Abstract
Malignant histiocytosis (MH) is an extremely rare neoplasm of the macrophage-dendritic cell lineage. We report the clinical characteristics, molecular aberrations, treatments, and outcomes of patients with MH seen at two referral centers from January 2000 to May 2023. We identified 43 patients with MH, of which 26 had histiocytic sarcoma (MH-H), 9 interdigitating dendritic cell sarcoma (MH-IDC), and 8 Langerhans cell sarcoma (MH-LC). The median age at diagnosis was 61 years (range, 3-83). Thirty-three patients (77%) had multifocal disease while 10 had unifocal involvement. Tumor specimens from 22 patients (51%) underwent targeted next generation sequencing, and 19 of 22 (86%) had at least 1 pathogenic mutation, including mutations in MAPK pathway genes (73%). The median overall survival (OS) among the entire cohort was 16 months (95% CI: 8–50). The outcomes of those with multifocal disease was significantly shorter than their unifocal counterpart: median OS of 10 months vs. 50 months (p=0.07). Patients with risk organ involvement (bone marrow, spleen, or liver) had significantly inferior outcomes. Chemotherapy and surgery were the most common first-line treatments for multifocal and unifocal disease, respectively. While the outcome for patients with multifocal disease was poor, there was a subset of patients who had durable responses to treatment. Our study highlights that MH has heterogeneous clinical presentation, frequent oncogenic mutations, and prognosis that is strongly tied to disease extent and type of organ involvement.
Keywords: BRAF, histiocytosis, KRAS, NRAS, organ, pembrolizumab, MEK-inhibitors, surgery, next-generation sequencing, chemotherapy
Graphical Abstract

Introduction
Malignant histiocytosis (MHs) is a rare, aggressive neoplasm of the macrophage-dendritic cell lineage, which comprises the M group in the 2016 Histiocyte Society revised classification of histiocytic disorders.1 In the World Health Organization (WHO) and International Consensus Classification (ICC), MH encompasses histiocytic sarcoma (MH-H), Langerhans cell sarcoma (MH-LC), and interdigitating dendritic cell sarcoma (MH-IDC).2-3 Patients with MH may present with involvement of the reticuloendothelial system (lymph nodes, liver, spleen), skin, lung, bone or other soft tissue.2,3
There is a paucity of data in MH due to lack of pathologically confirmed cases in the literature. Studies have shown that patients with MH-H, similar to the more indolent histiocytic neoplasms such as Erdheim-Chester disease and Langerhans cell histiocytosis (LCH), harbor diverse activating mutations involving the rat sarcoma virus (RAS), mitogen-activated protein kinase (MAPK), phosphoinositide-3 kinase (PI3K), protein kinase B, and mammalian target of rapamycin (mTOR) pathways.4 National database studies from the United States have reported a median overall survival (OS) of 6 and 19 months for MH-HS and MH-LC, respectively with potentially better outcomes among patients with localized disease.3,5,6 However, a standard of care has not been established as most of the existing treatment-specific information are derived from case reports and registry-based studies that do not provide granular data on treatments and molecular data.7-12 To fill this knowledge gap, our study describes the clinical characteristics, molecular aberrations, treatments, and outcomes of patients with MH seen at two tertiary care institutions.
Methods
After approval by the respective institutional review boards at Mayo Clinic and the University of Alabama at Birmingham, we retrospectively reviewed the health records of patients with a histologically confirmed diagnosis of MH who were seen at these two institutions from January 1, 2000 through May 31, 2023. The histopathologic findings of all cases were re-reviewed and confirmed by three expert hematopathologists at both sites (K.L.R., D.M., and A.R.). We included those cases that met diagnostic criteria for MH according to the classification of the Histiocyte Society: i) expression of two or more macrophage markers (CD68, CD163, lysozyme) or dendritic/Langerhans cell markers (S100, CD1a, langerin), and ii) anaplastic histology.1,13 After review, the cases were classified as MH-H, MH-LC, MH-IDC, or unclassifiable according to the current WHO and ICC definitions.14-16 Pathologic features of cases MH-1 through MH-22 were reported in a prior publication.15 By morphology, MH-H, MH-LC, and MH-IDC show similar histologic features (Supplementary Figures 1, 2, 3) characterized by large, pleomorphic nuclei, vesicular chromatin, distinct nucleoli, and abundant pale eosinophilic cytoplasm.
