Abstract
Historically hematopoietic stem cells are believed to be predominantly dormant but could be induced into active cell cycle under specific conditions. This review, coupled with years of research from our laboratory, challenges this belief by demonstrating a significant portion of hematopoietic stem cells are actively cycling rather than quiescent. This addresses a major heuristic error in the understanding of hematopoietic stem cells that has shaped this field for decades. By evaluating the cycle status of engraftable hematopoietic stem cells in whole unseparated bone marrow, we demonstrated that a significant portion of these cells are actively cycling, and further confirmed by tritiated thymidine suicide and bromodeoxyuridine labeling assays. Moreover, by analyzing both whole unseparated bone marrow and purified lineage-negative hematopoietic stem cells in murine models, our findings indicate that lineage-positive cells, usually discarded during purification, actually contain actively cycling stem cells. Taken together, our findings highlight that hematopoietic stem cells are characterized as actively cycling and expressing differentiation epitopes. This corrects a basic mistake in stem cell biology. Furthermore, these findings provide valuable insights for a better understanding of the actively cycling hematopoietic stem cells in the field of stem cell biology.
Keywords: hematopoietic stem cells, cell cycle, quiescent cell, stem cell differentiation
Our focus on quiescence comes from work on hematopoietic stem cells. A very large number of articles highlight the essential quiescence of hematopoietic stem cells. Since 1984, there have been over 1000 (Pubmed) references with the indicators quiescence and hematopoietic stem cells. In this context, quiescence refers to the state of the cell cycle of these cells. The basic definition of quiescence is a state of quietness or inactivity. This is reversible. It is assumed by most that hematopoietic stem cells are dormant or noncycling but can be induced into active cell cycle by a number of entities. This underlies the basic definition of hematopoietic stem cells (Fig. 1). We feel that this represents a major heuristic mistake.
FIG. 1.
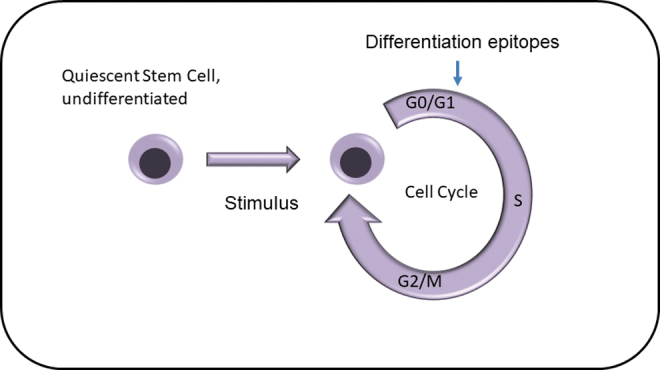
Old model of hematopoietic stem cell with the quiescent cell being induced into a cycling stem cell.
Well, now we need to define heuristics. In 1974, Tversky and Kahneman published “Judgment under Uncertainty: Heuristics and Biases: Biases in judgments reveal some heuristics of thinking under uncertainty” [1]. They outlined three major heuristics; in this instance, two are applicable to the quiescence issue. As per their abstract “representativeness, which is usually employed when people are asked to judge the probability that an object or event A belongs to class or process B; (ii) availability of instances or scenarios, which is often employed when people are asked to assess the frequency of a class or the plausibility of a particular development.”
These are practical approaches to problems that give ready answers but that are subject to significant error. The definition of hematopoietic stem cells as quiescent involves both representative and availability heuristics.
This is important because a large volume of scientific literatures have been based on this heuristic mistake. This has also taught us one major lesson; scientific investigators do not appreciate being informed that they are in error. As Max Plank put it many years ago, “A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it,” or a paraphrased variant is, “Science advances one funeral at a time.”
Albert Einstein famously stated, “Blind belief in authority is the greatest enemy of truth.” This statement highlights the critical need for the scientific community to encourage a spirit of questioning and open-mindedness, especially when faced with established dogmas.
