Abstract
Background:
Hyaluronic acid dermal fillers are used extensively in periocular aesthetic medicine, and the incidence of filler-related complications is increasing. This study aimed to investigate the optimal dosing strategy for hyaluronidase and to identify predictors of poor outcomes.
Methods:
We performed a retrospective review of 157 orbits of 90 patients treated with hyaluronidase over a 4-year period. Demographic data, indication, and details of hyaluronidase treatment and outcomes were recorded.
Results:
The primary indication for dissolving filler was swelling in 52%, lumpiness in 20%, and before surgical blepharoplasty in 17%. The most frequently used hyaluronidase concentration was 150 U per mL in 66%, followed by 75 U per mL in 31%, 37.5 U per mL in 3%, and 100 U per mL in 1%. Outcomes were characterized as follows: 59% with a satisfactory result; 24% as insufficient treatment requiring further hyaluronidase; and 18% complaining of facial changes such as hollowing, indicating a post hyaluronidase syndrome. There was no statistical difference in outcomes between the 75 and 150 U per mL dosage groups (P = 0.625). A significant correlation was identified between posthyaluronidase syndrome and duration of filler in situ (P = 0.00019) and volume of filler (P = 0.000017).
Conclusions:
The posthyaluronidase syndrome may be related to previous filler volume and duration, rather than the concentration or dose of hyaluronidase used. All patients should be informed about the risks of adverse effects after hyaluronidase treatment; patients with longer histories of filler use and higher total volumes should be advised of the increased risk.
Takeaways
Question: What causes the posthyaluronidase syndrome?
Findings: An estimated 18% of patients treated with hyaluronidase to dissolve fillers complained of negative aesthetic outcomes such as hollowing, indicating a posthyaluronidase syndrome. A significant correlation was identified between posthyaluronidase syndrome and duration of filler in situ and volume of filler. The concentration and dose of hyaluronidase had no effect on outcome.
Meaning: The risk of posthyaluronidase syndrome is real and is related to the volume and duration of the filler rather than the dose or concentration of hyaluronidase used.
INTRODUCTION
Hyaluronic acid (HA) dermal fillers are used extensively in aesthetic medicine in the periocular region to treat the tear trough deformity, mask lower eyelid fat prolapse and increase the cheek volume and augment the volume of the temple and brow fad pads.1,2 Fillers are modified naturally occurring HA polymers consisting of repeating disaccharide units linked by glucuronidic β bond.3 The physiochemical structure of different filler preparations determines properties such as cohesiveness, elasticity, lift capacity, and durability.4
As soft tissue augmentation has become increasingly popular, the incidence of complications has risen.5,6 Swelling, lumpiness, Tyndall effect, migration, and more rarely, infection may occur from fillers in any location but can be more noticeable in the periocular region, which is more unforgiving owing to the thinner tissues overlying the facial skeleton.7,8 The most serious complication is vision loss, with over 100 cases reported in the literature.9 When filler-related complications are identified, treatment with hyaluronidase may be performed to dissolve the filler. Another important indication for hyaluronidase therapy is to dissolve fillers before surgical blepharoplasty, as the filler-induced soft tissue modulation may confound surgical planning.10
There is currently no standardized protocol for the concentration and dosage of hyaluronidase. Doses may be adjusted if the filler properties are known as the resistance to degradation changes with modification of the polymer length and crosslinking.11 There is also growing concern regarding the potential for posttreatment adverse effects from hyaluronidase, which have been reported to include periocular hollowing, worsening rhytids, and adverse changes in skin quality.10
In this study, we investigate the incidence and associations of the posthyaluronidase syndrome. We present the demographics, indications, and outcomes of the largest series of hyaluronidase treatments in the literature to date. The relationships between patient characteristics, filler properties, and outcomes after filler dissolution are described to identify independent predictors of negative outcomes. In addition, we describe outcomes of different total dosing and concentrations of hyaluronidase to propose a range of efficacious and safe dosing.