Each case was categorized as unifocal (single region of disease involvement, biopsy-proven) or multifocal (>1 region of disease involvement with at least 1 region being biopsy-proven and the remaining suspicious for MH based on radiographic imaging) at the time of initial diagnosis. MH was considered secondary if there was a history of previous hematologic malignancy and primary if there was none.1
For organ involvement and treatment response assessment, we used data from the available imaging studies including radiographs, contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), and F-18 fluorodeoxyglucose (FDG) positron emission tomography–CT (PET-CT). Patients were considered to have risk organ involvement if there was biopsy-confirmed disease in the bone marrow or radiographically suspected or biopsy-confirmed disease in the spleen or liver. Data for next-generation sequencing (NGS) with an oncogene panel (ie, Caris Molecular intelligence [Caris Life Sciences], FoundationOne [Foundation Medicine, Inc], Invitae [Invitae Corp.], GeneDx [GeneDx], Strata NGS [Strata Oncology], Tempus xT [Tempus]), and in-house custom panels were abstracted where available.
For assessment of response to therapy, we used the clinical and radiographic response criteria previously published: complete response (complete resolution of symptoms or complete radiologic resolution of proven or suspected lesions due to MH), partial response (partial resolution of symptoms or partial radiologic resolution of proven or suspected lesions due to MH), stable disease (no change in symptoms or no substantial radiologic change in proven or suspected lesions due to MH for 3 months), and progressive disease (worsening of symptoms or worsening/new radiologic findings in proven or suspected lesions due to MH).17 Descriptive statistics, Fischer’s exact test to compare categorical variables, Wilcoxon rank sum and the Kruskal-Wallis tests to compare continuous variables, and Kaplan-Meier analyses for OS and progression-free survival (PFS) estimation were performed using BlueSky Statistics v7.40 (BlueSky Statistics LLC, Chicago, IL, USA). P value <0.05 was considered statistically significant. OS was defined as the time from diagnosis to death from any cause or to the date of last known follow-up if still alive. PFS was defined as the time from treatment start date to relapse/progression, death from any cause, or to the date of last known follow-up if still alive.
Results
Clinical Characteristics
Forty-three patients with histopathologically confirmed MH were included in our study. The median age at diagnosis was 61 years (range, 3-83) and 23 (53%) were males (Table 1, Supplementary Table 1). Most patients (26; 60%) had MH-H, while the rest had MH-IDC (9; 21%) or MH-LCS (8; 19%). Most (77%) had multifocal disease. The median duration from symptom onset to diagnosis was 2.5 months (range, 0.1-32). The median number of tissue biopsies to make a diagnosis was 2 (range, 1-10). The presenting signs and symptoms were variable and specific to the organs involved. Relevant laboratory values, the median at diagnosis (range), included the following: hemoglobin, 11.7 g/dL (range 7.4-15.6 g/dL); white blood cell count, 9.6×109/L (1.8-33.1); platelets 260×109/L (11-956); and serum lactate dehydrogenase (LDH), 183 U/L (99-707 [ref, 122-222 U/L]). Among the laboratory parameters, there were no significant differences noted between patients who had unifocal versus multifocal disease or MH subtype.
Table 1.
