Abstract
Blastomycosis is a disease caused by endemic fungi that ranges from severe pulmonary or disseminated to mild or asymptomatic. Environmental factors associated with it are not well described throughout the endemic area. We used the intramural State Inpatient Database from the Agency for Healthcare Research and Quality and ArcMap GIS to identify geographic high-risk clusters of blastomycosis hospitalizations in 13 states in the US endemic regions (AR, IA, IL, IN, KY, LA, MI, MN, MO, MS, OH, TN, and WI). We then used logistic regression to identify risk factors associated with these high-risk clusters. We describe six clusters of counties in which there was an elevated incidence of blastomycosis hospitalizations. We identified maximum mean annual temperature, percentage of persons aged ≥65 years, and mercury and copper soil content as being associated with high-risk clusters. Specifically, the odds of a county being part of a high-risk cluster was associated with increasing percentage of population over age 65, decreasing maximum temperature, increasing mercury, and decreasing copper soil content. Healthcare providers should be aware of these high-risk areas so that blastomycosis can be included, as appropriate, in a differential diagnosis for patients currently or previously residing in these areas.
Keywords: epidemiology, blastomycosis, Blastomyces dermatitidis, Blastomyces gilchristii
Introduction
The dimorphic fungi Blastomyces dermatitidis and the newly described Blastomyces gilchristii [1] are capable of causing infection in humans, dogs, and other mammals known as blastomycosis. The primary route of infection is through inhaling fungal conidia into the lungs [2], with the severity in humans varying greatly and which may contribute to death of the affected patient. As described in outbreak situations, as many as 50% of cases are asymptomatic, although infection can also appear as pulmonary, extrapulmonary, or severe disseminated form. An estimated 25–40% of symptomatic infections manifest as severe disseminated disease [2]. Cellular immunity may provide protection against subsequent infections [3].
The epidemiology of blastomycosis is poorly under-stood as the organism is difficult to recover from the environment [4] and a sensitive and specific skin test does not exist [5]. Reporting of the disease is currently mandatory in only a few US states (AR, LA, MI, MN, MS, and WI), and many estimates are limited to a single state or local area making comparisons difficult. Estimates of blastomycosis incidence in endemic and hyper-endemic areas, which include the regions surrounding the Mississippi and Ohio River Valleys, Great Lakes and southern parts of Canada [6–8], have been described as ranging from 0.5/100,000 to 100/100,000 persons [4,9–14]. State-specific hospitalization incidence reaching 2.9 hospitalizations per 100,000 person-years has been described [15]. Obtaining the etiologic agent in culture or observation of the organism in clinical specimens is recommended for diagnosis in order to avoid misdiagnosis and errors due to cross-reactivity with histoplasmosis as exists with other assays [3].
Much of the current knowledge regarding the association between environmental and climate variables and blastomycosis is from descriptions made during outbreak investigations. Multiple factors identified during these investigations have been implicated as factors for enhanced organism growth, as well as increased disease risk but questions remain. The fungus is presumed to exist in proximity to waterways [4,7,10], specific soil types [10,16] and has been isolated from areas high in decaying organic matter [17] such as a woodpile [18], decaying wood structures [19,20] and a beaver lodge [21]. The presence of conifers has been identified in areas of high-endemicity suggesting either that growth of the organism is encouraged under similar conditions as conifers or that the soil conditions created by conifer growth and decay is beneficial to organism [22]. However, even though Baumgardner et al. identified proximity to waterways as a risk factor in northern Wisconsin [10], they saw a different pattern in Milwaukee County [16]. While proximity to waterways in this county was not a significant factor, the incidence of infection differed among diverse watershed areas [16]. Other studies describe outbreaks where the infection was presumed to be from dust generated from construction sites [23,24] or other types of outdoor work areas [25,26]. Further, studies describing environmental variables associated with the growth of either species or blastomycosis infections are often limited to a few states [4,7,10,16,27] and multiple states within the endemic area are not represented in the literature. In contrast, environmental factors associated with coccidioidomycosis growth and risk have been well described [28,29]. Because of the large endemic area for blastomycosis and potential for variation in environmental factors, we used a single, multi-state dataset with standard methodology throughout the endemic area to provide new insights into the epidemiology of blastomycosis.
