Abstract
Viruses have evolved different strategies to interfere with host cell apoptosis. Herpesvirus saimiri (HVS) and other lymphotropic herpesviruses code for proteins that are homologous to the cellular antiapoptotic Bcl-2. In this study HVS-Bcl-2 was stably expressed in the human leukemia cell line Jurkat and in the murine T-cell hybridoma DO to assess its antiapoptotic spectrum and to gain further insight into its mode of action. HVS- Bcl-2 prevented apoptosis that occurs as a result of a disturbance of intracellular homeostasis by, for example, DNA damage or menadione, which gives rise to oxygen radicals. In Jurkat cells, HVS-Bcl-2 also inhibited apoptosis mediated by the death receptor CD95. In DO cells, HVS-Bcl-2 did not interfere with CD95-mediated apoptosis but blocked dexamethasone-induced cell death. Mitochondrial damage is a central coordinating event in apoptosis induced by different stimuli. To assess the integrity of mitochondria, we used rhodamine 123, which is released upon disturbance of the mitochondrial membrane potential, and determined the release of cytochrome c into the cytosol. Both signs of mitochondrial damage were prevented by HVS-Bcl-2. This viral protein also inhibited the generation of caspase-3-like DEVDase activity and blocked the cleavage of poly(ADP-ribose) polymerase, a natural substrate of caspase-3-like proteases. In conclusion, HVS-Bcl-2 protects against a great variety of apoptotic stimuli, stabilizes mitochondria, and acts upstream of the generation of caspase-3-like activity.
Apoptosis is used by the host as a defense mechanism to eliminate virus-infected cells. The programmed cell death of infected cells limits viral replication and may prevent virus-induced malignancies. Apoptosis is characterized by distinct biochemical and morphological changes, such as activation of caspases (formerly called ICE-like proteases), mitochondrial depolarization with release of cytochrome c, and nucleosomal DNA fragmentation. A central role in the execution of apoptosis is performed by caspases. Caspases are present in the cytosol as inactive proenzymes. They become activated upon intramolecular cleavage and are thought to execute apoptosis induced by different stimuli (45). To date, more than 10 caspases which differ in their substrate specificities and their susceptibility to protease inhibitors have been identified. Caspase-3 and related caspases are involved in nuclear apoptosis and in extranuclear apoptotic events such as the formation of apoptotic bodies and exposure of phosphatidylserine (24, 35). When caspase-3 is activated by apoptotic stimuli, it activates a DNase, named caspase-activated DNase (CAD), that degrades DNA during apoptosis. This endonuclease CAD is present in the cytosol complexed with its inhibitor ICAD. Caspase-3 cleaves ICAD, and this allows CAD to translocate to the nucleus and degrade DNA (13, 48).
During the apoptotic process, both cellular and viral proteins and nucleic acids are destroyed. Different viruses have developed a variety of strategies to interfere with host cell apoptosis (56, 58). Lymphotropic herpesviruses such as human herpesvirus 8 (HHV-8) (6, 49), Epstein-Barr virus (EBV) (21), and herpesvirus saimiri (HVS) (44) code for a protein that shows homology to cellular members of the Bcl-2 family. An important function of these antiapoptotic herpesvirus Bcl-2 homologs in the life cycle of these viruses is suggested by the finding that lymphotropic herpesviruses of distantly related species code for a Bcl-2 homolog (14, 61).
Bcl-2 family members are key regulators in the development of apoptosis. Cellular Bcl-2 is a multifunctional protein, and different mechanisms have been implicated in the protection of cells from apoptotic stimuli: cellular Bcl-2 or Bcl-xL can prevent the loss of mitochondrial membrane potential that is induced by a number of apoptotic stimuli (36, 55). In some experimental systems, Bcl-2 is also active when specifically targeted to the endoplasmic reticulum (67). In addition, Bcl-2 and Bcl-xL interact with several proteins participating in cell death regulation, such as the mammalian homolog of CED-4 (Apaf-1), Raf-1, BAG-1, calcineurin, p53BP-2, and other members of the Bcl-2 family (reviewed in references 32 and 46).
Homology of cellular Bcl-2 family members is restricted to distinct Bcl-2 homology regions, BH1, BH2, BH3, and BH4. Most of the cellular Bcl-2 members have a membrane anchor at their C terminus and are localized at the outer mitochondrial, outer nuclear, and endoplasmic membranes. The functions of the different Bcl-2 homology domains could be identified to some extent. BH1 and BH2 domains are involved in Bcl-2 homodimer formation. The BH3 domain of death agonists like Bax or Bak is required for heterodimerization with Bcl-xL and Bcl-2 and to promote apoptosis. The BH4 domain of Bcl-2 and Bcl-xL is involved in binding to death-regulatory proteins like Raf-1, Bag-1, calcineurin (32, 46), and CED-4 (27). The HVS-encoded Bcl-2 is shorter than cellular Bcl-2 or Bcl-xL and lacks a strong homology to the BH3 and BH4 domains but contains, like its cellular counterpart, a membrane anchor at the C terminus (44, 53). Cellular Bcl-2 can be converted to a death promoter upon cleavage by caspases (7), and this death-promoting activity is dependent on BH3. It was suggested that the lack of BH3 allows the viral Bcl-2 homologs to evade regulation by caspases (7).
