Abstract
Background:
Subjects with a fragility fracture have an increased risk of a new fracture and should receive effective strategies to prevent new events. The medium-term to long-term strategy should be scheduled by considering the mechanisms of action in therapy and the estimated fracture risk.
Objective:
A systematic review was conducted to evaluate the sequential strategy in patients with or at risk of a fragility fracture in the context of the development of the Italian Guidelines.
Design:
Systematic review and meta-analysis.
Data sources and methods:
PubMed, Embase, and the Cochrane Library were investigated up to February 2021 to update the search of a recent systematic review. Randomized clinical trials (RCTs) that analyzed the sequential therapy of antiresorptive, anabolic treatment, or placebo in patients with or at risk of a fragility fracture were eligible. Three authors independently extracted data and appraised the risk of bias in the included studies. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation methodology. Effect sizes were pooled in a meta-analysis using fixed-effects models. The primary outcome was the risk of refracture, while the secondary outcome was the bone mineral density (BMD) change.
Results:
In all, 17 RCTs, ranging from low to high quality, met our inclusion criteria. A significantly reduced risk of fracture was detected at (i) 12 or 24 months after the switch from romosozumab to denosumab versus placebo to denosumab; (ii) 30 months from teriparatide to bisphosphonates versus placebo to bisphosphonates; and (iii) 12 months from romosozumab to alendronate versus the only alendronate therapy (specifically for vertebral fractures). In general, at 2 years after the switch from anabolic to antiresorptive drugs, a weighted BMD was increased at the lumbar spine, total hip, and femoral neck site.
Conclusion:
The Task Force formulated recommendations on sequential therapy, which is the first treatment with anabolic drugs or ‘bone builders’ in patients with very high or imminent risk of fracture.
Keywords: anabolic, antiresorptive, fragility fracture, sequential therapy, systematic review
Plain language summary
A systematic review to evaluate the sequential therapy of antiresorptive (denosumab and bisphosphonate, such as alendronate, minodronate, risedronate, and etidronate), anabolic treatment (such as romosozumab, teriparatide), or placebo in patients with or at risk of a fragility fracture in the context of the development of the Italian Guidelines
Subjects with previous fragility fractures should promptly receive effective strategies to prevent the risk of subsequent events. Indeed, patients with a fragility fracture have a doubled risk of a new fracture. For this reason, it is essential to provide adequate sequential therapy based on the mechanisms and the rapidity of action. A systematic review was performed to identify the sequential strategy in patients at high- or imminent-risk of (re)fracture and to support the Panel of the Italian Fragility Fracture Guideline in formulating recommendations. Our systematic review included seventeen studies mostly focused on women and enabled us to strongly recommend the anabolic drugs as first-line treatment. Specifically, for the sequential therapy from anabolic to antiresorptive treatment, there was a significant reduction in the risk of different types of fractures after the switch from romosozumab to denosumab versus placebo to denosumab. These findings were confirmed at 24 months after the switch. Considering the sequential treatment from antiresorptive to anabolic medications, there was a decreased risk of fracture 12 months after the switch from placebo to teriparatide versus bisphosphonate or antiresorptive to teriparatide. Moreover, a greater bone mineral density increase after the switch from anabolic to antiresorptive medications was shown in the lumbar spine, total hip, and femoral neck. The results of this systematic review and meta-analysis confirm that initial treatment with anabolic drugs produces substantial bone mineral density improvements, and the transition to antiresorptive drugs can preserve or even amplify the acquired benefit. These findings support the choice to treat very high-risk individuals with anabolic drugs first, followed by antiresorptive drugs.
Introduction
In subjects with a history of fragility fracture(s), effective prevention strategies are warranted to prevent subsequent events.1,2 Indeed, patients with a fragility fracture have a doubled risk of a new fracture. 3 In particular, subjects who have recently had a fracture represent a concerning subset, defined by the term ‘imminent risk’, and are estimated to have a fivefold increased risk for a second fracture within 12–24 months.4,5 In addition, the risk of recurrence dramatically increases with the number of previous fractures, regardless of their location. 6
The resulting burden is significant, including limited walking, chronic pain, loss of independence, and reduced quality of life. 7 Therefore, patients should promptly receive an effective strategy to reduce the risk of new fractures. 8 Unfortunately, data show that many high-risk patients still do not receive any treatment.1,2 It is crucial to enact strategies aimed at both identifying individuals at significant fracture risk and granting them an effective pharmacological treatment. 9
Pharmacological therapies for the prevention and treatment of bone loss and mitigation of fracture risk have developed considerably in the last decades, with a significant increase in treatment options with different and innovative mechanisms of action available to the clinician. Among the antiresorptive drugs, denosumab represents the most recently developed compound, besides bisphosphonates, which are usually the first-line treatment. Denosumab is a monoclonal antibody directed against the receptor activator of NFkB ligand (RANKl) and acts by inhibiting the differentiation, activity, and survival of osteoclasts. 10 Meanwhile, classified as anabolics, teriparatide and abaloparatide are parathyroid hormone (PTH) analogs that act by stimulating osteoclast activity, favoring new bone formation. 11 Finally, romosozumab represents the last developed drug, recently licensed and introduced in clinical practice. Romosozumab is a novel agent that differs from prior anabolic drugs by blocking the action of sclerostin, an inhibitor of the Wnt pathway. 12 Romosozumab has been defined as a ‘bone builder’ as it assures a concurrent stimulation of neoformation and inhibition of bone resorption, leading to an accelerated and amplified anabolic therapeutic window. 13 The choice of therapy should be based on the estimated fracture risk, the mechanisms of action, and its rapidity of action as well as the medium-term to long-term strategy by scheduling combined or sequential approaches.12,14 For this reason, it is essential to provide adequate sequential therapy after suspending these treatments.
A comparative analysis of different treatments and therapeutic strategies, both in terms of fracture protection and the onset of the protective effect, is still an unmet need. This systematic review aims to identify the sequential strategy in patients at high risk or imminent risk of (re)fracture.
Materials and methods
We performed a systematic review to support the Panel of the Italian Fragility Fracture Guideline (published on the platform of the Italian National Institute of Health) 15 in formulating recommendations. In accordance with the GRADE-ADOLOPMENT methodology 16 and the standards elaborated by the Sistema Nazionale Linee Guida,17,18 the clinical question defined by the multidisciplinary panel was as follows: ‘Which therapeutic strategy should be recommended in the short- and long-term treatment of patients at high- or imminent-risk of (re) fracture?’ Specifically, we have updated a recent systematic review, 19 which assessed the sequential treatment in women with postmenopausal osteoporosis.
Inclusion and exclusion criteria
Randomized clinical trial (RCT) studies were detected if they met the following criteria: (1) population: patients who experienced a fragility fracture or were affected by osteoporosis; (2) intervention: antiresorptive (denosumab and bisphosphonate, such as alendronate, minodronate, risedronate, and etidronate), anabolic therapies (romosozumab, teriparatide, and PTH), or placebo; (3) comparison: sequential therapy of drugs abovementioned; (4) outcome: the primary outcome was a risk of the fracture using the dichotomized measure of risk ratio (RR), while the secondary outcome was mean change in bone mineral density (BMD) (at the lumbar spine, total hip, and femoral neck) considering the sequential treatment.
