Abstract
We defined the epitopes recognized by three influenza A virus-specific, H-2Kd-restricted CD8+ cytotoxic T-lymphocyte (CTL) clones: H1-specific clone A-12, H2-specific clone F-4, and H1- and H2-cross-reactive clone B7-B7. The A-12 and B7-B7 clones recognized the same peptide, which comprises amino acids 533 to 541 (IYSTVASSL) of A/PR/8 hemagglutinin (HA). The F-4 and B7-B7 clones both recognized the peptide which comprise amino acids 529 to 537 (IYATVAGSL) of A/Jap HA. Amino acids 533 to 541 of A/PR/8 HA are compatible with amino acids 529 to 537 of A/Jap HA. Amino acid S at positions 3 and 7 was responsible for recognition by H1-specific clone A-12, while amino acid G at position 7 was responsible for recognition by H2-specific clone F-4. Two conserved amino acids, T at position 4 and A at position 6, were responsible for recognition by H1-, and H2-cross-reactive clone B7-B7. These results indicate that a single nine-amino-acid region is recognized by HA-specific CTL clones of three different subtype specificities and that the amino acids responsible for the recognition by the CTL clones are different.
Infection with influenza A virus induces influenza A virus-specific CD8+ cytotoxic T lymphocytes (CTLs) (5). It is generally accepted that influenza A virus-specific CD8+ CTLs play an important role in recovery from influenza virus infection because they clear virus-infected cells from infected mice (8, 9). CTLs recognizing hemagglutinin (HA) are either subtype specific or subtype cross-reactive for H1, H2, and H3. We previously reported that H1- and H2-cross-reactive CTL clone B7-B7 was induced by stimulation with a hybrid protein which contained a portion of the NS1 subunit and the HA2 subunit of the A/PR/8 virus (1, 4) and that the epitope recognized by this CTL clone at amino acids (aa) 533 to 543 in the transmembrane region of HA (4).
In the present paper, we report H-2Kd-restricted, H1-specific and H2-specific CD8+ CTL clones. The epitopes recognized by these two CTL clones were determined to be in the same region on HA and also in the same region as that recognized by H-2Kd-restricted, H1- and H2-cross-reactive CD8+ CTL clone B7-B7. We then define the amino acids on the epitope which are responsible for recognition by the CTL clones with respective specificity or cross-reactivity.
Male BALB/c mice were purchased from Charles River Breeding Laboratories (Wilmington, Mass.) and used at 4 to 5 weeks of age. Influenza A viruses A/PR/8 (A/Puerto Rico/8/34, H1N1), A/Jap (A/Japan/305/57, H2N2), and A/PC (A/Port Chalmers/1/73, H3N2) were propagated in 9-day-old embryonated chicken eggs. P815 cells (H-2d, mastocytoma), CV-1 cells, and CVK-10 (H-2Kd-transfected CV-1 cell line) cells, kindly provided by T. J. Braciale, were used as target cells.
Peptides were prepared by the solid-phase method on an ABI 430A peptide synthesizer (Applied Biosystems, Inc., Foster City, Calif.) as previously described (2). For the amino acid sequences of these peptides, see Table 2. Mice were immunized intranasally with 100 PFU of the virus under methoxyflurane anesthesia as previously reported (1). Spleens of mice immunized with A/PR/8 or A/Jap were removed 3 or more weeks after immunization for in vitro stimulation.
TABLE 2.
Amino acid sequences of the peptides used in the experiments
| Peptide | Amino acid sequencea |
|---|---|
| f1 (A/Jap) | 529I Y A T V A G S L S L539 |
| f2 | – – S – – – G – – S – |
| f3 | – – A – – – S – – S – |
| f4 | – – A – – – G – – V – |
| f5 | – – S – – – S – – S – |
| f6 | – – S – – – G – – V – |
| f7 | – – A – – – S – – V – |
| f8 (A/PR/8) | – – S – – – S – – V – |
| g1 (A/Jap) | 529I Y A T V A G S L537 |
| g2 | A – – – – – – – – |
| g3 | – A – – – – – – – |
| g4 | – – – A – – – – – |
| g5 | – – – – A – – – – |
| g6 | – – – – – S – – – |
| g7 | – – – – – – – A – |
| g8 | – – – – – – – – A |
| g9 (A/PR/8) | – – S – – – S – – |
| h1 | – – – A A – – – – |
| h2 | – – – – A S – – – |
| h3 | – – – A – S – – – |
The residues are numbered based on the amino acid sequence of A/Jap HA. Dashes represent identical amino acids.
