Abstract
P2X7 receptors (P2X7Rs) are ATP-gated ion channels that display the unusual property of current facilitation during long applications of agonists. Here we show that facilitation disappears in chimeric P2X7Rs containing the C-terminus of the P2X2 receptor (P2X2R), and in a truncated P2X7R missing the cysteine-rich domain of the C-terminus. The chimeric and truncated receptors also show an apparent decreased permeability to N-methyl-d-glucamine+ (NMDG+). The effects of genetic modification of the C-terminus on NMDG+ permeability were mimicked by preapplication of the HSP90 antagonist geldanamycin to the wild-type receptor. Further, the geldanamycin decreased the shift in the reversal potential of the ATP-gated current measured under bi-ionic NMDG+/Na+ condition without affecting the ability of the long application of agonist to facilitate current amplitude. Taken together, the results suggest that HSP90 may be essential for stabilization and function of P2X7Rs through an action on the cysteine-rich domain of the cytoplasmic the C-terminus.
Introduction
P2X receptors (P2XRs) are a family of seven ATP-gated ion channels that underlie a diverse array of functions in both simple and complex organisms (North, 2002). All seven members of the family (P2X1R through P2X7R) are composed of three subunits and show a preference for cations over anions (Nicke et al., 1998; Barrera et al., 2005; Egan et al., 2006). Each subunit has intracellular N- and C-termini and two transmembrane domains (TM1 and TM2) separated by a large extracellular loop (Surprenant et al., 1996; Egan et al., 2004). By comparison with all other family members, the C-terminus of P2X7R is longer and contains a unique cysteine-rich domain (Egan et al., 2006; Erb et al., 2006; Costa-Junior et al., 2011). Channels with truncated C-termini missing this domain show an apparent decreased ability to transport large (up to 900 Da) polyatomic molecules during long applications of ATP (Surprenant et al., 1996; Khakh et al., 1999), suggesting that this cytoplasmic domain plays a role in regulation of current through the channel. Recently, high-resolution structures of the zebrafish P2X4.1 receptor (zfP2X4.1R) were obtained in the presence and absence of ATP, leading to a much better understanding of the conformational changes underlying channel gating (Kawate et al., 2009; Hattori and Gouaux, 2012). However, these structures lack the cytoplasmic N- and C-termini, and the manner in which the cytoplasmic domains regulate channel function, therefore, is still open to question. One possibility suggested by proteomic studies is that the C-terminus contains a binding site for regulatory proteins such as laminin α3, integrin kinase, and the heat shock proteins HSP70, HSP71, and HSP90 that are capable of altering channel properties (Kim et al., 2001; Gu et al., 2009). The presence of an HSP90 is particularly intriguing because this heat shock protein is thought to repress P2X7R function (Adinolfi et al., 2003) and play a role in caspase-dependent apoptosis of rodent macrophages and microglia (Levin et al., 2008; Chen et al., 2009).
In the present work, we sought to determine if HSP90 regulates ATP-gated current by interacting with the C-terminus of the P2X7R. Some (P2X2R, P2X4R, P2X7R) but not all P2XRs show an apparent time-dependent increase in permeability to large organic cations such as N-methyl-d-glucamine+ (NMDG+), ethidium+, and the carbocyanine nucleic acid dye YO-PRO-1 (Khakh et al., 1999; Virginio et al., 1999; Yan et al., 2008, 2010). In the case of the P2X7R, the time-course of the apparent change in cation permeability is associated with an increase in charge transfer across the membrane (called “current facilitation”) (Yan et al., 2008, 2010; Khadra et al., 2013; Rokic and Stojilkovic, 2013). In contrast, the long applications of ATP needed to induce the permeability changes in P2X2Rs result in a decrease in charge transfer because the receptor desensitizes (Khakh et al., 1999; Coddou et al., 2015). The different time-dependent effects of ATP on the size of P2X2R and P2X7R currents suggest that chimeras of these channels may be useful tools to determine the locus of the HSP90 effect on P2X7R function. Here, we report findings suggesting that HSP90 plays an important role for stabilization and function of P2X7R by interacting with the cysteine-rich domain of the C-terminus of the P2X7R.
Materials and Methods
Cell Culture and Heterologous Expression of Recombinant Receptors.
HEK293 or HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum and 100 IU/ml penicillin-streptomycin (Gibco/Thermo Fisher Scientific, Waltham, MA). These cells were plated at a density of 100,000 cells per 35-mm culture dish and kept at 37°C in a humidified atmosphere containing 5% CO2. Cells were cotransfected with 1.0 μg of cDNA encoding a rat wild-type or mutant P2X7R, and 0.5 μg of a DsRed expression plasmid using 4 μl of Attractene according to the manufacturer’s protocols (Qiagen, Sussex, UK). Large ATP-gated currents through functional receptors were studied 24–36 hours post-transfection.
Generation of Chimeric P2X7Rs.
Chimeras were constructed by performing two successive PCR amplifications using 5′ and 3′ primers containing overlapping regions between rat P2X2Rs and P2X7Rs in pcDNA3.1(+). To generate a chimera consisting of the P2X7R with the N-terminal domain of P2X2R, we used overlap oligonucleotides encoding the sequences RNRRL/GTIKWIL (corresponding to aa 25–29 for P2X2R and aa 27–33 for P2X7R). To generate a chimera consisting of P2X7R with the C-terminal domain of P2X2R, we used overlap oligonucleotides encoding aa LIINT/FMNKNKL (corresponding to aa 353–357 for P2X7R, aa 355–361 for P2X2R). Each 25-μl PCR reaction contained 0.1 μg of the template P2XR, 500 pmol of each primer, 125 μM dNTPs, and 2.5 units/ml PFU or GXL DNA polymerase (Takara Bio Inc., Shiga, Japan). PCR parameters included an initial denaturation at 95°C for 1 minute followed by 30 cycles (95°C for 10 seconds, 55 ∼ 60°C for 5 seconds, 68°C for 60 seconds and 3 minutes). The first PCR product was purified by MiniPrep (Qiagen, Sussex, UK), and 1 μl of the product was used as template DNA for the second PCR. All sequences were confirmed by DNA sequencing (FASMAC Co. Ltd., Kanagawa, Japan).
Immunocytochemistry.
