Abstract
Knee osteoarthritis (KOA) is a prevalent condition characterized by the progressive deterioration of the entire joint and has emerged as a prominent contributor to disability on a global scale. The nature of the disease and its impact on joint function significantly limit mobility and daily activities, highlighting its substantial influence on patients' overall well-being. Stromal vascular fraction (SVF) is a heterogenous, autologous cell product, containing mesenchymal stem cells, derived from the patient's subcutaneous adipose tissue with demonstrated safety and efficacy in the treatment of KOA patients. We conducted a single-arm, open-label, multisite, FDA approved clinical study in Kellgren–Lawrence severity grade 2–4 KOA patients. The cellular product was manufactured from patient-specific lipoaspirate in a centrally located FDA-compliant manufacturing facility. Twenty-nine subjects were treated with a quality tested single intra-articular injection of GMP manufactured SVF. Adverse events, laboratory values, vital signs, and physical examination findings were monitored during the study period. Robust tolerability, without any substantial safety issues, was demonstrated. Knee pain and function, assessed through the Knee Injury and Osteoarthritis Outcome Score (KOOS), demonstrated notable improvements. These positive benefits persisted for up to 12 months, and the majority of participants expressed satisfaction. SVF from each patient was stored in a liquid nitrogen freezer for future clinical treatments. Unique to this study of autologous cells is the shipment of lipoaspirate from the clinic to a central FDA-compliant manufacturing facility for cleanroom-controlled manufacturing. The cell product characterization data demonstrate that this method produces an equivalent product in terms of cell count and viability with the added benefit of further quality assurance testing, including sterility, endotoxin, and flow cytometry, before patient administration. Clinical Trial Registration Number: NCT 04043819.
Keywords: adult stem cells, mesenchymal stem cells, knee, osteoarthritis, stromal vascular fraction, intra-articular injection
Introduction
Arthritis is a leading cause of disability in the United States with annual costs for medical care and lost earnings exceeding $300 billion (https://www.cdc.gov/chronicdisease/resources/publications/factsheets/arthritis.htm). The most common form of arthritis is osteoarthritis (OA) with knee osteoarthritis (KOA) accounting for more than 80% of the burden of this disease [1,2]. Due to an aging population and rising rates of obesity, the number of Americans with arthritis is projected to increase to 78.4 million within the next 20 years [3].
Osteoarthritis is a progressive disease involving the entire joint [4]. An imbalance of anabolic and catabolic pathways leads to cartilage degeneration, synovial inflammation, and subchondral bone remodeling abnormalities. The ability of chondrocytes to self-renew diminishes with age [5]. Increased expression of inflammatory cytokines IL-1 β, TNF-α, and matrix metallopeptidase-13 [6] promotes the production of other pro-inflammatory factors, including IL-8, IL-6, leukotriene inhibiting factor, proteases, and prostaglandin E2. This array of inflammatory compounds leads to extracellular matrix degradation and the development of osteoarthritis [7].
There are currently no cures for OA. Symptoms are generally managed with a combination of weight loss, physical therapy, bracing, nonsteroidal anti-inflammatory medications (NSAIDS), analgesic medications, and injections of corticosteroids, hyaluronic acid, or platelet rich plasma [8]. These treatments often do not provide lasting improvement, changes in structural abnormalities, or restoration of function. In severe cases, total knee arthroplasty (TKA) is often recommended, but many patients are too young, have medical contraindications, or simply prefer to avoid surgery. It has been estimated that 3.6 million Americans suffer with pain and limited mobility without an effective treatment [9].
Adipose tissue has become important to the study of regenerative medicine in orthopedics [10]. Interest in adipose tissue as a source of cells useful for tissue repair has grown due to the abundance and availability of adipose through the safe and simple method of lipoaspiration [11,12]. Perivascular mesenchymal stem cells (MSCs) also known as “medicinal signaling cells” [13–15] have been identified in bone marrow, umbilical cord, skeletal muscle, and synovial tissue, but subcutaneous adipose tissue is the richest source of these regenerative cells [16]. Only a small portion of the nucleated cells derived from bone marrow aspirate (0.001–0.01%) consists of MSCs [17,18]. Whereas, in adipose tissue, up to 15%–30% may consist of MSCs (an adipose MSC commonly termed adipose stem or stromal cells, ASCs) [19] and are less likely to be influenced by a patient's age [20].
