Abstract
In comparison to wild-type herpesvirus saimiri, viral interleukin-17 gene knockout mutants have unaltered behavior regarding viral replication, T-cell transformation in vitro, and pathogenicity in cottontop tamarins. Thus, this gene is not required for T-cell lymphoma induction but may contribute to apathogenic viral persistence in the natural host, the squirrel monkey.
Herpesvirus saimiri does not cause disease in its natural host, the squirrel monkey (Saimiri sciureus). In other New World primate species, such as common marmosets (Callithrix jacchus) or cottontop tamarins (Saguinus oedipus), this virus induces acute T-cell lymphoma (6, 10, 15). Moreover, virus strain C488 is capable of transforming human T cells in cultures to stable interleukin-2 (IL-2)-dependent growth (2, 8). Virus gene expression in the transformed human T-cell lines is mostly restricted to the transforming gene stpC/tip and to the viral superantigen homolog gene ie14/vsag (9, 14, 22). Whereas stpC/tip is essential for transformation, deletion of vsag neither affects the capacity of the virus to transform T cells in cultures nor to induce T-cell lymphoma in cottontop tamarins (6, 14, 15). The neighboring viral gene ORF13 has a cellular homolog, initially called CTLA8 (18). Functional analysis of the ORF13 and CTLA8 products has led to their identification as viral IL-17 (vIL-17) and cellular IL-17. Cellular IL-17 is exclusively produced by activated CD4+ T cells. vIL-17 has been demonstrated in the supernatants of infected permissive owl monkey kidney (OMK) cultures by immunoprecipitation. Both cellular IL-17 and vIL-17 induce stroma cells to secrete prostaglandin E2, IL-6, IL-8, and granulocyte colony-stimulating factor (11, 19, 21, 23). IL-17 promotes the proliferation of CD34+ progenitor cells and their differentiation into neutrophil granulocytes (11). Moreover, IL-17 has been shown to support T-cell proliferation (21). Thus, the functional role of the viral homolog ORF13/vil-17 in T-cell transformation and virulence needed to be elucidated.
MATERIALS AND METHODS
Construction of virus mutants.
Knockout mutants of herpesvirus saimiri C488 (3, 5) were constructed by homologous recombination according to published procedures (8, 14). Plasmid x50 contains a 3,716-bp XbaI fragment of C488 including vil-17 at nucleotides (nt) 1055 to 600 (14; accession no. Y13183). The neor gene from pSV2neo was inserted in an antisense orientation relative to vil-17 into the XmnI cleavage site at nt 1040, shortly after the vIL-17 initiation codon at nt 1055. The genotype of the recombinant virus clones was confirmed by Southern blotting of HindIII- and BglII-digested virus DNA and by hybridization with both virus- and neor-specific radiolabeled probes. Moreover, the mutant viruses and transformed cells were analyzed by PCR for stpC, vsag, and vil-17 DNAs (14).
T-cell assays.
Virus culturing, T-cell culturing, and T-cell transformation assays were done according to published protocols (8, 14). Phytohemagglutinin (PHA)-activated primary T cells from peripheral blood of C. jacchus and Saguinus fuscicollis were repeatedly stimulated with irradiated human feeder cells (120 Gy) and PHA (5 μg/ml) at intervals of at least 1 month and expanded in the presence of low concentrations of IL-2 (10 U of Proleukin per ml; Chiron, Ratingen, Germany) in order to obtain sufficient material for parallel in vitro transformation experiments. In addition, experiments were performed with fresh blood cells of four C. jacchus donors. In this case, the transformation assay was done without exogenous IL-2. Human T cells which were transformed by wild-type or mutant viruses were analyzed by flow cytometry with directly labeled antibodies against CD3, CD4, CD8, CD45, CD56, and CD69 (Becton-Dickinson, Heidelberg, Germany) and were further tested for CD2 hyperreactivity according to published procedures (14, 16). For this purpose, transformed T cells (5 × 104) were incubated alone or in the presence of antibody and/or 5 × 104 stimulator cells in 200 μl of complete RPMI 1640 medium. The rat monoclonal antibody 39C1.5 (Immunotech, Marseille, France) recognizing the human T11.1 epitope on the CD2 molecule was used in stimulation and blocking assays at 1 μg/ml. Cell line L428 from human Hodgkin’s lymphoma was applied as a source of cell-bound CD58, which binds to CD2. Murine B-cell line A20 does not provide functional CD58 but carries large amounts of Fcγ receptors used for cross-linking the stimulatory antibody 39C1.5. Combined stimulation by PHA (1 μg/ml) and L428 cells served as a positive control. The supernatants were harvested after 24 h. Human gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) antibody pairs (Genzyme, Rüsselsheim, Germany) were applied to determine cytokine concentrations by triplicate enzyme-linked immunosorbent assays (ELISA) of 1:100-diluted culture supernatants.
