Abstract
Tax, the transforming protein of human T-cell leukemia virus type 1 (HTLV-1), is required for strong activation of HTLV-1 transcription. This activation is mediated through interaction with the KIX domain of the cellular coactivator CREB binding protein (CBP). In this study we examined the possibility that the Tax-KIX interaction may mediate effects on cellular gene transcription in vivo, as a growing number of cellular transcription factors have been shown to utilize CBP as a coactivator. We tested the ability of Tax to deregulate the activity of the cellular transcription factor, c-Myb, since both Tax and c-Myb interact with the KIX domain of CBP. Our results show that in vivo, Tax antagonizes the transcriptional activity of c-Myb and, reciprocally, c-Myb antagonizes the transcriptional activity of Tax. Furthermore, c-Myb competes for KIX binding to Tax in vitro, indicating that these two transcription factors bind CBP in a mutually exclusive manner. This novel mechanism of transcriptional interference by Tax may promote globally deregulated cellular gene expression in the HTLV-1-infected cell, possibly leading to leukemogenesis.
The human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus that is the causative agent of tropical spastic paraparesis and adult T-cell leukemia (ATL) (22, 24). ATL is characterized by clonal proliferation of a CD4+ T lymphocyte that typically carries a single copy of the HTLV-1 proviral genome (14). The HTLV-1-encoded Tax protein is critical for HTLV-1 pathogenesis (for a review, see reference 11). Tax is a 40-kDa transcriptional regulator protein required for viral transcription and has been shown to deregulate a wide variety of cellular genes (for a review, see references 10 and 11). Tax deregulation of cellular gene expression is widely believed to be the primary event in the initiation of HTLV-1-dependent leukemogenesis.
While the mechanism of Tax-mediated cellular transformation is poorly understood, several molecular steps in Tax transcriptional activation have recently been characterized. To activate transcription of the HTLV-1 genome, Tax interacts with the cellular transcription factor CREB bound to the three viral CRE promoter elements and also contacts nucleotides immediately flanking the CREB binding site (1, 4, 7, 12, 18, 33, 34). Tax, in the context of this stable promoter-bound complex, then serves as a high-affinity binding site for recruitment of the coactivator CREB binding protein (CBP) (5, 15, 17). Although the precise function of CBP in the context of Tax activation of HTLV-1 transcription has not been fully defined, several lines of evidence suggest that CBP functions as a coactivator through chromatin remodeling and recruitment of the general transcription machinery (2, 21, 32).
CBP is a large nuclear protein, 2,441 amino acids in length, that carries several discrete domains which bind many structurally unrelated transcription factors. One of these domains, called KIX, is located approximately between amino acids (aa) 450 and 700 of CBP. This region of KIX serves as the major binding site for HTLV-1 Tax, as well as the cellular transcription factors c-Myb, c-Jun, and serine-133-phosphorylated CREB (3, 9, 15, 17, 20, 23, 30). KIX aa 588 to 665 have been identified as the minimal region of KIX that is sufficient for interaction with Tax in vivo (31). This region of KIX contains a compact hydrophobic core structure composed of three interacting α helices (25). The observation that free Tax protein binds to this domain of KIX, together with the recognition of this domain by several cellular transcription factors, raises the possibility that Tax may compete with these transcriptional activator proteins for utilization of limiting CBP in an HTLV-1-infected cell. Occupancy of the KIX domain by Tax may block the binding of the other transcription factors, producing widespread deregulation of cellular gene expression.
In this study, we investigated the effect of Tax on the transcriptional activity of the cellular transcription factor c-Myb, a protein which has a primary role in regulation of hematopoietic cell growth, differentiation, and transformation (19, 28). c-Myb has previously been shown to interact with aa 590 to 669 of the KIX domain (9), a region which significantly overlaps the minimal region of the KIX domain required for Tax binding and corresponds to the hydrophobic core region. We demonstrate here that Tax represses the transcriptional activity of c-Myb and that the CBP binding region of c-Myb (aa 185 to 360) is sufficient for the repression by Tax. We further show that this CBP binding region of c-Myb effectively competes with Tax for KIX binding in vitro, suggesting that the binding of these two transcription factors to KIX is mutually exclusive. These data provide evidence for Tax repression of a cellular transcription factor through direct competition for CBP. This competition may promote global dysregulation of cellular genes in an HTLV-1-infected cell.
