Abstract
Gut dysbiosis contributes to deterioration of chronic kidney disease (CKD). Probiotics are a potential approach to modulate gut microbiota and gut-derived metabolites to alleviate CKD progression. We aim to provide a comprehensive view of CKD-related gut dysbiosis and a critical perspective on probiotic function in CKD. First, this review addresses gut microbial alterations during CKD progression and the adverse effects associated with the changes in gut-derived metabolites. Second, we conduct a thorough examination of the latest clinical trials involving probiotic intervention to unravel critical pathways via the gut–kidney axis. Finally, we propose our viewpoints on limitations, further considerations, and future research prospects of probiotic adjuvant therapy in alleviating CKD progression. Enhancing our understanding of host–microbe interactions is crucial for gaining precise insights into the mechanisms through which probiotics exert their effects and identifying factors that influence the effectiveness of probiotics in developing strategies to optimize their use and enhance clinical outcomes.
Keywords: gut-dysbiosis, chronic kidney disease, gut−kidney axis, probiotic adjuvant therapy, host−microbe interactions
1. Introduction
Chronic kidney disease (CKD) is characterized by a substantial loss of kidney function and is associated with consistent exposure to numerous risk factors such as hypertension, diabetes mellitus, and obesity, leading to the irreversible progressive decline of kidney excretory function. The accumulation of uremic toxins in the circulation that are normally excreted by healthy kidneys causes massive damage to the kidney. Typically, kidney fibrosis is involved in CKD development, leading to myofibroblast activation, migration, accumulation of extracellular matrix, and kidney failure.1 CKD also impacts other organs and tissues, especially the cardiovascular system. Left ventricular hypertrophy is highly prevalent in patients with CKD, which is strongly associated with systolic hypertension and eventually results in heart failure, a leading cause of morbidity and mortality in this population.2,3
The CKD diagnosis is based on kidney function and structure abnormalities that persist for >3 consecutive months. The Kidney Disease Improving Global Outcomes (KDIGO) classifies CKD based on the estimated glomerular filtration rate (eGFR) into five stages: >90 mL/min per 1.73 m2 (stage 1), 60–89 mL/min per 1.73 m2 (stage 2), 30–59 mL/min per 1.73 m2 (stage 3), 15–29 mL/min per 1.73 m2 (stage 4), and <15 mL/min per 1.73 m2 (stage 5). The extent of albuminuria is also an additional indicator of end-stage renal disease (ESRD) progression.4
The global CKD prevalence is increasing, affecting over 10% of the global population and accounting for over 843.6 million individuals worldwide.5 Unfortunately, there is no cure for CKD, with treatments, including lifestyle changes, medication, and dialysis, only assisting in relieving symptoms and delaying their progression. A kidney transplant may also be a treatment option for patients suffering from ESRD. However, the limited number of donated kidneys and lengthy waiting periods impede its accessibility. Therefore, CKD is becoming a severe public health issue, requiring a better solution to ameliorate and alleviate its progression.6
Accumulating clinical evidence supports the theory that gut dysbiosis significantly contributes to deteriorating CKD progression, generating gut-derived uremic toxin (GDUT) and aggravating kidney failure.7−9 Therefore, strategies based on microbiota-based interventions could be considered preventive and therapeutic approaches to modulate gut microbiota and their metabolites to alleviate CKD progression. Probiotics are “live microorganisms that, when administered in adequate amounts, exist a health benefit on the host”.10 They are well-recognized for modulating gut microbiota, and research is ongoing regarding their efficacy in preventing and managing CKD. The efficacy of probiotics in decreasing uremic toxin production and improving renal function has been investigated using in vitro models11,12 and various animal11,13 and human CKD studies.14
To provide a critical perspective on the potential of probiotics as preventive and therapeutic approaches in CKD, we first systematically delve into gut dysbiosis and its interplay with gut microbiota, associated metabolites, and CKD progression. Subsequently, we evaluate the latest studies examining the efficacy of probiotics in clinical cohorts and comprehensively review their mechanisms of action in CKD through the gut–kidney axis. Finally, we discuss the limitations, further considerations, and future research prospects regarding the utilization of probiotics as adjuvant therapy to enhance outcomes in patients with CKD.
2. Strong Link between CKD and Gut Microbial Dysbiosis
The intestinal microbiota is markedly altered in CKD due to multifactorial causes, including iatrogenic effects and altered physiological conditions.15,16 Antibiotics, phosphate binders, dietary restriction, and low fiber consumption lead to slow intestinal transit, impaired protein assimilation, metabolic acidosis, and constipation.17 CKD-induced adaptive secretion of uric acid by the colon also limits the increase in serum uric acid concentration.18 More undigested protein and urea/uric acid in the colon enrich the proliferation of proteolytic bacteria with urease, uricase, phenol-, and indole-forming enzymes, whereas the short-chain fatty acid-producing bacteria are decreased.9 The increased intestinal pH through the augmented secretion of urea into the gut, along with limited carbohydrate fermentation, favors disruption of the intestinal epithelial barrier, and the production of phenolic and indolic compounds by colonic bacteria.19 This CKD-induced gut dysbiosis increases the production of GDUT and decreases the level of short-chain fatty acid (SCFA) such as butyric acid. The alteration of the gut environment affects the gut barrier by disrupting the colonic epithelial tight junctions, which in turn facilitates the movement of endotoxin, pathogenic microbes, and bacterial fragments into the systemic circulation that triggers inflammation.20
Additionally, the bladder microbiotas also plays a pivotal role in urinary tract infections, interstitial cystitis, urinary incontinence, and kidney stones.21 To date, the literature discussing the relationship between gut microbiota and bladder microbiota is limited. However, kidney function undoubtedly impacts the bladder microbiota, as evidenced by Hrbacek et al. (2022), who found distinct overall microbial compositions between patients with CKD and healthy individuals.22 Furthermore, Kramer et al. (2018) discovered that among patients with CKD, those with better kidney function (higher eGFR) exhibited greater diversity in their urinary microbiota.23 The composition of a patient’s urine, which affects the urinary microbiota, can be influenced by kidney function. For instance, a significant decrease in eGFR leads to reduced concentrations of uromodulin (also known as Tamm–Horsfall glycoprotein), produced by renal tubules. Because uromodulin has bacteriostatic properties, its diminished presence affects bacterial growth.24 Therefore, CKD affects the diversity and composition of the bladder/urinary microbiota, subsequently influencing urinary tract symptoms and bladder health.
2.1. Gut-Derived Uremic Toxins Play a Significant Role in CKD Progression
Uremic retention solutes, so-called uremic toxins, are normally excreted by the healthy kidney but accumulate in patients with CKD and contribute to biological dysfunction.25 They can be categorized into three major types: (1) free water-soluble molecules with a molecular weight <500 Da that readily pass the dialysis filter with less toxicity (e.g., trimethylamine, urea, oxalate, creatinine); (2) middle molecules with a molecular weight ≥500 Da that have limited passing capacity due to the dialysis membrane characteristics (e.g., adiponectin, cystatin C, TNF-α); (3) low molecular weight protein-bound molecules which their dialytic removal predominantly depends on the equilibrium between bound and free molecules (e.g., indoxyl sulfate, p-cresyl sulfate) (Table 1).26 Uremic toxins can also be classified according to the site of origin,27 such as GDUT, produced from microbial metabolism. Gut microorganisms can deaminate or decarboxylate amino acids.28 Deamination of aromatic amino acids (tyrosine, tryptophan, and phenylalanine) leads to the formation of phenolic compounds (indole, p-cresol, and phenol). Consequently, gut microbial dysbiosis could increase the production of GDUT precursors and generate more uremic toxins.29
Table 1. Classification of Uremic Toxinsa,25,37.
| categories | numbers | MW | molecules |
|---|---|---|---|
| free water-soluble molecules | 45 | <500 Da | • creatinine |
| • hypoxanthine | |||
| • oxalate | |||
| • SDMA | |||
| • TMA/TMAO | |||
| • urea | |||
| • uric acid | |||
| middle molecules | 22 | ≥500 Da | • adiponectin |
| • cystatin C | |||
| • cytokine: IL-6, IL-8, IL-10, and TNF-α | |||
| • fibroblast growth factor-23 | |||
| • glutathione (oxidized) | |||
| • leptin | |||
| • retinol-binding protein | |||
| • VEGF | |||
| protein-bound molecules | 25 | <500 Da (most of them except for leptin and retinol-binding protein) | • 4-ethylphenyl sulfate |
| • CMPF | |||
| • hippuric acid | |||
| • indoleacetic acid | |||
| • indoxyl glucuronide | |||
| • indoxyl sulfate | |||
| • kynurenic acid | |||
| • leptin | |||
| • p-cresol | |||
| • p-cresyl glucuronide | |||
| • p-cresyl sulfate | |||
| • phenol | |||
| • phenyl glucuronide | |||
| • phenyl sulfate | |||
| • phenylacetic acid | |||
| • phenylacetyl glutamine | |||
| • retinol-binding protein | |||
| total | 90 | leptin and retinol-binding protein exhibit distinctive characteristics in two groups | |
Da, Dalton; SDMA, symmetric dimethylarginine; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; IL, interleukin; TNF-α, tumor necrosis factor α; CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid; VEGF, vascular endothelial growth factor.
GDUTs have recently gained attention for their role in CKD as they cause detrimental effects on renal, vascular, cardiac, and other tissues and organs.30 Currently, five GDUTs are associated with cardiovascular disease and mortality in CKD as well as other end-organ toxicity: indoxyl sulfate (IS), indole-3 acetic acid (IAA), p-cresyl sulfate (PCS), phenylacetylglutamine (PAG), and trimethylamine-N-oxide (TMAO).31−33 Dietary protein is the source of IS, IAA, PCS, and PAG, whereas IS and IAA are protein-bound uremic toxins generated from dietary tryptophan. Indole, a precursor of IS, is a product of tryptophan produced by intestinal microorganisms, which is converted into an IS through sulfation in the liver after intestinal absorption. IAA biosynthesis is linked to the metabolism of tryptophan and involves the elimination of amino and carboxyl groups from tryptophan α-carbon via intermediates such as indolepyruvate, indoleacetamide, or indoleacetonitrile.34 PCS and PAG are derived from tyrosine and phenylalanine, which are metabolized and converted into p-cresol by intestinal proteolytic microorganisms in the colon, then absorbed into the circulation and subsequently undergo oxidization and sulfation by the liver to produce PCS.35 PAG biosynthesis is also involved in phenylalanine metabolism. Gut microbiota fermented unabsorbed phenylalanine to produce phenylacetate and conjugate with glutamine to generate PAG in the liver.36 Trimethylamine (TMA), a precursor of TMAO, is produced by bacterial metabolism of quaternary amines such as choline, phosphatidylcholine, betaine, and l-carnitine. TMA is subsequently absorbed and oxidized by the hepatic enzyme flavin monooxygenase isoform 3 (FMO3) to form TMAO in the liver.3
IS, IAA, PCS, and PAG are the protein-bound molecules with high binding affinity for albumin.37 These albumin complexes are transported to proximal renal tubular cells and excreted via organic anion transporter (OAT) 1 and OAT3, located on the basolateral membrane of tubular cells.38 OATs are inhibited by high levels of GDUTs as kidney function declines.39 Accumulation of IS, IAA, PCS, and PAG in circulation is toxic due to the limited dialytic clearance by conventional hemodialysis and their adverse health impact, resulting in cardiovascular complications and mortality in patients with CKD.40 Additionally, IS and PCS increase oxidative stress in renal tubular cells associated with CKD progression and its comorbidities.41 TMAO is efficiently removed by dialysis, but it is a risk factor for CKD and cardiovascular disease (CVD).42 A clinical trial of 4007 patients concluded that elevated plasma TMAO levels are associated with an increased risk of incident major adverse cardiovascular events such as death, myocardial infarction, or stroke during three years of follow-up.43
2.2. Gut Microbial Alternation in Patients with CKD is a Potential Biomarker for the Detection of Kidney Disease
Numerous studies have reported CKD-associated changes in the human gut microbiome composition, indicating strong links between CKD and gut microbial dysbiosis (Table 2). Based on previous studies with more than 75% consistent findings, patients with kidney disease have a higher relative abundance of Proteobacteria, Enterobacteriaceae, Streptococcaceae, Streptococcus, Bilophila, Desulfovibrio, Klebsiella, Escherichia-Shigella, and lower abundance of Firmicutes, Prevotellaceae, Prevotella, Prevotella 9, Alcaligenaceae, Roseburia, Faecalibacterium, and Faecalibacterium prausnitzii in comparison to healthy subjects.44 Immunoglobulin A (IgA) nephropathy patients have increased Ruminococcaceae, Lachnospiraceae, Eubacteriaceae, and Streptococcaeae in Firmicutes, while Bifidobacterium and species of Clostridium, Enterococcus, and Lactobacillus were decreased compared to healthy controls.45
Table 2. Summary of the Current Literature Supporting Gut Dysbiosis in Patients with Chronic Kidney Diseasea.
