Abstract
To study the antigenic conservation of epitopes of human immunodeficiency virus type 1 (HIV-1) isolates of different clades, the abilities of human anti-HIV-1 gp120 and gp41 monoclonal antibodies (MAbs) to bind to intact HIV-1 virions were determined by a newly developed virus-binding assay. Eighteen human anti-HIV MAbs, which were directed at the V2, V3 loop, CD4-binding domain (CD4bd), C5, or gp41 regions, were used. Nine HIV-1 isolates from clades A, B, D, F, G, and H were used. Microtiter wells were coated with the MAbs, after which virus was added. Bound virus was detected after lysis by testing for p24 antigen with a noncommercial p24 enzyme-linked immunosorbent assay. The anti-V3 MAbs strongly bound the four clade B viruses and viruses from the non-B clades, although binding was weaker and more sporadic with the latter. The degrees of binding by the anti-V3 MAbs to CXCR4- and CCR5-tropic viruses were similar, suggesting that the V3 loops of these two categories of viruses are similarly exposed. The anti-C5 MAbs bound isolates of clades A, B, and D. Only weak and sporadic binding of all the viruses tested with anti-CD4bd, anti-V2, and anti-gp41 MAbs was detected. These results suggest that V3 and C5 structures are shared and well exposed on intact virions of different clades compared to the CD4bd, V2, and gp41 regions.
Immunochemical analysis of the reaction between monoclonal antibodies (MAbs) and soluble gp120 or oligomeric gp160 envelope glycoproteins has been used to study the degree of antigenic and structural conservation of human immunodeficiency virus type 1 (HIV-1) viral envelopes across clades. Both linear and conformational antigenic epitopes have been identified. Some MAbs to primarily linear epitopes (e.g., V3 loop) cross-react with several HIV-1 subtypes, indicating that such structures are shared by different HIV-1 clades (22). Similarly, MAbs to discontinuous structures that form conformational epitopes, such as the CD4 binding domain (CD4bd), cross-react with soluble gp120 and recombinant gp120 of different clades, confirming their conservation within and between clades (25).
eports indicate, however, that gp120 subunit proteins are not adequate mimics of the more complex structures present on virions (10, 35, 47, 49, 51). It has been shown that the patterns of reactivity of MAbs or sera with subunit proteins do not correspond with the ability to neutralize their respective viruses (34, 35, 43). Nevertheless, most of the antigenic and structural information available on HIV-1 has been obtained by immunochemical studies using soluble subunit proteins (35, 37). No study that investigated the nature of exposed epitopes on intact virus particles has been reported. Ultimately, antibodies induced by a vaccine against different HIV clades must target the native viral envelope. This indicates the need to study the antigenic conservation of epitopes shared among intact virions of different HIV-1 clades and to identify regions that are well exposed on the surfaces of these viruses.
Immunogold staining and electron microscopic studies have shown that class I major histocompatibility complex molecules and other host cell membrane proteins (such as various adhesion molecules) are incorporated into the membranes of retroviruses, including HIV, as they bud from host cells (17). A simple virus-binding enzyme-linked immunosorbent assay (ELISA), utilizing MAbs against host membrane proteins, was used to examine the acquisition of various cell-derived proteins by HIV and simian immunodeficiency virus as they bud from different host cells (6, 11, 44). This ELISA has now been adapted to the study of the epitopes of the viral glycoproteins exposed on intact HIV-1 virions.
A total of nine HIV-1 isolates were tested. They are classified into subtypes A (VI191), B (CA5, MNp, IIIB, and JR-FL), D (MAL), F (CA20), G (VI526), and H (CA13) (30, 33, 39, 40). Isolates IIIB and MAL are laboratory-adapted strains; the other seven viruses tested are primary isolates. Isolate MNp (donated by John Sullivan, University of Massachusetts Medical School, Worcester) is a primary isolate that was obtained from a patient’s spleen and cocultivated with donor peripheral blood mononuclear cells (PBMCs). This virus has never been passaged in a cell line. Virus stocks were prepared on human PBMCs from HIV-negative donors as previously described (42, 43). The PBMC-infected culture supernatants were aliquoted (1 ml/tube) and stored in liquid nitrogen. The p24 concentration in each virus stock was quantitated by a noncommercial p24 ELISA as previously described (6).
Eighteen human MAbs were tested for their abilities to bind HIV-1 virions of different clades. These MAbs included three anti-V2 MAbs (697-D, 1361, and 1357), six anti-V3 MAbs (447-52D, 419-D, 694/98D, 838-D, 412-D, and 1027-15D), four anti-CD4bd MAbs (654-D, 559/64-D, 588-D, and 1202-D), two anti-C5 MAbs (670-D and 1331A), and three anti-gp41 MAbs (98-6, 1342, and 1367). These MAbs were produced by using PBMCs derived from HIV-1-seropositive individuals, and most of them have been extensively described (19, 21, 23, 31, 32, 52, 55). Briefly, in producing the MAbs, the Epstein-Barr virus-transformed PBMCs that were positive for the desired antibodies were expanded and then fused with the SHM-D33 human X mouse heteromyeloma (19). The resulting heterohybridomas were rescreened for the production of the desired antibody, and the positive cultures were sequentially cloned at limiting cell concentrations until monoclonality was achieved.