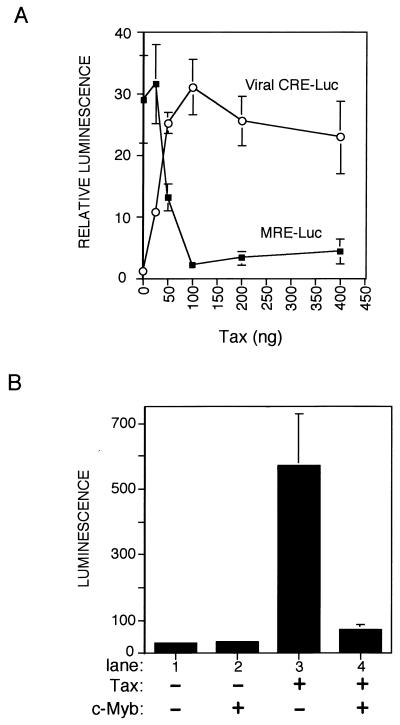
Tax represses the transcription function of c-Myb in vivo.
Since both Tax and c-Myb bind an extensively overlapping region of KIX, we hypothesized that large amounts of Tax may bind to CBP, thus antagonizing the transcription function of c-Myb. To test this idea, transient transfection experiments were performed with the human T-cell line Jurkat. The transcriptional activity of c-Myb was measured by using a Myb-responsive luciferase reporter construct (13), which carries five copies of a Myb-responsive element cloned immediately upstream of the minimal E1B promoter (MRE-luc). Figure 1A shows that MRE-luc was active in Jurkat cells, an expected result since the c-Myb protein is expressed at high levels in this cell line (data not shown). Cotransfection of increasing amounts of a Tax expression plasmid (pIEX [27]) produced a 15-fold repression of transcription from the Myb-responsive reporter plasmid. This repression by Tax appeared to be dependent upon c-Myb in the cell, as deletion of the Myb response elements abrogated the repression by Tax (data not shown). Under precisely the same conditions that produced strong repression of c-Myb-dependent gene expression, Tax strongly activated (15-fold) the Tax-responsive viral CRE reporter plasmid (viral CRE-luc [15]), indicating that Tax was functional in the assay and was not toxic to the cells (Fig. 1A). Although Tax and c-Myb have not previously been shown to interact, complex formation between these two transcription factors in vivo might explain the observed c-Myb repression by Tax. Cotransfection of a c-Myb expression plasmid, however, did not rescue c-Myb transcriptional activity, suggesting that the mechanism of Tax repression is indirect (data not shown).
FIG. 1.
Mutual transcriptional repression by Tax and the cellular transcription factor c-Myb. (A) Tax represses the transcriptional function of c-Myb. HTLV-1-negative Jurkat T cells were transiently cotransfected (with Lipofectamine) with 500 ng of either pMRE-luc (■) (13) or viral CRE-luc (○) (15) reporter plasmids and the indicated amount of the Tax expression plasmid (pIEX [27]). (B) c-Myb represses the transcriptional function of Tax. CV-1 cells (at 60% confluency on 60-mm plates) were transiently cotransfected (with calcium phosphate) with 1 μg of the HTLV-1 promoter reporter pLTR-luc (15) and 1 μg of either the c-Myb (8) or Tax expression plasmid. Cell extracts were assayed for luciferase activity. Reported values represent the means ± standard errors (error bars) from three independent experiments.
If Tax repression of c-Myb transcriptional activity occurs through competition for the KIX domain of CBP as hypothesized, then c-Myb should likewise inhibit the transcriptional activity of Tax. To test this hypothesis, we performed the reciprocal experiment with CV-1 cells. CV-1 cells were selected for this experiment, as endogenous c-Myb protein is undetectable, thus allowing direct measurement of c-Myb transcriptional activity following cotransfection of a c-Myb expression plasmid (8). Figure 1B shows that, as expected, transfection of the Tax expression plasmid produced a 17-fold increase in activation of the HTLV-1 promoter-luciferase reporter construct (lane 3). Cotransfection of the c-Myb expression plasmid in the presence of Tax produced an 8-fold attenuation of Tax transactivation of the HTLV-1 promoter (Fig. 1B, lane 4). Cotransfection of the c-Myb expression plasmid with the MRE-luc reporter plasmid produced a 14-fold increase in luciferase activity, indicating that the c-Myb expression plasmid produced functional protein in this assay (data not shown). Analogous to the reciprocal repression of c-Myb activity by Tax, transfection of additional Tax expression plasmid did not result in the recovery of repression, again consistent with an indirect mechanism of repression (data not shown). Together, these studies show that Tax represses the transcriptional activity of c-Myb and, reciprocally, c-Myb represses the transcriptional activity of Tax. These results are consistent with the hypothesis that Tax and c-Myb compete for utilization of limiting CBP in vivo.