Studies were excluded if they (i) were not published in English; (ii) did not report original findings (i.e. letters, case report); (iii) did not identify patients affected by a fragility fracture or osteoporosis; or (iv) did not consider a sequential drug treatment.
Data source and search strategy
We performed a PubMed, Embase, and Cochrane Library search from 2019 to February 2021 and identified publications on sequential therapy among patients with fragility fracture or osteoporosis. The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses,20,21 as reported in Supplemental Table S1. The search strategy (Supplemental Table S2) specified keywords and corresponding Medical Subject Headings (MeSH) terms related to fragility fracture/osteoporosis AND sequential therapy AND anabolic/antiresorptive drugs. We checked the reference lists of the studies and the systematic reviews identified during the search process.
Study selection and data extraction
Three independent authors (AB, GP, and RR) screened titles and abstracts according to the search strategy and then assessed the full text of the potentially relevant studies. Discrepancies between readers were discussed and resolved at the conference.
For each included RCT, the following information was extracted the name of the first author, year and country of publication, study setting, type of population, intervention and comparator, and follow-up period (Supplemental Table S3).
Studies quality
The updated systematic review was evaluated using the AMSTAR-2 checklist, 22 while the quality of each included publication was assessed using the Cochrane risk of bias (RoB) tool for RCTs. 23 The following domains of the Cochrane RoB tool were appraised: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias (such as funding bias). Each domain was classified as ‘high’, ‘low’, or ‘unclear’ RoB if the publication did not provide sufficient information to be classified (Supplemental Figure S1).
Quality of evidence
The quality of evidence of the primary outcome was judged through five dimensions (RoB, consistency of effect, imprecision, indirectness, and publication bias) by the GRADE approach. 24 The evidence was downgraded from ‘high quality’ by one level if serious or by two levels if very serious limitations were found for each of the five dimensions (Supplemental Table S4).
Statistical analysis
The measure of interest was the summary RR that evaluated the effect of sequential therapy (anabolic to antiresorptive, or vice versa) on the risk of fragility fracture. Estimates were summarized if at least three studies reported the association of interest. Heterogeneity between study-specific estimates was tested using χ2 statistics 25 and measured with the I2 index (a measure of the percentage variation across the studies). 26
Moreover, a pooled estimate of BMD (mean change, %) was obtained for each site (lumbar spine, total hip, and femoral neck). A weighted average of the BMD was obtained by considering the sample size of the ith study and summing them across all studies. 27 p Values less than 0.05 for all tests were considered statistically significant.
Results
Study selection
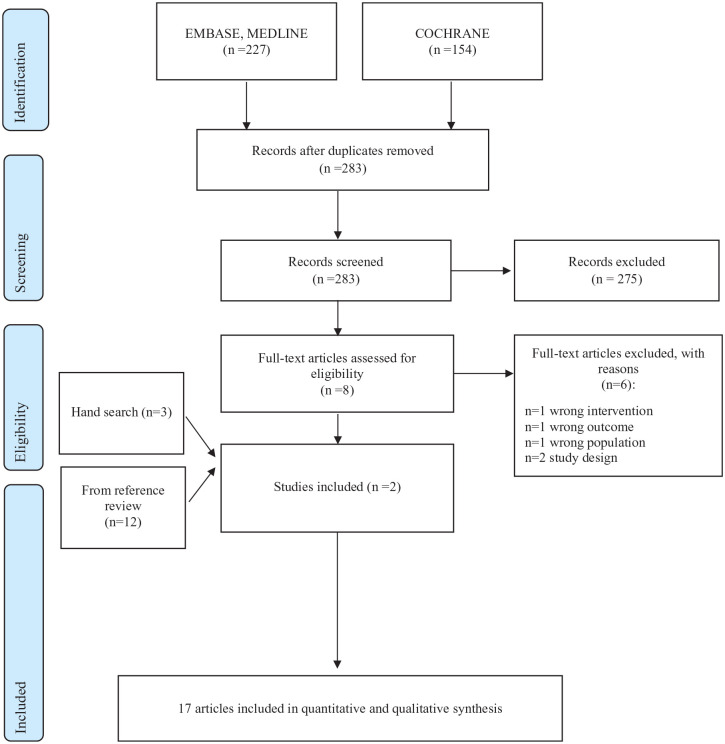
A total of 283 studies were detected, as shown in Figure 1. After screening the title and abstract, eight papers were eligible for inclusion. Subsequently, six studies were discarded reading the full texts because they were duplicates (1) or because the intervention (1), outcome (1), population (1), or study design (2) were incorrect. In all, 14 studies were also included after the review of the references28–41 and 3 by hand-searching.13,42,43 The main characteristics of the included studies are reported in Supplemental Material (Table S3).
Figure 1.
Flow chart of study selection.
Study characteristics
The included papers were conducted in the United States,29,33,43 Italy, 31 UK, 35 Japan, 37 Austria, and Czech Republic, 42 in addition to multicenter studies carried out in various countries.13,28,30,32,34,36,38–41 Nine studies considered the sequential therapy from anabolic to antiresorptive treatment,13,28,29,34,37,39–41,43 specifically (a) romosozumab to denosumab versus placebo to denosumab13,34,40; (b) teriparatide to denosumab or alendronate or minodronate 37 ; (c) romosozumab to alendronate versus only alendronate28,41; (d) teriparatide to bisphosphonate versus placebo to bisphosphonate 39 ; (e) PTH to bisphosphonate versus PTH to placebo 29 ; and (f) romosozumab to denosumab versus romosozumab to placebo. 43 Seven papers evaluated the sequential treatment from antiresorptive to anabolic medications,30–32,35,36,38,42 specifically (g) alendronate to teriparatide versus placebo to teriparatide 42 ; (h) antiresorptive to teriparatide versus no treatment to teriparatide35,38; (i) antiresorptive to teriparatide versus only antiresorptive 31 ; (j) alendronate to romosozumab or teriparatide 32 ; and (k) risedronate or alendronate30,36 or etidronate or non-bisphosphonate 30 to teriparatide.30,36 Finally, one study reported the sequential treatment from anabolic to antiresorptive medications and vice versa. 33
All studies, except one, 37 were focused on women. The proportion of patients with previous fragility fractures was reported by nine studies,28–30,32,33,36,37,41,42 whereas the nature and severity of prior fractures were documented in eight13,28,34–37,40,41 and five13,28,34,40,41 studies, respectively. Moreover, secondary osteoporosis was indicated as an exclusion criterion in eight studies30,31,33,35–38,42 (Supplemental Table S5).
RoB assessment and certainty of the evidence
According to the RoB assessment (Supplemental Figure S1), eight studies had an unclear risk for random sequence generation,29–31,38–40,42,43 nine for allocation concealment,29–31,35,38–40,42,43 one for blinding of participants and personnel, 35 four for incomplete outcome data,29,35,39,42 and other biases, such as funding bias.31,35,37,40 A high RoB was found for random sequence generation in one study 35 and other biases in seven papers.28,30,32,36,38,39,41
The certainty of evidence ranged from moderate to high RoB for the sequential therapy from anabolic to antiresorptive treatment, while a low RoB was attributed to the sequential treatment from antiresorptive to anabolic medications (Supplemental Figure S1).
