Abstract
γ-Secretase is a membrane protein complex that cleaves the β-amyloid precursor protein (APP) within the transmembrane region, after prior processing by β-secretase, producing amyloid β-peptides Aβ40 and Aβ42. Errant production of Aβ-peptides that substantially increases Aβ42 production has been associated with the formation of amyloid plaques in Alzheimer's disease patients. Biophysical and genetic studies indicate that presenilin-1, which contains the proteolytic active site, and three other membrane proteins [nicastrin, anterior pharynx defective-1 (APH-1), and presenilin enhancer-2 (PEN-2)] are required to form the core of the active γ-secretase complex. Here, we report the purification of the native γ-secretase complexes from HeLa cell membranes and the identification of an additional γ-secretase complex subunit, CD147, a transmembrane glycoprotein with two Ig-like domains. The presence of this subunit as an integral part of the complex itself was confirmed through coimmunoprecipitation studies of the purified protein from HeLa cells and of solubilized complexes from other cell lines such as neural cell HCN-1A and HEK293. Depletion of CD147 by RNA interference was found to increase the production of Aβ peptides without changing the expression level of the other γ-secretase components or APP substrates whereas CD147 overexpression had no statistically significant effect on Aβ-peptide production, other γ-secretase components or APP substrates, indicating that the presence of the CD147 subunit within the γ-secretase complex down-modulates the production of Aβ-peptides.
Keywords: aspartyl protease, membrane protein complex, membrane protein purification
The membrane protein complex γ-secretase was first recognized through its role in the production of the amyloid β (Aβ)-peptides that are pathogenic in Alzheimer's disease (AD) (1). γ-Secretase is a membrane protein complex with unusual aspartyl protease activity that cleaves a variety of type I membrane proteins, such as β-amyloid precursor protein (APP), CD44, deleted in colorectal cancer (DCC), ErbB4, E-cadherin, low-density lipoprotein (LDL) receptor-related protein (LRP), N-cadherin, Nectin-1, and Notch, within their transmembranous regions (2–11); therefore, in addition to its role in AD, γ-secretase has been found to participate in other important biological functions, such as intracellular signaling. γ-Secretase processing of APP requires prior removal of a major fragment of the APP extracellular domain (sAPPβ) by β-secretase to yield a membrane-bound fragment [APP C-terminal fragment (CTF)β]. Subsequent cleavage of this membrane-bound fragment by γ-secretase results in the release of the AD-associated Aβ-peptides (12). The proteolytic activity of γ-secretase is found to be critically dependent not on the specific sequence, but instead on the size of the extracellular domain (13); such sequence-independent characteristics of the substrate are reminiscent of those of the 26S proteasome complex that cleaves substrates in a non-sequence-specific manner. γ-Secretase is present in almost all animal species, vertebrates and invertebrates; it is expressed in many human organs and tissues.
Activation of γ-secretase is highly regulated. Upon formation of the core complex [presenilin-1 (Psn-1), nicastrin (Nct), anterior pharynx defective-1 (APH-1), and presenilin enhancer-2 (PEN-2)], the proteolytic component, Psn-1, is autocatalytically processed to form an active core complex. This processing entails cleavage of Psn-1 to yield an N-terminal fragment (Psn-1 NTF) and a C-terminal fragment (Psn-1 CTF), which remain stably within the complex. Psn-1 contains eight putative transmembrane (TM) domains, with the N-terminal and C-terminal ends as well as the region of autocatalytic processing on the hydrophilic loop between TM6 and TM7 located at the cytoplasmic face of the plasma membrane, according to a widely acknowledged topological model (14, 15), although alternative models have been proposed (16). Nct is a type I transmembrane glycoprotein with a large N-terminal extracellular domain and a short (20 residues) intracellular domain. The first evidence that Nct is an essential component of the γ-secretase complex came from RNA interference (RNAi) experiments in Caenorhabditis elegans, where deletion of the Nct gene produced an embryonic lethal phenotype highly reminiscent of those produced by reduction of the activity of genes involved in the Notch-signaling pathway or by reduction of Psn expression (11). The genetic screen in C. elegans designed to search for mutations that cause Notch-signaling defects led to the identification of APH-1 and PEN-2 membrane proteins that play critical roles in γ-secretase activity (17). APH-1 and PEN-2 were subsequently proven to be integral components of γ-secretase complex through coimmunoprecipitation and RNAi experiments, which indicated that they are physically and functionally associated with γ-secretase (18, 19). There is overwhelming evidence, both in vivo and in vitro, that the expression of the four components Psn-1, Nct, APH-1, and PEN-2 is sufficient to produce a complex with γ-secretase activity (20–23).
