Abstract
Purpose: :
Measure concentrations of the neurogenic, pro-neurogenic, pro-synaptogenic and anti-inflammatory mediator N-docosahexaenoylethanolamine (synaptamide) in relation to its precursor docosahexaenoic acid (DHA) in breast milk.
Design and methods: :
Postpartum women were recruited prior to discharge. We supplemented half the subjects with omega-3 fatty acids. Breast milk samples were collected at 1, 4 and 8 weeks. Synaptamide and DHA concentrations were determined by liquidchromatography/tandem mass spectrometry (LC-MS/MS) and gas chromatography, respectively.
Results: :
Synaptamide was detected in all breast milk samples. The concentration ranged from 44 to 257 fmol/mL. Omega-3 fatty acid supplementation did not affect DHA or synaptamide concentration in breast milk due to a high-DHA-containing diet self-selected by control mothers. Nevertheless, synaptamide levels significantly correlated with DHA concentration in breast milk (r = 0.624, P < 0.001).
Conclusion: :
This is the first demonstration of detectable concentrations of synaptamide in human breast milk. Although the attempt to raise the milk DHA content by omega-3 fatty acid supplementation was not successful in the current study, the positive correlation observed between synaptamide and DHA concentration suggests that synaptamide levels in human milk can be raised by proper omega-3 fatty acid supplementation that is known to increase DHA.
Keywords: Synaptamide, Docosahexaenoic acid (DHA), Gas chromatography, Liquid chromatography, Mass spectrometry
1. Introduction
Infants delivered preterm are at risk of being deficient in long-chain polyunsaturated fatty acids (LCPUFA). This is due to infants’ limited ability to synthesize LCPUFA from its precursors and hence reliance on enteral sources of LCPUFA [1]. Doxosahexaenoic acid (22:6n-3, DHA), a long chain omega-3 (n-3) fatty acid derived from its precursor, α-linolenic acid (18:3n-3), is highly enriched in the brain and is important for proper brain development [2,3]. The Western diet does not provide an appropriate amount of DHA for lactating mothers, and postnatal levels of DHA in preterm infants have been reported to fall by 40% during the first week of life [4].
Many studies have reported the effects of DHA supplementation in lactating mothers on the fatty acid composition in breast milk and in infant plasma. Maternal daily supplementation of 1200 mg of DHA increased the breastmilk and infant plasma DHA concentration to almost 12 and 2–3 times higher than the control group, respectively [2]. Also, significant increases in DHA were found in breast milk as well as in infant plasma after 6 weeks of maternal supplementation of DHA at 400 mg/day [3]. These studies confirmed that DHA supplementation to lactating mothers is safe and is effective in increasing DHA levels in the breast milk and in infants.
Postnatal deficiency in DHA is pro-inflammatory and has been associated with the development of neonatal diseases [4]. Infants born preterm are at risk for several disease processes including chronic lung disease (CLD), necrotizing enterocolitis (NEC) and retinopathy of prematurity, all of which are related to inflammation [5]. In infants with CLD, the pro-inflammatory cytokines intraleukin(IL)-1, IL-6, IL-8 and tumor necrosis factor (TNF)-α predominate in both plasma and airway fluids [5]. Of particular note, infants who developed CLD had lower mean DHA levels than control infants without CLD [6]. Studies performed in animal models have shown a decrease in NEC and lung injury in animals receiving DHA supplementation [5,7].
The mechanisms by which DHA supplementation to lactating mothers or directly to infants attenuates inflammation are not well understood. Recently, N-docosahexaenoylethanolamine (synaptamide), a neurogenic and synaptogenic metabolite of DHA, has been shown to be also anti-inflammatory, providing a potential mediator responsible for ameliorating neonatal proinflammatory conditions [8]. As synaptamide is endogenously derived from DHA, synaptamide production likely responds to its precursor DHA levels as altered by dietary omega-3 PUFA. Indeed, a direct link between synaptamide levels in the fetal hippocampus and omega-3 PUFA in the maternal diet has been demonstrated in a mouse model [9]. However, no studies have examined the relationship between maternal omega-3 supplementation and synaptamide levels in humans. Breast milk is the primary source of DHA in most neonates, whether born full term or preterm. Although prior studies have measured DHA concentrations in breast milk, synaptamide levels have not been assessed. The objective of this study is to measure synaptamide concentrations in relation to DHA in expressed breast milk from lactating mothers. To manipulate the DHA concentration in breast milk, we supplemented half the subjects with omega-3 fatty acids and evaluated DHA and synaptamide concentrations in the expressed breast milk from all mothers enrolled.
