Abstract
Background and Aims
This study assessed whether baseline triggering receptor expressed on myeloid cells [TREM-1] whole blood gene expression predicts response to anti-tumour necrosis factor [anti-TNF] therapy in patients with ulcerative colitis [UC] or Crohn’s disease [CD].
Methods
TREM-1 whole blood gene expression was analysed by RNA sequencing in patients with moderately to severely active UC or CD treated with adalimumab in the Phase 3 SERENE-UC and SERENE-CD clinical trials. The predictive value of baseline TREM-1 expression was evaluated and compared according to endoscopic and clinical response vs non-response, and remission vs non-remission, at Weeks 8 and 52 [SERENE-UC], and Weeks 12 and 56 [SERENE-CD].
Results
TREM-1 expression was analysed in 95 and 106 patients with UC and CD, respectively, receiving standard-dose adalimumab induction treatment. In SERENE-UC, baseline TREM-1 expression was not predictive of endoscopic response [p = 0.48], endoscopic remission [p = 0.53], clinical response [p = 0.58], or clinical remission [p = 0.79] at Week 8, or clinical response [p = 0.60] at Week 52. However, an association was observed with endoscopic response [p = 0.01], endoscopic remission [p = 0.048], and clinical remission [p = 0.04997] at Week 52. For SERENE-CD, baseline TREM-1 expression was not predictive of endoscopic response [p = 0.56], endoscopic remission [p = 0.33], clinical response [p = 0.07], or clinical remission [p = 0.65] at Week 12, or endoscopic response [p = 0.61], endoscopic remission [p = 0.51], clinical response [p = 0.62], or clinical remission [p = 0.97] at Week 56.
Conclusions
Baseline TREM-1 gene expression did not uniformly predict adalimumab response in SERENE clinical trials. Further research is needed to identify potential blood-based biomarkers predictive of response to anti-TNF therapy in patients with inflammatory bowel disease.
Clinicaltrials.gov identifiers
Keywords: Biomarkers, clinical trials
1. Introduction
Ulcerative colitis [UC] and Crohn’s disease [CD] are immune-mediated inflammatory bowel diseases [IBDs] with a complex interplay of genetic and environmental factors involved in their pathogenesis.1 Adalimumab is an anti-tumour necrosis factor [anti-TNF] agent that is approved by the US Food and Drug Administration2 and the European Medicines Agency3 for the treatment of moderately to severely active UC and CD. Although anti-TNF agents such as adalimumab play an important role in the management of IBD, primary non-response to anti-TNFs occurs in 10–40% of patients.4 Identifying factors that predict response to anti-TNF therapy may improve the success of the treat-to-target approach recommended in IBD management guidelines.5,6
Conflicting evidence exists regarding whether baseline triggering receptor expressed on myeloid cells [TREM-1] whole blood gene expression level is predictive of response to anti-TNF agents in patients with UC or CD.7–11 In one study, baseline whole blood TREM-1 expression was downregulated in a mixed population of patients with UC or CD who achieved endoscopic remission following anti-TNF treatment, and low gut mucosa and whole blood expression of TREM-1 demonstrated similar accuracy as biomarkers of anti-TNF responsiveness; in contrast, a low serum TREM-1 protein level was not predictive of response.7 Another study showed that low baseline whole blood TREM-1 levels could be used as a potential predictive biomarker of anti-TNF therapeutic efficacy, in terms of mucosal healing in patients with CD.8 Consistent with these results, further analysis found an association between high TREM-1 expression levels in cluster of differentiation 14-positive monocytes and decreased differentiation to M2-type regulatory macrophages, which are involved in mediating the anti-TNF response, indicating that response to anti-TNF agents may be linked to low TREM-1 levels in monocytes in patients with IBD.9 In contrast to these findings, significantly downregulated baseline TREM-1 whole blood expression was predictive for non-response to anti-TNF therapy in a small cohort of patients with CD, based on clinical and/or endoscopic improvement of IBD-related symptom response criteria.10 Gene expression data from a cell-centred meta-analysis conducted by the authors of this latter study suggested that, in individuals who do not respond to anti-TNF agents, an increase in inflammatory macrophages is associated with upregulation of TREM-1 and chemokine receptor type 2-chemokine ligand 7 axes.10 Further, results from a study of paediatric patients with IBD suggested that baseline TREM-1 whole blood expression was not predictive for response to anti-TNF therapy, with no differences seen between responders and non-responders.11 One potential explanation for these opposing findings is the widespread use of corticosteroid [CS] drugs among patients with IBD.12
The effect of CS use on TREM-1 expression is unclear and may be dependent on the individual CS. For example, in murine models, the CS dexamethasone has been shown to suppress TREM-1 expression on neutrophils, with this suppressive effect being mediated by TNF-alpha; in contrast, hydrocortisone had no effect on TREM-1 expression compared with controls.13 The differences in study designs, patient populations and their CS use, and findings mean that the potential utility of baseline TREM-1 whole blood gene expression as a predictive biomarker for response to anti-TNF therapy has yet to be elucidated.
The aim of the current study was to determine whether baseline TREM-1 whole blood gene expression was predictive of outcomes following standard adalimumab treatment in patients with UC or CD in the large, randomized, Phase 3 SERENE-UC and -CD studies.14,15
2. Methods
2.1. SERENE-UC and SERENE-CD study designs
SERENE-UC [NCT02065622]14 and SERENE-CD [NCT02065570]15 were Phase 3, double-blind, randomized, multicentre clinical trials that evaluated higher vs standard adalimumab induction dosing regimens [Table 1] followed by adalimumab maintenance therapy in adult patients with moderately to severely active UC [n = 852] or CD [n = 514]. The primary endpoint of SERENE-UC was clinical remission as per full Mayo score at Weeks 8 and 52. The co-primary endpoints of SERENE-CD were Clinical Disease Activity Index clinical remission at Week 4 and endoscopic response at Week 12. The full methods and results of SERENE-UC14 and SERENE-CD15 have been published previously.
Table 1.
Adalimumab induction dosing regimens in SERENE-UC and SERENE-CD.
| SERENE-UC14 | SERENE-CD15 | |
|---|---|---|
| Higher dose | 160 mg at Weeks 0, 1, 2, and 3, followed by 40 mg at Weeks 4 and 6 | 160 mg at Weeks 0, 1, 2, and 3, followed by 40 mg every other week from Weeks 4 to 12 |
| Standard dose | 160 mg at Week 0 followed by 80 mg at Week 2 and 40 mg at Weeks 4 and 6 | 160 mg at Week 0 followed by 80 mg at Week 2 and 40 mg every other week from Weeks 4 to 12 |
CD, Crohn’s disease; UC, ulcerative colitis.
2.2. Assessment of clinical outcomes
In the present study, clinical and endoscopic response and remission, defined in Table 2, were assessed at Weeks 8 and 52 in the SERENE-UC population, and at Weeks 12 and 56 in SERENE-CD participants.
