Abstract
Decay-accelerating factor (DAF) mediates cellular attachment for many human picornaviruses. In most cases, viral binding to DAF is itself insufficient to permit cell infectivity, with a second, functional internalization receptor being required to facilitate this process. Previously, we postulated that the role of DAF in enterovirus cell infection is as a sequestration receptor, maintaining a reservoir of bound virus in an infectious state, awaiting interaction with functional internalization receptors. Many of these functional receptors possess the capacity to induce relatively rapid changes in capsid conformations, resulting in the formation of altered particles (A-type particles). In this report, we show that antibody-cross-linked DAF, in contrast to endogenous surface-expressed forms, can act as a functional virus receptor to mediate coxsackie A21 virus (CAV21) lytic cell infection. In contrast to the situation with ICAM-1-mediated CAV21 infection, in which high levels of A-type particles are formed, cross-linked DAF-induced CAV21 replication occurs in the absence of detectable A-particle formation.
Many human enteroviruses bind to surface-expressed decay-accelerating factor (DAF), but for most of these viruses this interaction is insufficient to mediate cell infection (5, 6, 12, 38, 40, 41, 47). We have suggested that DAF functions as a sequestration receptor for these viruses (40, 41), with the implication that DAF is able to bind virus at the cell surface and maintain it in a conformationally unaltered state to await interaction with a functional internalization receptor. A common feature of the well-characterized functional picornavirus receptors, poliovirus receptor (PVR) and intercellular adhesion molecule-1 (ICAM-1), is a capacity to induce specific changes in viral capsid architecture, resulting in the formation of altered (A-type) particles (3, 10, 17, 18, 23). Whether formation of such particles is crucial for viral cell entry or is simply a redundant by-product of the internalization process is currently an area of much debate. Recent findings that cold-adapted poliovirus mutants can undergo replication at 25°C in the absence of A-particle formation (16) and that poliovirus type 1 A-particles formed independently of receptor interactions are infectious (15) highlight this controversy.
Data in support of the postulate that DAF functions as a sequestration receptor include the findings that DAF-binding coxsackie A21 virus (CAV21) requires interaction with ICAM-1 for cell entry (40) and also that a soluble form of DAF, while inhibiting echovirus 7 cell attachment, is unable to induce A-particle formation (31). Whether there is a causative link between the failure of DAF to induce a conformational change in the virus and also to permit cell infectivity is not known. Recently, we reported that pretreating rhabdomyosarcoma (RD) cells with an antibody to the third short consensus repeat (SCR3) of DAF enhanced the binding of CAV21 to these cells, and experiments with solid-phase-immobilized soluble DAF indicated that this effect was likely to be the result of an antibody-induced configuration change in DAF (42). Importantly, in that study we also recorded that the anti-DAF SCR3 monoclonal antibody (MAb) enhanced cell susceptibility to CAV21 and this MAb-induced infectivity appeared to be mediated through DAF.
In the present study, we investigated the nature of antibody-treated DAF-mediated CAV21 lytic infection of RD cells and showed that it is due to the specific action of extracellular cross-linking of surface DAF. In this environment, MAb cross-linking changes the role of DAF from that of a sequestration receptor to that of a functional uptake receptor. We show that CAV21 can enter RD cells via an ICAM-1 route accompanied by the formation of high levels of A-type particles, whereas entry by the cross-linked DAF route occurs in the absence of detectable levels of A-particle formation. In addition, viral uptake and infectivity mediated by cross-linked DAF are shown to be relatively slow processes, possibly indicating a different route of entry than that mediated by the classical uptake receptor, ICAM-1.
Antibody cross-linking of DAF does not induce ICAM-1 expression.
