Abstract
Cyclooxygenase inhibitors represented extremely promising novel anti-inflammatory drugs until one of them, rofecoxib (Vioxx), was found to be associated with increased cardiovascular morbidity; however, another such drug, celecoxib (Celebrex), suffers far less from this side effect for unknown reasons and is still widely used. In this issue, Brueggemann et al. (page p. 1053) suggest a hypothesis. Celecoxib, but not rofecoxib, is shown to act as an “opener” of voltage-gated KCNQ5 K+ channels and a blocker of “L-type” Ca2+ channels, causing a reduction in the excitability and contractility of vascular smooth-muscle cells (VSMCs). Furthermore, VSMC tone is shown to be selectively reduced by celecoxib, resulting in dilation of blood vessels and reduction in systemic blood pressure, suggesting that the reduced work load on the heart may counteract any other deleterious effects of this class of drugs. Here, these findings are discussed in light of the role of KCNQ K+ channels in control of excitability in general, the “lipid imbalance theory” of cyclooxygenase-2 risks, and the potential for novel therapeutic modalities for cardiovascular disease focused on ion channels in vascular smooth muscle.
Keywords: COX, cyclooxygenase; CV, cardiovascular; AVP, arginine vasopressin; VSMC, vascular smooth muscle cell; VGCC, voltage-gated Ca2+ channel; COXIB, cyclooxygenase-2-selective drugs.
The development of nonsteroidal anti-inflammatory drugs selective for the cyclooxygenase-2 (COX-2) enzyme 10 years ago seemed to be a major breakthrough for the amelioration of a variety of inflammatory disease states. Although the traditional nonsteroidal anti-inflammatory drugs are equally efficacious against inflammatory processes, they also suppress the COX-1 enzyme responsible for cytoprotective actions in the gastrointestinal system, making them ulcerogenic for many people. The two most popular COX-2-selective drugs (COXIBs) were rofecoxib (Vioxx; Merck, Whitehouse Station, NJ) and celecoxib (Celebrex; Pfizer, New York, NY), which garnered sales of $1.5 billion and $400 million, respectively, in their first year alone. Moreover, the COX-2 enzyme was shown to be heavily used by cancerous colonic cells, raising the prospect that the COXIBs could work as preventative anticancer drugs as well. However, trouble loomed for the COXIB success story. In the rofecoxib colon cancer prevention trial (APPROVe), whereas the patients receiving the drug experienced a 24% reduction in the recurrence of colonic polyps, they also suffered a 1.92-fold increase in cardiovascular (CV) side effects—mostly heart attacks and strokes (Bresalier et al., 2005). Within days of reports of these CV side effects of rofecoxib, Merck withdrew Vioxx from the market and has been subject to much litigation from patients taking the drug who experienced such CV events. Likewise, another selective COX-2 inhibitor, valdecoxib, suffers from the same problems (Nussmeier et al., 2005). On the other hand, the CV risk profile of celecoxib is much lower, with most studies suggesting the risk to be only minor (McGettigan and Henry, 2006; Dajani and Islam, 2008). What is the reason for this seeming discrepancy among COX-2-inhibitor drugs in their adverse CV effects?