Animal experiments.
The Institutional Animal Care and Use Committee at the Biomedical Research Centre (Rijswijk, The Netherlands) approved the study of vil-17 mutant viruses in cottontop tamarins (S. oedipus). Wild-type C488 as well as mutants 13-1.9 and 13-2.11 (107 PFU in 1 ml of cell-free culture supernatant in Dulbecco’s minimal essential medium) were intravenously injected each into two naive, purpose-bred S. oedipus monkeys in parallel to published experiments with ie14/vsag deletion mutants (15). A high dose was chosen to avoid limiting conditions of infection. Animals R207 and R217 received wild-type virus, B133 and R178 received mutant 13-1.9, and B198 and R226 received mutant 13-2.11. The animals were euthanatized when illness was evident. Autopsy and histological analysis were performed. Blood samples of 1.5 ml each were taken prior to infection, at weekly intervals, and prior to euthanasia. Virus isolation experiments were performed on all blood samples obtained after infection. Cytokine concentrations in monkey plasma were determined with ELISA for IFN-γ (Genzyme) and for IL-17 (Biosource, Fleurus, Belgium). The IL-17 test was found to recognize cellular IL-17 even from cottontop tamarins but did not recognize vIL-17. Cells from peripheral blood and autopsy samples were cultured without IL-2 in a 1:1 mixture of RPMI 1640 medium and CG medium (Vitromex, Selters, Germany) supplemented with fetal bovine serum (10%), glutamine, and gentamicin (8, 15). Stably growing cells were analyzed by genomic PCR for vil-17 and for the neighboring gene vsag as a positive control. Flow cytometry analysis was performed with the ex vivo cell lines and with fresh peripheral blood mononuclear cells (PBMC) by use of cross-reactive monoclonal antibodies which had been generated against human CD2, CD3, CD4, CD8, CD14, CD20, CD25, CD28, CD29, CD38, and HLA-DR (15). All assays for in vitro transformation and pathogenesis were performed in parallel to the tests of vsag deletion mutants (14, 15).
RESULTS
vil-17 is lytically expressed.
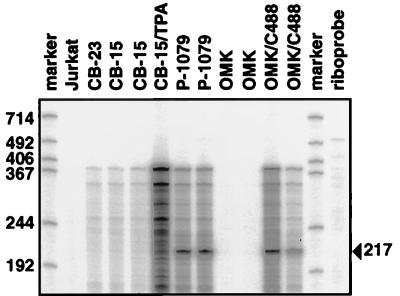
The pattern of expression of vil-17 of wild-type herpesvirus saimiri C488 was studied by various methods. Northern blots with total or polyadenylated RNA were negative, even with RNA from infected OMK cells. We further tried to demonstrate the viral protein by ELISA and Western blotting with cross-reactive monoclonal antibody 5 according to published procedures (11). Western blotting with chemiluminescence detection was not sufficiently sensitive to detect vIL-17. In ELISA, a weak signal was detected only in supernatants of semipermissive transformed marmoset T cells and not of transformed human T cells. Finally, we applied an RNase protection method (20) to enhance the sensitivity for the detection of vil-17 transcripts. The RNA probe spanned 378 bp of viral sequence starting at the SpeI restriction site within the vil-17 reading frame and including 213 nt upstream of the vIL-17 initiation codon. Protected fragments were observed in RNA samples from lytic cultures only. Signals were observed in virus-producing transformed C. jacchus P-1079 T cells (9) and in virus-infected OMK cells. Nonproductive human T cells (CB-15, CB-23) did not show this band, even after phorbol ester (tetradecanoyl phorbol acetate, 2 ng/ml) stimulation. The intensity of the vil-17 signals was generally low. The long exposure times explain the relatively high background seen in all cell types that contained viral DNA. The protected fragment of 217 nt corresponds to a transcription start site 52 nt upstream of the IL-17 initiation codon (Fig. 1).