METHODS
This study received internal approval from Moorfields Eye Hospital NHS Trust (study no.: 917) and was conducted in accordance with the Declaration of Helsinki. No formal ethics approval was required, as this was a retrospective chart review study. Medical records of consecutive patients treated with hyaluronidase by a single surgeon between January 2016 and July 2020 were retrospectively reviewed. Patients with a minimum of 1 week of follow-up after the initial hyaluronidase injection were included. Exclusion criteria included missing demographic data, indications for treatment, and hyaluronidase dosing. For each patient, age, gender, laterality, volume, location, treatment date, indication for dissolving filler, concentration and volume of hyaluronidase used, and date of last clinical follow-up were documented. Patients were surveyed after the initial hyaluronidase injection to determine the result, and posttreatment photographs were taken. Consent was obtained from patients to include photographs in this publication.
HYALURONIDASE INJECTION PROTOCOL
A detailed history was taken for each patient regarding past injectables, and pretreatment photographs were taken. The area of interest was first inspected and then palpated. Ultrasound imaging or magnetic resonance imaging (MRI) imaging was undertaken in some patients to assist with the delineation of the filler deposit when required.
The concentration and dose of hyaluronidase to be used was discussed with the patient. Patients were consented about the potential negative effects of hyaluronidase. Factors regarding the dose or concentration of hyaluronidase included the indication for dissolving (complete removal of fillers versus to treat a small problematic area), the volume of filler in situ, and the brand of filler used.
The skin was cleaned with chlorhexidine and allowed to air dry. A 1500 unit vial of hyaluornidase was diluted with 0.9% sodium chloride to achieve the appropriate concentration. Typically, a 1500 unit vial would be diluted with 10 mL of saline to create a concentration of 150 U per mL and 1–3 mL would be injected into each under-eye region to dissolve tear tough filler depending upon the extent of filler present. A blunt cannula method was used to deliver the hyaluronidase within the expected borders of the filler deposit. This treated area was then massaged to promote hyaluronidase dispersion and breakdown of the filler product.
OUTCOME MEASURES
Patient outcomes were categorized into three groups: satisfactory result, insufficient treatment, or posthyaluronidase syndrome. This was based upon patient’s perceptions correlated with assessment by a consultant oculoplastic surgeon and pre and posttreatment photographs.
Satisfactory Result
After first injection of hyaluronidase, there was no evidence of remaining filler and no adverse effects.
Insufficient Treatment
After the first hyaluronidase treatment, patients required additional units of hyaluronidase to further dissolve the primary filler.
Posthyaluronidase Syndrome
These patients reported a more negative effect on appearance after hyaluronidase treatment. This phenomenon will be referred as “posthyaluronidase syndrome,” which is defined as any of the following facial changes: hollowing of the facial tissues, loss of skin elasticity, or discoloration of the skin, whereby the deterioration in the appearance was deemed to be worse than the pretreatment appearance. This was defined based upon patient reports confirmed by pretreatment photographs that could be objectively correlated with symptoms.
DATA ANALYSIS
The association between two categorical variables was tested with the chi square test. The association between a categorical variable with more than two categories and a continuous variable was first tested using the Kruskal-Wallis test, and if there was a significant difference among the groups, the pair-wise Wilcoxon rank sum test was performed with multiple test correction using the Benjamini-Hochberg procedure to identify the groups with difference. The association with a binary variable and a continuous variable was tested using the Wilcoxon rank sum (Mann-Whitney) test. The correlation between two continuous variables was assessed with Kendall and Spearman rank correlation coefficients. Statistical analysis was performed using SPSS, version 22 (IBM Corp, Armonk, N.Y.) with a significance level of 5%.
RESULTS
Demographic Characteristics
Over the study period, 163 periorbital applications of 94 individuals were injected with hyaluronidase for dissolution of filler. Six cases were excluded due to lack of recorded demographic details lost to follow-up and undocumented dosages of hyaluronidase. In total, 157 periorbital applications of 90 individuals were included in this study.
