Increased awareness of nontuberculous mycobacterial pulmonary disease (NTM PD) has led to efforts to more fully describe the burden, epidemiological and clinical features, and cost of this condition in different populations globally. Approaches encompass various methodologies including surveillance, population-based studies, analysis of large linked datasets, surveys, laboratory-based convenience samples and combinations of these approaches. Surveillance has been defined as the “systematic collection, consolidation, and evaluation of morbidity and mortality reports, and… regular dissemination to all who need to know” [1]. For NTM PD, notifiable condition reporting varies. In Queensland, Australia, notification of NTM infections is required; reporting is electronic and laboratory-based [2]. In several states within the USA, NTM isolates or cases are listed as notifiable conditions [3, 4], although in Oregon, only extrapulmonary isolates are listed as notifiable [3]. Although reporting might not be complete, and the system may have biases related to healthcare-seeking behaviour and physician awareness, these data are essential to elucidating the epidemiology of NTM PD [3].
Unfortunately, in most of the world, NTM PD is not notifiable and therefore other approaches are needed to describe disease burden, temporal and geographic distribution and associated mortality. In selected countries in Europe, the availability of a centralised public health laboratory has served as a tremendous resource for describing the epidemiology of NTM PD [5–7]. In the UK, five mycobacteriology reference laboratories serve England, Wales and Northern Ireland, all of which report to Public Health England. All culture-positive isolates are reported, along with the patient’s age and sex, specimen source and NTM species. Although detailed clinical information is not available, these data are useful in describing burden and trends. A recent analysis for 2007–2012 identified a doubling of the incidence of pulmonary Mycobacterium avium complex (MAC) among men and women (figure 1) [5], continuing the trend reported previously [6]. In Scotland, although no temporal trend was observed, an increase in M. avium was noted during the period 2000–2010 [7]. Denmark has a national registry maintained at the Statens Serum Institute, holding data from all patients with NTM-positive specimens, which has been used to estimate incidence, prevalence and mortality [8]. A key aspect of the study was the linkage of microbiology information to the Danish National Registry of Patients, which allowed estimation of infection and disease rates by comorbidity status, and of 3- and 5-year cumulative survival by infecting species. The Public Health Ontario Laboratory includes approximately 95% of all mycobacteria tests conducted in the province; these data have been key to describing dramatic increases in both NTM isolation and NTM PD prevalence, with an increase in the 5-year prevalence of disease from 29.3 per 100 000 persons in 1998–2002 to 41.3 per 100 000 persons during 2006–2010 [9]. In North Carolina, USA, a recent study obtained reports on NTM isolates from nearly all laboratories serving three counties during 2006–2010, representing approximately 95% of all isolates in those counties during that time [10]. The reports had information on age, sex, race and site of isolation, and found an overall infection prevalence of 13.6 per 100 000 during that period, with the highest prevalence in the older age groups [10].
FIGURE 1.
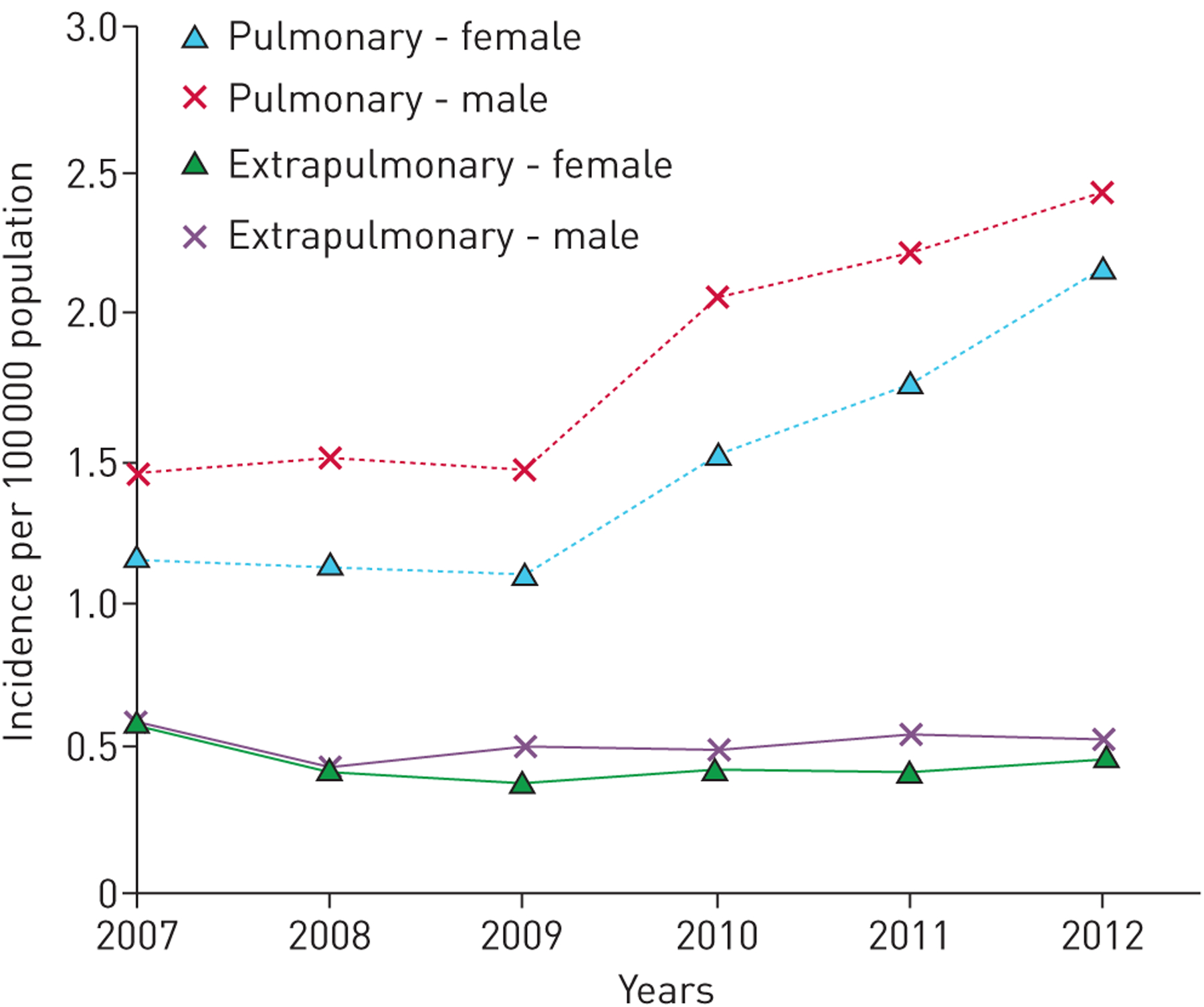
