Abstract
Esophageal cancer ranks among the most prevalent malignant tumors globally, primarily due to its highly aggressive nature and poor survival rates. According to the 2020 global cancer statistics, there were approximately 604000 new cases of esophageal cancer, resulting in 544000 deaths. The 5-year survival rate hovers around a mere 15%-25%. Notably, distinct variations exist in the risk factors associated with the two primary histological types, influencing their worldwide incidence and distribution. Squamous cell carcinoma displays a high incidence in specific regions, such as certain areas in China, where it meets the cost-effectiveness criteria for widespread endoscopy-based early diagnosis within the local population. Conversely, adenocarcinoma (EAC) represents the most common histological subtype of esophageal cancer in Europe and the United States. The role of early diagnosis in cases of EAC originating from Barrett's esophagus (BE) remains a subject of controversy. The effectiveness of early detection for EAC, particularly those arising from BE, continues to be a debated topic. The variations in how early-stage esophageal carcinoma is treated in different regions are largely due to the differing rates of early-stage cancer diagnoses. In areas with higher incidences, such as China and Japan, early diagnosis is more common, which has led to the advancement of endoscopic methods as definitive treatments. These techniques have demonstrated remarkable efficacy with minimal complications while preserving esophageal functionality. Early screening, prompt diagnosis, and timely treatment are key strategies that can significantly lower both the occurrence and death rates associated with esophageal cancer.
Keywords: Esophageal cancer, Screening, Early detection, Treatment, Endoscopic mucosal resection, Endoscopic submucosal dissection
Core Tip: Esophageal cancer, characterized by its aggressive nature and low survival rates, is one of the most common malignant tumors globally. Variations in risk factors between the two primary histological types, squamous cell carcinoma (ESCC) and adenocarcinoma (EAC), affect their incidence and distribution worldwide. While ESCC is prevalent in specific areas like China and meets criteria for cost-effective early diagnosis, EAC is predominant in Europe and the United States, with the role of early diagnosis in cases arising from Barrett's esophagus being a subject of controversy. The disparities in early-stage treatment across different regions are due to varying rates of early diagnosis, with countries like China and Japan advancing in endoscopic techniques for treatment. Early screening, diagnosis, and treatment significantly reduce the incidence and mortality of esophageal cancer.
INTRODUCTION
Esophageal carcinoma (EC), which ranks as the seventh most common cancer worldwide, was responsible for an estimated 604000 new cases in 2020, placing it in the sixth position in terms of global cancer mortality with approximately 544000 deaths[1]. These data underscore the critical impact of esophageal cancer, as it contributes to a substantial proportion of cancer-related deaths-approximately one in every 18. Notably, this malignancy disproportionately affects nearly 70% of male population. Additionally, there is a marked sex disparity in both the incidence and mortality rates of EC, with 2- to 3-fold greater prevalence and fatality rates observed in males than females[2,3]. EC incidence shows pronounced geographical disparities, with Eastern Asia emerging as the epicenter of the highest incidence for both sexes. This regional specificity is largely attributed to China, which bears a considerable burden of this disease. Remarkably, the incidence of EC in China is exceptionally high. In a global context, new cases and fatalities from China account for a staggering 53.70% and 55.35%, respectively, of the worldwide totals[1,4]. Furthermore, the geographic variability in the incidence of EC is strikingly divergent across its two primary histological subtypes: Squamous cell carcinoma (ESCC) and adenocarcinoma (EAC), each characterized by unique etiological factors. The prevalence of ESCC in specific regions of China is in sharp contrast to the prevalence of EAC predominantly observed in Europe and the United States[1,3]. This divergence greatly informs the development and execution of region-specific screening programs, emphasizing the need for tailored approaches in addressing this malignancy in diverse populations.
The etiology of EC involves a complex interplay of multifaceted factors, encompassing genetic predispositions, lifestyle choices, and environmental exposures. These influences collectively orchestrate alterations across a spectrum of molecular levels, including genomic, transcriptomic, methylation, and epigenomic domains[5]. Despite this knowledge, the exact causative factors for EC have not been fully elucidated. In Western regions, the primary risk factors for ESCC are predominantly associated with the combined effects of excessive alcohol consumption and tobacco smoking[1,6]. In contrast, in lower-income countries, where ESCC accounts for more than 90% of all esophageal cancer cases, the principal risk factors are less clear. However, dietary factors, including nutritional deficiencies and exposure to nitrosamines, have been suggested as potential contributors[7]. Lifestyle factors, encompassing tobacco and alcohol use, coupled with dietary habits, synergize to elevate the risk profile in certain populations[8]. Conversely, EAC accounts for roughly two-thirds of esophageal cancer cases in affluent regions. In these contexts, critical risk factors include excessive body weight, gastroesophageal reflux disease (GERD), and Barrett’s esophagus (BE). Additionally, the declining incidence of chronic Helicobacter pylori infection, which shows an inverse association with EAC, may also play a contributory role in the disease's epidemiology[9-12].
Despite a noticeable reduction in its incidence in recent years, EC continues to pose a substantial challenge to global health. Paradoxically, even as the incidence of new cases declines, the mortality rate associated with EC remains alarmingly high. This grim reality is highlighted by a 5-year relative survival rate that scarcely reaches 20%, making it the cancer with the second lowest survival rate, exceeded only by pancreatic cancer (10%)[13,14]. A further complicating feature of this situation is the elusive nature of early-stage EC, which often presents without specific clinical symptoms. This absence of distinct indicators results in a distressing scenario in which a substantial number of patients are diagnosed at an advanced stage and miss the window for early detection. Consequently, these individuals face a diminished quality of life and a dire prognosis[15,16]. This pressing situation has led to a surge of research initiatives focused on refining screening methods, enhancing early detection techniques, and improving therapeutic approaches. With this led, both the American and British Societies of Gastroenterology have developed a comprehensive set of guidelines for the screening and surveillance of BE and EAC[17-22]. Parallel to these efforts, China has also made substantial contributions to global endeavors, issuing a consensus on the screening of early-stage EC and its precancerous lesions[23,24]. In this literature review, we delve deeper into the multifaceted landscape of esophageal cancer, scrutinizing recent breakthroughs and key findings in the domains of screening, early detection, and treatment.
Screening approaches for esophageal cancer
The prevailing uncertainty about the primary etiological factors of EC has left the medical fraternity grappling with a lack of definitive intervention strategies. This ambiguity has resulted in a considerable knowledge gap regarding EC, thus limiting the available options for effective medical intervention. In response to this predicament, secondary prevention has emerged as a crucial strategy. Early diagnosis and prompt treatment are achieved through structured screening processes. This approach has increasingly been recognized as a practical means to diminish the risks associated with advanced stages of EC and to reduce its associated mortality. The customization of screening protocols necessitates a consideration of both genetic and environmental risk factors. A genetic predisposition, when combined with lifestyle elements such as tobacco use and alcohol intake, guides the formulation of specialized screening programs. These programs are designed to cater to the distinct risk profiles prevalent in specific demographic groups[8].
