Abstract
Background The underlying mechanisms of thrombosis in Lemierre's syndrome and other septic thrombophlebitis are incompletely understood. Therefore, in this case control study we aimed to generate hypotheses on its pathogenesis by studying the plasma proteome in patients with these conditions.
Methods All patients with Lemierre's syndrome in the Skåne Region, Sweden, were enrolled prospectively during 2017 to 2021 as cases. Age-matched patients with other severe infections were enrolled as controls. Patient plasma samples were analyzed using label-free data-independent acquisition liquid chromatography tandem mass spectrometry. Differentially expressed proteins in Lemierre's syndrome versus other severe infections were highlighted. Functions of differentially expressed proteins were defined based on a literature search focused on previous associations with thrombosis.
Results Eight patients with Lemierre's syndrome and 15 with other severe infections were compared. Here, 20/449 identified proteins were differentially expressed between the groups. Of these, 14/20 had functions previously associated with thrombosis. Twelve of 14 had a suggested prothrombotic effect in Lemierre's syndrome, whereas 2/14 had a suggested antithrombotic effect.
Conclusion Proteins involved in several thrombogenic pathways were differentially expressed in Lemierre's syndrome compared to other severe infections. Among identified proteins, several were associated with endothelial damage, platelet activation, and degranulation, and warrant further targeted studies.
Keywords: Lemierre's syndrome, septic thrombophlebitis, Fusobacterium necrophorum, mass spectrometry, thrombogenesis
Background
Lemierre's syndrome is a life-threatening disease often defined as Fusobacterium necrophorum bacteremia following an oropharyngeal infection with direct (internal jugular vein thrombosis) or indirect (septic pulmonary embolization) signs of septic thrombophlebitis. 1 2 Following the introduction of antibiotics and improvements in health care, case fatality rates have decreased from 90% when originally described to 2%. 2 3 Nevertheless, supportive intensive care admissions are required in 43% of the previously healthy adolescents and young adults affected. 2 While still rare, incidence rates of Lemierre's syndrome are perceived to increase, possibly in part explained by better diagnostics or due to decreased antibiotic treatment of nonstreptococcal pharyngotonsillitis (including potential cases of F. necrophorum pharyngotonsillitis). 2 4 5 6 7 8
F. necrophorum is an obligate anaerobic gram-negative rod. Historically, it has been described to be part of the normal tonsillar flora, 1 yet a more accurate description is likely that transient carriage occurs among teenagers and adolescents. 9 F. necrophorum is increasingly recognized as a primary pathogen in local infections, for example, pharyngotonsillitis 10 11 12 13 14 and peritonsillar abscess 15 16 in addition to its role as the typical pathogen seen in Lemierre's syndrome. 1 Little is known of its pathogenicity, including the very particular presentation with thrombosis in Lemierre's syndrome. 1 17 18 In invasive infection caused by F. necrophorum , direct or indirect signs of infection-induced thrombosis are remarkably common and can be seen in more than one-third of the patients. 2 17 While infection-associated thrombosis also exists in other types of infections, including COVID-19, the association is much weaker. 19 20 One of the main differences between infection-associated thrombosis and thrombosis in other circumstances is the inflammation-mediated component. Here, in a process referred to as “thromboinflammation,” inflammation secondary to infection is believed to trigger the activation of platelets, accompanied by endothelial damage, subsequent fibrin deposition, and the formation of thrombosis. 21
In Lemierre's syndrome, direct evidence of pus collections surrounding the internal jugular vein as well as pus within the thrombi has been described. 22 Most studies on prothrombotic activity have been performed on the subspecies F. necrophorum subsp. necrophorum , 23 24 25 mainly causing veterinary infections, while less is known about F. necrophorum subsp. funduliforme , causing human infections. 1
Given the known predilection of F. necrophorum to invade the internal jugular vein, it is likely that the extrinsic pathway of the coagulation cascade is involved starting with tissue factor exposure from endothelial injury. 26 Previous studies have shown the contact system to be activated on the surface of F. necrophorum subsp. funduliforme , 27 which then activates the intrinsic pathway and links inflammation and thrombosis. 28 F. necrophorum subsp. funduliforme has also been shown to bind and activate plasminogen, which has been suggested to be important for invasiveness after initial localized tonsillitis. 29 Based on clinical studies, thrombocytopenia, elevated PT-INR, and occasionally more severe forms of disseminated intravasal coagulation are known to occur in Lemierre's syndrome. Yet the pathogenesis underlying thrombogenesis remains unknown. 1 2 Consequently, to generate hypotheses about this pathogenesis, we designed a study investigating proteomic differences in patients with Lemierre's syndrome compared to patients with other severe infections without thrombosis. Our aim was to describe the proteomic profile of Lemierre's syndrome, with particular focus on proteins previously associated with any aspect of thrombogenesis to generate hypotheses for future studies.