Methods
We used the intramural State Inpatient Database (SID) from the US Agency for Healthcare Research and Quality (AHRQ), through an established collaborative effort. The AHRQ SID are produced as part of the Healthcare Cost and Utilization Project (HCUP) and include all inpatient encounters at community hospitals within participating states [30]. Community hospitals include nonfederal, short-term general, academic, and teaching hospitals [31]. According to the American Hospital Association, community hospitals make up 87% of all US registered hospitals and 95% of total US admissions [31].
Hospitalization records were identified and extracted from the AHRQ SID database using the International Classification of Diseases, 9th Revision, Clinical Modification codes (ICD-9-CM). A blastomycosis-associated hospitalization was defined as a hospital admission with a primary or secondary diagnosis of blastomycosis (ICD-9-CM: 116.0 and 116). Patient county of residence was extracted for each hospitalization record.
We included thirteen states within the US endemic area (AR, IA, IL, IN, KY, LA, MI, MN, MO, MS, OH, TN, and WI), which collectively have 1,174 counties. We included data from 2007 to 2011 for all thirteen states with the exception of Louisiana (2008–2011) and Mississippi (2010–2011), which had fewer years of data available. We calculated the average-annual county-specific blastomycosis-associated hospitalization incidence within these states by dividing the average annual number of blastomycosis-associated hospitalizations by the denominator population. The 2010 county-specific census data was used to approximate the total person-years contributed by each county in 2010.
We used ArcMap GIS 10.2.1 (Environmental Systems Research Institutes, Inc. Redlands CA) to calculate the Getis-Ord-Gi* (Gi*) statistic. The Gi* statistic provides the statistical probability of clusters of counties having a higher (hot spot) or lower (cold spot) hospitalization incidence than would be expected by random chance. We accounted for differences in county populations by using hospitalization incidence in each county instead of count of incident hospitalizations. The Gi* statistic is calculated as a Z-score and uses weighted data points from neighboring counties to identify clusters [32]. Weights were determined using Euclidean distance, with significant values (p < .05) indicate that a county is part of a low-risk or high-risk cluster. Shapefiles containing geographic information such as state and county boundaries were obtained from the US Census Topographically Integrated Geographically Encoding and Referencing (TIGER) products website [33]. Spatial data were projected using the North American Datum (NAD) Universal Transverse Mercator (UTM) Zone 16N projection.
We collected county-level environmental and demographic variables for modeling factors associated with high-risk and low-risk clusters. We evaluated environmental and geographic variables from the United States Forest Service (2001–2006 averaged data) [34], US Census Bureau (2010 data) [33], US Geological Survey (2008 data) [35], and population and socioeconomic factors from the US Census Bureau. Environmental variables included elevation, soil minerals, percentage of surface water area, temperature (maximum and minimum daily temperature, averaged over the years of data available), ratio of land area to water surface area and precipitation. Population and socioeconomic variables included age distributions, percentage of population with health insurance, population education level, and population density.
We described counties within significant clusters using the extracted county-level variables. Next, we used logistic regression to identify the factors associated with counties in significant clusters. Univariate logistic regression models were constructed to identify significant variables (p <. 05). All significant factors were then assessed for multicollinearity and included in multivariable models built by backward selection if not highly correlated. We assessed confounding where appropriate. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC).
This study was considered not to involve human subject by the National Institutes of Health Office of Human Subjects Research.
Results
There were 81 million inhabitants in 2010 in the thirteen states included in this analysis, that is 27% of the total US population. We identified 3,644 hospitalizations in the 13 states and 1,174 counties included in this investigation, with an average annual count of 746 hospitalizations. The overall average annual blastomycosis-associated hospitalization incidence was 0.911/100,000 person-years, but the county-specific incidence ranged from no blastomycosis hospitalizations to 37.8/100,000 person-years. We are unable to present a map with county-specific incidence because of low hospitalization counts in some counties, making data potentially identifiable.
Using the Gi* statistic, we found 96 counties that were part of six high-risk clusters, one of which was centered in northern Wisconsin and included counties in Michigan and Minnesota. A second cluster was in an area at the intersection of multiple states (Illinois, Kentucky, Missouri, Indiana) and a third, smaller, high-risk cluster was in Arkansas and Louisiana. Three single county, high-risk areas were in Illinois, Kentucky, and Minnesota (Fig. 1). Overall, 3.7% of our total study population was in part of a high-risk cluster. The cluster centered in northern Wisconsin included one county in Minnesota, five counties in Michigan and 29 counties in Wisconsin with a total population of 1,433,787 persons (Table 1). The cluster at the intersection of multiple states included 52 total counties, that is, 21 counties in Illinois, 23 counties in Kentucky, four counties in Missouri, and four counties in Indiana with a combined population of 1,477,493 persons. The third, smaller, high-risk area included five counties in Arkansas and one in Louisiana with a total population of 107,118 persons. Three high-risk areas, each consisting of one county, had population sizes ranging from 13,311 to 17,056 persons. Hospitalization incidence in these clusters ranged from zero to 7.4 hospitalizations per 100,000 person-years. The significant cluster with no hospitalizations (cluster 5) is a result of using distance-weighted hospitalization incidence from neighboring counties to calculate the Gi* statistic for each county feature. We found areas of lower risk but these areas were not statistically significant at p < .05.