FIG. 1.
Lytic transcription of vil-17. RNase protection analysis was performed to demonstrate vil-17 transcripts of wild-type herpesvirus saimiri C488. Protected fragments were observed in RNA samples from lytic cultures only. The signal was observed in virus-producing transformed C. jacchus P-1079 T cells (9) and in virus-infected OMK cells. Nonproductive human T cells (CB-15, CB-23) did not show this band, even after phorbol ester (tetradecanoyl phorbol acetate [TPA]) stimulation. The protected fragment of 217 nt corresponds to a transcription start site 52 nt upstream of the vIL-17 initiation codon. The probe template comprises 378 bp of viral sequence starting at the SpeI restriction site within the open reading frame and including 213 nt upstream of the vIL-17 ATG.
vil-17 mutant viruses have unaltered replication and T-cell transformation properties.
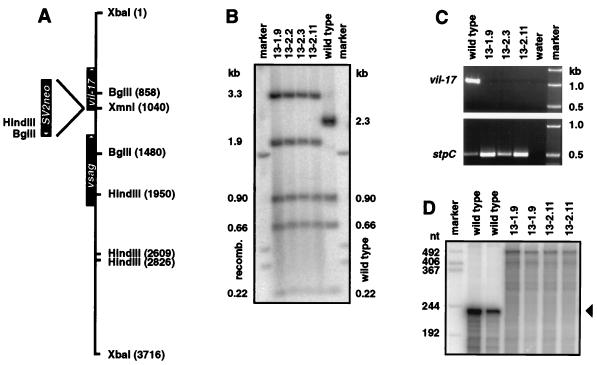
In order to study the functional relevance of the vil-17 gene, virus mutants 13-1.9 and 13-2.11 were selected from two independent experiments in order to minimize any bias from spontaneous mutations elsewhere in the herpesvirus genome. The viruses carried the antisense-oriented neor gene at a position close to the N terminus of vIL-17 (Fig. 2A). The mutation was confirmed by Southern hybridization and PCR (Fig. 2B and C). As the vil-17 coding sequence had been disrupted and not deleted, the transcription of the mutated vil-17 gene was analyzed in order to exclude artificial expression. Such expression seemed unlikely, as the end of the neor fragment did not provide promoter or initiation codon sequences. RNase protection assays were performed with a probe template which contained 229 nt of the vil-17 reading frame and 328 nt of the 5′ part of neor, thus spanning the junction site between vil-17 and neor. The full-length undigested RNA probe was detectable as a faint band in mutant virus-infected OMK cells. There were no indications of an artificial transcript of the mutant gene. Protected fragments were observed with wild-type virus-infected OMK cells at the expected sizes but not with cells infected with mutant viruses (Fig. 2D).
FIG. 2.