The virus-binding ELISA has previously been used to identify and quantitate adhesion molecules that are incorporated into the envelopes of HIV-1 virions (6, 11, 44). We have adapted this protocol to measure the abilities of anti-HIV-1-specific MAbs to bind to intact HIV-1 virions. In this modified protocol, 96-well microtiter plates were coated overnight at 4°C with 0.1 ml of the respective anti-HIV-1 antibodies at 10 μg per ml in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, 3 mM NaN3 [pH 9.6]). The plates were washed four times with phosphate-buffered saline (PBS) and blocked for 1 h at 37°C with 0.2 ml of 3% bovine serum albumin in PBS per well. The plates were then washed four times with RPMI 1640, and 0.1 ml of virus-containing supernatant with 50 to 200 ng of p24 per ml was added per well. The virus was allowed to bind to the MAbs for 1 h at 37°C. After four washes with RPMI 1640 to remove unbound virus, the bound virus was lysed by exposure to 0.25 ml of 1% Triton X-100 (Sigma) per well for 1 h at room temperature, and the p24 was quantitated by a noncommercial ELISA as described below. For each experiment, antibody and virus combinations were tested in duplicate wells.
To ascertain that the virus binding to the test MAb was specific, a series of controls were included in each experiment. Irrelevant MAb 860-55D to parvovirus B19 (1) and anti-HIV-1 pooled immunoglobulin (HIVIG) from infected persons (donated by A. Prince [HIVIG-US] and B. Jackson [HIVIG-UG]) were used as negative and positive controls, respectively. MAbs 860-55D and HIVIG were applied at 10 and 50 μg/ml, respectively. Virus-only and MAb-only wells were also included in each experiment. In each experiment, the actual p24 concentration that was added to each well was quantitated concurrently, and only assays with actual p24 inputs between 50 and 200 ng/ml were included in the data presented. To ensure the reproducibility of our method, 50% of the experiments were repeated.
The amount of virus p24 measured for each virus-MAb combination is a reflection of the extent of the virus bound to the MAb. A virus-MAb combination is considered reactive if the calculated average amount of p24 captured (in pg/ml) exceeds a threshold T. The threshold T is based on the average p24 capture amounts for the nine isolates in the presence of negative control MAb 860-55D plus 6 standard deviations. The data used for this calculation appear in Table 1. The threshold value obtained was 14 pg/ml. Thus, p24 values >14 pg/ml were considered positive and values ≤14 pg/ml were considered negative.
TABLE 1.
Binding of anti-gp120 and anti-gp41 MAbs to HIV-1 isolates of different clades
| Subtype | Isolate | Phenotype | p24 capture (pg/ml) by indicated MAbs:
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-V3
|
Anti-CD4bd
|
Anti-C5
|
Anti-V2
|
Anti-gp41
|
Control Aba
|
||||||||||||||||
| 447-52D | 419-D | 694/98D | 838-D | 412-D | 654-D | 559/64-D | 588-D | 1202-D | 670-D | 1331A | 697-D | 1361 | 1357 | 98-6 | 1367 | 1342 | 860-55D | HIVIG-US | |||
| B | CA5 | NSI | 264 | 50 | 80 | 55 | 37 | 1 | 0 | 1 | 3 | 86 | 74 | 22 | 20 | 7 | 0 | 4 | 2 | 4 | 114 |
| MNp | SI | 299 | 90 | 11 | 65 | 71 | 3 | 2 | 0 | 0 | 30 | 25 | 2 | 0 | 0 | 0 | 3 | 4 | 0 | 207 | |
| IIIB | SI | 169 | 0 | 52 | 51 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 139 | |
| JR-FL | NSI | 370 | 57 | 83 | 103 | 72 | 33 | 0 | 0 | 0 | 100 | 197 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 252 | |
| A | VI191 | NSI | 39 | 4 | 0 | 37 | 0 | 0 | 21 | 22 | 18 | 60 | 0 | 15 | 12 | 10 | 0 | 10 | 0 | 6 | 137 |
| D | MAL | SI | 276 | 64 | 22 | 21 | 72 | 0 | 42 | 27 | 60 | 162 | 189 | 38 | 23 | 33 | 6 | 11 | 0 | 6 | 286 |
| F | CA20 | NSI | 34 | 0 | 6 | 11 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 10 | 7 | 0 | 1 | 37 |
| G | VI526 | SI | 43 | 0 | 2 | 8 | 4 | 0 | 27 | 20 | 23 | 4 | 0 | 10 | 10 | 6 | 1 | 5 | 0 | 6 | 137 |
| H | CA13 | SI | 15 | 1 | 9 | 0 | 0 | 0 | 8 | 1 | 3 | 0 | 12 | 3 | 0 | 5 | 0 | 0 | 0 | 1 | 59 |
The levels of p24 binding by the negative (860-55D) and positive (HIVIG-US) control antibodies are the averages calculated from all experiments.