When ASCs are exposed to the synovial fluid from patients with KOA, their immunomodulatory properties are enhanced with reduced T cell proliferation and the generation of T regulatory cells [21,22]. Bioinformatics analysis of soluble factors and extracellular vesicles secreted by ASCs following exposure to an OA patient's synovial fluid provides the molecular basis for immunomodulation and cartilage protection in the osteoarthritic joint [23]. Long-term systemic immunomodulatory effects have also been demonstrated following intra-articular ASC administration [24]. These paracrine effects establish a regenerative microenvironment which promotes endogenous stem cell recruitment, activation, and differentiation [25].
The stromal vascular fraction (SVF) derived from adipose tissue is an abundant source of regenerative cells, including ASCs, pericytes, endothelial progenitor cells, macrophages, lymphocytes, fibroblasts, and smooth muscle cells [26–30], and obtained through enzyme-digested lipoaspirate without the need for culture expansion [16]. Standardized definitions of adipose stem cells and SVF have been proposed by the International Federation of Adipose Therapeutics (IFATS) and International Society of Cellular Therapy (ISCT) [26].
VetStem (VSB), the parent company and contract manufacturer for the Sponsor, has provided manufacturing for adipose-derived cell therapy for veterinarians since 2003 using a centralized FDA-compliant manufacturing model where adipose samples are collected in clinics and shipped refrigerated to a central laboratory for processing, storage, and return to clinics. Generally, PSC-01 is an enzymatically separated SVF similar to the originally published methods in the human [31] and utilized by the VSB facility in its canine and equine osteoarthritis research and development and clinical therapy programs as described in published studies [32,33].
The safety and efficacy of SVF cells have been previously reported in many human clinical trials [34–50]. Based on scientific and empirical evidence, we conducted an open-label, multisite, prospective clinical study designed to evaluate the safety of an intra-articular injection of autologous, adipose-derived SVF cells in patients with moderately severe KOA.
Unique to this clinical trial for autologous cells is this centralized FDA-compliant manufacturing method (current Good Manufacturing Practices, cGMP). Generally, in autologous SVF treatment publications, the method for adipose processing to SVF is using a point-of-care model and device where the cells are administered in the clinic without benefit of laboratory sterility testing and cell characterization. This clinical trial was designed to provide data for assessment of any impacts of the shipping and remote manufacturing.
Materials and Methods
Clinical study objectives
The primary objective of this study was to evaluate the safety of a single intra-articular dose of an investigational biologic product (IBP), PSC-01, an autologous, adipose-derived SVF for the treatment of KOA. The secondary objective was to obtain preliminary evidence of efficacy for PSC-01 in the target population. The data were submitted to the Food and Drug Administration (FDA) as part of the submission process to support potential regulatory approval.
Study design
This study was designed as a single-arm, open-label, clinical safety study in subjects diagnosed with KOA. It was conducted at seven sites within the United States and with nine investigators experienced in orthopedics. The study was conducted in compliance with FDA regulations for phase 1/2A clinical trials and was approved by an Institutional Review Board.
Eligibility
The target population included males and females, 18–80 years of age with Kellgren–Lawrence (K-L) grade 2, 3, or 4 in one knee, and at least weekly pain for a minimum duration of 3 months after failing conservative therapy. The subjects were included if they were otherwise healthy with no disease conditions that would impact safe participation in the study. Diagnosis was made with clinical and radiographic evaluation. Subjects were excluded if the contralateral knee had a K-L score greater than 2.
A total of 38 subjects were initially enrolled to achieve 29 subjects completing the study. Analgesics, NSAIDS, and supplements were allowed to be given during the study if the subject had been on that treatment for at least 30 days before enrollment and remained on that treatment at the same dose for the duration of the study. Steroids and injectable joint products were not allowed during the study or within 60 days before treatment.
Adipose tissue harvest and SVF isolation
The IBP was an autologous, adipose-derived cell product extracted from adipose tissue by lipoaspiration (PSC-01, SVF). Subjects were screened, enrolled, and an adipose harvest conducted in the investigators clinic to acquire the tissue for extraction of the SVF cells. Following tumescent anesthesia with standard Klein's solution, a minimum of 100 mL lipoaspirate was targeted for collection by the investigator, decanted for 15 min, and then shipped to the central FDA-compliant, manufacturing cleanroom facility. The sample was shipped overnight on priority using Federal Express in a validated temperature-controlled shipping container to the Sponsor's facility. Before processing, a sample was taken for sterility assessment of the incoming sample to properly assess the source of any contamination. All lipoaspirate samples were processed within 24 h of collection.