2. Material and methods
2.1. Subjects and experimental design
The inclusion criteria included female military health care beneficiaries ≥18 years old who delivered a live-born infant at Walter Reed National Military Medical Center, and planned to breastfeed for at least two months. Mothers with diabetes were excluded. Subjects were recruited from the postpartum ward prior to their discharge home. Mothers who consented were randomized to continue on their usual diet (non-supplemented cohort) or to be supplemented with one Barlean’s Ideal Omega-3 softgel capsule daily, containing 750 mg eicosapentaenoic acid (EPA) and 250 mg DHA (http://www.pharmaca.com).
Maternal demographic data and characteristics were collected from each enrolled mother, including age, race, education, pre-pregnancy body mass index (BMI), and any pregnancy complications. Infant characteristics included birth weight, gestational age, and Apgar scores. At enrollment and at 30 and 60 days after enrollment, mothers completed a food diary as a measure of omega-3 fatty acid dietary intake [10]. This dietary survey has been shown to have high reliability and validity. Mothers collected 1–2 teaspoons (5–10 mL) of breast milk at baseline and then weekly until the end of study at 60 days after enrollment. Breast milk storage bags were provided and the mothers were instructed to label and place the storage bags in their home freezer immediately after collection. The breast milk samples were collected from the mothers at study completion and were then stored at −80°C until analysis.
2.2. Fatty acid and synaptamide analysis
Human breast milk samples were thawed at 4°C and mixed to disperse fat which had partially separated. Samples were kept on ice whenever possible during analysis. For most samples, synaptamide analysis was accomplished using 3 mL of sample. In brief, 10 μL deuterated internal standard solution containing 4 pmol each d4-synaptamide in methanol was added to samples followed by vortexing for 1 min. Samples were spun for 11 min at 3000 g in a Sorval RTH 750 rotor at 15°C to partially separate the fat which formed a semi-solid disk on the top [11]. A plastic syringe with a 25 gauge hypopdermic needle was used to collect the lower liquid layer which was then transferred to a clean 15 mL polypropylene tube and the fat was discarded. Each sample was brought to 3 mL with water and 7 mL methanol containing butylated hydroxytoluene (BHT, 50 mg/L) was added, vortexed and centrifuged at 3300g for 20 min at 4°C to precipitate the protein pellet. Supernatants were subjected to solid phase extraction and LC-MS analysis as previously described with slight modifications [8]. In brief, the supernatant was loaded onto 30 mg Strata-X 33 μm polymeric reverse phase SPE cartridges (Phenomenex, Torrance, CA) that had been prewashed with 2.5 mL methanol followed by 2.5 mL water. Columns were washed with 5 mL water followed by 5 mL 50% methanol/BHT(50 mg/L) then dried by allowing air to pass through for 1 min. N-acyl ethanolamines were eluted with 2 mL methanol-BHT, dried under nitrogen, resuspended in 25 μL methanol/BHT and 8 μL was analyzed by reverse phase HPLC-MS-MS. For HPLC separation, a Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm, Agilent Technologies, Santa Clara, CA) was used with a linear gradient of acetonitrile/methanol/water changing from 16.3/30/53.7 to 48.7/15/36.3 in 5 min, and then to 18.1/68.4/13.5 in 22 min. The separated synaptamide was detected by a Q-Exactive mass spectrometer (Thermo Scientific, Waltham, MA) operating in the positive ion targeted MS-MS mode, and quantitated against the deuterated internal standard.