Incidence of Mycobacterium avium-intracellulare isolation (2007–2012). Reproduced with permission from the publisher [5].
A separate laboratory collaboration represents a unique effort to describe NTM species globally. In 2008, global partners in the NTM-Network European Trials Group provided species identification results for all patients from whom NTM was isolated from pulmonary samples. Species identification results were received from 20 182 patients from 62 laboratories in 30 countries [11], representing national or subnational reference laboratories. Based on these data, the predominant species showed marked differences across continents: MAC predominated in Australia and North America (table 1), whereas M. xenopi was quite common in Europe, and was the third most frequently isolated species after MAC and M. gordonae [11] (which is generally regarded as nonpathogenic [12]). Within Europe, M. xenopi was more frequently isolated in Southern than Northern Europe [11].
TABLE 1.
Distribution of respiratory nontuberculous mycobacteria (NTM) isolates.
| Laboratories | Patients with NTM isolated | MAC isolates# | |
|---|---|---|---|
| Europe | 43 | 6803 | 2500 (36.9) |
| North America | 4 | 4913 | 2553 (52.0) |
| South America | 3 | 393 | 123 (31.3) |
| Australia (Queensland) | 1 | 453 | 322 (71.1) |
| Asia | 3 | 1974 | 1062 (53.8) |
| South Africa | 2 | 5646 | 2849 (50.5) |
| Total | 56 | 20182 | 9421 (46.7) |
Data are presented as n or n (%). MAC: Mycobacterium avium complex.
percentage of all NTM. Reproduced with permission [11].
In countries such as the USA where NTM PD is typically not nationally notifiable, approaches to obtain national and regional population-based estimates have included analysis of large linked databases with microbiology data linked to clinical and demographic information [13, 14]. Linkage to discharge diagnosis codes (ICD9 or ICD10) allows estimation of the sensitivity of these diagnostic codes relative to microbiologic definitions [13, 15]. Another approach for national estimates has been to use national healthcare claims datasets for selected populations, and use ICD9 codes to estimate prevalence in each population. For example, the healthcare claims data from the Centers for Medicare and Medicaid Services (CMS) is representative of persons aged ⩾65 years, and analysis of CMS claims data demonstrated an increase in prevalence of NTM PD from 1997 to 2007 [16]. Prevalence estimates have been combined with survey data and adjusted for the known sensitivity of diagnostic codes to provide burden estimates across all age groups in the United States [17]. In Japan, surveys have also been used, with NTM prevalence estimated using the known ratio of tuberculosis cases to NTM cases [18]. A similar approach was used in France with sentinel site surveillance as part of the laboratory network in the French Mycobacteria Study Group [19]. A more recent nationally representative survey of physicians in five European countries (France, Germany, Italy, Spain and the UK) and Japan allowed intercountry comparisons of infecting species and comorbidities, as well as comparisons of diagnostic methods, disease severity and treatment practices [20]. Consistent with prior reports, the European patients with NTM PD (table 2, figure 2) [12] were more likely to be male, current or former smokers and have chronic obstructive pulmonary disease (COPD) (33%), whereas Japanese patients were more likely to be female (65%) and less likely than European patients to have COPD (13%) [20].