Geographical variations in esophageal cancer screening
The distinct geographic disparities in the incidence of ESCC and EAC necessitate a nuanced understanding of the development of effective screening strategies. Tailoring screening methods to align with the predominant histological subtype in a given region is critical for enhancing cost-effectiveness and early detection efficacy. In areas with a high incidence of ESCC, a cost-effective, endoscopy-based screening might be justified. Such a strategy is aimed at the broader population and focuses on identifying precancerous lesions and early-stage ESCC. In contrast, regions with a higher incidence of EAC require a more targeted approach, focusing on screening high-risk groups such as individuals with GERD or BE. This approach ensures optimal use of resources while increasing the likelihood of detecting early-stage EAC[8,25].
Given the aggressive nature of EC and the commonality of late-stage diagnoses, the implementation of early detection programs is critical, especially in high-incidence areas. Routine screening in these regions can markedly enhance survival rates[8]. For patients with a significant incidence of EAC, vigilant surveillance of BE is vital. Endoscopic screening, particularly for patients with multiple risk factors for BE-related cancer, is recommended. For those with BE but without atypical hyperplasia, endoscopic surveillance every 3 to 5 years is advised, and GERD management aligns with that for patients without BE[4]. Regular endoscopic examinations supplemented by biopsy sampling are need for the early detection of dysplastic changes. The geographical variations in esophageal cancer screening are indicated in Table 1.
Table 1.
Geographical variations in esophageal cancer screening
|
Country
|
Governmental/healthcare policies
|
Definition of high-risk groups
|
Screening strategies
|
| China | China guideline for the screening, early detection and early treatment of esophageal cancer (2022, Beijing)[26] | (1) Age ≥ 40 yr from areas with high prevalence of esophageal tumors; (2) Family history of esophageal tumors; and (3) Risk factors for esophageal cancer (smoking, heavy alcohol consumption, squamous carcinoma of the head and neck or respiratory tract, preference for high-temperature and preserved foods, poor oral hygiene, etc) | Endoscopic screening: (1) High-risk groups: endoscopic screening with iodine staining of the esophageal mucosa is recommended (45 yr ≤ age ≤ 75 yr, every 5 yr); (2) Low-grade intraepithelial neoplasia every 1-3 yr; (3) Low-grade intraepithelial neoplasia combined with endoscopic risk factors or lesions > 1 cm in length will undergo endoscopy once a year for 5 yr; (4) Endoscopy is recommended every 3 to 5 yr for patients with Barrett's esophagus without atypical hyperplasia; (5) Endoscopy is recommended every 1 to 3 yr for Barrett's esophagus patients with low-grade intraepithelial neoplasia; (6) A new type of esophageal cell collector is recommended for Barrett esophageal screening; (7) The new esophageal cell collector (Cytosponge) performs cytological examination combined with biomarker detection for effective primary screening of Barrett's esophagus-related dysplasia and early esophageal adenocarcinoma; and (8) Biomarker testing alone not recommended for esophageal cancer screening. Equipment: Lugol color endoscopy or NBI endoscopy is recommended as the first choice for esophageal cancer screening, ordinary white light endoscopy can be chosen for those with insufficient conditions, and magnifying endoscopy can be used in conjunction with NBI endoscopy for those with conditions |
| American | ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus[21] | (1) Male; (2) More than 5 yr or frequent (at least once per week) symptoms of gastroesophageal reflux disease; and (3) ≥ 2 risk factors for Barrett's esophagus or esophageal adenocarcinoma, risk factors including age > 50 yr, Caucasian ethnicity, centripetal obesity (waist circumference > 102 cm or waist-to-hip ratio > 0.9), history of smoking, and history of first-degree relatives with Barrett's esophagus or esophageal adenocarcinoma | (1) Unsedated transnasal endoscopy can be considered as an alternative to conventional upper endoscopy for Barrett's esophagus screening; (2) For BE patients without dysplasia, endoscopic surveillance should take place at intervals of 3 to 5 yr; and (3) Use of additional biomarkers for risk stratifi cation of patients with Barrett's esophagus is currently not recommended. Equipment: Surveillance should be performed with high-defi nition/high-resolution white light endoscopy |
| United Kingdom | British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus[18] | (1) White male; (2) Age > 50 yr; (3) Obese; (4) Chronic GERD symptoms for more than 3 yr; and (5) First-degree relative with history of Barrett's esophagus or esophageal adenocarcinoma years | (1) Endoscopic screening can be considered in patients with chronic GERD symptoms and multiple risk factors (at least three of age 50 yr or older, white race, male sex, obesity). However, the threshold of multiple risk factors should be lowered in the presence of family history including at least one first-degree relative with Barrett’s or OAC; (2) High-resolution endoscopy should be used in Barrett’s oesophagus surveillance; (3) Patients with Barrett’s oesophagus shorter than 3 cm, with IM, should receive endoscopic surveillance every 3-5 yr; (4) Patients with segments of 3 cm or longer should receive surveillance every 2-3 yr; and (5) Biomarker panels cannot yet be recommended as routine of screening |
NBI: Narrow band imaging; GERD: Gastroesophageal reflux disease; OAC: Oesophageal adenocarcinoma.
Strategic implementation of screening programs for esophageal cancer
Timely medical intervention facilitated by these screening strategies has the potential to halt the progression to invasive carcinoma. While advocating for screening within identified target populations, it is crucial to emphasize that routine endoscopic or non-endoscopic screening methods are not recommended for the general population. It is vital to acknowledge the distinct differences in the high-risk population characteristics for ESCC vs EAC. Screening guidelines for EAC specifically focus on particular demographic groups[26]. These groups include males, individuals of Caucasian descent, those with a family history of BE, individuals with a prolonged history of reflux symptoms, and people with additional risk factors such as smoking and obesity. In contrast, the target demographic for ESCC screening is primarily identified by geographical location, focusing on individuals residing in high-risk areas[27,28]. Regarding the recommended age for initiating and ceasing screening, the guidelines for BE and EAC suggest beginning routine screening at 50 years of age for those in high-risk categories. On the other hand, the latest guidelines for ESCC advise starting routine screening at 40 years of age and continuing until 75 years of age, unless the individual's life expectancy is estimated to be under five years[18-20,24]. This approach reflects the need for age-specific screening protocols tailored to the risk profiles of different populations, ensuring efficient and effective detection and management of esophageal cancer.
One important gap in EC screening is the lack of robust evidence to guide optimal surveillance intervals. A landmark endoscopic screening study, involving 33319 volunteers from a region with a high ESCC incidence in China showed notable findings. The cumulative mortality rate in the intervention group was significantly lower than that in the control group. Additionally, the cumulative incidence of ESCC was also significantly lower in the intervention group. These outcomes suggest that endoscopic screening and subsequent interventions can significantly diminish the incidence and mortality of esophageal cancer[29]. Guidelines from esteemed organizations such as the American Gastroenterological Association[17] and the British Society of Gastroenterology[18] , recommend a nuanced approach. The authors suggested that patients with BE measuring less than 3 cm or without dysplasia should undergo endoscopic surveillance every 3-5 years. Similarly, the Chinese consensus on ESCC screening presents a tailored perspective. That screening frequency for ESCC should be selectively adjusted, focusing on high-risk populations, with a recommended interval of once every five years. Additionally, in economically challenging regions facing medical resource limitations, extending endoscopic surveillance intervals to as long as a decade is recommended[24,30,31]. This thoughtful approach aims to balance the need for effective screening with practical considerations in regions with limited resources. This finding underscores the importance of customizing screening protocols to align with the specific risk profiles and resource availability of different populations, ensuring that screening efforts are both efficient and sustainable.