Gas chromatography was used to determine total DHA in the breast milk. Lipids were extracted from approximately 200 μL breast milk according to the method of Bligh and Dyer in the presence of 15 μg heneicosanoic acid as an internal standard [12]. The lipid extract was transmethylated with BF3-methanol, extracted in hexane, and injected into a gas chromatograph (Agilent, Palo Alto, CA) as described previously [13].
2.3. Food diary analysis
For analysis, the DHA contents of the fish included in the survey were determined using the USDA National Nutrient Database for Standard Reference [14]. As each type of fish contained in the food diary included several subtypes, both the average of the subtypes and the subtype with the maximal DHA content were used for analysis. By using both, at completion of the analysis each subject had both an average weekly DHA intake and a maximum weekly DHA intake. EPA and α-linolenic acid totals were also calculated using the same method as DHA for complete analysis of the food surveys.
2.4. Statistical methods
Pearson’s correlation was used to determine associations between synaptamide and DHA concentrations in breast milk.
3. Results
3.1. Population characteristics
Thirteen out of fourteen women enrolled completed the study. The demographic characteristics of the study participants are shown in Table 1. Most women (93%) were 26–34 years of age. Pre-pregnancy BMI was normal in 10 participants, one was overweight, and 3 women were obese (BMI ≥ 30 k/m2). There were no significant differences between the unsupplemented (control) and fish oil supplemented study participants for maternal age, pre-pregnancy BMI or education, or for infant birth weight, gestational age, or 5-min Apgar score.
Table 1.
Demographic characteristics of all enrolled subjects.
| Control | Fish Oil | |
|---|---|---|
| Women | ||
| Age | ||
| 18–25 | 0 | 1 |
| 26–34 | 6 | 6 |
| 35 and over | 1 | 0 |
| Pre-pregnancy BMI (kg/m2) | ||
| 18.5–24.9 | 5 | 5 |
| 25–29.9 | 1 | 0 |
| >30 | 1 | 2 |
| Education | ||
| High school | 1 | 0 |
| Some college | 0 | 1 |
| 4-year college graduate | 3 | 5 |
| Postgraduate | 3 | 1 |
| Infants | ||
| Birth weight (g)a | 3367 | 3305 |
| Gestational age (wks)a | 39 | 39 |
| 5 min Apgar scorea | 8.9 | 8.9 |
BMI body mass index.
Mean.
3.2. Food diary results
The food diary analyses are shown in Table 2. The weekly average intake of DHA among the women was consistent throughout the 60 days of study duration. At each analysis, these women on average met or exceeded the weekly DHA recommendation (1.4 g/week) from the Food and Agricultural Organization of the United Nations. [15] Analyses based on the dietary survey indicated that both average and maximum estimation of dietary DHA intake in the omega-3 fatty acid supplemented group were lower than in unsupplemented mothers, who apparently had self-selected to a diet high in DHA content. However, the total DHA intake did not differ in the supplemented group compared to the non-supplemented group.
Table 2.
DHA intake estimated from dietary survey and fish oil supplementation.
| Control | Supplemented A | Supplemented B | p A | p B | |
|---|---|---|---|---|---|
| Initial Ave | 1.37 ± 1.00 | 0.38 ± 0.66 | 2.13 ± 0.66 | 0.08 | 0.17 |
| Max | 2.68 ± 2.18 | 0.78 ± 1.40 | 2.53 ± 1.40 | 0.12 | 0.89 |
| Day 30 Ave | 1.25 ± 1.70 | 0.50 ± 0.93 | 2.25 ± 0.93 | 0.38 | 0.25 |
| Max | 2.47 ± 3.69 | 1.10 ± 2.11 | 2.85 ± 2.11 | 0.46 | 0.84 |
| Day 60 Ave | 1.73 ± 1.65 | 0.17 ± 0.16 | 1.92 ± 0.16 | 0.07 | 0.80 |
| Max | 3.42 ± 3.60 | 0.35 ± 0.32 | 2.10 ± 0.32 | 0.09 | 0.41 |
The weekly docosahexaenoic acid (DHA) intake was calculated based on maternal dietary long chain polyunsaturated fatty acid (LCPUFA) intake surveyed at baseline (initial) and at days 30 and 60. The data are reported as Mean ± SD grams of DHA intake per week. The DHA intake for the supplemented group is shown in two ways; before (A) or after (B) adding DHA intake from the fish oil supplement. Statistical significance of DHA intake is indicated for A and B compared to control as pA and pB, respectively.