Cells were plated onto glass slides, then transfected with cDNAs encoding wild-type and chimeric receptors as described above. The plated cells were then washed three times with 0.1 M sodium phosphate buffer (PBS), followed by fixation with 4% paraformaldehyde in PBS for 20 minutes at room temperature. After washing three times with PBS, cells were incubated with blocking solution (0.1% Triton X-100 and 5% donkey serum in PBS) for 30 minutes. Cells were then incubated with anti-P2X7R (1:1000; Alomone Labs, Jerusalem, Israel) in the blocking solution for 2 hours at room temperature. After being washed three times with PBS, cells were incubated with Alexa Fluor 488-conjugated donkey anti-rabbit (1:400; Molecular Probes/Thermo Fisher Scientific) for 1 hour at room temperature. After being washed three times with PBS and rinsed in distilled water, coverslips were mounted on slides with an antifading reagent.
Whole-Cell Current Recording and Application of Drugs.
Immediately preceding the start of an experiment (Migita et al., 2001), plated cells were mechanically dispersed using a fire-polished Pasteur pipette, and an aliquot of the dispersion was transferred to a recording chamber. Cells predicted to express P2XR protein were identified using fluorescence microscopy to detect the presence of Ds-Red. Whole-cell current was measured at room temperature with low resistance (1.5–3 MΩ), lightly fire-polished, and borosilicate electrodes from single cells held at –60 mV using a broken-patch method. The data were recorded with a Multiclamp 700B (Molecular Devices, Sunnyvale, CA), filtered at 1 kHz, and digitized at 10 kHz using a Digidata 1322A (Molecular Devices). Data acquisition and analysis were performed using pClamp9.2 software (Molecular Devices) and Igor Pro software (WaveMetrics, Inc., Lake Oswego, OR). The pipette solution contained (in mM): 140 CsCl, 1 MgCl2, 10 EGTA, and 10 HEPES (pH 7.4 with CsOH). The extracellular solution contained: 154 NaCl, 1 MgCl2, 1 CaCl2, 10 glucose, and 10 HEPES (pH 7.4 with NaOH). Drugs were applied for 1 second or 60 seconds once every 2–5 minutes using triple-barreled glass and a Perfusion Fast-Step System SF-77 (Warner Instruments, Hamden, CT). For estimates of activation and deactivation rates, cells were lifted from the substrate to increase speed of the concentration changes. The time-course of the concentration change was measured by switching the solution from normal external solution to solution containing 150 mM K+. The 10–90% time for change in holding current was 9.8 ± 0.2 milliseconds (switch into high K+ solution; n = 19) and 5.9 ± 0.4 milliseconds (switch back to normal bath solution; n = 19). Successive applications of ATP were separated by at least 3 minutes.
The current-voltage curve, obtained by ramping the voltage from –80 to 40 mV with 200 milliseconds at every other second for 60 seconds, was used to estimate changes in reversal potential during the application of either 30 μM (when studying P2X2R) or 3 mM (when studying P2X7R) ATP. To measure permeability changes, the extracellular solution contained no Ca2+ or Mg2+, and the 154 mM NaCl was replaced by 154 mM NMDG-Cl; MgCl2 was also removed from the pipette solution. From these data, we calculated the relative permeability as PNMDG/PCs = exp(VrevF/RT), where Vrev is the bi-ionic reversal potential measured in extracellular NMDG-Cl and intracellular CsCl, F is Faraday’s constant, R is the universal gas constant, and T is the absolute temperature.
To construct concentration-response curves, peak agonist-gated current was normalized to that measured during application of 10 mM ATP in each cell. Individual curves were then fit with the Hill equation (eq. 1) using the Levenberg-Marquardt algorithm implemented in IGOR Pro, where I is the peak amplitude of the agonist-gated current and X is the agonist concentration.
| (1) |
The fit was used to estimate the maximum current (Imax), the concentration of agonist needed to evoke a half-maximal current (Xhalf, also called the EC50), and the Hill coefficient (nH, equal to the rate). The values of each of these parameters were appropriately pooled to determine differences among groups.
Western Blots.
HEK293T cells expressing P2X7R and P2X7R[Δ18] were cultured in 10-cm plates and treated with geldanamycin (5 μM, 20 minutes). Cells were washed twice with PBS, scraped from the dish, and centrifuged at 1000g for 1 minute at room temperature. The pellets were homogenized with a lysis buffer composed of 20 mM Tris-HCl at pH 8.0, 10 mM KCl, 1 mM MgCl2, 1 mM EDTA, 10% glycerol, and a protease inhibitor cocktail (cOmplete Protease Inhibitor Cocktail tablets; Roche Applied Science, Indianapolis, IN). This was then incubated on ice for 20 minutes, followed by centrifugation at 1000g for 5 minutes at 4°C. The protein-rich supernatant was centrifuged again at 3300g for 5 minutes at 4°C, followed by an additional centrifugation at 6000g for 15 minutes at 4°C to remove mitochondria. Finally, the resulting supernatant was centrifuged at 20,000g for 1 hour at 4°C. The pellet was treated with a second lysis buffer (20 mM Tris-HCl at pH 8.0, 150 mM KCl, 1 mM MgCl2, 1 mM EDTA, 1% SDS, and protease inhibitor cocktail). Protein concentration in lysate was determined by Micro BCA Protein Assay Kit (Thermo Fisher Scientific). Protein samples (10 μg) were diluted into the sample buffer (100 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, and 10% β-mercaptoethanol), then boiled for 5 minutes, separated by SDS-PAGE on 10% gels, and transferred onto a methanol-activated polyvinylidene difluoride membrane (Immobilon-P, PVDF; MilliporeSigma/Merck, Darmstadt, Germany). After blocking with 5% nonfat milk/Tris-buffered saline solution containing 0.1% Tween 20 (TBS-T) for 1 hour at room temperature, the membrane was incubated with primary antibodies (anti-P2X7R, 1:10,000; Alomone Labs, anti-HSP90, 1:5,000; BD Transduction Laboratories, and anti-β-actin, 1:10,000; Sigma-Aldrich) at 4°C for overnight and washed three times with TBS-T. Horseradish peroxidase–conjugated anti-mouse IgG (1:5000; Jackson ImmunoResearch Laboratories, West Grove, PA) or anti-rabbit IgG (1:5000; Jackson ImmunoResearch Laboratories) was used as a secondary antibody. The signals were detected by using an ECL (Bio-Rad, Hercules, CA).