In a cleanroom environment with biosafety cabinets, the lipoaspirate was enzymatically digested (collagenase, Nordmark Pharma GmbH), washed, centrifuged, and separated into a stromal vascular cell mixture and cryopreserved in one or more dose vials at the cGMP facility. Quality control (QC) samples were taken and stored in the same manner as the IBP doses for lot release assessment. All samples were stored in liquid nitrogen at <130°C, generally for 1–4 weeks before scheduled patient treatment. Storage stability testing has demonstrated stability for a minimum of 2 years. Our veterinary SVF research program has demonstrated frozen stability of SVF for >15 years.
SVF assessment and lot release
After cryopreservation, the QC samples were removed from the storage freezers, thawed, and evaluated for cell count, viability, sterility, endotoxin, and by flow cytometry. In addition, the initial incoming sample was assessed for sterility to assess the collection quality and the shipping impacts.
The dose was determined by the total available SVF cells, with a minimum dose of 2 × 106 nucleated cells and a maximum of 10 × 106 nucleated cells. A cell counter (Nucleocounter by ChemoMetec, Denmark) using a validated propidium iodide cell counting method was used to measure the cell count and viability.
SVF dose delivery
Once passing QC lot release criteria, the participant was scheduled for knee injection. The cells were shipped in a liquid nitrogen (<−130°C) dry shipper and stored on-site at the investigator's clinic until use with a safe storage period of 7 days after shipment. After thawing at room temperature, PSC-01 was injected under ultrasound guidance into the lateral suprapatellar recess of the selected joint with a 22 gauge or greater needle. A photograph of the procedure and ultrasound image were reviewed by the Sponsor medical director to confirm proper needle placement. Each participant received only a single dose of PSC-01 cells. No repeat dosing was allowed during the study. Subjects were permitted to bear weight as tolerated on the treated knee immediately following the procedure, and adjunct treatments were not used (e.g., physical therapy).
Primary outcome safety
Collected safety assessment data included patient questionnaire, medical history, physical examination, vital signs, self-reported assessment, postlipoaspiration and postinjection observations, adverse events (AEs), and laboratory tests (complete blood count, blood chemistry panel, and urinalysis). An analysis of AEs and trends was conducted to determine the safety profile of the adipose harvest procedure and the IBP therapy. Particular attention was given to the days following adipose harvest and IBP injection for acute AEs. All treated subjects were followed poststudy for a 6-month and 12-month safety assessment. AEs were reported in terms of severity, resolution, and causality and were coded according to MedDRA (Medical Dictionary for Regulatory Activities) dictionary as to preferred term and SOC (MedDRA organ system classification).
Efficacy evaluation
Treatment efficacy was measured with the Knee Injury and Osteoarthritis Outcome Score (KOOS). The KOOS is a knee-specific instrument, validated, clinically relevant, and reliable self-administered instrument that can be used for follow-up of several types of knee injury, including osteoarthritis [51]. The KOOS consists of 42 items in 5 separately scored subscales: Pain, Symptoms, Function in Daily Living (ADL), Function in Sport and Recreation (Sport), and knee-related Quality of Life (QOL). A Likert scale is used to answer 42 items with five possible options scored from 0 (No Problems) to 4 (Extreme Problems). Each subscale is calculated as the sum of the items. Scores are transformed to a 0–100 scale, with zero representing extreme knee problems and 100 representing no knee problems.
KOOS scores are often compared to a clinically relevant improvement. Roos and Lohmander [51] provided the logic for the use of this scoring paradigm to access changes following treatment over time in patients with KOA. These authors also recommended a minimal important clinical change (MIC) of 8–10 in the absence of a more refined MIC for a particular study or intervention. In our analysis, we used an MIC of 8. In another method to evaluate patients 2 years following TKA, Lyman proposed a minimal clinically important difference (MCID) for each of the KOOS subscales as 9, 8, 9, 8, and 6 for Pain, Symptoms, ADL, Sport, and QOL, respectively [52]. The KOOS was assessed at screening, day of treatment, interim follow-up visit, final visit (Day 84), and the 12-month time points.
To measure the subject's satisfaction at 12-month assessment, subjects were asked to respond to the question, “How satisfied are you with the results of the investigational stem cell treatment for your knee?” with a five-point Likert scale (very satisfied, satisfied, neutral, dissatisfied, or very dissatisfied).
Results
Demographics
A total of 37 subjects satisfied the screening criteria and underwent lipoaspiration. IBP release criteria were not met for eight subjects who were then withdrawn from the study. A total of 29 subjects received a single IBP injection with an average age of 65.6 years, average body mass index (BMI) of 27.5 kg/m2, and average K-L severity score of 2.9 with 21% grade 2, 69% grade 3, and 10% grade 4 (Table 1). Thirty-one percent (N = 9) of the subjects were male, 69% (N = 20) were female. All 29 participants injected with the IBP completed the study period with no withdrawals and completed all follow-up visits through 12 months.