Table 2.
Definitions of clinical and endoscopic response and remission used in the analysis of data from SERENE-UC and SERENE-CD, and in previously published studies of the ability of baseline whole blood TREM-1 expression to predict response to anti-TNF treatment in patients with IBD.
| Endpoint | SERENE-UC | SERENE-CD | Verstockt et al.7 | Prins et al.9 | Gaujoux et al.10 | |||
|---|---|---|---|---|---|---|---|---|
| Week 8 | Week 52 | Week 12 | Week 56 | UC | CD | CD | UC/CD | |
| Week 8/14 | 6 months | 6 months | Week ≥14 | |||||
| Clinical response | Full Mayo score decrease of ≥3 points and ≥30% from baseline, plus a ≥1-point decrease in rectal bleeding subscore or an absolute bleeding score of ≤1 | Full Mayo score decrease of ≥3 points and ≥30% from baseline, plus a ≥1-point decrease in rectal bleeding subscore or an absolute bleeding score of ≤1 in Week 8 responders | ≥70-point decrease in CDAI score | ≥70-point decrease in CDAI score | — | — | — | Physician-defined clinical and/or endoscopic improvement of IBD symptoms plus a decision to continue anti-TNF therapy |
| Clinical remission | Full Mayo score ≤2 with no subscore >1 | Full Mayo score ≤2 with no subscore >1 in Week 8 remitters | CDAI <150 | CDAI <150 | — | — | — | — |
| Endoscopic responsea | Endoscopic subscore ≤1 | Endoscopic subscore ≤1 in Week 8 responders | >50% decrease from baseline in SES-CD [or a ≥2-point reduction in patients with a baseline SES-CD of 4] | >50% decrease from baseline in SES-CD [or a ≥2-point reduction in patients with a baseline SES-CD of 4] | — | — | — | — |
| Endoscopic remission | Endoscopic subscore 0 | Endoscopic subscore 0 in Week 8 remitters | SES-CD ≤4 plus ≥2-point decrease from baseline and no subscore >1 in any individual variable | SES-CD ≤4 plus ≥2-point decrease from baseline and no subscore >1 in any individual variable | Endoscopic subscore ≤1 | SES-CD ≤2 | Complete absence of ulcerations | — |
aDescribed as ‘endoscopic improvement’ in the SERENE-UC study.14
CD, Crohn’s disease; CDAI, Clinical Disease Activity Index; IBD, inflammatory bowel disease; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TNF, tumour necrosis factor; TREM-1, triggering receptor expressed on myeloid cells; UC, ulcerative colitis.
2.3. Evaluation of TREM-1 expression
In patients who provided consent for exploratory biomarker analyses, whole blood TREM-1 gene expression was assessed by RNA sequencing [RNA-seq] at baseline and Weeks 2, 4, and 8 for UC, and at baseline and Weeks 2, 4, and 12 for CD. Single-end RNA-seq was performed to generate 76-bp reads. The samples were enriched by poly-A selection and globulin depletion with 14 Universal Human Reference RNA controls in 24 sequencing flow cells. Unique molecular identifier tagging was also used to improve sensitivity. Average unique read numbers were 20.6 million [standard error (SE): 5.04 million] in UC and 15.2 million [SE: 7.2 million] in CD. Soluble TREM-1 protein levels at baseline were also assessed using the Olink inflammation panel.
2.4. Statistical considerations
As this was a post hoc analysis, the sample sizes for each analysis were not pre-specified, and were instead determined by the numbers of patients from each trial who provided consent for exploratory molecular biomarker analyses. Unless otherwise specified, analyses were conducted only in the subset of patients who received standard-dose induction adalimumab. A univariate multiple regression analysis corrected for multiple testing with baseline TREM-1 gene expression [log2 counts per million] as the response and baseline CS use as a predictor was performed using data from each trial separately to assess whether CS use impacted TREM-1 gene expression.
Baseline TREM-1 gene and soluble protein expression were analysed in patients evaluated for clinical outcomes at Weeks 8 and 52 in SERENE-UC, and at Weeks 12 and 56 in SERENE-CD. Two-sample t-tests were used to compare:
Baseline TREM-1 gene and soluble protein expression in clinical remitters vs clinical non-remitters, clinical responders vs clinical non-responders, endoscopic remitters vs endoscopic non-remitters, and endoscopic responders vs endoscopic non-responders. Definitions of all these endpoints are provided in Table 2.
Change in TREM-1 gene expression from baseline to Weeks 2, 4, and 8 for UC, and from baseline to Weeks 2, 4, and 12 for CD.
A logistic regression model was fitted to calculate area under the curve [AUC] values for baseline TREM-1 gene expression for clinical remission and response, and endoscopic remission and response at Weeks 8 and 52 [SERENE-UC] and Weeks 12 and 56 [SERENE-CD].
In addition, baseline TREM-1 gene expression was compared with a two-sample t-test in clinical and endoscopic remitters and responders vs non-remitters and non-responders stratified into quartiles based on Week 4 adalimumab levels [25%: 0‒2.5 mg/mL; 50%: 2.6‒5.4 mg/mL; 75%: 5.5‒8.8 mg/mL; 100%: 8.9‒33.2 mg/mL]. Adalimumab levels were measured using an enzyme-linked immunosorbent assay in SERENE-UC14 and a validated ligand-binding assay in SERENE-CD.15 The drug level stratification analyses included patients who received the higher dose of adalimumab, as well as those treated with the standard dose in SERENE-UC and SERENE-CD, to capture and assess data from patients with the widest possible range of drug exposure levels. Data for all these analyses are presented as box plots of the median [interquartile range], while the p-values were calculated using the baseline mean [standard deviation]. All p-values were adjusted for multiple testing correction using the Benjamini–Hochberg method.16 To evaluate whether the results of this analysis were impacted by non-responders with inadequate drug levels, this analysis was repeated in only patients receiving the standard or high dose of adalimumab with adequate drug levels [>5 µg/mL] at Week 8 [SERENE-UC] and Week 12 [SERENE-CD].
The relationships between baseline TREM-1 gene expression and change from baseline levels, and neutrophil percentage as a proportion of the total leucocyte population, neutrophil absolute counts, and faecal calprotectin were assessed, grouped by endoscopic outcomes, and statistically tested using Pearson’s correlation. Neutrophil percentage analyses were also performed stratified by prior CS use at baseline, given the possible impact of CS use on TREM-1 expression.13 The neutrophil analyses were performed using complete blood count levels, with neutrophils chosen because of their relatively high expression of TREM-1 compared with other leucocytes. All p-values reported throughout are nominal. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
2.5. Ethical statement
The independent ethics committee or institutional review board at each study site approved the study protocol, informed consent forms, recruitment materials, and all other patient information related to both SERENE studies. Examples of ethics committees/institutional review boards for both studies include Advarra Inc. [formerly Quorum Review Inc., USA], Helsinki Committee [Israel], Western University Health Science Research Ethic Board [Canada], and Comitato Etico IRCCS [Italy]. All approvals were obtained before study drug shipment to a site; however, approval reference numbers for each site are not available because the sponsor’s standard operating procedure is to archive studies within 1 year of completion of the clinical study report. All patients in both studies provided written informed consent ahead of screening; informed consent was also provided by patients for optional testing [i.e. by the subset of patients whose samples were used for the exploratory biomarker analyses that are presented here].