Establishment and characterization of CTL clone B7-B7 were previously reported (2–4). The A-12 and F-4 CTL clones were established in the same way with a minor modification. Limiting dilution was carried out to establish CTL clones. The A-12 and F-4 clones described in this paper grew in wells in which two and four responder cells had been seeded, respectively. CTL assays were performed as previously reported (3). Specific lysis was calculated by the formula 100 × [(release by CTL − spontaneous release)/(maximum release − spontaneous release)]. CTL clones were stained with fluorescein isothiocyanate-conjugated anti-L3T4 (CD4) or phycoerythrin-conjugated anti-Lyt2 (CD8) antibodies.
We established two CTL clones (A-12 and F-4) from spleen cells of BALB/c mice immunized with the A/PR/8 or A/Jap virus. CTL clone A-12 lysed A/PR/8 (H1N1)-infected P815 target cells but did not lyse A/Jap (H2N2) or A/PC (H3N2) virus-infected P815 cells (data not shown). CTL clone F-4 lysed A/Jap-infected P815 cells but did not lyse A/PR/8 or A/PC virus-infected P815 cells (data not shown). CTL clone B7-B7 lysed P815 cells infected with the A/PR/8 or A/Jap virus. Thus, clone A-12 is H1 subtype specific, clone F-4 is H2 subtype specific, and clone B7-B7 is cross-reactive for H1 and H2 (2–4). Clones A-12, F-4, and B7-B7 had a CD8+ CD4− phenotype.
L929 cells (H-2k) transfected with genes coding for H-2Dd (DM1 cells) and H-2Ld (LM1 cells) and CV-1 and CVK-10 cells (CV-1 cell line transfected with genes coding for H-2Kd cells) were used to examine major histocompatibility complex restriction. Clone A-12 lysed A/PR/8 virus-infected P815 and infected CVK-10 cells; however, it did not lyse A/PR/8 virus-infected DM1 cells, infected LM1 cells, infected L929 cells, or infected CV-1 cells (Table 1). Clone F-4 lysed A/Jap virus-infected P815 and CVK-10 cells but did not lyse A/Jap virus-infected DM1, LM1, L929, or CV-1 cells. Clone B7-B7 lysed P815 and CVK-10 cells infected with the A/PR/8 or A/Jap virus (Table 1). These results indicate that the recognition of the target cells by these three CTL clones was restricted by the H-2Kd allele.
TABLE 1.
Influenza A virus subtype specificities and H-2Kd restriction of CTL clones
| Target cells | Virus used for infection | % Specific 51Cr releasea
|
||
|---|---|---|---|---|
| A-12 | F-4 | B7-B7 | ||
| Expt 1 | ||||
| P815 | A/PR/8 | 37.7 | −6.6 | 66.1 |
| A/Jap | 6.7 | 56.4 | 72.1 | |
| None | 5.9 | 14.5 | −5.2 | |
| Ld-L929 (H-2Ld) | A/PR/8 | 1.1 | 1.7 | 2.4 |
| A/Jap | −4.2 | −1.7 | 2.1 | |
| None | 1.8 | 1.8 | 1.3 | |
| Dd-L929 (H-2Dd) | A/PR/8 | 1.4 | 6.3 | 0.6 |
| A/Jap | −2.5 | −0.6 | −0.3 | |
| None | −8.6 | 0.4 | −2.5 | |
| L929 | A/PR/8 | −0.6 | 0.9 | 21.0 |
| A/Jap | −2.4 | −4.4 | 14.7 | |
| None | −4.1 | −3.0 | 15.6 | |
| Expt 2 | ||||
| CV-K10 (H-2Kd) | A/PR/8 | 37.5 | −0.1 | 31.8 |
| A/Jap | 6.4 | 28.1 | 34.9 | |
| None | 2.3 | 0.8 | 1.8 | |
| CV1 | A/PR/8 | −0.7 | −0.5 | −1.1 |
| A/Jap | 3.6 | 2.5 | −0.5 | |
| None | −1.8 | −1.7 | −0.4 | |
The effector-to-target cell ratio was 5:1 in a 4-h assay.