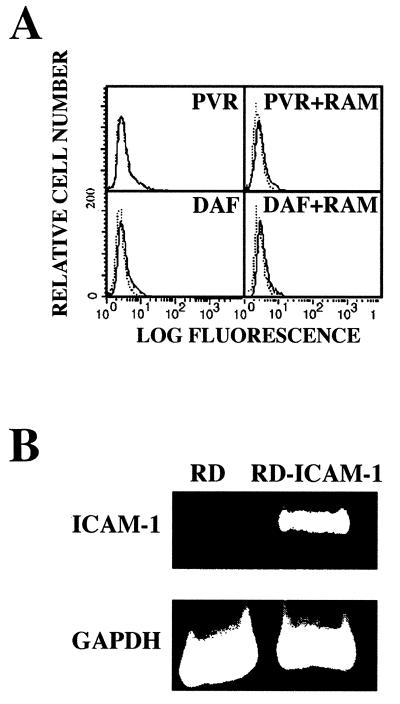
Previously, we have shown that the RD cells used in our studies lack ICAM-1 surface expression and that pretreating these cells with an anti-DAF MAb directed against the DAF SCR3 renders them susceptible to CAV21 lytic infection (42). DAF and ICAM-1 share a spatial association on the surfaces of HeLa cells (40), and DAF can induce signal transduction when cross-linked with murine anti-DAF SCR3 MAbs and rabbit anti-mouse immunoglobulin G (IgG) antibodies (RAM) (13, 28, 43). Therefore, we investigated whether the combined action of MAb binding to DAF SCR3 in association with RAM or MAb binding to DAF SCR3 together with CAV21 binding to DAF SCR1 could induce ICAM-1 expression, thus facilitating CAV21 lytic infection. The fluorescence histograms in Fig. 1A indicate that, as with the control anti-PVR MAb (27), no detectable ICAM-1 expression at 24 h posttreatment was observed on the surfaces of RD cells that had DAF cross-linked with an anti-DAF SCR3 MAb, when tested alone or in combination with RAM. Furthermore, no ICAM-1 was detected on the surfaces of cells exposed to both DAF SCR3 MAbs and CAV21, even when a low level of cytopathic infection was evident (data not shown). At the RNA level, no ICAM-1 mRNA was detected by reverse transcription-PCR from RD cells with antibody-cross-linked DAF compared with that amplified from RD cells stably transfected with ICAM-1 cDNA (RD-ICAM) (Fig. 1B).
FIG. 1.
Detection of ICAM-1 surface and mRNA expression in RD cells after MAb cross-linking of DAF. (A) Flow cytometric analysis of ICAM-1 expression. RD cells were incubated with anti-DAF SCR3 MAb (IH4) or anti-PVR MAb (27) alone or in combination with rabbit-anti-mouse IgG (RAM) for 1 h at room temperature and then washed to remove residual antibody. Following incubation at 37°C for 24 h, cell monolayers were dispersed by treatment with EDTA and cells were incubated with either fluorescein isothiocyanate (FITC)-conjugated anti-ICAM-1 (8) or anti-CD4 (Becton-Dickenson, Sydney, Australia) MAb for 30 min on ice. The cells were then washed and pelleted, resuspended in phosphate-buffered saline–bovine serum albumin, and analyzed with a FACStar analyzer (Becton-Dickenson). The solid histogram represents binding of the FITC-anti-CD4 MAb; the dashed histogram represents binding of the FITC-anti-ICAM-1 MAb. (B) Detection of ICAM-1 mRNA by reverse transcription-PCR. Total cellular RNA from RD cells that were incubated overnight at 37°C in the presence of an anti-DAF MAb and RAM was reverse transcribed using avian myeloblastosis virus reverse transcriptase and oligo(dT) priming. PCR amplification of ICAM-1 cDNA was performed by employing standard methodologies, using the following ICAM-1-specific primers; sense, 5′-AGAACCTTACCCTACGCTGC-3′, and antisense, 5′-CAGTATTACTGCACACGTCAGC-3′.
F(ab′)2 fragments not Fab fragments to DAF SCR3 mediate CAV21 lytic infection.