Figure 1.
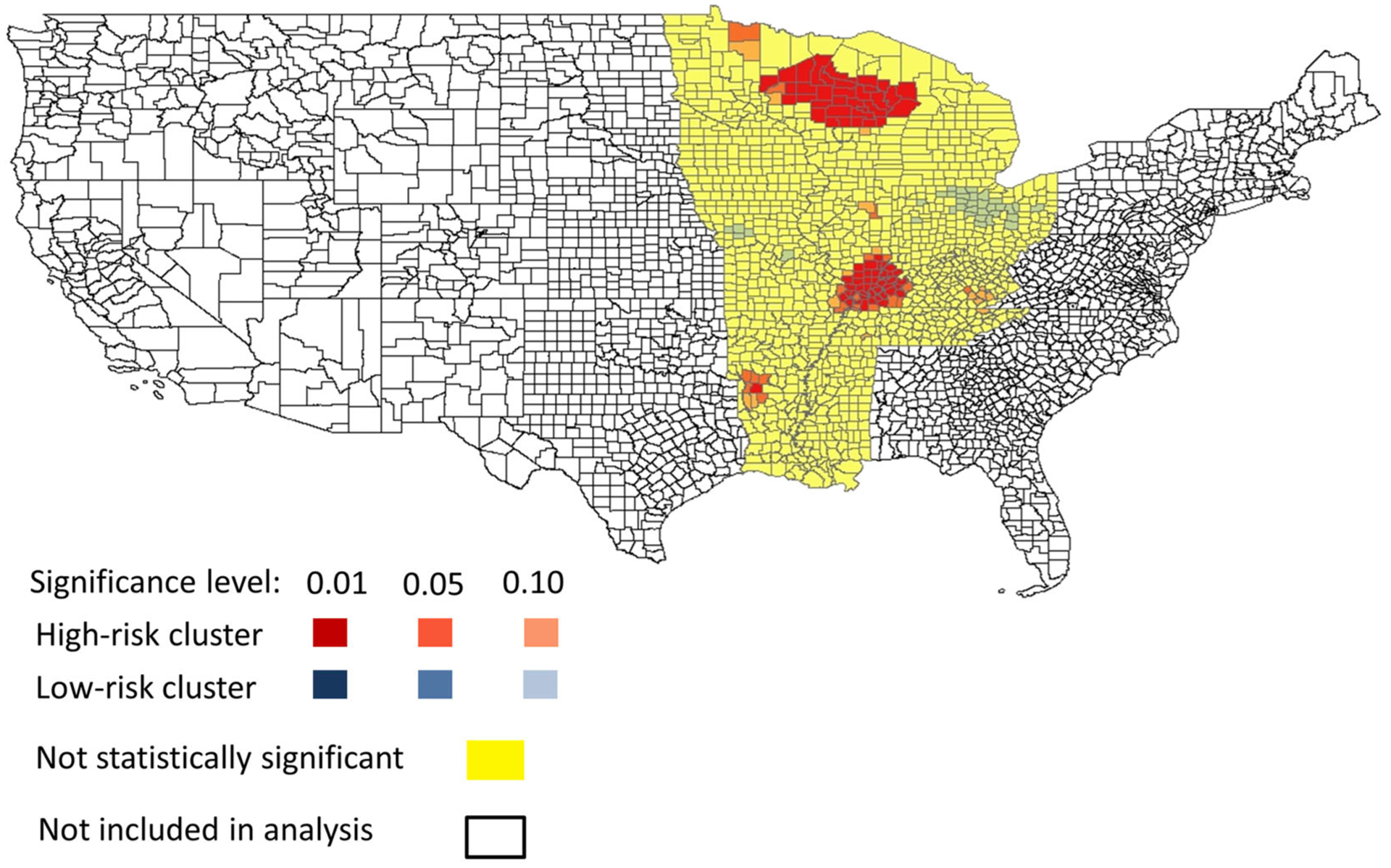
Map of blastomycosis-associated hospitalization clusters calculated by Getis-Ord-Gi* statistic in United States endemic area.