Construction and confirmation of vil-17 mutants of herpesvirus saimiri C488. (A) The neor gene from plasmid pSV2neo was inserted in the antisense orientation into the XmnI cleavage site of plasmid x50 (14; accession no. Y13183) a few nucleotides downstream of the start codon for vIL-17. (B) Southern blots were prepared with HindIII-digested viral DNA from wild-type virus and from cloned vil-17 mutants from two independent recombination experiments. After hybridization with the radioactively labeled insert from plasmid x50, the expected band sizes for both the wild-type (given on the right) and the mutant (given on the left) viruses were observed. (C) The presence of the mutation in recombinant viruses was confirmed by PCR for vil-17, whereas the stpC gene was present in all samples. (D) In order to exclude potential artificial transcripts initiated within the SV2neo cassette, RNase protection assays were performed. The riboprobe comprised the transition region from the neor gene to the vil-17 open reading frame. Whereas wild-type viruses showed the protected fragment corresponding to the viral sequence (229 nt), protected fragments were not observed in RNA from mutant virus-infected OMK cells.
Replication at low titers was unchanged, as the mutant viruses had been easily cloned by plaque purification. The viruses were grown to titers of 107 PFU/ml and used for in vitro transformation and pathogenicity tests. T cells of humans and monkeys (C. jacchus and S. fuscicollis) were efficiently transformed in cultures in the presence of 10 U of IL-2 per ml (marmoset cells) or 50 U of IL-2 per ml (human cells) (Table 1). The presence of the mutation in transformed T cells was confirmed by PCR for the vil-17 and vsag genes.
TABLE 1.
In vitro transformation of human and marmoset T cells
| Donora | Species | Mob | No. of successful transformation/ no. of attempts for virus:
|
||
|---|---|---|---|---|---|
| C488 (wild type) | 13-1.9 (Δvil-17) | 13-2.11 (Δvil-17) | |||
| 5772774 | Human | 12 | 4/4 | 3/4 | 3/4 |
| 120-CB | Human | 6 | 2/2 | 2/2 | 2/2 |
| RA-CB | Human | 6 | 2/2 | 2/2 | 2/2 |
| SU | C. jacchus | 6 | 1/1 | NDc | 1/1 |
| 87 | S. fuscicollis | 6 | 2/2 | 2/2 | 2/2 |
| 89 | S. fuscicollis | 6 | 2/2 | 2/2 | 2/2 |
| Total | 13/13 | 11/12 | 12/13 | ||
CB, cord blood.
Months of culture.
ND, not done.
The surface phenotype of transformed T cells without the vil-17 gene was not altered compared to that of wild-type virus- transformed cells. Human T cells expressed CD4 or CD8, CD3, CD45, CD56, and CD69, as expected. Moreover, the typical hyperreactivity to CD2 stimulation (14, 16) was not altered by the mutation. This hyperreactivity is not found in untransformed T cells. The mutant and wild-type virus-transformed cells reacted equally on CD2.1 stimulation by secreting IFN-γ and TNF-α (Table 2).
TABLE 2.
CD2 hyperreactivity of transformed human T cells resulting in the production of IFN-γ or TNF-α
| Cell line/ stimulanta | Cytokine production by cells transformed with the indicated virusb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ (ng/ml)
|
TNF-α (ng/ml)
|
|||||||
| C488A | C488B | 13-1.9A | 13-2.11A | C488A | C488B | 13-1.9A | 13-2.11A | |
| None (medium) | 1.84 | 2.01 | 2.87 | 3.51 | 0.11 | 0.13 | 0.19 | 0.15 |
| L428/PHA | 37.54 | 43.24 | 54.64 | 52.55 | 2.61 | 2.13 | 2.44 | 2.53 |
| L428 | 25.60 | 28.50 | 38.66 | 36.10 | 2.32 | 1.67 | 1.71 | 1.55 |
| L428/αCD2 | 4.91 | 2.42 | 3.58 | 3.41 | 1.25 | 0.72 | 0.83 | 0.67 |
| A20 | 3.00 | 1.71 | 1.88 | 4.25 | 0.25 | 0.12 | 0.14 | 0.08 |
| A20/αCD2 | 16.09 | 12.37 | 13.35 | 20.43 | 1.02 | 0.80 | 0.74 | 0.78 |
The cell lines resulted from the experiment with donor 120-CB (Table 1).
Suffix A or B identifies independent parallel cell lines obtained with the indicated viruses.
Mutation of vil-17 does not block pathogenicity.