3. Results
In SERENE-UC, whole blood TREM-1 gene expression was analysed by RNA-seq in 95 and 70 patients who were receiving the standard adalimumab dose at Weeks 8 and 52, respectively. In SERENE-CD, TREM-1 gene expression was analysed in 106 patients who were receiving the standard dose at Week 12, and in 50 and 48 patients for endoscopic and clinical outcomes, respectively, at Week 56 [clinical data were not available at Week 56 for two patients]. Patient demographics and baseline characteristics are reported for the TREM-1 subsets and the overall study cohorts by endoscopic and clinical response/non-response and remission/non-remission outcomes in SERENE-UC [Weeks 8 and 52; Supplementary Tables 1 and 2] and SERENE-CD [Weeks 12 and 56; Supplementary Tables 3 and 4]. The baseline demographics and clinical characteristics of both the UC and CD patient subsets analysed by endoscopic and clinical outcomes were generally representative of the overall study cohorts that have been reported previously.14,15 An exception to this was noted in SERENE-UC, where across all endpoints analysed, the proportion of male patients was higher in the overall population than in the RNA-seq subgroup [Supplementary Tables 1 and 2]. Additionally, in SERENE-CD, a higher proportion of RNA-seq patients reported aminosalicylate use vs the overall population, and Clinical Disease Activity Index scores were slightly lower in the RNA-seq subgroup vs the overall population across all endpoints analysed [Supplementary Tables 3 and 4]. Importantly, all 95 [100%] patients in the SERENE-UC TREM-1 subset were White; in SERENE-CD, 91.8% of the 106 patients were White, 6.4% were Black or African American, and 1.8% were Asian.
Finally, baseline CS use was associated with significantly lower baseline TREM-1 gene expression in SERENE-UC [p = 0.008], but not in SERENE-CD [p > 0.05].
3.1. Baseline TREM-1 expression levels by endoscopic and clinical outcomes
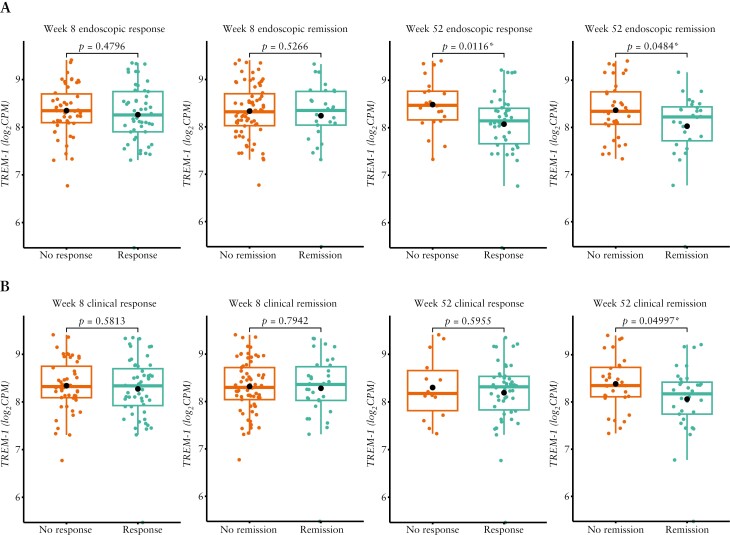
Among patients with UC who received the standard adalimumab dose, baseline TREM-1 gene expression did not differ in those who achieved endoscopic response at Week 8 vs non-responders at this time point; the same was true of endoscopic remitters vs non-remitters at Week 8 [Figure 1A]. Significant but minor differences were observed based on endoscopic response [p = 0.0116] and endoscopic remission [p = 0.0484] at Week 52 [Figure 1A]. Regarding clinical outcomes, baseline TREM-1 gene expression did not differ between patients with UC who achieved clinical response vs non-response at Week 8 or 52, or between clinical remitters vs non-remitters at Week 8; however, there was a significant but minor difference for clinical remission at Week 52 [p = 0.05; Figure 1B]. The AUC values in patients with UC were low and ranged from 0.48 to 0.69.
Figure 1.
Baseline TREM-1 whole blood expression in patients with UC treated with the standard dose of adalimumab in SERENE-UC who had [A] endoscopic response/non-response and remission/non-remission at Weeks 8 and 52 and [B] clinical response/non-response and remission/non-remission at Weeks 8 and 52.a,b Data are median [interquartile range], with the p-values calculated using the baseline mean [standard deviation]. aDefinitions of endoscopic and clinical outcomes are listed in Table 2. bn = 95 at Week 8; n = 70 at Week 52. *p < 0.05. TREM-1, triggering receptor expressed on myeloid cells; UC, ulcerative colitis.
In patients with UC, baseline soluble TREM-1 protein levels did not differ in endoscopic or clinical remitters or responders at Week 8 or 52 vs non-remitters and non-responders, respectively [p > 0.05].
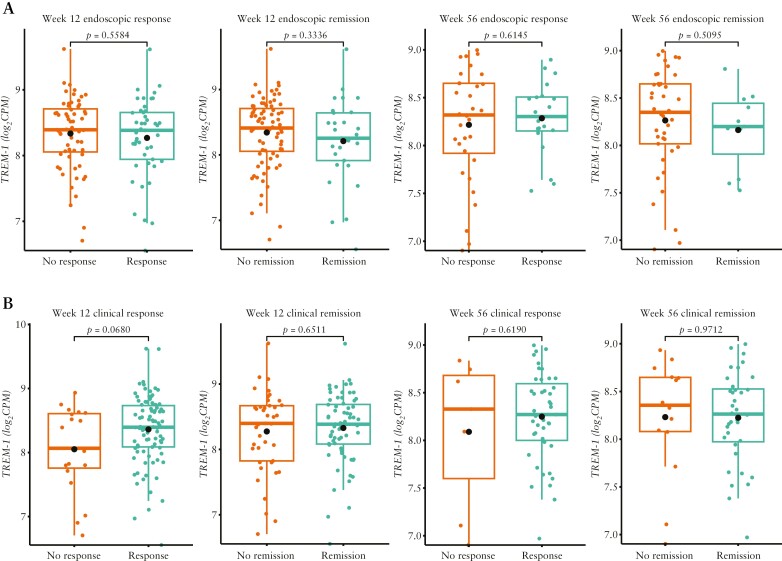
In patients with CD, baseline TREM-1 gene expression did not differ in endoscopic remitters or responders at Week 12 or 56 vs non-remitters and non-responders, respectively, at these time points [Figure 2A]. As with the UC population, there was also no significant difference in baseline TREM-1 gene expression in patients with CD who were clinical remitters vs non-remitters, or clinical responders vs non-responders, at either evaluation time point following standard adalimumab dosing [Figure 2B]. The AUC values in patients with CD were low and ranged from 0.48 to 0.63.