| subjects | alteration of gut microbiota | main findings | ref |
|---|---|---|---|
| immunoglobulin (Ig) A nephropathy patients | • elevation of some genera/species of Ruminococcaceae, Lachnospiraceae, Eubacteriaceae, Streptococcaeae, Sutterellaceae, and Enterobacteriaceae | • IgAN patients had an altered fecal microbiota | (45) |
| • reduction of Bifidobacterium and species of Clostridium, Enterococcus and Lactobacillus | |||
| HD patients | • elevation of enterobacteria (Klebsiella and Escherichia coli), enterococci, and Clostridium perfringens | • an overgrowth of aerobes of the fecal microbiota in HD patients is responsible for a high accumulation of p-cresol and indole | (81) |
| • reduction of bifidobacteria | |||
| CKD stage 2–3 patients | • elevation of the phyla Actinobacteria and Proteobacteria | • lower intestinal flora diversity and abundances | (49) |
| • elevation of the genera Bacteroides, Escherichia, Ruminococcus, Blautia, Enterococcus, Clostridium, Eubacterium, Klebsiella, Sarcina, Eggerthella, Turicibacter, Bilophila and Pseudoramibacter | • Ruminococcus and Roseburia displayed the highest diagnostic values for distinguishing CKD patients from healthy controls | ||
| • reduction of the genera Roseburia, Prevotella, Faecalibacterium, Megamonas, Coprococcus, Burkholderia, Dialister, Lachnospira, Streptococcus, Megasphaera, Sutterella, Collinsella, Stenotrophomonas, Haemophilus, Odoribacter, Butyricimonas, Acidaminococcus, and Granulicatella | |||
| ESRD patients | • elevation of Bacteroides, Escherichia/Shigella, Subdoligranulum, Fusobacterium | • this reduction in beneficial bacteria may play an important role in the pathogenic processes of CKD | (106) |
| • reduction of Prevotella, Roseburia, Faecalibacterium, Clostridium, Coprococcus, Dorea. | |||
| HD patients and nondialyzed CKD patients | • elevation of the phyla Bacteroidetes and Proteobacteria | • the differential gut microbiota has the potential to guide noninvasive diagnosis and targeted interventions | (119) |
| • elevation of the genera Bacteroides, Escherichia Shigella, Parabacteroides, Ruminococcus gnavus group, Ruminococcus torques group, Weissella, Flavonifractor, Ruminiclostridium 5, Sellimonas, Erysipelatoclostridium, Eggerthella, and Clostridium innocuum group | • Holdemanella, Megamonas, and Prevotella2 were exhibited the highest abundance, whereas Dielma and Scardovia were absent in healthy controls; these genera could be an indicator of the progression of CKD and HD | ||
| • reduction of the phyla Firmicutes | |||
| • reduction of the genera Dialister, Eubacterium rectale group, Carnobacterium, Lachnospira, Subdoligranulum, Eubacterium coprostanoligenes group, Coprococcus 2, Roseburia, RuminococcaceaeUCG 009, Ruminococcaceae NK4A214 group, Lachnospiraceae FCS020 group, Ruminococcus1, Romboutsia, Butyricicoccus, Collinsella, RuminococcaceaeUCG 003, Eubacterium hallii group, Tyzzerella3, and LachnospiraceaeUCG 001 | |||
| ESRD patients | • elevation of the families Enterobacteriaceae, Halomonadaceae, Moraxellaceae, Polyangiaceae, Pseudomonadaceae, and genera Brachybacterium, Catenibacterium, Nesterenkonia, and Thiothrix were markedly increased in patients with ESRD | • ESRD significantly modifies the composition of gut microbiome in humans; uremia and the strict dietary restrictions must have contributed to the observed changes in their microbial flora | (111) |
| • reduction of the families Sutterellaceae, Bacteroidaceae, and Lactobacillaceae | |||
| CKD and ESRD patients | • at the phylum level, the relative abundance of Proteobacteria was enriched in the ESRD group, whereas Euryarchaeota was more abundant in the healthy control group | • a decrease in 14 SCFA-producing bacteria in the CKD group, including R. bromii, R. callidus, R. hominis, E. rectale, F. prausnitzii, C. comes, C. eutactus, C. sporogenes, S. variabile, D. succinatiphilus, B. adolescentis, L. crispatus, A. indistinctus, and A. inops, which could promote CKD progression by impairing intestinal barrier function and stimulating excessive inflammation | (7) |
| • at the family level, Veillonellaceae, Lactobacillaceae, and Eubacteriaceae significantly decreased, but Enterobacteriaceae gradually increased in the ESRD group. Rikenellaceae, Lactobacillaceae, and Clostridiaceae decreased in the moderate CKD group, and Selenomonadaceae increased in the mild CKD group | • the distinct changes in gut microbiota were associated with alterations in the metabolic functions of arginine and proline, arachidonic acid, and glutathione, as well as in the biosynthesis pathways of ubiquinone and other terpenoid-quinone compounds during CKD progression | ||
| • at the genus level, Roseburia, Faecalibacterium, Eubacterium rectale, Eubacterium, and Ruminococcus considerably decreased in the moderate CKD and ESRD groups, whereas Flavonifractor and Citrobacter increased in these groups | • the disrupted microbiota in relation to CKD severity may be implicated in an imbalanced toxic and pro-oxidant metabolism within both the gut and host, ultimately accelerating the CKD progression, which may represent a valuable early diagnostic and therapeutic target for CKD | ||
| • Species varied across CKD progress: | |||
| Four species increased (Citrobacter freundii, Citrobacter werkmanii, Flavonifractor plautii, and Anaerostipes caccae) during CKD progression | |||
| Fourteen species decreased (Methanobrevibacter smithii, Coprococcus comes, Coprococcus eutactus, Clostridium sporogenes, Ruminococcus callidus, Ruminococcus bromii, Roseburia hominis, Faecalibacterium prausnitzii, Veillonella parvula, Megasphaera elsdenii, Dialister succinatiphilus, Acidaminococcus intestini, Faecalicoccus pleomorphus, and Subdoligranulum variabile) during CKD progression | |||
| • Species varied in only a specific CKD group: | |||
| Megasphaera micronuciformis level increased in the mild CKD group | |||
| Alistipes indistinctus, Alistipes inops, and Bacteroides uniformis levels decreased in the moderate CKD group | |||
| Turicibacter sanguinis level increased, but the Streptococcus mutans, Bifidobacterium adolescentis, and Lactobacillus crispatus levels decreased in the ESRD group | |||
| ESRD patients | • the most enriched species in patients included Eggerthella lenta, Flavonifractor spp. (mainly F. plautii), Alistipes spp. (mainly A. finegoldii and A. shahii), Ruminococcus spp. and Fusobacterium spp. | • over a half of the species were significantly altered in ESRD patients, suggesting ESRD strongly affects the microbiome | (120) |
| • depleted species included Prevotella spp. (mainly P. copri), Clostridium spp. and several butyrate producers (Roseburia spp., Faecalibacterium prausnitzii and Eubacterium rectale) | • the enrichment of uremic toxins in patients with ESRD is associated with gut microbiome-mediated aromatic amino acids degradation and microbial secondary bile acids biosynthesis | ||
| ESRD patients | • elevation of Alteromonadaceae, Clostridiaceae, Dermabacteraceae, Enterobacteriaceae, Halomonadaceae, Polyangiaceae, Moraxellaceae, Methylococcaceae, Micrococcaceae, Cellulomonadaceae, Pseudomonadaceae, Xanthomonadaceae, Verrucomicrobiaceae | • patients with ESRD exhibited significant expansion of bacteria possessing urease, uricase, and indole- and p-cresol-forming enzymes and reduced bacterial families possessing butyrate-forming enzymes | (9) |
| • reduction of Lactobacillaceae, and Prevotellaceae. | |||
| ESRD patients | • four decreased (Prevotella sp. 885, Weissella confuse, Roseburia faecis, and Bacteroides eggerthii) and three elevated species (Alloscardovia omnicolens, Merdibacter massiliensis, and Clostridium glycyrrhizinilyticum) were altered across non-CKD, early to advanced stages | • these results implicate specific gut microorganisms as useful biomarkers for early CKD diagnosis and prognosis monitoring | (118) |
| • Cetobacterium somerae (mild CKD), Candidatus Stoquefichus sp. KLE1796 (mild CKD), Fusobacterium mortiferum (moderate CKD), Bariatricus massiliensis (moderate CKD), Bacteroides stercorirosoris (moderate CKD), and Merdimonas faecis (advanced CKD) were altered only in particular stages | |||
| CKD stage 4–5 patients | • elevation of Proteobacteria, Enterobacteriaceae, Corynebacteriaceae, Enterococcus | • CKD patients have increased plasma TMAO levels due to contributions from impaired renal functions and gut microbiota dysbiosis | (8) |
| • reduction of Ruminococcaceae, Prevotella, Roseburia, Coprococcus | |||
| CKD stage 2–4 cats | • reduction of Holdemania, Adlercreutzia, Eubacterium, Slackia, and Mogibacterium | • decreased fecal microbiome diversity and richness are associated with CKD in cats | (121) |
HD, hemodialysis; ESRD, end stage renal disease; CKD, chronic kidney disease; TMAO, trimethylamine-N-oxide.