In this issue of Molecular Pharmacology, Brueggemann et al. (2009) provide a hypothesis based on drug-selective actions on two critical ion channels in vascular smooth-muscle cells (VSMCs). The channels are voltage-gated K+ channels of the KCNQ (Kv7, “M-type”) class, and “L-type” voltage-gated Ca2+ channels (VGCCs). Like all smooth muscle, the tone of VSMCs depends on intracellular [Ca2+], which is largely determined by the opening of VGCCs, which in turn is determined by membrane potential. M-type K+ channels, so named for their suppression by muscarinic agonists in nerve (Constanti and Brown, 1981), are expressed in a variety of excitable cells in which they play a dominant role in the regulation of the resting potential and action potential firing (Brown, 2008). Thus, suppression of M currents in the nervous system by neurotransmitters and hormones results in increased neuronal firing, inherited mutations of KCNQ2 and KCNQ3 genes cause epileptic syndromes in human infants as a result of neuronal hyperexcitability, and dysfunctional KCNQ1-containing channels in the heart are responsible for cardiac arrhythmias (Hernandez et al., 2008; Maljevic et al., 2008; Peroz et al., 2008). With the discovery by several laboratories of several KCNQ subtypes in smooth muscle arose the possibility of the control of vascular tone by M-channel activity (Mackie and Byron, 2008). Indeed, KCNQ1, KCNQ4, and KCNQ5 have been identified in VSMCs, and KCNQ5 activity seems instrumental to the vasoconstrictor response to the hormone arginine vasopressin (AVP) (Yeung et al., 2007; Mackie et al., 2008). How does this fit in with the COXIB story? In a stroke of breathtaking serendipity, Brueggemann et al. (2009) show that celecoxib, but not rofecoxib or diclofenac (another COXIB), acts as a KCNQ channel opener. Moreover, only celecoxib reversed AVP-induced Ca2+ spiking and vasoconstriction. Thus, celecoxib would act in smooth muscle similarly to other KCNQ openers (retigabine, flupirtine, and meclofenamic acid) (Yeung et al., 2007), resulting in a hyperpolarization of the resting potential, vasodilation, and a brake-on vasopressin-induced constriction. Indeed, celecoxib seems to act in VSMCs similarly as neuronal M-channel openers: the reduction of excitability, and brake-on action potentials (Lerche et al., 2001; Gamper et al., 2006).
However, we must not forget about the second part of this two-part discovery. Indeed, Brueggemann et al. (2009) also show that celecoxib, but again not rofecoxib or diclofenac, are potent inhibitors of L-type VGCCs. Thus, celecoxib not only opens the K+ channels that slow depolarizations and action potentials, but it also inhibits the Ca2+ channels whose activity is required for increases in intracellular Ca2+, excitation/contraction coupling, and vasoconstriction. The end result of this one-two punch is celecoxib-mediated relaxation of the VSMCs, vasodilation, and presumably a reduction in systemic blood pressure. The relationship between systemic blood pressure and myocardial infarction is well known, with the CV risk doubling for each increment of 20/10 mm Hg of the systolic/diastolic pressures greater than 115/75 mm Hg (Chobanian et al., 2003). The effect of celecoxib in blockade of AVP actions in mesenteric artery shown in Brueggemann et al. (2009) is particularly interesting in light of the heavy association of cerebral vasospasm with high AVP levels in the brain (Delgado et al., 1988; Trandafir et al., 2004). Because vasospasm in the brain often leads to cerebrovascular infarct (stroke), celecoxib may well reduce the risk of stroke via its KCNQ5-mediated influence on the deleterious AVP response. Thus, the ion channel hypothesis put forward by these authors to explain the differential CV risk of celecoxib versus the other COXIB drugs can be summed up in this way: acting as an M-channel opener and a VGCC blocker, celecoxib causes vasodilation, a reduction in systemic vascular resistance, and a decrease in systemic blood pressure, reducing the stress on the heart and vascular system, which lowers the risk of heart attack. In the brain, the reduced tension in the cerebral vasculature, combined with antivasospasm activity, reduces the risk of cerebral stroke. Thus, the overall CV risk of COXIBs, which might increase because of differential actions on lipids (e.g., the “lipid imbalance theory,” see below) is postulated to be balanced by a reduction in CV events via direct actions on ion channels. Because rofecoxib is inactive against both KCNQ and VGCC channels, its negative effects are not balanced by the beneficial ones, leading to more adverse CV events.