Figure 2.
Baseline TREM-1 whole blood expression in patients with CD treated with the standard dose of adalimumab in SERENE-CD who had [A] endoscopic response/non-response and remission/non-remission at Weeks 12 and 56 and [B] clinical response/non-response and remission/non-remission at Weeks 12 and 56.a,b Data are median [interquartile range], with the p-values calculated using the baseline mean [standard deviation]. aDefinitions of endoscopic and clinical outcomes are listed in Table 2. bn = 106 at Week 12; n = 50 and n = 48 at Week 56 for endoscopic and clinical outcomes, respectively. CD, Crohn’s disease; TREM-1, triggering receptor expressed on myeloid cells.
In patients with CD, baseline soluble TREM-1 protein levels did not differ in endoscopic remitters or responders at Week 12 or 56 vs non-remitters and non-responders, or clinical remitters at Week 56 vs non-remitters [all p > 0.05]; however, there was a nominal difference between clinical responders and non-responders at Week 56 [p = 0.04].
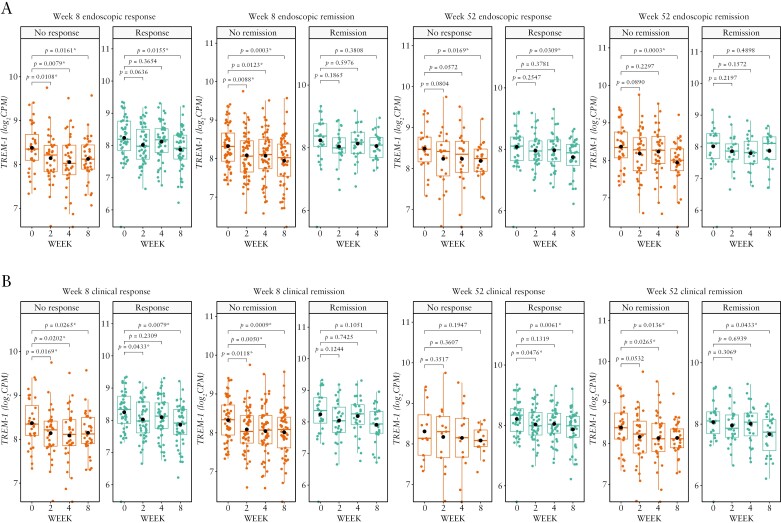
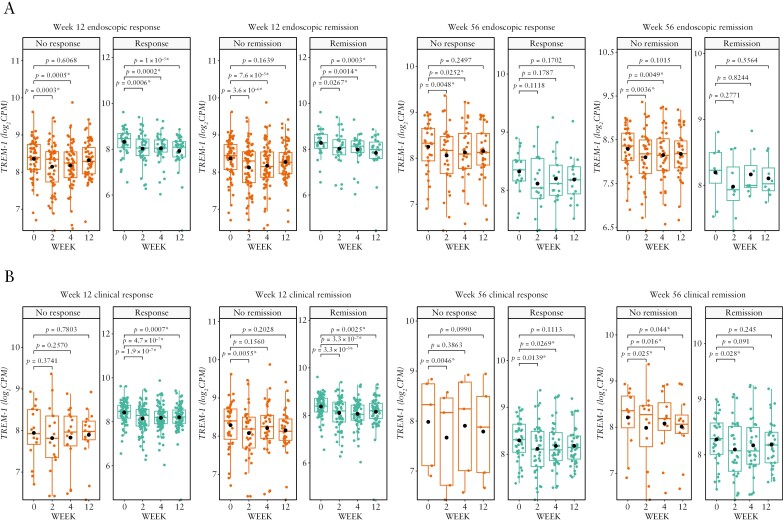
Regardless of endoscopic or clinical outcome at any time point, TREM-1 gene expression generally decreased from baseline over time in both studies [Figures 3 and 4]. In SERENE-UC, the decrease was particularly marked in endoscopic and clinical non-remitters [p < 0.001 at Week 8 among patients who were endoscopic or clinical non-remitters at Week 8 or endoscopic non-remitters at Week 52, and p < 0.05 at Week 8 among clinical non-remitters at Week 52; Figure 3]. By contrast, in SERENE-CD, the greatest decreases from baseline in TREM-1 gene expression were seen in endoscopic and clinical remitters and responders [p < 0.001 at Week 12 among patients who were endoscopic or clinical responders, or endoscopic remitters, and p < 0.01 among patients who were clinical remitters at Week 12; Figure 4].
Figure 3.
TREM-1 whole blood expression over time in patients with UC treated with the standard dose of adalimumab in SERENE-UC who had [A] endoscopic response/non-response and remission/non-remission at Weeks 8 and 52 and [B] clinical response/non-response and remission/non-remission at Weeks 8 and 52.a,b Data are median [interquartile range], with the p-values calculated using the baseline mean [standard deviation]. aDefinitions of endoscopic and clinical outcomes are listed in Table 2. bn = 95 at Week 8; n = 70 at Week 52. *p < 0.05. TREM-1, triggering receptor expressed on myeloid cells; UC, ulcerative colitis.
Figure 4.
TREM-1 whole blood expression over time in patients with CD treated with the standard dose of adalimumab in SERENE-CD who had [A] endoscopic response/non-response and remission/non-remission at Weeks 12 and 56 and [B] clinical response/non-response and remission/non-remission at Weeks 12 and 56.a,b Data are median [interquartile range], with the p-values calculated using the baseline mean [standard deviation]. aDefinitions of endoscopic and clinical outcomes are listed in Table 2. bn = 106 at Week 12; n = 50 and n = 48 at Week 56 for endoscopic and clinical outcomes, respectively. *p < 0.05. CD, Crohn’s disease; TREM-1, triggering receptor expressed on myeloid cells.