Ren et al. investigated differences in the microbial structure based on different CKD stages. Linear discriminant analysis (LDA) indicated that Tenericutes and Mollicutes were enhanced in CKD stages 1–2, Parasutterella was enriched in CKD stages 3–4, and Akkermansia, Blautia, and Verrucomicrobia were augmented in CKD stage 5.46Alphaproteobacteria, Streptococcaceae, and Streptococcus were more abundant in adults receiving hemodialysis or peritoneal dialysis than in controls.44 Guirong et al. evaluated the gut microbiota composition of kidney transplant (KT) recipients reporting that their gut microbial profiles were similar to patients with CKD stages 3–4, with increased Bacteroidetes, Proteobacteria, Clostridiales, and Enterobacteriaceae but decreased Firmicutes, Lachnospiraceae, Ruminococcaceae, and Faecalibacterium compared to healthy controls.47
These differences in bacterial phyla and genera between patients with CKD and healthy controls suggest that the alterations in bacterial taxa may offer valuable insights into predicting CKD progression. Akkermansia (the area under the receiver operating characteristic curve (AUC) = 0.753),48Lactobacillus (AUC = 0.792),48Ruminococcus (AUC = 0.771),49 and Roseburia (AUC = 0.803)49 differentiated adults with CKD from controls. Furthermore, Escherichia-Shigella and Prevotella 9 (AUC = 0.86) could be used to accurately distinguish patients with diabetic nephropathy from age/gender-matched diabetes mellitus.50
2.3. Gut Microbial Dysbiosis Affects CKD Progression
The alteration of gut microbial profiles significantly affects CKD progression through the following mechanisms:
2.3.1. Bacterial Families Possessing the Enzymes to Synthesize Uremic Toxins
Three bacterial families (Clostridiaceae, Enterobacteriaceae, and Verrucomicrobiaceae) contain the tryptophanase gene to produce indole. Five families (Cellulomonadaceae, Dermabacteraceaea, Micrococcaceae, Polyangiaceae, and Xanthomonadaceae) possess the uricase gene, and two (Clostriadiaceae and Enterobacteriaceae) can deaminate tyrosine into p-cresol.9 These bacterial families are dominant in ESRD patients. Gryp et al. reported that the gut microbiome of patients with CKD was dominated by GDUT-precursor producers including Actinomycetaceae, Bacteroidaceae, Clostridiaceae, Enterococcaceae, Lachnospiraceae, Staphylococcaceae, Tannerellaceae, Bacteroides uniformis, Odoribacter splanchnicus, and Oscillibacter sp., generating p-cresol, phenol, and IAA.51 Other families, such as Bifidobacteriaceae, Coriobacteriaceae, Enterobacteriaceae, Propionibacteriaceae, and Rikenellaceae, are involved in indole production.51 A high abundance of bacterial families with urease, uricase, and indole and p-cresol-forming enzymes could accelerate CKD progression by affecting the synthesis of uremic toxins.9
2.3.2. Bacterial Families Possessing the Ability to Synthesize SCFA
Lactobacillaceae and Prevotellaceae are butyrate-producing bacteria with phosphotransbutyrylase and butyrate kinase activity, which are reduced in ESRD patients.9 Butyrate exerts the most biological activity in SCFA and stimulates mucin synthesis to protect intestinal homeostasis and intact antibacterial barrier.52 Integration of the intestinal epithelium is crucial to prevent the systemic translocation of microbial toxins and pathogens into the systemic circulation with widespread damage throughout the body.53 Gryp et al. revealed a lower abundance of SCFA-producing bacteria, Bifidobacterium spp., and Streptococcus spp. in patients with CKD.51 Similarly, Wang et al. reported a decrease in SCFA-producing bacteria in the CKD group, including Ruminococcaceae, Eubacteriaceae, Oscillospiraceae, Lachnospiraceae, Clostridiaceae, Oscillospiraceae, Veillonellaceae, Rikenellaceae, Bifidobacteriaceae, and Lactobacillaceae.7
2.3.3. Bacterial Families Possessing/Producing Lipopolysaccharide (LPS)
LPS constitutes the outer membranes of most Gram-negative bacteria.54 Gram-negative bacterial families, such as Corynebacteriaceae, Pseudomonadaceae, and Enterobacteriaceae, are enriched in the CKD population.8,9 LPS activates the NF-κB pathway and mTOR signaling in macrophages to stimulate the production of proinflammatory cytokines (IL-β1, TGF-β1, MCP-1, and TNF-α), leading to the CKD progression with kidney inflammatory injuries and fibrosis.55,56
2.4. The Causal Relationship Between CKD and Gut Dysbiosis Remains Unclear
Clinical studies have highlighted microbial composition and its bidirectional relationship with CKD, contributing to disease progression.57 However, the causality between CKD progression and gut dysbiosis has not been fully elucidated; therefore, further exploration of the causal relationship between gut dysbiosis and CKD is required to clarify the potential pathogenesis of CKD progression. Xu et al. transferred fecal microbiota from patients with CKD and healthy controls into antibiotic-treated C57BL/6 mice, showing that the CKD fecal samples resulted in significantly higher plasma TMAO levels in mice with increased Clostridium and Parabacteroides, along with decreased Ruminococcaceae and Megamonas compared to the group that received healthy fecal microbes.8 Wang et al. revealed that ESRD-specific gut microbiota induced systemic inflammation and colonic epithelial barrier defects in germ-free rats with a significant increase in fecal phenol and phenol-producing bacteria (Bacteroides and Escherichia), suggesting that gut microbiota from ESRD patients led to gut barrier defects by excessive phenol production.58
Until now, manipulating gut microbiota via fecal microbiota transplantation (FMT) from patients with ESRD or CKD into germ-free animals has not provided convincing evidence to illustrate the detrimental effect on renal function. The parameters of FMT operation, including frequency, dosage of fecal microbiota, and duration, should be considered in future investigations to establish the causative relationship between gut dysbiosis and CKD. However, several studies demonstrated that the gut microbiota significantly aggravate or alleviate CKD progression. Fecal microbiota from CKD rats transplanted into 5/6 nephrectomy rats increased protein-bound uremic toxins and a decline in renal function compared to the nontransplanted 5/6 nephrectomy rats. However, these adverse effects were significantly mitigated when fecal microbiota from healthy recipients was transplanted.59 Similarly, CKD mice that underwent FMT from healthy mice demonstrated a noticeable improvement in gut microbiota disturbance and a reduction in PCS accumulation.60 Collectively, these studies showed that manipulating the gut microbiota in CKD is a potential approach to alleviate disease progression.
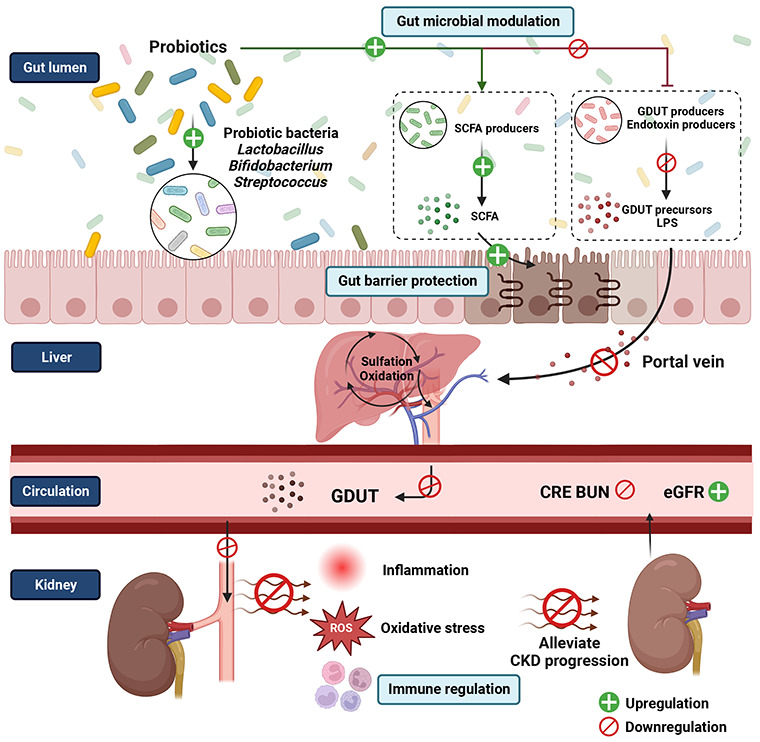
3. Alleviating Effects of Probiotics on Ckd via the Gut–Kidney Axis
The strong linkage between CKD and gut microbial dysbiosis suggests that modifying the gut microbiota could potentially diminish uremic toxin levels and associated adverse effects. Probiotics, defined as “live microorganisms that, when administered in adequate amounts, exist a health benefit on the host”10 exert a positive impact on CKD alleviation by attenuating gut microbiome disturbances.
3.1. In Vitro Preselection Platforms with in Vivo Verification are Feasible Strategies for Potential CKD-Alleviating Probiotic Screening
Preselection of suitable probiotic strains based on their beneficial attributes is crucial because their physiological functions are highly strain-specific.61 However, most studies have not elucidated the probiotic selection in detail, so the development of an in vitro screening platform is essential for providing an opportunity to mass-screen potential microorganisms.11 A previous study created a probiotic screening platform based on gut-derived uremic toxin-reducing probiotics and successfully preselected three probiotic strains, Lactobacillus paracasei subsp. paracasei BCRC 12188, Streptococcus salivarius subsp. thermophiles BCRC 13869, and Lactobacillus plantarum subsp. plantarum BCRC 12251 due to their ability to reduce IS in vitro.12In vivo, oral administration of a combination of the three strains (Pm1) significantly suppressed IS accumulation in the serum, kidneys, and liver in a cisplatin-induced acute kidney injury mouse model.12 A cisplatin-induced minipig model also demonstrated a lower incidence of lesions, including atrophy, mononuclear inflammation, cell infiltration, and interstitial fibrosis in renal tubules in the high-dosage Pm1 group.62 A significant reduction of blood urea nitrogen (BUN) and creatinine (CRE) was also observed compared with the cisplatin group. The possible mechanism involves the downregulation of inflammatory cytokine production and the upregulation of plasma superoxide dismutase activity. The modulation of gut microbiota was also observed after Pm1 intervention, with a decreasing abundance of Gram-negative bacteria contributing to reduced inflammation and apoptosis in the kidney, preventing CKD progression.62
A recent study preselected two potential strains (Lactobacillus plantarum subsp. plantarum MFM 30–3 and Lactobacillus paracasei subsp. paracasei MFM 18) using a modified screening platform to determine the ability to remove uremic toxin precursors in simulated intestinal juice.11 Furthermore, an in vivo study in an adenine-induced renal injury mouse model demonstrated that the renal dysfunction features, including high levels of BUN, CRE, IS, and PCS in plasma, interstitial fibrosis, and kidney injuries, were reduced by intervention with the preselected probiotics, which was accompanied by improvement of gut dysbiosis and prevention of intestinal barrier disruption via modulation of metabolite production.11
Developing an in vitro screening platform to identify strains that can reduce uremic toxin levels with further in vivo verification is a feasible strategy for potential CKD-alleviating probiotic screening. However, the speed of removal of a toxic compound is strain-specific and affected by the physiological state of the strain, pH, and nutrients.63 Other selection criteria, including decreasing indole production by Escherichia coli,64 antipathogenic,64,65 antioxidant,66,67 anti-inflammatory,68,69 and gut barrier protection70,71 are highly recommended to increase the therapeutic potentials for CKD.
3.2. The Positive Effects on CKD Alleviation after Probiotics Intervention
Table 3 summarizes the clinical trials examining the use of probiotics in patients with CKD. A total of 12 trials each were conducted for both dialysis patients and nondialyzed patients with stage 2–5 CKD. One trial was conducted on kidney transplantation patients. The sample size ranged between 9 and 70 patients, and the study duration varied from 2 to 24 weeks. Probiotic agent dosages ranged from 1.1 × 107 to 2.0 × 1012 CFU. The probiotic agents were in various forms, including sachets, powders, and capsule.