Statistics.
Statistical analyses of data were performed using the Student’s t test for comparisons between two groups, or analysis of variance followed by Turkey’s test for multiple comparisons, using the statistics routines of Igor Pro. Results are expressed as means and standard errors of the mean. The level of significance was set at P < 0.05.
Chemicals.
Geldanamycin was obtained from SERVA Electrophoresis GmbH (Heidelberg, Germany). 17-AAG and 17-DMAG were obtained from TOCRIS Bioscience (Bristol, UK). Other chemicals were purchased from Sigma-Aldrich.
Results
HSP90 Interacts with P2XRs.
P2X2Rs, P2X4Rs, and P2X7Rs, but not P2X3Rs, show apparent time-dependent changes in polyatomic cation permeability when exposed to agonist for several seconds (Khakh et al., 1999; North, 2002; Yan et al., 2008, 2010). Interestingly, P2X2R currents desensitize, whereas P2X4R and P2X7R currents facilitate during extended exposure to agonists. Lalo et al. (2012) showed that human P2X1R currents were blocked and human P2X2R currents were potentiated by preapplication of the HSP90 antagonist geldanamycin. We investigated the effects of geldanamycin on rat P2XRs (Fig. 1A) and found that currents through both rat P2X4Rs (amplitude of the 2nd ATP-gated current in the absence and presence of geldanamycin divided by that of the 1st equaled 0.67 ± 0.07 and 4.81 ± 1.29, respectively) and rat P2X7R (ratios in the absence and presence of geldanamycin equaled 0.72 ± 0.10 and 2.28 ± 0.73, respectively) were potentiated by geldanamycin, whereas currents through the P2X2R and P2X3R were not (Fig. 1B). The ability of geldanamycin to potentiate human P2X2Rs (Lalo et al., 2012) but not rat P2X2Rs (Fig. 1) demonstrates important yet previously unappreciated species-dependent actions of HSPs on P2X receptors and suggest that unique sites may be involved in HSP90 regulation of individual P2XRs.
Fig. 1.
Effect of the HSP90 inhibitor geldanamycin on other P2XRs-mediated currents. (A) Representative currents evoked by 30 μM ATP for P2X2R, P2X3R, and P2X4R or 3 mM ATP for P2X7R in the absence (upper figures) or presence (lower figures) of 5 μM geldanamycin (20 minutes). (B) Mean ratio showed 2nd ATP currents in the absence (black bar) or presence (white bar) of geldanamycin divide by 1st ATP current in P2XRs (n = 4–6). *P < 0.05, **P < 0.01 compared with the corresponding values from the absence of geldanamycin (Student’s t test).
Functional P2X2/7 Chimeras Are Targeted to the Cell Surface Membrane.
We wondered if this difference of HSP90 regulation of rat P2X2Rs (no effect) and P2X7Rs (potentiation) reflects the variations in sequences of the N- and/or the C-terminus. To investigate this hypothesis, we made P2X2/7 chimeric receptors in which parts of the P2X7R were replaced with parts of the P2X2R (Fig. 2A). We transfected HEK293 cells with genes encoding the chimeras and studied them using immunohistochemistry and electrophysiology. All of the chimeric proteins were expressed in transfected cells (Fig. 2B), and in all cases, the transfected cells showed measurable currents when challenged with ATP (Fig. 3). Current through the X7-X7-X2 chimera was smaller than that seen for the other constructs, but still large enough to allow accurate measurements of its properties (see below).
Fig. 2.
Property of P2X7 chimeras. (A) Schematic representations of the wild-type and chimeric receptors used in this study. (B) Immunofluorescence staining of wild-type and chimeric receptors in HEK-293T cells transiently expressing these receptors.
Fig. 3.
P2X7 and its chimeras receptors currents during long-term stimulation with ATP. Representative traces showing the whole-cell currents elicited by a 60-second application of 3 mM ATP for P2X7 and its chimeras receptors and 30 μM ATP for P2X2R.
A Role of the Intracellular Region of P2X7R in Current Facilitation.
The wild-type P2X7R shows a biphasic response to long applications of ATP composed of an initial fast inward current called I1 and a second, slower inward current called I2 that grows larger in the continued presence of ATP (upper left panel of Fig. 3). All of the constructs showed similar densities of I1 and I2 currents with two exceptions (Table 1). First, the P2X7R chimera containing the C-terminus of the P2X2R (X7-X7-X2) showed a monophasic current that lacked an obvious I2 component. Second, I2 was significantly larger in the P2X7R containing the N-terminus of the P2X2R (X2-X7-X7). Although these results are interesting, the effects of domain swaps and mutations on the densities of I1 and I2 could reflect changes in protein assembly, protein trafficking, or channel kinetics. We did not pursue these distinctions further because the investigation of I1 and I2 per se was not the main goal of this study. More central to our project was the finding that the two mutant constructs with altered C-termini (X7-X7-X2 and X2-X7-X2) showed significantly less facilitation than the wild-type receptor (see Table 1). Taken together, these results support the contention that facilitation of P2X7R current requires a full-length native C-terminal tail (Yan et al., 2008, 2010; Liang et al., 2015).
TABLE 1.
Sixty-second application of ATP in P2X7 chimeras
| Chimera | 10–90% Rise Time | Decay Time Constant | I 1 | I 2 | n |
|---|---|---|---|---|---|
| ms | ms | pA/pF | pA/pF | ||
| X7-X7-X7 | 32 ± 3 | 252 ± 27 | −102 ± 31 | −213 ± 56 | 8 |
| X2-X7-X7 | 38 ± 6 | 833 ± 200 | −247 ± 38 | −425 ± 30* | 6 |
| X7-X7-X2 | 81 ± 15** | 945 ± 204 | −11 ± 4 | −9 ± 3* | 5 |
| X2-X7-X2 | 44 ± 5 | 187 ± 24 | −333 ± 126 | −124 ± 42 | 6 |
| X7[Δ18] | 54 ± 5 | 1439 ± 603** | −124 ± 37 | −95 ± 40 | 5 |
| X2-X2-X2 | 97 ± 7** | 121 ± 22 | −1212 ± 299** | −195 ± 96 | 4 |
Values are mean ± S.E.M.