The mutant viruses were further studied for their pathogenetic potential in vivo. At day 15 or 16 after infection with wild-type or mutant viruses, all six S. oedipus monkeys developed severe disease. Animals R207, B133, R178, and R226 had profound diarrhea. At necropsy, severely enlarged mesenteric lymph nodes were observed in animals R207, R217, B133, B198, and R226. The kidneys in animals R207, R217, B133, and B198 had an irregular red and white speckled appearance and, upon histological analysis, revealed infiltration of neoplastic lymphoid cells. The adrenal glands of animals R217 and B198 were hemorrhagic. Animals R207, R217, R178, B198, and R226 had signs of enteropathy. Whole-blood flow cytometry yielded moderately increased numbers of memory-type CD4+ CD29+ cells. Double staining for CD14/CD4, CD20/HLA-DR, CD2/HLA-DR, CD2/CD28, and CD2/CD38 did not reveal significant changes, nor did the absolute numbers of T cells (CD2+) and B cells (CD20+). The absolute numbers of granulocytes and monocytes tended to decrease during the course of infection. Giemsa- stained blood smear slides indicated a terminal relative increase in lymphocyte numbers and a decrease in granulocyte counts regardless of the virus genotype. The IL-17 and IFN-γ plasma levels were measured by ELISA. Animals with terminal disease had increased cellular IL-17 titers and strongly elevated levels of IFN-γ (Table 3).
TABLE 3.
Plasma INF-γ and IL-17 levels in infected S. oedipus monkeys
| Virus | Animal | Level (pg/ml) on the indicated day of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ
|
IL-17
|
||||||||
| 0 | 8 | 15 | 16 | 0 | 8 | 15 | 16 | ||
| Wild type | R207 | <30 | <30 | 196 | 304 | 38 | <30 | 193 | 195 |
| R217 | <30 | <30 | 700 | —a | <30 | <30 | 200 | — | |
| 13-1.9 | B133 | <30 | <30 | 132 | 248 | <30 | <30 | 40 | 58 |
| R178 | <30 | <30 | 104 | 100 | 106 | 31 | 157 | 150 | |
| 13-2.11 | B198 | <30 | <30 | 668 | — | <30 | <30 | 906 | — |
| R226 | <30 | <30 | 130 | 226 | <30 | <30 | 97 | 117 | |
—, Animals had died.
Cell cultures from blood samples and from samples of various organs (thymus, spleen, liver, kidney, and axillary, mesenteric, and inguinal lymph nodes) were used to establish lymphoma cell lines and to isolate virus. At day 7 after infection, ex vivo T-cell lines were established, whereas virus isolations failed. At day 14 after infection, virus was isolated from PBMC of all animals (8, 15). Ex vivo T-cell lines were regularly obtained from PBMC (day 14 and terminal) and from thymus, spleen, and lymph nodes at autopsy. These IL-2-independent cell lines expressed CD2, CD3, CD4, CD8, CD25, and major histocompatibility class II antigen. All the cell lines expressed CD8 with variable coexpression of CD4 (10 to 100%).
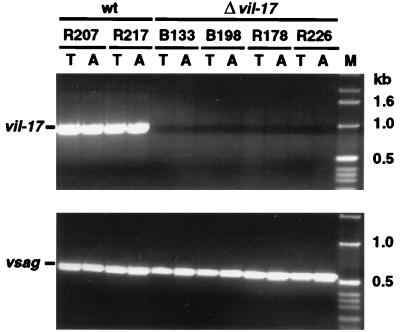
PCR with DNA from cell lines of each animal demonstrated the presence of viral genomes and verified the respective genotype (wild type or mutant). Whereas cells from wild-type virus-infected animals were positive for both vil-17 and vsag, those from mutant virus-infected monkeys contained vsag but not vil-17 (Fig. 3). The presence of viral genomes was confirmed by hybridization of Gardella in situ lysis Southern blots (8).
FIG. 3.