The average patient age was 46 years (median 45; range 22–79), and 81 patients (90%) were women (Table 1). Patients were from outside referral sources, and the full details regarding the volume of filler, brand, and the number of filler injections were often unobtainable. The volume of preexisting filler to be dissolved was identified from records in 45% of patients with an average of 2.1 mL (median 1.5; range 0.2–10) within each periorbital and cheek region, and in the remaining 55%, the volume was unknown. The average duration of filler in situ was 30 months (median 12; range 0–156). The majority of patients received Juvederm (Allergan, Irvine, Calif.; 36%) followed by Teosyal PureSense Redensity 2 (Teoxane SA, Switzerland; 17%) and Restylane (Galderma Laboratories, L.P., Fort Worth, Tex.; 13%) families of filler. Most of these patients were unsure of the particular type of filler beyond the manufacturer name. The remaining patients (35%) did not have any further details regarding the types of fillers but only HA fillers were included in this study.
Table 1.
Patient Demographics, Filler Details, and Indication for Dissolving Filler
| Patient and Filler Characteristics | |
|---|---|
| Proportion of females | 90% (81/90) |
| Age at presentation (y) | Mean 46 (median 45; range 22–79) |
| Volume of filler (mL) | Mean 2.1 (median 1.5; range 0.2–10) |
| Duration of filler in situ (mo) | Mean 30 (median 12; range 0–156) |
| Filler Brand | |
| Juvederm | 36% (56/157) |
| Teosyal Redensity | 17% (26/157) |
| Restylane | 13% (20/157) |
| Unknown | 35% (55/157) |
| Filler Location | |
| Tear trough only | 62% (98/157) |
| Tear trough and cheek | 24% (37/157) |
| Periorbital area | 14% (22/157) |
| Primary Indication for Filler Dissolving | |
| Swelling | 52% (81/157) |
| Lumpiness | 20% (31/157) |
| Festoons | 6% (9/157) |
| Tyndall effect | 4% (6/157) |
| Infection | 1% (2/157) |
| Filler migration | 1% (2/157) |
| In preparation for blepharoplasty surgery | 17% (26/157) |
The tear trough was the most common location for previous filler treatment (86%) in this series; 62% to the tear trough only and 24% to both tear trough and cheek. The remaining 14% had filler injected to cheek, brow, or other periorbital areas (temple and upper lid sulcus). This reflects the nature of the investigator’s practice, focused on tertiary aesthetic oculoplastics.
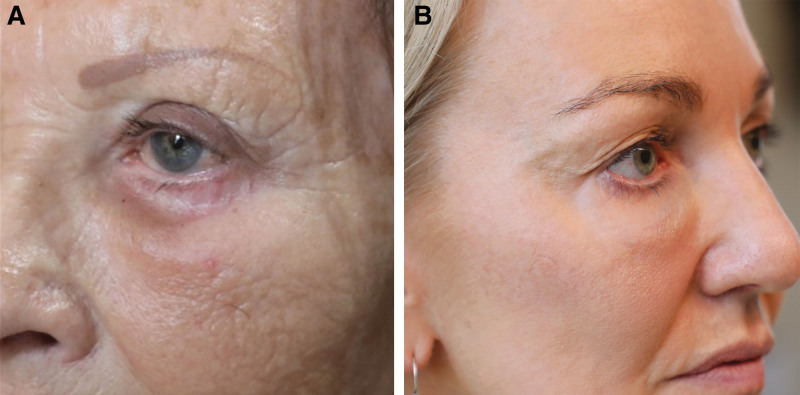
The primary indication for dissolving filler was swelling in 52%, lumpiness in 20%, festoons or malar edema in 6%, Tyndall effect in 4%, and infection or migration in 1% each (Fig. 1). An estimated 17% of cases involved hyaluronidase administration in the preoperative phase to prepare for blepharoplasty.