Baseline TREM-1 gene expression was not predictive of endoscopic outcomes at Week 8 or 52 when patients with UC receiving either the high [n = 152 at Week 8 and n = 113 at Week 52] or the standard [n = 95 and n = 70, respectively] dose of adalimumab were stratified by Week 4 per-quartile drug levels [Supplementary Figure 1]. Similarly, baseline TREM-1 gene expression was not predictive of endoscopic outcomes at Week 12 or 56 in SERENE-CD when stratified by per-quartile drug levels in patients receiving the high [n = 150 at Week 12 and n = 61 at Week 56] or standard [n = 106 and n = 50, respectively] dose of adalimumab [Supplementary Figure 2]. Regardless of adalimumab dose, baseline TREM-1 gene expression was not predictive of clinical outcomes at Week 8 or 52 in patients with UC [Supplementary Figure 3], or in patients with CD at Week 12 or 56 [Supplementary Figure 4], when stratified by per-quartile drug levels. Similar results were found when the analysis was repeated only in patients with adequate adalimumab levels [>5 µg/mL] at Week 8 [SERENE-UC] and Week 12 [SERENE-CD]. Baseline TREM-1 gene expression did not predict endoscopic outcomes at Week 8 or 52 in patients with UC, or Week 12 or 56 in patients with CD. For clinical outcomes, baseline TREM-1 gene expression was not predictive of clinical response at Week 8 or 52 in patients with UC, or clinical remission at Week 8; however, unlike the Week 4 drug level analysis, it was predictive of clinical remission at Week 52. For patients with CD, baseline TREM-1 gene expression was not predictive of clinical response or remission at Week 12 or 56 [data not shown].
3.2. Association of baseline TREM-1 gene expression with baseline neutrophil percentage and faecal calprotectin grouped by endoscopic outcomes
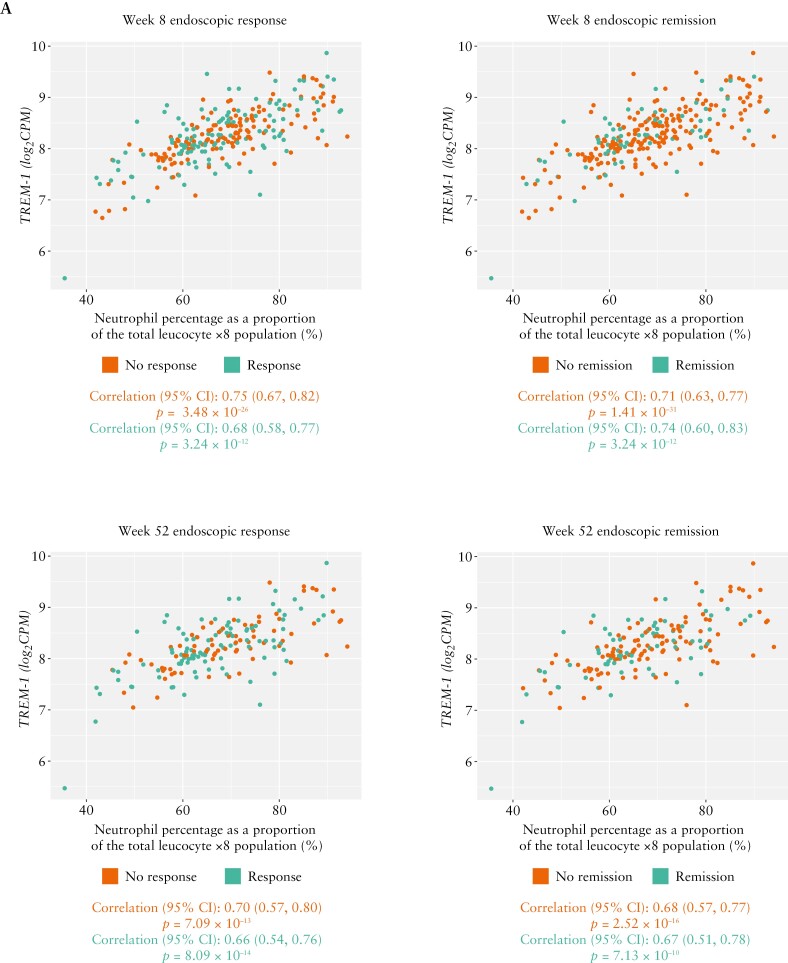
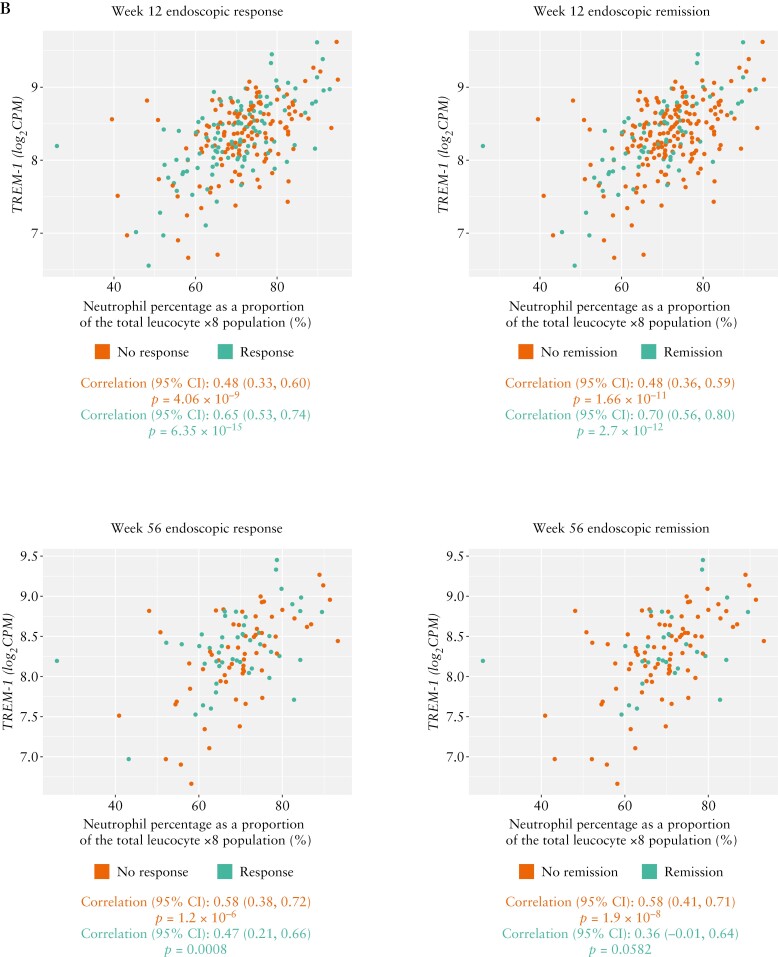
In patients treated with standard-dose adalimumab, baseline neutrophil percentage was highly positively correlated with baseline TREM-1 gene expression in responders and non-responders, and remitters and non-remitters across endoscopic outcomes in patients with UC [Week 8: r = 0.68 for response vs r = 0.75 for non-response, and r = 0.74 for remitters vs r = 0.71 for non-remitters; Week 52: r = 0.66 for response vs r = 0.70 for non-response, and r = 0.67 for remitters vs r = 0.68 for non-remitters; Figure 5A] and CD [Week 12: r = 0.65 for response vs r = 0.48 for non-response, and r = 0.70 for remitters vs r = 0.48 for non-remitters; Week 56: r = 0.47 for response vs r = 0.58 for non-response, and r = 0.36 for remitters vs r = 0.58 for non-remitters; Figure 5B]. All correlations were significant [p < 0.001] except for remitters at Week 56 in SERENE-CD [p = 0.06]. No notable differences were observed between remitters vs non-remitters or responders vs non-responders. Similar results were seen when stratified further by patients with and without steroid exposure history, with no notable differences between groups. In addition, similar patterns were seen when analysing the relationships between absolute neutrophil counts instead of percentages and baseline TREM-1 expression, as well as change from baseline TREM-1 expression and neutrophil percentage [data not shown].