The p24 from virus lysates was quantitated by a modification of a previously described noncommercial ELISA (6). Briefly, 0.1 ml of test samples was added overnight onto 96-well plates that had been coated with 0.1 μg of anti-p24 human MAb 91-5 (that maps to position 198 to 210 on the recombinant p24 protein) used at a concentration of 0.5 μg per ml (19). After the plates were washed, biotinylated anti-p24 human MAb 241-D (an anti-p24 MAb that maps to position 210 to 381 on recombinant p24 protein) was added for 2.5 h and detected by incubation of the plates with strepavidin-alkaline phosphatase (ELISA amplification system kit; Gibco BRL, Gaithersburg, Md.) for 1 h at 37°C. After six washes with 0.05 M Tris–0.15 M NaCl (BioWhittaker, Walkersville, Md.), the plates were developed with the ELISA amplification system and read at 490 nm (6).
To determine the optimum amount of MAb to use, concentrations varying between 0 and 50 μg/ml were tested with 0.1 ml of virus stocks containing approximately 100 ng of p24 per ml. As shown in Fig. 1a, MAb concentrations between 2.5 and 10 μg/ml showed optimum binding to the viruses tested. The MAbs were then applied at 10 μg/ml and further tested with virus at various p24 concentrations ranging between 0 and 200 ng/ml. As shown on Fig. 1b, the amount of virus that bound to the MAbs increased with increasing virus p24 input. For subsequent experiments, virus at approximately 100 ng/ml was used. Binding of ≤6 pg of p24 per ml was observed with negative control MAb 860-55D, irrespective of the virus input. HIVIG showed positive binding of the viruses tested in preliminary experiments (Fig. 1) and subsequently bound all the isolates tested, irrespective of clades (when applied at 50 μg/ml), and was therefore used as a positive control (Table 1). The virus binding assay was highly reproducible (as shown in Table 2).
FIG. 1.
Binding patterns with varying concentrations of MAbs (a) and virus (b). Each curve represents the binding achieved by the designated combination of monoclonal or polyclonal antibody and virus. In panel a, the amount of MAb used varied from 0 to 20 μg/ml while the amount of virus added per well was held constant (100 ng/ml). In panel b, the amount of virus used varied from 0 to 200 ng of p24/ml while the amount of MAb added per well was held constant (10 μg/ml).
TABLE 2.
Test variability of the virus binding assay
| Isolate | Expt no. | p24 capture (pg/ml) by MAb:
|
|||
|---|---|---|---|---|---|
| 447-52D | 838-D | 412-D | 654-D | ||
| CA5 | 1 | 248 | 50 | 31 | 0 |
| 2 | 249 | 46 | 32 | 5 | |
| 3 | 264 | 55 | 37 | 1 | |
| MNp | 1 | 299 | 65 | 71 | 3 |
| 2 | 260 | 56 | NDa | 0 | |
| CA20 | 1 | 34 | 11 | 0 | 0 |
| 2 | 37 | 4 | 0 | 0 | |
ND, not done.
Virus culture supernatant at 100 ng of p24/ml was used as the standard input in the binding assay. To determine if the presence of non-virion-associated p24 and shed gp120 in the virus culture supernatants affected the binding of intact virions to anti-Env MAbs, 1-ml stocks of four viruses (VI526, IIIB, VI191, and CA5) were prepared at 100 ng of p24/ml and pelleted. Non-virion-associated p24 and gp120 present in the supernatants of the pellets were decanted. The pellets were then resuspended in 1 ml of RPMI 1640 medium and tested in binding assays with MAbs, and the binding patterns were compared with those of the unpelleted viruses. The p24 concentrations of the pelleted viruses ranged between 54 and 83 ng/ml. Overall, the pelleted viruses bound to MAbs with the same pattern as the unpelleted viruses. For example, MAb 447-52D bound comparably to both pelleted and unpelleted IIIB, with p24 concentrations of 148 and 169 pg/ml, respectively. MAbs 838-D and 654-D did not bind to either pelleted or unpelleted VI526. MAb 654-D did not bind to CA5 irrespective of whether it was pelleted or not. MAb 838-D bound to both pelleted and unpelleted CA5 with p24 concentrations of 53 and 55 pg/ml, respectively. These results suggest that non-virion-associated p24 and gp120 had little or no influence on the binding of these viruses to the antibodies tested.
The abilities of five anti-V3 MAbs to bind to four clade B and five non-B-clade HIV-1 viruses were examined. The anti-V3 MAbs strongly bound the four clade B viruses and bound viruses from the non-B clades, although binding was weaker and more sporadic than with clade B strains (Table 1). MAb 447-52D was the most potent MAb, binding all of the isolates tested, with p24 concentrations captured ranging between 15 and 370 pg/ml. MAb 419-D was the weakest in binding. Only four isolates, three from clade B and one from clade D, bound to the 419-D MAb. Isolates JR-FL (clade B) and MAL (clade D) were the most sensitive in binding to anti-V3 MAbs (ranges, 57 to 370 and 21 to 276 pg/ml, respectively). Three isolates, CA20 (clade F), CA13 (clade H), and VI526 (clade G) only bound to one of the anti-V3 MAbs (447-52D).