3.3. DHA and synaptamide concentration in breast milk
In agreement with the estimated DHA intake from the food diary, no differences in average DHA or synaptamide concentrations were observed in the breast milk from omega-3 supplemented compared to non-supplemented (control) groups at each sampling time point (Table 3). When the DHA content was expressed as a percentage, no significant difference was found either between control and omega-3 supplemented group at the baseline (0.75 ± 1.10 and 0.34 ± 0.15%; p = 0.34), 4th week (0.31 ± 1.15 and 0.30 ± 0.09%; p = 0.92) and 7th week (0.32 ± 0.31 and 0.26 ± 0.07%; p = 0.66). Regardless, a wide within-group concentration spread was observed for both synaptamide (44–246 fmol/mL for control; 65–257 fmol/mL for supplemented group) and DHA (0.13–1.66 μmol/mL or 0.07–2.99% for control; 0.12–1.15 μmol/mL or 0.17–0.65% for supplemented group). Despite the absence of an omega-3 supplementation effect, we found that synaptamide levels significantly correlated with the DHA concentration in the breast milk as illustrated in the Fig. 1 (r = 0.624, P < 0.001). The correlation of synaptamide concentration was weaker but remained significant (r = 0.342, p < 0.05) when analyzed against DHA percentage.
Table 3.
Docosahexaenoic acid (DHA) and synaptamide concentrations in breast milk from fish-oil supplemented and non-supplemented control groups (Mean ± SD).
| Docosahexaenoic acid (DHA)a (μmoles/mL) | |||
|---|---|---|---|
| Group | Baseline | 4th weekc | 7th weekd |
|
| |||
| Control | 0.64 ± 0.52 | 0.48 ± 0.39 | 0.34 ± 0.18 |
| Fish Oil Supplemented | 0.60 ± 0.31 | 0.43 ± 0.14 | 0.32 ± 0.16 |
| p-value | 0.86 | 0.77 | 0.85 |
| Synaptamideb (fmoles/mL) | |||
|
| |||
| Group | Baseline | 4th week | 7th week |
|
| |||
| Control | 181.3 ± 46.3 | 110.3 ± 55.2 | 85.5 ± 33.3 |
| Fish Oil Supplemented | 142.5 ± 33.9 | 134.3 ± 63.1 | 119.9 ± 40.0 |
| p-value | 0.11 | 0.48 | 0.12 |
DHA concentration determined by GC/FID.
Synaptamide concentration determined by LC-MS/MS.
3.3 ± 0.8 weeks
6.9 ± 0.5 weeks.
Fig. 1.