Although the complexes made up of the four components have been found to be enzymatically active, it is not yet clear whether such complexes constitute the native form of the γ-secretase complex. Interestingly, experiments in which these four subunits were overexpressed in yeast (21), Drosophila cells (20), mammalian cells (22), and budded virus particles from Sf9 cells (24) produced differing levels of γ-secretase activity, with the budding virus particles having by far the highest level of activity. It has been suggested that such different levels of activity might be due to the presence of unknown cofactors or integral components that modulate the activities of the γ-secretase complex in vivo (24, 25). In the present study, we identified a regulatory subunit of γ-secretase, CD147, through the purification of native γ-secretase complex from detergent-solubilized HeLa cell membranes. We confirmed that CD147 is an integral component of γ-secretase by coimmunoprecipitation experiments conducted on both the purified complex and cell membranes from various cell lines. Through RNAi and overexpression investigations in CHO-APP695 cells, we demonstrated that the presence of CD147 in the γ-secretase complex down-modulates the production of Aβ-peptides.
Materials and Methods
Purification of γ-Secretase Complex and Identification of CD147 Subunit. Cells from 50 liters of suspension HeLa cell culture were homogenized by using a glass homogenizer; unbroken cells and cell debris were removed by low speed centrifugation, followed by high-speed centrifugation to collect the membranes. The membranes were solubilized with FOS-CHOLINE-12 detergent (Anatrace, Maumee, OH) and subjected to an additional high-speed centrifugation step to remove the insoluble material. The solubilized membranes were then applied to a Q-Sepharose HP column (Amersham Pharmacia Biosciences). The bound proteins were eluted with a NaCl step gradient (100 mM, 200 mM, 300 mM, 400 mM, 500 mM, and 1 M). Fractions were evaluated by Western blot with antibodies against Nct and Psn-1 CTF. Nct and Psn-1 were found in fractions that eluted at a salt concentration of 200 mM. These fractions were pooled and loaded onto a lentil lectin column (Amersham Pharmacia Biosciences). The bound proteins eluted at 200 mM methyl α-d-mannopyranoside. The eluted fractions were pooled, concentrated, and applied to a Superdex 200 molecular sieve column (Amersham Pharmacia Biosciences). The peak fractions, centered at a molecular mass of ≈250–300 kDa based on a column calibration using various molecular weight standards, were pooled and concentrated. An aliquot of the concentrated sample was analyzed by SDS/PAGE (4–20% Tris·HCl, Bio-Rad), and the gel was stained with Coomassie blue. The concentrated sample was also analyzed for the presence of Psn-1 (NTF and CTF), Nct, PEN-2, and APH-1 by Western blot. After in-gel trypsin digestion, peptides generated from the unidentified 50-kDa band were extracted and analyzed to obtain their amino acid sequences (Molecular Structure Facility, University of California, Davis). A GenBank blast search revealed the homology of the peptides with corresponding fragments of the CD147 sequence.
Antibodies, Quantitative Western Blot, and Coimmunoprecipitation. Anti-Psn-1 CTF (MAB5232), anti-human CD147 (CBL535), anti-APP N-terminal 66–81 aa (MAB348), and anti-Aβ 1–17 aa (MAB1560) monoclonal antibodies were purchased from Chemicon; rabbit anti-Nct (N1660) and goat anti-CD147 (E4029) polyclonal antibodies were purchased from Sigma; anti-Psn-1 NTF (SC-1245) and goat anti-mouse CD147 antibody (T-18, SC9756) were purchased from Santa Cruz Biotechnology; anti-APH-1 antibody H2-D2 and anti-PEN-2 antibodies were kindly provided by G. Yu and T. W. Kim, respectively. Antibody 192, which specifically recognizes sAPPβ, was a gift from P. Schubert (Elan Pharmaceuticals, South San Francisco, CA).
Quantitative Western blots were performed as described (26). Briefly, the blots were developed with the ECL-Plus detection system (Amersham Pharmacia Biosciences), after which the labeled protein was visualized on a FluorChem 8900 digital imaging system (Alpha Innotech, San Leandro, CA). Band intensities were measured densitometrically by using the alphaeasefc software package (Alpha Innotech).
HeLa, HEK293, and neural cell membrane samples for coimmunoprecipitation experiments were prepared by solubilization with FOS-CHOLINE-12 detergent at 4°C for 30 min. After ultra-centrifugation at 120,000 × g for 60 min at 4°C, the supernatants were collected as the solubilized membrane protein. The coimmunoprecipitation was performed as described (18, 19, 27). Briefly, the purified proteins and solubilized cell membranes were incubated with specific antibodies and protein G beads (Amersham Pharmacia Biosciences) at 4°C overnight. Control experiments were carried out by using nonimmunized animal serum from the same species as each specific antibody was developed from. Immunoprecipitates were washed, eluted with reduced 2× SDS Laemmli sample buffer, and then applied to a 4–20% SDS/PAGE gel (Bio-Rad) for immunoblotting.
Cell Lines. Suspension HeLa cells were obtained from the National Cell Culture Center, and HCN-1A cells were purchased from American Type Culture Collection and cultured by using the protocols provided. HEK293 cells were cultured in DMEM containing 10% FBS. CHO-APP695 cells (kindly provided by S. Sisodia) were maintained in F-12K culture medium (Gibco 21127-022) containing 10% FBS, supplemented with 200 μg/ml G418.