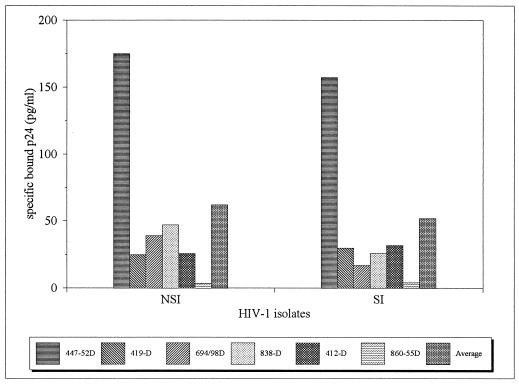
The V3 loop of HIV-1 has been implicated in the induction of syncytia in MT2 cells (13, 28, 48). Recently, the non-syncytium-inducing (NSI) and syncytium-inducing (SI) viral phenotypes have been shown to correlate well with coreceptor usage in that NSI isolates are CCR5-tropic and SI isolates are CXCR4-tropic (7, 12, 54). Some reports indicate that the V3 loops of NSI isolates are not well exposed compared to the V3 loops of SI isolates (9). Of the nine isolates studied, four were NSI (CA5, JR-FL, VI191, and CA20) and five were SI (MNp, IIIB, MAL, VI526, and CA13) (12, 41). Overall, degrees of binding by anti-V3 MAbs to NSI (CCR5-tropic) and SI (CXCR4-tropic) viruses were similar, suggesting that the V3 loops of these two categories of viruses are similarly exposed on the surfaces of these viruses (Fig. 2 and Table 1).
FIG. 2.
Binding of anti-V3 MAbs to NSI (CCR5-tropic) and SI (CXCR4-tropic) viruses. Four NSI viruses (CA5, JR-FL, VI191, and CA20) and five SI viruses (MNp, IIIB, MAL, VI526, and CA13) were tested. The average degrees of binding of viruses from each category to each anti-V3 MAb are shown, as is the average binding of each category to all anti-V3 MAbs. The values of specific bound p24 were calculated by subtracting the amounts of p24 in the wells coated with anti-parvovirus B19 MAb 860-55D, which acted as the negative control, from the amounts of p24 in the wells of the test MAbs. The data on which the averages depicted in this figure are based are shown in Table 1.
The five anti-V3 MAbs tested have previously been mapped with V3 peptides derived from MNp, IIIB, and RF (22, 23). However, interaction with these linear core epitopes accounts for only about 10% of the binding energy of the MAbs for the intact molecule (18). These data suggest that the conformation of the V3 loop plays a critical role in the recognition of viral structures by these MAbs. This finding was confirmed in studies where MAb 447-52D was shown to neutralize primary isolates that lacked the exact core epitope (12). In Table 3, the presence or absence of the linear core epitope of each anti-V3 MAb in each test virus is indicated. In most cases, when the core epitope was present, the MAb bound to the virus (Table 3). For example, the V3 sequence of MNp contained the epitopes of four of the anti-V3 MAbs (447-52D, 419-D, 694/98D, and 412-D) and bound to three of the four MAbs (447-52D, 419-D, and 412-D). However, binding was also noted when the linear core epitope was not present. For example, isolate MAL lacked the core epitopes of all the MAbs tested but bound to each of them. These results confirm that the conformation of the V3 loop is critical in binding to MAbs.
TABLE 3.
Correlation between presence of a V3 linear core epitope in an HIV-1 isolate and the isolate’s ability to bind to the MAb recognizing that epitope
| Isolate | Result for anti-V3 MAb:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 447-52D
|
419-D
|
694/98D
|
838-D
|
412-D
|
||||||
| Epitopea | Bindingb | Epitope | Binding | Epitope | Binding | Epitope | Binding | Epitope | Binding | |
| CA5 | + | + | − | + | + | + | − | + | − | + |
| MNp | + | + | + | + | + | − | − | + | + | + |
| IIIB | + | + | − | − | + | + | − | + | − | + |
| JR-FL | + | + | − | + | + | + | + | + | − | + |
| VI191 | + | + | − | − | + | − | − | + | − | − |
| MAL | − | + | − | + | − | + | − | + | − | + |
| CA20 | + | + | − | − | + | − | − | − | − | − |
| CA13 | − | + | − | − | − | − | − | − | − | − |
The presence (+) or absence (−) in the test viruses of the linear core epitopes recognized by the anti-V3 MAbs.
The ability of the MAb to bind to the isolate. +, binding of >14 pg/ml; −, binding of <14 pg/ml (see the text).