Methods
Study Design and Setting
Cases and controls were defined and enrolled as follows:
When F. necrophorum was identified in blood cultures at the Department of Clinical Microbiology, Skåne University Hospital, Lund, Sweden, the researchers were rapidly notified, and patients were screened for inclusion. All patients diagnosed with Lemierre's syndrome, defined as F. necrophorum -bacteremia following an oropharyngeal infection within the last 4 weeks and presence of either direct or indirect signs of septic thrombophlebitis, 2 at any hospital ( n = 9) in the Skåne Region, Sweden (population 1.4 million 2021), were enrolled during 2017 to 2021 as cases. Septic thrombophlebitis was defined as a visualized thrombus in any of the jugular veins in the vicinity of an infection and visualized by imaging or as septic pulmonary emboli or multifocal pneumonia seen on chest radiology.
Controls, constituting patients with other severe infections, were enrolled in three ways and defined as patients with F. necrophorum -bacteremia who did not develop Lemierre's syndrome ( n = 3), patients presenting through a sepsis alert system, where sepsis is defined as suspected infection with organ dysfunction (enrolled through a partner study, n = 5), and patients enrolled via convenience sampling by the first author during clinical shifts, defined as patients with acute infections requiring hospital treatment at the Department of Infectious Diseases ( n = 7). All controls were enrolled at the Skåne University Hospital Lund, Sweden, during the years 2017 to 2021. Age criterion for all controls was set to 13 to 50 years to match patients with Lemierre's syndrome.
Sample Preparation
Citrate plasma samples were drawn in all patients, within 2 hours centrifuged for 10 minutes at 2,000 to 2,200 g and then stored in –70 to –80°C pending analysis. Hereafter, all sample preparation steps, including protein digestion and desalting were performed on Agilent AssayMAP Bravo Platform. Each plasma sample was diluted 10 times with 100-mM ammonium bicarbonate (AmBic) and 10 µL of each diluted plasma sample was transferred to a 96-well plate where 40 µL of 4 M urea in 100 mM AmBic was added for a final volume of 50 µL. The proteins were reduced with 10 µL of 60 mM dithiothreitol (DTT; final concentration of 10 mM) for 1 hour at 37°C followed by alkylation with 20 µL of 80 mM iodoacetamide (IAA; final concentration of 20 mM) for 30 minutes in the dark at room temperature. The plasma samples were digested with 2 µg Lys-C for 5 hours at room temperature and further digested with 2 µg trypsin overnight at room temperature. 30 The digestion was stopped by pipetting 20 µL of 10% trifluoroacetic acid (TFA) and the digested peptides were desalted on Bravo platform. To prime and equilibrate the AssayMAP C18 cartridges, 90% acetonitrile (ACN) with 0.1% TFA and 0.1% TFA were used, respectively. The samples were loaded into the cartridges at the flow rate of 5 µL/min. The cartridges were washed with 0.1% TFA before the peptides were eluted with 80% ACN/0.1% TFA. The eluted peptides were dried in a SpeedVac and resuspended in 25 μL of 2% ACN/0.1% TFA. The peptide concentration was measured using the Pierce Quantitative Colorimetric Peptide Assay (Thermo Fisher Scientific). The samples, 10 µL, were diluted with 10 µL 2% ACN/0.1% TFA and spiked with 2 µL of diluted iRT peptides (JPT Peptide Technologies) before liquid chromatography–mass spectrometry (LC-MS/MS) analysis.
LC MS Analysis
The samples, 3 µL, were analyzed on hybrid mass spectrometer Exploris 480 (Thermo Fisher Scientific) coupled with an Ultimate 3000 UHPLC system (Thermo Fisher Scientific). A two-column setup was used on the HPLC system and peptides were loaded into an Acclaim PepMap 100 C18 precolumn (75 μm × 2 cm, Thermo Scientific) and then separated on an EASY spray column (75 μm × 50 cm, nanoViper, C18, 2 μm, 100 Å) with the flow rate of 300 nL/min. The column temperature was set to 60°C. Solvent A (0.1% FA in water) and solvent B (0.1% FA in 80% ACN) were used to create gradient, and 120-minute linear gradient from 3 to 38% of solvent B in solvent A was used to elute the peptides.
Data-Independent Acquisition Data Acquisition
The Exploris 480 was operated in the data-independent acquisition (DIA) mode. Full MS survey scans from m/z (mass-to-charge ratio) 350 to 1,650 with a resolution 120,000 were performed in the Orbitrap detector. The normalized automatic gain control (AGC) target was set to 300% with the maximum injection time of 45 milliseconds. One segment for MS1 was kept constant and 44 segments with variable isolation windows were acquired for MS2 with the window overlap of 1 m/z. The resolution of MS2 was set 30,000. The stepped normalized collision energy (NCE) for HCD was set at 27, 30, and 32 and the normalized AGC target for MS2 was set at 300%. The maximum injection time was set to auto. See the mass list in Supplementary Material 1 (available in the online version).