Table 3. Summary of Clinical Trials Examining the Use of Probiotics in Patients with Chronic Kidney Diseasea.
| study design/intervention duration/country | probiotics | dosage | main findings | ref |
|---|---|---|---|---|
| a retrospective study | Lactobacillus (Lactiplantibacillus) plantarum MFM30-3 | 1 × 1010 CFU/day | • reduction of creatinine | (77) |
| kidney transplatation patients (n = 24) | Lactobacilus (Lacticaseibacillus) paracasei MFM 18 | • improvement of eGFR | ||
| 3 months | ||||
| Taiwan | ||||
| a single-arm pilot study | • powder sachet (2 g): | 2 sachets/day | • fecal SCFAs increased significantly | (73) |
| hemodialysis patients (n = 18) | Bifidobacterium bifidum BGN4: 7 × 109 CFU | • reduction of serum calprotectin, a marker of acute inflammation, decreased significantly | ||
| 3 months | Bifidobacterium longum BORI: 2 × 109 CFU | • serum TNF-α and IL-6 upon LPS stimulation significantly decreased | ||
| Korea | • reduced systemic inflammatory responses were associated with an increase in Tregs and a decrease in proinflammatory monocytes | |||
| a randomized, single-blind, placebo-controlled pilot trial | • synbiotic bag: | 2 bags/day | • reduction of serum free IS | (76) |
| CKD stage 3b-4 patients (n = 47) | Lactobacillus (Lacticaseibacillus) casei LC4P1: 2.4 × 109 | • reduction of small intestinal permeability | ||
| 2 months | Bifidobacterium animalis BLC1: 2.4 × 109 | • amelioration of abdominal pain and constipation syndrome | ||
| Italy | fructooligosaccharides 2.5 g | |||
| inulin 2.5 g | ||||
| natural antioxidants (quercetin 0.064 g, resveratrol 0.023 g, and proanthocyanidins 0.013 g) | ||||
| a randomized, double-blinded, placebo-controlled clinical trial | Lactobacillus acidophilus | 2 × 1012 CFU/day | • improvement of gastrointestinal symptoms | (98) |
| hemodialysis patients (n = 18) | Bifidobacterium bifidum | 2.31 g/day | • elevation of Bifidobacterium | |
| 2 months | inulin | |||
| Mexico | ||||
| a double-blind, randomized clinical trial | Lactobacillus (Lactiplantibacillus) plantarum A87 | 4 × 1010 CFU/day | • reduction of serum CRP, glucose, and syndecan-1 (endothelial lesion marker) | (100) |
| hemodialysis patients (n = 70) | Lactobacillus (Lacticaseibacillus) rhamnosus | |||
| 3 months | Bifidobacterium bifidum A218 | |||
| Brazil | Bifidobacterium longum A101 | |||
| a double-blind, randomized, placebo-controlled clinical trial | Lactobacilus (Lacticaseibacillus) casei | 1 g/day | • reduction of BUN and trend to decrease serum CRE | (78) |
| CKD stage 3–4 patients (eGFR = 15–59 mL/min per 1.73 m2) (n = 66) | Lactobacilus acidophilus | |||
| 6 weeks | Lactobacilus bulgarigus | |||
| Iran | Lactobacilus (Lacticaseibacillus) rhamnosus | |||
| Bifidobacterium breve | ||||
| Bifidobacterium longum | ||||
| Sterptococus thermophilus | ||||
| Fructooligosaccharides | ||||
| a double-blind, randomized, placebo-controlled clinical trial | Lactobacillus (Lacticaseibacillus) rhamnosus | 1.6 × 107 CFU/day | • reduction of serum p-cresol and phenol | (79) |
| hemodialysis patients (n = 42) | ||||
| 4 weeks | ||||
| Iran | ||||
| a double-blind, randomized, placebo-controlled trial | • powder packets (5 g): | 3 packets/day | • reduction of plasma p-cresol | (80) |
| nondialyzed CKD stage 3–4 patients (eGFR = 15–60 mL/min per 1.73 m2) (n = 30) | Lactobacillus (Lactiplantibacillus) plantarum: 5 × 1010 | |||
| 4 weeks | Lactobacillus (Lacticaseibacillus) casei subsp. rhamnosus: 2 × 109 | |||
| Italy | Lactobacillus gasseri: 2 × 109 | |||
| Bifidobacterium infantis: 1 × 109 | ||||
| Bifidobacterium longum: 1 × 109 | ||||
| Lactobacillus acidophilus: 1 × 109 | ||||
| Lactobacillus (Ligilactobacillus) salivarius: 1 × 109 | ||||
| Lactobacillus sporogenes: 1 × 109 | ||||
| Streptococcus thermophiles: 5 × 109 | ||||
| inulin: 2.2 g | ||||
| tapioca-resistant starch:1.3 g | ||||
| a single-arm pilot study | Bifidobacterium infantis | 6 × 108 CFU/day | reduction of fecal indole and p-cresol, and plasma indican | (81) |
| hemodialysis patients (n = 20) | Lactobacillus acidophilus | |||
| 4 weeks | Enterococcus faecalis | |||
| Japan | the ratio of probiotic strain: 1:1:1 | |||
| a prospective, single-arm study | Lactobacillus acidophilus La-14 | 1.5 × 1010 65 mg | • reduction of serum PCS, IS, IL-6, and MDA | (74) |
| hemodialysis patients (n = 30) | fructooligosaccharides | |||
| 8 weeks | ||||
| Bulgaria | ||||
| a randomized, double-blinded, placebo-controlled clinical trial | Lactococcus lactis subsp. lactis LL358 | 2 sachets/day | • reduction of serum IS | (82) |
| hemodialysis patients (n = 50) | Lactobaccillus (Ligilactobacillus) salivarius LS159 | 1 × 1011 CFU/day | ||
| 6 months | Lactobaccillus (Lactiplantibacillus) pentosus LPE588 | |||
| Taiwan | ||||
| a randomized, double-blinded, placebo-controlled clinical trial | Bifidobacterium longum NQ1501 | B. longum | • probiotics did not significantly alter species diversity (Chao1 and Shannon) of the fecal microbiome, but induced a rearrangement in the microbial composition | (83) |
| hemodialysis patients (n = 45) | Lactobacillus acidophilus YIT2004 | 3.7 × 109 CFU/day | ||
| 6 months | Enterococcus faecalis YIT0072 | L. acidophilus | ||
| China | 8.9 × 108 CFU/day | • probiotics reduced serum indole-3-acetic acid-O-glucuronide, m-cresol, p-cresol, and phenol of nondiabetic patients | ||
| E. faecalis | ||||
| 1.8 × 109 CFU/day | ||||
| a simple randomized, controlled clinical trial | Lactobacillus (Lacticaseibacillus) casei shirota 8 × 109 CFU in fermented dairy drink | 8 × 109 CFU/day | • reduction of blood urea in 16 × 109 CFU group | (84) |
| CKD stage 3–4 patients (eGFR = 15–59 mL/min per 1.73 m2) (n = 30) | 16 × 109 CFU/day | |||
| 2 months | ||||
| Mexico | ||||
| a double-blind, randomized, placebo-controlled trial | • synbiotic pill: | 2 pills/day | • reduction of serum IS and trend to decrease serum PCS and TMAO | (14) |
| nondialyzed CKD patients (eGFR = 15 −45 mL/min per 1.73 m2) (n = 34) | Lactobacillus acidophilus CBT LA1: 4 × 109 | • improvement of eGFR | ||
| 12 weeks | Lactobacillus (Lacticaseibacillus) casei CBT LC5: 4 × 109 | • decreased level of high-sensitivity CRP | ||
| Serbia | Bifidobacterium lactis CBT BL3: 8 × 109 | • enrichment of Bifidobacteria, Lactobacillus, and Subdoligranulum | ||
| inulin: 1.6 g | ||||
| a preliminary study | Lactobacillus (Lacticaseibacillus) casei Shirota (LcS) | 3 × 108 CFU/day | • reduction of serum p-cresol | (85) |
| hemodialysis patients (n = 9) | Bifidobacterium breve Yakult (BbY) | 5.01 g | • improvement of difficulty in defecation and increased stool quantity | |
| 2 weeks | galacto-oligosaccharides (GOS) | |||
| Japan | ||||
| a double-blind, randomized, placebo-controlled crossover study | Streptococus thermophilus KB19 | 1.8 × 1012 CFU/day | • the trend of reductions of serum CRP and total indoxyl glucuronide | (86) |
| hemodialysis patients (n = 22) | Lactobacillus acidophilus KB27 | |||
| 2 months | Bifidobacterium longum KB31 | |||
| USA | ||||
| a pilot-scale, randomized, double-blind, placebo-controlled trial | Lactobacillus acidophilus KB31 | 9 × 1010 CFU/day | • reduction of serum BUN and uric acid | (87) |
| CKD stage 3–4 patients (n = 13) | Bifidobacterium longum KB35 | • elevation of Lactobacillus and Streptococcus | ||
| 3 months | Streptococus thermophilus KB27 | • reduction of fecal pH | ||
| Canada | ||||
| a prospective, randomized, double-blind, placebo-controlled, crossover trial at five institutions | Streptococus thermophilus KB19 | 9 × 1010 CFU/day | • reduction of BUN and trend to decrease serum CRE and uric acid | (88) |
| CKD stage 3–4 patients (n = 46) | Lactobacillus acidophilus KB27 | • improvement of quality of life | ||
| 3 months | Bifidobacterium longum KB31 | |||
| USA, Canada, Nigeria, and Argentina | ||||
| a double-blinded, placebo-controlled, randomized clinical trial | Lactobacillus genera | 9 × 109 CFU/day | • reduction of serum PCS and trend to decrease serum IS | (89) |
| nondialyzed CKD stage 4–5 patients (eGFR = 10–30 mL/min per 1.73 m2) (n = 31) | Bifidobacteria genera | 15 g | • elevation of Bifidobacterium and trend to increase Lactobacillus | |
| 6 weeks | Streptococcus genera | |||
| Australia | with nine different strains | |||
| inulin | ||||
| fructo-oligosaccharides | ||||
| galacto-oligosaccharides (GOSs) | ||||
| a single-center, open-label, randomized, placebo-controlled study | • Enterelle capsule (0.377 g): | • week 1: Enterelle ×3/day | • probiotics-treated patients exhibited a significant reduction of urinary indican and 3-methyl-indole. | (90) |
| CKD stage 3a patients (eGFR = 45–60 mL/min per 1.73 m2) (n = 28) | Enterococcus faecium (UBEF-41) | • week 2–3 Bifiselle ×3/day | • Lactobacillales and bifidobacteria concentrations were increased in the probiotics group | |
| 15 weeks | Ramnoselle ×3/day | |||
| Italy | Lactobacillus acidophilus (LA-14) Saccharomyces cerevisiae subsp. Boulardii (MTCC-5375) | • Week 4–15: | ||
| • bifidobacteria capsule (0.455g): | Bifiselle ×2/day | |||
| Bifidobacterium brevis (BB03) | Ramnoselle ×2/day | |||
| Bifidobacterium bifidum (BB06) | ||||
| Bifidobacterium longum (BL05) | ||||
| • Ramnoselle capsule (0.455 g): | ||||
| Lactobacillus (Lacticaseibacillus) rhamnosus (HN-001) | ||||
| Lactobacillus (Lacticaseibacillus) rhamnosus (LR-32) | ||||
| Lactobacillus acidophilus (LA-14) | ||||
| • a preliminary study | Bifidobacterium longum | 3 × 109 CFU/day | reduction of serum IS | (91) |
| hemodialysis patients (n = 11) | ||||
| 5 weeks | ||||
| Japan | ||||
| a double-blinded, placebo-controlled, randomized clinical trial | Lactobacillus acidophilus NCFM | 11 × 106 CFU/day | • improvement of gastrointestinal symptoms | (99) |
| hemodialysis patients (n = 42) | Bifidobacterium lactis Bi-07 | 2.31 g/day | • the trend to decrease CRP | |
| 2 months | inulin | |||
| Mexico | ||||
| a randomized, double-blinded, placebo-controlled trial | Bifidobacterium bifidum A218 | 4 × 109 CFU/day | • reduction of serum endotoxin and proinflammatory cytokine (TNF-α, IL-6, and IL-5) | (75) |
| peritoneal dialysis patients (n = 39) | Bifidobacterium catenulatum A302 | • elevation of anti-inflammatory cytokine (IL-10) | ||
| 6 months | Bifidobacterium longum A101 | |||
| Taiwan | Lactobacillus (Lactiplantibacillus) plantarum A87 | |||
| the ratio of probiotic strain: 1:1:1:1 | ||||
| a single-arm pilot study | Lactobacillus acidophilus TYCA06 | 1 × 1010 CFU/day | • eGFR decline was significantly slower | (64) |
| nondialyzed CKD stage 3–5 patients (n = 38) | Bifidobacterium longum subsp. infantis BLI-02 | • serum TNF-α, IL-6, IL-18, and endotoxin were significantly decreased | ||
| 6 months | Bifidobacterium bifidum VDD088 | • improvement of gastrointestinal symptoms (borborygmus and flatulence) | ||
| Taiwan | the ratio of probiotic strain:1:1:1 | • the abundance of B. bifidum and B. breve increased significantly | ||
| a randomized, double-blinded, placebo-controlled clinical trial | Lactobacillus (Lacticaseibacillus) casei Zhang | 4 × 109 CFU/day | • log10 urine albumin-to-creatinine ratio (UACR) mildly increased in the probiotic group compared to a significant increase in the placebo group | (92) |
| nondialyzed CKD stage 3–5 patients (n = 62) | • the amplitude of the increase in CRE was lower in the probiotic group after 1 year observation | |||
| 3 months | • eGFR decline was slower in the probiotic group during the intervention and follow-up 7 months | |||
| China | • the diversity of intestinal flora did not change among groups | |||
CFU, colony-forming unit; CKD, chronic kidney disease; CRE, creatinine; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rates; IS, indoxyl sulfate; PCS, P-cresyl sulfate; TMAO, trimethylamine-N-oxide; SCFA, short-chain fatty acid; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-5, interleukin-5; IL-6, interleukin-6; IL-10, interleukin-10; IL-18, interleukin-18; MDA, malondialdehyde.