Table 1.
Description of significant blastomycosis-associated hospitalization incidence high-risk clusters in United States endemic area identified by Getis-Ord-Gi* statistic.
| Cluster | States | Number of counties | Total population | Percent of study population | Average annual hospitalization incidence per 100,000 person-years |
|---|---|---|---|---|---|
| 1 | Wisconsin, Minnesota and Michigan | 35 | 1,433,787 | 1.75 | 7.41 |
| 2 | Minnesota | 1 | 13,311 | 0.02 | 6.01 |
| 3 | Illinois | 1 | 14,081 | 0.02 | 1.42 |
| 4 | Illinois, Kentucky, Missouri, Indiana | 52 | 1,477,493 | 1.80 | 2.92 |
| 5 | Kentucky | 1 | 17,056 | 0.02 | 0 |
| 6 | Arkansas, Louisiana | 6 | 107,118 | 0.13 | 5.88 |
| Not part of a cluster | 13 states | 1078 | 78,799,984 | 96.26 | 0.75 |
| Total | 13 states | 1174 | 81,862,830 | 100 | 0.911 |
Minimum and maximum mean annual temperatures, percentage of population aged ≥65 years, population density, and specific soil minerals (mercury, zinc, and copper in parts per million (ppm)) were found to be significant in univariate logistic regression models (Table 2). Minimum and maximum mean annual temperatures were highly correlated, so we did not include minimum mean annual temperature in subsequent models. In contrast, we did not note that precipitation, elevation, population density, percentage of surface water area, or ratio of land area to water surface area were significantly associated with high-risk clusters in univariate analysis. Percentage of population with at least a bachelor’s degree and percentage of population with health insurance were assessed as potential confounding variables but were not found to be associated with high-risk cluster counties.
Table 2.
Environmental and demographic variables by blastomycosis-associated hospitalization incidence high-risk cluster in the United States endemic area.
| Cluster | Percentage of population ≥65 years | Median population per square mile | Mean minimum temperature (Celsius) | Mean maximum temperature (Celsius) | Mean mercury (ppm) | Mean zinc (ppm) | Mean copper (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | 17.0 | 24.3 | 0.19 | 11.78 | 0.04 | 52.4 | 13.5 |
| 2 | 18.1 | 4.6 | −2.29 | 10.20 | 0.03 | 53.4 | 13.2 |
| 3 | 19.4 | 29.3 | 4.92 | 16.58 | 0.02 | 83.0 | 17.1 |
| 4 | 16.1 | 41.8 | 8.31 | 20.04 | 0.10 | 51.3 | 11.5 |
| 5 | 13.3 | 52.1 | 7.5 | 19.14 | 0.02 | 41.8 | 6.2 |
| 6 | 16.4 | 27.4 | 10.96 | 23.69 | 0.025 | 18.6 | 5.5 |
| Not a cluster | 14.6 | 52.1 | 6.39 | 18.13 | 0.04 | 59.8 | 13.8 |
Note: ppm is defined as parts per million.
Variables retained in multivariable models after backward selection modeling techniques included maximum temperature, percentage of population ≥65 years and two soil minerals (mercury, and copper) (Table 3). For every 1 degree Celsius increase in maximum mean annual temperature, the odds of a county being in a high-risk cluster decreased by 7%. For every 1% increase in total population over 65 years, the odds of a county being in a high-risk cluster increased by 16%. For every one-hundredth of a part per million increase in mercury soil content, the odds of a county being in a high-risk cluster increased by 4%. For every one part per million increase in copper soil content, the odds of a county being in a high-risk cluster decreased by 6%.
Table 3.
Results of multivariable logistic regression model used to describe environmental and demographic factors associated with high-risk clusters of blastomycosis hospitalization incidence in the United States endemic area.
| Variables | Odds ratio (95%CI) | P-value |
|---|---|---|
| Maximum mean annual temperature, per 1 degree Celsius | 0.93 (0.87, 0.98) | 0.02 |
| Percentage of population 65 years and older, per 1% | 1.16 (1.09, 1.24) | <.0001 |
| Soil content: mercury per .01 ppm | 1.04(1.01, 1.07) | 0.008 |
| Soil content: copper, per 1 ppm | 0.94 (0.90, 0.97) | 0.001 |
Note: ppm is defined as parts per million.