Data Analysis
The DIA data were analyzed with DirectDIA workflow by using Spectronaut 16.0.220606. Data were extracted based on maximum intensity for both precursors and fragment ions. The default settings were applied for the peptide and protein identification and quantification. In brief, q -value of 0.01 was used to estimate false discovery rates for both precursor and protein identification and the p -value was calculated by the kernel-density estimator. For quantification, interference correction was activated and a minimum of three fragment ions and two precursor ions were kept. The peak area of the MS2 level was used for quantitation. Peptide (stripped sequence) quantity was measured by the mean of one to three most intensive precursors, and protein quantity was calculated accordingly by the mean of one to three best peptides. Data were filtered by q -value and cross-run normalization was inactive. Data were exported from Spectronaut and downstream statistical analysis was performed with R 4.2.2. All the scripts are available through https://github.com/gtorisson/MS_Lemierre_2023 and a working document is attached in the Supplementary Material 2 (available in the online version).
Statistical Analysis
Label-free quantification DIA data were log2-transformed. If more than 30% of values were missing, the proteins were discarded. Data were then normalized to median value of each sample, after which imputation based on normal distribution with a down shift/left censoring of 1.8 standard deviations was performed. Thereafter differential expression of proteins between patients with Lemierre's syndrome and other severe infections were investigated using t -test of independent samples with Benjamini–Hochberg false discovery rate correction for multiple comparisons. A q -value of <0.05 was considered significant if log2 fold change was at least 1. Volcano plots and heat maps were performed, highlighting differentially expressed proteins. Relative differences of protein abundance (fold change) and q -values were presented for all differentially expressed proteins in tables or text. Uniprot 31 was used to identify proteins expressed and literature searches were performed of each differentially expressed protein to ascertain previously known associations with thrombosis and direction of suggested effect (pro- or antithrombotic). Functions of all differentially expressed proteins are described in Supplementary Table S1 (available in the online version) with references provided.
This study was approved by the local Ethical Review Board in Lund, Sweden (number 2017/740). The sepsis alert partner study was approved by the Ethical Review Board in Lund (number 2016/271).
Results
Baseline Characteristics
In total, 23 patients were enrolled, including 8 cases with Lemierre's syndrome and 15 patients with other severe infections (controls). Among controls, 53% had bacteremia with the following bacteria identified: F. necrophorum (n = 3, who did not fulfill the criteria of Lemierre's syndrome), Escherichia coli ( n = 2), polymicrobial bacteremia ( n = 1), Streptococcus agalactiae ( n = 1), S. dysgalactiae ( n = 1), and S. anginosus ( n = 1).
Baseline characteristics were similar between groups in terms of age and rarity of comorbidities. Slight differences were seen in gender, sepsis on presentation, and time from presentation to inclusion. Most notably, only 25% of patients with Lemierre's syndrome were females, compared to 47% among controls. Cases and controls were relatively well matched in terms of inflammation as measured by C-reactive protein (CRP), with moderate levels of elevation seen in both groups with substantial overlap and considerable variance ( Table 1 ).
Table 1. Baseline characteristics of enrolled patients in the primary analysis; Lemierre's syndrome and other severe infections (controls).
| Lemierre's syndrome ( N = 8) | Other severe infections ( N = 15) | |
|---|---|---|
| Age, y, median (range) | 22 (16–46) | 24 (17–50) |
| Female, n (%) | 2 (25) | 7 (47) |
| Any comorbidity, a n (%) | 0 | 2 (13) |
| Days from presentation to enrollment, d, median (range) | 2 (1–3) | 1 (1–4) |
| C-reactive protein at the time of sampling, mean [SD] | 182 [81] | 124 [107] |
| Sepsis on presentation, b n (%) | 5 (63) | 4 (40) |
| Septic shock on presentation, b n (%) | 1 (13) | 0 |
| Bacteremia, n (%) | 8 (100) | 8 (53) |
| Head and neck-infection, n % | 8 (100) | 10 (67) |
| V. jugularis thrombosis, n (%) | 5 (63) | 0 |
| Anticoagulant treatment started when samples were drawn, c n (%) | 1 (13) | 0 |
In the plasma samples analyzed by quantitative proteomics mass spectrometry, a total of 654 proteins were identified, of which 205 were excluded due to greater than 30% missing values. Data on missing values, as well as imputed values, can be seen in Supplementary Material 2 ( Figs. S2 , S4 , and S5 ; available in the online version). Hence, 449 proteins remained in the analysis.
Primary Analysis: Differentially Expressed Proteins in Lemierre's Syndrome versus Other Severe Infections
Twenty of 449 proteins were differentially expressed in patients with Lemierre's syndrome compared to controls. These were visualized with groupwise comparisons ( Figs. 1 and 2 ). Based on a literature search, 14/20 of differentially expressed proteins were identified as previously associated with thrombosis, either suggested to be causal or implied as biomarkers without known pathogenesis (see Supplementary Material 1 , Table S1 ; available in the online version for short descriptions of functions of proteins).
Fig. 1.

Volcano plot of differentially expressed proteins in patients with Lemierre's syndrome ( n = 8; right hand side) versus other severe infections ( n = 15; left hand side). Differentially expressed proteins were defined by independent samples t -test with Benjamini–Hochberg multiple comparison correction with significance defined as q < 0.05 and log2 fold change of at least 1. Red dots indicate differentially expressed proteins.
Fig. 2.