Fig. 1.
Indications for dissolving filler. A, Swelling of the tear trough region. B, Swelling with Tyndall effect.
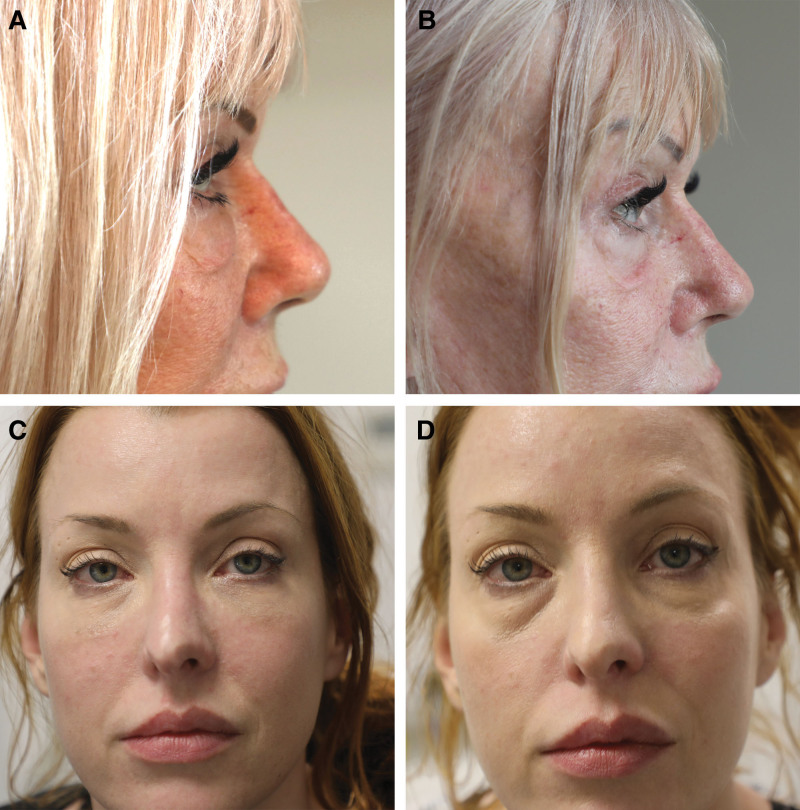
Of the 157 orbits treated with hyaluronidase, 59% were categorized as a satisfactory result, 24% as insufficient treatment, including residual lumps or swelling, and 18% with posthyaluronidase syndrome (Fig. 2).
Fig. 2.
Posthyaluronidase syndrome. A, Pretreatment photograph showing edema of the lower eyelids. B, Posttreatment photograph displaying hollowing of the lower lids and cheek with loose skin. C, Pretreatment photograph showing edema of both lower eyelids. D, Posttreatment photograph displaying significant hollowing of the lower lids with dark circles.
HYALURONIDASE CONCENTRATION
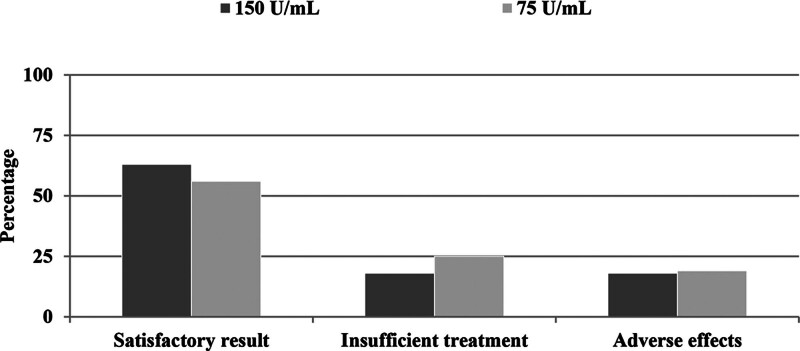
The most frequently used hyaluronidase concentration was 150 U per mL in 66% of orbits followed by 75 U per mL in 31% of cases (Table 2). Only 3% received 37.5 U per mL and 1% 100 U per mL. There was no statistical difference in outcomes between the 75 and 150 U per mL dosage groups (P = 0.625). In the 75 U per mL group, 56% (27 of 48) had a satisfactory result and 25% (12 of 48) had an insufficient response, compared with a satisfactory outcome in 63% (65 of 103) and insufficient response in 18% (19 of 103) in the 150 U per mL group (Fig. 3). In the 37.5 U per mL group, all patients had an insufficient response.