The various probiotic strains evaluated were within nine genera (Bifidobacterium, Enterococcus, Lacticaseibacillus, Lactiplantibacillus, Lactobacillus, Lactococcus, Ligilactobacillus, Saccharomyces, and Streptococcus) according to new Lactobacillus taxonomy.72 These probiotic species have been proven to function in many pathological and physiological processes, including the regulation of immune capacity,64,73−75 alleviation of oxidative stress,74 protection of the gastrointestinal tract,76 reduction of plasma BUN, CRE, and renal toxins.74,76−92 It is worth noting that intervention with multiple probiotic strains in human trials is preferred (20 of 25 studies) due to a broader range of health-promoting effects and synergistic mechanisms of action,93 suggesting a higher opportunity for success.94 However, insufficient evidence supports a better health-promoting effect when using multistrain probiotics than single-strain probiotics.95 Therefore, the selection of probiotic strains for human trials must be based on in vitro and in vivo scientific evidence.
Most studies reported positive effects such as reduced uremic toxins (IS, PCS, TMAO, indoxyl glucuronide) or precursors of uremic toxin (indole, p-cresol, and phenol) after the probiotic intervention.14,74,76,79−83,85,86,89−91 However, only seven trials14,64,77,78,87,88,92 observed an improved renal function index, including reduced BUN and CRE, and slowing eGFR decline. The primary function of specific probiotics is an adjuvant strategy used to restore microbial balance and suppress circulating levels of uremic toxins,96 so improved renal function indicators may not be observed at this stage. Additionally, BUN and CRE levels are influenced by dietary and physiologic conditions unrelated to renal function,97 which might affect the statistical significance of clinical studies. Besides renal function, some studies also observed improved gastrointestinal symptoms (borborygmus, flatulence, abdominal pain, and constipation syndrome),64,76,85,98,99 gut microbial modulation (upregulation of lactobacilli and bifidobacteria),14,64,87,89,90,98 and anti-inflammatory effects.64,73−75,14,86,99,100 Inflammatory biomarkers were evaluated in seven studies, with decreased inflammatory cytokines (TNF-α, IL-5, IL-6, IL-18), CRP, and LPS in serum compared to controls after probiotics intervention.
Synbiotic supplementation also positively affected renal function and gut microbiota in patients with CKD.14,78,89,98 Synbiotic therapy significantly reduced serum IS and PCS in patients with CKD, with the enrichment of Bifidobacterium and Lactobacillus and depletion of Ruminococcaceae. Moreover, a significant negative correlation was observed between changes in the relative abundance of Bifidobacterium and serum PCS and IS concentrations.89
Attempts to restore the desired microbiome by introducing favorable microorganisms seem feasible for CKD alleviation. However, the limited size of the study cohorts and the relatively short follow-up period hinder a comprehensive understanding of probiotics’ efficacy in treating individuals with CKD. Therefore, further long-term basic and clinical studies are needed in the future.
3.3. The Gut–Kidney Axis Plays a Vital Role in the Preventive/Therapeutic Mechanisms of Probiotics in CKD
The probiotics’ potential efficacy in managing CKD is through the gut–kidney axis by modulating gut microbial composition and improving gut dysbiosis, which further reduces uremic toxins, increases SCFA, enhances gut barrier integrity, ameliorates gastrointestinal symptoms, and decreases the inflammatory response, contributing to alleviating CKD progression.
3.3.1. Modulating Gut Microbial Composition and Improving Gut Dysbiosis
Numerous studies reveal that orally administered probiotics in animal models slow the progression of kidney disease by correcting the intestinal microbial imbalance.11,13,101 Six clinical studies analyzed gut microbiota after probiotics intervention in patients with CKD using high throughput sequencing, and the results support the importance of the gut–kidney axis in alleviating CKD.14,64,73,83,89,92 In a single-center double-blind, randomized, placebo-controlled study, administration of Bifco capsules, which is a mixture of viable bacteria (Enterococcus faecalis, Bifdobacterium longum, and Lactobacillus acidophilus), for six months induced microbial composition changes and reduced the relative abundance of Ruminococcaceae, Halomonadaceae, Peptostreptococcaceae, and Erysipelotrichacease and elevated Bacteroidaceae and Enterococcaceae in nondiabetic hemodialysis patients.83 In another 6-month single-arm pilot study, the dominant genera of the intestinal microbiome changed during the probiotic intervention.64 In a double-blind, randomized, placebo-controlled trial, intervention with synbiotic pills (Lactobacillus acidophilus CBT LA1, Lacticaseibacillus casei CBT LC5, Bifidobacterium lactis CBT BL3, and inulin), the relative abundance of Bifidobacterium, Lactobacillus, and Subdoligranulum significantly increased compared to the placebo group.14 In a small-scale study with a probiotics intervention (mixture of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI) for three months, the population of Prevotella, Enterococcus, Alistipes, Clostridia, Escherichia-Shigella, Klebsiella, and Bifidobacterium increased while Bacteroides, Faecalibacterium, Eubacterium siraeum, Tyzzerella, Sutterella, and Akkermansia reduced.73
Through analyzing the microbial composition based on the reviewed clinical studies, the CKD alleviating effect of probiotics on modulating gut microbiota involves:
3.3.1.1. Upregulating the Beneficial Bacterial Genus Bifidobacterium
Among the clinical trials, six studies noted significant increases in Bifidobacterium, which parallels other fecal microbial analyses in patients with CKD using culture-dependent, qPCR quantification or next-generation sequencing approaches.14,64,73,89,90,98Bifidobacterium, decreased in patients with CKD,45 has been reported to promote colon health by increasing the production of microbial metabolites, such as SCFA,102 and the slow CKD progression positively relates to the eGFR improvement.14,51
3.3.1.2. Upregulating Bacterial Families Possessing the Enzymes to Synthesize SCFA
Other bacterial genera possessing enzymes to synthesize SCFA are enriched after probiotic intervention, such as Lactobacillus and Subdoligranulum.14,103 These bacteria convert dietary fiber in the gut into monosaccharides through a series of reactions mediated by specific enzymes.104 A reduction in Bifidobacterium, Lactobacillus, and SCFA producers, such as Prevotella spp., Clostridium spp., Roseburia spp., Enbacterium spp., Coprococcus spp., and Faecalibacterium prausnitzii have been observed in the patients with CKD.9,45,105−107 Furthermore, SCFA producers (Butyricicoccus spp., F. prausnitzii, Roseburia spp., and Bifidobacterium spp.) showed an inverse correlation with the severity of CKD progression and increased with higher eGFR.51 Additionally, SCFAs produced by gut bacteria and delivered to the kidney through the peripheral circulation could protect tubular cells from oxidative cellular stress, mitigate renal ischemia-reperfusion injury, reduce inflammation, lower the infiltration of immune cells, and diminish apoptotic cells in injured kidneys.108 Thus, increased SCFA-producing bacteria in the gut after probiotic intervention could improve gut health and positively affect CKD.109,110
3.3.1.3. Downregulating the Bacterial Families Possessing the Enzymes to Synthesize Uremic Toxins
A high abundance of gut bacterial families with enzymes to synthesize uremic toxins could accelerate CKD progression. Indole, phenol, and p-cresol are aromatic compounds produced by intestinal bacteria via aromatic amino acids (tryptophan and tyrosine).9,51,110 Upregulating nonphenol-producing bacteria, such as Subdoligranulum, genera of the Ruminococcaceae family,14 and downregulating indole-producing bacteria, such as Escherichia spp.,64 were observed in patients with CKD after the probiotic intervention. Reducing the Ruminococcaceae family was also reported in HD patients supplemented with probiotics.83 Some bacteria from Ruminococcaceae can ferment tyrosine to p-cresol,110 and modulation of Ruminococcaceae appears to correlate with a healthier gut environment in CKD patients.
3.3.1.4. Downregulating the Bacterial Families Associated with Inflammation
Reduction of endotoxin-producing Gram-negative bacteria, such as Megamonas, Escherichia-Shigella, and Halomonadaceae, in patients with CKD with probiotic intervention indicates decreasing chronic immune responses associated with inflammation in the human gut. Both studies show a reduction in the serum levels of endotoxin.64,75,83 Several reports found more Halomonadaceae in ESRD patients.9,111 The observed decline in these bacteria suggests that probiotics may potentially restrain their overgrowth in CKD patients.
3.3.2. Reducing Serum Uremic Toxins/Endotoxin and Increasing Fecal SCFA via Modulating Gut Microbiota
Probiotic intervention modulates gut microbial composition, thereby down-regulating the bacterial families that synthesize uremic toxins. Reduced precursors of uremic toxins (indole, p-cresol, and phenol)79−81,83,85,90 or uremic toxins (IS, PCS, TMAO, indoxyl glucuronide)14,74,76,81−83,86,89−91 were observed in 13 trials of patients with CKD after probiotic intervention. Probiotic intervention also reduces the levels of endotoxin/LPS by inhibiting Gram-negative bacteria. LPS, a major component of the outer membrane in Gram-negative bacteria.112 Serum endotoxin levels were significantly decreased in patients with CKD receiving probiotics.64,75,83 Additionally, the probiotic intervention also upregulates bacterial families possessing the enzymes to synthesize SCFA.73
3.3.3. Reducing Inflammation, Oxidative Stress, and Intestinal Barrier Injury via Reducing Serum Uremic Toxins/Endotoxin and Increasing SCFAs
SCFAs produced by the intestinal microbiome can reduce inflammation and oxidative stress. The mechanisms involved in the anti-inflammatory response include decreasing the proliferation of immune cells and cytokine levels, inhibiting NF-κB, reducing neutrophil recruitment,113 and a direct effect on T cells through binding to specific receptors (GPR41, GPR43, and GPR109A).104 SCFAs can modulate the Keap1-Nrf2-dependent cellular signaling pathway to maintain redox homeostasis.114,115 Detrimental effects of GDUTs, including oxidative stress, inflammation, fibrosis, kidney failure, and insulin resistance, have been well documented,. Thus, by downregulating GDUTs and upregulating SCFAs, probiotics reduce inflammation, oxidative stress, and intestinal barrier injury in patients with CKD. Probiotics have been shown to decrease serum proinflammatory cytokines,64,73,75 increase T regulatory cytokines,75 reduce small intestinal permeability,76 increase fecal SCFAs,73 and reduce serum GDUTs14,76,81−83,86,89−91 and endotoxins64,75 in patients with CKD. These findings verify that the gut–kidney axis significantly impacts the preventive/therapeutic mechanisms of probiotics in CKD.