Okay, what is the basis for our contention here? Bear with me here, for the complexity, but in the end, it is fairly simple. First, what is the dogma (for most) about the marrow-based hematopoietic stem cell? In general, most feel that the hematopoietic stem cell is lineage negative, that is, devoid of distinguishing differentiation lineage markers, which usually include GR-1 (granulocytes), Ter119 (erythroid cells), B220 (mostly B lymphocytes), Lyt-2 and 3,4 (T cells), and Mac-1 (monocytes/macrophages). This, as we shall see, is a major error.
The stem cell is also characterized by the presence of stem cell specific markers such as Sca-1, c-kit, and CD150, this may be correct. It is felt that the stem cell is quiescent or dormant, but easily induced into an active cell cycle and a fount of differentiation potential [2–4]. Our studies have supported this phenotype for purified lineage negative stem cells assayed in lethally irradiated murine hosts, but critically not when whole unseparated marrow is assayed in lethally irradiated host mice [5,6]. Here lies the critical error that has pervaded the field. The differentiation marked cells that are removed in the stem cell “purification” are in fact replete with hematopoietic stem cells and these cells are actively cycling (Fig. 2).
FIG. 2.
The depletion of lineage-positive cells also removes stem cells. The purified stem cells are missing the depleted stem cells. FACS, fluorescence-activated cell sorting; SLAM, signaling lymphocyte activation molecule.
This indicates that hematopoietic stem cells have an actively cycling component and thus are not defined by quiescence. Since cycling stem cells will be continually changing their phenotype, this also explains the manifest heterogeneity of even the most purified stem cells [7–14]. But back to the data indicating that hematopoietic stem cells are not quiescent. Experiments carried out over 20 plus years studying marrow cells whole or purified by various approaches and stimulated with different cytokine cocktails indicated that their phenotype changed with cycle passage as to engraftment and homing into lethally irradiated mice, progenitor-to-stem cell ratios, differentiation into megakaryocytes and granulocytes, and various aspects of gene expression [15–21].
Study by Passegue et al. [22], however, indicated that only G0-purified stem cells showed long-term engraftment into lethally irradiated mice, the gold standard defining pluripotent hematopoietic stem cells. This raised questions as to the meaning of our own data. Could we be dealing with in vitro tissue culture artifacts? We proceeded to carry out experiments along the lines of Passegue et al. [22] and, in fact, confirmed their data. However, we noted that all the previous data on stem cell cycle status had been carried out on purified stem cells.
No one had bothered to evaluate the cycle status of engraftable hematopoietic stem cells in whole unseparated murine bone marrow (a heuristic mistake). We proceeded to separate whole marrow cells into G0, G1, and S/G2/M cohorts and engrafted these into lethally irradiated mice [5]. The results were quite startling at or over 50% of engraftable stem cells were in the S/G2/M cohort. This suggested that these stem cells were cycling, not quiescent. In further experiments, we employed a tritiated thymidine suicide approach in which cycling cells exposed to high specific radioactive tritiated thymidine would be selectively killed.
Thus, the decrease in in vivo engraftment in the tritiated thymidine-exposed cells would indicate the number of cycling cells in the population, since cells going through the S-phase would incorporate the tritiated thymidine and be killed. Innocent bystander effects were ruled out by specific cell mixture experiments. In addition, the path length of beta emitter rules against innocent bystander effects. The results of these experiments also indicated that the stem cells were cycling. In lethally irradiated mice infused with whole marrow, the marrow cells exposed to tritiated thymidine showed a 65%–80% reduction in engraftment up to 12 months after cell infusion (25–28 mice per time point, 4 experiments, P < 0.001) [5].