I1, initial peak current; I2, inward current at 60 sec; nd, not determined; n, number of experiments.
P < 0.05, **P < 0.01 versus wild-type P2X7 (Tukey’s test).
NMDG+ Permeability in P2X7 Chimeric Receptors.
Next, we examined the effect of altering the structure of the P2X7R on the time-dependent change in the reversal potentials of the ATP-gated currents, measured when NMDG+ or Cs+ is the dominant carrier of cationic current, which coincides with the generation of I2 during long applications of ATP (Fig. 4). We measured reversal potentials at the start (during I1) and end (during I2) of a 60-second application of 3 mM ATP. For the wild-type P2X7R, the reversal potential shifted from –38.9 ± 1.7 to –13.0 ± 5.9 mV (n = 6) as PNMDG/PCs increased from 0.21 ± 0.01 to 0.67 ± 0.12. The shift in the reversal potential was smaller for the wild-type P2X2R (15.8 ± 5.3 mV, n = 6), indicating that PNMDG/PCs increased from 0.15 ± 0.01 to 0.28 ± 0.03. Most importantly, all of the chimeras showed much smaller changes in Erev and PNMDG/PCs, with the smallest changes occurring in the chimeric receptors containing the C-terminus of the P2X2R (Table 2). If indeed the shift in reversal potential reflects a change in NMDG+ permeability (see below), then these data support the contention that the C-terminus plays a significant role in NMDG+ permeability of the P2X7Rs (Jiang et al., 2005; Yan et al., 2008, 2010).
TABLE 2.
Permeability of the wild-type P2X7R and its mutants to NMDG+
Reversal potential (Erev) and relative permeability (PNMDG/PCs) for wild-type P2X7R and its chimeras at the beginning (1 second) and end (60 seconds) of 60-secons application of ATP. Values are mean ± S.E.M.
| Chimera | Erev |
Δvrev | PNMDG/PCs |
n | ||
|---|---|---|---|---|---|---|
| 1 sec | 60 sec | 1 sec | 60 sec | |||
| mV | mV | mV | ||||
| X7-X7-X7 | −38.9 ± 1.7 | −13.0 ± 5.9b | 25.9 ± 5.7 | 0.21 ± 0.01 | 0.67 ± 0.12b | 6 |
| + geldanamycin | −39.4 ± 3.0 | −31.9 ± 2.7a′ | 7.7 ± 1.2a | 0.22 ± 0.03 | 0.29 ± 0.04a | 7 |
| X2-X7-X7 | −45.4 ± 1.1 | −35.9 ± 1,4ab | 9.5 ± 1.7a | 0.17 ± 0.01 | 0.24 ± 0.01a | 5 |
| + geldanamycin | −39.1 ± 0.6 | −34.9 ± 2.1a | 4.3 ± 2.0a | 0.21 ± 0.01 | 0.26 ± 0.02a | 7 |
| X7-X7-X2 | −41.8 ± 7.3 | −39.4 ± 6.6a | 2.4 ± 2.5a | 0.23 ± 0.07 | 0.25 ± 0.06a | 6 |
| + geldanamycin | −42.5 ± 2.0 | −43.2 ± 2.9a | 2.6 ± 2.0a | 0.19 ± 0.02 | 0.19 ± 0.02a | 5 |
| X2-X7-X2 | −46.6 ± 3.9 | −39.5 ± 6.5a | 8.8 ± 2.3a | 0.16 ± 0.02 | 0.24 ± 0.05a | 6 |
| + geldanamycin | −37.6 ± 2.0 | −37.4 ± 3.5a | 1.3 ± 1.7a | 0.23 ± 0.02 | 0.24 ± 0.03a | 5 |
| X7[A18] | −33.5 ± 2.0 | −33.5 ± 1,0a′ | 2.1 ± 0.9a | 0.27 ± 0.02 | 0.27 ± 0.01a | 6 |
| + geldanamycin | −31.5 ± 1.5 | −35.1 ± 3.4a | −3.6 ± 2.6a | 0.29 ± 0.02 | 0.26 ± 0.04a | 5 |
| X2-X2-X2 | −47.9 ± 1.0 | −32.6 ± 2.4a′b | 15.8 ± 5.3 | 0.15 ± 0.01 | 0.28 ± 0.03ab | 6 |
n, number of experiments.
′P < 0.05, aP < 0.01 (Tukey’s test), significant differences between wild-type P2X7R and others.
′P < 0.05, bP < 0.01 (Student’s t test), significant shifts between 1 second and 60 seconds.
Fig. 4.
Permeability to NMDG+ in P2X7 and its chimeras receptors. Voltage ramps of –80 mV to 40 mV were applied to the cells during 60-second activation of P2X7R and its mutants by 3 mM ATP or P2X2R by 30 μM ATP in extracellular NMDG+. The currents initially (1 second) show a low permeability to NMDG+ that is followed by a progressively rightward-shift in the reversal potential of the ATP-activated current in P2X7 and P2X2Rs.
Geldanamycin Potentiates I1 through an Action on C-Terminus.
The C-terminus of the P2X7R binds HSP90 in pull-down assays (Kim et al., 2001; Gu et al., 2009). Thus, we investigated the functional consequences of this interaction by determining the effect of the HSP90 antagonist geldanamycin on ATP-gated current (Fig. 5A). We found that the peak I1 evoked by a 1-second application of 3 mM ATP in the wild-type P2X7R increased 2.1 ± 0.5 fold after a 20-minute application of geldanamycin (Fig. 5B, Table 3). Similar effects were seen using other HSP90 antagonists; peak I1 evoked by a 1-second application of 3 mM ATP in the wild-type P2X7R increased 2.0 ± 0.2- and 1.5 ± 0.1-fold after a 20-minute application of 5 nM 17-AAG and 100 nM 17-DMAG, respectively (Supplemental Fig. 1). Further, geldanamycin tended to increase I1 through the sole chimeric receptor with an intact P2X7 C-terminus (X2-X7-X7) (see I1/Icontrol of Table 3). In contrast, the chimera with non-native (X7-X7-X2 and X2-X7-X2) C-termini failed to show increased ATP-evoked currents in the presence of geldanamycin.
TABLE 3.