Viral DNA in ex vivo T-cell lines from virus-infected monkeys. Tumor cell lines from all virus-infected S. oedipus animals were subjected to genomic viral PCR analysis. The mutation was confirmed in cell lines from animals carrying mutant viruses with a disrupted vil-17 gene. In contrast, the T-cell lines from wild-type (wt) control animals had PCR signals for both virus genes analyzed (vil-17 and vsag). T, cell line from thymus; A, cell line from axillary lymph nodes. The sizes of marker DNA fragments are given on the right.
The rapid onset of disease argues for polyclonal transformation events due to simultaneous infection of T cells. Tumor clonality was not investigated, because essential species-specific reagents are not available. Histology revealed peripheral pleomorphic T-cell lymphoma with characteristic follicular lysis in lymph nodes and infiltration of multiple organs. No apparent differences in tumor morphology or metastasis were caused by viruses with or without vil-17.
Genes 12, 13, and 14 are nonessential for replication and transformation.
We recently isolated a virus mutant (488CD- 1) which had a spontaneous deletion of the vil-17 gene. The deletion was defined by Southern blotting and PCR sequencing of products generated with primers in ORF10 (position 24069, according to the sequence of strain A11; accession no. X64346) and downstream of ORF15 (position 29820). The deletion border sites mapped to nt 25654 and 29022, thus eliminating ORF12, ORF13, and ORF14 entirely and truncating ORF11 by one third at the C terminus (286 of 505 amino acids were retained). In cultures, this virus mutant was capable of transforming T cells from humans and cottontop tamarins. In addition, mutant 488CD-1 was able to transform nonstimulated primary T cells of four different common marmoset (C. jacchus) donors in the absence of exogenous IL-2. Concerning the transformation potential of 488CD-1, differences were not observed from wild-type virus C488 and from individual ORF13 or ORF14 mutants (13-1.9, 13-2.11, 14-3.10, and 14-4.6). Thus, all three virus genes (ORF12, ORF13, and ORF14) are dispensable for virus replication and in vitro T-cell transformation.
DISCUSSION
The transforming and pathogenic properties of wild-type virus were abolished by deletion of the transforming gene stpC (12, 13, 17) and of tip, whose gene product specifically interacts with tyrosine kinase Lck of T cells (4). While transforming capacity was lost, virus replication was not influenced (6, 7, 14). In addition to the transformation-associated genes stpC, tip, and vsag (14), vil-17 was one of the main candidates to contribute to transformation and pathogenesis. However, as demonstrated in this study, disruption of this gene did not influence the virus functions analyzed. We conclude that vil-17 is dispensable for lytic virus replication, in vitro transformation of simian and human T cells, and pathogenicity in cottontop tamarins. Our previous work with similar assays showed that the neighboring gene vsag is also nonessential (14, 15). The results for the separate mutations of vil-17 and vsag were confirmed by the spontaneous combined deletion of genes 12 to 14 in mutant 488CD-1. In contrast to vsag, which is strongly transcribed in stimulated transformed human T cells (14), vil-17 is expressed only during lytic virus replication. Low levels of transcripts and protein were detected in lytically infected OMK cells and in semipermissive transformed marmoset T cells. Since vIL-17 is able to induce IL-8 in fibroblasts and epithelial cells (11), positive feedback regulation might be possible via the lytically expressed vIL-8 receptor (1, 9, 14). Although the presence of vil- 17 does not seem to be critical for pathogenicity, it remains to be seen whether vil-17 plays a role in perinatal transmission or apathogenic persistence in squirrel monkeys (S. sciureus), which are regularly infected by the virus.
ACKNOWLEDGMENTS
We thank A. Filatov (Moscow, Russia) and P. Rieber (Dresden, Germany) for providing monoclonal antibodies, D. De Groote (Biosource, Fleurus, Belgium) for the IL-17 ELISA kit, P. van Eerd and P. Frost (Rijswijk, The Netherlands) for veterinary care for the animals, N. Deuerling (München, Germany) and D. Labahn (Erlangen, Germany) for providing monkey blood samples, A. Ensser (Erlangen, Germany) for providing a PCR primer binding to ORF10 of C488, and E. Meinl (Erlangen, Germany) for valuable suggestions.