Baseline Characteristics of Patients with Malignant Histiocytosis
| Combined (n=43) |
MH-H (n=26) |
MH-IDC (n=9) |
MH-LC (n=8) |
p-value | |
|---|---|---|---|---|---|
| Median age in years (range) | 61 (3-83) | 61 (3-79) | 61 (38-83) | 60 (24-75) | 0.87 |
| Male (%) | 23 (53%) | 13 (50%) | 5 (56%) | 5 (63%) | 0.67 |
| Unifocal (%) | 10 (23%) | 4 (15%) | 4 (44%) | 2 (25%) | |
| Multifocal (%) | 33 (77%) | 22 (85%) | 5 (56%) | 6 (75%) | 0.32 |
| Secondary MH with previous history of hematologic malignancy (%) | 12 (28%) | 4 (15%) | 5 (56%) | 3 (38%) | 0.07 |
| CLL | 2 | 0 | 0 | 2 | |
| Marginal zone lymphoma | 2 | 1 | 1 | 0 | |
| CLL with Richter transformation | 2 | 0 | 2 | 0 | |
| B-ALL | 1 | 1 | 0 | 0 | |
| DLBCL | 1 | 0 | 0 | 1 | |
| Erdheim-Chester disease | 1 | 1 | 0 | 0 | |
| Follicular lymphoma | 1 | 0 | 1 | 0 | |
| Leukemia, unknown subtype | 1 | 0 | 1 | 0 | |
| MPN-U | 1 | 1 | 0 | 0 | |
| Laboratory values at diagnosis | |||||
| Hemoglobin, g/dL (range) | 11.7 (7.4-15.6) | 11.2 (7.4-14.3) | 12.8 (10.4-13.9) | 12.6 (8.9-15.6) | 0.13 |
| White blood cell count, 109/L (range) | 9.6 (1.8-33.1) | 9.6 (2.5-33.1) | 8.2 (5.6-12.3) | 9.2 (1.8-14.6) | 0.88 |
| Platelets, 109/L (range) | 260 (11-956) | 252 (11-956) | 244 (216-370) | 244 (41-809) | 0.91 |
| Serum LDH, U/L (range) | 183 (99 - 707) | 186 (99-707) | 182 (169-188) | 206 (139-595) | 0.96 |
| Patients with PD-L1 testing (%) | 18 | 6 | 7 | 5 | |
| ≥50% | 7 (39%) [range 0-95%] |
1 (17%) | 4 (57%) | 2 (40%) | 0.52 |
| Next-generation sequencing | 22/22 | 9/22 | 6/22 | 7/22 | |
| KRAS | 6 | 3 | 2 | 1 | |
| TP53 | 6 | 4 | 1 | 1 | |
| Non-BRAFV600E | 3 | 1 | 1 | 1 | |
| BRAFV600E | 2 | 0 | 1 | 1 | |
| Other | 5 | 1 | 1 | 3 | |
| Unifocal, First line Treatment | |||||
| Resection | 5 | 4 | 0 | 1 | |
| Observation | 2 | 0 | 1 | 1 | |
| Radiation | 1 | 0 | 1 | 0 | |
| Systemic therapy | 1 | 0 | 1 | 0 | |
| Unknown | 1 | 0 | 1 | 0 | |
| Multifocal, first line treatment: | |||||
| Chemotherapy | 17 | 12 | 3 | 2 | |
| Radiation therapy | 4 | 2 | 0 | 2 | |
| Surgery | 2 | 2 | 0 | 0 | |
| No treatment | 3 | 2 | 0 | 1 | |
| Unknown | 3 | 2 | 1 | 0 | |
| MEK inhibitor | 1 | 0 | 0 | 1 | |
| mTOR inhibitor | 1 | 1 | 0 | 0 | |
| Radiation and systemic therapy | 1 | 1 | 0 | 0 | |
| Observation | 1 | 0 | 1 | 0 | |
| Time period of diagnosis (%) | |||||
| Pre-2010 | 4 | 4 | 0 | 0 | |
| Post-2010 | 39 | 22 | 9 | 8 |
Abbreviations: B-ALL, B-acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; CRP, C-reactive protein, DLBCL, diffuse large B-cell lymphoma; LDH, lactate dehydrogenase; MH-H, malignant histiocytosis of histiocytic subtype; MH-LC, malignant histiocytosis of Langerhans cell subtype; MPN-U, myeloproliferative neoplasm, unclassifiable; NS, not statistically significant.
Most patients (31; 72%) had primary MH. Among those who had secondary MH, previous hematologic malignancies included chronic lymphocytic leukemia (CLL) (2), marginal zone lymphoma (2), B-cell acute lymphoblastic leukemia (B-ALL, 1), CLL with Richter transformation (2), DLBCL (1), Erdheim-Chester disease (1), follicular lymphoma (1), leukemia of unknown subtype (1), and myeloproliferative neoplasm, unclassifiable (1). The median time between prior hematologic malignancy and MH diagnosis was 21 months (range, 5-181). Seven of 10 cases with secondary MH had evidence of clonal relationship with the lymphoid neoplasm proven by either B-cell immunoglobulin gene rearrangements, next generation sequencing or fluorescence in situ hybridization studies. These seven cases were therefore transdifferentiated MH and amongst these three were MH-LCS, three were MH-IDC, and one was MH-H (Appendix).