Primary outcome
The risk of refracture was measured after the switch from anabolic to antiresorptive or vice versa (Table 1). Regarding the sequential therapy from anabolic to antiresorptive treatment, there was a significant reduction in the risk of different types of fractures (vertebral, nonvertebral, major nonvertebral, and major osteoporotic fracture) after the switch from romosozumab to denosumab versus placebo to denosumab (RR from 0.25 to 0.75; 95% CI, 0.16–0.99), while a nonsignificant risk reduction was only detected for hip fracture (RR, 0.50; 95% CI, 0.24–1.04). 13 These findings were confirmed at 24 months after the switch.34,40 Moreover, a reduced risk of nonvertebral fracture was reported 30 months after the switch from teriparatide to bisphosphonates versus placebo to bisphosphonates (RR, 0.54; 95% CI, 0.32–0.91). 39 Finally, there was a significant decrease in risk of vertebral and hip fractures (RR 0.52 and 0.60, respectively; 95% CI, 0.40–0.97)28,41 12 months after the switch from romosozumab to alendronate versus the only alendronate therapy, while a nonsignificant risk reduction in nonvertebral fracture was detected (RR, 0.81; 95% CI, 0.63–1.04). 41
Table 1.
Risk of refracture: switching from anabolic to antiresorptive, or vice versa.
| First author, year | Months from baseline | Months from switch | Site of fracture | Incidence of fracture | |||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | RR (95% CI) | |||||
| Group 1: Romo to Dmab; Group 2: placebo to Dmab | |||||||
| Cosman 2016 FRAME Study |
24 | 12 | Vertebral fracture | 21/3325 (0.6%) | 84/3327 (2.5%) | 0.25 (0.16–0.40) | |
| Nonvertebral fracture | 96/3589 (2.7%) | 129/3591 (3.6%) | 0.75 (0.57–0.97) | ||||
| Major nonvertebral fracture | 67/3589 (1.9%) | 101/3591 (2.8%) | 0.67 (0.49–0.91) | ||||
| Hip fracture | 11/3589 (0.3%) | 22/3591 (0.6%) | 0.50 (0.24–1.04) | ||||
| Major osteoporotic fracture | 68/3589 (1.9%) | 110/3591 (3.1%) | 0.62 (0.46–0.84) | ||||
| Lewiecki 2019 Extension of FRAME study |
36 | 24 | Vertebral fracture | 32/3325 (1.0%) | 94/3327 (2.8%) | 0.34 (0.23–0.51) | |
| Nonvertebral fracture | 139/3589 (3.9%) | 176/3591 (4.9%) | 0.79 (0.64–0.98) | ||||
| Major nonvertebral fracture | 100/3589 (2.8%) | 138/3591 (3.8%) | 0.73 (0.56–0.93) | ||||
| Hip fracture | 18/3589 (0.5%) | 31/3591 (0.9%) | 0.58 (0.33–1.04) | ||||
| Major osteoporotic fracture | 103/3589 (2.9%) | 147/3591 (4.1%) | 0.70 (0.55–0.90) | ||||
| Miyuachi 2019 Subgroup analysis of FRAME Study |
36 | 24 | Vertebral fracture | 4/237 (1.7%) | 11/243 (4.5%) | 0.37 (0.12–1.15) | |
| Nonvertebral fracture | 7/247 (2.8%) | 15/245 (6.1%) | 0.46 (0.19–1.12) | ||||
| Major nonvertebral fracture | 4/247 (1.6%) | 7/245 (2.9%) | 0.57 (0.17–1.91) | ||||
| Hip fracture | 0/247 (0.0%) | 2/245 (0.8%) | 0.20 (0.01–4.11) | ||||
| Major osteoporotic fracture | 5/247 (2.0%) | 8/245 (3.3%) | 0.62 (0.21–1.87) | ||||
| Group 1: Romosozumab to ALN; Group 2: only ALN | |||||||
| Cosman 2020
Post hoc analysis of ARCH Study |
24 | 12 | Nonvertebral fracture | 105/1739 (6.0%) | 127/1726 (7.4%) | 0.81 (0.63–1.05) a | |
| Hip fracture | 25/1739 (1.4%) | 42/1726 (2.4%) | 0.60 (0.37–0.99) a | ||||
| Saag 2017 ARCH Study |
24 | 12 | Vertebral fracture | 127/2046 (6.2%) | 243/2047 (11.9%) | 0.52 (0.40–0.66) b | |
| Group 1: Teriparatide 20 µg to BPs; Group 2: teriparatide 40 µg to BPs; Group 3: placebo to BPs | |||||||
| Prince 2005 | 30 | Nonvertebral fracture | Group 1 30/436 (6.9%) | Group 2 22/412 (5.3%) | Group 3 38/414 (9.2%) | 20 µg: 0.70 (0.43–1.13)
c
40 µg: 0.54 (0.32–0.91) c |
|
| Group 1: BPs or AR to teriparatide; Group 2: placebo to teriparatide | |||||||
| Obermayer-Pietsch 2008 EUROFORS Study |
24 | Any fracture | 3/134 (2.2%) | 5/84 (5.9%) | 0.38 (0.09–1.53) | ||
Adjusted for baseline BMD, age strata, and the presence of severe vertebral fracture at baseline.
Adjusted for age (<75 versus ⩾75 years), the presence or absence of severe vertebral fracture at baseline, and baseline bone mineral density T score at the total hip.
Adjusted for the duration of osteoporosis drug treatment.
ALN, alendronate; AR, antiresorptive; BPs, bisphosphonates; BMD, bone mineral density; CI, confidence interval; RR, risk ratio.
Considering the sequential treatment from antiresorptive to anabolic medications, there was a decreased risk of fracture 12 months after the switch from placebo to teriparatide versus bisphosphonate or antiresorptive to teriparatide (RR, 0.38; 95% CI, 0.09–1.53). 38
Secondary outcome
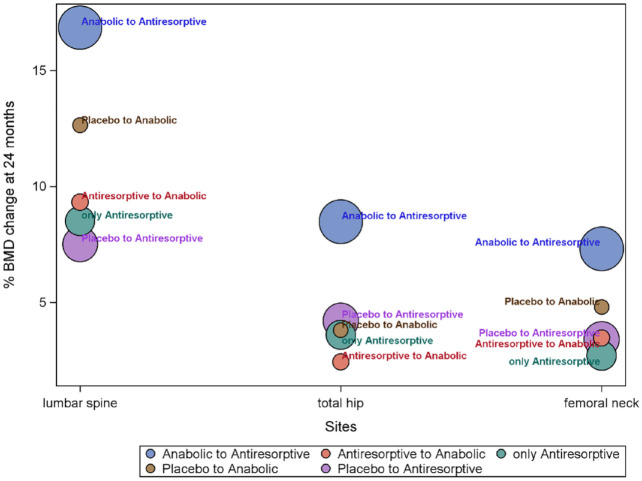
Table 2 reports the change in BMD 2 years after the sequential therapy from anabolic to antiresorptive medications or vice versa. A greater BMD increase after the switch from anabolic to antiresorptive medications was shown in the lumbar spine (16.84%), total hip (8.47%), and femoral neck (7.31%).28,34 Conversely, a lower BMD increase occurred in the (i) lumbar spine (7.50%) after the switch from placebo to antiresorptive drugs 34 ; (ii) total hip (2.42%) after switching from antiresorptive to anabolic treatment 30 ; and (iii) femoral neck (2.70%) for the only antiresorptive therapy 28 (Figure 2).