Discussion
We identified areas within the recognized blastomycosis endemic region where the hospitalization incidence was significantly higher than expected. Nearly 4% of our study population resided in areas identified as being high risk for increased hospitalizations due to this infection. Of note, is the fact that three of these clusters (clusters 3, 4, and 5) occurred in states that do not currently have mandatory reporting. While we did not observe any significant areas of low risk, we did find significant factors that were predictive of counties in which the populace were at high risk for blastomycosis hospitalizations. Variables retained in the multivariable logistic regression model and identified as predictive of high-risk counties were maximum temperature, mercury and copper soil content, and percentage of population aged ≥65 years. A description of blastomycosis hospitalizations throughout the United States using the AHRQ SID database was recently published [15], and the present report we believe adds to the literature by describing detailed information on high-risk areas within the US endemic area and describing factors associated with clustering of cases within counties.
We found an inverse relationship between maximum temperature and being part of a high risk cluster. A lower percentage of counties with higher maximum temperature were part of a high-risk cluster. Our use of the annual mean maximum temperature might be most closely related to latitude rather than climate since it does not allow for variations among seasons or other variability within the year. While seasonality may play a role in organism growth and frequency of infection, seasonal patterns have not been consistently assessed [11,22,27,36]. Assessment of seasonality as a risk factor is complicated by delays in diagnosis [2,37], as well as an incubation period of around 30–45 days or longer [2,17,21]. Although we identified high-risk clusters throughout the endemic region, the two largest clusters were in the northern half of this area, which could contribute to the association with latitude. A large outbreak in 2010 [38] might have contributed to the high-risk cluster centered in Wisconsin. Another large outbreak linked to highway construction occurred in Indianapolis from 2005 to 2008 [26]. However, the other large, high-risk cluster identified in our analysis was located to the southwest of Indianapolis, and we were unable to find a reported outbreak which might have contributed to this cluster.
We identified a relationship between age structure of a county’s population and being part of a high-risk cluster, that is, counties with a higher proportion of persons ≥65 years were more likely to be part of a high-risk cluster. This may be related to our use of blastomycosis hospitalizations for this analysis as age is not thought to affect the susceptibility to this infection. Differences in age groups have been observed, specifically higher rates among middle-aged men [7], but these are likely a reflection of the population most often exposed to the organism [39]. The association with older populations that we identified may be due to older individuals being at increased risk for either more severe disease or more complicated infections due to the presence of other health conditions, resulting in more hospitalizations. Higher mortality rates due to blastomycosis have been observed in persons aged ≥65 years [37].
To our knowledge, the association between mercury and copper soil content and organism growth has not been previously reported. However, in a report from 1905, copper salt is described as potential treatment for reducing blastomycosis lesions [40], suggesting that copper salt may play a role in inhibiting organism growth. Also, previous studies have suggested that some inorganic compounds and chemicals may be related to organism growth [41]. Previous studies have also identified slightly acidic soil in areas where the organism was isolated [17,18] and soil pH is known to affect mineral retention [42]. Additionally, presence of conifers has been identified in areas of high endemicity, suggesting that the preference for slightly acidic or other soil composition is shared or that organism growth is favored in areas where specific conditions created by conifer growth and decay exist [22]. Further, mercury is known to be adsorbed by clay minerals, oxides and organic matter [42] and the etiologic agent of blastomycosis is thought to exist in soils high in decaying organic matter [8].
Percent of population with at least a bachelor’s degree or with health insurance were not found to be associated with a county being part of a high-risk cluster. This suggests that these are not likely to be confounders and our results are not likely to be biased by regions with varying education or health insurance coverage. Furthermore, these factors are not likely to be confounders in the association between percentage ≥65 years and odds of a county being part of a high risk cluster. However, we did not control for the potential of confounding from co-morbid conditions in the association between percentage of population ≥65 years and odds of a county being part of a high risk cluster. Population density, precipitation, elevation, percentage of surface water area, or ratio of land area to water surface area were also not significantly associated with high-risk clusters in univariate analysis. Although blastomycosis outbreaks have been frequently described in groups of people engaging in outdoor recreations activities such as hiking or boating [21,43], outbreaks of blastomycosis have also been observed in nonrural and urban areas [7,44]. Consistent with this, the high-risk areas that we identified were not significantly associated with population density. We were not able to assess distance to waterways since we did not have specific residential locations of patients and this analysis was conducted at the county level. However, water related variables that we assessed were not significantly associated with odds of the county being part of a high-risk cluster.