We previously reported that an H1- and H2-cross-reactive CTL epitope is located within the transmembrane region (aa 533 to 543) of HA (7). We found that aa 533 to 543 of A/PR/8 HA are comparable to aa 529 to 539 of A/Jap HA and that there are three amino acid substitutions within this region (Table 2). In the present paper, amino acid residues are numbered based on the amino acid sequence of A/Jap HA.
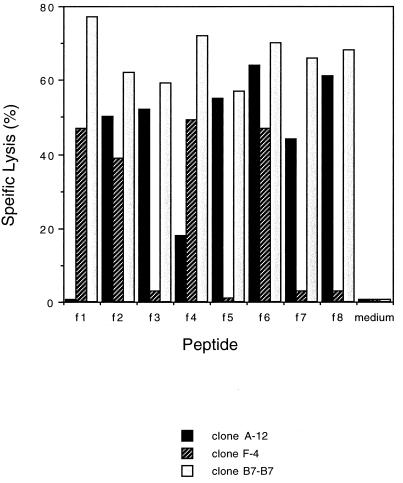
Clones A-12 and F-4, respectively, recognized aa 533 to 543 of A/PR/8 HA and aa 529 to 537 of A/Jap HA (data not shown). We synthesized peptides with an A-S substitution at position 531, a G-S substitution at position 535, and an S-V substitution at position 538 or a peptide with two amino acid substitutions (Table 2). Clone A-12 lysed P815 cells pulsed with peptides f2, f3, f5, f6, f7, and f8 but did not lyse target cells pulsed with peptide f1 or f4 (Fig. 1). Clone F-4 lysed P815 cells pulsed with f1, f2, f4, and f6 but not cells pulsed with f3, f5, f7, or f8 (Fig. 1). The results were similar at peptide concentrations of 100, 10, 1, and 0.1 μg/ml (data not shown). These results indicate that S residues at the positions 531 and 535 are essential for recognition by clone A-12, while the G at position 535 is essential for recognition by clone F-4.
FIG. 1.
P815 cells were pulsed with each of peptides f1 to f8 at 10 μg/ml and used as target cells for H1-specific CTL clone A-12, H2-specific CTL clone F-4, and the H1- and H2-cross-reactive CTL clone B7-B7 in 4-h CTL assays. The effector-to-target cell ratios were 8:1 for the A-12 clone, 6:1 for the F-4 clone, and 7:1 for the B7-B7 clone.
Clone B7-B7 lysed P815 cells pulsed with each of the eight peptides (f1 through f8) (Fig. 2). We then synthesized another set of peptides (Table 2). In this 9-mer region, there were seven amino acids (IY-TVA-SL) conserved between A/PR/8 and A/Jap. Each amino acid was replaced with A, except the amino acid residue at position 6, which was replaced with S. Clone B7-B7 did not lyse P815 cells pulsed with peptide g3 at a concentration of 10 μg/ml and peptide g8 at a concentration of 1 μg/ml but did lyse those pulsed with other peptides at concentrations of 10, 1, and 0.1 μg/ml (Fig. 2). It has been suggested that Y at position 2 and L at position 9 are anchor residues; therefore, it is likely that peptides g3 and g9 did not bind to H-2Kd.
FIG. 2.
P815 cells were pulsed with each of peptides g1 to g8 and h1 to h3 at various concentrations and used as target cells for H1- and H2-cross-reactive CTL clone B7-B7 in a 4-h CTL assay. The effector-to-target cell ratio was 7:1 when peptides g1 to g8 were used and 4:1 when peptides h1 to h3 were used.
We next synthesized three other peptides which have A and S substitutions at two of positions 4, 5, and 6 (Table 2). Clone B7-B7 lysed P815 cells pulsed with peptide h1 or h2 at concentrations of 10, 1, and 0.1 μg/ml (Fig. 2). Clone B7-B7 did not lyse target cells pulsed with peptide h3. However, P815 cells pulsed with peptide h3 were lysed by the A-12 clone (data not shown). These results indicate that amino acids at positions 4 and 6 are essential for recognition by clone B7-B7.