Table 2.
Bone mineral density changes from the switching: anabolic to antiresorptive, or vice versa.
| First author, year | Months from baseline | Months from switch | Comparative LS BMD change from baseline (mean%) | Comparative TH BMD change from baseline (mean%) | Comparative FN BMD change from baseline (mean%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p Value | Group 1 | Group 2 | p Value | Group 1 | Group 2 | p Value | |||||||||
| Group 1: Romo to Dmab; Group 2: placebo to Dmab | |||||||||||||||||
| Cosman 2016 FRAME Study |
18 | 6 |
N = 65 15.1 |
N = 61 3.3 |
p < 0.001 |
N = 66 8.4 |
N = 62 1.6 |
p < 0.001 |
N = 66 6.7 |
N = 62 −0.2 |
p < 0.001 | ||||||
| 24 | 12 | 17.6 | 5.0 | p < 0.001 | 8.8 | 2.9 | p < 0.001 | 6.6 | 0.6 | p < 0.001 | |||||||
| Lewiecki 2019 Extension of FRAME study |
24 | 12 |
N = 3169 16.6 |
N = 3176 5.5 |
p < 0.001 |
N = 3237 8.5 |
N = 3256 3.2 |
p < 0.001 |
N = 3237 7.3 |
N = 3256 2.3 |
p < 0.001 | ||||||
| 36 | 24 | 18.1 | 7.5 | p < 0.001 | 9.4 | 4.2 | p < 0.001 | 8.2 | 3.4 | p < 0.001 | |||||||
| Miyauchi 2019 Subgroup analysis of FRAME Study |
24 | 12 |
N = 205 20.2 |
N = 190 7.3 |
p < 0.001 |
N = 205 7.9 |
N = 200 2.9 |
p < 0.001 |
N = 205 7.8 |
N = 200 3.6 |
p < 0.001 | ||||||
| 36 | 24 |
N = 207 22.1 |
N = 195 9.5 |
p < 0.001 |
N = 218 8.7 |
N = 210 4.5 |
p < 0.001 |
N = 218 8.6 |
N =2 10 4.4 |
p < 0.001 | |||||||
| Group 1: Teriparatide to Dmab; Group 2: TPTD to ALN; Group 3: TPTD to minodronate | |||||||||||||||||
| Niimi 2018 | 12 | Group 1 N = 100 4.3 ± 3.5 |
Group 2 N = 100 1.3 ± 5.1 |
Group 3 N = 100 0.5 ± 4.6 |
p < 0.01 Dmab versus Mino or ALN |
Group 1 N = 100 1.4 ± 3.4 |
Group 2 N = 100 0.7 ± 4.6 |
Group 3 N = 100 0.2 ± 4.6 |
p = 0.16 | ||||||||
| Group 1: Romosozumab to ALN; Group 2: only ALN | |||||||||||||||||
| Saag 2017 Extension of ARCH study |
36 | 24 |
N = 2046 14.9 |
N = 2047 8.5 |
p < 0.001 |
N = 2046 7.0 |
N = 2047 3.6 |
p < 0.001 |
N = 2046 5.9 |
N = 2047 2.7 |
p < 0.001 | ||||||
| Cosman 2020 ARCH Study |
24 | 12 |
N = 1739 15.5 ± 0.4 |
N = 1726 7.3 ± 0.3 |
p < 0.001 |
N = 1739 7.3 ± 0.2 |
N = 1726 3.5 ± 0.2 |
p < 0.001 |
N = 1739 6.1 ± 0.4 |
N = 1726 2.3 ± 0.3 |
p < 0.001 | ||||||
| Group 1: PTH to BPs; Group 2: PTH to placebo | |||||||||||||||||
| Black 2005 PATH study |
24 | 12 |
N = 12 12.1 |
N = 7 4.0 |
p < 0.05 |
N = 12 4.0 |
N = 7 0.0 |
p < 0.05 |
N = 12 4.0 |
N = 7 1.0 |
p < 0.05 | ||||||
| Group 1: Romosozumab to Dmab; Group 2: Romosozumab to placebo | |||||||||||||||||
| Kendler 2019 | 24–36 | 0–12 |
N = 16 2.5 ± 1.5 |
N = 19 −9.1 ± 1.6 |
N = 16 2.0 ± 1.3 |
N = 19 −5.3 ± 2.0 |
N = 16 1.3 ± 1.3 |
N = 19 −4.3 ± 2.3 |
|||||||||
| Group 1: ALN to Teriparatide; Group 2: treatment naïve to TPTD | |||||||||||||||||
| Fahrleitner-Pammer 2016 | 12 |
N = 29 1.1 |
N = 16 6.2 |
p = 0.004 | |||||||||||||
| 24 | 5.3 | 10.2 | p = 0.077 | ||||||||||||||
| Group 1: BisP or AR to TPTD; Group 2: no treatment to TPTD | |||||||||||||||||
| Middleton 2007 | 12 |
N = 38 9.0 |
N = 14 7.8 |
p = 0.54 |
N = 38 1.0 |
N = 14 −0.3 |
p = 0.36 | ||||||||||
| 18 | 9.8 | 6.1 | p = 0.30 | 2.8 | 1.3 | p = 0.44 | |||||||||||
| Obermayer-Pietsch 2008 EUROFORS Study |
6 |
N = 134 3.5 |
N = 84 5.8 |
p < 0.001 |
N = 134 −0.3 |
N = 84 0.5 |
N = 134 −0.3 |
N = 84 1.2 |
p < 0.05 | ||||||||
| 12 | 6.6 | 9.3 | p < 0.001 | 0.6 | 1.8 | p < 0.05 | 1.1 | 2.2 | |||||||||
| 18 | 8.6 | 11.1 | p < 0.01 | 0.6 | 2.7 | p < 0.05 | 2.1 | 3.1 | |||||||||
| 24 | 10.2 | 13.1 | p < 0.01 | 2.3 | 3.8 | p < 0.01 | 3.4 | 4.8 | |||||||||
| Group 1: AR to Teriparatide; Group 2: no change AR | |||||||||||||||||
| Gonnelli 2006 | 6 |
N = 27 5.6 ± 6.7 |
N = 28 1.2 ± 3.4 |
p < 0.05 |
N = 27 −2.1 ± 3.5 |
N = 28 0.20 ± 2.9 |
NS |
N = 27 −1.8 ± 8.7 |
N = 28 1.6 ± 3.1 |
NS | |||||||
| 12 | 7.1 ± 5.9 | 1.5 ± 4.3 | p < 0.05 | −0.8 ± 2.7 | 1.2 ± 4.2 | NS | 2.6 ± 5.1 | 1.1 ± 3.8 | NS | ||||||||
| Group 1: ALN to Romosozumab; Group 2: ALN to TPTD | |||||||||||||||||
| Langdhal 2017 STRUCTURE study |
6 |
N = 206 7.2 (6.6–7.8) |
N = 209 3.5 (2.9–4.0) |
p < 0.0001 |
N = 206 2.3 (1.9–2.7) |
N = 209 −0.8 (−1.2 to −0.4) |
p = 0.0001 |
N = 206 2.1 (1.6–2.7) |
N = 209 −1.1 (−1.6 to −0.5) |
p = 0.0003 | |||||||
| 12 | 9.8 (9.0–10.5) | 5.4 (4.7–6.1) | p < 0.0001 | 2.9 (2.5–3.4) | −0.5 (−0.9 to −0.0) | p = 0.0357 | 3.2 (2.6–3.8) | −0.2 (−0.8 to 0.4) | p = 0.4566 | ||||||||
| Group 1: RIS to Teriparatide; Group 2: ALN to TPTD | |||||||||||||||||
| Miller 2008 | 6 |
N = 158 3.0 |
N = 166 2.0 |
N = 158 −1.2 |
N = 166 −1.9 |
p = 0.07 | |||||||||||
| 12 | 5.1 | 3.6 | p < 0.05 | −0.3 | −1.7 | p = 0.07 | |||||||||||
| Group 1: RIS to Teriparatide; Group 2: ALN to TPTD; Group 3: ETN to TPTD; Group 4: Non-BPs to TPTD | |||||||||||||||||