Our results highlight specific geographic regions within the endemic area where healthcare clinicians should be especially aware of the disease and to include it on their list of differential diagnoses for a patient with compatible symptoms. Additionally, all healthcare clinicians should be aware of these high-risk areas for the potential risk of disease among their patients who have previously resided in these areas. This analysis only included the endemic area and the term high-risk is describing counties with higher than expected blastomycosis hospitalization incidence as compared to the remainder of the endemic area. By limiting our analysis to a recognized endemic area, it permitted us the ability to focus on specific risk factors at a finer scale than would have otherwise been possible had we evaluated the entire United States for this rare disease. Our results indicate the importance of increased awareness among patients and their healthcare clinicians, especially in these areas. Although the majority of the study area was not identified as part of a high-risk cluster, healthcare clinicians should still be aware of the disease throughout the endemic area as our term “high-risk” is relative to the entire endemic area and it may be appropriate to consider these areas as “highest-risk”.
Several limitations should be considered regarding the interpretation of these results. One limitation is the use of hospitalization record level data. Because a single patient could be represented multiple times for the same illness due to readmissions or transfers, we may be overestimating the incidence of blastomycosis-associated hospitalizations from individual patients who are hospitalized multiple times for blastomycosis. Second, hospitalization data represent only patients with severe infection or with other conditions severe enough to warrant hospitalization and not all blastomycosis cases are hospitalized. Percentage of blastomycosis cases that are hospitalized have been described as ranging from 59% to 70% [7,14,38,45]. The percentage of cases hospitalized may vary between regions with and without mandatory reporting; increased awareness of the disease may result in earlier diagnosis, thereby avoiding progression to severe disease. Although our estimates should not be used to estimate blastomycosis case incidence, we feel that the relative patterns of hospitalization incidence are valuable for describing the epidemiology of the disease and its impact on populations. Defining areas of high-risk or higher than expected hospitalization incidence and comparing relative differences between high-risk and non-high-risk counties provides novel epidemiologic information for blastomycosis. However, it is important to note that hospitalized blastomycosis cases may not experience the same risk factors as nonhospitalized blastomycosis cases, possibly limiting the detection of some important exposures not experienced by persons who are ultimately hospitalized with blastomycosis. An additional limitation when using hospitalization data and ICD-9-CM coding is the potential for misclassification, especially with histoplasmosis, a fungal disease with overlapping endemic area. Specifically, we are unable to determine the diagnostic method used and the degree of misdiagnosis may differ by region, depending on awareness and frequency of histoplasmosis infections.
A further limitation is related to the size of the geographic units and temporal periods used for the analysis. We conducted our analysis using annual county-level data. We were limited to this level of aggregated data because of privacy issues and the rare nature of the disease. However, many of the social and environmental variables that we assessed may vary greatly geographically within counties and temporally within a single year. Additionally, the organism is thought to be transient in the environment [8,27] and measuring environmental variables annually may not reflect the specific conditions present during organism growth. The association between coccidioidomycosis cases and seasonal wet and dry cycles in the United States has been well described [28,29]. Further investigation into similar factors and their potential associations with blastomycosis cases is warranted. Because we are using summary variables in which large variations in smaller geographic areas or temporal variations could be attenuated through the process of averaging across larger geographic regions and time periods, the significant associations that we identified are likely to be important factors for increased blastomycosis hospitalizations.
Despite the limitations with using hospitalization data, the AHRQ SID data include nearly all hospitalizations from community hospitals in participating states, and community hospitals account for 87% of all US registered hospitals and 95% of total US hospital admissions [31] and provide a comprehensive and consistent data collection methodology throughout the states in the endemic area, making this dataset uniquely valuable for studying rare disease such as this. Additionally, we applied the same analytic methodology to all states in our analysis, allowing for an epidemiologic description of a disease throughout the endemic area.
In this study, we describe six areas with the highest risk of increased blastomycosis-associated hospitalizations in the US endemic area. Our analysis was limited to the endemic area for blastomycosis and areas that we identified as high risk should be considered as the highest-risk areas within the high-risk endemic area. Knowledge about these areas of highest risk is important so that blastomycosis may be higher on a healthcare provider’s list of differential diagnoses for a patient currently or previously residing in these areas. This could reduce delays in diagnosis and result in improved outcomes for patients.