TABLE 2.
Predisposing factors for mycobacteriosis
| Condition | Examples |
|---|---|
| Pre-existing lung disease | COPD, asthma, bronchiectasis, lung cancer, smoking, recurrent aspiration with gastro-oesophageal reflux disease, inhaled corticosteroids, pulmonary fibrosis, cystic fibrosis, thoracic deformities such as ankylosing spondylitis, scoliosis, previous tuberculosis, postinfectious residual cavitation, bulla or cyst, pneumoconiosis (silicosis), alveolar proteinosis |
| Systemic immune deficiency | HIV infection (in particular peripheral CD4+ cell count <50 μL−1), status after organ transplantation, malignancies (haematological cancer), alcoholism, cachexia, immunosuppressive therapy (e.g. tumour necrosis factor-alpha inhibitors, corticosteroids), teething (in children with cervical lymphadenitis), age (young children and the elderly), male gender#, diabetes mellitus#, renal failure# |
| Increased genetic susceptibility due to mutations of the following genes | Interferon gamma receptor gene, interleukin-12 receptor gene, signal transducer and activator of transcription 1 (STAT 1) transmembrane conductance regulator gene (cystic fibrosis), alpha 1-antitrypsin gene |
suspected or possible risk factors. Reproduced with permission from the publisher [12].
FIGURE 2.
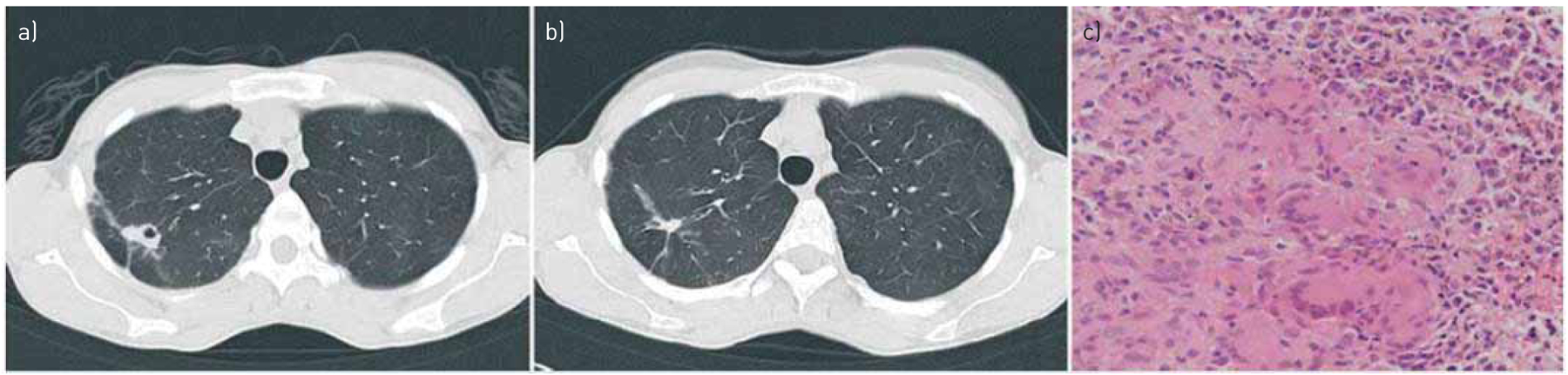
a) Nontuberculous mycobacteria right upper lobe. b) Residual scar left after antibiotic therapy. c) Computed tomography-guided needle biopsy revealed Mycobacterium avium and histologically epithelial granulomas without necrosis. Reproduced with permission from the publisher [12].