The full-length of CHO CD147 cDNA was cloned into pcDNA3.1/zeo(–) expression vector (Invitrogen) and transfected to CHO-APP695 cells by using Lipofectamine 2000 (Invitrogen). Cell lines with stably overexpressed CD147 were selected and cloned by using the antibiotic zeocin. The stably CD147-overexpressed CHO-APP695 cell lines were maintained in F-12K culture medium (Gibco 21127-022) containing 10% FBS, supplemented with 200 μg/ml G418 and 150 μg/ml zeocin.
RNA Interference. Three Stealth small interfering RNA (siRNA) duplex oligoribonucleotides against CHO cell CD147 (GenBank no. AF 320819) were synthesized by Invitrogen. The sequences were as follows: (i) sense 5′-UAUGUCAAGGUUGCUGAUGGUCAGC-3′, antisense 5′-GCUGACCAUCAGCAACCUUGACAUA-3′; (ii) sense 5′-AAAGAGCAGGUAAGGUGUCUUGG-3′, antisense 5′ CCAAGACACACCUUACCUGCUCUUU-3′; and (iii) sense 5′-UUGUCGUUCAUGUGAUGACCACUGC-3′, antisense 5′-GCAGUGGUCAUCACAUGAACGACAA-3′. siRNA oligos were transfected into CHO-APP695 cells by using the BLOCK-iT transfection kit (Invitrogen) according to the manufacturer's protocol. The BLOCK-iT fluorescent oligo that is not homologous to any known genes was used as transfection efficiency detector and a negative control to ensure against induction of nonspecific cellular events caused by introduction of the oligo into cells. Among the three siRNA oligo duplexes against CD147, we selected the one that required the smallest concentration to achieve the desired knock-down effect to perform the titration experiments. Titration of the CD147 siRNA duplex was performed by using graded concentrations as described (28–30). Culture media were replaced with fresh media after 64 h; cells and conditioned media were harvested 8 h later. CD147 and other protein expression levels were examined by quantitative Western blot. sAPPα, sAPPβ, and total sAPP levels in culture media were also examined by quantitative Western blot, whereas Aβ40 and Aβ42 were measured by ELISA.
Aβ40 and Aβ42 Quantitative Assay. Aβ40 and Aβ42 levels in the cell culture media were quantitatively measured by ELISA (catalogue nos. 8940 and 8942; Signet Laboratories, Dedham, MA) according to the protocol provided by the manufacturer. For measuring Aβ40, the culture media were diluted 1:5 with diluents supplied in the kit because of its high concentration. Undiluted culture media were used for detecting Aβ42. The data were statistically analyzed with one-way ANOVA and the Student t test.
Results
Identification of CD147 in the Purified Native γ-Secretase Complex. For the purification of endogenous γ-secretase complex, we developed a multistep chromatographic protocol to obtain purified native γ-secretase complex from detergent-solubilized HeLa cell membranes. The purified sample was analyzed by SDS/PAGE (Fig. 1A) and yielded six strong bands, five of which had apparent molecular masses expected for the previously identified components of the γ-secretase complex (Psn-1 NTF, Psn-1 CTF, Nct, APH-1, and PEN-2) and one unexpected at a molecular mass of ≈50 kDa. The presence of the known components of the γ-secretase complex was confirmed by Western blot (Fig. 1B). The unexpected 50-kDa band was initially identified through amino acid sequencing of proteolytic fragments as CD147, a highly glycosylated membrane protein with an apparent molecular mass of ≈ 50 kDa. The identity of this band as CD147 was confirmed by polyclonal goat anti-human CD147 antibody and monoclonal mouse anti-human CD147 antibody (Fig. 1C).
Fig. 1.
CD147 is found in the purified γ-secretase complex. (A) Purified sample was analyzed by SDS/PAGE (4–20% Tris·HCl gel) where the gel was stained with Coomassie blue. (B) The presence of the previously identified components of the γ-secretase complex was confirmed by Western blot. (C) The 50-kDa band, initially identified as CD147 through amino acid sequencing, was confirmed by Western blot by using two different anti-CD147 antibodies.
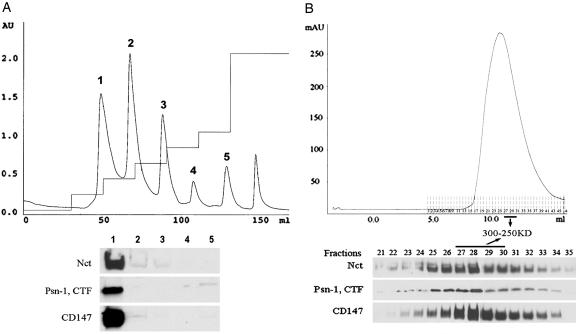
To assess whether or not CD147 is a copurifying contaminant, we evaluated the relative presence of both CD147 and known members of the complex during the entire course of the purification. CD147 was found to coelute with the previously identified γ-secretase components Nct and Psn-1 CTF throughout the purification process, as monitored by immunoblot analysis. In the Q-Sepharose HP chromatographic run, Nct, Psn-1 CTF, and CD147 were found in fractions eluting at a salt concentration of 200 mM (Fig. 2A); in molecular sieve chromatography, these proteins were found to coelute at an apparent molecular mass of 250–300 kDa (Fig. 2B). This molecular mass is consistent with a complex consisting of CD147, Nct, Psn-1, PEN-2, and APH-1.