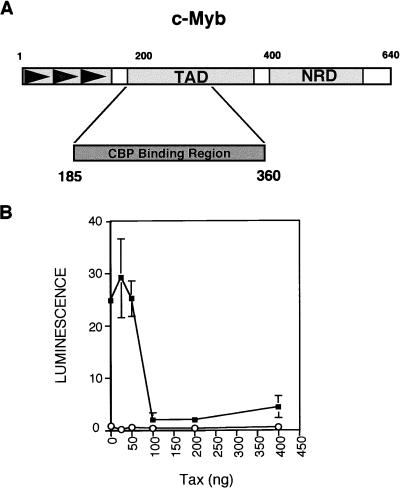
The CBP binding region of c-Myb is sufficient for Tax repression.
A region of the c-Myb transcriptional activation domain has previously been shown to directly bind to the KIX domain to recruit CBP to c-Myb-responsive promoters (Fig. 2A) (9, 20). To more directly examine whether competition for CBP might account for Tax repression of c-Myb transcriptional activity, we fused the CBP binding region of c-Myb to the DNA binding domain of GAL4 (GAL4 [aa 1 to 147]–c-Myb [aa 185 to 360]; referred to hereafter as GAL4–c-Mybaa185–360). We hypothesized that this chimeric protein would activate transcription of a GAL4-responsive reporter construct through direct recruitment of CBP. Moreover, increasing amounts of Tax should compete for the available intracellular CBP, resulting in repression of GAL4-c–Mybaa185–360-dependent transcription. Figure 2B shows the results of a transient transfection assay in which pGAL4/c-Mybaa185–360 was cotransfected with a reporter plasmid carrying five copies of the GAL4-responsive element (p5× GAL4-luc) in Jurkat cells. Interestingly, the GAL4–c-Mybaa185–360 chimera activated transcription from the GAL4 reporter construct, indicating that the CBP binding region of c-Myb was sufficient for transcriptional activity. However, titration of the Tax expression plasmid resulted in repression of c-Mybaa185–360-dependent transcription in a dose-dependent fashion (Fig. 2B). As previously observed (Fig. 1A), maximal transcriptional repression occurred when 100 ng of the Tax expression plasmid, a concentration of Tax which should not cause squelching or toxicity to the cells, was cotransfected into the reaction mixture (Fig. 1A). These results strongly suggest that Tax inhibition of c-Myb transcriptional activity occurs through a mechanism dependent upon the c-Myb–CBP interaction.
FIG. 2.
The CBP-binding region of c-Myb is sufficient for Tax repression. (A) Schematic representation of the functional domains of c-Myb. Arrowheads indicate the DNA binding domains of c-Myb. The transcriptional activation domain (TAD) and negative regulatory domain (NRD) are also indicated. aa 185 to 360 includes the CBP binding region of c-Myb (9, 20). (B) Tax represses c-Myb transcription through the CBP binding domain of c-Myb. Jurkat T cells were transiently cotransfected with 200 ng of the p5× GAL4-luc reporter plasmid and the indicated amounts of the Tax expression plasmid. Values for reporter alone (○) or reporter in the presence of 200 ng of cotransfected pGAL-Mybaa185–360 expression plasmid (■) are indicated. Cell extracts were assayed for luciferase activity. Reported values represent the means ± standard errors (error bars) from three independent experiments.
Tax and c-Myb compete for KIX in vitro.
The observation that Tax and c-Myb both bind to the KIX domain provides strong support for the hypothesis that their binding to CBP is mutually exclusive, thus explaining their reciprocal repression in vivo. To directly test this hypothesis, we used the electrophoretic mobility shift assay (EMSA) to measure whether the CBP binding region of c-Myb can compete with Tax for binding to KIX. We have previously used the EMSA to show that purified Tax (35), in the context of CREB and the HTLV-1 viral CRE DNA, forms a high-affinity binding site for KIX (15). Under the conditions of the EMSA, KIX binds to the Tax-CREB-viral CRE ternary complex to form a slower-migrating quaternary complex (Fig. 3, lane 4). To test whether c-Myb can compete with Tax for binding to KIX, we titrated glutathione S-transferase (GST)–c-Mybaa185–360 into binding reaction mixtures containing the KIX-Tax-CREB-viral CRE quaternary complex. Figure 3 shows that increasing concentrations of purified GST–c-Mybaa185–360 in the binding reaction mixtures produced a dose-dependent reduction in the amount of KIX-containing quaternary complex, without affecting the Tax-CREB-viral CRE ternary complex (lanes 5 to 9). GST alone did not significantly affect the quaternary complex, indicating that c-Mybaa185–360 was specifically required for the KIX competition. These results show that the CBP binding region of c-Myb is sufficient for competition with Tax for KIX binding in vitro. The observation that GST–c-Mybaa185–360 did not form more slowly migrating complexes with the KIX-Tax-CREB-DNA complex indicates that Tax and c-Myb binding to KIX is mutually exclusive. Furthermore, GST–c-Mybaa185–360 did not affect the ternary complex containing Tax, indicating that Tax and c-Myb do not form a detectable complex in this assay. Together, these results suggest that through their common recognition of the KIX domain, Tax and c-Myb compete for utilization of CBP in vivo.