Table 1.
Baseline Data of Included Subjects
| Average age (years) | Average BMI (kg/m2) | K-L severity score |
Total participants treated | |||
|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Average grade | |||
| 65.6 | 27.5 | 21% | 69% | 10% | 2.9 | 29 |
SVF characterization
The average delivered dose in this study was 4.0 ± 1.8 × 106 nucleated cells with an average viability post-thaw of 72.7% ± 7.1%. The average percentage regenerative cell composition (ASC and pericyte) was 24.3% of the total viable cells, and all delivered cells were no growth on sterility testing.
One patient sample had an incoming positive sterility result and that product was quarantined, and the patient removed from the study and not treated. This is a high value component of the central laboratory model when you know dose, sterility, and purity before patient treatment occurs.
Safety outcomes
During the study period, no clinically significant changes in laboratory values, vital signs, BMI, or physical examination were identified following lipoaspiration or IBP injection.
Following lipoaspiration, AEs were assessed in all 37 subjects. A total of 13 grade 1 and 11 grade 2 AEs were reported. Of these, 17 were deemed related to the lipoaspiration procedure and included mild to moderate pain, bruising, subcutaneous hematoma, or numbness. All the reported AEs resolved before the end of the study without the need for ongoing treatment, and there were no serious AEs.
Following treatment with IBP injection, AEs were assessed in all 29 subjects. A total of 16 grade 1 and 15 grade 2 AEs were reported (Table 2). Of these, 6 were deemed related to the IBP injection and included mild to moderate pain or itching. All the reported AEs resolved before the end of the study without the need for ongoing treatment and there were no serious AEs.
Table 2.
Adverse Events Reported During the Study by Body System Code (SOC) and Grade
| Body system (SOC) | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| R/RP | NR | R/RP | NR | R/RP | NR | R/RP | NR | R/RP | NR | |
| Musculoskeletal and connective tissue disorders | 1 | 5 | 1 | 9 | 2 | 14 | ||||
| General disorders and administration site conditions | 3 | 3 | 0 | |||||||
| Infections and infestations | 1 | 1 | 0 | 2 | ||||||
| Injury poisoning and procedural complications | 1 | 1 | 0 | 2 | ||||||
| Investigations | 1 | 1 | 0 | 2 | ||||||
| Nervous system disorders | 1 | 1 | 1 | 1 | ||||||
| Metabolism and nutrition | 1 | 0 | 1 | |||||||
| Endocrine disorders | 1 | 0 | 1 | |||||||
| Renal and urinary disorders | 1 | 0 | 1 | |||||||
| Surgical and medical procedures | 1 | 0 | 1 | |||||||
| Totals | 5 | 11 | 1 | 14 | 0 | 0 | 0 | 0 | 6 | 25 |
R/RP, related/probably related; NR, not related.
Poststudy safety outcomes
During the time period following the end of study (Day 84) to the 12-month follow-up, there were a total of nine grade 1 and eight grade 2 AEs, none of which was deemed related to the lipoaspiration or IBP treatment (Table 3). There were four grade 3 AEs reported, three of which were orthopedic issues not related to the treatment and one subject underwent a TKA due to continued osteoarthritis pain in the treated knee. All treatment-related AEs resolved before the 12-month follow-up, and there were no treatment-related serious AEs.
Table 3.