Molecular Aberrations
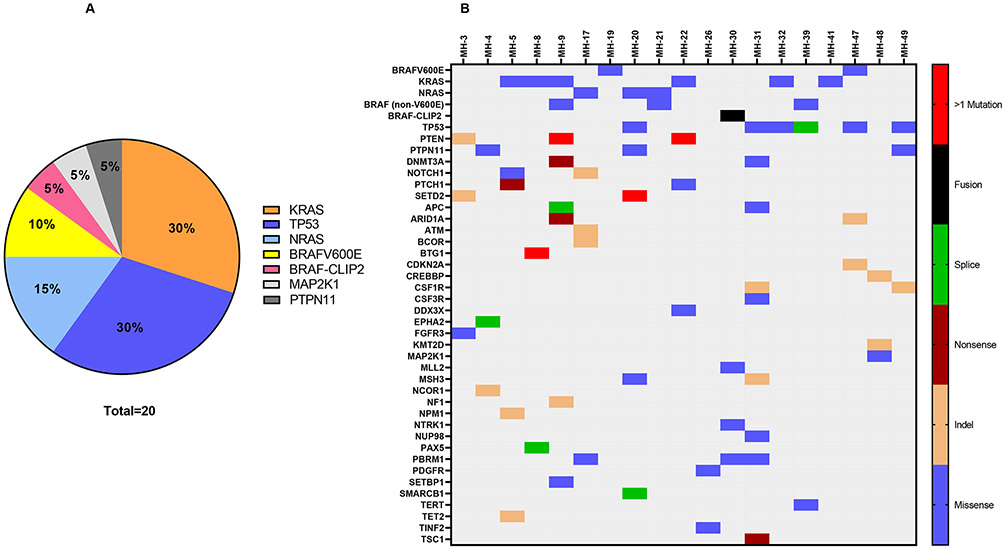
The tissue specimens from 22 patients (51%) underwent targeted NGS. Nineteen (86%) had at least 1 genomic alteration and among these patients, 17 patients had >1 mutations while 2 patients had only one mutation identified (Figure 1, Supplementary Table 2-3). Mutations involving genes of the MAPK pathway were identified in 16 sequenced cases (73%). The most frequently mutated genes were KRAS (6), TP53 (6), and BRAF (5) (Figure 1). Among the KRAS mutations, those involving exon 2 included KRASQ61R, KRASQ61H, and KRASG12D, mutations involving exon 1 included KRASG12R and KRASG12V; mutations involving exon 3 included KRASK117N. Alterations in BRAF included the BRAFV600E mutation, BRAFD594G, BRAFK01N, BRAFN581S, and CLIP2-BRAF fusion. Mutations involving genes of the PI3K pathway were identified in 1 case, with the mutated gene being PTEN. The two patients who had an isolated mutation involved BRAFV600E and KRASG12V respectively.
Figure 1A.
Pathogenic mutations and fusions involved in malignant histiocytosis.
Figure 1B. Heat map illustrating the mutations involved in patients with malignant histiocytosis who underwent next-generation sequencing.
Among patients who underwent targeted NGS, there was no difference in overall survival based on presence or absence of KRAS, TP53, or BRAF mutations (Supplementary Figure 4). There was also no association with extent of disease, pattern of organ involvement, or subtype of MH based on presence or absence of KRAS, TP53, or BRAF mutations (p > 0.05).
Organ and Site of Involvement
For staging of disease in the entire cohort, the following modalities were utilized: FDG PET-CT (32; 74%), CT chest/abdomen/pelvis (11; 26%), and MRI of the brain (4;11%). The most common sites of involvement were lymph nodes (22, 51%), bones (13, 30%), and lungs (10, 23%). Other disease sites included skin (8), liver (7), bone marrow (6), central nervous system (5), spleen (6), sinus and nasal cavity (3), adrenal (2), small bowel (2), cardiovascular (1), renal (1), gastric cardia (1), pancreatic head (1), and other soft tissue (n=2) [Supplementary Figure 5A-F].