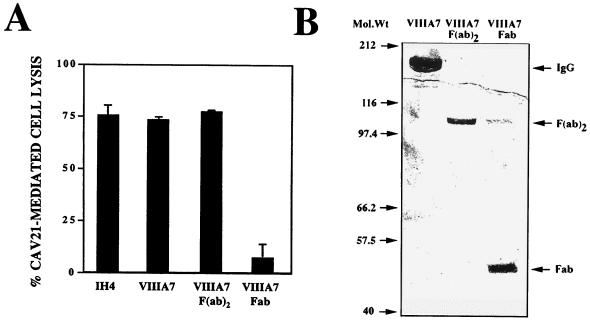
Internalization of DAF is likely to require cross-linking of the molecule (26, 28). We investigated the relative capacity of DAF to mediate CAV21 cell entry and infection after pretreatment with F(ab′)2 or Fab fragments of an anti-DAF SCR2/3 MAb (MAb VIIIA7) (24). MAb VIIIA7 and MAb IH4 (anti-DAF SCR3 [13]) were both shown to increase the infection of RD cells by CAV21, while no lytic infection was observed in cells pretreated with an irrelevant MAb (42). The data in Fig. 2A revealed that CAV21 lytically infects RD cells pretreated with either whole antibody or with F(ab′)2 fragments to DAF SCR2/3 but not with Fab fragments. The small, residual CAV21 lytic infection mediated by the Fab fragments is most probably due to the action of trace contaminating levels of F(ab′)2 fragments in this preparation (Fig. 2B).
FIG. 2.
Induction of CAV21 lytic infection by anti-DAF SCR3 F(ab′)2 fragment binding. (A) F(ab′)2 fragments not Fab fragments to DAF SCR3 mediate CAV21 lytic infection. F(ab′)2 antibody fragments were prepared from whole MAb VIIIA7 (IgG1) by digestion with resin-immobilized ficin and protein G gel chromatography according to the manufacturer’s protocol (Immunopure Kit no. 44880; Pierce, Rockford, Ill.). Confluent monolayers of RD cells in 96-well culture plates were preincubated with an anti-DAF SCR3 MAb (IH4), anti-DAF SCR2/3 (VIIIA7), or VIIIA7 F(ab′)2 or Fab fragments (0.5 μg/ml) prior to challenge with CAV21 (104 50% tissue culture infective doses/well). After incubation for 48 h at 37°C, cell lysis was assessed by staining the monolayers with a crystal violet-methanol solution and then measuring the absorbance at 540 nm on a multiscan enzyme-linked immunosorbent assay plate reader (Flow Laboratories). Results are expressed as the mean percentage of cell lysis relative to untreated controls of triplicate wells ± standard deviation. (B) Polyacrylamide gel electrophoretic analysis of VIIIA7 F(ab′)2 or Fab fragments. Fifty micrograms of whole MAb VIIIA7 or MAb VIIIA7 F(ab′)2 or Fab fragments was separated on a 10% slab sodium dodecyl sulfate-polyacrylamide gel run under nonreducing conditions. Specific protein bands were visualized by staining with Coomassie brilliant blue.
Growth rates of CAV21 in RD-DAF-cross-linked or RD-ICAM-1-expressing cells.