In a third approach, purified lineage negative, c-kit, and Sca-1+, and Flk2 negative stem cells were determined in mice given bromodeoxyuridine (BrdU) both orally and intraperitoneally to label cells going through the cell cycle. At 12, 24, and 48 h from BrdU exposure, 39%, 65%, and 72% of the long-term hematopoietic stem cell were labeled, and 31%, 58%, and 67% of these were in the G0/G1 phase of the cell cycle. These data indicated cell cycle passage over 48 h. These three approaches all indicate that hematopoietic stem cells are an actively cycling population and not quiescent (Fig. 3).
FIG. 3.
Old versus new stem cell models. In the new model, stem cells are cycling and expressing differentiation epitopes.
More recent studies have additionally confirmed the true nature of marrow hematopoietic stem cells. As to the presence of differentiation epitopes on stem cells, previous study suggested that CD4 [23] and Mac 1 [24] were present on purified stem cells. We have extended these data showing that stem cells were present in the lineage-positive separated cells from a stem cell purification [6].
Employing a limiting dilution competitive repopulation analysis, we have estimated that the frequency of stem cells in whole bone marrow was 1 × 9.6 × 104, whereas that in the lineage-positive population was 1 in 1.55 × 105 and furthermore when marrow cells were isolated into different differentiated fractions positive for either B220, Gr-1, Ter119, Mac-1 or Lyt-2 and 3,4, we found significant number of stem cells in each fraction [6].
When stem cells are purified by current classic approaches, the lineage-positive cells with their cycling stem cell component are discarded. Thus, the purified stem cells on which most cell studies are performed are not representative of the total marrow stem cell population. This represents the major heuristic stem cell mistake.
We have focused here on the issue of quiescence of stem cells. Other studies suggest that the differentiation potential of stem cells may also not have been appropriately addressed and that issue is addressed in a separate study on universal stem cells [25].
Finally, we acknowledge that a fraction of stem cells at any point in time will show a quiescent phenotype. However, this phenotype will be constantly changing and does not represent the total stem cell population. This should be kept in mind when interpreting hematopoietic stem cell studies.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated in this article.
Author Disclosure Statement
The authors declare no competing interests.
Funding Information
This study was supported by NIH/NIGMS, 1P30GM145500, 1 P20 GM119943, and NIDDK, R01DK112808
References
- 1. Tversky A and Kahneman D. (1974). Judgment under uncertainty: heuristics and biases. Science 185:1124–1131. [DOI] [PubMed] [Google Scholar]
- 2. Morrison SJ, Wandycz AM, Hemmati HD, Wright DE and Weissman IL. (1997). Identification of a lineage of multipotent hematopoietic progenitors. Development 124:1929–1939. [DOI] [PubMed] [Google Scholar]
- 3. Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M and Jacobsen SE. (2005). Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121:295–306. [DOI] [PubMed] [Google Scholar]
- 4. Forsberg EC, Serwold T, Kogan S, Weissman IL and Passegue E. (2006). New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell 126:415–426. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg LR, Dooner MS, Johnson KW, Papa EF, Pereira MG, Del Tatto M, Adler DM, Aliotta JM and Quesenberry PJ. (2014). The murine long-term multi-lineage renewal marrow stem cell is a cycling cell. Leukemia 28:813–822. [DOI] [PubMed] [Google Scholar]
- 6. Goldberg LR, Dooner MS, Papa E, Pereira M, Del Tatto M, Cheng Y, Wen S and Quesenberry PJ. (2022). Differentiation epitopes define hematopoietic stem cells and change with cell cycle passage. Stem Cell Rev Rep 18:2351–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abkowitz JL, Golinelli D, Harrison DE and Guttorp P. (2000). In vivo kinetics of murine hemopoietic stem cells. Blood 96:3399–3405. [PubMed] [Google Scholar]