Treatment
Unifocal Disease
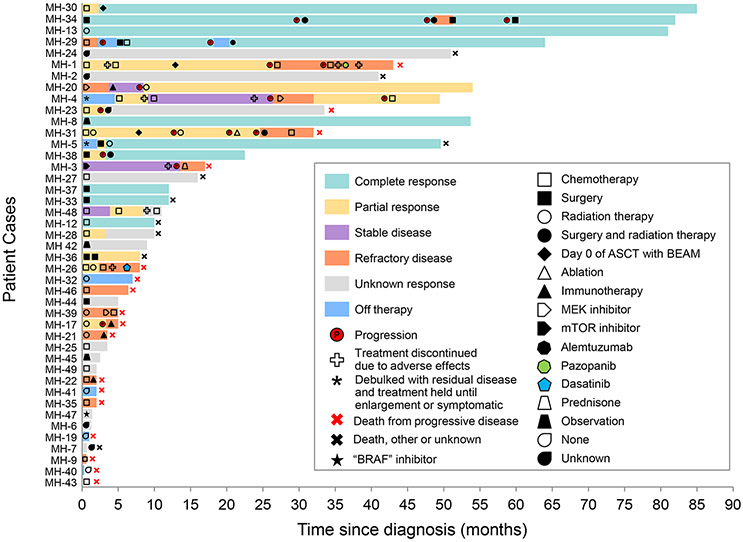
Ten patients in our cohort had unifocal disease and received the following first-line treatments for their MH: surgical resection (5), radiation therapy (2), observation (n=1), systemic therapy (1), and unknown (1). (Figure 2, Supplementary table 1 and 4). The median PFS was NR (95% CI: 11-NR) Among the patients who had surgery, two achieved a sustained complete response (mean follow up: 12 months). Two had partial responses requiring adjunctive treatment with radiation therapy or repeat surgery, while one patient had an unknown response. Among the patients who had radiation therapy, one had a complete response durable over 5 years without disease recurrence, while another had a partial response for only 2 months and required subsequent treatment with pembrolizumab. One patient with MH-LC (MH-42) had spontaneous resolution and continues to do well over 9 months from diagnosis.
Figure 2. Swimmer plot depicting disease course, treatment outcomes, and adverse effects in patients with malignant histiocytosis.
The bar graphs show the time from date of diagnosis to date of last known follow up. Additional details on the specific treatments utilized can be found in Supplementary Table 1 and 4.
Multifocal Disease
Among the 34 patients with multifocal disease, 17 had systemic chemotherapy as first-line treatment for their MH with a median PFS of 3 months (95% CI: 1–NR). Chemotherapy regimens included platinum-based, etoposide-based, and anthracycline-based, cladribine, and intrathecal therapy for isolated CNS disease (Supplementary Table 1 and 4). Two patients received consolidation therapy with carmustine, etoposide, cytarabine, and melphalan followed by autologous stem cell rescue.
Besides chemotherapy as a first-line treatment, MH-20 received trametinib as a targeted therapy due to the presence of an NRAS gain of function mutation but progressed within 2 months of initiation of treatment. Three patients were treated with palliative RT, two patients with surgery, one sirolimus and prednisone, and one observed. Three patients died before receiving systemic treatment due to acute illness and/or poor performance status. Treatment for three patients was unknown.
In the one patient who was observed (MH-8), there was MH-H involvement of abdominal lymph nodes. The patient’s initial CT of the abdomen and pelvis showed numerous enlarged lymph nodes, and the largest mass measured 5.5×2.6 cm. The patient had an excisional biopsy of one node (Supplementary Figure 5D) but otherwise chose to be observed because of lack of symptoms. Curiously, this patient had spontaneous tumor regression. On his latest CT, his largest lymph node had decreased to 1 cm.
Elevent patients received subsequent treatments including binimetinib, pembrolizumab, surgery, surgery with adjuvant cladribine, surgery with adjuvant RT, RT alone, etoposide, and gemcitabine and docetaxel followed by consolidation with autologous stem cell transplant [Figure 2, Supplementary Table 4]. Binimetinib was given to MH-39 but the patient progressed within 1 month of treatment.
Four patients received treatment with a PD-1 inhibitor. Three patients had progressive disease while one patient had stable disease. Among the patients who progressed, the PD-L1 expressions in tumor cells by immunohistochemistry were ≤ 30% and the disease progressions were rapid resulting in death within a month of starting treatment. One patient had PD-L1 expression of 95% and pembrolizumab was able to control the disease for 2 months; at 4 months, there was a slight increase in local disease burden and radiation therapy was administered while continuing the pembrolizumab. After radiation therapy, she had a near complete response durable for over 2 years from treatment initiation.18
Salvage therapies included cladribine, gemcitabine/docetaxel, pazopanib, cobimetinib, dasatinib, alemtuzumab, or selinexor and choline salicylate (MH-4, phase I clinical trial, NCT04640779). None of these treatments were durable and further details can be found in Figure 2 and Supplementary Table 4. Palliative localized therapy included surgical resection with or without radiation therapy, radiation therapy alone, or ablation.