Challenges and debates in the early detection of esophageal cancer
The intricate relationship between BE and esophageal EAC highlights the critical importance of vigilant surveillance in individuals at heightened risk. However, the optimal frequency and methods of this surveillance are contentious topics that continue to spark debate within the medical community. Addressing these controversies is pivotal in refining early detection strategies and ensuring effective and timely interventions. The American College of Gastroenterology (ACG) has updated its guidelines for the diagnosis and management of BE. These guidelines now recommend a one-time screening endoscopy for patients with chronic GERD symptoms who also present with three or more additional risk factors. These risk factors include male sex, age older than 50 years, Caucasian ethnicity, tobacco use, obesity, and a family history of BE or EAC in a first-degree relative. Alongside these recommendations, an innovative, non-endoscopic capsule sponge device, which can be swallowed and integrated with a biomarker, has emerged as a promising and more acceptable alternative to traditional endoscopy. This device is particularly suited for screening individuals who exhibit chronic reflux symptoms alongside other risk factors associated with BE[22]. While BE is acknowledged as an important precursor to EAC, with individuals diagnosed with BE facing an increased risk of developing esophageal cancer, the exact role of BE in the early detection of EAC continues to be a topic of intense discussion and research[25]. This ongoing debate underscores the complexity of esophageal cancer screening and the need for continued research to establish the most effective strategies for early detection and prevention.
Navigating the challenges of surveillance in BE
A primary challenge in managing BE is determining the most appropriate surveillance intervals. The diverse rates at which BE progresses to cancer, coupled with concerns about overdiagnosis, fuel ongoing debates about the frequency of endoscopic surveillance. It is critical to find a balance between the early detection of cancers and the avoidance of unnecessary medical procedures[25]. The ACG guidelines recommend that the frequency of endoscopic surveillance in patients with BE should be based on the degree of dysplasia observed in previous biopsies[22]. This approach aims to tailor surveillance to the individual patient's risk profile.
Another contentious area is the histological grading of dysplasia within BE tissue. Assessing the risk of progression to EAC based on the severity of dysplasia is complex. The development and utilization of reliable predictive markers are essential for improving risk stratification and guiding early intervention strategies[25]. Recent advancements in imaging technologies are offering new avenues for addressing these challenges. Innovations such as advanced endoscopic techniques and confocal laser endomicroscopy (CLE) provide a more detailed examination of BE. These technologies enhance the ability to accurately stratify risk and detect early changes, potentially transforming the approach to surveillance in BE[32,33]. These developments represent an important step forward in the management of BE, offering the potential for more precise, personalized surveillance strategies that can better identify patients at increased risk of progression to esophageal EAC. As these technologies evolve, the outcomes of patients with BE may substantially improve by the facilitation of earlier and more targeted interventions.
The role of advanced imaging technology in the early detection of esophageal cancer
The evolution of imaging and diagnostic technologies plays a crucial role in enhancing the early detection of esophageal cancer. Innovations in endoscopy and non-invasive imaging modalities are instrumental in identifying precancerous lesions and early-stage tumors, thereby improving patient prognosis. In recent years, remarkable advancements have been made in endoscopic technologies, which markedly aid in the early diagnosis of esophageal cancer. High-definition endoscopy, magnification endoscopy, and virtual chromoendoscopy have substantially improved the visualization of suspicious lesions. These advancements enable clinicians to detect abnormalities at earlier stages, potentially leading to more effective treatments[32]. A notable study highlighting these advancements is a randomized crossover trial that evaluated the effectiveness of high-definition white light endoscopy, following the Seattle protocol, against narrow-band imaging with targeted biopsies for detecting neoplasia. The study involved a cohort of 123 patients with BE. These findings revealed a greater detection rate of dysplasia using narrow-band imaging than using high-definition white light endoscopy (30% vs 21%, P = 0.01)[34]. Significantly, all the detected dysplastic or carcinoma cases displayed distinctive mucosal or vascular irregularities, which were identifiable through narrow-band imaging.
CLE
CLE has become a key tool for real-time, in vivo histopathological imaging, providing cellular-level resolution during endoscopy. Moreover, endoscopic ultrasound-fine needle aspiration enhances the ability to distinguish neoplastic changes, aiding in the accurate diagnosis of early-stage esophageal cancer[32]. This technique employs blue laser light following the intravenous administration of fluorescein, enabling high-magnification, real-time imaging and the acquisition of targeted optical biopsies[22]. A systematic review and meta-analysis incorporating seven studies with 473 patients demonstrated a high sensitivity (89%) and specificity (83%) for CLE compared to histopathology[35]. Despite these encouraging results, challenges persist, including the substantial cost, the necessity for intravenous fluorescein, the requisite training for image interpretation, and the prolonged duration of the examination. Moreover, the applicability of these findings to the broader surveillance population remains uncertain, given that most studies were conducted in centers with a high prevalence of dysplasia/neoplasia. While confocal endoscopy shows promise for guiding biopsies and directing therapy, particularly in centers characterized by a high incidence of tumors or dysplasia, its added value compared to high-definition white-light and electron-stained endoscopy remains ambiguous[36].
Volumetric laser endomicroscopy
Volumetric laser endomicroscopy, utilizing optical coherence tomography, captures high-resolution, cross-sectional images of esophageal tissue. It allows for detailed 2-dimensional visualization of the mucosa and submucosa up to a depth of 3 mm[37,38]. This non-invasive imaging modality facilitates the detection of early-stage lesions and helps distinguish between benign and malignant conditions[32]. A study with 29 full scan videos from patients with and without neoplasia demonstrated that experts correctly identified 73% of neoplastic cases and 52% of nondysplastic cases[39].
Artificial intelligence in diagnosis
The use of artificial intelligence (AI) in image analysis has revolutionized early diagnosis. AI algorithms, trained on vast datasets, can identify subtle abnormalities in endoscopic images and aid in the rapid interpretation of diagnostic information. This approach not only enhances the accuracy of early detection but also addresses challenges related to interobserver variability[32,40]. Machine learning and deep learning (DL) are important parts of AI[41]. A clinical study in the Netherland showed that a DL system could detect tumors in patients with BE more accurately than could endoscopists (sensitivity 90%/specificity 88%) by analyzing 1704 unique high-resolution images of the esophagus from 669 patients[41]. This study demonstrated the high sensitivity and accuracy of deep neural network-based methods for detecting SCC and differentiating from non-cancerous lesions[42]. Additionally, the computer-aided detection algorithm demonstrated enhanced performance compared to that of a group of 53 nonexpert endoscopists. A pilot study examining this system during live endoscopy sessions produced encouraging outcomes[43]. The ongoing development of imaging technologies and AI for esophageal cancer diagnosis holds great promise. Future directions include refining existing techniques, expanding accessibility, and integrating these technologies into routine clinical practice for widespread and effective early detection. These findings underscore the importance of advanced imaging techniques in the early detection of esophageal cancer. Narrow-band imaging, in particular, demonstrates a superior ability to identify subtle changes indicative of dysplasia or early-stage carcinoma. As these technologies continue to evolve and become more widely adopted in clinical practice, they hold the potential to significantly improve the early detection and management of esophageal cancer, ultimately leading to better patient outcomes.