Acknowledgments
We would like to thank the state data organizations that contribute data to the Healthcare Cost and Utilization Project, without whom this study would not be possible. The authors would also like to thank Sean Cleary for his careful review and suggestions. This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One 2013. March; 8: e59237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman SW, Dismukes WE, Proia LA et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008; 46: 1801–1812. [DOI] [PubMed] [Google Scholar]
- 3.Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infec Dis Clin N Am 2003; 17: 21–40. [DOI] [PubMed] [Google Scholar]
- 4.Reed KD, Meece JK, Archer JR, Peterson AT. Ecologic niche modeling of Blastomyces dermatitidis in Wisconsin. PLoS One 2008; 3: e2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies SF, Sarosi GA. Epidemiological and clinical features of pulmonary blastomycosis. Semin Respir Infect 1997; 12: 206–218. [PubMed] [Google Scholar]
- 6.Watts B, Argekar P, Saint S, Kauffman CA. Building a diagnosis from the ground up. New Engl J Med 2007; 356: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 7.Pfister JR, Archer JR, Hersil S et al. Non-rural point source blastomycosis outbreak near a yard waste collection site. Clin Med and Res 2011; 9: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann JA, Kostiuk SL, Dworkin MS, Johnson YJ. Temporal and spatial distribution of blastomycosis cases among humans and dogs in Illinois (2001–2007). J Am Vet Med Assoc 2011; 239: 335–343. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Blastomycosis–Wisconsin, 1986–1995. MMWR 1996; 45: 601–603.8676851 [Google Scholar]
- 10.Baumgardner DJ, Steber D, Glazer R et al. Geographic information system analysis of blastomycosis in northern Wisconsin, USA: waterways and soil. Med Mycol 2005; 43: 117–125. [DOI] [PubMed] [Google Scholar]
- 11.Chapman SW, Lin AC, Hendricks KA et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Resp Infect 1997; 12: 219–228. [PubMed] [Google Scholar]
- 12.Furcolow ML, Busey JF, Menges RW, Chick EW. Prevalence and incidence studies of human and canine blastomycosis. Am J Epidemiol 1970; 92: 121–131. [DOI] [PubMed] [Google Scholar]
- 13.Klein BS, Davis JP. A laboratory-based surveillance of human blastomycosis in Wisconsin between 1973 and 1982. Am J Epidemiol 1985; 122: 897–903. [DOI] [PubMed] [Google Scholar]
- 14.Cano MV, Ponce-De-Leon GF, Tippen S, Lindsley MD, Warwick M, Hajjeh RA. Blastomycosis in Missouri: epidemiology and risk factors for endemic disease. Epidemiol Infect 2003; 131: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seitz AE, Younes N, Steiner CA, Prevots DR. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PLoS One 2014; 9: e105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgardner DJ, Knavel EM, Steber D, Swain GR. Geographic distribution of human blastomycosis cases in Milwaukee, Wisconsin, USA: association with urban watershed. Mycopathologia 2006; 161: 275–282. [DOI] [PubMed] [Google Scholar]
- 17.Klein BS, Vergeront JM, Disalvo AF, Kaufman L, Davis JP. Two outbreaks of blastomycosis along rivers in Wisconsin. Isolation of Blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am Rev Respir Dis 1987; 136: 1333–1338. [DOI] [PubMed] [Google Scholar]
- 18.Baumgardner DJ, Paretsky DP. The in vitro isolation of Blastomyces dermatitidis from a woodpile in north central Wisconsin, USA. Med Mycol 1999; 37: 163–168. [PubMed] [Google Scholar]
- 19.Denton JF, Disalvo AF. Isolation of Blastomyces dermatitidis from natural sites at Agusta, Georgia. Am J Trop Med Hyg 1964; 13: 716–722. [DOI] [PubMed] [Google Scholar]
- 20.Denton JF, Disalvo AF. Additional isolations of Blastomyces dermatitidis from natural sites. Am J Trop Med Hyg 1979; 28: 697–700. [PubMed] [Google Scholar]
- 21.Klein BS, Vergeront JM, Weeks RJ et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med 1986; 314: 529–534. [DOI] [PubMed] [Google Scholar]