Binding of anti-C5 MAbs to the different clades was strong and comparable to binding by the anti-V3 MAbs (Table 1). Of the 18 test combinations of anti-C5 MAbs and viruses examined, 9 (50%) combinations showed positive binding of >14 pg/ml. Isolates JR-FL (clade B) and MAL (clade D) bound best, with p24 levels greater than 100 pg/ml. Overall, both MAbs (670-D and 1331A) bound the same isolates. Isolates IIIB, CA20, and VI526 did not bind to either anti-C5 MAbs.
Four anti-CD4bd MAbs and three MAbs each to V2 and gp41 were tested with nine HIV-1 isolates of the different clades. The results shown in Table 1 indicate that binding of the viruses to these MAbs was weak and sporadic compared with binding to anti-V3 and anti-C5 MAbs. These results also show that binding was not clade specific. Though binding of all three groups of MAbs (anti-CD4bd, anti-V2, and anti-gp41) to the nine isolates was weak, the three groups bound fairly well to MAL (clade D) as compared to the remaining eight isolates.
Of 36 test combinations of the four anti-CD4bd MAbs with the nine viruses, 10 (28%) combinations showed positive binding (>14 pg/ml); MAb 1202 demonstrated the strongest binding with virus MAL (60 pg/ml; Table 1). Five viruses, CA5, MNp, IIIB, CA20, and CA13, did not bind any of the four anti-CD4bd MAbs, while MAL was the most sensitive, binding to three of four MAbs. MAb 654-D was the poorest MAb, binding only to one isolate, JR-FL (clade B).
Of 27 test combinations of the three anti-V2 MAbs with the nine viruses, 6 (22%) combinations showed positive binding (Table 1). Virus MAL was again the most sensitive in binding to all three anti-V2 MAbs. MAb 697-D was the most potent in binding to three viruses (CA5, VI191, and MAL). Six viruses, MNp, IIIB, JR-FL, CA20, VI526, and CA13, did not bind to any of the three MAbs.
Twenty-seven test combinations involving three anti-gp41 MAbs and the nine viruses were examined (Table 1). None of these combinations showed a binding level >14 pg/ml with any of the viruses tested.
To ascertain if the failure of MAbs to bind to envelope proteins on virions was simply the result of their inability to bind the protein, we examined the binding patterns of three anti-CD4bd MAbs (654-D, 559/64, and 1202) and three anti-V2 MAbs [697-D, 1361-D, and 1357D(A)] for binding to soluble gp120 proteins from four viruses (CA5, JR-FL, MAL, and CA13). Briefly, 0.1 ml of 10% Triton X-100 was added to 0.9 ml of virus culture supernatant to inactivate the virus and solubilize the virus proteins. Then, 5 ng of the HIV-1 gp120 proteins per ml was captured on ELISA plates with a sheep antibody specific for the C terminus of gp120. The captured gp120 molecules were reacted with the anti-V2 and anti-CD4bd MAbs as previously described (29). HIVIG-US and 860-55D were used as positive and negative controls, respectively. All the anti-CD4bd and anti-V2 MAbs reacted with the solubilized gp120 of the viruses tested irrespective of the clade of the virus, with the exception of MAb 697-D (an anti-V2 MAb), which did not react with MAL (a subtype D virus) (data not shown). This extensive reactivity with soluble gp120 demonstrates that the anti-CD4bd and anti-V2 MAbs recognize the epitopes on the soluble gp120 proteins of these viruses but that these epitopes are not exposed on the gp120 protein of the intact virions.
Thus, with this new assay, we show for the first time that MAbs directed at different HIV-1 envelope regions are able to bind to intact HIV-1 virions. Anti-V3 and anti-C5 MAbs bind strongly to different HIV-1 clades, while anti-V2, anti-CD4bd, and anti-gp41 MAbs weakly bind these isolates.
Early studies of V3 Abs against divergent clade B laboratory strains suggested that V3 Abs were “type specific” (24, 45, 46). However recently, several human anti-V3 MAbs derived from clade B-infected patients and one from a clade E-infected patient were shown to cross-react extensively with the V3 loops of diverse clades (20, 22, 36). Other studies have shown that anti-V3 MAbs are able to bind to cells infected with isolates of different HIV-1 clades and that these antibodies can neutralize diverse isolates (14, 16, 18, 27, 55). Our studies using V3 MAbs and intact virions demonstrate that V3 Abs are neither type specific nor clade specific since some anti-V3 MAbs (447-52D and 838-D) bind virions from several clades. In addition, we have observed that the anti-V3 MAbs bind similarly to both NSI and SI isolates. Taken together, these results suggest that, although there is sequence heterogeneity in the V3 loops of different clades, antigenic similarities exist among isolates of different clades of HIV-1 and that these similarities are conferred by both sequence and conformational homologies. Moreover, this region is well exposed on the surfaces of these viruses, irrespective of their genetic subtypes or phenotypes (NSI or SI).