Figure 5.
Association of baseline TREM-1 whole blood expression with baseline neutrophil percentage by endoscopic response/non-response and remission/non-remissiona in patients with UC and CD treated with the standard dose of adalimumab in [A] SERENE-UC and [B] SERENE-CD. All correlations were statistically significant [p < 0.05]. aDefinitions of endoscopic outcomes are listed in Table 2. CD, Crohn’s disease; CI, confidence interval; TREM-1, triggering receptor expressed on myeloid cells; UC, ulcerative colitis.
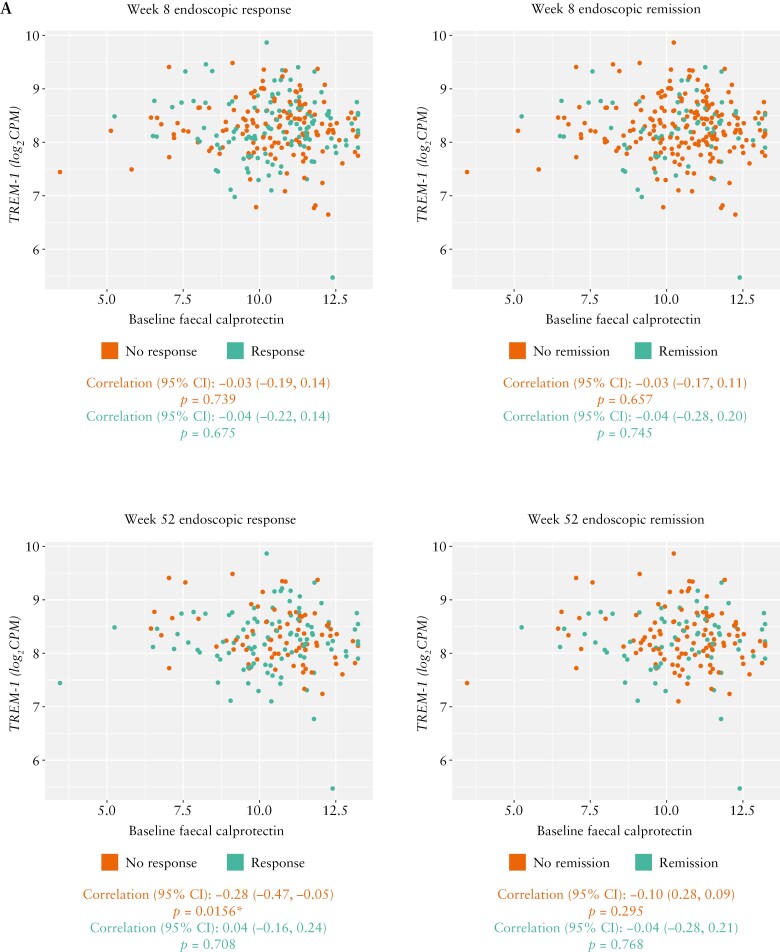
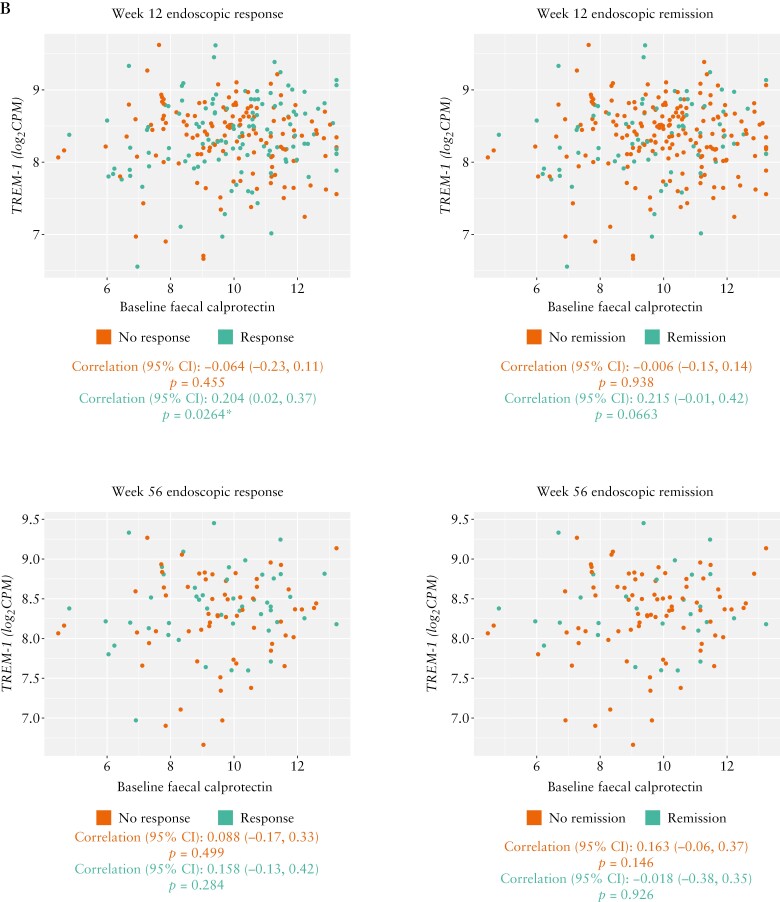
In contrast to the association seen with neutrophils, no strong association was observed between faecal calprotectin levels and baseline TREM-1 gene expression for any endoscopic outcome in patients with either UC [Week 8: r = −0.04 for both responders and remitters vs r = −0.03 for both non-responders and non-remitters; Week 52: r = 0.04 for responders vs r = −0.28 for non-responders, and r = −0.04 for remitters vs r = −0.10 for non-remitters; Figure 6A] or CD [Week 12: r = 0.20 for responders vs r = −0.06 for non-responders, and r = 0.215 for remitters vs r = −0.006 for non-remitters; Week 56: r = 0.16 for responders vs r = 0.09 for non-responders, and r = −0.02 for remitters vs r = 0.16 for non-remitters; Figure 6B]. All correlations were non-significant [p > 0.05], except for endoscopic non-responders at Week 52 in SERENE-UC [p = 0.02] and endoscopic responders at Week 12 [p = 0.03] in SERENE-CD. Similar results were seen for the relationships between change from baseline in faecal calprotectin levels and baseline TREM-1 expression [data not shown].
Figure 6.