- 22.Baumgardner DJ, Buggy BP, Mattson BJ, Burdick JS, Ludwig D. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis 1992; 15: 629–635. [DOI] [PubMed] [Google Scholar]
- 23.Kitchen MS, Reiber CD, Eastin GB. An urban epidemic of North American blastomycosis. Am Rev Respir Dis 1977; 115: 1063–1066. [DOI] [PubMed] [Google Scholar]
- 24.Baumgardner DJ, Burdick JS. An outbreak of human and canine blastomycosis. Rev Infect Dis 1991; 13: 898–905. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Blastomycosis acquired occupationally during prairie dog relocation- Colorado, 1998. MMWR 1999; 48: 98–100. [PubMed] [Google Scholar]
- 26.Carlos WG, Rose AS, Wheat LJ et al. Blastomycosis in Indiana: digging up more cases. Chest 2010; 138: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 27.Baumgardner DJ, Paretsky DP, Baeseman ZJ, Schreiber A. Effects of season and weather on blastomycosis in dogs: northern Wisconsin, USA. Med Mycol 2011; 49: 49–55. [DOI] [PubMed] [Google Scholar]
- 28.Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Persp 2005; 113: 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comrie AC, Glueck MF. Assessment of climate-coccidioidomycosis model. Model sensitivity for assessing climatologic effects on the risk of acquiring coccidioidomycosis. Ann NY Acad Sci 2007; 1111: 83–95. [DOI] [PubMed] [Google Scholar]
- 30.HCUP SID Database Documentation. Healthcare Cost and Utilization Project (HCUP). August 2014. US Agency for Healthcare Research and Quality, Rockville, Maryland. www.hcup-us.ahrq.gov/db/state/siddbdocumentation.jsp. [Google Scholar]
- 31.American Hospital Association Resource Center. Fast Facts on US Hospitals. Chicago, IL; 2012. www.aha.org/research/rc/stat-studies/fast-facts.shtml. [Google Scholar]
- 32.ESRI. Hot Spot Analysis (Getis-Ord Gi*) (Spatial Statistics). ArcGIS Resource Center; 2012. Available from: http://resources.arcgis.com/en/help/main/10.2/index.html#//005p00000010000-00010. [Google Scholar]
- 33.US Census Bureau. Geography: maps and data. [cited 2014] Available from: http://www.census.gov/geo/maps-data/.
- 34.US Forest Service. Publications and data. [cited 2013]; Available from: http://nrs.fs.fed.us/data/urban/.
- 35.US Geologic Survey. Mineral resources on-line spatial data. [cited 2013]; Available from: http://tin.er.usgs.gov/geochem/doc/averages/countydata.htm.
- 36.Lowry PW, Kelso KY, McFarland LM. Blastomycosis in Washington Parish, Louisiana, 1976–1985. Am J Epidemiol 1989; 130: 151–159. [DOI] [PubMed] [Google Scholar]
- 37.Dworkin MS, Duckro AN, Proia L, Semel JD, Huhn G. The Epidemiology of blastomycosis in Illinois and factors associated with death. Clin Infect Dis 2005; 41: e107–111. [DOI] [PubMed] [Google Scholar]
- 38.Roy M, Benedict K, Deak E et al. A large community outbreak of blastomycosis in Wisconsin with geographic and ethnic clustering. Clin Infect Dis 2013; 57: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev 2010; 23: 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bevan A Treatment of actinomycosis and blastomycosis with copper salts. JAMA. 1905; 45: 1492–1493. [Google Scholar]
- 41.Baumgardner DJ. Microecology of Blastomyces dermatitidis: the ammonia hypothesis. Med Mycol 2009; 47: 745–752. [DOI] [PubMed] [Google Scholar]
- 42.McLean J, Bledsoe B. US Environmental Protection Agency. ed. Issues Papers. Ground water issue: behavior of metals in soils; 1992. EPA/540/S-92/01. 1992. 1–25. Available from: http://www.epa.gov/superfund/remedytech/tsp/download/issue14.pdf. [Google Scholar]
- 43.Cockerill FR, Roberts GD, Rosenblatt JE, Utz JP, Utz DC. Epidemic of pulmonary blastomycosis (Namekagon fever) in Wisconsin canoeists. Chest 1984; 86: 688–692. [DOI] [PubMed] [Google Scholar]
- 44.Lemke MA, Baumgardner DJ, Brummitt CF et al. Blastomycosis in urban southeastern Wisconsin. Wisc Med J 2009; 108: 407–410. [PubMed] [Google Scholar]
- 45.Meece JK, Anderson JL, Gruszka S, Sloss BL, Sullivan B, Reed KD. Variation in clinical phenotype of human infection among genetic groups of Blastomyces dermatitidis. J Infect Dis 2013; 207: 814–822. [DOI] [PubMed] [Google Scholar]