Fig. 2.
CD147 coelutes with γ-secretase components during chromatographic purification. (A) In the Q-Sepharose HP chromatographic step, CD147, together with Psn-1 CTF and Nct, was found only on fractions eluting at a salt concentration of 200 mM. (Elution steps: 100 mM, 200 mM, 300 mM, 400 mM, 500 mM, and 1 M.) (B) In molecular sieve chromatography, CD147, Psn-1 CTF, and Nct coeluted at a molecular mass of 250–300 kDa.
CD147 Is an Integral Component of the Active γ-Secretase Complex in Native Membranes. The association of CD147 with γ-secretase complex was further explored through coimmunoprecipitation experiments on the purified protein with antibodies to CD147, Psn-1 CTF, and Nct. As anticipated, we found that CD147 antibody immunoprecipitated Psn-1 CTF and Nct (Fig. 3A). Conversely, Psn-1 CTF and Nct antibodies were found to immunoprecipitate each other, as well as CD147 (Fig. 3 B and C). Only the processed form of Psn-1 (Psn-1 cut to form Psn-1 NTF and Psn-1 CTF) was found to coimmunoprecipitate with CD147 antibody. The results of the coimmunoprecipitation experiments strongly indicate that CD147 is an integral component of the γ-secretase complex, not merely a transiently interacting binding partner.
Fig. 3.
Coimmunoprecipitation experiments using purified proteins indicate that CD147 is an integral member of the γ-secretase complex. (A) In purified γ-secretase complex, antibody to human CD147 coimmunoprecipitates Psn-1 CTF and Nct. (B) Anti-Psn-1 CTF antibody coimmunoprecipitates CD147 and Nct. (C) Anti-Nct antibody coimmunoprecipitates CD147 and Psn-1 CTF. The negative controls are shown in lane 3. Asterisks correspond to immunoglobulins.
In addition to purified protein, we conducted similar coimmunoprecipitation experiments with human neural cells (HCN-1A cell line) (Fig. 6A, which is published as supporting information on the PNAS web site) and HEK293 cells (Fig. 6B); HeLa cell membranes were used as a reference (Fig. 6C). It was found that CD147 antibodies could also coimmunoprecipitate Psn-1 and Nct in neural and HEK293 cells. These experiments yielded results similar to that observed for purified protein and showed that CD147 is an integral component of the γ-secretase complex and whose presence is not limited to complexes obtained from HeLa cells.
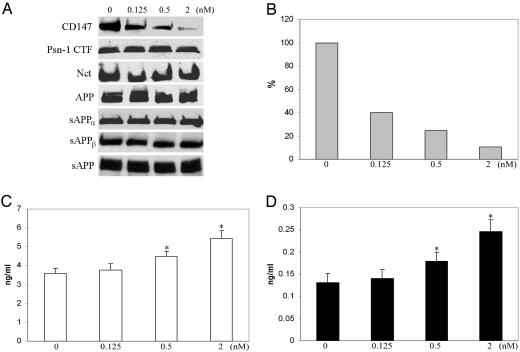
Depletion of CD147 in the CHO-APP695 Cell Line Increases the Production of Aβ-Peptides. The effect of CD147 deletion on γ-secretase activity was examined through silencing studies of the CD147 gene; silencing was accomplished through the transfection of Stealth siRNA against CD147 into CHO-APP695 cells that stably overexpresses human APP695. Titration experiments with graded concentrations of Stealth siRNA duplexes showed that siRNA mediates the down-regulation of CD147 expression in a dose-dependent manner (Fig. 4 A and B) without changing the expression level of other components of γ-secretase, such as Psn-1 CTF and Nct, or the level of soluble APPα(sAPPα), sAPPβ and total sAPP (Fig. 4A). The maximal reduction in CD147 expression was observed at 2 nM of siRNA duplexes. Higher concentrations of CD147 siRNA duplex did not further detectably decrease CD147 expression (Fig. 7, which is published as supporting information on the PNAS web site). Stealth siRNA at a concentration of 2 nM produced ≈90% reduction in CD147 expression; 0.5 nM caused ≈75% reduction and 0.125 nM yielded ≈60% reduction (Fig. 4B). Reduction of 90% CD147 expression surprisingly increased Aβ40 and Aβ42 levels by 52% and 87%, respectively, as measured by ELISA; reduction of 75% CD147 expression increased Aβ40 and Aβ42 levels by 25% and 38% respectively (Fig. 4 C and D); reduction of 60% CD147 expression did not significantly increase the levels of Aβ40 and Aβ42 as compared with controls using transfection reagents without siRNA (Fig. 4 C and D). BLOCK-iT fluorescent oligo was used as the detector of transfection efficiency and another negative control; application of up to 80 nM did not change the expression level of CD147 or the production of Aβ40 and Aβ42.