Parts of this study were supported by the Wilhelm Sander-Stiftung (Neustadt, Germany), the Bayerische Forschungsstiftung (München, Germany), the German-Israeli-Foundation (Jerusalem, Israel), and the Bundesministerium für Bildung und Forschung (Bonn, Germany).
REFERENCES
- 1.Ahuja S, Murphy P. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. [PubMed] [Google Scholar]
- 2.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R, Fleckenstein B. Stable growth transformation of human T-lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biesinger B, Trimble J, Desrosiers R, Fleckenstein B. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990;176:505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- 4.Biesinger B, Tsygankov A, Fickenscher H, Emmrich F, Fleckenstein B, Bolen J, Bröker B. The product of the herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem. 1995;270:4729–4734. doi: 10.1074/jbc.270.9.4729. [DOI] [PubMed] [Google Scholar]
- 5.Desrosiers R, Falk L. Herpesvirus saimiri strain variability. J Virol. 1982;43:352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duboise S, Guo J, Czajak S, Desrosiers R, Jung J. STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;71:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duboise S, Guo J, Desrosiers R, Jung J. Use of virion DNA as a cloning vector for the construction of mutant and recombinant herpesviruses. Proc Natl Acad Sci USA. 1996;93:11389–11394. doi: 10.1073/pnas.93.21.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fickenscher H, Fleckenstein B. Growth-transformation of human T cells. Methods Microbiol. 1998;25:573–602. [Google Scholar]
- 9.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleckenstein B, Desrosiers R. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Press; 1982. pp. 253–331. [Google Scholar]
- 11.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin J, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung J, Trimble J, King N, Biesinger B, Fleckenstein B, Desrosiers R. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knappe A, Hiller C, Thurau M, Wittmann S, Hofmann H, Fleckenstein B, Fickenscher H. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J Virol. 1997;71:9124–9133. doi: 10.1128/jvi.71.12.9124-9133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knappe A, Thurau M, Niphuis H, Hiller C, Wittmann S, Kuhn E-M, Rosenwirth B, Fleckenstein B, Heeney J, Fickenscher H. T-cell lymphoma caused by herpesvirus saimiri C488 independently of ie14/vsag, a viral gene with superantigen homology. J Virol. 1998;72:3469–3471. doi: 10.1128/jvi.72.4.3469-3471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittrücker H, Müller-Fleckenstein I, Fleckenstein B, Fleischer B. CD2-mediated mutual stimulation of herpesvirus saimiri-transformed human T lymphocytes. J Exp Med. 1992;176:909–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy C, Kretschmer C, Biesinger B, Beckers J, Jung J, Desrosiers R, Müller-Hermelink H, Fleckenstein B, Rüther U. Epithelial tumors induced by a herpesvirus oncogene in transgenic mice. Oncogene. 1994;9:221–226. [PubMed] [Google Scholar]
- 18.Rouvier E, Luciani M, Mattei M, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 19.Spriggs M K. Interleukin-17 and its receptor. J Clin Immunol. 1997;17:366–369. doi: 10.1023/a:1027360106635. [DOI] [PubMed] [Google Scholar]
- 20.Stammiger T, Fickenscher H, Fleckenstein B. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J Gen Virol. 1990;71:105–113. doi: 10.1099/0022-1317-71-1-105. [DOI] [PubMed] [Google Scholar]
- 21.Yao Z, Fanslow W, Seidin M, Rousseau A, Painter S, Comeau M, Cohen J, Spriggs M. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 22.Yao Z, Maraskovsky E, Spriggs M, Cohen J, Armitage R, Alderson M. Herpesvirus saimiri open reading frame 14, a protein encoded by a T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J Immunol. 1996;156:3260–3266. [PubMed] [Google Scholar]
- 23.Yao Z, Painter S, Fanslow W, Ulrich D, Macduff B, Spriggs M, Armitage R. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]