| Boonen 2008 EUROFORS study |
6 | Group 1 N = 59 2.3 |
Group 2 N = 107 3.0 |
Group 3 N = 30 5.8 |
Group 4 N = 49 4.0 |
p < 0.05 ETN versus RIS, ALN |
Group 1 N = 59 −1.6 |
Group 2 N = 107 −1.2 |
Group 3 N = 30 −0.7 |
Group 4 N = 49 −0.3 |
NS | Group 1 N = 59 −1.1 |
Group 2 N = 107 −1.8 |
Group 3 N = 30 −0.9 |
Group 4 N = 49 −1.4 |
NS | |
| 12 | 5.6 | 5.4 | 8.8 | 5.3 |
p < 0.05 ETN versus RIS, ALN |
−0.4 | −0.6 | 1.1 | −0.4 | NS | 0.2 | −0.5 | 1.5 | −0.3 | NS | ||
| 18 | 7.7 | 7.8 | 11.6 | 8.2 |
p < 0.05 ETN versus NON-BPs |
0.9 | 0.6 | 2.4 | 1.4 | NS | 1.6 | 1.3 | 3.8 | 2.3 |
p < 0.005 ALN versus ETI |
||
| 24 | 9.4 | 9.2 | 13.5 | 9.3 |
p < 0.05 ETN versus NON-BPs |
2.9 | 2.1 | 3.7 | 1.8 | NS | 4.1 | 3.4 | 3.7 | 2.7 | NS | ||
ALN, alendronate; AN, anabolic; AR, antiresorptive; BMD, bone mineral density; ETN, etidronate; FN, femoral neck; LS, lumbar spine; PTH, parathyroid hormone; RIS, risedronate; TH, total hip; TPTD, teriparatide.
Figure 2.
BMD change (mean % change) 24 months after the switch from anabolic, antiresorptive, or placebo.
Circles represent all studies related to the therapies and have a diameter proportional to the sample size.
AN, anabolic; AR, antiresorptive.
Discussion
This systematic review evaluated a clinical question for the Italian Guidelines, 15 and a panel of experts formulated recommendations through a structured and transparent process. Specifically, we conducted a systematic review and meta-analysis on sequential therapy in patients at very high risk of or with a fragility fracture that enabled us to strongly recommend the anabolic drugs as first-line treatment (moderate quality of evidence) as confirmed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. 44
We found a relevant reduction in fracture risk at almost every skeletal site, especially when we considered the effects of the sequence: (i) romosozumab to denosumab versus placebo to denosumab; (ii) romosozumab to alendronate versus alendronate by alone; and (iii) teriparatide to bisphosphonates versus placebo to bisphosphonates. On the contrary, the sequence from bisphosphonate or antiresorptive to teriparatide versus no treatment to teriparatide 38 did not find a significant reduction of fracture risk 12 months after the switch (RR, 0.38; 95% CI, 0.09–1.53). Furthermore, the percent BMD changes were calculated by merging studies that evaluated the different therapeutic strategies. Greater improvement in BMD has been obtained using an anabolic by alone, such as romosozumab, or (a better choice) an anabolic followed by an antiresorptive drug, such as denosumab. We confirmed a significantly greater BMD benefit for the sequence: (i) romosozumab to denosumab versus placebo to denosumab; (ii) teriparatide to denosumab versus teriparatide to oral bisphosphonates; (iii) romosozumab to alendronate versus alendronate by alone; (iv) PTH to bisphosphonates versus PTH to placebo; and (v) romosozumab to denosumab versus romosozumab to placebo. In a representative open study, the DATA study, 33 postmenopausal women starting with teriparatide for 24 months and subsequently switching to denosumab for 24 months showed greater spine and hip BMD gains compared to the opposite treatment sequence. Notably, the results seen in the combination group (teriparatide plus denosumab followed by denosumab alone) showed an early BMD gain at the spine and hip sites. 45 This negative influence of antiresorptive pretreatment seems to be related to the antiresorptive potency of the used drug; the higher the antiresorptive potency (alendronate > risedronate > risedronate > etidronate > non-bisphosphonates), the lower the BMD gains induced by teriparatide.30,36 Moreover, the study of Gonnelli et al. 31 showed an increase in spine BMD after switching from antiresorptive to teriparatide compared to only antiresorptive therapy, despite the limited sample size. Thus, the impact of post-anabolic treatment on the retention of densitometric gains is substantial, as evidenced by studies on both teriparatide 37 and romosozumab. 41 We believe the analysis by Cosman provides a valuable perspective. For patients at low risk, antiresorptive therapy on its own suffices, whereas the subsequent strategy for high-risk patients post-anabolic therapy should be contingent upon the densitometric outcome attained. Should the densitometric increase be deemed satisfactory, maintenance with bisphosphonates is appropriate; however, if the desired endpoint is not achieved, continuation with denosumab may be advisable until the target T-score (possibly −2.5) is reached. At that juncture, transitioning to bisphosphonates may be considered.