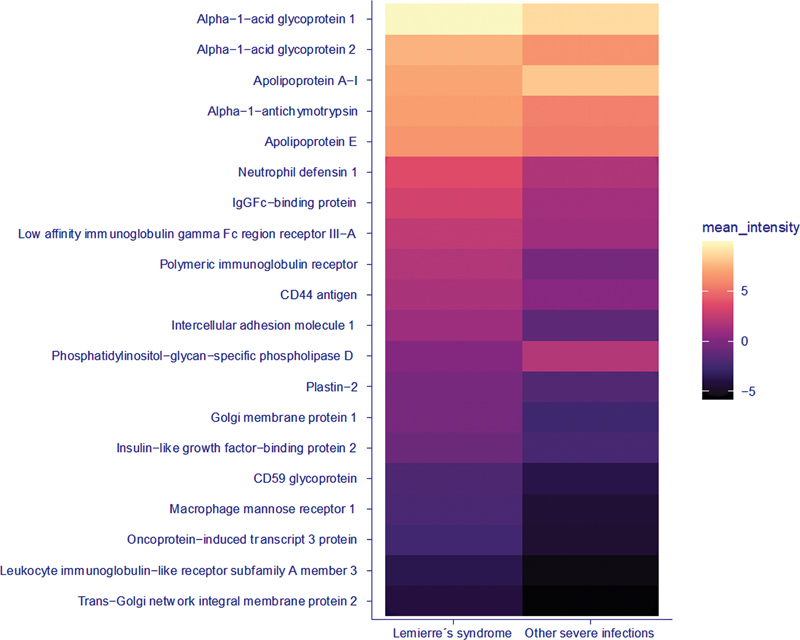
Heat map visualizing 20 differentially expressed proteins in Lemierre's syndrome (LS; n = 8) versus other severe infections (sepsis; n = 15). Values represent protein abundance (log2-transformed and median-normalized) with darker colors equaling lower intensity and vice versa.
Twelve proteins were differentially expressed with a suggested prothrombotic effect in Lemierre's syndrome, and consisted of proteins defined as prothrombotic, which were upregulated, or as antithrombotic proteins, which were downregulated. Three prothrombotic proteins identified as elevated in Lemierre's syndrome, that is, neutrophil defensin 1, CD44-antigen, and intercellular adhesion molecule 1 (ICAM-1), are highlighted due to previously described and particularly interesting associations with thrombogenesis (details in Table 2 and Supplementary Material 1 , Table S1 [available in the online version only]).
Table 2. Differentially expressed proteins in Lemierre's syndrome ( n = 8) versus other severe infections ( n = 15) with a previously suggested pro- or antithrombotic effect ( n = 16 proteins) .
| Proteins with suggested prothrombotic effect in Lemierre's syndrome (LS) | Uniprot ID | Fold change LS vs. other severe infections | q |
|---|---|---|---|
| Intercellular adhesion molecule 1 | P05362 | 5.4 | 0.008 |
| Macrophage mannose receptor 1 | P22897 | 4.6 | 0.017 |
| Neutrophil defensin 1 | P59665 | 3.6 | 0.024 |
| IgGFc-binding protein | Q9Y6R7 | 3.5 | 0.005 |
| Trans-Golgi network integral membrane protein 2 | O43493 | 3.4 | 0.030 |
| Insulinlike growth factor-binding protein 2 | P18065 | 2.8 | 0.030 |
| CD44 antigen | P16070 | 2.3 | 0.008 |
| Low-affinity IgGγ Fc receptor III-A | P08637 | 2.3 | 0.038 |
| Alpha-1-acid glycoprotein 1 | P02763 | 2.3 | 0.016 |
| Alpha-1-acid glycoprotein 2 | P19652 | 2.2 | 0.010 |
| Apolipoprotein E (Apo-E) | P02649 | 2.0 | 0.025 |
| Apolipoprotein A-I | P02647 | 0.47 | 0.030 |
| Proteins with suggested antithrombotic effect in LS | Uniprot accession ID | Fold change LS vs. other severe infections | q |
| CD59 glycoprotein (1F5 antigen) | P13987 | 3.3 | 0.01 |
| Plastin-2 | P13796 | 2.9 | 0.001 |
Note: Statistically significant differences were determined by independent samples t -test with Benjamini–Hochberg multiple comparison correction used ( q < 0.05) and log2 fold change of at least 1.
Two proteins were differentially expressed with a suggested antithrombotic effect in Lemierre's syndrome, and consisted of proteins defined as antithrombotic, which were upregulated ( Table 3 ), of which Plastin-2 is highlighted as the most interesting finding due to its association with development of thrombocytopenia ( Supplementary Material 1 , Table S1 [available in the online version only]).