Adverse Events Reported from Day 85 Through Month 12 by Body System Code (SOC) and Grade
| Body system (SOC) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| Cardiac disorders | 1 | 1 | |||
| Gastrointestinal disorders | 1 | 1 | |||
| Musculoskeletal and connective tissue disorders | 6 | 3 | 4 | 13 | |
| Neoplasms, benign, malignant, and unspecified | 1 | 1 | |||
| Nervous system disorders | 2 | 2 | |||
| Renal and urinary disorders | 1 | 1 | |||
| Respiratory, thoracic, and mediastinal disorders | 1 | 1 | |||
| Skin and subcutaneous tissue disorders | 1 | 1 | |||
| 9 | 8 | 4 | 0 | 21 |
Efficacy outcomes
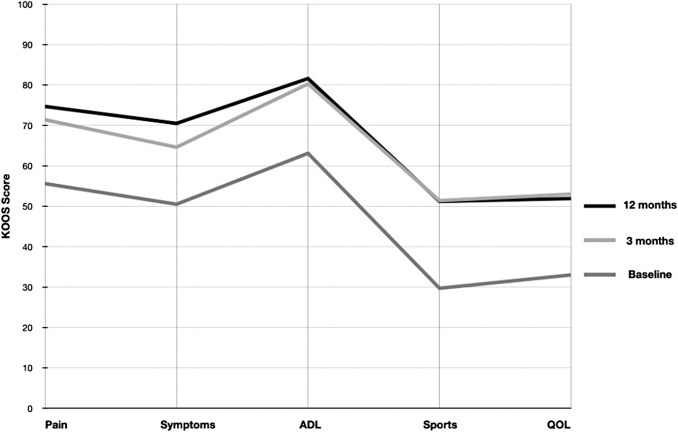
All 29 subjects completed the middle and end of study (Day 84) KOOS assessments. The baseline KOOS subscales were Pain (55.6), Symptoms (50.5), Daily Function (63.1), Sports (29.7), QOL (33.0), and average KOOS (52.5). All KOOS subscales improved at both the middle and end of study assessments with Pain (13.5 and 15.8), Symptoms (12.5 and 14.1), Daily Function (16.1 and 17.2), Sports (19.2 and 21.7), QOL (16.3 and 20), and average KOOS (15.4 and 17.8) noted in Table 4. At the end of the study, the mean of every KOOS subscale exceeded the recommended clinical MIC and MCID (Fig. 1).
Table 4.
Changes in KOOS Subscale Scores
| Category | N | Pain | Symptoms | Daily function | Sports | QOL | Average |
|---|---|---|---|---|---|---|---|
| Baseline | 29 | 55.6 | 50.5 | 63.1 | 29.7 | 33.0 | 52.5 |
| Scores at Day 84 | 29 | 71.4 | 64.6 | 80.3 | 51.4 | 53.0 | 69.8 |
| Score changes from baseline to Day 84 | 15.8 | 14.1 | 17.2 | 21.7 | 20.0 | 17.3 | |
| % Improvement from baseline to Day 84 | 28.4% | 28.0% | 27.3% | 73.3% | 60.8% | 32.9% | |
| 12-month follow-up | 26 | 74.7 | 70.5 | 81.6 | 51.2 | 51.9 | 70.6 |
| Score changes from baseline to 12 months | 19.1 | 20.0 | 18.5 | 21.5 | 18.9 | 18.1 | |
| % Improvement from baseline to 12 months | 34.4% | 39.6% | 29.3% | 72.7% | 57.5% | 34.4% | |
| % Improvement from day 84 to 12 months | 4.7% | 9.0% | 1.5% | −0.3% | −2.1% | 1.1% |
QOL, quality of life.
Fig. 1.
Improvement in Knee Injury and Osteoarthritis Outcome Score (KOOS) subscales (Pain, Symptoms, ADL, Sports and QOL) in response to treatment. Improvements were seen in all five KOOS subscales at 3 month followup (light gray) and maintained at 12 month followup (dark gray). Medium gray = baseline scores.
Poststudy efficacy outcomes
Three participants did not provide a KOOS evaluation as a result of having undergone knee joint replacement surgery, leaving a total of 26 participants for the 12-month poststudy efficacy analysis.
At 12 months, the mean of every KOOS subscale continued to exceed the suggested MIC. Compared to baseline, 79.3% of the subjects exceeded the average KOOS MIC. The proportion of subjects that exceeded the MIC for Pain, Symptoms, Daily Function, Sports, and QOL was 76%, 66%, 69%, 79%, and 76%, respectively.
At 12 months, the mean of every KOOS subscale continued to exceed the suggested MCID. Compared with the Day 84 assessment, the KOOS Pain, Symptoms, and ADL subscales all continued to improve at 12 months. The KOOS Sport and QOL subscales decreased slightly. The proportion of subjects that exceeded the MCID for Pain, Symptoms, Daily Function, Sports, and QOL was 69%, 66%, 69%, 79%, and 83%.
Furthermore, we evaluated the distribution of the KOOS outcomes by baseline radiographic severity (K-L grade) and baseline BMI. The proportion of subjects with an average KOOS exceeding the MIC for K-L grade 2, 3, and 4 was 83%, 85%, and 33%, respectively. The proportion of subjects with average KOOS exceeding the MIC for BMI rated as normal, overweight, and obese was 57%, 88%, and 83%, respectively.
There was no correlation between the proportion of subjects with an average KOOS exceeding the MIC and total nucleated cell count or with the percentage of adipose stem cells in the IBP.