FIG. 3.
The CBP binding region of c-Myb (aa 185 to 360) competes with Tax for KIX binding in vitro. Binding reaction mixtures contained 4 fmol of 32P-end-labeled viral CRE DNA probe (lane 1) (7) with purified recombinant CREB (0.03 pmol; lanes 2 to 15) (12), with purified recombinant Tax (1 pmol; lanes 3 to 15) (35), and with purified recombinant KIX (1 pmol; lanes 4 to 15) (15). Binding reaction mixtures also contained 2, 4, 12, 20, or 28 pmol of purified GST–c-Mybaa185–360 (lanes 4 to 9) or GST (lanes 10 to 15), as indicated. Binding reaction mixtures were electrophoresed on a 5% nondenaturing gel, as previously described (7). The positions of the relevant protein-DNA complexes are shown.
In summary, we provide evidence in support of the hypothesis that the HTLV-1-encoded oncoprotein Tax competes with cellular protein c-Myb for utilization of intracellular CBP. c-Myb is a DNA binding cellular transcription factor that is expressed in hematopoietic cells and appears to play a role in proliferation, differentiation, and malignant transformation (19, 28). We demonstrate that Tax expression interferes with the transcriptional activity of c-Myb in transient transfection assays. Evidence presented both in vivo and in vitro strongly suggests that the transcriptional interference occurs through competition for CBP, as the binding of Tax and c-Myb to the KIX domain of CBP is mutually exclusive. To extend these studies and to provide biological relevance for the observation, we are attempting to identify endogenous c-Myb-regulated genes that are repressed in the presence of Tax.
Competition for limiting intracellular CBP has potentially significant implications for the deregulation of gene expression in an HTLV-1-infected cell. In the early transcription phase of the viral life cycle, Tax protein is expressed at very high levels. During this time, Tax binding at the KIX domain would effectively sequester available CBP away from cellular transcription factors, likely resulting in the disruption of at least some of the transcription regulatory networks in which CBP is involved and possibly initiating a pathway toward leukemogenesis. A role for CBP in leukemogenesis is supported by studies suggesting that dysregulation of CBP by chromosomal translocation is a hallmark of acute myeloid leukemias (6, 16, 26, 29). The high-affinity binding of Tax to CBP, together with the evidence that Tax is the oncoprotein responsible for HTLV-1-associated ATL, supports a direct link between CBP, Tax, and hematopoietic malignancies. Further characterization of the Tax-CBP interaction, and the intracellular consequences of the interaction, will likely provide insights into the mechanisms of CBP-dependent leukemogenesis.
Acknowledgments
We thank Linda Boxer for the Myb expression plasmid and MRE-luc reporter plasmid, K. T. Jeang for the Tax expression plasmid, and Ken Escudero for construction of the p5× GAL4-luc reporter plasmid. We also thank members of the laboratory for discussions and critical reading of the manuscript.
This work was supported by a grant (VM-170) from the American Cancer Society (to J.K.N.).