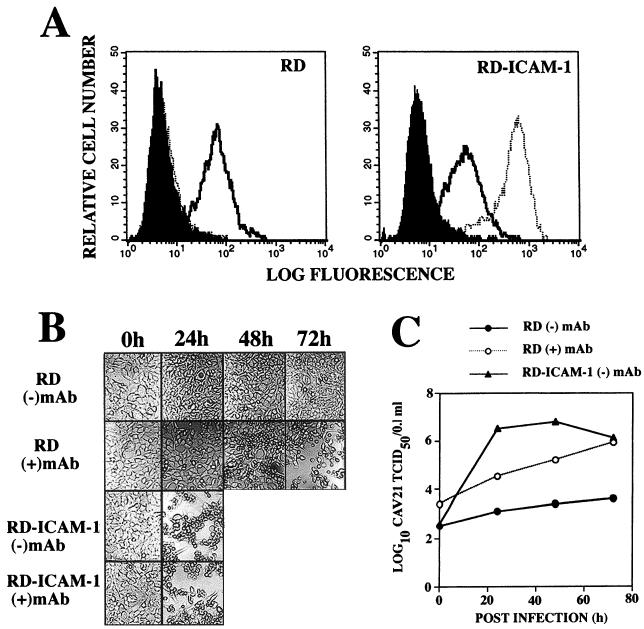
CAV21 can infect cells that use ICAM-1 as a functional receptor in the absence of human DAF (39). We compared the relative growth rates of CAV21 in 24-well monolayers of RD cells stably transfected with ICAM-1 cDNA with that of RD cells in which the surface DAF was cross-linked with an anti-DAF SCR3 MAb. The fluorescence histograms in Fig. 3A confirmed that both RD cells and ICAM-1-expressing RD cells express comparable levels of surface DAF. CAV21 induced complete lytic infection of RD-ICAM cells within 24 h postinfection, in the presence or absence of an anti-DAF SCR3 MAb; while at this time only a low level of lytic infection was evident in the SCR3 MAb-treated RD cells (Fig. 3B). The lytic infection in the SCR3 MAb-treated RD cells continued to progress, and at 72 h postinfection, total destruction of the cell monolayer was observed (Fig. 3B). No visible lytic infection of non-MAb-treated RD cells was evident from 0 to 72 h postinfection (Fig. 3B). The infectious CAV21 yields illustrated in Fig. 3C indicate that CAV21 replication in RD-ICAM cells was more rapid than that observed in MAb-DAF-cross-linked RD cells for which the yields increased with time in an almost linear fashion.
FIG. 3.
Comparison of CAV21 growth rates in RD cells, as mediated by ICAM-1 or cross-linked DAF. (A) Flow cytometric analysis of surface levels of DAF and ICAM-1 on RD and RD-ICAM cells. RD and RD-ICAM cells were incubated with an anti-DAF SCR3 MAb (IH4) or an anti-ICAM-1 MAb (8) diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) (PBS-BSA) on ice for 30 min, after which the cells were washed with 5.0 ml of PBS-BSA. The cells were then pelleted at 1,000 × g for 5 min and resuspended in 100 μl of fluorescein isothiocyanate (FITC)-goat anti-mouse IgG (heavy and light chains) diluted in PBS-BSA. After incubation on ice for 30 min, the cells were washed and pelleted as above, resuspended in PBS-BSA, and analyzed with a FACStar analyzer. The solid histograms represent binding of the FITC-anti-mouse conjugate; the open histograms represent binding of the anti-DAF MAb; the dashed histogram represents the anti-ICAM-1 MAb binding. (B) CAV21-induced lytic infection. Monolayers of RD and RD-ICAM cells in 24-well culture plates were preincubated in the presence or absence of an anti-DAF SCR3 MAb (IH4, 5.0 μg/ml) for 1 h at 37°C prior to challenge with CAV21 (106 50% tissue culture infective doses). After incubation at 37°C for 1 h, the CAV21 inoculum was removed and cell monolayers were washed four times with PBS and then overlaid with 1.0 ml of Dulbecco modified Eagle medium containing 1% fetal calf serum. Cell monolayers were examined for signs of CAV21-induced lytic infection at the times indicated and photographed at a magnification of ×20 with Kodak Technical Pan 100 ASA film. (C) RD-ICAM monolayers in 96-well plates were inoculated with 100 μl of 10-fold serial dilutions of the viral lysates (Fig. 3B) and incubated at 37°C for 48 h. To quantitate cell lysis, monolayers were processed as described in the legend for Fig. 2B. Fifty percent endpoint titers were calculated by the method of Reed and Muench (33), in which a well was scored as positive if its absorbance was less than that of the no virus control − three standard deviations.