At the 12-month mark, the majority of subjects (75.8%) were “very satisfied” or “satisfied” with their treatment. A total of 17.2% of participants were not satisfied with the treatment.
Discussion
In this study, we explored the safety and efficacy of an autologous, adipose-derived SVF GMP-manufactured product in subjects with moderately severe KOA. Our findings are similar to other published clinical trials that reported on the safety of intra-articular SVF injection. Our subjects reported good procedure tolerability and only mild to moderate AEs. At 12-month follow-up, there were no serious treatment-related AEs, and most subjects were satisfied with their results. Functional and symptomatic improvements exceeded the suggested MCID at 3 months and persisted up to 12 months. Although our study did not include a control group, the magnitude of clinical improvement seen in this study is consistent with previously published clinical trials as shown in meta-analysis by Anil et al. [53].
To the best of our knowledge, this is the first US FDA-approved clinical trial of an autologous SVF product manufactured in an FDA-compliant, cGMP facility. The use of an autologous cell product enhances safety as there is no need to identify an HLA-matched donor and no risk of contamination from transmissible diseases or clonogenic tumor cells. Compliance with FDA cGMP guidelines assures the consistent production of an autologous cellular product with known identity, purity, and potency. Sterility testing and packaging further enhances product safety by minimizing the risk of propagation of pathogenic agents that might otherwise occur with point-of-care devices or other non-GMP manufacturing methods.
Furthermore, the question of degradation of viability of cells due to shipping was answered in that the viability of the central laboratory model SVF was equivalent to the literature reported viability for the same-day point-of-care methods while providing additional quality and safety testing [54,55].
Pain, stiffness, instability, and weakness are all seen in KOA patients because of degeneration and inflammation in both intra-articular and extra-articular joint tissues. Knee pain has been shown to correlate with the levels of inflammatory mediators such as IL-1β, IL-6, and TNF-α in the early stages of KOA [56]. Impaired regulation of angiogenesis in the synovium and osteochondral junction contributes to chronic inflammation, neo-innervation, and pain [57]. The involvement of multiple joint tissues complicates the treatment of this serious medical disease.
The infiltration of SVF into a pro-inflammatory environment can activate ASCs to modulate immune cells mainly through the production of IL-1Ra, IDO, IL-4, IL-10, prostaglandin 2, and TGF-β [45]. Polarization of macrophages to anti-inflammatory type M2 would express IL-4, IL-10, and IGF-1 and inhibit production of TNF-α. This has the effect of reducing metalloproteinase levels stopping the pro-degenerative effects and restoring tissue homeostasis.
The present study has some limitations. First, we did not include a placebo group as our intention was to measure safety. Furthermore, we included a small number of subjects. Future investigations on SVF efficacy will require larger samples to measure effect size. Long-term follow-up would be warranted to fully understand the durability of this treatment.
Conclusions
KOA is a serious medical condition for which there are no treatments offering long-term symptomatic relief. We were able to demonstrate good tolerability, safety profile, and preliminary efficacy at 12 months with a single injection of autologous, adipose derived, cGMP manufactured SVF in subjects with moderately severe KOA. The results of this study are consistent with previously reported safety and efficacy in a number of published clinical trials. These authors believe that the unique approach of providing a central cGMP laboratory derived autologous cell therapy, with cells stored for future possible treatments, is worthy of further development. This scientific evidence provides strong support for the development of a placebo-controlled, randomized clinical trial with long-term follow-up.
Acknowledgments
The authors are grateful to the Personalized Stem Cells, Inc., staff for their efforts in the successful management of the clinical study. The authors are particularly grateful for the efforts of Sue Harman, Manely Yafeh, and Tania Rodriquez for management of the investigators, study sites, and clinical data.
Personalized Stem Cells, Inc., provided financial and administrative support for the conduct of this study and arranged for the tested SVF product. This study was conducted as part of an FDA Investigational New Drug Application.
Ethics Approval and Consent to Participate
The institutional review board (International Cellular and Regenerative Medicine Institute) reviewed and approved the study and provided safety oversight during conduct of the study.
Author Disclosure Statement
R.H. and C.R. are employees of Personalized Stem Cells, Inc., R.H., C.R., G.M., and P.H. are shareholders of Personalized Stem Cells, Inc. The other authors have no conflicts of interest to declare.
Funding Information
Personalized Stem Cells, Inc., funded the study and the writing, editing, and publishing of this article.