Biomarker integration in screening programs
Recognizing the limitations of traditional screening methods, the incorporation of biomarkers into screening programs represents a promising avenue for enhancing early detection precision. Biomarkers such as microRNA signatures, circulating tumor DNA (ctDNA), and protein markers hold potential for identifying high-risk individuals and detecting esophageal cancer at its earliest, most treatable stages[44-46]. MicroRNA profiling can reveal specific expression patterns linked to esophageal cancer[45,47]. For example, the level of plasma microRNA-192-5p has been shown to predict the response to chemotherapy and the prognosis in patients with esophageal cancer[48]. ctDNA analysis is a non-invasive method for detecting genetic mutations associated with esophageal cancer, and studies have shown that this method has substantial sensitivity and specificity[49-51]. A quantitative analysis encompassing 15 studies on the application of ctDNA technology, along with 8 studies involving 414 patients, revealed that the overall sensitivity and specificity of ctDNA-based diagnostic methods were 71.0% (ranging from 55.7% to 82.6%) and 98.6% (ranging from 33.9% to 99.9%), respectively, while those of ctDNA-based diagnostic methods were 48.9% (29.4%-68.8%) and 95.5% (90.6%-97.9%), respectively[52]. Certain proteins, epitomized by distinct antigens and enzymes, have emerged as potent indicators in identifying esophageal cancer. Protein marker analysis, often conducted through blood tests, can complement imaging techniques and contribute to a more comprehensive screening approach. Ide et al[53] found that angiopoietin-like protein 2 may be a novel biomarker for the diagnosis and prognosis of patients with esophageal cancer[54]. Given the heterogeneity of esophageal cancer, utilizing multimodal biomarker panels can enhance diagnostic accuracy. Integrating different types of biomarkers into a unified screening program allows for a more nuanced assessment, increasing the chances of early cancer detection. Despite these advancements, challenges such as standardization, cost, and validation across diverse populations remain. Ongoing research and collaborative efforts are crucial to refine and integrate these technologies into routine clinical practice, aiming to improve early detection and treatment outcomes for esophageal cancer patients.
Therapeutic management disparities in early-stage EC
The heterogeneities observed in the therapeutic orchestration of incipient EC are intricately linked with the divergences in early diagnostic frequencies discernible across various geographies. This aspect is particularly evident in high-incidence areas such as China and Japan, where advanced early detection capabilities have led to the development of specialized endoscopic techniques for both diagnosis and treatment, Table 2.
Table 2.
The pros and cons of diagnostic and therapeutic protocols in esophageal carcinoma
|
Diagnostic and therapeutic protocols
|
Indications
|
Pros
|
Cons
|
| (1) For patients with early-stage EC who meet the absolute and relative indications for endoscopic resection, ESD being the first choice; (2) When the long diameter of the lesion is ≤ 10 mm, if the whole piece can be guaranteed resection, EMR treatment can also be considered; (3) For patients with early-stage EAC after EMR resection, ablation treatment is recommended; (4) Endoscopic radiofrequency ablation (RFA) can be used to treat ESCC limited to the lamina propria of the mucosa; and (5) For patients with lesions infiltrating to a depth of submucosal (> 200 μm) T1b stage EC patients with lymph node or vascular invasion and tumor low differentiation (≥ G3), esophagectomy should be performed. Those who refuse surgery or are intolerant to surgery should be treated with concurrent radiotherapy and chemotherapy[26] | (1) The absolute indication of endoscopic resection of EC is that the lesion is limited to the epithelial layer and lamina propria of T1a esophageal cancer, and the risk of lymph node metastasis is low; (2) The relative indications of endoscopic resection: the lesion extends to the muscularis mucosa or slightly infiltrates the submucosa (the depth of submucosal infiltration is less than 200 μm), the range is ≥ 3/4 of esophageal circumference, and the risk of stenosis after resection is high. However, patients should be fully informed of postoperative stenosis and other risks; (3) Lesions with infiltration depths (> 200 μm) up to the submucosal layer (T1b) are associated with metastasis, in which case they should be treated in the same way as advanced cancers, even if they are classified as superficial[26,55] | (1) EMR: Easy to operate and less invasive. Short operation time, low risk and quick recovery; (2) ESD: Larger tumors, especially those larger than 2 cm in diameter, can be completely removed at once, reducing the likelihood of recurrence; (3) RFA has a relatively short recovery time and demonstrates a low recurrence rate; (4) Surgery: Completely remove the tumor, thereby reducing the risk of recurrence, and providing exact pathological staging information, which helps to assess the progression of the cancer and plan subsequent treatment; and (5) CRT: Providing good local lesion control and helping to reduce tumor size, which may make surgery easier or, in some cases, avoid it; being effective in reducing the rate of tumor recurrence for some patients; and making CRT an effective treatment option for patients who can't afford to have surgery due to health issues | (1) EMR: Difficulty in removing large or poorly defined tumors at one time, which may require multiple treatments and a relatively high rate of recurrence; (2) Technically demanding, the procedure takes longer and may increase the risk of complications, such as bleeding or perforation; (3) RFA: Narrow range of indications; may increase risks and complications, include pain, bleeding, esophageal stricture or perforation, requiring specialized equipment and trained physicians; (4) Surgery: There are risks associated with the surgery itself (e.g., infection, bleeding, anesthesia complications, etc.), possible postoperative complications (e.g., esophageal stricture, dysphagia, malnutrition, etc.), and negative impact on the patient's quality of life, especially in terms of digestive function and eating habits. In addition, the long postoperative recovery time may require additional nutritional support and rehabilitation; and (5) CRT: Include a wide range of possible side effects from chemotherapy and radiation (e.g., nausea, vomiting, hair loss, fatigue, loss of appetite, etc.); the potential for a long-term decline in quality of life, such as digestive problems and difficulty swallowing; and the potential for a wide-ranging impact on a patient's overall health status, especially for patients who are older or who have other health problems |
EC: Esophageal carcinoma; ESD: Endoscopic submucosal dissection; EMR: Endoscopic mucosal resection; CRT: Concurrent radiotherapy; RFA: Radiofrequency ablation.
Region-specific advances
In locales such as China and Japan, characterized by a heightened prevalence of esophageal cancer, there has been a notable advancement in the infrastructure for early detection[55]. This advancement has translated into distinct treatment approaches tailored to the regional disease profile[56]. The understanding and adoption of these region-specific methodologies can offer insights for improving global treatment outcomes. The disparities in therapeutic management of early-stage EC are intricately linked to regional variations in incidence and the effectiveness of early diagnosis programs. Regions with a higher incidence, such as China and Japan, often exhibit more advanced early detection infrastructures, leading to distinct treatment approaches[57]. Particularly in these high-incidence regions, advanced early diagnosis has elevated the role of specialized endoscopic techniques as a cornerstone in the therapeutic regimen for esophageal cancer[56-58].