Table 2.
Outcomes of Different Concentrations of Hyaluronidase
| Hyaluronidase Concentration (U/mL) |
Percentage of Orbits Treated | Total Dose in Units, Mean ± SD (range) |
Outcome | ||
|---|---|---|---|---|---|
| Satisfactory Result | Insufficient Treatment | Adverse Effects | |||
| 150 | 66% (103/157) | 495 ± 145 (105–900) | 63% (65/103) | 18% (19/103) | 18% (19/103) |
| 100 | 1% (2/157) | 80 ± 0 | 0% | 100% (2/2) | 0% |
| 75 | 31% (48/157) | 219 ± 95 (37.5–375) | 56% (27/48) | 25% (12/48) | 19% (9/48) |
| 37.5 | 3% (4/157) | 70 ± 39 (19–113) | 0% | 100% (4/4) | 0% |
| Total | 395 ± 193 (19–900) | 59% (92/157) | 24% (37/157) | 18% (28/157) | |
Fig. 3.
Comparison of outcomes between 150 U per mL and 75 U per mL hyaluronidase concentrations.
There was no difference in the incidence of posthyaluronidase syndrome between the two main concentration groups. In patients who received 75 U per mL, 19% (nine of 48) experienced adverse effects compared with 18% (19 of 103) who received 150 U per mL (P > 0.999).
HYALURONIDASE TOTAL DOSE
The mean total-dose of hyaluronidase was 403 ± 191 units with a large range of 19–900 units. A significant difference was not found between any of the outcome groups and the total dose of hyaluronidase (satisfactory result 389 ± 191 units; insufficient treatment 382 ± 192 units; adverse effects 423 ± 189 units; P = 0.161)
In the cases where the volume of filler was not known due to no records being available, a significantly greater amount of hyaluronidase was used (mean 444 ± 191 units versus 368 ± 191 units; P = 0.012). An average of 35 units of hyaluronidase (range 22.5–225) was used to treat every 0.1 mL of filler, where the filler volume was known.
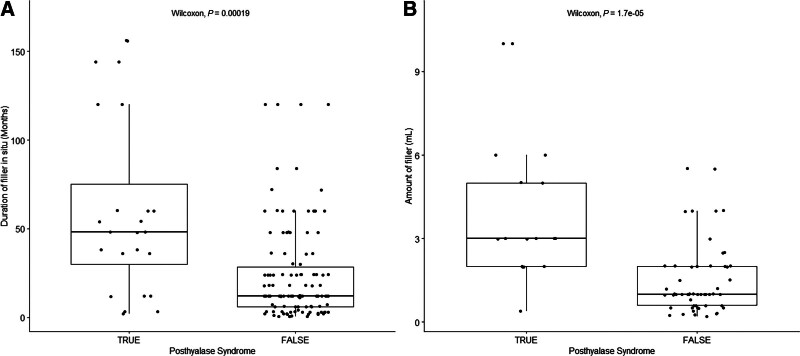
DURATION AND VOLUME OF PREVIOUS FILLER PREDICT POSTHYALURONIDASE SYNDROME
Univariate analysis was performed to evaluate the effect of patient age, duration of filler in situ, filler brand, location of filler, type of complication, and volume of filler on developing excessive hollowing after hyaluronidase treatment. Figure 4A shows that a statistically significant correlation was identified between duration of filler in situ and a reported posthyaluronidase syndrome (P = 0.00019). Mean duration of filler in situ before hyaluronidase dissolution was 60 ± 35 months (median 48; range 2–156) in patients who reported adverse outcomes versus 24 ± 35 months (median 12; range 0–120) in asymptomatic patients.