We have defined the epitopes recognized by three influenza A virus-specific, H-2Kd-restricted CD8+ CTL clones, A-12, F-4, and B7-B7, in the present paper. Clones A-12 and B7-B7 recognized the same peptide region, which comprises aa 533 to 541 of A/PR/8 HA. H2-specific clone F-4 and clone B7-B7 both recognized the peptide which comprised aa 529 to 537 of A/Jap HA. We found that aa 533 to 541 of A/PR/8 HA are compatible with aa 529 to 537 of A/Jap HA. Therefore, one compatible region of HA was recognized by HA-specific CTL clones of three different subtype specificities.
Amino acid residues responsible for recognition by CTLs were defined. Clone A-12 recognized the peptides which had S at either position 3 or 7 but not those which have A at position 3 and G at position 7. On the other hand, F-4 recognized peptides which had a G at position 7 but not those which have an S at that position. Thus, the amino acids at positions 3 and 7 were important for recognition by clone A-12, and the amino acid at position 7 was important for recognition by clone F-4. Amino acids at positions 4 and 6 were essential for recognition by clone B7-B7.
The results in the present paper, along with those reported in our previous papers (6, 10), suggest that the virus specificity of T lymphocytes is not determined solely by the degree of amino acid conservation in the epitopes among viruses. It is likely that the degree of T-cell receptor (TCR) stringency on T lymphocytes is also responsible for virus specificity or virus cross-reactivity. It will be interesting to analyze the TCR of these T-cell clones to further determine the interactions between TCR and peptides. The results indicate that recognition of a single peptide by T lymphocytes is heterogeneous and provide an interesting model for understanding T-lymphocyte recognition of epitopes.
REFERENCES
- 1.Kuwano K, Scott M, Young J F, Ennis F A. Active immunization against virus infections due to antigenic drift by induction of crossreactive cytotoxic T lymphocytes. J Exp Med. 1989;169:1361–1371. doi: 10.1084/jem.169.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuwano K, Tamura M, Ennis F A. Cross-reactive protection against influenza A infections by an NS1-specific CTL clone. Virology. 1990;178:174–179. doi: 10.1016/0042-6822(90)90391-4. [DOI] [PubMed] [Google Scholar]
- 3.Kuwano K, Braciale T J, Ennis F A. Cytotoxic T lymphocytes recognize a cross-reactive epitope on the transmembrane region of influenza H1 and H2 hemagglutinins. Viral Immunol. 1989;3:163–173. doi: 10.1089/vim.1989.2.163. [DOI] [PubMed] [Google Scholar]
- 4.Kuwano K, Reyes V E, Humphreys R E, Ennis F A. Recognition of disparate HA and NS1 peptides by an H-2Kd-restricted influenza specific CTL clone. Mol Immunol. 1991;3:163–173. doi: 10.1016/0161-5890(91)90080-4. [DOI] [PubMed] [Google Scholar]
- 5.Linn Y L, Askonas B A. Biological property of influenza A virus-specific killer T cell clone: inhibition of virus replication in vivo and induction of delayed type hypersensitivity reaction. J Exp Med. 1981;154:225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston P G, Kurane I, Lai C-J, Okamoto Y, Men R, Karaki S, Takiguchi M, Ennis F A. Dengue virus-specific, HLA B35-restricted, human CD8+ cytotoxic T lymphocyte (CTL) clones: recognition of NS3 amino acids 500–508 by CTL clones of two different serotype specificities. J Immunol. 1995;154:1287–1295. [PubMed] [Google Scholar]
- 7.Saikh K U, Tamuta M, Kuwano K, Dai L-C, West K, Ennis F A. Protective cross-reactive epitope on the nonstructural protein NS1 of influenza A virus. Viral Immunol. 1993;4:229–236. doi: 10.1089/vim.1993.6.229. [DOI] [PubMed] [Google Scholar]
- 8.Wells M A, Ennis F A, Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol. 1981;126:1042–1046. [PubMed] [Google Scholar]
- 9.Yap K L, Ada G L, McKenzie I F C. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature (London) 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 10.Zeng L, Kurane I, Okamoto Y, Ennis F A, Brinton M A. Identification of amino acids involved in recognition by dengue virus NS3-specific, HLA-DR15-restricted cytotoxic CD4+ T-cell clones. J Virol. 1996;70:3108–3117. doi: 10.1128/jvi.70.5.3108-3117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]