Radiolabeled CAV21 binding studies on confluent wells of 24-well tissue culture plates revealed that RD-ICAM cells bound approximately eightfold more virus than MAb-DAF-cross-linked cells (data not shown). However, when the CAV21 growth curve experiment was repeated with 10-fold less virus inoculum on RD-ICAM cells, viral yields almost identical to those shown in Fig. 3C were observed (data not shown). An interesting result was that even though RD-ICAM cells possessed the capacity to bind approximately 8-fold more CAV21 inoculum than MAb-treated RD cells, the infectious viral yield at zero hour postinfection was approximately 10-fold lower than that of MAb-treated RD cells, comparable to that of non-MAb-treated RD cells (Fig. 3C). This finding suggests that DAF-sequestered CAV21 remains significantly more infectious than ICAM-1-bound CAV21.
Conformational change of CAV21.
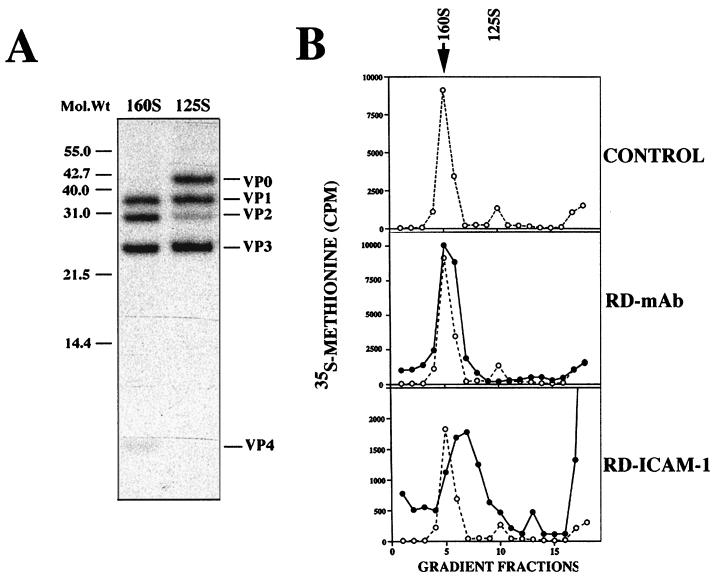
Surface-expressed ICAM-1 mediates the conformational change of intact CAV21 160S virions to 135S (A-type) particles (39), although whether this is important for infectivity is not known. Therefore, we examined whether MAb-cross-linked surface DAF engaged in the process of facilitating CAV21 cell infection could convert CAV21 160S particles to 135S (A-type) particles. Intact CAV21 160S particles and 125S provirions (Fig. 4A) were used as internal migration controls. CAV21 160S particles were bound at 0°C to either ICAM-1 or MAb-cross-linked DAF on the surfaces of RD cells, followed by incubation for 2 h at 37°C. An incubation period of 0.5 to 1.0 h at 37°C is sufficient to convert the majority of cell-bound/internalized poliovirus type 1 and human rhinovirus 14 160S virions to 135S particles (3, 10, 17, 23). Cell-bound or internalized CAV21 particles were released by multiple cycles of freezing and thawing, and their sedimentation was analyzed by sucrose gradient centrifugation. The gradient profiles in Fig. 4B indicate that DAF-bound/internalized CAV21 particles migrated in parallel with the control 160S particles, suggesting no receptor-mediated conversion to 135S particles. In contrast, the majority of the ICAM-1-bound or internalized CAV21 particles migrated in fractions between the intact CAV21 160S and 125S provirion controls at approximately 135S and a small amount migrated at 80S; both of these populations are consistent with receptor-mediated conformational change (3, 10, 14, 17, 23).
FIG. 4.
Receptor-mediated conformational change of CAV21 (A). Electrophoretic analysis of the polypeptide composition of CAV21 160S and 125S particles. 35S-labeled preparations of CAV21 160S and 125S particles were separated on a 15% slab sodium dodecyl sulfate-polyacrylamide gel, and individual proteins were identified by autoradiography. (B) Sedimentation of cell bound/internalized CAV21 virions. Suspensions of RD-MAb (pretreated with an anti-DAF SCR3 MAb; 5.0 μg/ml) and RD-ICAM cells were incubated with radiolabeled CAV21 160S virions for 1 h at 0°C, washed with phosphate-buffered saline (PBS), incubated for 2 h at 37°C, washed again with PBS, and then submitted to five cycles of freezing and thawing. Cell membrane debris was removed by centrifugation, and the supernatants were layered on 5 to 30% linear sucrose gradients (12 ml) and centrifuged for 90 min at 4°C in an SW 41Ti rotor at 36,000 rpm. Fractions (∼700 μl) were collected from the bottom of the gradient, and radioactivity was determined by liquid scintillation counting. The control 160S/125S sedimentation profile has been overlaid on both the gradient profiles from RD-ICAM and RD-MAb cell preparations.