Outcomes
The median OS among the entire cohort was 16 months (95% CI: 8–50) and the median PFS among the entire cohort was 5 months (95% CI: 2-16) [Figure 3]. The median follow-up duration of the entire cohort was 54 months. The multifocal cohort trended toward a shorter median OS at 10 months (95% CI: 6-43) compared with unifocal cohort for which the median OS was 50 months (95% CI: 50-not reached; p=0.07) (Figure 3). The multifocal cohort also trended toward a shorter median PFS of 2.5 months (95% CI: 1.9– 10) compared with the unifocal cohort in which the median PFS was not reached (11.7 months vs. not reached; p=0.085) (Supplementary Figure 6). Primary MH had a median OS of 32 months (95% CI: 10-NR) compared to secondary MH, which had a median OS of 6 months (95% CI: 4-NR; p=0.24) (Figure 3). There was no statistically significant difference between the median OS for MH-H (17 months, 95% CI: 18 – 51) and MH-LCS (5 months, 95% CI: 3.6 – not reached), and MH-IDC (not reached, 95% CI: 10 months – not reached) (p=0.66). There was also no statistically significant difference between the median PFS for MH-H (5 months, 95% CI: 2-17) and MH-LCS (2.1 months, 1.6–not reached), and MH-IDC (not reached, 95% CI: 10 months–not reached) (p=0.2; Supplementary Figure 7).
Figure 3.
Survival analysis of patients with malignant histiocytosis. (A): Overall survival of the entire cohort. (B) Progression-free survival of the entire cohort. (C) Overall survival comparing patients with unifocal vs. multifocal involvement. (D) Overall survival comparing patients with primary vs. secondary malignant histiocytosis.
Patients with risk organ involvement had significantly shorter median OS of 5 months [95% CI: 1–not reached] vs. 41 months [95% CI: 10 – not reached] respectively (p=0.009) respectively and significantly shorter median PFS of 2 months [95% CI: 1–not reached] vs. 10 months [95% CI: 2–not reached] respectively (p=0.027) [Supplementary Figure 8].
Discussion
We report the clinical characteristics, molecular aberrations, treatments, and outcomes of the largest contemporary cohort of MH. The key findings include the presence of MAPK pathway mutations in 73% of the patients, potential role of surgical excision and radiation in unifocal disease, overall poor outcome among those with multifocal disease or risk organ involvement (bone marrow, spleen, and/or liver), and the limited efficacy of combination chemotherapy as well as targeted agents.
The frequencies of mutations in our MH cohort are consistent with three other recent case series, which showed that 57-100% of patients with histiocytic sarcoma had somatic alterations within the MAPK signaling pathway.4,19,20 Regarding outcome data for MH, national database studies have suggested that patients with localized disease have better outcomes, and this was confirmed in our study.3,5,6 While others report that patients with multifocal disease have dismal outcomes,2 our study suggests that a subset of patients with multifocal disease can have long-term survival. For example, in our study, we had one patient with multifocal disease who had a durable remission following ASCT with BEAM. Another patient with multifocal disease had a durable response to radiation therapy and pembrolizumab, and one patient even showed spontaneous regression.18 Future studies should investigate whether the tumor microenvironment or degree of PD-L1 expression may have prognostic value. Another potential explanation for why a subset of patients with multifocal disease have better outcomes is that the prognosis of the disease is dependent on the site of organ involvement. Our data indicates that patients with risk organ involvement including bone marrow, spleen, and/or liver had significantly inferior outcomes compared to patients without such involvement, which is also seen in other histiocytic neoplasms such as Erdheim-Chester disease and Langerhans cell histiocytosis.21,22 23 More data is needed to identify other prognostic factors that will guide treatment in patients with MH.
Given the poor outcomes seen in many cases of multifocal MH, it is important to provide effective treatment options in the first line setting. However, the rarity of the disease has precluded a standard of care, even for first line treatment. Historically, chemotherapy has been utilized in the first line setting, but our data suggest that chemotherapeutic regimens demonstrated limited efficacy with short median PFS. Even with autologous stem cell transplant with intensive chemotherapy conditioning, only 1 out of 3 patients in our cohort were able to maintain a durable remission.