The C5 region is known to be well conserved (39, 50). Some models of gp120 propose that some epitopes in the C5 domain are buried (37, 38); however, most of the studies upon which this conclusion was based were performed with gp120 monomers (37) or oligomers (38) rather than native gp120 in intact virions. Our studies circumvented these limitations and investigated the exposure of C5 on virus particles. In our virus binding ELISA, we observed that the binding of anti-C5 MAbs to virions of different clades was strong and comparable to that of the anti-V3 MAbs. Eighteen test combinations of anti-C5 MAbs and viruses were examined, nine (50%) of which showed good binding to isolates of clades A, B, and D. These results suggest that the structure of the C5 region is shared among isolates of these clades and that this region is well exposed on the surfaces of these viruses.
Binding by MAbs to three additional envelope regions, V2, CD4bd, and gp41, was weak and sporadic but not clade restricted. The poor reactivities of the three anti-V2 MAbs could be attributed to the high level of variability and frequent insertions and deletions of varying lengths that characterize this region (39). In contrast, the CD4bd region is thought to be conserved; yet anti-CD4bd MAbs, while binding strongly to solubilized gp120, bound weakly and sporadically to the nine viruses tested. This could be due to the masking of this region when the envelope gp120 molecule adopts its native quaternary structure in the assembled virion. The apparent unavailability of these epitopes on the surfaces of free intact virions suggests that the envelope of the virus may undergo conformational changes upon binding to the CD4 receptor on cells. This could lead to exposure of new epitopes on the virus particle to which antibodies to V2, CD4bd, and gp41 could then bind. These antibodies could then act to neutralize at a postbinding step, which has been described for several antibodies to these and other epitopes (2, 3).
Carbohydrate moieties present on the HIV-1 envelope are known to mask antigenic epitopes on the virus (8, 26), thereby making the virus more resistant to neutralizing antibodies (4, 15). This may provide an additional, or alternative, explanation for the failure of epitopes in V2, the CD4bd, and gp41 to bind MAbs. When the envelope is deglycosylated, the virus often becomes susceptible to neutralizing antibodies (4, 15). Thus, the glycosylation patterns around the V2, CD4bd, and gp41 regions may differ from those covering the V3 and C5 regions, thereby making the former inaccessible for binding with the respective antibodies tested.
The binding patterns of anti-gp120 and anti-gp41 MAbs to the native, oligomeric viral envelope glycoproteins expressed on the surfaces of human PBMCs infected in vitro with HIV-1 primary isolates of different clades were previously examined by flow cytometry (55). The results of these studies corroborate those presented above in that anti-V3 and anti-C5 MAbs bound well to cells infected with isolates of different genetic subtypes; in addition, the anti-V3 MAbs bound most strongly to clade B-infected cells, while the anti-V2, anti-gp41, and anti-CD4bd MAbs bound weakly. In addition, both studies of infected cells and virus particles demonstrate that the binding of many MAbs does not correlate with the genetic subtypes of the virions. This observation is also in agreement with previous observations showing that sera from patients infected with a particular clade do not preferentially neutralize viruses of that clade (34, 42, 53). Moreover, immunochemical analysis of the reaction between polyclonal or monoclonal antibodies and V3 peptides or soluble gp120 molecules has shown that binding patterns do not correspond to clades (5, 35). Whether or not the patterns of binding we observe with MAbs and virions will correlate with the abilities of the MAbs to neutralize the viruses remains to be demonstrated. These analyses are under way and will be described elsewhere.
In conclusion, we have demonstrated the abilities of anti-V3 and anti-C5 MAbs to strongly bind to HIV-1 isolates of different genetic subtypes, showing that the V3 and C5 structures are shared and are well exposed on the surfaces of these viruses. The similar patterns of binding of the anti-V3 MAbs to both CCR5- and CXCR4-tropic viruses indicate that the V3 loops of these viruses are similarly exposed. The binding of the anti-CD4bd, anti-V2, and anti-gp41 MAbs to the different isolates was weak and sporadic, suggesting that these regions are not well exposed on the surfaces of intact virions.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institutes of Health (AI 32424, AI 36085, and HL 59725), from the department of Veterans Affairs, from Fonds Wetenschappelijk Onderzoek (grant G 0134.97), and from the European Commission (grants IC 18-CT96-0110 and IC 18 CT97-02476).
We thank Arthur Nádas for the statistical analysis and his cooperation in our studies. We also thank Suman Laal for critical review of this manuscript.