References
- 1. Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, et al. (2000). Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 133:635–646. [DOI] [PubMed] [Google Scholar]
- 2. Vos T and Collaborators GDaIIaP. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hootman JM, Helmick CG, Barbour KE, Theis KA and Boring MA. (2016). Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheum 68:1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loeser RF, Goldring SR, Scanzello CR and Goldring MB. (2012). Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta PK, Das AK, Chullikana A and Majumdar AS. (2012). Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wojdasiewicz P, Poniatowski LA and Szukiewicz D. (2014). The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm 2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blasioli DJ and Kaplan DL. (2014). The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev 20:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Testa G, Giardina SMC, Culmone A, Vescio A, Turchetta M, Cannavo S and Pavone V. (2021). Intra-articular injections in knee osteoarthritis: A review of literature. J Funct Morphol Kinesiol 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. London NJ, Miller LE and Block JE. (2011). Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses 76:887–892. [DOI] [PubMed] [Google Scholar]
- 10. Vallee M, Cote JF and Fradette J. (2009). Adipose-tissue engineering: taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol Biol (Paris) 57:309–317. [DOI] [PubMed] [Google Scholar]
- 11. Bunnell BA. (2021). Adipose tissue-derived mesenchymal stem cells. Cells 10:3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK and Hedrick MH. (2005). Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54:132–141. [DOI] [PubMed] [Google Scholar]
- 13. Caplan AI. (2017). Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caplan AI. (2019). There is no “Stem Cell Mess”. Tissue Eng Part B Rev 25:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AI. C. (2019). Medicinal signalling cells: they work, so use them. Nature 566:39. [DOI] [PubMed] [Google Scholar]
- 16. Bora P and Majumdar AS. (2017). Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. [DOI] [PubMed] [Google Scholar]
- 18. Martin DR, Cox NR, Hathcock TL, Niemeyer GP and Baker HJ. (2002). Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol 30:879–886. [DOI] [PubMed] [Google Scholar]
- 19. Kim GB and Shon OJ. (2020). Current perspectives in stem cell therapies for osteoarthritis of the knee. Yeungnam Univ J Med 37:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dufrane D. (2017). Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant 26:1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cifu A, Domenis R, Pozzi-Mucelli M, Di Benedetto P, Causero A, Moretti M, Stevanato M, Pistis C, Parodi PC, Fabris M and Curcio F. (2020). The exposure to osteoarthritic synovial fluid enhances the immunomodulatory profile of adipose mesenchymal stem cell secretome. Stem Cells Int 2020:4058760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Domenis R, Cifu A, Quaglia S, Pistis C, Moretti M, Vicario A, Parodi PC, Fabris M, Niazi KR, Soon-Shiong P and Curcio F. (2018). Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep 8:13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ragni E, Colombini A, Vigano M, Libonati F, Perucca Orfei C, Zagra L and de Girolamo L. (2021). Cartilage protective and immunomodulatory features of osteoarthritis synovial fluid-treated adipose-derived mesenchymal stem cells secreted factors and extracellular vesicles-embedded miRNAs. Cells 10:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pers YM, Quentin J, Feirreira R, Espinoza F, Abdellaoui N, Erkilic N, Cren M, Dufourcq-Lopez E, Pullig O, et al. (2018). Injection of adipose-derived stromal cells in the knee of patients with severe osteoarthritis has a systemic effect and promotes an anti-inflammatory phenotype of circulating immune cells. Theranostics 8:5519–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Im GI. (2016). Endogenous cartilage repair by recruitment of stem cells. Tissue Eng Part B Rev 22:160–171. [DOI] [PubMed] [Google Scholar]
- 26. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K and Gimble JM. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molnar V, Pavelic E, Vrdoljak K, Cemerin M, Klaric E, Matisic V, Bjelica R, Brlek P, Kovacic I, Tremolada C and Primorac D. (2022). Mesenchymal stem cell mechanisms of action and clinical effects in osteoarthritis: A narrative review. Genes (Basel) 13:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lana J, Lana A, da Fonseca LF, Coelho MA, Marques GG, Mosaner T, Ribeiro LL, Azzini GOM, Santos GS, Fonseca and Ede Andrade MAP. (2022). Stromal vascular fraction for knee osteoarthritis - an update. J Stem Cells Regen Med 18:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P and Zuk P. (2013). Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp 79:e50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228. [DOI] [PubMed] [Google Scholar]
- 31. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick MH. (2002). Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black L, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA and Harman R. (2007). Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Vet Ther 8:272–284. [PubMed] [Google Scholar]