However, this issue begs a brief discussion of why the COXIBs should increase the risk of CV events in the first place. The leading hypothesis is called the “lipid imbalance theory.” It is based on the opposing effects on thrombosis of prostanoids consisting of thromboxanes and prostacyclins, believed to be produced by the COX-1 and COX-2 enzymes, respectively (Fitzgerald, 2004). In particular, prostacyclin is a strong vasodilator and reduces the aggregation response of platelets, whereas thromboxane A2 is highly thrombogenic. Thus, selective blockade of COX-2 activity would then increase vascular thrombosis in the absence of other ameliorating factors. However pleasing this theory is in its parsimony, it may have shortcomings (Flavahan, 2007). First, the COX subtype responsible for prostacyclin production in the endothelia remains controversial, with data pointing to the COX-1 enzyme, not the COX-2, as largely responsible; second, platelet activity may well not be increased in patients taking COX-2 inhibitors; and finally, the studies identifying rofecoxib as increasing CV risk did not show that risk decreased by concomitant aspirin use (Bresalier et al., 2005), as might be expected if increased thrombogenic activity were responsible. Thus, this fascinating and important issue seems to be enigmatic.
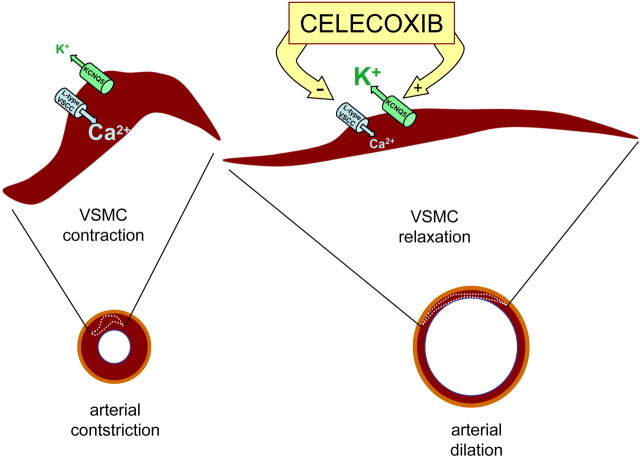
Whatever the underlying cause(s) may be of the CV risk of the COXIBs in general, that of celecoxib seems be uniquely lower. Figure 1 summarizes the hypothesis put forward by Brueggemann et al. (2009) to explain this finding. The two ion channel types involved are KCNQ5 K+ channels and “L-type” VGCCs. The channel-opening action of celecoxib on the former type slows cellular depolarization in response to excitatory inputs, as do M-type channels in neurons, resulting in VSMCs in less opening of VGCCs and less Ca2+ spiking. The inhibitory action is reinforced by direct blockade of the VGCCs, which acts against increases in intracellular Ca2+, Ca2+-induced Ca2+ release from intracellular stores, and smooth muscle contraction. Brueggemann et al. (2009) suggest that the overall effects are a decrease in systemic vascular resistance and blood pressure and reduced vasospasm in the brain, both of which contributing to fewer heart attacks and strokes and lower overall CV-mediated morbidity. Is this scenario plausible? Quite definitely. Is it entirely true? Only further study will tell, but this article indicates that such further investigation is highly warranted. It is noteworthy that the celecoxib analog 2,5-dimethyl-celecoxib, which has no activity against the COX-2 enzyme (Schönthal, 2006), had the same effect on both channels as did celecoxib, indicating that the ion channel mechanism reported here has nothing to do with cyclooxygenase activity.
This study reinforces the potential therapeutic potential of altering vascular ion channel activity for CV disease. Although Ca2+ channel blocking drugs are currently being used as antihypertensives, drugs acting on KCNQ channels have not yet been explored seriously. This is probably because the KCNQ2–5 group of channels has been associated with neuronal function, with the vast majority of their study being in the nervous system. However, it is increasingly clear that these channels play critical roles in the vasculature, in which their regulation by the myriad signaling pathways worked out in neurons (Delmas and Brown, 2005) probably exerts strong control over vascular smooth muscle tone and consequently may be important targets for pharmaceutical interventions. The celecoxib study presented in this issue may thus be the proverbial “tip of the iceberg,” and we will be expecting additional discoveries that shed light on the mechanisms conferring safety to anti-inflammatory drugs such as the COXIBs and on novel modes of therapeutic interventions in general for cardiovascular and cerebrovascular disease.
Fig. 1.