REFERENCES
- 1.Arakelov S, Gorny M K, Williams C, Riggin C H, Brady F, Collett M S, Zolla-Pazner S. Generation of neutralizing anti-B19 parvovirus human monoclonal antibodies from patients infected with human immunodeficiency virus. J Infect Dis. 1993;168:580–585. doi: 10.1093/infdis/168.3.580. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong S J, Dimmock N J. Varying temperature-dependence of post-attachment neutralization of human immunodeficiency virus type 1 by monoclonal antibodies to gp120: identification of a very early fusion-independent event as a neutralization target. J Gen Virol. 1996;77:1397–1402. doi: 10.1099/0022-1317-77-7-1397. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong S J, McInerney T L, McLain L, Wahren B, Hinkula J, Levi M, Dimmock N J. Two neutralizing anti-V3 monoclonal antibodies act by affecting different functions of human immunodeficiency virus type 1. J Gen Virol. 1996;77:2931–2941. doi: 10.1099/0022-1317-77-12-2931. [DOI] [PubMed] [Google Scholar]
- 4.Back N K T, Smith L, Dejong J J. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology. 1994;2:431–438. doi: 10.1006/viro.1994.1141. [DOI] [PubMed] [Google Scholar]
- 5.Barin F, Lahbabi Y, Buzelay L, LeJeune B, Baillou-Beaufils A, Denis F, Mathiot C, M’Boup S, Vithayasai V, Dietrich U, Goudeau A. Diversity of antibody binding to V3 peptides representing consensus sequences of HIV type 1 genotypes A to E: an approach for HIV type 1 serological subtyping. AIDS Res Hum Retroviruses. 1996;12:1279–1289. doi: 10.1089/aid.1996.12.1279. [DOI] [PubMed] [Google Scholar]
- 6.Bastiani L, Laal S, Zolla-Pazner S, Kim M. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyo E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolmstedt A, Olofsson S, Sjogren-Jansson E, Jeansson S, Sjoblom I, Akerblom L, Hansen J S, Hu S. Carbohydrate determinant NeuAc-GalB(1-4) of N-linked glycans modulates the antigenic activity of human immunodeficiency virus type 1 glycoprotein pg120. J Gen Virol. 1992;73:3099–3105. doi: 10.1099/0022-1317-73-12-3099. [DOI] [PubMed] [Google Scholar]
- 9.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broder C C, Earl P L, Long D, Abedon S T, Moss B, Doms R W. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capobianchi M R, Fais S, Castilletti C, Gentile M, Amelglio F, Dianzani F. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J Infect Dis. 1994;169:886–889. doi: 10.1093/infdis/169.4.886. [DOI] [PubMed] [Google Scholar]
- 12.Cecilia D, KewalRamani V N, O’Leary J, Volsky B, Nyambi P, Burda S, Xu S, Littman D R, Zolla-Pazner S. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng-Mayer C, Shioda T, Levy J A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991;65:6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley A J, Gorny M K, Kessler II J A, Boots L J, Ossorio-Castro M, Lineberger D W, Emini E A, Koenig S, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis D, Stephens M, Willers C, Lachmann P. Glycosylation governs the binding of antipeptide antibodies to regions of hypervariable amino acid sequence within recombinant gp120 of human immunodeficiency virus type 1. J Gen Virol. 1990;71:2889–2898. doi: 10.1099/0022-1317-71-12-2889. [DOI] [PubMed] [Google Scholar]
- 16.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelderblom H R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991;5:617–637. [PubMed] [Google Scholar]
- 18.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorny M K, Gianakakos V, Sharpe S, Zolla-Pazner S. Generation of human monoclonal antibodies to HIV. Proc Natl Acad Sci USA. 1989;86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorny M K, Mascola J R, Israel Z R, VanCott T C, Williams C, Balfe P, Hioe C, Brodine S, Burda S, Zolla-Pazner S. A human monoclonal antibody specific for the V3 loop of HIV type 1 clade E cross-reacts with other HIV type 1 clades. AIDS Res Hum Retroviruses. 1998;14:213–221. doi: 10.1089/aid.1998.14.213. [DOI] [PubMed] [Google Scholar]
- 21.Gorny M K, Moore J P, Conley A J, Karwowska S, Sodroski J, Williams C, Burda S, Boots L J, Zolla-Pazner S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorny M K, VanCott T C, Hioe C, Israel Z R, Michael N L, Conley A J, Williams C, Kessler II J A, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and inter-clade cross-reactivity. J Immunol. 1997;159:5114–5122. [PubMed] [Google Scholar]
- 23.Gorny M K, Xu J, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150:635–643. [PubMed] [Google Scholar]
- 24.Goudsmit J, Debouck C, Meloen R H, Smit L, Bakker M, Asher D M, Wolff A V, Gibbs C J, Jr, Gajdusek D C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci USA. 1988;85:4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haigwood N L, Barker C B, Higgens K W, Skiles P V, Moore G K, Mann K A, Lee D R, Eichberg J W, Steimer K S. Evidence for neutralizing antibodies directed against conformational epitopes of HIV-1 gp120. In: Brown F, Chanock R M, Ginsberg H, Luner R A, editors. Vaccines 90: modern approaches to new vaccines including prevention of AIDS. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1990. pp. 313–320. [Google Scholar]