In this issue of the European Respiratory Journal, Diel et al. [21] estimate the incidence of NTM PD using administrative healthcare claims data, and provide the first cost estimates for NTM PD in Europe. One prior analysis in Germany using national hospitalisation data found an increasing trend for NTM PD-associated hospitalisations during the period 2005–2011 [22], but no analysis that includes clinical information on outpatients or cost has been conducted in Germany or elsewhere in Europe. In the work by Diel et al. [21], more than 80 German companies provided administrative health insurance claims data from more than 7 million members. The authors define NTM PD cases using ICD10 codes, where a case is defined as any person with one occurrence of the code A31.0 as an inpatient, or two occurrences of the code as an outpatient, during the study period 2010–2011. A novel feature of this work is the ability to demarcate a risk-free period, here defined as four consecutive quarters with no NTM PD code identified.
Because NTM PD is typically a chronic condition, with treatment lasting several years and patients at risk of reinfection or relapse [23], the prevalence in any given year will be higher than the incidence. Having incidence rates as well as estimates of the duration of infection allows more precise estimation of the future burden of NTM PD. The authors found an annual incidence rate of 1.1–1.5 per 100 000 population, similar to the average annual incidence estimated for Denmark of 1 per 100 000 population during 1997–2008 [8] but lower than the available average age-adjusted (to the US population) annual incidence of 3.8 per 100000 from Oregon, USA [24]. However, case definitions in both the Danish and Oregon studies were based on American Thoracic Society microbiologic criteria, which are 50–73% more sensitive than ICD codes [13, 15, 25]. Given these methodological differences, we can say the estimates are similar, but it is difficult to know the actual magnitude of the difference, as adjustment for undercoding in Germany would be likely to yield higher incidence estimates. Further studies with similar methodologies are needed to ascertain actual differences in incidence.
Another unique contribution of this research is the estimation of cost – both direct and indirect – as well as disease-specific incremental costs to allow estimation of attributable costs. Included in the estimation were costs related to inpatient care, outpatient visits, diagnostic tests, prescribed pharmaceuticals and sick pay based on the German Social Code. Indirect costs were estimated using standard methods according to the Hanover Consensus to estimate productivity losses from sick leave. Comprehensive costs from a single dataset have not previously been estimated. Moreover, to obtain attributable cost (that attributed specifically to NTM PD), the authors chose a nested case–control design whereby they matched cases to non-cases from the same claims dataset. Matching was based on age, sex and comorbidities; the latter were quantified using the Charleston Comorbidity Index, which considers the number of comorbid conditions. This measure has been used in other studies of NTM, most notably in a Danish population-based study of NTM PD [8]. In addition, because COPD is such a common comorbidity associated with NTM, cases were also matched with regard to COPD status, and mortality was compared across groups. A striking finding is that the cost of NTM PD was four-fold higher than that for matched controls, with hospitalisation comprising 63% of the total cost. Not surprisingly, pharmaceuticals were expensive: the remaining drug costs were 21.8% of the total direct costs. Overall, the cost exceeded that estimated for COPD in the European Lung White Book [26], emphasising the societal and individual burden of this disease.
Another important and novel contribution of this work is the estimation of mortality associated with NTM PD relative to matched controls: NTM PD patients had a 3.6-fold increased risk of death during the 3-year follow up period compared with controls. The risk of death was highest in the first quarter after diagnosis, suggesting diagnosis later in the course of illness when the disease is often more severe. Of particular interest is that mortality among NTM PD patients with COPD was 41%, compared with 14% among those without NTM PD. Although not directly comparable, all-cause mortality was similar in a population-based study of NTM PD (defined based on microbiologic criteria) in Denmark, with mortality ranging from 39% to 46% among patients with medium or high comorbidity levels [8].
Finally, Diel et al. [21] provide a detailed and comprehensive view of antibiotic use and the variety of antibiotic regimens prescribed. Such information has, to date, been quite limited, not just for Europe but globally, and not available for large, representative populations. An interesting debate is ongoing regarding the use of old and new antibiotics to manage NTM [27, 28]. DIEL et al. [21] found that 54% had begun treatment at the time of diagnosis and 74% received antibiotics at some point during the past 3 years, with 34.5% receiving a standard regimen and 11.8% receiving macrolide monotherapy for at least 6 months. The only other recent data from Europe are from a recently published multicountry survey, which found that only 4.3% of patients in Germany received a recommended regimen (rifamycin, ethambutol and macrolide) for >6 months. The reasons for the discrepancy are not clear and it may be that patients enrolled in a statutory health insurance program are more likely to receive a standard regimen.