Effect of geldanamycin on current facilitation evoked by 60-second application of ATP in wild-type P2X7R and its mutants
Values are mean ± S.E.M.; n, number of experiments.
| Chimera | I1(pA/pF) | I2(pA/pF) | I 1 /I control | I 2 /I control | n |
|---|---|---|---|---|---|
| X7-X7-X7 | −110 ± 24 | −239 ± 43 | 0.9 ± 0.0 | 2.4 ± 0.4 | 14 |
| + geldanamycin | −198 ± 45 | −253 ± 65 | 2.1 ± 0.5* | 2.5 ± 0.4 | 11 |
| X2-X7-X7 | −474 ± 194 | −650 ± 232 | 0.7 ± 0.1 | 1.0 ± 0.1 | 5 |
| + geldanamycin | −427 ± 121 | −557 ± 117 | 1.0 ± 0.1 | 1.4 ± 0.2 | 5 |
| X7-X7-X2 | −2 ± 1 | −1 ± 1 | 0.8 ± 0.4 | 0.4 ± 0.2 | 3 |
| + geldanamycin | −5 ± 2 | −4 ± 1 | 1.1 ± 0.0 | 0.7 ± 0.1 | 3 |
| X2-X7-X2 | −74 ± 34 | −18 ± 8 | 0.5 ± 0.2 | 0.1 ± 0.0 | 6 |
| + geldanamycin | −273 ± 108 | −56 ± 17 | 0.5 ± 0.1 | 0.1 ± 0.0 | 3 |
| X7[Δ18] | −101 ± 25 | −78 ± 26 | 0.8 ± 0.1 | 0.6 ± 0.1 | 8 |
| + geldanamycin | −278 ± 89 | −188 ± 66 | 0.7 ± 0.1 | 0.5 ± 0.1 | 4 |
P < 0.05, **P < 0.01 versus Normal solution (Student’s t test).
Fig. 5.
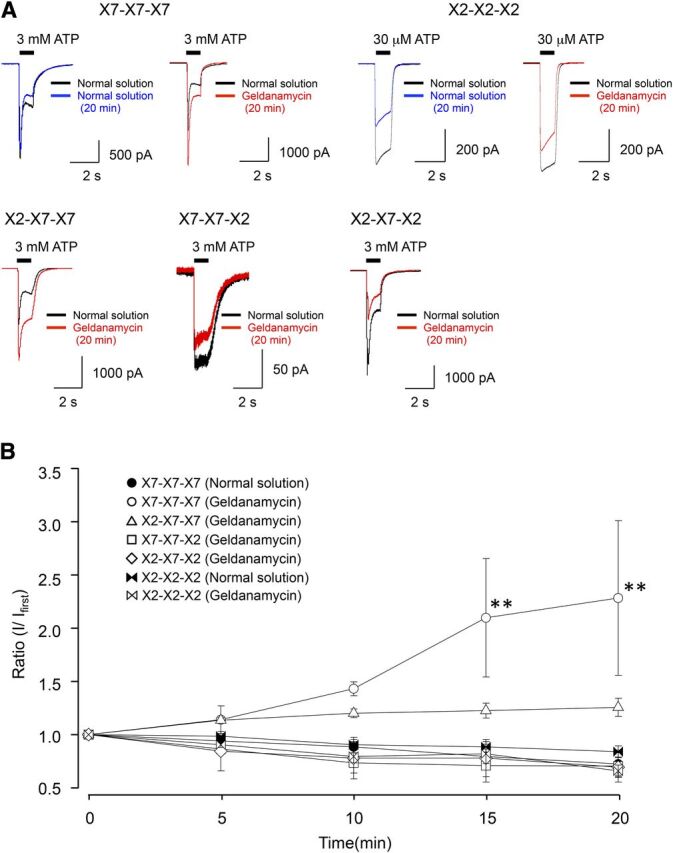
Effect of the HSP90 inhibitor geldanamycin on P2X7 and its chimeras’ receptor-mediated currents. (A) Representative currents evoked by 3 mM ATP recorded in cells expressing P2X7R and its chimeras’ receptors or 30 μM ATP in P2X2R in the absence or presence of 5 μM geldanamycin (20 minutes). (B) Mean normalized data of P2X7 and its chimeras’ receptor-mediated 3 mM ATP currents in normal HEPES solution and in the presence of 5 μM geldanamycin (n = 4–8). Statistical analysis was performed by one-way analysis of variance followed by Tukey’s test (**P < 0.01).
We considered the following possible causes of the effect of geldanamycin on the initial phase of the P2X7R current. First, geldanamycin might alter the sensitivity of the P2X7R to ATP. This seems unlikely because we used supermaximal concentrations of ATP to evoke I1 in these experiments (Fig. 5), and because the EC50s measured in the presence and absence of geldanamycin were nearly identical (Table 4). Second, the increase in I1 amplitude might result from an increase in protein expression of either the P2X7R channel or the putative accessory protein HSP90. However, we saw no changes in total or surface protein expression using Western blot analysis (Fig. 9, A and B, Supplemental Fig. 2). The third possibility, that geldanamycin affects the ability of C-terminal domain of the P2X7R to interact with HSP90, therefore, remains the most likely explanation for the change in I1 amplitude.
TABLE 4.
ATP pharmacology of P2X7 chimeras
Values are mean ± S.E.M.
| Chimera | EC50(μM) | Hill coefficient | n |
|---|---|---|---|
| X7-X7-X7 | 1473 ± 40 | 1.7 | 12 |
| X7-X7-X7 (geld) | 1377 ± 253 | 5.4 | 7 |
| X2-X7-X7 | 574 ± 22** | 1.9 | 6 |
| X7-X7-X2 | 304 ± 90** | 0.9 | 5 |
| X2-X7-X2 | 543 ± 40** | 2.0 | 6 |
| X7[Δ18] | 265 ± 51** | 0.9 | 5 |
| X7[Δ18] (geld) | 480 ± 197** | 1.1 | 8 |
| X2-X2-X2 | 13 ± 0** | 3.4 | 7 |
geld, geldanamycin; n, number of experiments.
P < 0.01 versus wild-type P2X7 (Tukey’s test).
Geldanamycin Also Alters Apparent Pore Dilation, but Not Current Facilitation.