Association of baseline TREM-1 whole blood expression with faecal calprotectin by endoscopic response/non-response and remission/non-remissiona in patients with UC and CD treated with the standard dose of adalimumab in [A] SERENE-UC and [B] SERENE-CD. aDefinitions of endoscopic outcomes are listed in Table 2. *p < 0.05. CD, Crohn’s disease; CI, confidence interval; TREM-1, triggering receptor expressed on myeloid cells; UC, ulcerative colitis.
4. Discussion
The findings of this study demonstrate that baseline TREM-1 whole blood gene expression was not a predictor of clinical or endoscopic outcomes following adalimumab treatment in the Phase 3 SERENE studies in patients with moderately to severely active UC or CD. In SERENE-UC, baseline TREM-1 gene expression was weakly associated with endoscopic response and remission after 52 weeks of adalimumab treatment, but this was not sufficiently robust for TREM-1 to be considered a predictive biomarker of response to adalimumab. Similarly, baseline TREM-1 gene expression did not predict clinical outcomes in SERENE-UC, or clinical or endoscopic outcomes in SERENE-CD. Largely similar results were observed when analysed using TREM-1 soluble protein levels. Stratification by per-quartile drug levels did not increase the ability of baseline TREM-1 gene expression to predict outcomes [including when only patients with adalimumab levels >5 µg/mL were analysed], suggesting that these results are not influenced by adalimumab dose.
The results of the current study are consistent with those of both the Personalising Anti-TNF Therapy in Crohn’s disease study of patients with CD [NCT03088449]17 and a small study in 33 children with IBD, in which baseline TREM-1 whole blood gene expression did not predict response to either adalimumab or infliximab.11 However, they contrast with the findings of other studies where TREM-1 appeared to be predictive of clinical and/or endoscopic outcomes in patients with IBD, albeit with opposing signals for low vs high baseline TREM-1 expression levels linked to response.7,8,10
The discrepancy between the results of our study and those of previous studies may be attributable to variations in study designs and patient populations. For example, in the current study, samples were analysed from a relatively large number of patients from the SERENE randomized trials [95 and 106 patients with UC and CD, respectively]. In contrast, previous studies analysed smaller samples of patients with IBD [54,7 22,10 and 3311 patients], generally treated in real-world clinical practice settings. Additionally, the methods used to measure whole blood TREM-1 gene expression differed between studies: we used RNA-seq to measure the full transcript, whereas real-time reverse-transcription polymerase chain reaction was used in the studies by Verstockt and Salvador-Martin, with the full transcript and three TREM-1 isoforms being studied by Verstockt et al. [TREM-1-mb, TREM-1-sv, and TREM-1-x2].7,11 Finally, the definitions of clinical and endoscopic outcomes and the timing of assessments differed between studies [Table 2].
Regardless of clinical or endoscopic remission/non-remission and response/non-response, TREM-1 gene expression generally decreased over time following adalimumab treatment in both SERENE-UC and SERENE-CD. This suggests that TREM-1 could be a marker of anti-TNF treatment. Indeed, the link between downregulation of TREM-1 and response was specific to patients treated with anti-TNF agents in one of the studies by Verstockt et al.7 It is possible that treatment of IBD with anti-TNF agents could reduce signalling through receptors such as TREM-1 and lead to general downregulation, even if this does not result in clinical or endoscopic response or remission.
Of note, a highly positive correlation was observed between baseline TREM-1 gene expression and the baseline percentage of neutrophils in both endoscopic remitters/non-remitters and responders/non-responders in both SERENE-UC [Weeks 8 and 52] and SERENE-CD [Weeks 12 and 56], except for remitters at Week 56 in SERENE-CD. This correlation was unaffected by steroid treatment history, and supports previous reports of TREM-1 activation modulating the function of neutrophils in models of IBD.18 While faecal calprotectin has been related to anti-TNF response and is usually monitored in clinical practice,19,20 we did not find a strong correlation between TREM-1 expression and absolute baseline or change from baseline in faecal calprotectin levels. This is consistent with the findings of Verstockt et al.7
Our analysis was limited in that it focused solely on TREM-1 expression, which has been suggested previously as a potential blood-based biomarker for anti-TNF response. Further analyses, using an unbiased, genome-wide approach as well as genetic studies at the individual level, may allow for identification of novel predictive biomarkers in whole blood and at the cellular level. However, findings generated from such a genome-wide approach would need confirmation in independent cohorts. Another limitation is that all patients in the subset of patients analysed from SERENE-UC and 92% of patients from SERENE-CD were White; this limited diversity means that the analysis may not be generalizable to other racial groups. Finally, dichotomous [response vs non-response]rather than continuous outcomes were analysed, which can lead to a loss of power for detecting a difference between groups; however, these outcomes were based on protocol-defined primary and secondary endpoints used for the primary analysis of each trial.
In summary, the results of this robust analysis in patients with moderately to severely active UC or CD from the large, randomized, Phase 3 SERENE studies demonstrate that baseline TREM-1 whole blood gene expression does not predict endoscopic or clinical outcomes following adalimumab treatment. Consequently, further exploration of potential predictors of response to anti-TNF therapy is needed to identify non-invasive biomarkers that could be used in clinical practice to allow personalized treatment and optimize treat-to-target management of IBD.
Supplementary Material
Acknowledgements
The authors would like to thank Tariq Ahmad for his contributions to the planning/design of the analysis, the statistical analyses, and data interpretation. AbbVie and the authors thank the participants, study sites, and investigators who participated in this clinical trial. Medical writing support was provided by Fraser Harris on behalf of 2 the Nth [Cheshire, UK], and was funded by AbbVie.
Contributor Information
Bram Verstockt, Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium; Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium.
Valerie Pivorunas, Precision Medicine Immunology, AbbVie Bioresearch Centre, Worcester, MA, USA.
Naim Al Mahi, Genomic Research Center, AbbVie, North Chicago, IL, USA.
Nizar Smaoui, Genomic Research Center, AbbVie, North Chicago, IL, USA.
Heath Guay, Precision Medicine Immunology, AbbVie Bioresearch Centre, Worcester, MA, USA.
Nicholas A Kennedy, Department of Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK.
James R Goodhand, Department of Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK.
Simeng Lin, Department of Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK.
Benjamin Y H Bai, Genomics of Inflammation and Immunity Group, Wellcome Sanger Institute, Hinxton, UK.
Stephen B Hanauer, Department of Medicine, Northwestern University Feinberg School of Medicine, Evanston, IL, USA.
Marc Ferrante, Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium; Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium.
Julian Panés, Hospital Clinic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain.
Séverine Vermeire, Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium; Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium.
Conference presentation
Part of this work was presented at the 17th Congress of the European Crohn’s and Colitis Organisation, 16–19 February 2022, Vienna, Austria.