Despite the high frequency of mutations seen in the MAPK pathway, our data shows limited efficacy of single agent targeted therapy. Interestingly, other case reports have described the successful use of BRAF- and MEK-inhibitors in select cases of MH but others have reported limited efficacy.7,8,12,24-28 It is possible that MEK-inhibition was not effective in our cohort becauseour patients had more complex mutational profiles. Combination of targeted agents with or without chemotherapy has to be explored in the future.
Beyond targeting driver mutations in MH, immune checkpoint inhibitor therapy has been tried after recent studies showed a high rate of PD-L1 expression in histiocytic disorders.9,10 One report described a case of multifocal histiocytic sarcoma with PD-L1 expression of 75% and a favorable response to nivolumab.11 However, another report described a patient with PD-L1 positive (15-20%) multifocal histiocytic sarcoma and had progressive disease despite treatment with nivolumab.29 In our case series, PD-1 inhibition alone had a low overall response rate but the addition of radiation therapy to pembrolizumab for one of our patients (PD-L1 expression 95%) led to a near complete response that has been durable for more than 2 years.18
We also report two cases of spontaneous partial regression, including the multifocal MH case mentioned above (MH-IDC) and one unifocal bone lesion (MH-LC). Within the M-group, spontaneous regression has been reported in only one other case report of interdigitating dendritic cell sarcoma.30 The mechanism for spontaneous regression is unknown.
The strengths of our study include the largest cohort that has been histopathologically reviewed centrally, a large proportion of patients having undergone NGS, our median follow-up data of 5 years, and data on 4 patients treated with PD-1 inhibitors and 3 patients treated with MEK-inhibitors. Our study also has some limitations, such as its retrospective nature and small sample size.
Conclusion
Our study shows that MH represents a clinically heterogeneous neoplasm with a median overall survival of less than 2 years. However, the clinical outcome can be variable among disease subgroups. In general, multifocal and risk organ involvement (bone marrow, liver, and/or spleen) are associated with poor outcomes regardless what type of systemic treatment is used, while unifocal disease may potentially be controlled with surgery or radiation resulting in durable remissions. Spontaneous remission can occur but is very rare. Most patients have somatic oncogenic alterations involving the genes of the MAPK pathway. Traditional chemotherapeutic regimens have variable response rates that are mostly not durable. Targeted agents, such as BRAF- or MEK-inhibitor, also have limited efficacy. The role of PD-1 inhibitors should be further studied, especially when used in conjunction with radiation. Finally, we report that a small subset of patients with multifocal disease can have a relatively good outcome and spontaneous remission can occur, although very rarely.
Supplementary Material
Acknowledgments of grants:
Supported in part by the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) CA97422-21. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This was presented in part as a poster at the American Society of Clinical Oncology Annual Meeting at Chicago, Illinois, May 31-June 4, 2019 and at the Histiocyte Society in Memphis, TN, Nov 3-5, 2019.
Contributor Information
Gordon J. Ruan, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
Saurabh Zanwar, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
Aishwarya Ravindran, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota; Division of Laboratory Medicine-Hematopathology, Department of Pathology, The University of Alabama at Birmingham, Birmingham, Alabama..
Susan Schram, Sawtooth Epidemiology & Infectious Diseases, Boise, ID..
Jithma P. Abeykoon, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
Antonious Hazim, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
Jason R. Young, Department of Radiology, Mayo Clinic Jacksonville, Florida..
Mithun V. Shah, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
N. Nora Bennani, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
Liuyan Jiang, Department of Laboratory Medicine and Pathology, Mayo Clinic Jacksonville, Florida..
Diana Morlote, Division of Laboratory Medicine-Hematopathology, Department of Pathology, The University of Alabama at Birmingham, Birmingham, Alabama..
Karen L. Rech, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota..
Gaurav Goyal, Division of Hematology, Mayo Clinic, Rochester, Minnesota; Department of Hematology-Oncology, The University of Alabama at Birmingham, Birmingham, Alabama..
Ronald S. Go, Division of Hematology, Mayo Clinic, Rochester, Minnesota..