- 33. Black L, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA and Harman R. (2008). Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dog. Vet Ther 9:192–200. [PubMed] [Google Scholar]
- 34. Pak J. (2011). Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep 5:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pak J, Lee JH and Lee SH. (2013). A novel biological approach to treat chondromalacia patellae. PLoS One 8:e64569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bui KH-T, Duong TD, Nguyen NT, Nguyen TD, Le VT, Mai VT, Phan NL-C, Le DM and Ngoc NK. (2014). Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed Res Ther 1:2–8. [Google Scholar]
- 37. Garza J, Maria D, Palomera T, Dumanian G and Dos-Anjos S. (2015). Use of autologous adipose- derived stromal vascular fraction to treat osteoarthritis of the knee: A feasibility and safety study. J Regen Med 03:1–6. [Google Scholar]
- 38. Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D and Tucker BS. (2020). Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. Am J Sports Med 48:588–598. [DOI] [PubMed] [Google Scholar]
- 39. Pak J, Lee JH, Park KS, Jeong BC and Lee SH. (2016). Regeneration of cartilage in human knee osteoarthritis with autologous adipose tissue-derived stem cells and autologous extracellular matrix. Biores Open Access 5:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fodor PB and Paulseth SG. (2016). Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J 36:229–236. [DOI] [PubMed] [Google Scholar]
- 41. Yokota N, Yamakawa M, Shirata T, Kimura T and Kaneshima H. (2017). Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther 6:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yokota N, Hattori M, Ohtsuru T, Otsuji M, Lyman S, Shimomura K and Nakamura N. (2019). Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am J Sports Med 47:2577–2583. [DOI] [PubMed] [Google Scholar]
- 43. Michalek J, Moster R, Lukac L, Proefrock K, Petrasovic M, Rybar J, Chaloupka A, Darinskas A, Michalek J, et al. (2017). Stromal vascular fraction cells of adipose and connective tissue in people with osteoarthritis: A case control prospective multi-centric non-randomized study. Global Surg 3:1–9. [Google Scholar]
- 44. Michalek J, Vrablikova A, Darinskas A, Lukac L, Prucha J, Skopalik J, Travnik J, Cibulka M and Dudasova Z. (2019). Stromal vascular fraction cell therapy for osteoarthritis in elderly: Multicenter case-control study. J Clin Orthop Trauma 10:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lapuente JP, Dos-Anjos S and Blazquez-Martinez A. (2020). Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res 15:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H and Kuroda R. (2020). The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord 21:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H and Kuroda R. (2021). Comparison of clinical and imaging outcomes of different doses of adipose-derived stromal vascular fraction cell treatment for knee osteoarthritis. Cell Transplant 30:9636897211067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yokota N, Lyman S, Hanai H, Shimomura K, Ando W and Nakamura N. (2022). Clinical safety and effectiveness of adipose-derived stromal cell vs stromal vascular fraction injection for treatment of knee osteoarthritis: 2-year results of parallel single-arm trials. Am J Sports Med 50:2659–2668. [DOI] [PubMed] [Google Scholar]
- 49. Zhang S, Xu H, He B, Fan M, Xiao M, Zhang J, Chen D, Tong P and Mao Q. (2022). Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: a minimum 5-year follow-up study. Stem Cell Res Ther 13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hong Z, Chen J, Zhang S, Zhao C, Bi M, Chen X and Bi Q. (2019). Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 43:1123–1134. [DOI] [PubMed] [Google Scholar]
- 51. Roos EM and Lohmander LS. (2003). The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lyman S, Lee YY, McLawhorn AS, Islam W and MacLean CH. (2018). What Are the Minimal and Substantial Improvements in the HOOS and KOOS and JR Versions After Total Joint Replacement? Clin Orthop Relat Res 476:2432–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anil U, Markus DH, Hurley ET, Manjunath AK, Alaia MJ, Campbell KA, Jazrawi LM and Strauss EJ. (2021). The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee 32:173–182. [DOI] [PubMed] [Google Scholar]
- 54. Liu P, Gurung B, Afzal I, Santin M, Sochart DH, Field RE, Kader DF and Asopa V. (2022). The composition of cell-based therapies obtained from point-of-care devices/systems which mechanically dissociate lipoaspirate: a scoping review of the literature. J Exp Orthop 9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodriguez J, Pratta AS, Abbassi N, Fabre H, Rodriguez F, Debard C, Adobati J, Boucher F, Mallein-Gerin F, et al. (2017). Evaluation of three devices for the isolation of the stromal vascular fraction from adipose tissue and for ASC culture: a comparative study. Stem Cells Int 2017:9289213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li L, Li Z, Li Y, Hu X, Zhang Y and Fan P. (2020). Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskelet Disord 21:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bonnet CS and Walsh DA. (2005). Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 44:7–16. [DOI] [PubMed] [Google Scholar]