We studied the effect of geldanamycin on current facilitation and apparent pore dilation by measuring I2 and the shift in the reversal potential of the ATP-gated current before and after application of the HSP90 antagonist. As shown in Fig. 6, geldanamycin significantly increased the I1 phase of the P2X7R current. In contrast, the total I2 current, measured 60 seconds after the start of the ATP application, was not significantly different in the presence and absence of geldanamycin (–253 ± 65 pA/pF and –239 ± 43 pA/pF, respectively). Similar effects were seen in the X2-X7-X7 chimera. We also measured a smaller shift in the reversal potential (∼10 mV; Table 2) in the presence of geldanamycin in comparison with measurements made in its absence (compare raw data of Figs. 4 and 7, Table 2). The fact that geldanamycin increases total current density but causes a smaller shift in the reversal potential of the ATP-gated current measured under bi-ionic NMDG+/Na+ conditions argues against the recent suggestion that the altered Erev results entirely from an effect of ion accumulation (Li et al., 2015). Rather, the data support the direct influence of HSP90 on the ability of the P2X7R pore via the C-terminus to adopt a conformational state with altered permeability to NMDG+.
Fig. 6.

Effect of geldanamycin on current facilitation in P2X7R and its mutants. Representative traces showing the whole-cell currents elicited by a 60-second application of 3 mM ATP for P2X7R in the presence and absence of 5 μM geldanamycin (20 minutes).
Fig. 7.
Effect of geldanamycin on permeability to NMDG+ in P2X7R and its mutants. Voltage ramps of –80 mV to 40 mV were applied to the cells during 60-second activation of P2X7R by 3 mM ATP in extracellular NMDG+ under the presence of 5 μM geldanamycin (20 minutes).
Inhibition of Geldanamycin-Potentiated I1 Current with Deleted Cysteine-Rich Juxtamembrane Domain of P2X7R.
Previous studies have shown that the C-terminus of P2X7R involves the 18-amino acid cysteine-rich juxtamembrane domain contributing to current facilitation, apparent pore dilation, and palmitoylation (Jiang et al., 2005; Yan et al., 2008, 2010; Gonnord et al., 2009; Roger et al., 2010). Resh (2006) showed that palmitoylation can influence membrane binding of the modified proteins and the trafficking of ion channels. We thus investigated the effect of geldanamycin on ATP-induced currents using a deletion mutant (P2X7R[Δ18]) that lacks the 18-amino acid (362–379: CCRSRVYPSCKCCEPCAV) cysteine-rich site (Jiang et al., 2005). The ability of geldanamycin to potentiate the I1 current in wild-type P2X7R completely disappeared in the truncated P2X7R[Δ18] (Fig. 8A). The same results were obtained using 17-AAG and 17-DMAG (Supplemental Fig. 1). Further, using Western blot analysis of protein expression, we saw no changes in total or surface P2X7R[Δ18] expression in the presence and absence of geldanamycin, 17-AAG, and 17-DMAG (Fig. 9, Supplemental Fig. 2). In addition, I2 current and the shift in the reversal potential in the presence and absence of geldanamycin was unchanged in P2X7R[Δ18] (Fig. 8, B–D). Taken together, these data suggest that HSP90 prevents facilitation of the initial ion influx by an action on the cysteine-rich domain of the C-terminus of the wild-type P2X7R.
Fig. 8.

Effect of geldanamycin on ATP-induced currents in P2X7R[Δ18]. (A) Representative currents evoked by 3 mM ATP recorded in cells expressing P2X7R and P2X7R[Δ18] in the absence or presence of 5 μM geldanamycin (20 minutes). Mean normalized data of P2X7- and P2X7R[Δ18]-mediated 3 mM ATP currents in normal HEPES solution and in the presence of 5 μM geldanamycin (n = 4–8). Statistical analysis was performed by one-way analysis of variance followed by Tukey’s test (**P < 0.01). (B) Representative traces showing the whole-cell currents elicited by a 60-second application of 3 mM ATP for P2X7R[Δ18] in the presence and absence of 5 μM geldanamycin (20 minutes). (C) Voltage ramps of –80 mV to 40 mV were applied to the cells during 60-second activation of P2X7R[Δ18] by 3 mM ATP in extracellular NMDG+. (D) Voltage ramps of –80 mV to 40 mV were applied to the cells during 60-second activation of P2X7R[Δ18] by 3 mM ATP in extracellular NMDG+ under the presence of 5 μM geldanamycin (20 minutes).
Fig. 9.
Effect of geldanamycin on P2X7R and P2X7R[Δ18] expression. (A) Western blots showing total or plasma membrane of P2X7R, P2X7R[Δ18], HSP90, GAPDH, and β-actin proteins in HEK293T cells transfected with P2X7R (X7) and P2X7R[Δ18] (Δ18) treated or untreated with 5 μM geldanamycin for 20 minutes. (B) Summary of the amount of P2X7R and P2X7R[Δ18] relative to β-actin. Data indicate no difference in the total (n = 6) or surface (n = 3) level of P2X7R, P2X7R[Δ18], and HSP90 between treated and untreated with geldanamycin.
Discussion
Site-directed mutagenesis of the C-terminus has numerous effects on P2XR channel function; among these, the best known is a change in the apparent ability of the pore to permeate large organic cations during long applications of ATP (Khakh et al., 1999; Egan et al., 2006; Erb et al., 2006; Yan et al., 2008, 2010). Further, the C-terminus has been shown to interact with a number of intracellular and cytoskeletal proteins, including HSP90 (Kim et al., 2001), in a manner that localizes the receptor to the plasma membrane and regulates the gating of the P2X1R (Lalo et al., 2011, 2012). In the present paper, we show that the HSP90 antagonist geldanamycin significantly changes the channel properties of the ATP-gated currents of a number of P2XRs (P2X2R, P2X4R, and P2X7R), suggesting that HSP90 indeed plays an important role in the regulation of P2XR function.