Discussion.
In this study we have shown that CAV21 can lytically infect RD cells by two independent cellular receptors: (i) via ICAM-1, with rapid exponential viral growth and efficient conversion of intact viral particles to A-type particles, and (ii) via MAb-cross-linked DAF, with relatively slow linear viral growth and no or undetectable generation of altered particles. These findings raise the interesting possibility that ICAM-1 and cross-linked DAF use different mechanisms of cell entry to enable CAV21 cell infection. However, an alternate explanation for the reduced rate of CAV21 infection mediated by cross-linked DAF compared with that mediated by ICAM-1 that cannot be discarded is that DAF may induce low levels of A-particle formation, which for this cell entry mechanism is a rate-limiting step; therefore, A-particles are not able to accumulate sufficiently to be detected.
For picornaviruses in general relatively little is known about how the viral genome is delivered into the cytoplasm following conformational capsid alteration. One possibility is that PVR-altered poliovirus type 1 and ICAM-1-altered human rhinovirus 14 particles are internalized by the cell via clathrin-coated pit endocytosis (19, 48). Therefore, ICAM-1-altered CAV21 virions may be expected to enter cells via a similar route (19). However, a recent study has shown that poliovirus infection is not inhibited in susceptible cells expressing a dominant-negative dynamin mutant which interferes with the conversion of clathrin-coated pits to clathrin-coated vesicles (32). In contrast, adenovirus infection requires dynamin and this is consistent with adenovirus entry via the clathrin-coated pit pathway (46). A further area of much debate is whether acidification of the endosome is required for uncoating of the virus (30). This may also be the mechanism employed by picornavirus-integrin receptor complexes (1, 4, 34). All known integrins contain internalizing signals located in the cytoplasmic domain of the β-subunit (22). The internalizing role of integrins in viral infection is postulated to be due to the NPXY amino acid consensus sequences known to play a crucial role in coated-pit-mediated internalization of many cell surface receptors (11).
A further mode of membrane internalization that is less well studied as a potential viral transport system is that through cellular structures referred to as caveolae (35, 36). We would suggest that MAb-cross-linked DAF is likely to mediate CAV21 cell infection through caveolae. Caveolae are specialized cell surface invaginations with several known functions, including endocytosis of macromolecules using mechanisms independent of clathrin-coated pits (26, 35). Caveolae are highly enriched in glycosyl-phosphatidylinositol (GPI)-linked proteins and intracellular signalling molecules (37), while cellular coated-pit structures appear to selectively exclude cholesterol and some GPI-linked proteins (9). It has been shown recently that antibody-induced clustering of one such GPI-linked molecule, DAF, leads to the incorporation of the DAF-antibody complex into closed caveolae which are then endocytosed (26); further, caveolae are 60 to 100 nm in diameter, three to five times larger than the diameter of human enteroviruses. Recently, simian virus 40 has been shown to enter cells via caveolae by using the clustering of major histocompatibility complex class I molecules (2, 44). The RD cells used in the present study may possess caveolae, as evidenced by the content of the caveola marker protein, caveolin (unpublished data), and further studies are under way to test whether MAb-cross-linked DAF translocates sequestered virus to caveolae. It is interesting to note that dynamin is required for normal caveola-mediated internalization (21), therefore, as poliovirus entry does not require dynamin (32), it may be postulated that poliovirus cell entry is not mediated via caveolae.