Funding
This work was supported by AbbVie, who funded this study and participated in the study design, research, analysis, data collection, interpretation of data, and review and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Conflict of Interest
BV received research support from AbbVie, Biora Therapeutics, Landos, Pfizer, Sosei Heptares, and Takeda; speaker’s fees from AbbVie, Biogen, BMS, Celltrion, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, Truvion, and Viatris; and consultancy fees from AbbVie, Alimentiv, Applied Strategic, Atheneum, Biora Therapeutics, BMS, Galapagos, Guidepont, Landos, Mylan, Inotrem, Ipsos, Janssen, Progenity, Sandoz, Sosei Heptares, Takeda, Tillotts and Viatris. NAM, VP, NS, and HG are employees of AbbVie and may own stock/options. NAK received research grants from AbbVie, Biogen, Celgene, Celltrion, Galapagos, MSD, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; speaker and/or advisory board member for Allergan, Amgen, BMS, Celltrion, Falk, Galapagos, Janssen, Mylan, Pharmacosmos, Takeda, and Tillotts; and support to attend meetings from AbbVie, Falk, Janssen, Norgine, and Tillotts. JRG received research grants from Biogen, Celltrion, Galapagos, and Roche and non-financial support from Immunodiagnostik. SL received non-financial support outside the submitted work from Ferring and Pfizer. BYHB has no competing interests to declare. SBH reports consultancy and speaker fees, and institutional support, from AbbVie. MF received research grants from AbbVie, Amgen, Biogen, Janssen, Pfizer, Takeda, and Viatris; consultancy fees from AbbVie, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Janssen-Cilag, Medtronic, MSD, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Takeda, and Thermo Fisher; and speaker fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Falk, Ferring, Janssen-Cilag, Lamepro, MSD, Pfizer, Sandoz, Takeda, Truvion Healthcare, and Viatris. JP received research grants from AbbVie and Pfizer; and consultancy and/or speaker fees from Abbott, AbbVie, Arena Pharmaceuticals, Athos, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Galapagos, Genentech-Roche, GSK, Janssen, Mirum, Morphic, Nestlé, Origo, Pandion, Pfizer, Progenity, Protagonist, Revolo, Robarts, Takeda, Theravance, and Wasserman. SV received research grants from AbbVie, Galapagos, J&J, MSD, Pfizer, and Takeda; and consultancy and/or speaker fees from AbbVie, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Cytoki Pharma, Falk, Ferring, Galapagos, Genentech/Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, MSD, Mundipharma, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillotts, and Zealand Pharma.
Data Availability
The data underlying this article cannot be shared publicly due to patient consent and privacy sharing limitations.
Author Contributions
BV, VP, NAM, NS, and HG contributed to the planning/design of the analysis. All authors contributed to the statistical analyses, data interpretation, and/or drafting or critical review of the manuscript content. All authors approved the final manuscript.
References
- 1. Kuhnen A. Genetic and environmental considerations for inflammatory bowel disease. Surg Clin North Am 2019;99:1197–207. [DOI] [PubMed] [Google Scholar]
- 2. US Food and Drug Administration. Humira Prescribing Information 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125057s417lbl.pdfAccessed 18 July 2022.
- 3. European Medicines Agency. Humira summary of product characteristics 2022. https://www.medicines.org.uk/emc/product/2150/smpcAccessed 18 July 2022.
- 4. Marsal J, Barreiro-de Acosta M, Blumenstein I, Cappello M, Bazin T, Sebastian S.. Management of non-response and loss of response to anti-tumor necrosis factor therapy in inflammatory bowel disease. Front Med 2022;9:897936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022;16:2–17. [DOI] [PubMed] [Google Scholar]
- 6. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 7. Verstockt B, Verstockt S, Dehairs J, et al. Low TREM-1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMedicine 2019;40:733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verstockt B, Verstockt S, Blevi H, et al. TREM-1, the ideal predictive biomarker for endoscopic healing in anti-TNF-treated Crohn’s disease patients? Gut 2019;68:1531–3. [DOI] [PubMed] [Google Scholar]
- 9. Prins MM, Verstockt B, Ferrante M, Vermeire S, Wildenberg ME, Koelink PJ.. Monocyte TREM-1 levels associate with anti-TNF responsiveness in IBD through autophagy and Fcγ receptor signaling pathways. Front Immunol 2021;12:627535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaujoux R, Starosvetsky E, Maimon N, et al. ; Israeli IBD research Network (IIRN). Cell-centred meta-analysis reveals baseline predictors of anti-TNFα non-response in biopsy and blood of patients with IBD. Gut 2019;68:604–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salvador-Martín S, Raposo-Gutiérrez I, Navas-López VM, et al. Gene signatures of early response to anti-TNF drugs in pediatric inflammatory bowel disease. Int J Mol Sci 2020;21:3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raine T, Melmed GY, Finney-Hayward T, et al. Trends in corticosteroid [CS] use over time and following diagnosis in patients with inflammatory bowel disease [IBD], using IBM® Marketscan®. J Crohns Colitis 2022;16:i454–55. [Google Scholar]
- 13. Mihailidou I, Pelekanou A, Pistiki A, et al. Dexamethasone down-regulates expression of triggering receptor expressed on myeloid cells-1: evidence for a TNFα-related effect. Front Public Health 2013;1:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panés J, Colombel J-F, D’Haens GR, et al. Higher vs standard adalimumab induction and maintenance dosing regimens for treatment of ulcerative colitis: SERENE UC trial results. Gastroenterology 2022;162:1891–910. [DOI] [PubMed] [Google Scholar]
- 15. D’Haens GR, Sandborn WJ, Loftus EV Jr, et al. Higher vs standard adalimumab induction dosing regimens and 2 maintenance strategies: randomized SERENE CD trial results. Gastroenterology 2022;162:1876–90. [DOI] [PubMed] [Google Scholar]
- 16. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. J R Statist Soc B 1995;57:289–300. [Google Scholar]
- 17. Bai BYH, Reppell M, Smaoui N, et al. Baseline expression of immune gene modules in blood is associated with primary response to anti-TNF therapy in Crohn's disease patients. J Crohns Colitis. 2024;18:431–445. doi: 10.1093/ecco-jcc/jjad166. Epub ahead of print. PMID: 37776235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo DH, Che X, Kim S, et al. Triggering receptor expressed on myeloid cells-1 agonist regulates intestinal inflammation via Cd177+ neutrophils. Front Immunol 2021;12:650864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laserna-Mendieta E, Lucendo A.. Faecal calprotectin in inflammatory bowel diseases: a review focused on meta-analyses and routine usage limitations. Clin Chem Lab Med 2019;57:1295–307. [DOI] [PubMed] [Google Scholar]
- 20. Ma C, Battat R, Khanna R, Parker CE, Feagan BG, Jairath V.. What is the role of C-reactive protein and fecal calprotectin in evaluating Crohn’s disease activity? Best Pract Res Clin Gastroenterol 2019;38-39:101602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to patient consent and privacy sharing limitations.