Table 3. Differentially expressed proteins without previously known association to thrombosis in Lemierre's syndrome (LS; n = 8) versus other severe infections ( n = 15) .
| Proteins without known anti- or prothrombotic effects | Uniprot ID | Fold change LS vs. other severe infections | q |
|---|---|---|---|
| Polymeric Ig receptor | P01833 | 4.7 | 0.038 |
| Golgi membrane protein 1 | Q8NBJ4 | 4.6 | 0.01 |
| Oncoprotein-induced transcript 3 protein | Q8WWZ8 | 3.9 | 0.022 |
| Leukocyte Ig-like receptor subfamily A member 3 | Q8N6C8 | 3.9 | 0.01 |
| Alpha-1-antichymotrypsin | P01011 | 2.4 | 0.015 |
| Phosphatidylinositol-glycan-specific phospholipase D | P80108 | 0.30 | 0.010 |
Note: Statistically significant differences were determined by independent samples t -test with Benjamini–Hochberg multiple comparison correction used ( q < 0.05) and log2 fold change of at least 1.
Finally, a further six proteins without identified suggested pro- or antithrombotic effect were differentially expressed in Lemierre's syndrome ( Table 3 and Supplementary Material 1 , Table S1 [available in the online version]).
Discussion
We aimed to investigate proteomic differences in patients with Lemierre's syndrome to generate hypotheses on thrombogenic pathways. Based on quantitative proteomics mass spectrometry, several interesting and differentially expressed proteins in Lemierre's syndrome were identified in plasma when compared to controls with other severe infections. Most of the proteins had previous direct or indirect links to thrombosis and included proteins expressed following endothelial injury, proteins causing platelet activation or degranulation, and proteins linking innate immunity with thrombotic pathways. While these findings are novel and interesting, the explorative study design and small sample size limits interpretation to hypothesis generation for future studies on proposed specific proteins and mechanisms.
Few studies on the pathogenesis of Lemierre's syndrome have been performed despite its remarkable presentation with septic thrombophlebitis. 1 3 Yet, a few virulence mechanisms of F. necrophorum subsp. funduliforme have been identified, and consist of inactivation of factor H (inhibiting complement system activation), 32 leukotoxin production, 33 binding and activation of plasminogen, 29 and activation of the contact system. 27 Since F. necrophorum invades tissues adjacent to tonsils and subsequently the internal jugular vein, 22 it is likely that endothelial injury and presentation of tissue factor play a role in thrombogenesis. In this work, endothelial markers (e.g., ICAM-1 34 35 36 ) were shown to be elevated and a potential role in thrombogenesis is proposed. Furthermore, Plastin-2 was elevated in patients with Lemierre's syndrome, which has previously been suggested to inhibit platelet production. 37 This is in line with the common finding of thrombocytopenia as a part of Lemierre's syndrome 2 and could potentially be one of the mechanisms through which it develops. Several proteins (e.g., Apolipoprotein A-I, 38 Alpha-1-acid glycoprotein 1 and 2 39 ) identified as differentially expressed in Lemierre's syndrome have been shown to be involved in platelet activation, aggregation, and degranulation. CD44 antigen 40 has been described to upregulate tissue factor, which activates both the extrinsic pathway and platelets, 26 and has been hypothesized to be of importance in adhesion of activated platelets to endothelial cells. 41 Proteins previously described to link innate immunity and thrombosis were also elevated in Lemierre's syndrome (e.g., neutrophil defensin-1 42 43 44 ). Hence, multiple potential thrombogenic pathways are hypothesized, with particular emphasis on the potential interplay between endothelial injury, platelet activation, and activation of coagulation by interaction with innate immunity. Moreover, a potential role of Plastin-2 in the thrombocytopenia commonly seen in Lemierre's syndrome is highlighted. While the majority of findings suggest a prothrombotic expression of proteins in Lemierre's syndrome, two proteins were identified with suggested antithrombotic effects (i.e., Plastin-2 37 and CD59 glycoprotein 28 ). Given the complexity of blood coagulation 26 and thromboinflammation 28 as well as the known presence of, for example, thrombocytopenia in Lemierre's syndrome, these findings are not surprising.
The main difficulty of this study was to design a matched control group. In the primary analysis, we chose to not use a healthy control group, since most differentially expressed proteins between Lemierre's syndrome and healthy controls or patients with nonseptic venous thromboembolism would likely have been related to inflammation as such, rather than thromboinflammation. 28 Since Lemierre's syndrome occurs in a healthy and young age group rarely affected by sepsis, we aimed to match the control group to cases with Lemierre's syndrome by including both patients with F. necrophorum -bacteremia without Lemierre's syndrome and young and previously healthy patients with other severe infections. Controls were age-matched and either enrolled by a partnering (not yet published) sepsis-alert study or through convenience sampling of patients requiring hospital treatment for other severe infections that were encountered during clinical shifts. In addition to the case control study design, the main limitation of the study is the small sample size of participants with Lemierre's syndrome. Yet, given that 5 years of population-based prospective inclusion was necessary to enroll 8 patients with Lemierre's syndrome in a population of 1.4 million, a markedly prolonged study period would have been necessary to increase power. Notably, no previous similar population-based prospective studies of Lemierre's syndrome exist. Since the main aim was to generate hypotheses, the study size was deemed sufficient. Furthermore, baseline characteristics differed slightly, with longer time to enrollment in Lemierre's syndrome patients compared to other severe infections. In part, this was due to the case definition of Lemierre's syndrome requiring identification of bacteremia, which generally takes slightly less than 24 hours. 45 Patients with Lemierre's syndrome were also more commonly septic on presentation, which might have impacted results and the level of inflammation. CRP levels were slightly higher in patients with Lemierre's syndrome yet with substantial overlap between groups. Yet, differences in time from admission to enrollment between groups might be of importance, since CRP is known to peak after a few days. 46 Importantly, dynamics of any of the differentially expressed proteins are not known and might impact findings, yet differences in timing of enrollment were minor. Finally, handling of missing values in the proteomics dataset was strict, as proteins with more than 30% missing values were excluded from the analysis. A stricter-than-usual approach was chosen due to the small sample size, since imputation was based on left-censored normal distribution with missing values predicted to be missing not at random (MNAR). This limits the depth of our results since we will not detect proteins potentially elevated in certain groups and not discovered in others. However, if a higher percentage of missing data was allowed and subsequently imputed, additional proteins would be identified although these findings would then be based mainly on imputed results. 47 While this study was designed to be explorative, the risk of type 1 errors would increase with expanded imputation. This was the reason why a more robust statistical approach was chosen given the small sample size.