Overall, in Europe, information on the burden of NTM is fragmentary, and available information and estimates vary greatly between countries. Moreover, the lack of a standardised methodology limits intercountry comparisons. In addition, data on treatment costs are scarce. This article presents an important addition to the available knowledge about NTM PD by using administrative data linked to clinical data to estimate burden, cost and mortality, adjusted for comorbid conditions. In light of the increasing burden of NTM PD globally, and the high costs associated with the disease, obtaining ongoing estimates of the incidence and prevalence of NTM PD are needed. To this end, initiating NTM infection notification could be considered as part of the public health system and integrated into existing laboratory-based electronic notification systems for tuberculosis, where feasible. These notifications could be supplemented by continued analysis of linked healthcare claims data from different countries to understand the clinical features of NTM PD, including data that had been mostly lacking up to now on the course and outcome of the disease with or without treatment [12], and its cost. Diel et al. [21] have shown us the way forward.
Footnotes
Conflict of interest: None declared.
References
- 1.Langmuir AD. The surveillance of communicable diseases of national importance. N Engl J Med 1963; 268: 182–192. [DOI] [PubMed] [Google Scholar]
- 2.Thomson R, Donnan E, Konstantinos A. Notification of nontuberculous mycobacteria: an Australian perspective. Ann Am Thorac Soc 2017; 14: 318–323. [DOI] [PubMed] [Google Scholar]
- 3.Winthrop KL, Henkle E, Walker A, et al. On the reportability of nontuberculous mycobacterial disease to public health authorities. Ann Am Thorac Soc 2017; 14: 314–317. [DOI] [PubMed] [Google Scholar]
- 4.Donohue MJ, Wymer L. Increasing prevalence rate of nontuberculous mycobacteria infections in five states, 2008–2013. Ann Am Thorac Soc 2016; 13: 2143–2150. [DOI] [PubMed] [Google Scholar]
- 5.Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis 2016; 16: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JE, Kruijshaar ME, Ormerod LP, et al. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC Public Health 2010; 10: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell CD, Claxton P, Doig C, et al. Non-tuberculous mycobacteria: a retrospective review of Scottish isolates from 2000 to 2010. Thorax 2014; 69: 593–595. [DOI] [PubMed] [Google Scholar]
- 8.Andrejak C, Thomsen VO, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010; 181: 514–521. [DOI] [PubMed] [Google Scholar]
- 9.Marras TK, Mendelson D, Marchand-Austin A, et al. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerging Infect Dis 2013; 19: 1889–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GS, Ghio AJ, Stout JE, et al. Epidemiology of nontuberculous mycobacteria isolations among central North Carolina residents, 2006–2010. J Infect 2016; 72: 678–686. [DOI] [PubMed] [Google Scholar]
- 11.Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013; 42: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 12.Schoenfeld N, Haas W, Richter E, et al. Recommendations of the German Central Committee against Tuberculosis (DZK) and the German Respiratory Society (DGP) for the diagnosis and treatment of non-tuberculous mycobacterioses. Pneumologie 2016; 70: 250–276. [DOI] [PubMed] [Google Scholar]
- 13.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adjemian J, Frankland TB, Daida YG, et al. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerging Infect Dis 2017; 23: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis 2013; 72: 37–42. [DOI] [PubMed] [Google Scholar]
- 16.Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strollo SE, Adjemian J, Adjemian MK, et al. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc 2015; 12: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan(1). Emerg Infect Dis 2016; 22: 1116–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maugein J, Dailloux M, Carbonnelle B, et al. Sentinel-site surveillance of Mycobacterium avium complex pulmonary disease. Eur Respir J 2005; 26: 1092–1096. [DOI] [PubMed] [Google Scholar]
- 20.Van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017; 49: 1601855. [DOI] [PubMed] [Google Scholar]
- 21.Diel RL, Niklas L, Loebinger M, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J 2017; 49: 1602109. [DOI] [PubMed] [Google Scholar]
- 22.Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalisations associated with pulmonary non-tuberculous mycobacterial infections in Germany, 2005–2011. BMC Infect Dis 2013; 13: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace RJ Jr, Brown-Elliott BA, McNulty S, et al. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014; 146: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henkle E, Hedberg K, Schafer S, et al. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc 2015; 12: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winthrop KL, Baxter R, Liu L, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf 2011; 20: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B, eds. European Lung White Book. Sheffield, European Respiratory Society, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Pontali E, D’Ambrosio L, Centis R, et al. Multidrug-resistant tuberculosis and beyond: an updated analysis of the current evidence on bedaquiline. Eur Respir J 2017; 49: 1700146. [DOI] [PubMed] [Google Scholar]
- 28.Vesenbeckh S, Schönfeld N, Krieger D, et al. Bedaquiline as a potential agent in the treatment of M. intracellulare and M. avium infections. Eur Respir J 2017; 49: 1601969. [DOI] [PubMed] [Google Scholar]