Fig. 4.
Boxplots of the duration of filler in situ and the volume of filler in patients who did and did not develop the posthyalase syndrome. A, Patients with a posthyaluronidase syndrome (true) were associated with significantly longer duration of filler in situ than patients who did not report a posthyaluronidase syndrome (false; P = 0.00019). B, Patients with a posthyaluronidase syndrome (true) were associated with significantly higher volume of filler than patients who did not report a posthyaluronidase syndrome (false; P = 0.000017).
A larger volume of filler was significantly associated with hollowing (P = 0.000017) and is shown in Figure 4B. The mean filler volume was 4 ± 2.2 mL (median 3 mL, range 0.4–10) in patients who reported posthyaluronidase syndrome versus 1.5 ± 2.0 mL (median 1, range 0.2–5.5) in those who had either satisfactory or insufficient outcomes. No other significant associations were identified.
DISCUSSION
This is the largest study of hyaluronidase treatment and importantly allows us to evaluate different concentrations and doses and to be able to present our patients with success rates and rates of adverse effects. Fillers are increasingly popular and may be dissolved for a number of reasons; in this study, the commonest indication was swelling, accounting for 52% of patients. It is often difficult to differentiate between overfill and swelling, but all patients in this study defined as having swelling reported a history of significant variability, being worse in the morning, which is pathognomonic of an edematous etiology resulting from the hydrophilic nature of the filler and diurnal changes in body fluid distribution.
The scientific literature presents a growing body of evidence supporting the long-term persistence of HA fillers. Numerous studies have documented instances of fillers persisting for many years.12–14 Recently, a study utilizing MRI imaging reported that HA fillers remained detectable for as long as 12 years in certain patients, with durability influenced by the injection site and the specific product used.15 Our study also observed complications arising from fillers administered many years ago. One male patient presented with malar edema secondary to tear trough and cheek fillers injected 13 years earlier. These findings highlight the potential for prolonged effects and complications associated with HA fillers.
This study also defines for the first time a “posthyaluronidase syndrome,” consisting of either hollowing and volume loss, loss of skin elasticity, or skin pigmentation exceeding the prefiller treatment appearance. Posthyaluronidase syndrome was reported after 18% of hyaluronidase applications. Univariate analysis found that posthyaluroniase syndrome was related to the volume of filler previously injected and the duration the filler has been in situ, rather than being an effect of hyaluronidase dosing or concentration. In the context of reconstructive surgery, fillers may be used as tissue expanders, effectively creating relaxed skin.16,17 Consequently, upon removal of the fillers, the skin may exhibit an appearance of looseness and loss of elasticity.18 A confounding factor is the natural age-related changes that may have occurred whilst the filler has been in situ, and this study is not designed to control for this. There may be effects of hyaluronidase on endogenous HA. Further studies will be required to determine which of these components are clinically significant.
The concentration of hyaluronidase did not have a statistically significant effect on outcomes. Importantly, the 75 U per mL concentration was not associated with a significant increase in insufficient response, and there was no difference in posttreatment hollowing when compared with the 150 U per mL group. This implies that most patients were treated with an effective dose of hyaluronidase and suggests that a dose-response curve may be seen at lower concentrations. Unfortunately, there were only four patients in the 37.5 U per mL group, which does not allow for robust analysis. The total dose of hyaluronidase also did not affect outcomes although there were many confounding factors, which limit the reliability of this conclusion. These results may be confounded by the different fillers in situ, their volumes, and their susceptibility to hyaluronidase. Other limitations include the retrospective design with the absence of a standardized protocol for hyaluronidase dosing.