Endoscopic resection methods
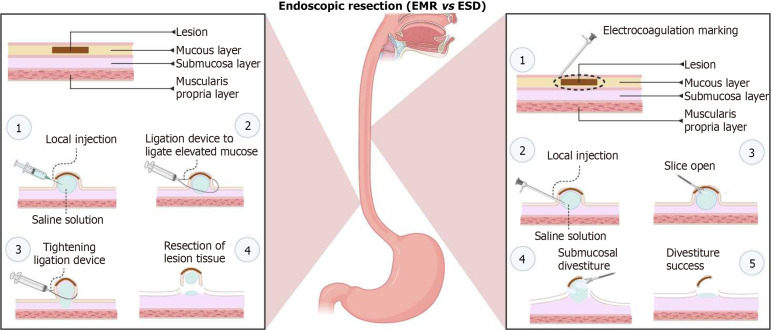
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have demonstrated remarkable efficacy in treating early-stage esophageal cancer while preserving esophageal functionality[59]. EMR[60,61] and ESD[62] are increasingly recognized for their minimally invasive nature and potential in providing effective treatment with reduced morbidity compared to traditional surgical approaches (Figure 1).
Figure 1.
Endoscopic resection methods (endoscopic mucosal resection vs endoscopic submucosal dissection). EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection. Created with BioRender.com.
EMR: EMR stands as a pivotal technique in the treatment of early-stage esophageal cancer. This minimally invasive approach involves the removal of cancerous or precancerous tissue from the mucosal layer while preserving the underlying structures and esophageal function. European guidelines recommend EMR for lesions under 10 mm, highlighting the importance of en bloc resection to improve disease-free survival due to the risk of lymph node metastasis in ESCC[63]. The judicious selection of candidates for EMR is critical to avoid poor prognosis. In Japan, EMR has been meticulously guided by the Japanese Society for Gastrointestinal Endoscopy's guidelines, addressing various clinical questions from preoperative diagnosis to postoperative surveillance[58]. Derived from a comprehensive review of scientific literature, this guideline offers insights on 18 clinical queries encompassing aspects such as preoperative diagnosis, indications for surgery, resection techniques, evaluating the potential for cure, and monitoring patients who have undergone endoscopic esophagectomy for esophageal cancer. Empirical evidence from a retrospective study, which scrutinized 44 patients with clinical stage I (T1a-MM ,T1bcN0M0, in Japan) esophageal cancer using EMR demonstrated that EMR provides both disease control and adequate treatment of the lesion[64]. In addition, EMR is an effective treatment for superficial esophageal cancer. However, a study of 30 cT2N0M0 patients treated with EMR showed that EMR successfully cleared all known disease in 17/30 patients. In the 17 patients where EMR successfully eradicated all detectable disease, there were neither recurrences nor cancer-related fatalities. This outcome indicates that EMR is a safe diagnostic approach for cT2N0M0 esophageal cancer. Additionally, EMR tends to be favored for older patients presenting with smaller tumors and lower SUVmax values[65].
ESD: ESD, a technique originating and perfected in Japan, has primarily been applied in the management of superficial ESCC. As an advanced endoscopic method, ESD facilitates the en-bloc resection of larger lesions, epitomizing precision and meticulousness. This precise and meticulous procedure is particularly effective in treating early-stage EC, providing a greater chance of complete tumor removal with minimal impact on surrounding tissues. Over the preceding decade, an escalating scholarly interest in esophageal ESD has manifested in Western medical circles. Notably, the Western landscape is characterized by the prevalence of EAC, overshadowing ESCC, which has exhibited a discernible decline over recent decades. The utility of ER in BE has garnered substantiated recognition as a pivotal modality, affording comprehensive insights into staging, prognosis, and the extirpation of neoplastic manifestations associated with BE. This approach uniformly receives commendation across authoritative societal guidelines, especially in the context of managing high-grade dysplasia (HGD) and tumors confined to the muscularis mucosa (T1a)[19,66,67].
ESD has been widely used to treat superficial esophageal cancer, advantages of ESD include high rates of whole-mount resection and accurate pathologic diagnosis. Even in cases with clinical T1b-SM cancer, subsequent treatments can potentially lead to a radical cure, depending on the risk of metastasis. The role of ESD in the treatment of esophageal cancer will become increasingly important[68]. A study involving 66 patients with pathologic staging of T1b from 7 United State centers showed that 54 patients underwent preoperative staging, 27 patients (50%) were Tis/T1a, and 27 patients (50%) were T1b. The rate of complete resection was 92.4% (61/66) and the rate of R0 resection was 54.5% (36/66). 10 patients who underwent ESD resection within the criteria for cure had no recurrence after 13 months of follow-up. Ten patients who underwent ESD resection had no recurrence after 13 months of follow-up. Ten of the 39 patients (25.6%) who underwent noncurative resection had residual/recurrent disease[69]. Consequently, these findings from this study should alleviate persistent worries about prolonged procedure durations and increased rates of adverse events associated with ESD in the esophagus. Ongoing improvements in ESD techniques, exemplified by innovations such as countertraction, hold promise for achieving even more profound dissection planes and favorable outcomes, though further investigation is warranted in this regard. Prospective studies are imperative to explore the potential efficacy of adjuvant chemotherapy and radiation post-ESD, particularly for patients ineligible for surgical options, are imperative. Furthermore, the evolving landscape anticipates a pivotal role for advanced imaging and AI. Their application is envisaged to discern Tis/HGD/T1a from T1b disease, particularly given the current dearth of precise staging methods preceding the ER of early esophageal cancer[70]. Global standardization of treatment protocols, informed by successes in high-incidence areas, is crucial. Sharing best practices, lessons learned, and technological advancements can contribute to the development of universally effective therapeutic strategies for early-stage EC.
In geographical areas marked by elevated incidences of EC, such as China and Japan, endoscopic techniques have become integral components of the therapeutic landscape. Their success is underscored by the ability to treat a substantial number of cases at an early stage, contributing to improved overall survival rates. The efficacy of endoscopic techniques lies not only in their ability to effectively remove early-stage tumors but also in their potential to minimize complications. By avoiding the need for major surgery, patients undergoing EMR or ESD experience shorter recovery times and reduced postoperative morbidity. A crucial advantage of endoscopic techniques is the preservation of esophageal functionality. Unlike more invasive surgical procedures, EMR and ESD are designed to remove cancerous tissue while maintaining the structural integrity and function of the esophagus.
Challenges in esophageal cancer screening and future perspectives
Implementing population-wide screening programs for EC is fraught with challenges, including resource constraints and the need for well-established infrastructure. Overcoming these challenges requires a multidisciplinary approach involving healthcare policymakers, clinicians, and researchers to design effective and sustainable screening initiatives.
Resource constraints: Population-based screening programs are hindered by financial, infrastructural, and human resource limitations. Addressing these constraints is critical to enable widespread and equitable access to screening services.
Infrastructure development: The establishment and maintenance of infrastructure for screening are complex tasks, especially in resource-limited settings. This involves setting up facilities, training healthcare professionals, and ensuring the availability of diagnostic tools.