Importantly, the findings of this study are solely aimed to guide future studies. These should evaluate reproducibility of these findings with different methods, and in targeted studies investigating suggested thrombogenic pathways. As such, these results speculate on thrombogenic pathways but mainly provide a smorgasbord for follow-up studies to better understand causes of septic thrombogenesis and Lemierre's syndrome.
In conclusion, we provide data on the plasma proteomic expression in Lemierre's syndrome that support the hypotheses that endothelial injury, platelet activation, and degranulation are important thrombogenic pathways in Lemierre's syndrome and warrant future targeted studies.
Funding Statement
Funding This work was supported by The Royal Physiographic Society in Lund, Region Skåne and the Swedish Government Funds for Clinical Research (ALF). In addition, support from the Swedish National Infrastructure for Biological Mass Spectrometry (BioMS) is gratefully acknowledged.
Footnotes
Conflict of Interest J.E. reports consulting fees from LEO Pharma and honoraria for lectures for LEO Pharma, Pfizer/BMS and Bayer AB. None of the other authors have conflicting interests.
What is known about this topic?
Lemierre's syndrome is characterized by the development of septic thrombophlebitis after an oropharyngeal infection with F. necrophorum , followed by embolization and septic disease in previously healthy adolescents and young adults.
Little is known on why patients with Lemierre's syndrome develop septic thrombophlebitis.
What does this paper add?
This is a unique sample of prospectively enrolled patients with Lemierre's syndrome that generate hypotheses on the causes of septic thrombophlebitis. Differentially expressed proteins were identified when the plasma proteome of patients with Lemierre's syndrome was compared to other severe infections using mass spectrometry.
Several proteins involved in thrombogenic pathways were found to be differentially expressed in Lemierre's syndrome compared to other severe infections, including proteins involved in endothelial damage, platelet activation, and degranulation.
Supplementary Material
Supplementary Material 2
Supplementary Material 2
References
- 1.Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre's syndrome . Clin Microbiol Rev. 2007;20(04):622–659. doi: 10.1128/CMR.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nygren D, Holm K. Invasive infections with Fusobacterium necrophorum including Lemierre's syndrome: an 8-year Swedish nationwide retrospective study . Clin Microbiol Infect. 2020;26(08):1.089E10–1.089E15. doi: 10.1016/j.cmi.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Lemierre A. On certain septicæmias due to anaerobic organisms. Lancet. 1936;227(5874):701–703. [Google Scholar]
- 4.Bank S, Jensen A, Nielsen H M, Kristensen L H, Voldstedlund M, Prag J. Fusobacterium necrophorum findings in Denmark from 2010 to 2014 using data from the Danish microbiology database . APMIS. 2016;124(12):1087–1092. doi: 10.1111/apm.12606. [DOI] [PubMed] [Google Scholar]
- 5.Hagelskjaer Kristensen L, Prag J. Lemierre's syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey . Eur J Clin Microbiol Infect Dis. 2008;27(09):779–789. doi: 10.1007/s10096-008-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazier J S, Hall V, Yusuf E, Duerden B I. Fusobacterium necrophorum infections in England and Wales 1990-2000 . J Med Microbiol. 2002;51(03):269–272. doi: 10.1099/0022-1317-51-3-269. [DOI] [PubMed] [Google Scholar]
- 7.Hagelskjaer L H, Prag J, Malczynski J, Kristensen J H. Incidence and clinical epidemiology of necrobacillosis, including Lemierre's syndrome, in Denmark 1990-1995. Eur J Clin Microbiol Infect Dis. 1998;17(08):561–565. doi: 10.1007/BF01708619. [DOI] [PubMed] [Google Scholar]
- 8.Tyrstrup M, Beckman A, Mölstad S et al. Reduction in antibiotic prescribing for respiratory tract infections in Swedish primary care: a retrospective study of electronic patient records. BMC Infect Dis. 2016;16(01):709. doi: 10.1186/s12879-016-2018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nygren D, Brorson E, Musonda M, Wasserstrom L, Johansson Å, Holm K. Geographical differences in tonsillar carriage rates of Fusobacterium necrophorum : a cross-sectional study in Sweden and Zambia . Anaerobe. 2021;69:102360. doi: 10.1016/j.anaerobe.2021.102360. [DOI] [PubMed] [Google Scholar]
- 10.Centor R M, Atkinson T P, Xiao L. Fusobacterium necrophorum oral infections: a need for guidance . Anaerobe. 2022;75:102532. doi: 10.1016/j.anaerobe.2022.102532. [DOI] [PubMed] [Google Scholar]