Fig. 4.
CD147 as a member of the γ-secretase complex regulates the γ-secretase proteolytic activity in the production of Aβ40 and Aβ42 peptides. (A) CD147 RNAi specifically reduces CD147 expression in CHO-APP695 cells without changing the expression of other γ-secretase components. The expression levels were measured by quantitative Western blots. CD147 RNAi does not change the amount of sAPPα, sAPPβ, and sAPP in culture media. (B) Graded concentrations of Stealth CD147 siRNA duplexes show a dose-dependent reduction in CD147 expression. The levels of Aβ40 (C) and Aβ42 (D) peptides in culture media were measured by ELISA, showing that the depletion of CD147 increased the production of Aβ-peptides. Error bars represent standard deviation. Asterisks indicate a significant difference from control (in absence of siRNA), as determined by one-way ANOVA (P < 0.01). The results in B–D were from six repeated experiments.
Overexpression of CD147 Does Not Affect the Production of Aβ-Peptide in CHO-APP695 Cells. To complement the RNAi studies, we conducted experiments to overexpress CD147 in the same cell line, CHO-APP695, used for the silencing studies. We established stable CD147 overexpression cell lines by transfecting the full-length CD147 pcDNA 3.1/zeo(–) construct plasmid DNA into CHO-APP695 cells. We compared the production of Aβ40 and Aβ42 in CD147-overexpressing and non-CD147-overexpressing CHO-APP695 cell lines. From these experiments, it was found that CD147 overexpression had no statistically significant effect on amyloid β-peptide production (Fig. 5 B and C) and caused no significant change in the expression levels of APP or γ-secretase components Psn-1 CTF and Nct (Fig. 5A).
Fig. 5.
Overexpression of CD147 on CHO-APP695 cells does not lead to significant changes in the production of Aβ40 and Aβ42 or in the expression of other γ-secretase components and APP. (A) The expression levels of CD147, APP, Nct, and Psn-1 CTF were measured by Western blots. The expression level of CD147 was substantially increased in CD147 overexpressed CHO-APP695 (lane 2) compared with CHO-APP695 (lane 1) cell line; the expression levels of APP, Nct, and Psn-1 CTF in the CHO-APP695 (lane 1) and CD147 overexpressed CHO-APP695 cell lines (lane 2) were comparable. The levels of Aβ40 and Aβ42 are shown in B and C, respectively. Error bars represent standard deviation. There are no statistically significant differences in the production levels of Aβ40 and Aβ42 by the CHO-APP695 and CD147 overexpressed CHO-APP695 cell lines, based on Student t test analysis (P > 0.05).
Discussion
Our efforts to purify endogenous γ-secretase from detergent-solubilized HeLa cell membranes led to the discovery of an additional component of the native complex. Samples of the purified complex were found to contain the previously identified components of γ-secretase (Psn-1 NTF, Psn-1 CTF, Nct, APH-1, and PEN-2) and an additional membrane protein, CD147. The finding that CD147 is an integral subunit of the γ-secretase complex and not a transient binding partner (or copurifying contaminant) was confirmed through coimmunoprecipitation experiments conducted on the purified complex with antibodies to CD147, Psn-1 CTF, and Nct. Coimmunoprecipitation experiments with solubilized membranes from human neural cells (HCN-1A) and HEK293 cells also demonstrated that CD147 is an integral component of γ-secretase complexes in cells other than HeLa cells.
The role of CD147 within the γ-secretase complex was examined through silencing experiments. Suppression of CD147 expression resulted in specific dosage-dependent increased levels of Aβ-peptide production without changing the expression level of the other γ-secretase components or available APP substrates. The results of the silencing experiments also suggested that a fraction of the CD147 population is not integrally associated with γ-secretase; this notion is supported by reports that CD147 participates in a range of biological responses (31–37). The presence of such forms of CD147 is also apparent in the results of the coimmunoprecipitation studies conducted for this effort where, after multiple immunoprecipitation cycles for the γ-secretase complex using Psn-1 CTF or Nct antibodies, a substantially higher amount of CD147 compared with Psn-1 or Nct remained in detergent solubilized membrane preparations (data not shown).