In this study, an average of 35 units of hyaluronidase was used to treat every 0.1 mL of filler with a wide range of 22.5–225 units and the overall rate of satisfactory outcome was 59%. Juhasz et al found no significant difference between the use or 20 or 40 units of hyaluronidase in dissolving 0.2 mL (4–6 mg of HA) of various fillers in a study with 15 participants.19 They examined fillers of various crosslinking and polymer lengths, which all showed similar susceptibility to hyaluronidase, although highly crosslinked HA fillers were slower to dissolve. Other groups have recommended variable hyaluronidase concentrations between 5 and 30 U per 0.1 mL (2 mg of HA) of filler.20,21
We are unable to draw conclusions on the optimal hyaluronidase dosing strategy based upon our results. In practice, each patient must be assessed on a case-by-case approach with a detailed history, examination, and investigation to confirm the volume and distribution of HA fillers. The patient’s presenting complaint and desired outcomes should be considered when treatment planning including consenting the patient about the possible side effects. In the authors’ experience, lower concentrations in larger volumes are a useful tool to be able to flood a larger area to dissolve any filler present.
With the introduction of new imaging technologies, such as ultrasound and MRI segmentation, better targeting of well-defined filler deposits may mitigate the need for higher doses of hyaluronidase. In addition, insufficient response should not be seen as treatment failure and dissolving filler may be seen as a process rather than a single potent treatment. However, the global effect of multiple smaller doses on the incidence of posthyaluronidase syndrome remains unknown.
We report that higher total doses of hyaluronidase were used when the volume and nature of previous fillers was unknown. This reflects the need to ensure that the fillers are effectively dissolved. The rate of insufficient treatment requiring further injections in this study (24%) was lower than the 35% rate reported by Zoumalan.10 These studies are not directly comparable because Zoumalan et al targeted the lower eyelid only, whereas patients in our study often had more extensive applications of filler involving the cheek and wider periocular areas, requiring greater doses of hyaluronidase (61 units per eyelid compared with 403 units in this study). In contrast to the United States, where hyaluronidase preparations are in 150 or 200 unit vials, in the United Kingdom, vials contain 1500 units of hyaluronidase22,23 and differences may exist in the potency of the formulations.
The strengths of this study include the large treatment numbers, but limitations include the retrospective design and the subjective definition of posthyaluronidase syndrome. Although pre- and postinjection photographs were used to mitigate this by confirming reported changes, the lack of standardized imaging and objective assessments makes this definition less accurate. Further prospective studies performing volumetric analysis comparing the periorbital regions prefiller and postfiller dissolution would help quantify this effect.
In conclusion, posthyaluronidase syndrome occurs in 18% of patients at the level of dosing described. Our results suggest that posthyaluronidase syndrome is related to duration and volume of filler, but not the dosage of hyaluronidase. Further research is warranted to investigate the long-term effects of filler on facial tissues. Patients with longer histories of filler use and high total volumes should be warned of the potentially higher risk of posttreatment hollowing and posthyaluronidase syndrome. Conversely, patients with smaller volumes of filler can be advised that hyaluronidase is less likely to be associated with adverse effects.
DISCLOSURES
The authors have no financial interest to declare in relation to the content of this article. Sieun Lee receives funding from Precision Imaging Beacon, University of Nottingham.
PATIENT CONSENT
Patients provided written consent for the use of their images.
ACKNOWLEDGMENTS
The authors thank Karen Papier, Alison Shalson, Mandy Saffer, Louise Leventhal, and Julie Davidson.