Education and awareness: Public awareness about esophageal cancer, its risk factors, and the significance of early detection is often lacking. Educational campaigns are crucial to enhance understanding and participation in screening programs.
Overcoming cultural and social barriers: Cultural beliefs and social norms can impede participation in screening initiatives. It's important to design culturally sensitive programs and engage communities to overcome these barriers.
Ensuring equity and accessibility: Ensuring equitable access to screening, particularly for underserved populations, is essential. This requires targeted efforts to reach rural areas and marginalized groups.
Monitoring and evaluation: Effective monitoring and evaluation mechanisms are essential for assessing the impact of screening programs, identifying areas for improvement, and optimizing resource allocation.
CONCLUSION
Addressing the global burden of esophageal cancer requires an understanding of regional variances in incidence, risk factors, and histological types. The development of tailored screening strategies, resolving debates in early detection, and adopting advanced treatment methods are key to enhancing patient outcomes. Future efforts should focus on: Global collaboration: Sharing best practices, research findings, and technological advancements on an international scale is vital for progress in prevention, early detection, and treatment of esophageal cancer. Technological innovation: Continued investment in innovative diagnostic and treatment technologies, including advanced endoscopic techniques, AI-driven diagnostic tools, and novel biomarkers, will play a significant role in advancing esophageal cancer care. Holistic approach: Combining medical advancements with public health initiatives, including lifestyle modification campaigns and risk factor management, is necessary for a comprehensive approach to esophageal cancer prevention and treatment. Personalized medicine: Emphasizing personalized screening and treatment strategies based on individual risk profiles and genetic predispositions will enhance the effectiveness of interventions. Research and development: Ongoing research into the etiology, pathogenesis, and optimal management of esophageal cancer is crucial to uncover new insights and improve clinical practices.
In summary, a multifaceted and collaborative approach, integrating medical advances with public health strategies, is essential for effectively tackling the complexities of esophageal cancer globally.
Footnotes
Conflict-of-interest statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 19, 2023
First decision: December 27, 2023
Article in press: February 19, 2024
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gragnaniello V, Italy S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
Contributor Information
Hong-Tao Qu, Department of Emergency, Yantai Mountain Hospital, Yantai 264000, Shandong Province, China.
Qing Li, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Liang Hao, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Yan-Jing Ni, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Wen-Yu Luan, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Zhe Yang, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Xiao-Dong Chen, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Tong-Tong Zhang, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Yan-Dong Miao, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China.
Fang Zhang, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, Shandong Province, China. zhangfang_0127@bzmc.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wild CP, Weiderpass E, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention. Lyon. 2020. France: International Agency for Research on Cancer. Available from: http://publications.iarc.fr/586 . [PubMed]
- 3.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310:627–636. doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 5.Chang J, Zhao X, Wang Y, Liu T, Zhong C, Lao Y, Zhang S, Liao H, Bai F, Lin D, Wu C. Genomic alterations driving precancerous to cancerous lesions in esophageal cancer development. Cancer Cell. 2023;41:2038–2050.e5. doi: 10.1016/j.ccell.2023.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed F. Esophageal cancer. N Engl J Med. 2004;350:1363–4; author reply 1363. doi: 10.1056/NEJMc033106. [DOI] [PubMed] [Google Scholar]
- 7.McCormack VA, Menya D, Munishi MO, Dzamalala C, Gasmelseed N, Leon Roux M, Assefa M, Osano O, Watts M, Mwasamwaja AO, Mmbaga BT, Murphy G, Abnet CC, Dawsey SM, Schüz J. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: A review of setting-specific exposures to known and putative risk factors. Int J Cancer. 2017;140:259–271. doi: 10.1002/ijc.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jankowski JA, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the west. Gastroenterology. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Petrick JL, Steck SE, Bradshaw PT, McClain KM, Niehoff NM, Engel LS, Shaheen NJ, Risch HA, Vaughan TL, Wu AH, Gammon MD. A pooled analysis of dietary sugar/carbohydrate intake and esophageal and gastric cardia adenocarcinoma incidence and survival in the USA. Int J Epidemiol. 2017;46:1836–1846. doi: 10.1093/ije/dyx203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbeek RE, Spittuler LF, Peute A, van Oijen MG, Ten Kate FJ, Vermeijden JR, Oberndorff A, van Baal JW, Siersema PD. Familial clustering of Barrett's esophagus and esophageal adenocarcinoma in a European cohort. Clin Gastroenterol Hepatol. 2014;12:1656–63.e1. doi: 10.1016/j.cgh.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol. 2017;112:1247–1255. doi: 10.1038/ajg.2017.155. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 14.Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, Posner MC, Bentrem DJ. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju133. [DOI] [PubMed] [Google Scholar]
- 15.Thrumurthy SG, Chaudry MA, Thrumurthy SSD, Mughal M. Oesophageal cancer: risks, prevention, and diagnosis. BMJ. 2019;366:l4373. doi: 10.1136/bmj.l4373. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Xu J, Zheng Y, Gao Y, He S, Li H, Zou K, Li N, Tian J, Chen W, He J. Esophageal cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. 2021;33:535–547. doi: 10.21147/j.issn.1000-9604.2021.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Gastroenterological Association. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O'Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 19.ASGE Standards of Practice Committee; Qumseya B, Sultan S, Bain P, Jamil L, Jacobson B, Anandasabapathy S, Agrawal D, Buxbaum JL, Fishman DS, Gurudu SR, Jue TL, Kripalani S, Lee JK, Khashab MA, Naveed M, Thosani NC, Yang J, DeWitt J, Wani S ASGE Standards of Practice Committee Chair. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc. 2019;90:335–359.e2. doi: 10.1016/j.gie.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TH, Thrift AP, Rugge M, El-Serag HB. Prevalence of Barrett's esophagus and performance of societal screening guidelines in an unreferred primary care population of U.S. veterans. Gastrointest Endosc. 