We took advantage of the fact that, whereas both P2X2R and P2X7R show a change in the reversal potential of the ATP-gated currents measured under bi-ionic conditions during long applications of agonist, only the P2X7R shows current facilitation (Khakh et al., 1999; Yan et al., 2008, 2010). Although the structural bases of apparent pore dilation and current facilitation are unknown (but see below), it is tempting to speculate that functional differences in the P2X2R and the P2X7R arise from their divergent C-termini. Specifically, the C-terminus of the P2X7R is much longer than that of the P2X2R, and contains a distinct cysteine-rich domain (North, 2002). Truncated P2X7Rs that lack this domain fail to show pore dilation and current facilitation (Yan et al., 2008, 2010), suggesting that the C-terminus plays a pivotal role in producing these phenomena. Further, the cysteine-rich domain is absent in P2X2Rs that show clear changes in reversal potentials in response to prolonged applications of agonist, albeit at a slower rate and to a lesser degree than the wild-type P2X7R (Khakh et al., 1999; Virginio et al., 1999; Yan et al., 2008, 2010; Mio et al., 2009; Khadra et al., 2012).
Using chimeric constructs of the P2X2R and P2X7R, we found that a full-length P2X7R C-terminus is required to achieve the maximum degree of facilitation; that is, neither the chimeric P2X7Rs containing the C-terminus of P2X2R nor the truncated P2X7R[Δ18] show the same degree of facilitation as that seen in the full-length wild-type P2X7R. These engineered receptors also showed a smaller shift in the reversal potential during long applications of ATP, indicative of lesser apparent pore dilation. Recently, Li et al. (2015) suggested that the shift in equilibrium potential induced by prolonged P2X2 channel activation does not result from pore dilation but rather from time-dependent alterations in the concentration of intracellular ions. However, our experiments clearly show that the geldanamycin decreased the shift in the reversal potential of the ATP-gated current measured under bi-ionic NMDG+/Na+ condition without affecting the ability of the long application of agonist to facilitate current amplitude. The smaller shift in the reversal potential paired to a larger inward current would seem to argue against an effect of ion accumulation. However, our data do not conclusively prove that HSP90 is capable of directing conformational changes in the P2X7R protein channel, and additional experiments derived from the study of Li and coworkers (2015) are needed to confidently rule out the possibility that a change in the intracellular concentrations of ions explains the effect of geldanamycin on the change in the reversal potential. Although further experiments are clearly needed, our results suggest that C-terminal of the P2X7R, especially the cysteine-rich region, may regulate ion permeability by promoting the adoption of a unique open channel conformation.
We also found that the X2-X7-X7 chimera showed a decrease NMDG+ permeability during long applications of ATP. In fact, this result is not surprising; others have shown that mutating a highly conserved threonine (Thr15) or nonconserved Serine (Ser23) in the N-terminus of the rat P2X7R significantly alters NMDG+ permeability (Yan et al., 2008) and ethidium bromide uptake (Allsopp and Evans, 2015). While it is tempting to speculate on how the N- and C-termini influences conformational states of the pore, firm determination awaits the solution of high-resolution structures of fully formed P2X7Rs.
P2X7Rs interact with a number of intracellular and membrane-spanning proteins (Kim et al., 2001; Gu et al., 2009). Adinolfi et al. (2003) used geldanamycin to show that tyrosine phosphorylation of HSP90 within the P2X7R complex negatively regulates channel function by lowering the affinity of the P2X7R for Bz-ATP through an action on a specific tyrosine (Tyr550) in the C-terminal tail. In our study, geldanamycin potentiated the initial I1 component of the ATP-induced current of the wild-type P2X7R, at the same time inhibiting the shift in the reversal potential. The potentiation of I1 current by geldanamycin was not present in cells expressing the X7-X7-X2, X2-X7-X2, and P2X7R[Δ18] chimera. Further, preapplication of geldanamycin prevented the increase in NMDG+ permeability in the wild-type receptor. All of these effects may reflect a constitutive action of HSP90 on P2X7R function as the result of an interaction with the C-terminus. We do not think that geldanamycin exerts its effects by acting as an ATPase (Panaretou et al., 1998), because the broken-patch method we use to record membrane current is expected to remove unbound intracellular ATP by dilution of the cytoplasm with the contents of the recording pipette, thereby effectively making the action of an ATPase redundant. Rather, geldanamycin may act to displace ATP from a critical binding site. Further experiments are needed to more precisely define the action of geldanamycin at the molecular level. Nevertheless, our data clearly show that the C-terminus of the P2X7R is somehow involved in geldanamycin effect. This should not be surprising because several reports highlight the importance of this domain in regulation of channel function. For example, Smart et al. (2003) showed that the distal C-terminal domain regulates surface expression of the P2X7R and is essential for the apparent pore dilation and current facilitation reported here. Further, separate domains within the C-terminus contribute to the Ca2+-dependent (Roger et al., 2008; Yan et al., 2011) and -independent (Roger et al., 2010) components of current facilitation of the P2X7R. Moreover, Gonnord et al. (2009) have indicated that the cysteine-rich site is important for the P2X7 palmitoylation that plays a critical role in the association with the lipid microdomains of plasma membrane. Now, our work with wild-type and chimeric receptors suggest the HSP90 facilitates P2X7R current through a physical interaction with the juxtamembrane cysteine-rich domain. However, how HSP90 causes facilitation is unknown. One possibility is that the binding of HSP90 at or near the cysteine-rich domain bends the C-terminus into a unique conformation that is capable of changing channel kinetics in a way that promotes NMDG+ permeability. Again, proof of this hypothesis awaits the arrival of full-length, high-resolution structures in complex with HSP90.
Ample data suggest that the C-terminus plays a significantly role in the regulation of inflammatory response (Adriouch et al., 2002), apoptosis (Wiley et al., 2002; Le Stunff et al., 2004; Feng et al., 2006), and pain (Sorge et al., 2012) by the P2X7R. Our experiments extend this work and suggest that one role for the C-terminus is to act as an interaction site for an accessory regulatory protein, HSP90. If so, then drugs that modulate the binding of HSP90 to the C-terminus may be useful therapies for neuropathic pain, apoptosis, and inflammation.
Acknowledgments
The authors thank Shiwori Osanai for technical assistance.
Abbreviations
- 17-AAG
17-N-allylamino-17-demethoxygeldanamycin
- 17-DMAG
17-dimethylaminoethylamino-17-demethoxygeldanamycin
- Bz-ATP
2′ (3′)-O-(4-benzoylbenzoyl)adenosine-5′-triphosphate
- HEK
human embryonic kidney
- HSP
heat shock protein
- NMDG+
N-methyl-d-glucamine+
- PBS
sodium phosphate buffer
- P2XR
P2X receptor
Authorship Contributions
Participated in research design: Migita.