4. Further Considerations and Concluding Remarks
Gut dysbiosis contributes to deteriorating CKD progression; thus, probiotics are a potential strategy to restore the desired microbiome and treat CKD. Clinical studies have revealed the various physiological functions of probiotics in patients with CKD including reduction of uremic toxins and related precursors, modulation of gut microbiota, regulation of immune capacity, protection of the gastrointestinal tract, and improvement of gastrointestinal symptoms. However, only approximately 25% of human cohorts have shown a positive effect on renal function, which is the most critical outcome demonstrating the efficacy of probiotics in improving CKD. The considerable heterogeneity in the responses to probiotic treatment in CKD may be due to individual factors such as diet, age, physiological condition, immune response, and indigenous gut microbiota,116 as well as differing expression of mucosal immune-related genes in gastrointestinal organs, leading to personalized colonization patterns.117
Nonetheless, a strong relationship exists between CKD-related specific gut microbial profiles and metabolites and their interplay with probiotics. Thus, it is necessary to investigate alterations in the bacterial communities and bacterial-related metabolic functions to select appropriate probiotics to treat CKD and understand their underlying mechanisms of action. Human clinical studies should involve the integrated analysis of microbial configurations and metabolite profiles during probiotics intervention using a combination of multiomic technologies, such as metagenomics, metabolomics, and transcriptomics, which could provide more precise insights into the mechanisms of probiotics function.
CKD severity shapes varying statuses of gut dysbiosis, indicating a descending trend of gut diversity and abundance with CKD progression.7,118 Clinical studies are usually conducted with specific CKD populations, which make it interesting whether disease severity influences gut modulation and subsequent probiotics effects. Such investigations could help suggest favorable timing of probiotic interventions for better outcomes. A large-scale prospective longitudinal clinical study of varying levels of impaired kidney function and a long follow-up period should be conducted to comprehensively understand probiotics’ efficacy in treating CKD. In addition, it is recommended that commensal bacteria be identified as novel microbiota-based biomarkers to monitor disease progression and facilitate the development of next-generation probiotics or precision probiotics to prevent CKD progression.
In conclusion, although several clinical studies have demonstrated the positive impacts of probiotics on various outcomes in patients with CKD, their effectiveness in CKD treatment remains a subject of debate and requires further verification. It is anticipated that future large-scale, long-term studies will confirm the potential benefits of utilizing probiotics as an adjuvant therapy in CKD.
Glossary
Abbreviations
- AUC
area under curve
- BUN
blood urea nitrogen
- CRE
creatinine
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- FMO
flavin monooxygenase isoform
- FMT
fecal microbiota transplantation
- GDUT
gut-derived uremic toxin
- IAA
indole-3 acetic acid
- IgA
immunoglobulin A
- IS
indoxyl sulfate
- KDIGO
Kidney Disease Improving Global Outcomes
- KT
kidney transplant
- LDA
linear discriminant analysis
- Lm
Lactobacillus mix
- LPS
lipopolysaccharide
- OAT
organic anion transporter
- PAG
phenylacetylglutamine
- PCS
p-cresyl sulfate
- SCFA
short-chain fatty acid
- TMA
trimethylamine
- TMAO
trimethylamine-N-oxide
Author Contributions
Hsiao-Wen Huang: Conceptualization, Data curation, Investigation, Writing - original draft. Ming-Ju Chen: Conceptualization, Investigation, Supervision, Writing–review and editing.
This work was supported by grants from the Council of Agriculture of Taiwan, Executive Yuan (110AS-10.1.7-AD-U1).
The authors declare no competing financial interest.
References
- Humphreys B. D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- Nardi E.; Mulè G.; Giammanco A.; Mattina A.; Geraci G.; Nardi C.; Averna M. Left ventricular hypertrophy in chronic kidney disease: A diagnostic criteria comparison. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 137–144. 10.1016/j.numecd.2020.08.028. [DOI] [PubMed] [Google Scholar]
- Tomlinson J. A. P.; Wheeler D. C. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int. 2017, 92, 809–815. 10.1016/j.kint.2017.03.053. [DOI] [PubMed] [Google Scholar]
- Romagnani P.; Remuzzi G.; Glassock R.; Levin A.; Jager K. J.; Tonelli M.; Massy Z.; Wanner C.; Anders H. J. Chronic kidney disease. Nat. Rev. Dis. Primers. 2017, 3, 17088. 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- Jager K. J.; Kovesdy C.; Langham R.; Rosenberg M.; Jha V.; Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. 10.1016/j.kint.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Tsai M. H.; Hsu C. Y.; Lin M. Y.; Yen M. F.; Chen H. H.; Chiu Y. H.; Hwang S. J. Incidence, prevalence, and duration of chronic kidney disease in Taiwan: Results from a community-based screening program of 106,094 individuals. Nephron. 2018, 140, 175–184. 10.1159/000491708. [DOI] [PubMed] [Google Scholar]
- Wang H.; Ainiwaer A.; Song Y.; Qin L.; Peng A.; Bao H.; Qin H. Perturbed gut microbiome and fecal and serum metabolomes are associated with chronic kidney disease severity. Microbiome. 2023, 11, 3. 10.1186/s40168-022-01443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K. Y.; Xia G. H.; Lu J. Q.; Chen M. X.; Zhen X.; Wang S.; You C.; Nie J.; Zhou H. W.; Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. 10.1038/s41598-017-01387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.; Piceno Y. M.; DeSantis T. Z.; Pahl M.; Andersen G. L.; Vaziri N. D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C.; Guarner F.; Reid G.; Gibson G. R.; Merenstein D. J.; Pot B.; Morelli L.; Canani R. B.; Flint H. J.; Salminen S.; Calder P. C.; Sanders M. E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Huang H.; Li K.; Lee Y.; Chen M. Preventive effects of Lactobacillus mixture against chronic kidney disease progression through enhancement of beneficial bacteria and downregulation of gut-derived uremic toxins. J. Agric. Food Chem. 2021, 69, 7353–7366. 10.1021/acs.jafc.1c01547. [DOI] [PubMed] [Google Scholar]
- Fang C. Y.; Lu J. R.; Chen B. J.; Wu C.; Chen Y. P.; Chen M. J. Selection of uremic toxin-reducing probiotics in vitro and in vivo. J. Funct. Foods. 2014, 7, 407–415. 10.1016/j.jff.2014.01.018. [DOI] [Google Scholar]
- Yang J.; Lim S. Y.; Ko Y. S.; Lee H. Y.; Oh S. W.; Kim M. G.; Cho W. Y.; Jo S. K. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 419–428. 10.1093/ndt/gfy172. [DOI] [PubMed] [Google Scholar]
- Mitrović M.; Stanković-Popović V.; Tolinački M.; Golić N.; Soković Bajić S.; Veljović K.; Nastasijević B.; Soldatović I.; Svorcan P.; Dimković N. The impact of synbiotic treatment on the levels of gut-derived uremic toxins, inflammation, and gut microbiome of chronic kidney disease patients-a randomized trial. J. Ren. Nutr. 2023, 33, 278–288. 10.1053/j.jrn.2022.07.008. [DOI] [PubMed] [Google Scholar]
- Sumida K.; Yamagata K.; Kovesdy C. P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134. 10.1016/j.ekir.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A.; Massy Z. A.; Meijers B.; Evenepoel P.; Vanholder R.; Raj D. S. Role of the gut microbiome in uremia: a potential therapeutic target. Am. J. Kidney Dis. 2016, 67, 483–498. 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing M. R.; Patel S. S.; Ramezani A.; Raj D. S. Gut microbiome in chronic kidney disease. Exp. Physiol. 2016, 101, 471–477. 10.1113/EP085283. [DOI] [PubMed] [Google Scholar]
- Hatch M.; Vaziri N. D. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin. Sci. 1994, 86, 511–516. 10.1042/cs0860511. [DOI] [PubMed] [Google Scholar]
- Lau W. L.; Vaziri N. D. The Leaky Gut and Altered Microbiome in Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 458–461. 10.1053/j.jrn.2017.02.010. [DOI] [PubMed] [Google Scholar]
- de Andrade L. S.; Ramos C. I.; Cuppari L. The cross-talk between the kidney and the gut: implications for chronic kidney disease. Nutrire. 2017, 42, 27. 10.1186/s41110-017-0054-x. [DOI] [Google Scholar]
- Kim D. S.; Lee J. W. Urinary tract infection and microbiome. Diagnostics (Basel). 2023, 13, 1921. 10.3390/diagnostics13111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrbacek J.; Tlaskal V.; Cermak P.; Hanacek V.; Zachoval R. Bladder microbiota are associated with clinical conditions that extend beyond the urinary tract. Microorganisms. 2022, 10, 874. 10.3390/microorganisms10050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H.; Kuffel G.; Thomas-White K.; Wolfe A. J.; Vellanki K.; Leehey D. J.; Bansal V. K.; Brubaker L.; Flanigan R.; Koval J.; Wadhwa A.; Zilliox M. J. Diversity of the midstream urine microbiome in adults with chronic kidney disease. Int. Urol. Nephrol. 2018, 50, 1123–1130. 10.1007/s11255-018-1860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffi H. S.; Bates J. M. Jr; Laszik Z.; Kumar S. Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J. Urol. 2009, 181, 2332–2338. 10.1016/j.juro.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholder R.; De Smet R.; Glorieux G.; Argilés A.; Baurmeister U.; Brunet P.; Clark W.; Cohen G.; De Deyn P. P.; Deppisch R.; Descamps-Latscha B.; Henle T.; Jörres A.; Lemke H. D.; Massy Z. A.; Passlick-Deetjen J.; Rodriguez M.; Stegmayr B.; Stenvinkel P.; Tetta C.; Wanner C.; Zidek W. European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- Sampaio-Maia B.; Simões-Silva L.; Pestana M.; Araujo R.; Soares-Silva I. J. The role of the gut microbiome on chronic kidney disease. Adv. Appl. Microbiol. 2016, 96, 65–94. 10.1016/bs.aambs.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Mishima E.; Fukuda S.; Mukawa C.; Yuri A.; Kanemitsu Y.; Matsumoto Y.; Akiyama Y.; Fukuda N. N.; Tsukamoto H.; Asaji K.; Shima H.; Kikuchi K.; Suzuki C.; Suzuki T.; Tomioka Y.; Soga T.; Ito S.; Abe T. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 2017, 92, 634–645. 10.1016/j.kint.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Smith E. A.; Macfarlane G. T. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. 1997, 3, 327–337. 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- Vaziri N. D.; Zhao Y. Y.; Pahl M. V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2016, 31, 737–746. 10.1093/ndt/gfv095. [DOI] [PubMed] [Google Scholar]
- Vanholder R.; Schepers E.; Pletinck A.; Nagler E. V.; Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigarran Guldris S.; González Parra E.; Cases Amenós A. Gut microbiota in chronic kidney disease. Nefrologia. 2017, 37, 9–19. 10.1016/j.nefro.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Evenepoel P.; Meijers B. K.; Bammens B. R.; Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. Suppl. 2009, 76, S12–S19. 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- Ottosson F.; Brunkwall L.; Smith E.; Orho-Melander M.; Nilsson P. M.; Fernandez C.; Melander O. The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J. Hypertens. 2020, 38, 2427–2434. 10.1097/HJH.0000000000002569. [DOI] [PubMed] [Google Scholar]