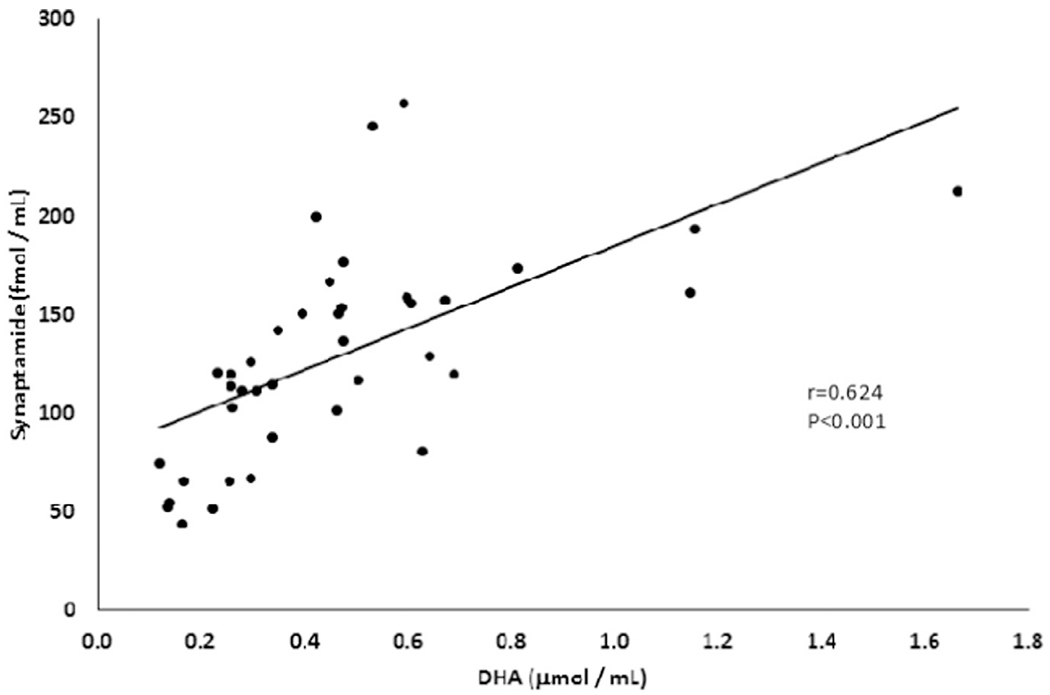
Correlation between synaptamide (fmol/mL) and docosahexaenoic acid (DHA) (μmol/mL) concentrations in breast milk from 13 mothers.
4. Discussion
Synaptamide is an endogenous metabolite of DHA recently identified as a mediator of the neurogenic, neuritogenic, synaptogenic, and anti-inflammatory effects conferred by omega-3 LCPUFA [8,9,16–18]. Despite its developmental importance, synaptamide has not previously been measured in human milk. In this study, we demonstrate the presence of synaptamide in human breast milk. Furthermore, this study represents the first clinical demonstration that there is a significant positive correlation between synaptamide and DHA concentrations in human milk.
The main strength of our study is the demonstrated ability to detect and quantify synaptamide concentrations in breast milk. However, the study has several limitations. First, the sample size is small, but we were nevertheless able to document a significant correlation between DHA and synaptamide concentrations in breast milk in each subject at multiple time points. Second, our study design did not include a placebo, and therefore was not blinded with regards to supplementation, resulting in a self-selected high dietary intake of DHA-containing food in non-supplemented control women. In addition, the compliance was not determined. Third, no measurements of synaptamide and DHA were obtained in the infants, which would have enabled a correlative determination of these compounds in infant blood samples compared to breast milk. A critical next step will be to evaluate synaptamide concentrations in relation to inflammatory responses in infants, particularly in preterm infants at risk for inflammation-associated morbidity.
5. Conclusion
We have documented for the first time that synaptamide is present at a quantifiable level in breast milk from lactating women. Of particular importance, and despite the limited sample size, there is a significant correlation between DHA and synaptamide concentrations. Considering that maternal DHA supplementation can elevate the DHA concentration in breast milk, the positive correlation between synaptamide and DHA suggests this neurogenic, synaptogenic and antiinflammatory mediator can also be increased in breast milk by maternal DHA supplementation. The clinical importance of this increase needs to be assessed by assessing the relationship between infant synaptamide concentrations and inflammatory biomarkers and, most importantly, the effect on inflammation-related morbidities in infants, in particular infants born preterm.
Acknowledgments
This study was supported by Intramural Program of NIAAA, NIH and the Neonatology Fellowship at Walter Reed National Military Medical Center, Uniformed Services University of Health Sciences (USUHS).
We thank Judith Fitzpatrick, MSN for her significant contributions to the successful conduct of this study.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Drs. Smith, Rouse, Hunt and Kim participated in design and conduct of the study, collection of data and data analysis, and review of the manuscript. Dr. Cunningham participated in study design and study implementation. Mr. Kevala was directly involved in the DHA and synaptamide analyses, as was Dr. Smith. All authors approved the final manuscript for submission. Drs. Smith, Hunt and Kim have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. Dr. Smith wrote the first draft of the manuscript. None of the authors received an honorarium, grant, or any form of payment to produce the manuscript.