2021;93:409–419.e1. doi: 10.1016/j.gie.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaheen NJ, Falk GW, Iyer PG, Gerson LB American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30–50; quiz 51. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, Wani S. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117:559–587. doi: 10.14309/ajg.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Clinical Research Center for Digestive Diseases Chinese Digestive Endoscopy Society; Chinese Digestive Doctor Association. [The Chinese consensus for screening, diagnosis and management of Barrett's esophagus and early adenocarcinoma(2017, Wanning)] Zhonghua Nei Ke Za Zhi. 2017;56:701–711. doi: 10.3760/cma.j.issn.0578-1426.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Early Diagnosis and Treatment Group of the Chinese Medical Association Oncology Branch. [Chinese expert consensus on early diagnosis and treatment of esophageal cancer] Zhonghua Zhong Liu Za Zhi. 2022;44:1066–1075. doi: 10.3760/cma.j.cn112152-20220220-00114. [DOI] [PubMed] [Google Scholar]
- 25.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 26.He J, Chen WQ, Li ZS, Li N, Ren JS, Tian JH, Tian WJ, Hu FL, Peng J Expert Group of China Guideline for the Screening, Early Detection and Early Treatment of Esophageal Cancer; Work Group of China Guideline for the Screening,Early Detection and Early Treatment of Esophageal Cancer. [China guideline for the screening, early detection and early treatment of esophageal cancer (2022, Beijing)] Zhonghua Zhong Liu Za Zhi. 2022;44:491–522. doi: 10.3760/cma.j.cn112152-20220517-00348. [DOI] [PubMed] [Google Scholar]
- 27.He Z, Ke Y. Precision screening for esophageal squamous cell carcinoma in China. Chin J Cancer Res. 2020;32:673–682. doi: 10.21147/j.issn.1000-9604.2020.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubenstein JH, Omidvari AH, Lauren BN, Hazelton WD, Lim F, Tan SX, Kong CY, Lee M, Ali A, Hur C, Inadomi JM, Luebeck G, Lansdorp-Vogelaar I. Endoscopic Screening Program for Control of Esophageal Adenocarcinoma in Varied Populations: A Comparative Cost-Effectiveness Analysis. Gastroenterology. 2022;163:163–173. doi: 10.1053/j.gastro.2022.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei WQ, Chen ZF, He YT, Feng H, Hou J, Lin DM, Li XQ, Guo CL, Li SS, Wang GQ, Dong ZW, Abnet CC, Qiao YL. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J Clin Oncol. 2015;33:1951–1957. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18:2493–2501. doi: 10.3748/wjg.v18.i20.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hur C, Choi SE, Kong CY, Wang GQ, Xu H, Polydorides AD, Xue LY, Perzan KE, Tramontano AC, Richards-Kortum RR, Anandasabapathy S. High-resolution microendoscopy for esophageal cancer screening in China: A cost-effectiveness analysis. World J Gastroenterol. 2015;21:5513–5523. doi: 10.3748/wjg.v21.i18.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Sommen F, Zinger S, Curvers WL, Bisschops R, Pech O, Weusten BL, Bergman JJ, de With PH, Schoon EJ. Computer-aided detection of early neoplastic lesions in Barrett's esophagus. Endoscopy. 2016;48:617–624. doi: 10.1055/s-0042-105284. [DOI] [PubMed] [Google Scholar]
- 33.Fockens KN, Jong MR, Jukema JB, Boers TGW, Kusters CHJ, van der Putten JA, Pouw RE, Duits LC, Montazeri NSM, van Munster SN, Weusten BLAM, Alvarez Herrero L, Houben MHMG, Nagengast WB, Westerhof J, Alkhalaf A, Mallant-Hent RC, Scholten P, Ragunath K, Seewald S, Elbe P, Baldaque-Silva F, Barret M, Ortiz Fernández-Sordo J, Villarejo GM, Pech O, Beyna T, van der Sommen F, de With PH, de Groof AJ, Bergman JJ Barrett's Oesophagus Imaging for Artificial Intelligence (BONS-AI) consortium. A deep learning system for detection of early Barrett's neoplasia: a model development and validation study. Lancet Digit Health. 2023;5:e905–e916. doi: 10.1016/S2589-7500(23)00199-1. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, Singh M, Hall M, Mathur SC, Wani SB, Hoffman B, Gaddam S, Fockens P, Bergman JJ. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15–21. doi: 10.1136/gutjnl-2011-300962. [DOI] [PubMed] [Google Scholar]
- 35.Xiong YQ, Ma SJ, Zhou JH, Zhong XS, Chen Q. A meta-analysis of confocal laser endomicroscopy for the detection of neoplasia in patients with Barrett's esophagus. J Gastroenterol Hepatol. 2016;31:1102–1110. doi: 10.1111/jgh.13267. [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A, Gupta N, Michalek JE, Lauwers GY, Wallace MB. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465–472. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houston T, Sharma P. Volumetric laser endomicroscopy in Barrett's esophagus: ready for primetime. Transl Gastroenterol Hepatol. 2020;5:27. doi: 10.21037/tgh.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faruqi SA, Arantes V, Bhutani MS. Barrett's esophagus: current and future role of endosonography and optical coherence tomography. Dis Esophagus. 2004;17:118–123. doi: 10.1111/j.1442-2050.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 39.Struyvenberg M, Kahn A, Fleischer D, Swager AF, Bouma B, Ganguly EK, Konda V, Lightdale CJ, Pleskow D, Sethi A, Smith M, Trindade AJ, Wallace MB, Wang K, Wolfsen HC, Tearney GJ, Curvers WL, Leggett CL, Bergman JJ. Expert assessment on volumetric laser endomicroscopy full scans in Barrett's esophagus patients with or without high grade dysplasia or early cancer. Endoscopy. 2021;53:218–225. doi: 10.1055/a-1194-0397. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Li L, You P, Wei Y, Xu B. Towards artificial intelligence to multi-omics characterization of tumor heterogeneity in esophageal cancer. Semin Cancer Biol. 2023;91:35–49. doi: 10.1016/j.semcancer.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Huang LM, Yang WJ, Huang ZY, Tang CW, Li J. Artificial intelligence technique in detection of early esophageal cancer. World J Gastroenterol. 2020;26:5959–5969. doi: 10.3748/wjg.v26.i39.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohmori M, Ishihara R, Aoyama K, Nakagawa K, Iwagami H, Matsuura N, Shichijo S, Yamamoto K, Nagaike K, Nakahara M, Inoue T, Aoi K, Okada H, Tada T. Endoscopic detection and differentiation of esophageal lesions using a deep neural network. Gastrointest Endosc. 2020;91:301–309.e1. doi: 10.1016/j.gie.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 43.de Groof AJ, Struyvenberg MR, Fockens KN, van der Putten J, van der Sommen F, Boers TG, Zinger S, Bisschops R, de With PH, Pouw RE, Curvers WL, Schoon EJ, Bergman JJGHM. Deep learning algorithm detection of Barrett's neoplasia with high accuracy during live endoscopic procedures: a pilot study (with video) Gastrointest Endosc. 2020;91:1242–1250. doi: 10.1016/j.gie.2019.12.048. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Z, Wang X, Geng X, Li Y, Mu J, Tan F, Xue Q, Gao S, He J. Liquid biopsy for esophageal cancer: Is detection of circulating cell-free DNA as a biomarker feasible? Cancer Commun (Lond) 2021;41:3–15. doi: 10.1002/cac2.