- Patten C. L.; Blakney A. J.; Coulson T. J. Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit. Rev. Microbiol. 2013, 39, 395–415. 10.3109/1040841X.2012.716819. [DOI] [PubMed] [Google Scholar]
- Briskey D.; Tucker P.; Johnson D. W.; Coombes J. S. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin. Exp. Nephrol. 2017, 21, 7–15. 10.1007/s10157-016-1255-y. [DOI] [PubMed] [Google Scholar]
- Aronov P. A.; Luo F. J.; Plummer N. S.; Quan Z.; Holmes S.; Hostetter T. H.; Meyer T. W. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol. 2011, 22, 1769–1776. 10.1681/ASN.2010121220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y.; Ezawa A.; Kikuchi K.; Tsuruta Y.; Niwa T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. 10.1007/s00216-012-5929-3. [DOI] [PubMed] [Google Scholar]
- Wu W.; Bush K. T.; Nigam S. K. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci. Rep. 2017, 7, 4939. 10.1038/s41598-017-04949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopienko A. J.; Nolin T. D. Microbiota-derived uremic retention solutes: perpetrators of altered nonrenal drug clearance in kidney disease. Expert Rev. Clin. Pharmacol. 2018, 11, 71–82. 10.1080/17512433.2018.1378095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekawanvijit S. Role of gut-derived protein-bound uremic toxins in cardiorenal syndrome and potential treatment modalities. Circ. J. 2015, 79, 2088–2097. 10.1253/circj.CJ-15-0749. [DOI] [PubMed] [Google Scholar]
- Edamatsu T.; Fujieda A.; Itoh Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PloS one. 2018, 13, e0193342 10.1371/journal.pone.0193342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Klipfell E.; Bennett B. J.; Koeth R.; Levison B. S.; Dugar B.; Feldstein A. E.; Britt E. B.; Fu X.; Chung Y. M.; Wu Y.; Schauer P.; Smith J. D.; Allayee H.; Tang W. H.; DiDonato J. A.; Lusis A. J.; Hazen S. L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011, 472, 57–63. 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H.; Wang Z.; Levison B. S.; Koeth R. A.; Britt E. B.; Fu X.; Wu Y.; Hazen S. L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford J.; Charlton K.; Stefoska-Needham A.; Ibrahim R.; Lambert K. The gut microbiota profile of adults with kidney disease and kidney stones: a systematic review of the literature. BMC Nephrol. 2020, 21, 215. 10.1186/s12882-020-01805-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M.; Montemurno E.; Piccolo M.; Vannini L.; Lauriero G.; Maranzano V.; Gozzi G.; Serrazanetti D.; Dalfino G.; Gobbetti M.; Gesualdo L. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PloS one. 2014, 9, e99006 10.1371/journal.pone.0099006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z.; Fan Y.; Li A.; Shen Q.; Wu J.; Ren L.; Lu H.; Ding S.; Ren H.; Liu C.; Liu W.; Gao D.; Wu Z.; Guo S.; Wu G.; Liu Z.; Yu Z.; Li L. Alterations of the human gut microbiome in chronic kidney disease. Adv. Sci. 2020, 7, 2001936. 10.1002/advs.202001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G.; Zhou M.; Yu L.; Ye J.; Yao L.; Shi L. Gut microbiota in renal transplant recipients, patients with chronic kidney disease and healthy subjects. J. South. Med. Univ. 2018, 38, 1401–1408. 10.12122/j.issn.1673-4254.2018.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Wang M.; Wang J.; Li R.; Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front. Cell. Infect. Microbiol. 2019, 9, 206. 10.3389/fcimb.2019.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q.; Wu K.; Pan W.; Zeng Y.; Hu K.; Chen D.; Huang X.; Zhang Q. Intestinal flora alterations in patients with early chronic kidney disease: a case-control study among the Han population in southwestern China. J. Int. Med. Res. 2020, 48, 926033. 10.1177/0300060520926033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.; Li L.; Li L.; Liu Y.; Ren Q.; Shi M.; Liu J.; Jiang J.; Ma H.; Huang Z.; Xia Z.; Pan J.; Wei T.; Wang Y.; Li P.; Lan T.; Tang X.; Zeng X.; Lei S.; Tang H.; Ma L.; Fu P. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol. 2019, 56, 581–592. 10.1007/s00592-019-01316-7. [DOI] [PubMed] [Google Scholar]
- Gryp T.; Huys G. R. B.; Joossens M.; Van Biesen W.; Glorieux G.; Vaneechoutte M. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int. J. Mol. Sci. 2020, 21, 1986. 10.3390/ijms21061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T. H.; Han K. S.; Park J. H.; Hwang H. J. Butyrate modulates mucin secretion and bacterial adherence in LoVo cells via MAPK signaling. PloS one 2022, 17, e0269872 10.1371/journal.pone.0269872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. S.; Wang J.; Yannie P. J.; Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020, 4, bvz039. 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani B.; Ruiz N. Function and biogenesis of lipopolysaccharides. EcoSal Plus. 2018, 8, 1128. 10.1128/ecosalplus.esp-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Zhu J.; Liu Y.; Dong Z.; Liu H.; Liu Y.; Zhou X.; Liu F.; Chen G. Lipopolysaccharide induces chronic kidney injury and fibrosis through activation of mTOR signaling in macrophages. Am. J. Nephrol. 2015, 42, 305–317. 10.1159/000441506. [DOI] [PubMed] [Google Scholar]
- Yamate J.; Machida Y.; Ide M.; Kuwamura M.; Sawamoto O.; LaMarre J. Effects of lipopolysaccharide on the appearance of macrophage populations and fibrogenesis in cisplatin-induced rat renal injury. Exp. Toxicol. Pathol. 2004, 56, 13–24. 10.1016/j.etp.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Al Khodor S.; Shatat I. F. Gut microbiome and kidney disease: a bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. 10.1007/s00467-016-3392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Hao Y.; Liu X.; Yu S.; Zhang W.; Yang S.; Yu Z.; Ren F. Gut microbiota from end-stage renal disease patients disrupt gut barrier function by excessive production of phenol. J. Genet. Genomics. 2019, 46, 409–412. 10.1016/j.jgg.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Liu X.; Zhang M.; Wang X.; Liu P.; Wang L.; Li Y.; Wang X.; Ren F. Fecal microbiota transplantation restores normal fecal composition and delays malignant development of mild chronic kidney disease in rats. Front. Microbiol. 2022, 13, 1037257. 10.3389/fmicb.2022.1037257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba C.; Soulage C. O.; Caggiano G.; Glorieux G.; Fouque D.; Koppe L. Effects of fecal microbiota transplantation on composition in mice with CKD. Toxins 2020, 12, 741. 10.3390/toxins12120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana R.; van Hemert S.; Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12. 10.1186/s13099-017-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J.; Li K. Y.; Wang P. J.; Huang H. W.; Chen M. J. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced Lanyu pig model. J. Food Drug Anal. 2020, 28, 103–114. 10.1016/j.jfda.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Kinoshita H.; Sohma Y.; Ohtake F.; Ishida M.; Kawai Y.; Kitazawa H.; Saito T.; Kimura K. Biosorption of heavy metals by lactic acid bacteria and identification of mercury binding protein. Res. Microbiol. 2013, 164, 701–709. 10.1016/j.resmic.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Wang I. K.; Yen T. H.; Hsieh P. S.; Ho H. H.; Kuo Y. W.; Huang Y. Y.; Kuo Y. L.; Li C. Y.; Lin H. C.; Wang J. Y. Effect of a probiotic combination in an experimental mouse model and clinical patients with chronic kidney disease: a pilot study. Front. Nutr. 2021, 8, 661794. 10.3389/fnut.2021.661794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J.; Bai J.; Luo B.; Ni Y.; Tian F.; Yan W. In vitro evaluation of probiotic properties and antioxidant activities of Bifidobacterium strains from infant feces in the Uyghur population of northwestern China. Ann. Microbiol. 2022, 72, 14. 10.1186/s13213-022-01670-y. [DOI] [Google Scholar]
- Vougiouklaki D.; Tsironi T.; Tsantes A. G.; Tsakali E.; Van Impe J. F. M.; Houhoula D. Probiotic properties and antioxidant activity in vitro of lactic acid bacteria. Microorganisms. 2023, 11, 1264. 10.3390/microorganisms11051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Gong W.; Xu C.; Zhu Z.; Peng Y.; Xie C. Probiotic assessment and antioxidant characterization of Lactobacillus plantarum GXL94 isolated from fermented chili. Front. Microbiol. 2022, 13, 997940. 10.3389/fmicb.2022.997940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Kim J. S.; Kim Y.; Jeong Y.; Kim J. E.; Paek N. S.; Kang C. H. Antioxidant and probiotic properties of Lactobacilli and Bifidobacteria of human origins. Biotechnol. Bioprocess Eng. 2020, 25, 421–430. 10.1007/s12257-020-0147-x. [DOI] [Google Scholar]
- Kim S.; Lee J. Y.; Jeong Y.; Kang C. H. Antioxidant activity and probiotic properties of lactic acid bacteria. Ferment. 2022, 8, 29. 10.3390/fermentation8010029. [DOI] [Google Scholar]
- Liu Y.; Peng J.; Zhu S.; Yu L.; Tian F.; Zhao J.; Zhang H.; Chen W.; Zhai Q. A screening model for probiotics against specific metabolic diseases based on Caco-2 monolayer membrane. Engineering. 2023, 25, 222–233. 10.1016/j.eng.2022.02.014. [DOI] [Google Scholar]
- Thananimit S.; Pahumunto N.; Teanpaisan R. Characterization of short chain fatty acids produced by selected potential probiotic Lactobacillus strains. Biomolecules. 2022, 12, 1829. 10.3390/biom12121829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.; Wittouck S.; Salvetti E.; Franz C. M. A. P.; Harris H. M. B.; Mattarelli P.; O’Toole P. W.; Pot B.; Vandamme P.; Walter J.; Watanabe K.; Wuyts S.; Felis G. E.; Gänzle M. G.; Lebeer S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- Choi E.; Yang J.; Ji G. E.; Park M. S.; Seong Y.; Oh S. W.; Kim M. G.; Cho W. Y.; Jo S. K. The effect of probiotic supplementation on systemic inflammation in dialysis patients. Kidney Res. Clin. Pract. 2022, 41, 89–101. 10.23876/j.krcp.21.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuskunov T.; Tilkiyan E.; Doykov D.; Boyanov K.; Bivolarska A.; Hristov B. The effect of synbiotic supplementation on uremic toxins, oxidative stress, and inflammation in hemodialysis patients-results of an uncontrolled prospective single-arm study. Medicina. 2023, 59, 1383. 10.3390/medicina59081383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I. K.; Wu Y. Y.; Yang Y. F.; Ting I. W.; Lin C. C.; Yen T. H.; Chen J. H.; Wang C. H.; Huang C. C.; Lin H. C. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef. Microbes. 2015, 6, 423–430. 10.3920/BM2014.0088. [DOI] [PubMed] [Google Scholar]
- Cosola C.; Rocchetti M. T.; di Bari I.; Acquaviva P. M.; Maranzano V.; Corciulo S.; Di Ciaula A.; Di Palo D. M.; La Forgia F. M.; Fontana S.; De Angelis M.; Portincasa P.; Gesualdo L. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins. 2021, 13, 334. 10.3390/toxins13050334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. N.; Ho D. R.; Huang Y. C.; Lin J. H.; Liu Y. L.; Chen M. J.; Chen C. S. A pilot study of nephrogenic probiotics to further improve an already stabilized graft function after kidney transplantation. Transplant. Proc. 2023, 55, 2090–2094. 10.1016/j.transproceed.2023.08.011. [DOI] [PubMed] [Google Scholar]