The prospect of anti-DAF antibodies cross-linking DAF in vivo is unlikely. More probable is the ability of DAF-ligand interactions to cross-link DAF, thus altering DAF structure and/or distribution and, thereby, cell susceptibility to enterovirus infection. Potential cellular DAF cross-linking candidates are CD97, identified as a cellular ligand for DAF (20) and ICAM-1, recently shown to share a spatial association with DAF (40). It can also be postulated that the binding of many enteric pathogens to DAF may have a direct regulatory effect on DAF function. For example, the binding of Escherichia coli-bearing adhesins of the Dr family to DAF SCR3 (29) may cross-link DAF to a level comparable to that induced by anti-DAF MAbs.
Clearly, the primary requirement for viral cell entry is receptor binding, which enables the virus to attach to the cell without being swept away by bodily fluid flow. Previously, this has been assumed to be a function of the specific viral receptor. However, recent work has shown that several enteroviruses bind to the ubiquitously expressed DAF molecule, yet many have quite distinct tissue tropism. This latter feature may be attributable to the use of discrete secondary functional receptors which are essential for entry or replication. For example, CAV21 and coxsackievirus B3 bind to DAF but require interaction with ICAM-1 and the coxsackievirus-adenovirus receptor, respectively, to mediate cell infection (7, 40, 41, 45). By analogy with leukocyte extravasation across endothelial cell barriers (25), we have suggested that the function of the primary DAF receptor is that of a low-affinity sequestration receptor, able to slow down viral motion and facilitate the virus’ encounter with a specific functional receptor (40, 41). While this notion has yet to be tested experimentally, the present results indicate that in some circumstances DAF binding can facilitate viral cell entry and, therefore, this receptor molecule may have functions additional to passive sequestration.
Acknowledgments
The author thanks Andrew Boyd (anti-ICAM-1), Philip Minor (anti-PVR), and Taroh Kinoshita (anti-DAF) for the generous gifts of the MAbs used in this study; Margery Kennett and Kerri Anne Brussen for the stock CAV21 preparation and for many helpful discussions; Gordon Burns for critical review of the manuscript; and Rebecca Ingham and Craig Koina for excellent technical assistance.
This research was supported by a project grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Agrez M V, Shafren D R, Gu X, Cox K, Sheppard D, Barry R D. Integrin αvβ6 enhances coxsackievirus B1 lytic infection of human colon cancer cells. Virology. 1997;239:71–77. doi: 10.1006/viro.1997.8831. [DOI] [PubMed] [Google Scholar]
- 2.Anderson H A, Chen Y, Norkin L C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arita M, Koike S, Aoki J, Horie H, Nomoto A. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J Virol. 1998;72:3578–3586. doi: 10.1128/jvi.72.5.3578-3586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, St. John N, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson J M, Chan B M, Solomon K R, St. John J N, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6249. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson J M, Mohanty J G, Crowell R L, St. John N F, Lublin D M, Finberg R W. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R C, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 8.Boyd A W, Wawryk S O, Burns G F, Fecondo J V. Intercellular adhesion molecule-1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci USA. 1988;85:3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bretscher M S, Thomson J N, Pearse B M. Coated pits act as molecular filters. Proc Natl Acad Sci USA. 1980;77:4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casasnovas J M, Springer T M. Pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): an intermediator in which ICAM-1 is bound and RNA is released. J Virol. 1994;68:5882–5889. doi: 10.1128/jvi.68.9.5882-5889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W J, Goldstein J L, Brown M S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 12.Clarkson N A, Kaufman R, Lublin D M, Ward T, Pipkin P A, Minor P D, Evans D J, Almond J W. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J Virol. 1995;69:5497–5501. doi: 10.1128/jvi.69.9.5497-5501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne K E, Hall S E, Thompson E S, Arce M A, Kinoshita T, Fujita T, Anstee D J, Rosse W, Lublin D M. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992;149:2906–2913. [PubMed] [Google Scholar]