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuda Y, Shimura T, Iwasaki H, Fukusada S, Nishigaki R, Kitagawa M, Katano T, Okamoto Y, Yamada T, Horike SI, Kataoka H. Urinary microRNA biomarkers for detecting the presence of esophageal cancer. Sci Rep. 2021;11:8508. doi: 10.1038/s41598-021-87925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kausar T, Ahsan A, Hasan MR, Lin L, Beer DG, Ralhan R. Sperm protein 17 is a novel marker for predicting cisplatin response in esophageal squamous cancer cell lines. Int J Cancer. 2010;126:1494–1503. doi: 10.1002/ijc.24828. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y, Ren KM. Development of a prognostic prediction model based on microRNA-1269a in esophageal cancer. World J Gastrointest Oncol. 2021;13:943–958. doi: 10.4251/wjgo.v13.i8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furuke H, Konishi H, Arita T, Kataoka S, Shibamoto J, Takabatake K, Takaki W, Shimizu H, Yamamoto Y, Komatsu S, Shiozaki A, Fujiwara H, Otsuji E. Plasma microRNA-192-5p can predict the response to neoadjuvant chemotherapy and prognosis in esophageal cancer. Cancer Sci. 2023;114:1686–1696. doi: 10.1111/cas.15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujisawa R, Iwaya T, Endo F, Idogawa M, Sasaki N, Hiraki H, Tange S, Hirano T, Koizumi Y, Abe M, Takahashi T, Yaegashi M, Akiyama Y, Masuda M, Sasaki A, Takahashi F, Sasaki Y, Tokino T, Nishizuka SS. Early dynamics of circulating tumor DNA predict chemotherapy responses for patients with esophageal cancer. Carcinogenesis. 2021;42:1239–1249. doi: 10.1093/carcin/bgab088. [DOI] [PubMed] [Google Scholar]
- 50.Min J, Zhou H, Jiang S, Yu H. A Review of Circulating Tumor DNA in the Diagnosis and Monitoring of Esophageal Cancer. Med Sci Monit. 2022;28:e934106. doi: 10.12659/MSM.934106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creemers A, Krausz S, Strijker M, van der Wel MJ, Soer EC, Reinten RJ, Besselink MG, Wilmink JW, van de Vijver MJ, van Noesel CJM, Verheij J, Meijer SL, Dijk F, Bijlsma MF, van Oijen MGH, van Laarhoven HWM. Clinical value of ctDNA in upper-GI cancers: A systematic review and meta-analysis. Biochim Biophys Acta Rev Cancer. 2017;1868:394–403. doi: 10.1016/j.bbcan.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Chidambaram S, Markar SR. Clinical utility and applicability of circulating tumor DNA testing in esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2022;35 doi: 10.1093/dote/doab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ide S, Toiyama Y, Shimura T, Kawamura M, Yasuda H, Saigusa S, Ohi M, Tanaka K, Mohri Y, Kusunoki M. Angiopoietin-Like Protein 2 Acts as a Novel Biomarker for Diagnosis and Prognosis in Patients with Esophageal Cancer. Ann Surg Oncol. 2015;22:2585–2592. doi: 10.1245/s10434-014-4315-0. [DOI] [PubMed] [Google Scholar]
- 54.Powell AGMT, Eley C, Chin C, Coxon AH, Christian A, Lewis WG South East Wales Oesophagogastric Cancer Collaborative. Prognostic significance of serum inflammatory markers in esophageal cancer. Esophagus. 2021;18:267–277. doi: 10.1007/s10388-020-00772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Nemoto K, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20:343–372. doi: 10.1007/s10388-023-00993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu X, Chen J, Yuan Z, Liu H, Liu F, Liu Y, Xue L, He S, Zhang Y, Dou L, Liu X, Zhao D, Li J, Wang S, Zhang P, Lu N, Wang G. Endoscopic resection techniques for squamous premalignant lesions and early carcinoma of the esophagus: ER-Cap, MBM, and ESD, how do we choose? A multicenter experience. Therap Adv Gastroenterol. 2020;13:1756284820909172. doi: 10.1177/1756284820909172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nihei K, Minashi K, Yano T, Shimoda T, Fukuda H, Muto M JCOG-GIESG Investigators. Final Analysis of Diagnostic Endoscopic Resection Followed by Selective Chemoradiotherapy for Stage I Esophageal Cancer: JCOG0508. Gastroenterology. 2023;164:296–299.e2. doi: 10.1053/j.gastro.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Ishihara R, Arima M, Iizuka T, Oyama T, Katada C, Kato M, Goda K, Goto O, Tanaka K, Yano T, Yoshinaga S, Muto M, Kawakubo H, Fujishiro M, Yoshida M, Fujimoto K, Tajiri H, Inoue H Japan Gastroenterological Endoscopy Society Guidelines Committee of ESD/EMR for Esophageal Cancer. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020;32:452–493. doi: 10.1111/den.13654. [DOI] [PubMed] [Google Scholar]
- 59.Deng C, Wu S, Xu F, Zhou Z, Zhu Y, Mei Z, He S. Precutting and trimming-assisted underwater endoscopic mucosal resection for large early esophageal cancer. Endoscopy. 2023;55:E494–E495. doi: 10.1055/a-2025-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24. doi: 10.1007/s10388-018-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe S, Ishihara R, Takahashi H, Ono H, Fujisaki J, Matsui A, Takahashi A, Goda K, Kawada K, Koike T, Takeuchi M, Tsuji Y, Hirasawa D, Oyama T. Long-term outcomes of endoscopic resection and metachronous cancer after endoscopic resection for adenocarcinoma of the esophagogastric junction in Japan. Gastrointest Endosc. 2019;89:1120–1128. doi: 10.1016/j.gie.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 63.Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591–622. doi: 10.1055/a-1811-7025. [DOI] [PubMed] [Google Scholar]
- 64.Yoshii T, Tamai S, Aoyama N, Minamide J, Takagi S, Motohashi O, Nakayama N, Nishimura K, Takata K, Kameda Y. Clinical outcome of endoscopic mucosal resection (EMR) in clinical stage I (cSt I) esophageal cancer. J Clin Oncol. 2009:27. [Google Scholar]
- 65.Nelson DB, Mitchell KG, Weston BR, Betancourt S, Maru D, Rice DC, Mehran RJ, Sepesi B, Antonoff MB, Walsh GL, Swisher SG, Roth JA, Vaporciyan AA, Blum M, Hofstetter WL. Should endoscopic mucosal resection be attempted for cT2N0 esophageal cancer? Dis Esophagus. 2019;32:1–6. doi: 10.1093/dote/doz016. [DOI] [PubMed] [Google Scholar]
- 66.Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, Pech O, Repici A, Bergman J, di Pietro M. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191–198. doi: 10.1055/s-0042-122140. [DOI] [PubMed] [Google Scholar]
- 67.Sharma P, Shaheen NJ, Katzka D, Bergman JJGHM. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology. 2020;158:760–769. doi: 10.1053/j.gastro.2019.09.051. [DOI] [PubMed] [Google Scholar]
- 68.Okubo Y, Ishihara R. Endoscopic Submucosal Dissection for Esophageal Cancer: Current and Future. Life (Basel) 2023;13 doi: 10.3390/life13040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joseph A, Draganov PV, Maluf-Filho F, Aihara H, Fukami N, Sharma NR, Chak A, Yang D, Jawaid S, Dumot J, Alaber O, Chua T, Singh R, Mejia-Perez LK, Lyu R, Zhang X, Kamath S, Jang S, Murthy S, Vargo J, Bhatt A. Outcomes for endoscopic submucosal dissection of pathologically staged T1b esophageal cancer: a multicenter study. Gastrointest Endosc. 2022;96:445–453. doi: 10.1016/j.gie.2022.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu K, Aadam AA. T1b esophageal cancer: Is it time for endoscopic submucosal dissection to enter the stage? Gastrointest Endosc. 2022;96:454–456. doi: 10.1016/j.gie.2022.05.004. [DOI] [PubMed] [Google Scholar]