Conducted experiments: Migita, Shimoyama.
Performed data analysis: Migita, Shimoyama.
Wrote or contributed to the writing of the manuscript: Migita, Ozaki, Shimoyama, Yamada, Nikaido, Furukawa, Shiba, Egan, Ueno.
Footnotes
This work was supported by Grant-in-Aid for Scientific Research (C) [No. 26460693] and for Exploratory Research [No. 25670663] from the Japan Society for the Promotion of Science and by a Priority Research Grant for Young Scientists Designated by the President of Hirosaki University.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Adinolfi E, Kim M, Young MT, Di Virgilio F, Surprenant A. (2003) Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem 278:37344–37351. [DOI] [PubMed] [Google Scholar]
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. (2002) Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol 169:4108–4112. [DOI] [PubMed] [Google Scholar]
- Allsopp RC, Evans RJ. (2015) Contribution of the juxtatransmembrane intracellular regions to the time course and permeation of ATP-gated P2X7 receptor ion channels. J Biol Chem 290:14556–14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM. (2005) Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem 280:10759–10765. [DOI] [PubMed] [Google Scholar]
- Chen H, Xia Y, Fang D, Hawke D, Lu Z. (2009) Caspase-10-mediated heat shock protein 90 beta cleavage promotes UVB irradiation-induced cell apoptosis. Mol Cell Biol 29:3657–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Stojilkovic SS. (2015) Role of domain calcium in purinergic P2X2 receptor channel desensitization. Am J Physiol Cell Physiol 308:C729–C736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Junior HM, Sarmento Vieira F, Coutinho-Silva R. (2011) C terminus of the P2X7 receptor: treasure hunting. Purinergic Signal 7:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Cox JA, Voigt MM. (2004) Molecular structure of P2X receptors. Curr Top Med Chem 4:821–829. [DOI] [PubMed] [Google Scholar]
- Egan TM, Samways DS, Li Z. (2006) Biophysics of P2X receptors. Pflugers Arch 452:501–512. [DOI] [PubMed] [Google Scholar]
- Erb L, Liao Z, Seye CI, Weisman GA. (2006) P2 receptors: intracellular signaling. Pflugers Arch 452:552–562. [DOI] [PubMed] [Google Scholar]
- Feng YH, Li X, Zeng R, Gorodeski GI. (2006) Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids 25:1271–1276. [DOI] [PubMed] [Google Scholar]
- Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, Kanellopoulos JM. (2009) Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J 23:795–805. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS. (2009) Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol 297:C430–C439. [DOI] [PubMed] [Google Scholar]
- Hattori M, Gouaux E. (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, North RA. (2005) N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. Am J Physiol Cell Physiol 289:C1295–C1302. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra A, Tomić M, Yan Z, Zemkova H, Sherman A, Stojilkovic SS. (2013) Dual gating mechanism and function of P2X7 receptor channels. Biophys J 104:2612–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra A, Yan Z, Coddou C, Tomić M, Sherman A, Stojilkovic SS. (2012) Gating properties of the P2X2a and P2X2b receptor channels: experiments and mathematical modeling. J Gen Physiol 139:333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Bao XR, Labarca C, Lester HA. (1999) Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci 2:322–330. [DOI] [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. (2001) Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 20:6347–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Jones S, Roberts JA, Mahaut-Smith MP, Evans RJ. (2012) Heat shock protein 90 inhibitors reduce trafficking of ATP-gated P2X1 receptors and human platelet responsiveness. J Biol Chem 287:32747–32754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Roberts JA, Evans RJ. (2011) Identification of human P2X1 receptor-interacting proteins reveals a role of the cytoskeleton in receptor regulation. J Biol Chem 286:30591–30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Auger R, Kanellopoulos J, Raymond MN. (2004) The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem 279:16918–16926. [DOI] [PubMed] [Google Scholar]
- Levin TC, Wickliffe KE, Leppla SH, Moayeri M. (2008) Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell Microbiol 10:2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Toombes GE, Silberberg SD, Swartz KJ. (2015) Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nat Neurosci 18:1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Samways DS, Wolf K, Bowles EA, Richards JP, Bruno J, Dutertre S, DiPaolo RJ, Egan TM. (2015) Quantifying Ca2+ current and permeability in ATP-gated P2X7 receptors. J Biol Chem 290:7930–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K, Haines WR, Voigt MM, Egan TM. (2001) Polar residues of the second transmembrane domain influence cation permeability of the ATP-gated P2X(2) receptor. J Biol Chem 276:30934–30941. [DOI] [PubMed] [Google Scholar]
- Mio K, Ogura T, Yamamoto T, Hiroaki Y, Fujiyoshi Y, Kubo Y, Sato C. (2009) Reconstruction of the P2X(2) receptor reveals a vase-shaped structure with lateral tunnels above the membrane. Structure 17:266–275. [DOI] [PubMed] [Google Scholar]
- Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17:3016–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067. [DOI] [PubMed] [Google Scholar]
- Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. (1998) ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J 17:4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. (2006) Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE 2006:re14. [DOI] [PubMed] [Google Scholar]
- Roger S, Gillet L, Baroja-Mazo A, Surprenant A, Pelegrin P. (2010) C-terminal calmodulin-binding motif differentially controls human and rat P2X7 receptor current facilitation. J Biol Chem 285:17514–17524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger S, Pelegrin P, Surprenant A. (2008) Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28:6393–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokic MB, Stojilkovic SS. (2013) Two open states of P2X receptor channels. Front Cell Neurosci 7:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart ML, Gu B, Panchal RG, Wiley J, Cromer B, Williams DA, Petrou S. (2003) P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem 278:8853–8860. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan M, et al. (2012) Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med 18:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. (1999) Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2:315–321. [DOI] [PubMed] [Google Scholar]
- Wiley JS, Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, Taper J, Gallo J, Manoharan A. (2002) A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study. Lancet 359:1114–1119. [DOI] [PubMed] [Google Scholar]
- Yan Z, Khadra A, Li S, Tomić M, Sherman A, Stojilkovic SS. (2010) Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 30:14213–14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Li S, Liang Z, Tomić M, Stojilkovic SS. (2008) The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol 132:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Khadra A, Sherman A, Stojilkovic SS. (2011) Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol 138:437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]