- 26.Hansen J S, Nielsen C, Arendrup M, Olofsson S, Mathiesen L, Nielsen J O, Clausen H. Broadly neutralizing antibodies targeted to mucin-type carbohydrate epitopes of human immunodeficiency virus. J Virol. 1991;65:6461–6467. doi: 10.1128/jvi.65.12.6461-6467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hioe C E, Xu S, Chigurupati P, Burda S, Williams C, Gorny M K, Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int Immunol. 1997;9:1281–1290. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- 28.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 29.Israel Z R, Gorny M K, Palmer C, McKeating J A, Zolla-Pazner S. Prevalence of a V2 epitope in clade B primary isolates and its recognition by sera from HIV-1 infected individuals. AIDS. 1997;11:128–130. [PubMed] [Google Scholar]
- 30.Janssens W, Heyndrickx L, Fransen K, Mitte J, Peeters M, Nkengasong J N, Ndumbe P M, Delaporte E, Perret J, Atende C, Piot P, van der Groen G. Genetic and phylogenetic analysis of env subtypes G and H in Central Africa. AIDS Res Hum Retroviruses. 1994;10:877–879. doi: 10.1089/aid.1994.10.877. [DOI] [PubMed] [Google Scholar]
- 31.Karwowska S, Gorny M K, Buchbinder A, Gianakakos V, Williams C, Fuerst T, Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retroviruses. 1992;8:1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- 32.Karwowska S, Gorny M K, Culpepper S, Burda S, Laal S, Samanich K, Zolla-Pazner S. The similarities and diversity among human monoclonal antibodies to the CD4-binding domain of HIV-1. In: Brown F, Sinsberg H S, Lerner R, editors. Vaccines 93: modern approaches to vaccine including prevention of AIDS. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 229–232. [Google Scholar]
- 33.Louwagie J, McCutchan F E, Peeters M, Brennan T P, Sanders-Buell E, Eddy G A, van der Groen G, Fransen K, Gershy-Damet G-M, Deleys R, Burke D S. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore J P, McCutchan F E, Poon S, Mascola J, Liu J, Cao Y, Ho D D. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68:8350–8364. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore J P, Sattentau Q, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers G, Korber B, Foley B, Smith R F, Jeang K, Mellors J W, Wain-Hobson A, editors. Human retroviruses and AIDS. Theoretical biology and biophysics. Los Alamos, N. Mex: Los Alamos National Laboratories; 1996. [Google Scholar]
- 40.Nkengasong J N, Janssens W, Heyndrickx L, Fransen K, Ndumbe P M, Motte J, Leonaers A, Ngolle M, Ayuk J, Piot P, van der Groen G. Genetic subtypes of HIV-1 in Cameroon. AIDS. 1994;8:1405–1412. doi: 10.1097/00002030-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Nyambi, P. N., L. Heyndrickx, W. Janssens, F. Daeyaert, P. Lewi, K. Fransen, B. Willems, S. Coppens, K. Vereecken, P. Piot, and G. van der Groen. Identification of specific amino acid motifs and biophenotypic properties among primary HIV-1 group M (subtype A-H) and O isolates belonging to different neutralization clusters. Submitted for publication.
- 42.Nyambi P N, Nkengasong J, Lewi P, Andries K, Janssens W, Fransen K, Heyndrickx L, Piot P, van der Groen G. Multivariate analysis of human immunodeficiency virus type 1 neutralization data. J Virol. 1996;70:6235–6243. doi: 10.1128/jvi.70.9.6235-6243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyambi P N, Nkengasong J, Peeters M, Simon F, Eberle J, Janssens W, Fransen K, Willems B, Vereecken K, Heyndrickx L, Piot P, van der Groen G. Reduced capacity of antibodies from patients infected with human immunodeficiency virus type-1 (HIV-1) group O to neutralize primary isolates of HIV-1 group M viruses. J Infect Dis. 1995;172:1228–1237. doi: 10.1093/infdis/172.5.1228. [DOI] [PubMed] [Google Scholar]
- 44.Orentas R J, Hildreth J E K. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 45.Palker T J, Clark M E, Langlois A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palker T J, Clark M E, Langlois A L, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes G. Type-specific neutralization of HIV with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1758–1762. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 49.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starcich B, Hahn B, Shaw G, McNeely P, Modrow S, Wolf H, Parks E, Parks W, Josephs S, Gallo R, Wong-Staal F. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986;45:637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanCott T C, Bethkes F R, Polonis V R, Gorny M G, Zolla-Pazner S, Redfield R R, Birx D L. Dissociation rate of antibody-gp120 binding interactions is predictive of V3-mediated neutralization of HIV-1. J Immunol. 1994;153:449–459. [PubMed] [Google Scholar]
- 53.Weber J, Fenyo E M, Beddows S, Kaleebu P, Björndal Å the WHO Network for HIV Isolation and Characterization. Neutralization serotypes of human immunodeficiency type 1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Carruthers C D, He T, Huang Y, Cao Y, Wang G, Hahn B, Ho D D. HIV type 1 subtypes, coreceptor usage, and CCR5 polymorphism. AIDS Res Hum Retroviruses. 1997;13:1357–1366. doi: 10.1089/aid.1997.13.1357. [DOI] [PubMed] [Google Scholar]
- 55.Zolla-Pazner S, O’Leary J, Burda S, Gorny M K, Kim M, Mascola J, McCutchan F. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J Virol. 1995;69:3807–3815. doi: 10.1128/jvi.69.6.3807-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]