Could the increase in Aβ40 and Aβ42 production attributable to CD147 silencing be due to reduction of potential non-γ-secretase-associated CD147? This question was addressed by experiments in which CD147 was overexpressed in the same cell type used for the silencing studies (CHO-APP695). Overexpression of CD147 in these cells did not lead to statistically significant alterations in the production of Aβ40 and Aβ42 or the expression of the other γ-secretase subunits or available APP substrates, indicating that the effect of silencing CD147 on Aβ-peptide production is through those molecules of CD147 that are part of the γ-secretase complex. Taken together, the results of the coimmunoprecipitation studies and the silencing and overexpession experiments demonstrate that CD147 is an integral regulatory subunit of the native γ-secretase complex whose deletion increases the production of Aβ-peptides.
The presence of an integral member of γ-secretase membrane protein complex with a regulatory effect on γ-secretase activity was not completely unexpected. Although the overexpresed complexes made up of Psn-1, Nct, APH-1, and PEN-2 have been demonstrated to be enzymatically active, it has not, however, been shown that four subunits alone constituted the complete native form of the γ-secretase complex. Experiments in which these four subunits were overexpressed in different eukaryotic cell types [yeast, Drosophila, and mammalian cells (20–22)] yielded significantly varied levels of γ-secretase activity. From such observations, it has been suggested that unknown cofactors may be modulating the activities of the γ-secretase complex in vivo (25).
The molecular mechanism by which CD147 exerts its effect on the activity of the γ-secretase complex remains to be elucidated. CD147, also referred to as basigin and EMMPRIN, is ubiquitously expressed in a variety of cells and tissues (31). It is predicted to have a short cytoplasmic domain consisting of ≈40 aa, a putative transmembrane region ≈25 aa long and a large extracellular region that contains two Ig-like domains (32). In addition, the amino acid sequence of the putative transmembrane region and its neighboring residues are highly conserved. Noteworthily, a charged residue, glutamic acid, is present in the middle of the putative transmembrane domain and is not found in any known γ-secretase substrates. Such charged residues are not commonly found in proteins that span the membrane only once; the placement of a charged residue in the middle of the lipid bilayer would be highly energetically unfavorable. This structural feature suggests that CD147 associates with other membrane proteins to exist in an energetically stable state. It should be noted that Psn-1 contains two negatively charged residues, aspartic acids, within its putative transmembrane region; how the charged residues of Psn-1 and CD147 are accommodated within the context of the lipid bilayer is not clear and the answer must await the high-resolution structure determination of the complex. CD147 is known to be an antigen or antigen carrier (32, 33) and is also believed to be involved in neural-glial cell interactions (34). It has been reported to be a membrane protein with multiple roles supporting a diverse range of biological activities, such as reproduction, neural function, inflammation, protein trafficking and tumor invasion (35–39); such multiple functional characteristics attributed to a single protein is not unusual and has been observed in other protein complexes, for example the 26S proteasome, where a subcomplex of the regulatory particle has also been found to participate in markedly different biological processes (40). The connection of many of the previously described functional characteristics of CD147 to its role in γ-secretase activity is not clear; however, the results of the CD147 silencing and overexpression studies on Aβ-peptide production presented here do not seem to support a mechanism of CD147 regulation of γ-secretase activity involving protein trafficking.
CD147 has been found to interact with several proteins, including members of the cyclophilin family, monocarboxylate transporters, and matrix metalloproteinase type 1 (MMP 1) (37, 41, 42). For example, cyclophilin A, a peptidyl prolyl cis/trans isomerase, binds to a proline in the second Ig-like domain of CD147. Determination of whether and, if so, how the interactions of these proteins with CD147 can affect the nature of CD147's structural conformation and/or interaction with the other members of the γ-secretase complex (Psn-1, Nct, APH-1, and PEN-2) is an obvious direction for future research.
An increase in the production of Aβ-peptides, especially Aβ42, by removal of CD147 or selected mutations of Psn-1 is expected to increase the formation of Aβ-peptide plaque, a hallmark of AD. Interestingly, deletion of CD147 in mice was found to result in various neurological abnormalities, including severe defects in nervous system development, pronounced spatial learning deficits in the Morris water maze testing, and working memory deficits (43), a behavior phenotype similar to those observed in transgenic mouse models of AD (44, 45). With the discovery of CD147 as an integral subunit of the native γ-secretase complex, obtaining details of the molecular mechanism of CD147 through atomic structure determination of the complex and subsequent functional studies of selected structure-directed mutants will be a crucial step in understanding the molecular processes involved in multiple-site cleavage of a constellation of substrates and in the design of AD therapeutics.
Supplementary Material
Acknowledgments
We thank E. Blakely and K. Bjornstad (Lawrence Berkeley National Laboratory) for kindly providing support for the culturing of HCN-1A neural cells, and S. Sisodia (University of Chicago, Chicago) for providing us with CHO-APP695 cells. We also would like to thank G. Yu (University of Texas Southwestern Medical Center, Dallas) for gifts of anti-APH-1 antibody H2-D2, T. W. Kim (Columbia University, New York) for gifts of anti-PEN-2 antibody, and P. Schubert (Elan Pharmaceuticals) for the antibody 192. This document was prepared as an account of work sponsored by the United States Government. This work was supported by funding from the National Institutes of Health and by the Office of Biological and Environmental Research, U.S. Department of Energy.