- 11.Holm K, Bank S, Nielsen H, Kristensen L H, Prag J, Jensen A. The role of Fusobacterium necrophorum in pharyngotonsillitis: a review . Anaerobe. 2016;42(42):89–97. doi: 10.1016/j.anaerobe.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Centor R M, Atkinson T P, Ratliff A E et al. The clinical presentation of Fusobacterium -positive and streptococcal-positive pharyngitis in a university health clinic: a cross-sectional study . Ann Intern Med. 2015;162(04):241–247. doi: 10.7326/M14-1305. [DOI] [PubMed] [Google Scholar]
- 13.Hedin K, Bieber L, Lindh M, Sundqvist M. The aetiology of pharyngotonsillitis in adolescents and adults: Fusobacterium necrophorum is commonly found . Clin Microbiol Infect. 2015;21(03):2630–2.63E9. doi: 10.1016/j.cmi.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nygren D, Wasserstrom L, Holm K, Torisson G. Associations between findings of Fusobacterium necrophorum or β-hemolytic streptococci and complications in pharyngotonsillitis: a registry-based study in southern Sweden . Clin Infect Dis. 2023;76(03):e1428–e1435. doi: 10.1093/cid/ciac736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers Klug T, Rusan M, Fuursted K, Ovesen T. Fusobacterium necrophorum : most prevalent pathogen in peritonsillar abscess in Denmark . Clin Infect Dis. 2009;49(10):1467–1472. doi: 10.1086/644616. [DOI] [PubMed] [Google Scholar]
- 16.Wikstén J E, Laakso S, Mäki M, Mäkitie A A, Pitkäranta A, Blomgren K. Microarray identification of bacterial species in peritonsillar abscesses. Eur J Clin Microbiol Infect Dis. 2015;34(05):905–911. doi: 10.1007/s10096-014-2301-x. [DOI] [PubMed] [Google Scholar]
- 17.Nygren D, Elf J, Torisson G, Holm K. Jugular vein thrombosis and anticoagulation therapy in Lemierre's syndrome - a post hoc observational and population-based study of 82 patients . Open Forum Infect Dis. 2020;8(01):ofaa585. doi: 10.1093/ofid/ofaa585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valerio L, Zane F, Sacco C et al. Patients with Lemierre syndrome have a high risk of new thromboembolic complications, clinical sequelae and death: an analysis of 712 cases. J Intern Med. 2021;289(03):325–339. doi: 10.1111/joim.13114. [DOI] [PubMed] [Google Scholar]
- 19.Smeeth L, Cook C, Thomas S, Hall A J, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367(9516):1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 20.Burn E, Duarte-Salles T, Fernandez-Bertolin S et al. Venous or arterial thrombosis and deaths among COVID-19 cases: a European network cohort study. Lancet Infect Dis. 2022;22(08):1142–1152. doi: 10.1016/S1473-3099(22)00223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beristain-Covarrubias N, Perez-Toledo M, Thomas M R, Henderson I R, Watson S P, Cunningham A F. Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol. 2019;10:2569. doi: 10.3389/fimmu.2019.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charles K, Flinn W R, Neschis D G. Lemierre's syndrome: a potentially fatal complication that may require vascular surgical intervention. J Vasc Surg. 2005;42(05):1023–1025. doi: 10.1016/j.jvs.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Beerens H. Procédé de différenciation entre Spherophorus necrophorus (Schmorl 1891) et Spherophorus funduliformis (Halle 1898) . Ann Inst Pasteur (Paris) 1954;86(03):384–386. [PubMed] [Google Scholar]
- 24.Forrester L J, Campbell B J, Berg J N, Barrett J T. Aggregation of platelets by Fusobacterium necrophorum. J Clin Microbiol. 1985;22(02):245–249. doi: 10.1128/jcm.22.2.245-249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanoe M, Yamanaka M, Inoue M. Effects of Fusobacterium necrophorum on the mesenteric microcirculation of guinea pigs . Med Microbiol Immunol (Berl) 1989;178(02):99–104. doi: 10.1007/BF00203305. [DOI] [PubMed] [Google Scholar]
- 26.Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(05):515–523. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holm K, Frick I M, Björck L, Rasmussen M. Activation of the contact system at the surface of Fusobacterium necrophorum represents a possible virulence mechanism in Lemièrre's syndrome . Infect Immun. 2011;79(08):3284–3290. doi: 10.1128/IAI.05264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawish E, Sauter M, Sauter R, Nording H, Langer H F. Complement, inflammation and thrombosis. Br J Pharmacol. 2021;178(14):2892–2904. doi: 10.1111/bph.15476. [DOI] [PubMed] [Google Scholar]
- 29.Holm K, Rasmussen M. Binding and activation of plasminogen at the surface of Fusobacterium necrophorum. Microb Pathog. 2013;59–60:29–32. doi: 10.1016/j.micpath.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Betancourt L H, Sanchez A, Pla I et al. Quantitative assessment of urea in-solution Lys-C/trypsin digestions reveals superior performance at room temperature over traditional proteolysis at 37°C. J Proteome Res. 2018;17(07):2556–2561. doi: 10.1021/acs.jproteome.8b00228. [DOI] [PubMed] [Google Scholar]