- Dehghani H.; Heidari F.; Mozaffari-Khosravi H.; Nouri-Majelan N.; Dehghani A. Synbiotic supplementations for azotemia in patients with chronic kidney disease: a randomized controlled trial. Iran. J. Kidney Dis. 2016, 10, 351–357. [PubMed] [Google Scholar]
- Eidi F.; Poor-Reza Gholi F.; Ostadrahimi A.; Dalili N.; Samadian F.; Barzegari A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: A double blind randomized clinical trial. Clin. Nutr. ESPEN. 2018, 28, 158–164. 10.1016/j.clnesp.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Guida B.; Germanò R.; Trio R.; Russo D.; Memoli B.; Grumetto L.; Barbato F.; Cataldi M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. 10.1016/j.numecd.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Hida M.; Aiba Y.; Sawamura S.; Suzuki N.; Satoh T.; Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996, 74, 349–355. 10.1159/000189334. [DOI] [PubMed] [Google Scholar]
- Lim P. S.; Wang H. F.; Lee M. C.; Chiu L. S.; Wu M. Y.; Chang W. C.; Wu T. K. The efficacy of Lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J. Ren. Nutr. 2021, 31, 189–198. 10.1053/j.jrn.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Liu S.; Liu H.; Chen L.; Liang S. S.; Shi K.; Meng W.; Xue J.; He Q.; Jiang H. Effect of probiotics on the intestinal microbiota of hemodialysis patients: a randomized trial. Eur. J. Nutr. 2020, 59, 3755–3766. 10.1007/s00394-020-02207-2. [DOI] [PubMed] [Google Scholar]
- Miranda Alatriste P. V.; Urbina Arronte R.; Gómez Espinosa C. O.; del Espinosa Cuevas M. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014, 29, 582–590. 10.3305/nh.2014.29.3.7179. [DOI] [PubMed] [Google Scholar]
- Nakabayashi I.; Nakamura M.; Kawakami K.; Ohta T.; Kato I.; Uchida K.; Yoshida M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol. Dial. Transplant. 2011, 26, 1094–1098. 10.1093/ndt/gfq624. [DOI] [PubMed] [Google Scholar]
- Natarajan R.; Pechenyak B.; Vyas U.; Ranganathan P.; Weinberg A.; Liang P.; Mallappallil M. C.; Norin A. J.; Friedman E. A.; Saggi S. J. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed. Res. Int. 2014, 2014, 568571. 10.1155/2014/568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan N.; Friedman E. A.; Tam P.; Rao V.; Ranganathan P.; Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. 10.1185/03007990903069249. [DOI] [PubMed] [Google Scholar]
- Ranganathan N.; Ranganathan P.; Friedman E. A.; Joseph A.; Delano B.; Goldfarb D. S.; Tam P.; Rao A. V.; Anteyi E.; Musso C. G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. 10.1007/s12325-010-0059-9. [DOI] [PubMed] [Google Scholar]
- Rossi M.; Johnson D. W.; Morrison M.; Pascoe E. M.; Coombes J. S.; Forbes J. M.; Szeto C. C.; McWhinney B. C.; Ungerer J. P.; Campbell K. L. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): A randomized trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. 10.2215/CJN.05240515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni M.; Citraro M. L.; Cerantonio A.; Deodato F.; Provenzano M.; Cianfrone P.; Capria M.; Corrado S.; Libri E.; Comi A.; Pujia A.; Abenavoli L.; Andreucci M.; Cocchi M.; Montalcini T.; Fuiano G. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur. J. Nutr. 2019, 58, 2145–2156. 10.1007/s00394-018-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama F.; Taki K.; Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am. J. Kidney Dis. 2003, 41, S142–S145. 10.1053/ajkd.2003.50104. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Cao C.; Wu Z.; Zhang H.; Sun Z.; Wang M.; Xu H.; Zhao Z.; Wang Y.; Pei G.; Yang Q.; Zhu F.; Yang J.; Deng X.; Hong Y.; Li Y.; Sun J.; Zhu F.; Shi M.; Qian K.; Ye T.; Zuo X.; Zhao F.; Guo J.; Xu G.; Yao Y.; Zeng R. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942. 10.1016/j.cmet.2021.06.014. [DOI] [PubMed] [Google Scholar]
- Zoppi G.; Cinquetti M.; Benini A.; Bonamini E.; Minelli E. B. Modulation of the intestinal ecosystem by probiotics and lactulose in children during treatment with ceftriaxone. Curr. Ther. Res. Clin. Exp. 2001, 62, 418–435. 10.1016/S0011-393X(01)89006-8. [DOI] [Google Scholar]
- Timmerman H. M.; Koning C. J.; Mulder L.; Rombouts F. M.; Beynen A. C. Monostrain, multistrain and multispecies probiotics--A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. 10.1016/j.ijfoodmicro.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Ouwehand A. C.; Invernici M. M.; Furlaneto F. A.; Messora M. R. Effectiveness of multi-strain versus single-strain probiotics: current status and recommendations for the future. J. Clin. Gastroenterol. 2018, 52, S35–S40. 10.1097/MCG.0000000000001052. [DOI] [PubMed] [Google Scholar]
- Evenepoel P.; Poesen R.; Meijers B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. 10.1007/s00467-016-3527-x. [DOI] [PubMed] [Google Scholar]
- Hosten A. O.BUN and creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker H. K, Hall W. D., Hurst J. W., Eds.; Butterworths: Boston, MA, 1990. [PubMed] [Google Scholar]
- Cruz-Mora J.; Martínez-Hernández N. E.; Martín del Campo-López F.; Viramontes-Hörner D.; Vizmanos-Lamotte B.; Muñoz-Valle J. F.; García-García G.; Parra-Rojas I.; Castro-Alarcón N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Ren. Nutr. 2014, 24, 330–335. 10.1053/j.jrn.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Viramontes-Hörner D.; Márquez-Sandoval F.; Martín-del-Campo F.; Vizmanos-Lamotte B.; Sandoval-Rodríguez A.; Armendáriz-Borunda J.; García-Bejarano H.; Renoirte-López K.; García-García G. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J. Ren. Nutr. 2015, 25, 284–291. 10.1053/j.jrn.2014.09.008. [DOI] [PubMed] [Google Scholar]
- de Araújo É. M. R.; Meneses G. C.; Carioca A. A. F.; Martins A. M. C.; Daher E. F.; da Silva Junior G. B. Use of probiotics in patients with chronic kidney disease on hemodialysis: a randomized clinical trial. J. Bras. Nefrol. 2023, 45, 152–161. 10.1590/2175-8239-jbn-2022-0021en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.; Niu J.; Tang X. C.; Shan L. H.; Xiao L.; Wang Y. Q.; Yin L. Y.; Yu Y. Y.; Li X. R.; Zhou P. The effects of probiotic Lactobacillus rhamnosus GG on fecal flora and serum markers of renal injury in mice with chronic kidney disease. Front. Biosci. (Landmark Ed). 2023, 28, 226. 10.31083/j.fbl2809226. [DOI] [PubMed] [Google Scholar]
- Rivière A.; Selak M.; Lantin D.; Leroy F.; De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hul M.; Le Roy T.; Prifti E.; Dao M. C.; Paquot A.; Zucker J. D.; Delzenne N. M.; Muccioli G.; Clément K.; Cani P. D. From correlation to causality: the case of Subdoligranulum. Gut Microbes 2020, 12, 1849998. 10.1080/19490976.2020.1849998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliocca G.; Mone P.; Di Iorio B. R.; Heidland A.; Marzocco S. Short-chain fatty acids in chronic kidney disease: focus on inflammation and oxidative stress regulation. Int. J. Mol. Sci. 2022, 23, 5354. 10.3390/ijms23105354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.; Xie S.; Lv D.; Zhang Y.; Deng J.; Zeng L.; Chen Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek. 2016, 109, 1389–1396. 10.1007/s10482-016-0737-y. [DOI] [PubMed] [Google Scholar]
- Jiang S.; Xie S.; Lv D.; Wang P.; He H.; Zhang T.; Zhou Y.; Lin Q.; Zhou H.; Jiang J.; Nie J.; Hou F.; Chen Y. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. 10.1038/s41598-017-02989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Y.; Chen T. W.; Lu W. L.; Liang S. S.; Huang H. D.; Tseng C. P.; Tarng D. C. Synbiotics alleviate the gut indole load and dysbiosis in chronic kidney disease. Cells. 2021, 10, 114. 10.3390/cells10010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Oliveira V.; Amano M. T.; Correa-Costa M.; Castoldi A.; Felizardo R. J.; de Almeida D. C.; Bassi E. J.; Moraes-Vieira P. M.; Hiyane M. I.; Rodas A. C.; Peron J. P.; Aguiar C. F.; Reis M. A.; Ribeiro W. R.; Valduga C. J.; Curi R.; Vinolo M. A.; Ferreira C. M.; Câmara N. O. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshifuji A.; Wakino S.; Irie J.; Tajima T.; Hasegawa K.; Kanda T.; Tokuyama H.; Hayashi K.; Itoh H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol. Dial. Transplant. 2016, 31, 401–412. 10.1093/ndt/gfv353. [DOI] [PubMed] [Google Scholar]
- Saito Y.; Sato T.; Nomoto K.; Tsuji H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125. 10.1093/femsec/fiy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri N. D.; Wong J.; Pahl M.; Piceno Y. M.; Yuan J.; DeSantis T. Z.; Ni Z.; Nguyen T. H.; Andersen G. L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- Maldonado R. F.; Sá-Correia I.; Valvano M. A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.; Wang L.; Bhat O. M.; Lohner H.; Li P. L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. 10.1016/j.redox.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.; Liu J.; Sun J.; Gong Q.; Ma H.; Kan X.; Cao Y.; Wang J.; Fu S. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radic. Biol. Med. 2020, 152, 728–742. 10.1016/j.freeradbiomed.2020.01.016. [DOI] [PubMed] [Google Scholar]
- Yaku K.; Enami Y.; Kurajyo C.; Matsui-Yuasa I.; Konishi Y.; Kojima-Yuasa A. The enhancement of phase 2 enzyme activities by sodium butyrate in normal intestinal epithelial cells is associated with Nrf2 and p53. Mol. Cell. Biochem. 2012, 370, 7–14. 10.1007/s11010-012-1392-x. [DOI] [PubMed] [Google Scholar]
- Suez J.; Zmora N.; Elinav E. Probiotics in the next-generation sequencing era. Gut Microbes. 2020, 11, 77–93. 10.1080/19490976.2019.1586039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmora N.; Zilberman-Schapira G.; Suez J.; Mor U.; Dori-Bachash M.; Bashiardes S.; Kotler E.; Zur M.; Regev-Lehavi D.; Brik R. B.; Federici S.; Cohen Y.; Linevsky R.; Rothschild D.; Moor A. E.; Ben-Moshe S.; Harmelin A.; Itzkovitz S.; Maharshak N.; Shibolet O.; Shapiro H.; Pevsner-Fischer M.; Sharon I.; Halpern Z.; Segal E.; Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018, 174, 1388–1405. 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- Wu I. W.; Gao S. S.; Chou H. C.; Yang H. Y.; Chang L. C.; Kuo Y. L.; Dinh M. C. V.; Chung W. H.; Yang C. W.; Lai H. C.; Hsieh W. P.; Su S. C. Integrative metagenomic and metabolomic analyses reveal severity-specific signatures of gut microbiota in chronic kidney disease. Theranostics. 2020, 10, 5398–5411. 10.7150/thno.41725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun H.; Yang W.; Zhao S.; Jiang M.; Xu M.; Liu F.; Wang Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. MicrobiologyOpen 2019, 8, e00678 10.1002/mbo3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Yang S.; Li S.; Zhao L.; Hao Y.; Qin J.; Zhang L.; Zhang C.; Bian W.; Zuo L.; Gao X.; Zhu B.; Lei X. G.; Gu Z.; Cui W.; Xu X.; Li Z.; Zhu B.; Li Y.; Chen S.; Guo H.; Zhang H.; Sun J.; Zhang M.; Hui Y.; Zhang X.; Liu X.; Sun B.; Wang L.; Qiu Q.; Zhang Y.; Li X.; Liu W.; Xue R.; Wu H.; Shao D.; Li J.; Zhou Y.; Li S.; Yang R.; Pedersen O. B.; Yu Z.; Ehrlich S. D.; Ren F. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. 10.1136/gutjnl-2019-319766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers S. C.; Quimby J. M.; Isaiah A.; Suchodolski J. S.; Lunghofer P. J.; Gustafson D. L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Int. Med. 2019, 33, 662–669. 10.1111/jvim.15389. [DOI] [PMC free article] [PubMed] [Google Scholar]