Author contributions: S.Z., H.Z., P.J.W., and B.K.J. designed research, performed research, analyzed data, and wrote the paper.
Abbreviations: AD, Alzheimer's disease; APP, β-amyloid precursor protein; Aβ, amyloid β; Psn-1, presenilin-1; NTF, N-terminal fragment; CTF, C-terminal fragment; Nct, nicastrin; APH-1, anterior pharynx defective-1; PEN-2, presenilin enhancer-2; siRNA, small interfering RNA; RNAi, RNA interference.
References
- 1.Selkoe, D. J. (2001) Physiol. Rev. 81, 741–766. [DOI] [PubMed] [Google Scholar]
- 2.Zhang, Z., Nadeau, P., Song, W., Donoviel, D., Yuan, M., Bernstein, A. & Yankner, B. A. (2000) Nat. Cell Biol. 2, 463–465. [DOI] [PubMed] [Google Scholar]
- 3.Lammich, S., Okochi, M., Takeda, M., Kaether, C., Capell, A., Zimmer, A. K., Edbauer, D., Walter, J., et al. (2002) J. Biol. Chem. 277, 44754–44759. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi, Y., Kim, S. H. & Sisodia, S. S. (2003) J. Biol. Chem. 278, 30425–30428. [DOI] [PubMed] [Google Scholar]
- 5.Ni, C. Y., Murphy, M. P., Golde, T. E. & Carpenter, G. (2001) Science 294, 2179–2181. [DOI] [PubMed] [Google Scholar]
- 6.Marambaud, P., Shioi, J., Serban, G., Georgakopoulos, A., Sarner, S., Nagy, V., Baki, L., Wen, P., Efthimiopoulos, S., Shao, Z., et al. (2002) EMBO J. 21, 1948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May, P., Reddy, Y. K. & Herz, J. (2002) J. Biol. Chem. 277, 18736–18743. [DOI] [PubMed] [Google Scholar]
- 8.Marambaud, P., Wen, P. H., Dutt, A., Shioi, J., Takashima, A., Siman, R. & Robakis, N. K. (2003) Cell 114, 635–645. [DOI] [PubMed] [Google Scholar]
- 9.Kim, D. Y., Ingano, L. A. & Kovacs, D. M. (2002) J. Biol. Chem. 277, 49976–49981. [DOI] [PubMed] [Google Scholar]
- 10.Strooper, B. D. & Annaert, W. (2001) Nat. Cell Biol. 3, E221–E225. [DOI] [PubMed] [Google Scholar]
- 11.Yu, G., Nishimura, M, Arawaka, S., Levitan, D., Zhang, L., Tandon, A., Song, Y. Q., Rogaeva, E., Chen, F., Kawarai, T., et al. (2000) Nature 407, 48–54. [DOI] [PubMed] [Google Scholar]
- 12.Hardy, J. & Selkoe, D. J. (2002) Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- 13.Struhl, G. & Adachi, A. (2000) Mol. Cell 6, 625–636. [DOI] [PubMed] [Google Scholar]
- 14.Li, X., Greenwald, I. (1998) Proc. Natl. Acad. Sci. USA 95, 7109–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doan, A., Thinakaran, G., Borchelt, D. R., Slunt, H. H., Ratovitsky, T., Podlisny, M., Selkoe, D. J., Seeger, M., Gandy, S. E., Price, D. L., et al. (1996) Neuron 17, 1023–1030. [DOI] [PubMed] [Google Scholar]
- 16.Dewji, N. N., Valdez, D. & Singer, S. J. (2004) Proc. Natl. Acad. Sci. USA 101, 1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis, R., McGrath, G., Zhang, J., Ruddy, D. A., Sym, M., Apfeld, J., Nicoll, M., Maxwell, M., Hai, B., Ellis, M. C., et al. (2002) Dev. Cell 3, 85–97. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. F., Shah, S., Li, H., Yu, C., Han, W. & Yu, G. (2002) J. Biol. Chem. 277, 45013–45019. [DOI] [PubMed] [Google Scholar]
- 19.Steiner, H., Winkler, E., Edbauer, D., Prokop, S., Basset, G., Yamasaki, A., Kostka, M. & Haass, C. (2002) J. Biol. Chem. 277, 39062–39065. [DOI] [PubMed] [Google Scholar]
- 20.Takasugi, N., Tomita, T., Hayashi, I., Tsuruoka, M., Niimura, M., Takahashi, Y., Thinakaran, G. & Iwatsubo, T. (2003) Nature 422, 438–441. [DOI] [PubMed] [Google Scholar]
- 21.Edbauer, D., Winkler, E., Regula, J. T., Pesold, B., Steiner, H. & Haass, C. (2003) Nat. Cell Biol. 5, 486–488. [DOI] [PubMed] [Google Scholar]
