SMaLL) for One-Step, Multimaterial 3D Printing. Adv. Mater. 2018, 30 (31), 1800364. 10.1002/adma.201800364. [DOI] [PubMed] [Google Scholar]
- Schwartz J. J.; Boydston A. J. Multimaterial actinic spatial control 3D and 4D printing. Nat. Commun. 2019, 10 (1), 791. 10.1038/s41467-019-08639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Kottisch V.; McLoughlin E. A.; Rouse Z. W.; Supej M. J.; Baker S. P.; Fors B. P. Photoswitching Cationic and Radical Polymerizations: Spatiotemporal Control of Thermoset Properties. J. Am. Chem. Soc. 2021, 143 (50), 21200–21205. 10.1021/jacs.1c09523. [DOI] [PubMed] [Google Scholar]
- Hsu T.-W.; Kempel S. J.; Michaudel Q. All-cis poly(p-phenylene vinylene)s with high molar masses and fast photoisomerization rates obtained through stereoretentive ring-opening metathesis polymerization of [2,2]paracyclophane dienes with various aryl substituents. J. Polym. Sci. 2022, 60, 569. 10.1002/pol.20210556. [DOI] [Google Scholar]
- Chiba K.; Miura T.; Kim S.; Kitano Y.; Tada M. Electrocatalytic Intermolecular Olefin Cross-Coupling by Anodically Induced Formal [2 + 2] Cycloaddition between Enol Ethers and Alkenes. J. Am. Chem. Soc. 2001, 123 (45), 11314–11315. 10.1021/ja016885b. [DOI] [PubMed] [Google Scholar]
- Ogawa K. A.; Goetz A. E.; Boydston A. J. Metal-Free Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc. 2015, 137 (4), 1400–1403. 10.1021/ja512073m. [DOI] [PubMed] [Google Scholar]
- Goetz A. E.; Boydston A. J. Metal-Free Preparation of Linear and Cross-Linked Polydicyclopentadiene. J. Am. Chem. Soc. 2015, 137 (24), 7572–7575. 10.1021/jacs.5b03665. [DOI] [PubMed] [Google Scholar]
- Yang X.; Gitter S. R.; Roessler A. G.; Zimmerman P. M.; Boydston A. J. An Ion Pairing Approach to Stereoselective Metal Free Ring Opening Metathesis Polymerization. Angew. Chem. 2021, 133 (25), 14071–14077. 10.1002/ange.202016393. [DOI] [PubMed] [Google Scholar]
- Yang X.; Murphy L. M.; Haque F. M.; Grayson S. M.; Boydston A. J. A highly efficient metal-free protocol for the synthesis of linear polydicyclopentadiene. Polym. Chem. 2021, 12 (19), 2860–2867. 10.1039/D1PY00191D. [DOI] [Google Scholar]
- Lu P.; Alrashdi N. M.; Boydston A. J. Bidirectional metal free ROMP from difunctional organic initiators. J. Polym. Sci., Part A: Polym. Chem. 2017, 55 (18), 2977–2982. 10.1002/pola.28704. [DOI] [Google Scholar]
- Mandal A.; Kilbinger A. F. Catalytic living ROMP: block copolymers from macro-chain transfer agents. Polym. Chem. 2023, 14 (23), 2797–2802. 10.1039/D3PY00387F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A.; Mandal I.; Kilbinger A. F. Catalytic Living Ring Opening Metathesis Polymerization Using Vinyl Ethers as Effective Chain Transfer Agents. Angew. Chem. 2023, 135 (4), e202211842. 10.1002/ange.202211842. [DOI] [PubMed] [Google Scholar]
- Mandal I.; Mandal A.; Kilbinger A. F. M. Macrochain Transfer Agents for Catalytic Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2022, 11 (12), 1384–1389. 10.1021/acsmacrolett.2c00684. [DOI] [PubMed] [Google Scholar]
- Mandal I.; Mandal A.; Rahman M. A.; Kilbinger A. F. M. Chain transfer agents for the catalytic ring opening metathesis polymerization of norbornenes. Chemical Science 2022, 13 (42), 12469–12478. 10.1039/D2SC04078F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. J.; Lien H. M.; Prine N.; Burns C.; Rylski A. K.; Gu X.; Cox L. M.; Mangolini F.; Freeman B. D.; Page Z. A. Multimorphic materials: spatially tailoring mechanical properties via selective initiation of interpenetrating polymer networks. Adv. Mater. 2023, 35 (9), 2210208. 10.1002/adma.202210208. [DOI] [PubMed] [Google Scholar]