REFERENCES
- 1.Adya N, Zhao L-J, Huang W, Boros I, Giam C-Z. Expansion of CREB’s DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at position 282–284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzrides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 4.Baranger A M, Palmer C R, Hamm M K, Giebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA binding enhancement by the HTLV-I transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 5.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow J, Stanton V P, Andresen M, Becher R, Behm F G, Chaganti R S K, Civin C I, Disteche C, Dube I, Frischauf A M, Horsman D, Mitelman F, Volinia S, Watmore A E, Housman D E. The translocation t(8;16)(p11;p13) of acute myeloid leukemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 7.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for HTLV-I latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M F, Kukowska-Latallo J F, Westin E, Smith M, Prochownik E V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988;8:884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai P, Akimaru H, Tanaka Y, Hou D, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 10.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 11.Franklin A A, Nyborg J K. Mechanisms of tax regulation of human T-cell leukemia virus type I gene expression. J Biomed Sci. 1995;2:17–29. doi: 10.1007/BF02257921. [DOI] [PubMed] [Google Scholar]
- 12.Franklin A A, Kubik M F, Uittenbogaard M N, Brauweiler A, Utaisincharoen P, Matthews M, Dynan W S, Hoeffler J P, Nyborg J K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) and cAMP element-binding protein (CREB) J Biol Chem. 1993;268:21225–21231. [PubMed] [Google Scholar]
- 13.Fu S L, Lipsick J S. FAETL motif required for leukemic transformation by v-Myb. J Virol. 1996;70:5600–5610. doi: 10.1128/jvi.70.8.5600-5610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo R C. Human T-cell leukemia-lymphoma virus and T-cell malignancies in adults. Cancer Surv. 1984;3:113–159. [Google Scholar]
- 15.Giebler H A, Loring J E, Van Orden K, Colgin M A, Garrus J E, Escudero K E, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type-1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles R H, Dauwerse J G, Higgins C, Petrij F, Wessels J W, Beverstock G C, Dohner H, Jotterand-Bellomo M, Falkenburg J H. Detection of CBP rearrangements in acute myelogenous leukemia with t(8;16) Leukemia. 1997;11:2087–2096. doi: 10.1038/sj.leu.2400882. [DOI] [PubMed] [Google Scholar]
- 17.Kwok R P S, Laurance M E, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the coactivator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 18.Lenzmeier B A, Giebler H A, Nyborg J K. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol Cell Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsick J S. One billion years of Myb. Oncogene. 1996;13:223–235. [PubMed] [Google Scholar]
- 20.Oelgeschlager M, Janknecht R, Krieg J, Schreek S, Luscher B. Interaction of the co-activator CBP with Myb proteins: effects on Myb-specific transactivation and on the cooperativity with NF-M. EMBO J. 1996;15:2771–2780. [PMC free article] [PubMed] [Google Scholar]
- 21.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 22.Osame M, Usuku K, Izumo S, Ijichi N, Amitini H, Igata A. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 23.Parker D, Ferreri K, Nakajima T, LaMorte V J, Evans R, Koerber S C, Hoeger C, Montminy M. Phosphorylation of CREB at Ser133 induces complex formation with CBP via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radhakrishnan I, Perez-Alvarado G C, Parker D, Dyson H J, Montminy M R, Wright P E. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- 26.Rowley J D, Reshmi S, Sobulo O, Musvee T, Anastasi J, Raimondi S, Schneider N R, Barredo J C, Cantu E S, Schlegelberger B, Behm F, Doggett N A, Borrow J, Zeleznik L N. All patients with the t(11;16)(q23;p13.3) that involves MLL and CBP have treatment-related hematolig disorders. Blood. 1997;90:535–541. [PubMed] [Google Scholar]
- 27.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type 1 Tax: regions necessary for function determined with 47 point mutants. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slater R M, van Ommen G J, Hagemeijer A, van der Reijden B A, Breuning M H, Golay J, Basilico L, Loffarelli L, Songia S, Brocolli V, Introna M. Regulation of the hematopoietic cell proliferation and differentiation by the myb oncogene family of transcription factors. Int J Clin Lab Res. 1996;26:24–32. doi: 10.1007/BF02644770. [DOI] [PubMed] [Google Scholar]
- 29.Sobulo O M, Borrow J, Tomek R, Reshmi S, Harden A, Schlegelberger B, Housman D, Doggett N A, Rowley J D, Zeleznik-Le N J. MLL is fused to CBP, a histone acetyltransferase, in therapy-related acute myeloid leukemia with a t(11;16)(q23;p13.3) Proc Natl Acad Sci USA. 1997;94:8732–8737. doi: 10.1073/pnas.94.16.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Orden, K., J.-P. Yan, A. Ulloa, and J. K. Nyborg. Binding of HTLV-I tax to the coactivator CBP disrupts the transcription activity of c-Jun. Submitted for publication.
- 31.Yan J-P, Garrus J, Giebler H A, Stargell L, Nyborg J K. Molecular interactions between the human T-cell leukemia virus Tax protein and the cellular coactivator CBP. J Mol Biol. 1998;281:395–400. doi: 10.1006/jmbi.1998.1951. [DOI] [PubMed] [Google Scholar]
- 32.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 33.Yin M-J, Paulssen E J, Seeler J S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type 1 tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin M-J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L-J, Giam C-Z. Interaction of the human T-cell lymphotrophic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]