Schematic representation of the proposed antihypertensive effects of celecoxib. Complementary actions of celecoxib on different classes of ion channels in VSMCs (enhancement of KCNQ5 potassium channel activity and suppression of L-type voltage-sensitive calcium channel activity) result in the relaxation of arterial myocytes and vasodilation.
Footnotes
Please see the related article on page 1053 .
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.059683
ABBREVIATIONS:
References
- BresalierRS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA and Baron JA Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators ( 2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352:1092–1102. [DOI] [PubMed] [Google Scholar]
- BrownDA (2008) Kv7 (KCNQ) potassium channels that are mutated in human diseases. J Physiol 586:1781–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrueggemannLI, Mackie AR, Mani BK, Cribbs LL and Byron KL (2009) Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol 76:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChobanianAV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al.. ( 2003) Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- ConstantiA and Brown DA (1981) M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett 24:289–294. [DOI] [PubMed] [Google Scholar]
- DajaniEZ and Islam K (2008) Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol 59 ( Suppl 2): 117–133. [PubMed] [Google Scholar]
- DelgadoTJ, Arbab MA, Warberg J and Svendgaard NA (1988) The role of vasopressin in acute cerebral vasospasm. Effect on spasm of a vasopressin antagonist or vasopressin antiserum. J Neurosurg 68:266–273. [DOI] [PubMed] [Google Scholar]
- DelmasP and Brown DA (2005) Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6:850–862. [DOI] [PubMed] [Google Scholar]
- FitzgeraldGA (2004) Prostaglandins: modulators of inflammation and cardiovascular risk. J Clin Rheumatol 10:S12–S17. [DOI] [PubMed] [Google Scholar]
- FlavahanNA (2007) Balancing prostanoid activity in the human vascular system. Trends Pharmacol Sci 28:106–110. [DOI] [PubMed] [Google Scholar]
- GamperN, Zaika O, Li Y, Martin P, Hernandez CC, Perez MR, Wang AY, Jaffe DB and Shapiro MS (2006) Oxidative modification of M-type K+ channels as a mechanism of cytoprotective neuronal silencing. EMBO J 25:4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HernandezCC, Zaika O, Tolstykh GP and Shapiro MS (2008) Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol 586:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LercheH, Jurkat-Rott K and Lehmann-Horn F (2001) Ion channels and epilepsy. Am J Med Genet 106:146–159. [DOI] [PubMed] [Google Scholar]
- MackieAR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE and Byron KL (2008) Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther 325:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MackieAR and Byron KL (2008) Cardiovascular KCNQ (Kv7) potassium channels: physiological regulators and new targets for therapeutic intervention. Mol Pharmacol 74:1171–1179. [DOI] [PubMed] [Google Scholar]
- MaljevicS, Wuttke TV and Lerche H (2008) Nervous system Kv7 disorders: breakdown of a subthreshold brake. J Physiol 586:1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettiganP and Henry D (2006) Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 296:1633–1644. [DOI] [PubMed] [Google Scholar]
- NussmeierNA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW and Verburg KM (2005) Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352:1081–1091. [DOI] [PubMed] [Google Scholar]
- PerozD, Rodriguez N, Choveau F, Baró I, Mérot J and Loussouarn G (2008) Kv7.1 (KCNQ1) properties and channelopathies. J Physiol 586:1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchönthalAH (2006) Antitumor properties of dimethyl-celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase-2: implications for glioma therapy. Neurosurg Focus 20:E21. [DOI] [PubMed] [Google Scholar]
- TrandafirCC, Nishihashi T, Wang A, Murakami S, Ji X and Kurahashi K (2004) Participation of vasopressin in the development of cerebral vasospasm in a rat model of subarachnoid haemorrhage. Clin Exp Pharmacol Physiol 31:261–266. [DOI] [PubMed] [Google Scholar]
- YeungSY, Pucovský V, Moffatt JD, Saldanha L, Schwake M, Ohya S and Greenwood IA (2007) Molecular expression and pharmacological identification of a role for Kv7 channels in murine vascular reactivity. Br J Pharmacol 151:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]