Data Sharing Statement:
For original data, please contact go.ronald@mayo.edu
References
- 1.Emile JF, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 127, 2672–2681 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard JE, Dwivedi RC, Masterson L & Jani P Langerhans cell sarcoma: a systematic review. Cancer Treat Rev 41, 320–331 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Kommalapati A, Tella SH, Durkin M, Go RS & Goyal G Histiocytic sarcoma: a population-based analysis of incidence, demographic disparities, and long-term outcomes. Blood 131, 265–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanmugam V., et al. Identification of diverse activating mutations of the RAS-MAPK pathway in histiocytic sarcoma. Mod Pathol 32, 830–843 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Kommalapati A, Tella SH, Go RS & Goyal G Predictors of survival, treatment patterns, and outcomes in histiocytic sarcoma. Leuk Lymphoma 60, 553–555 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Tella SH, Kommalapati A, Rech KL, Go RS & Goyal G Incidence, Clinical Features, and Outcomes of Langerhans Cell Sarcoma in the United States. Clin Lymphoma Myeloma Leuk 19, 441–446 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Gounder MM, Solit DB & Tap WD Trametinib in Histiocytic Sarcoma with an Activating MAP2K1 (MEK1) Mutation. N Engl J Med 378, 1945–1947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voruz S., et al. Response to MEK inhibition with trametinib and tyrosine kinase inhibition with imatinib in multifocal histiocytic sarcoma. Haematologica 103, e39–e41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J., et al. Expression of Programmed Cell Death 1 Ligands (PD-L1 and PD-L2) in Histiocytic and Dendritic Cell Disorders. Am J Surg Pathol 40, 443–453 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Gatalica Z., et al. Disseminated histiocytoses biomarkers beyond BRAFV600E: frequent expression of PD-L1. Oncotarget 6, 19819–19825 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bose S., et al. Favorable response to nivolumab in a young adult patient with metastatic histiocytic sarcoma. Pediatr Blood Cancer 66, e27491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branco B., et al. Targeted therapy of BRAF V600E-mutant histiocytic sarcoma: A case report and review of the literature. Eur J Haematol 103, 444–448 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Ravindran A., et al. Malignant Histiocytosis Comprises a Phenotypic Spectrum that Parallels the Lineage Differentiation of Monocytes, Macrophages, Dendritic Cells, and Langerhans Cells. Mod Pathol, 100268 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Khoury JD, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 36, 1703–1719 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravindran A., et al. Malignant Histiocytosis Comprises a Phenotypic Spectrum That Parallels the Lineage Differentiation of Monocytes, Macrophages, Dendritic Cells, and Langerhans Cells. Mod Pathol 36, 100268 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Campo E., et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood 140, 1229–1253 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal G., et al. Clinical and Radiologic Responses to Cladribine for the Treatment of Erdheim-Chester Disease. JAMA Oncol 3, 1253–1256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanwar S., et al. Prolonged remission with pembrolizumab and radiation therapy in a patient with multisystem Langerhans cell sarcoma. Haematologica (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durham BH, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med 25, 1839–1842 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan C., et al. Genomic profiling of primary histiocytic sarcoma reveals two molecular subgroups. Haematologica 105, 951–960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal G., et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood 135, 1929–1945 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Goyal G., et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood 139, 2601–2621 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal G., et al. Long-term outcomes among adults with Langerhans cell histiocytosis. Blood Adv (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branco B., et al. Targeted therapy of BRAF V600E-mutant histiocytic sarcoma: A case report and review of the literature. Eur J Haematol (2019). [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman V, Massoth LR, Sullivan RJ & Friedmann AM Secondary histiocytic sarcoma with BRAF(V600E) mutation after T-cell acute lymphoblastic leukemia in a very young child with dramatic response to dabrafenib and trametinib. Pediatr Blood Cancer 67, e28200 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Hu B., et al. Near Complete Response to Trametinib Treatment in Histiocytic Sarcoma Harboring a Somatic KRAS Mutation. J Natl Compr Canc Netw, 1–4 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Kumamoto T., et al. A case of recurrent histiocytic sarcoma with MAP2K1 pathogenic variant treated with the MEK inhibitor trametinib. Int J Hematol 109, 228–232 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Idbaih A., et al. Dramatic response of a BRAF V600E-mutated primary CNS histiocytic sarcoma to vemurafenib. Neurology 83, 1478–1480 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Voruz S., et al. Comment on "MEK inhibition with trametinib and tyrosine kinase inhibition with imatinib in multifocal histiocytic sarcoma". Haematologica 103, e130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khashab T, Sehgal L, Medeiros LJ & Samaniego F Spontaneous regression of interdigitating dendritic sarcoma in a patient with concurrent small lymphocytic lymphoma. BMJ Case Rep 2015(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For original data, please contact go.ronald@mayo.edu