Footnotes
Published online 23 April 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Lee S, Yen MT. Nonsurgical rejuvenation of the eyelids with hyaluronic acid gel injections. Semin Plast Surg. 2017;31:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustak H, Fiaschetti D, Goldberg RA. Filling the periorbital hollows with hyaluronic acid gel: long-term review of outcomes and complications. J Cosmet Dermatol. 2018;17:611–616. [DOI] [PubMed] [Google Scholar]
- 3.Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molliard SG, Betemps JB, Hadjab B, et al. Key rheological properties of hyaluronic acid fillers: from tissue integration to product degradation. Plast Aesthet Res. 2018;5:17. [Google Scholar]
- 5.Ibrahim O, Overman J, Arndt KA, et al. Filler nodules: inflammatory or infectious? A Review of biofilms and their implications on clinical practice. Dermatol Surg. 2017;44:53–60. [DOI] [PubMed] [Google Scholar]
- 6.Chiang YZ, Pierone G, Al-Niaimi F. Dermal fillers: pathophysiology, prevention and treatment of complications. J Eur Acad Dermatol Venereol. 2017;31:405–413. [DOI] [PubMed] [Google Scholar]
- 7.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy R, Roos JCP, Goldberg RA. Periocular hyaluronic acid fillers: applications, implications, complications. Curr Opin Ophthalmol. 2019;30:395–400. [DOI] [PubMed] [Google Scholar]
- 9.Beleznay K, Carruthers JDA, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097–1117. [DOI] [PubMed] [Google Scholar]
- 10.Zoumalan CI. Managing periocular filler-related syndrome prior to lower blepharoplasty. Aesthetic Plast Surg. 2019;43:115–122. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Kwon SB, Whang KU, et al. The duration of hyaluronidase and optimal timing of hyaluronic acid (HA) filler reinjection after hyaluronidase injection. J Cosmet Laser Ther. 2018;20:52–57. [DOI] [PubMed] [Google Scholar]
- 12.Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection. Ophthal Plast Reconstr Surg. 2017;33:S116–S118. [DOI] [PubMed] [Google Scholar]
- 13.Kopp S, Lawrence N, Donofrio L, et al. Delayed migration of hyaluronic acid fillers: a new complication? Dermatol Surg. 2014;40:85–87. [DOI] [PubMed] [Google Scholar]
- 14.Nathoo NA, Rasmussen S, Dolman PJ, et al. Periocular mass lesions secondary to dermatologic fillers: report of 3 cases. Can J Ophthalmol. 2014;49:468–472. [DOI] [PubMed] [Google Scholar]
- 15.Master M. Hyaluronic acid filler longevity and localization: magnetic resonance imaging evidence. Plast Reconstr Surg. 2021;147:50e–53e. [DOI] [PubMed] [Google Scholar]
- 16.Inbal A, Lemelman BT, Millet E, et al. Tissue expansion using hyaluronic acid filler for single-stage ear reconstruction: a novel concept for difficult areas. Aesthet Surg J. 2017;37:1085–1097. [DOI] [PubMed] [Google Scholar]
- 17.Fezza JP. Nonsurgical treatment of cicatricial ectropion with hyaluronic acid filler. Plast Reconstr Surg. 2008;121:1009–1014. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Yan Z, Zhang H, et al. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A. 2011;17:2981–2997. [DOI] [PubMed] [Google Scholar]
- 19.Juhasz M, Levin M, Marmur E. The kinetics of reversible hyaluronic acid filler injection treated with hyaluronidase. Dermat Surg. 2017;43:841–847. [DOI] [PubMed] [Google Scholar]
- 20.King M, Convery C, Davies E. This month’s guideline: the use of hyaluronidase in aesthetic practice (v2.4). J Clin Aesthet Dermatol. 2018;11:E61–E68. [PMC free article] [PubMed] [Google Scholar]
- 21.Quezada-Gaón N, Wortsman X. Ultrasound-guided hyaluronidase injection in cosmetic complications. J Euro Acad Dermatol Venereol. 2016;30:e39–e40. [DOI] [PubMed] [Google Scholar]
- 22.Prescribers’ Digital Reference. Hyaluronidase. Available at https://www.pdr.net/search-results?q=hyaluronidase. Published 2022. Accessed July 4, 2022.
- 23.British National Formulary. Hyaluronidase. Available at https://bnf.nice.org.uk/drugs/hyaluronidase/. Published 2022. Accessed July 4, 2022.