- 31.UniProt Consortium UniProt: the universal protein knowledgebase in 2023 Nucleic Acids Res 202351(D1):D523–D531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friberg N, Carlson P, Kentala E et al. Factor H binding as a complement evasion mechanism for an anaerobic pathogen, Fusobacterium necrophorum. J Immunol. 2008;181(12):8624–8632. doi: 10.4049/jimmunol.181.12.8624. [DOI] [PubMed] [Google Scholar]
- 33.Holm K, Collin M, Hagelskjær-Kristensen L, Jensen A, Rasmussen M. Three variants of the leukotoxin gene in human isolates of Fusobacterium necrophorum subspecies funduliforme . Anaerobe. 2017;45:129–132. doi: 10.1016/j.anaerobe.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Isogai N, Tanaka H, Asamura S. Thrombosis and altered expression of intercellular adhesion molecule-1 (ICAM-1) after avulsion injury in rat vessels. J Hand Surg [Br] 2004;29(03):230–234. doi: 10.1016/j.jhsb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Pierangeli S S, Espinola R G, Liu X, Harris E N. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88(02):245–250. doi: 10.1161/01.res.88.2.245. [DOI] [PubMed] [Google Scholar]
- 36.Nagashima S, Mendes M C, Camargo Martins A P et al. Endothelial dysfunction and thrombosis in patients with COVID-19-brief report. Arterioscler Thromb Vasc Biol. 2020;40(10):2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Bhatlekar S, Jacob S P et al. Actin bundling protein L-plastin regulates megakaryocyte membrane rigidity and platelet spreading. Blood. 2022;140 01:5510–5511. [Google Scholar]
- 38.Jones W L, Ramos C R, Banerjee A et al. Apolipoprotein A-I, elevated in trauma patients, inhibits platelet activation and decreases clot strength. Platelets. 2022;33(08):1119–1131. doi: 10.1080/09537104.2022.2078488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiedel B A, Costello M, Gewurz H, Hussissian E. Effects of heparin and α 1-acid glycoprotein on thrombin or Activated Thrombofax Reagent-induced platelet aggregation and clot formation. Haemostasis. 1983;13(02):89–95. doi: 10.1159/000214709. [DOI] [PubMed] [Google Scholar]
- 40.Villard A V, Genna A, Lambert J et al. Regulation of tissue factor by CD44 supports coagulant activity in breast tumor cells. Cancers (Basel) 2022;14(13):3288. doi: 10.3390/cancers14133288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Štok U, Blokar E, Lenassi M et al. Characterization of plasma-derived small extracellular vesicles indicates ongoing endothelial and platelet activation in patients with thrombotic antiphospholipid syndrome. Cells. 2020;9(05):1211. doi: 10.3390/cells9051211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Healy L D, McCarty O JT. Contact system sends defensins to the rescue. Blood. 2019;133(05):385–386. doi: 10.1182/blood-2018-12-887547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu-Fanne R, Stepanova V, Litvinov R I et al. Neutrophil α-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood. 2019;133(05):481–493. doi: 10.1182/blood-2018-07-861237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, Gauer J S, Baker S R, Philippou H, Connell S D, Ariëns R AS. Neutrophils can promote clotting via FXI and impact clot structure via neutrophil extracellular traps in a distinctive manner in vitro. Sci Rep. 2021;11(01):1718. doi: 10.1038/s41598-021-81268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nygren D, Oldberg K, Holm K. Short blood culture time-to-positivity in Fusobacterium necrophorum bacteremia is associated with Lemierre's syndrome . Anaerobe. 2022;73:102474. doi: 10.1016/j.anaerobe.2021.102474. [DOI] [PubMed] [Google Scholar]
- 46.Markanday A. Acute phase reactants in infections: evidence-based review and a guide for clinicians. Open Forum Infect Dis. 2015;2(03):ofv098. doi: 10.1093/ofid/ofv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGurk K A, Dagliati A, Chiasserini D et al. The use of missing values in proteomic data-independent acquisition mass spectrometry to enable disease activity discrimination. Bioinformatics. 2020;36(07):2217–2223. doi: 10.1093/bioinformatics/btz898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charlson M E, Pompei P, Ales K L, MacKenzie C R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(05):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 49.Singer M, Deutschman C S, Seymour C W et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(08):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 2
Supplementary Material 2