- 14.Crowell R L, Philipson L. Specific alteration of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971;8:509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry S, Chow M, Hogle J M. The poliovirus 135S particles is infectious. J Virol. 1996;70:7125–7131. doi: 10.1128/jvi.70.10.7125-7131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dove A W, Racaniello V R. Cold-adapted poliovirus mutants bypass a postentry replication block. J Virol. 1997;71:4728–4735. doi: 10.1128/jvi.71.6.4728-4735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fricks C E, Hogle J M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez Yafal A, Kaplan G, Racaniello V R, Hogle J M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 19.Grunert H P, Wolf K U, Langner K D, Sawitzky D, Habermehl K O, Zeichhardt H. Internalization of human rhinovirus 14 into HeLa and ICAM-1-transfected BHK cells. Med Microbiol Immunol. 1997;186:1–9. doi: 10.1007/s004300050039. [DOI] [PubMed] [Google Scholar]
- 20.Hamann J, Vogel B, Van Schijndel G M W, van Lier R A W. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF) J Exp Med. 1996;184:1185–1189. doi: 10.1084/jem.184.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henley J R, Krueger E W, Oswald B J, McNiven M A. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan G, Freistadt M S, Racaniello V R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990;64:4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita T, Medof M E, Silber R, Nussenzweig V. Distribution of decay accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162:75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence M B, Springer T A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 26.Mayor S, Rothberg K G, Maxfield F R. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 27.Minor P D, Pipkin P A, Hockley D, Schild G C, Almond J W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1:203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson-Weller A, Wang C E. Structure and function of decay accelerating factor CD55. J Lab Clin Med. 1994;123:485–491. [PubMed] [Google Scholar]
- 29.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesion in a model of a cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell R M, Ward T, Evans D J, Almond J W. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J Virol. 1997;71:9306–9312. doi: 10.1128/jvi.71.12.9306-9312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racaniello, V. R. 1998. Personal communication.
- 33.Reed L J, Muench H A. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–496. [Google Scholar]
- 34.Roivainen M, Piirainen L, Hovi T, Virtanen I, Riikonen T, Heino J, Hyypia T. Entry of coxsackievirus A9 into host cells: specific interactions with αvβ3 integrin, the vitronectin receptor. Virology. 1994;203:357–365. doi: 10.1006/viro.1994.1494. [DOI] [PubMed] [Google Scholar]
- 35.Rothberg K G, Ying Y S, Kolhouse J F, Kamen B A, Anderson R G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990;110:637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothberg K G, Heuser J E, Donzell W C, Ying Y S, Glenney J R, Anderson R G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 37.Sargiacomo M, Sudol M, Tang Z, Lisanti M P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafren D R, Dorahy D J, Greive S J, Burns G F, Barry R D. Mouse cells expressing human intercellular adhesion molecule-1 are susceptible to infection by coxsackievirus A21. J Virol. 1997;71:785–789. doi: 10.1128/jvi.71.1.785-789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafren D R, Dorahy D J, Ingham R A, Burns G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule-1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafren D R, Williams D T, Barry R D. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J Virol. 1997;71:9844–9848. doi: 10.1128/jvi.71.12.9844-9848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafren D R, Dorahy D J, Thorne R F, Kinoshita T, Barry R D, Burns G F. Antibody binding to individual short consensus repeats of decay-accelerating factor enhance enterovirus binding and cell infection. J Immunol. 1998;160:2318–2323. [PubMed] [Google Scholar]
- 43.Shenoy-Scaria A M, Kwong J, Fujita T, Olszowy M W, Shaw A S, Lublin D M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 44.Stang E, Kartenbeck J, Parton R G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward T, Pipkin P A, Clarkson N A, Stone D M, Minor P D, Almond J W. Decay accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 1994;13:5070–5074. doi: 10.1002/j.1460-2075.1994.tb06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willingmann P, Barnert H, Zeichhardt H, Habermehl K O. Recovery of structurally intact and infectious poliovirus type 1 from HeLa cells during receptor-mediated endocytosis. Virology. 1989;168:417–420. doi: 10.1016/0042-6822(89)90286-9. [DOI] [PubMed] [Google Scholar]