In general, antiresorptive agents, such as bisphosphonates, were the first developed drugs, and only later did an anabolic agent, such as teriparatide, become available. 46 Some pharmacological therapies can be used only for a limited timeframe, such as romosozumab and PTH analogs, which require a treatment cycle of 12 and 24 months, respectively. Furthermore, the discontinuation of certain treatments is followed by an undesired rebound effect, characterized by rapid bone loss that can undermine most of the densitometric benefits obtained over time and, consequently, loss of the clinical benefit in terms of fracture prevention. This can occur with anabolic, but it is especially alarming for denosumab. 47 Drugs currently available for the treatment of osteoporosis are classified by their mechanism of action. Antiresorptive drugs reduce osteoclastic bone resorption, and they include estrogens and selective estrogen receptor modulators, bisphosphonates, and denosumab. In addition, anabolic drugs increase osteoblastic bone formation activity, including teriparatide and abaloparatide. Finally, dual-action drugs, such as romosozumab, increase osteoblastic bone formation activity and reduce osteoclastic bone resorption.
Anabolic drugs, such as teriparatide, abaloparatide, and romosozumab, reduce the risk of fracture more rapidly and to a greater extent than antiresorptive medications. 41 Several head-to-head studies28,48–50 showed that anabolic agents are more effective in reducing fracture incidence than oral bisphosphonates in the next 1 or 2 years, which have a higher refracture risk.6,51–53 Anabolic medications not only provide bone mass accrual but are also associated with microstructural improvement, resulting in greater skeletal strength and resistance to fracture. Then, the sequential antiresorptive agents could sustain the BMD and strength gains, extending the protection against fractures over time. 54 With the newer drugs (denosumab and romosozumab), only head-to-head comparisons on densitometric effects are available. 55 However, there is increasing consensus on the appropriateness of estimating fracture risk reduction obtained with the densitometric gain associated with the therapy. 55 Unfortunately, anabolic drugs are widely underutilized in clinical practice, mostly due to their higher costs. For this reason, they are often used in high-risk patients, especially after the failure of a previous antiresorptive treatment for a new fracture or refracture. 45
Sequential therapy in osteoporosis offers a pivotal means to augment the efficacy of individual treatments, provided the proper sequence (from anabolic to antiresorptive) is employed. Yet, it is regrettable that the reverse order is still prevalent in clinical settings, which is counterproductive and is thus inadvisable. It is now acknowledged that the increase in bone density achieved through therapy can be translated into a reduced risk of subsequent fractures. Consequently, the study’s conclusions are pertinent to both high-risk and low-risk populations. Of course, in absolute numbers, the prevention of fractures will be considerably greater in the higher-risk cohort. This assertion is further corroborated by studies on romosozumab, where the romosozumab–alendronate sequence has demonstrated superiority over the alendronate–alendronate sequence in fracture outcomes, even though the study participants were of medium–low risk.
Limitations and strengths
Some limitations must be acknowledged. First, most studies were conducted in Europe, which may limit the generalizability of the results. Second, we have some concerns regarding the characteristics of patients and fracture sites at baseline. Moreover, few RCTs were included in each sequential therapeutic strategy, which might affect the interpretability of our findings. Third, the certainty of the evidence ranged from moderate to high RoB when considering anabolic as a first-line treatment. Conversely, a low RoB was attributed to sequential treatment from antiresorptive to anabolic medications.
Despite the above limitations, this study presents some strengths. The exhaustive search strategy identified an overview of RCTs focused on the sequential therapy from anabolic to antiresorptive and vice versa. In addition, the internal validity of the included studies was assessed using the RoB tool for RCTs.
Conclusion
The results of this systematic review and meta-analysis confirm that initial treatment with anabolic drugs produces substantial BMD improvements, both at the spine and at the hip sites, and the transition to antiresorptive drugs can preserve or even amplify the acquired benefit. These findings support the choice to treat very high-risk individuals with anabolic drugs first, followed by antiresorptive drugs, rather than using a reverse sequence.
Since osteoporosis is a chronic disease often requiring long-term treatments, clinicians are now asked not only to start a single drug but also to schedule long-term strategies based on the current and future fracture risk.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X241234584 for The sequential antifracturative treatment: a meta-analysis of randomized clinical trials by Angelo Fassio, Davide Gatti, Annalisa Biffi, Raffaella Ronco, Gloria Porcu, Giovanni Adami, Rosaria Alvaro, Riccardo Bogini, Achille P. Caputi, Luisella Cianferotti, Bruno Frediani, Stefano Gonnelli, Giovanni Iolascon, Andrea Lenzi, Salvatore Leone, Raffaella Michieli, Silvia Migliaccio, Tiziana Nicoletti, Marco Paoletta, Annalisa Pennini, Eleonora Piccirilli, Maurizio Rossini, Maria Luisa Brandi, Giovanni Corrao and Umberto Tarantino in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X241234584 for The sequential antifracturative treatment: a meta-analysis of randomized clinical trials by Angelo Fassio, Davide Gatti, Annalisa Biffi, Raffaella Ronco, Gloria Porcu, Giovanni Adami, Rosaria Alvaro, Riccardo Bogini, Achille P. Caputi, Luisella Cianferotti, Bruno Frediani, Stefano Gonnelli, Giovanni Iolascon, Andrea Lenzi, Salvatore Leone, Raffaella Michieli, Silvia Migliaccio, Tiziana Nicoletti, Marco Paoletta, Annalisa Pennini, Eleonora Piccirilli, Maurizio Rossini, Maria Luisa Brandi, Giovanni Corrao and Umberto Tarantino in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We thank Charlesworth Author Services for the English Academic Editing.
Footnotes
ORCID iDs: Angelo Fassio  https://orcid.org/0000-0001-9187-232X
https://orcid.org/0000-0001-9187-232X
Giovanni Adami  https://orcid.org/0000-0002-8915-0755
https://orcid.org/0000-0002-8915-0755
Giovanni Iolascon  https://orcid.org/0000-0002-0976-925X
https://orcid.org/0000-0002-0976-925X
Salvatore Leone  https://orcid.org/0000-0003-1690-8147
https://orcid.org/0000-0003-1690-8147
Silvia Migliaccio  https://orcid.org/0000-0002-4563-6630
https://orcid.org/0000-0002-4563-6630
Marco Paoletta  https://orcid.org/0000-0002-3291-9738
https://orcid.org/0000-0002-3291-9738
Eleonora Piccirilli  https://orcid.org/0000-0002-1570-6482
https://orcid.org/0000-0002-1570-6482
Maurizio Rossini  https://orcid.org/0000-0001-9692-2293
https://orcid.org/0000-0001-9692-2293
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Angelo Fassio, Rheumatology Unit, University of Verona, Piazzale A Scuro, Policlinico GB Rossi, Verona 37134, Italy.
Davide Gatti, Rheumatology Unit, University of Verona, Verona, Italy.
Annalisa Biffi, Department of Statistics and Quantitative Methods, National Centre for Healthcare Research and Pharmacoepidemiology, University of Milano–Bicocca, Milan, Italy; Unit of Biostatistics, Epidemiology, and Public Health, Department of Statistics and Quantitative Methods, University of Milano–Bicocca, Milan, Italy.
Raffaella Ronco, Department of Statistics and Quantitative Methods, National Centre for Healthcare Research and Pharmacoepidemiology, University of Milano–Bicocca, Milan, Italy; Unit of Biostatistics, Epidemiology, and Public Health, Department of Statistics and Quantitative Methods, University of Milano–Bicocca, Milan, Italy.