- 8. Muller-Sieburg CE, Cho RH, Thoman M, Adkins B and Sieburg HB. (2002). Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood 100:1302–1309. [PubMed] [Google Scholar]
- 9. Keller G. (1992). Clonal analysis of hematopoietic stem cell development in vivo. Curr Top Microbiol Immunol 177:41–57. [DOI] [PubMed] [Google Scholar]
- 10. Jordan CT and Lemischka IR. (1990). Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev 4:220–232. [DOI] [PubMed] [Google Scholar]
- 11. Guenechea G, Gan OI, Dorrell C and Dick JE. (2001). Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol 2:75–82. [DOI] [PubMed] [Google Scholar]
- 12. Uchida N, Dykstra B, Lyons KJ, Leung FY and Eaves CJ. (2003). Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol 31:1338–1347. [DOI] [PubMed] [Google Scholar]
- 13. Morita Y, Ema H and Nakauchi H. (2010). Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med 207:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colvin GA, Berz D, Liu L, Dooner MS, Dooner G, Pascual S, Chung S, Sui Y and Quesenberry PJ. (2010). Heterogeneity of non-cycling and cycling synchronized murine hematopoietic stem/progenitor cells. J Cell Physiol 222:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Habibian HK, Peters SO, Hsieh CC, Wuu J, Vergilis K, Grimaldi CI, Reilly J, Carlson JE, Frimberger AE, Stewart FM and Quesenberry PJ. (1998). The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med 188:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerny J, Dooner M, McAuliffe C, Habibian H, Stencil K, Berrios V, Reilly J, Carlson J, Cerny AM, d'Hondt L, Benoit B, Lambert JF, Colvin G, Nilsson S, Becker P and Quesenberry P. (2002). Homing of purified murine lymphohematopoietic stem cells: a cytokine-induced defect. J Hematother Stem Cell Res 11:913–922. [DOI] [PubMed] [Google Scholar]
- 17. Colvin GA, Lambert JF, Moore BE, Carlson JE, Dooner MS, Abedi M, Cerny J and Quesenberry PJ. (2004). Intrinsic hematopoietic stem cell/progenitor plasticity: Inversions. J Cell Physiol 199:20–31. [DOI] [PubMed] [Google Scholar]
- 18. Becker PS, Nilsson SK, Li Z, Berrios VM, Dooner MS, Cooper CL, Hsieh CC and Quesenberry PJ. (1999). Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Exp Hematol 27:533–541. [DOI] [PubMed] [Google Scholar]
- 19. Berrios VM, Dooner GJ, Nowakowski G, Frimberger A, Valinski H, Quesenberry PJ and Becker PS. (2001). The molecular basis for the cytokine-induced defect in homing and engraftment of hematopoietic stem cells. Exp Hematol 29:1326–1335. [DOI] [PubMed] [Google Scholar]
- 20. Lambert JF, Liu M, Colvin GA, Dooner M, McAuliffe CI, Becker PS, Forget BG, Weissman SM and Quesenberry PJ. (2003). Marrow stem cells shift gene expression and engraftment phenotype with cell cycle transit. J Exp Med 197:1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colvin GA, Dooner MS, Dooner GJ, Sanchez-Guijo FM, Demers DA, Abedi M, Ramanathan M, Chung S, Pascual S and Quesenberry PJ. (2007). Stem cell continuum: directed differentiation hotspots. Exp Hematol 35:96–107. [DOI] [PubMed] [Google Scholar]
- 22. Passegue E, Wagers AJ, Giuriato S, Anderson WC and Weissman IL. (2005). Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med 202:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wineman JP, Gilmore GL, Gritzmacher C, Torbett BE and Muller-Sieburg CE. (1992). CD4 is expressed on murine pluripotent hematopoietic stem cells. Blood 80:1717–1724. [PubMed] [Google Scholar]
- 24. Ishida A, Zeng H and Ogawa M. (2002). Expression of lineage markers by CD34+ hematopoietic stem cells of adult mice. Exp Hematol 30:361–365. [DOI] [PubMed] [Google Scholar]
- 25. Quesenberry PJ, Wen S, Goldberg LR and Dooner MS. (2022). The universal stem cell. Leukemia 36:2784–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated in this article.