- 22.Kimberly, W. T., LaVoie, M. J., Ostaszewski, B. L., Ye, W., Wolfe, M. S. & Selkoe, D. J. (2003) Proc. Natl. Acad. Sci. USA 100, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraering, P. C., Ye, W., Strub, J. M., Dolios, G., LaVoie, M. J., Ostaszewski, B. L., van Dorsselaer, A., Wang, R., Selkoe, D. J. & Wolfe, M. S. (2004) Biochemistry 43, 9774–9789. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi, I., Urano, Y., Fukuda, R., Isoo, N., Kodama, T., Hamakubo, T., Tomita, T. & Iwatsubo, T. (2004) J. Biol. Chem. 279, 38040–38046. [DOI] [PubMed] [Google Scholar]
- 25.Iwatsubo, T. (2004) Mol. Psychiatry 9, 8–10. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, S., Witzig, T. E., Timm, M., Haug, J., Wellik, L., Kimlinger, T. K., Greipp, P. R. & Rajkumar, S. V. (2004) Blood 104, 1159–1165. [DOI] [PubMed] [Google Scholar]
- 27.Li, Y. M., Xu, M., Lai, M. T., Huang, Q., Castro, J. L., DiMuzio-Mower, J., Harrison, T., Lellis, C., Nadin, A., Neduvelil, J. G., et al. (2000) Nature 405, 689–694. [DOI] [PubMed] [Google Scholar]
- 28.Ji, J. M., Wernli, M., Klimkait, T. & Erb, P. (2003) FEBS Lett. 552, 247–252. [DOI] [PubMed] [Google Scholar]
- 29.Kosciolek, B. A., Kalantidis, K., Tabler, M. & Rowley, P. T. (2003) Mol. Cancer Ther. 2, 209–210. [PubMed] [Google Scholar]
- 30.Phipps, K. M., Martinez, A., Lu, J., Heinz, B. A. & Zhao, G. (2004) Antiviral Res. 61, 49–55. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu, T. & Miyauchi, T. (2003) Histol. Histopathol. 18, 981–987. [DOI] [PubMed] [Google Scholar]
- 32.Miyauchi, T., Kanekura, T., Yamaoka, A., Ozawa, M., Miyazawa, S. & Muramatsu, T. (1990) J. Biochem. 107, 316–323. [DOI] [PubMed] [Google Scholar]
- 33.Miyauchi, T., Masuzawa, Y. & Muramatsu, T. (1991) J. Biochem. 110, 770–774. [DOI] [PubMed] [Google Scholar]
- 34.Fadool, J. M. & Linser, P. J. (1993) Dev. Dyn. 196, 252–262. [DOI] [PubMed] [Google Scholar]
- 35.Saxena, D. K., Oh-Oka, T., Kadomatsu, K., Muramatsu, T. & Toshimori, K. (2002) Reproduction 123, 435–444. [DOI] [PubMed] [Google Scholar]
- 36.Fan, Q. W., Yuasa, S., Kuno, N., Senda, T., Kobayashi, M., Muramatsu, T. & Kadomatsu, K. (1998) Neurosci. Res. 30, 53–63. [DOI] [PubMed] [Google Scholar]
- 37.Yurchenko, V., Zybarth, G., O'Connor, M., Dai, W. W., Franchin, G., Hao, T., Guo, H., Hung, H. C., Toole, B., Gallay, P., et al. (2002) J. Biol. Chem. 277, 22959–22965. [DOI] [PubMed] [Google Scholar]
- 38.Sun, J. & Hemler, M. E. (2001) Cancer Res. 61, 2276–2281. [PubMed] [Google Scholar]
- 39.Wilson, M. C., Meredith, D. & Halestrap, A. P. (2002) J. Biol. Chem. 277, 3666–3672. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez, F., Delahodde, A., Kodadek, T. & Johnston, S. A. (2002) Science 296, 548–550. [DOI] [PubMed] [Google Scholar]
- 41.Kirk, P., Wilson, M. C., Heddle, C., Brown, M. H., Barclay, A. N. & Halestrap, A. P. (2000) EMBO J. 19, 3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo, H., Li, R., Zucker, S. & Toole, B. P. (2000) Cancer Res. 60, 888–891. [PubMed] [Google Scholar]
- 43.Naruhashi, K., Kadomatsu, K., Igakura, T., Fan, Q. W., Kuno, N., Muramatsu, H., Miyauchi, T., Hasegawa, T., Itoh, A., Muramatsu, T., et al. (1997) Biochem. Biophys. Res. Commun. 236, 733–737. [DOI] [PubMed] [Google Scholar]
- 44.Richardson, J. C., Kendal, C. E., Anderson, R., Priest, F., Gower, E., Soden, P., Gray, R., Topps, S., Howlett, D. R., Lavender, D., et al. (2003) Neuroscience 122, 213–228. [DOI] [PubMed] [Google Scholar]
- 45.Higgins, G. A. & Jacobsen, H. (2003) Behav. Pharmacol. 14, 419–438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.