Gloria Porcu, Department of Statistics and Quantitative Methods, National Centre for Healthcare Research and Pharmacoepidemiology, University of Milano–Bicocca, Milan, Italy; Unit of Biostatistics, Epidemiology, and Public Health, Department of Statistics and Quantitative Methods, University of Milano–Bicocca, Milan, Italy.
Giovanni Adami, Rheumatology Unit, University of Verona, Verona, Italy.
Rosaria Alvaro, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Rome, Italy.
Riccardo Bogini, Local Health Unit Umbria, Perugia, Italy.
Achille P. Caputi, Department of Pharmacology, School of Medicine, University of Messina, Messina, Italy
Luisella Cianferotti, Italian Bone Disease Research Foundation, Florence, Italy.
Bruno Frediani, Rheumatology Unit, Department of Medicine, Surgery and Neurosciences, Azienda Ospedaliero-Universitaria Senese, University of Siena, Siena, Italy.
Stefano Gonnelli, Department of Medicine, Surgery and Neuroscience, Policlinico Le Scotte, University of Siena, Siena, Italy.
Giovanni Iolascon, Department of Medical and Surgical Specialties and Dentistry, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Andrea Lenzi, Department of Experimental Medicine, Sapienza University of Rome, Viale del Policlinico, Rome, Italy.
Salvatore Leone, AMICI Onlus, Associazione Nazionale per le Malattie Infiammatorie Croniche dell’Intestino, Milan, Italy.
Raffaella Michieli, Italian Society of General Medicine and Primary Care, Florence, Italy.
Silvia Migliaccio, Department of Movement, Human and Health Sciences, Foro Italico University, Rome, Italy.
Tiziana Nicoletti, Coordinamento Nazionale delle Associazioni dei Malati Cronici e rari di Cittadinanzattiva, Rome, Italy.
Marco Paoletta, Department of Medical and Surgical Specialties and Dentistry, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Annalisa Pennini, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Rome, Italy.
Eleonora Piccirilli, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy; Department of Orthopedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Maurizio Rossini, Rheumatology Unit, University of Verona, Verona, Italy.
Maria Luisa Brandi, Italian Bone Disease Research Foundation, Florence, Italy.
Giovanni Corrao, Department of Statistics and Quantitative Methods, National Centre for Healthcare Research and Pharmacoepidemiology, University of Milano–Bicocca, Milan, Italy; Unit of Biostatistics, Epidemiology, and Public Health, Department of Statistics and Quantitative Methods, University of Milano–Bicocca, Milan, Italy.
Umberto Tarantino, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy; Department of Orthopedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Declarations
Ethics approval and consent to participate: This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Consent for publication: Not applicable.
Author contributions: Angelo Fassio: Conceptualization; Data curation; Investigation; Project administration; Supervision; Writing – original draft.
Davide Gatti: Conceptualization; Data curation; Investigation; Project administration; Writing – original draft.
Annalisa Biffi: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft.
Raffaella Ronco: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft.
Gloria Porcu: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft.
Giovanni Adami: Conceptualization; Data curation; Writing – review & editing.
Rosaria Alvaro: Conceptualization; Data curation; Writing – review & editing.
Riccardo Bogini: Conceptualization; Data curation; Writing – review & editing.
Achille P. Caputi: Visualization; Writing – review & editing.
Luisella Cianferotti: Conceptualization; Data curation; Writing – review & editing.
Bruno Frediani: Visualization; Writing – review & editing.
Stefano Gonnelli: Conceptualization; Data curation; Writing – review & editing.
Giovanni Iolascon: Conceptualization; Data curation; Writing – review & editing.
Andrea Lenzi: Conceptualization; Data curation; Writing – review & editing.
Salvatore Leone: Conceptualization; Data curation; Writing – review & editing.
Raffaella Michieli: Conceptualization; Data curation; Writing – review & editing.
Silvia Migliaccio: Conceptualization; Data curation; Writing – review & editing.
Tiziana Nicoletti: Conceptualization; Data curation; Writing – review & editing.
Marco Paoletta: Conceptualization; Data curation; Writing – review & editing.
Annalisa Pennini: Conceptualization; Data curation; Writing – review & editing.
Eleonora Piccirilli: Conceptualization; Data curation; Writing – review & editing.
Maurizio Rossini: Conceptualization; Data curation; Writing – review & editing.
Maria Luisa Brandi: Conceptualization; Data curation; Writing – original draft.
Giovanni Corrao: Conceptualization; Data curation; Methodology; Writing – original draft; Writing – review & editing.
Umberto Tarantino: Conceptualization; Data curation; Investigation; Project administration; Supervision; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Italian guideline was funded by ALTIS Omnia Pharma Service, which did not affect the content of the document.
GA declares personal fees from Theramex, Amgen, BMS, Lilly, Fresenius Kabi, and Galapagos. LC declares personal fees from UCB Pharma, Abiogen Pharma, Bruno Farmaceutici, Sandoz, and Metagenics. DG has received honoraria as a consultant for Eli-Lilly, Organon, and MSD Italia. SG has received honoraria as a consultant for UCB Pharma. SM has received honoraria as a consultant for UCB, Eli-Lilly, and Amgen. MLB has received (i) honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB, (ii) grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma, (iii) consultant: Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB Pharma. GC received research support from the European Community (EC), the Italian Agency of Drug (AIFA), and the Italian Ministry for University and Research (MIUR). He took part in a variety of projects that were funded by pharmaceutical companies (i.e. Novartis, GSK, Roche, AMGEN, and BMS). He also received honoraria as a member of the Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MR declares personal fees from Amgen, ABBvie, BMS, Eli Lilly, Galapagos, Menarini, Novartis, Pfizer, Sandoz, Theramex, and UCB outside the submitted work. RM took part in a project funded by Abiogen Pharma. GI received honoraria as a speaker from Eli-Lilly, Menarini, and UCB Pharma. The other authors declare that they have no conflict of interest. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author).
Availability of data and materials: No additional data are available.
Transparency declaration: The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Wang P, Li Y, Zhuang H, et al. Influence of bone densitometry on the anti-osteoporosis treatment after fragility hip fracture. Aging Clin Exp Res 2019; 31: 1525–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keshishian A, Boytsov N, Burge R, et al. Examining the treatment gap and risk of subsequent fractures among females with a fragility fracture in the US Medicare population. Osteoporos Int 2017; 28: 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004; 35: 375–382. [DOI] [PubMed] [Google Scholar]
- 4. Johansson H, Siggeirsdóttir K, Harvey NC, et al. Imminent risk of fracture after fracture. Osteoporos Int 2017; 28: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borgström F, Karlsson L, Ortsäter G, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 2020; 15: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balasubramanian A, Zhang J, Chen L, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int 2019; 30: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1726–1733. [DOI] [PubMed] [Google Scholar]
- 8. Lewiecki EM. Secondary fracture prevention via a fracture liaison service. Womens Health (Lond) 2015; 11: 269–271. [DOI] [PubMed] [Google Scholar]
- 9. Gupta MJ, Shah S, Peterson S, et al. Rush fracture liaison service for capturing ‘missed opportunities’ to treat osteoporosis in patients with fragility fractures. Osteoporos Int 2018; 29: 1861–1874. [DOI] [PubMed] [Google Scholar]
- 10. Fassio A, Rossini M, Viapiana O, et al. New strategies for the prevention and treatment of systemic and local bone loss; from pathophysiology to clinical application. Curr Pharm Des 2017; 23: 6241–6250. [DOI] [PubMed] [Google Scholar]
- 11. Anagnostis P, Gkekas NK, Potoupnis M, et al. New therapeutic targets for osteoporosis. Maturitas 2019; 120: 1–6. [DOI] [PubMed] [Google Scholar]
- 12. Cheng C, Wentworth K, Shoback DM. New frontiers in osteoporosis therapy. Annu Rev Med 2020; 71: 277–288. [DOI] [PubMed] [Google Scholar]
- 13. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532–1543. [DOI] [PubMed] [Google Scholar]
- 14. Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017; 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sistema Nazionale Linee Guida (SNLG). Diagnosi, Stratificazione del rischio e continuità assistenziale delle Fratture da Fragilità, 2021. https://snlg.iss.it/.