Abbreviations
- DHA
docosahexaenoic acid
- LCPUFA
long-chain polyunsaturated fatty acids
- CLD
chronic lung disease
- NEC
necrotizing enterocolitis
- IL
interleukins
Footnotes
Conflicts of interest
Declarations of interest: None.
Disclaimers
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army or Department of the Air Force, Department of Defense, NIAAA, nor the U.S. Government. Some authors are military service members or a U.S. Government employee. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
References
- [1].Meldrum SJ, D’Vaz N, Casadio Y, et al. Determinants of DHA levels in early infancy: differential effects of breast milk and direct fish oil supplementation, Prostaglandins Leukot. Essent. Fatty Acids 86 (2012) 233–239. [DOI] [PubMed] [Google Scholar]
- [2].Marc I, Plourde M, Lucas M, et al. Early docosahexaenoic acid supplementation of mothers during lactation leads to high plasma concentrations in very preterm infants, J. Nutr 141 (2011) 231–236. [DOI] [PubMed] [Google Scholar]
- [3].Sherry CL, Oliver JS, Marriage BJ, Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio, Prostaglandins Leukot. Essent. Fatty Acids 95 (2015) 63–69. [DOI] [PubMed] [Google Scholar]
- [4].Martin CR, Dasilva DA, Cluette-Brown JE, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities, J. Pediatr 159 (2011) 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valentine CJ, Maternal dietary DHA supplementation to improve inflammatory outcomes in the preterm infant, Adv. Nutr 3 (2012) 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].De Dooy JJ, Mahieu LM, Van Bever HP, The role of inflammation in the development of chronic lung disease in neonates, Euro. J. Paediatr 160 (2001) 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ma L, Li N, Liu X, et al. Arginyl-glutamine dipeptide or docosahexaenoic acid attenuate hyperoxia-induced lung injury in neonatal mice, Nutrition 28 (2012) 1186–1191. [DOI] [PubMed] [Google Scholar]
- [8].Park T, Chen H, Kevala K, Lee JW, Kim HY, N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling, J Neuroinflamm 13 (2016) 284–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim HY, Lee J, Moon HS, et al. N-docosahexaenoylethanolamine promotes development of hippocampal neurons, Biochem. J 435 (2011) 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ritter-Gooder PK, Lewis NM, Barber-Heidal K, Waltz-Hill M, Development and pilot testing of an omega-3 fatty acid food frequency questionnaire, J. Food Composition. Anal 21 (2007) S43–S49. [Google Scholar]
- [11].Gouveia-Figueira S, Nording ML, Development and validation of a sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of 15 endocannabinoids and related compounds in milk and other biofluids, Anal. Chem 86 (2014) 1186–1195. [DOI] [PubMed] [Google Scholar]
- [12].Bligh EG, Dyer WJ, A rapid method for total lipid extraction and purification, Can. J. Biochem. Physiol 37 (1959) 911–917. [DOI] [PubMed] [Google Scholar]
- [13].Lozada LE, Desai A, Kevala K, Lee JW, Kim HY, Perinatal brain docosahexaenoic acid concentration has a lasting impact on cognition in mice, J. Nutr 147 (2017) 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].USDA Food Composition Database. United States Department of Agriculture-Agricultural Research Service. https://ndb.nal.usda.gov/ndb/search/list. Accessed August 8, 2017. [Google Scholar]
- [15].Global Recommendations for EPA and DHA Intake (Rev 16 April 2014). https://www.goedomega3.com/index.php/files/download/304. Accessed 10 June 2017.
- [16].Lee JW, Huang BX, Kwon H, et al. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function, Nat. Commun 19 (2016) 13123–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rashid MA, Katakura M, Kharebava G, Kevala K, Kim HY, N-Docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation, J. Neurochem 125 (2013) 869–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim HY, Spector A, N-Docosahexaenoylethanolamine: a neurotrophic and neuroprotective metabolite of docosahexaenoic acid, Mol. Aspects Med (2018) in press. [DOI] [PubMed] [Google Scholar]