- 16. Schünemann HJ, Wiercioch W, Brozek J, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol 2017; 81: 101–110. [DOI] [PubMed] [Google Scholar]
- 17. Manuale metodologico per la produzione di linee guida di pratica clinica, v. 1.3.2 April 2019. Centro Nazionale per l’Eccellenza Clinica, la Qualità e la Sicurezza delle Cure. Istituto Superiore di Sanità. [Google Scholar]
- 18. SNLG ISS. Sistema nazionale per le linee guida – Istituto superiore di sanità. Come produrre, diffondere e aggiornare raccomandazioni per la pratica clinica. Manuale metodologico. Roma: PNLG, 2019. [Google Scholar]
- 19. Anastasilakis AD, Polyzos SA, Yavropoulou MP, et al. Combination and sequential treatment in women with postmenopausal osteoporosis. Expert Opin Pharmacother 2020; 21: 477–490. [DOI] [PubMed] [Google Scholar]
- 20. Page MJ, Moher D. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and extensions: a scoping review. Syst Rev 2017; 6: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 25. Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. [Google Scholar]
- 28. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017; 377: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 29. Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 2005; 353: 555–565. [DOI] [PubMed] [Google Scholar]
- 30. Boonen S, Marin F, Obermayer-Pietsch B, et al. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2008; 93: 852–860. [DOI] [PubMed] [Google Scholar]
- 31. Gonnelli S, Martini G, Caffarelli C, et al. Teriparatide’s effects on quantitative ultrasound parameters and bone density in women with established osteoporosis. Osteoporos Int 2006; 17: 1524–1531. [DOI] [PubMed] [Google Scholar]
- 32. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 2017; 390: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 33. Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 2015; 386: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res 2019; 34: 419–428. [DOI] [PubMed] [Google Scholar]
- 35. Middleton ET, Steel SA, Doherty SM. The effect of prior bisphosphonate exposure on the treatment response to teriparatide in clinical practice. Calcif Tissue Int 2007; 81: 335–340. [DOI] [PubMed] [Google Scholar]
- 36. Miller PD, Delmas PD, Lindsay R, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 2008; 93: 3785–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Niimi R, Kono T, Nishihara A, et al. Efficacy of switching from teriparatide to bisphosphonate or denosumab: a prospective, randomized, open-label trial. JBMR Plus 2018; 2: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 2008; 23: 1591–1600. [DOI] [PubMed] [Google Scholar]
- 39. Prince R, Sipos A, Hossain A, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 2005; 20: 1507–1513. [DOI] [PubMed] [Google Scholar]
- 40. Miyauchi A, Dinavahi RV, Crittenden DB, et al. Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension. Arch Osteoporos 2019; 14: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res 2020; 35: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fahrleitner-Pammer A, Burr D, Dobnig H, et al. Improvement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatment. Bone 2016; 89: 16–24. [DOI] [PubMed] [Google Scholar]
- 43. Kendler DL, Bone HG, Massari F, et al. Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos Int 2019; 30: 2437–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Curtis EM, Reginster JY, Al-Daghri N, et al. Management of patients at very high risk of osteoporotic fractures through sequential treatments. Aging Clin Exp Res 2022; 34: 695–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 2017; 32: 198–202. [DOI] [PubMed] [Google Scholar]
- 46. Gatti D, Fassio A. Pharmacological management of osteoporosis in postmenopausal women: The current state of the art. J Popul Ther Clin Pharmacol 2019; 26: e1–e17. [DOI] [PubMed] [Google Scholar]
- 47. Elbers LPB, Raterman HG, Lems WF. Bone mineral density loss and fracture risk after discontinuation of anti-osteoporotic drug treatment: a narrative review. Drugs 2021; 81: 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-induced osteoporosis intervention study group. N Engl J Med 1998; 339: 292–299. [DOI] [PubMed] [Google Scholar]
- 49. Hadji P, Zanchetta JR, Russo L, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int 2012; 23: 2141–2150. [DOI] [PubMed] [Google Scholar]
- 50. Leder BZ, Mitlak B, Hu MY, et al. Effect of abaloparatide vs alendronate on fracture risk reduction in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2020; 105: 938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roux C, Briot K. Imminent fracture risk. Osteoporos Int 2017; 28: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 52. van Geel TACM, Huntjens KMB, van den Bergh JPW, et al. Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep 2010; 8: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007; 297: 387–394. [DOI] [PubMed] [Google Scholar]
- 54. Kanis JA, Cooper C, Rizzoli R, et al.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2019; 30: 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bouxsein ML, Eastell R, Lui LY, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 2019; 34: 632–642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X241234584 for The sequential antifracturative treatment: a meta-analysis of randomized clinical trials by Angelo Fassio, Davide Gatti, Annalisa Biffi, Raffaella Ronco, Gloria Porcu, Giovanni Adami, Rosaria Alvaro, Riccardo Bogini, Achille P. Caputi, Luisella Cianferotti, Bruno Frediani, Stefano Gonnelli, Giovanni Iolascon, Andrea Lenzi, Salvatore Leone, Raffaella Michieli, Silvia Migliaccio, Tiziana Nicoletti, Marco Paoletta, Annalisa Pennini, Eleonora Piccirilli, Maurizio Rossini, Maria Luisa Brandi, Giovanni Corrao and Umberto Tarantino in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X241234584 for The sequential antifracturative treatment: a meta-analysis of randomized clinical trials by Angelo Fassio, Davide Gatti, Annalisa Biffi, Raffaella Ronco, Gloria Porcu, Giovanni Adami, Rosaria Alvaro, Riccardo Bogini, Achille P. Caputi, Luisella Cianferotti, Bruno Frediani, Stefano Gonnelli, Giovanni Iolascon, Andrea Lenzi, Salvatore Leone, Raffaella Michieli, Silvia Migliaccio, Tiziana Nicoletti, Marco Paoletta, Annalisa Pennini, Eleonora Piccirilli, Maurizio Rossini, Maria Luisa Brandi, Giovanni Corrao and Umberto Tarantino in Therapeutic Advances in Musculoskeletal Disease