HVS is a lymphotropic and oncogenic herpesvirus. This virus persists for life in its natural host, the squirrel monkey, without causing apparent disease. It causes leukemia and lymphoma in other New World primate species. HVS transforms T cells from New World monkeys (50), rhesus monkeys (39), and humans (3) to stable growth in cell culture (reviewed in references 17 and 40). HVS-Bcl-2 is expressed predominantly during lytic replication of HVS (31) and can inhibit apoptosis induced by Sindbis virus (44).
The purpose of this study was to analyze the repertoire of apoptotic stimuli against which HVS-Bcl-2 is protective, to compare the functional properties of HVS-Bcl-2 and cellular Bcl-xL, and to gain further insight into the mode of action of this viral antiapoptotic effector. We report that HVS-Bcl-2 protected against apoptosis induced by irradiation, dexamethasone, and menadione, which gives rise to oxygen radicals. Inhibition of CD95-mediated cell death was seen in Jurkat cells but not in DO cells. HVS-Bcl-2 protected against this broad range of apoptotic stimuli by stabilizing mitochondria and by functioning upstream of the activation of caspase-3.
MATERIALS AND METHODS
Cell lines, plasmids, and transfections.
The complete sequence of open reading frame 16 of HVS strain C488 (30), which codes for HVS-Bcl-2, was amplified by PCR, cloned with the pCR2.1 vector (Invitrogen, De Schelp, The Netherlands), and confirmed by sequencing by the Dye Dideoxy terminator method (ABI, Weiterstadt, Germany). HVS-Bcl-2 was excised with Asp718 and NotI from pCR2.1 and then inserted into the eukaryotic expression vector pCEP4 (Invitrogen). The cDNA of Bcl-XL (4) (kindly provided by C. Thompson and L. Boise) was excised from pBluescript with PvuII and NotI and subsequently also inserted into pCEP4.
The human T-cell leukemia line Jurkat was transfected by electroporation. The conditions were as follows. A total of 5 × 106 cells were suspended in 250 μl of CG medium (Vitromex, Selters, Germany) with 25 μg of DNA of pCEP4-HVS-Bcl-2, pCEP4-Bcl-xL, or the unmodified pCEP4 and electroporated at 210 V and 960 μF. Additionally, the murine T-cell hybridoma line DO was transfected by electroporation (230 V and 960 μF) with pCEP4-HVS-Bcl-2 or unmodified control vector. Jurkat cells and DO cells were kept in RPMI supplemented with 10% fetal bovine serum, 2 mM glutamine, and 50 μg of gentamicin per ml.
Stable transfectants were isolated with hygromycin B (Boehringer Mannheim) at a final concentration of 500 μg/ml for Jurkat cells and 1,200 μg/ml for DO cells. Transfected clones were generated by seeding 1 to 100 cells in 96-well plates.
Transcript analysis.
The expression of HVS-Bcl-2 in stably transfected cell lines was analyzed by RNase protection. To construct a riboprobe template for RNase protection, a 561-bp fragment of pCEP4-HVS-Bcl-2 was excised with SnaBI and PvuII and cloned in pBluescript. This fragment consists of 250 bp of HVS-bcl-2, 263 bp of pCEP4, and 48 bp of pCR2.1. As a positive control for the expression of HVS-Bcl-2, we used the transformed T-cell lines Ha-S-T and Hk-S-T, which were derived from Callithrix jacchus and produce infectious virus (39). Total cellular RNA was prepared by the acidic phenol extraction method (10). RNase protection was carried out as described in standard protocols (54).
Induction of apoptosis.
Dexamethasone (Sigma, Deisenhofen, Germany), the anti-CD95 monoclonal antibody (MAb) CH-11 (Immunotech, Marseille, France), FLAG-CD95 ligand (57), irradiation, and menadione (Sigma) were applied to induce cell death. Menadione was dissolved in phosphate-buffered saline (PBS) at 100 mM and stored in aliquots at −20°C. MAb CH-11 was used for human Jurkat cells, while the FLAG-CD95 ligand was chosen for murine DO cells, since MAb CH-11, directed to human CD95, does not cross-react with murine CD95. The FLAG-CD95 ligand was cross-linked with the anti-FLAG MAb M2 (Integra, Fernwald, Germany).
Isolation of the cytosolic fraction.
The cytosolic fraction was obtained essentially as described previously (29). Cells were washed twice in ice-cold PBS and counted. Subsequently, 106 cells were suspended in 50 μl of ice-cold buffer A (20 mM HEPES KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg each of aprotinin and leupeptin [Sigma] per ml). After incubation for 15 min on ice, the cells were disrupted by four strokes in a 2-ml Kontes Dounce homogenizer with the B pestle (Kontes Glass Co., Vineland, N.J.). First, the material was centrifuged at 750 × g for 10 min at 4°C. The resulting supernatant was further centrifuged at 14,000 × g for 15 min at 4°C. The supernatant from this final centrifugation represents the cytosolic fraction, of which 20 μg of protein was used for Western blotting to detect cytochrome c.
Quantification of caspase activity.
The cells were treated with the anti-CD95 MAb CH-11 at the indicated concentrations (see Fig. 7) for 4 h at 37°C. After treatment, 5 × 105 cells were washed and resuspended in chilled lysis buffer (1% Triton X-100, 130 mM NaCl, 10 mM Tris [pH 7.4]). DEVD-aminomethylcoumarin (Alexis, Gruenberg, Germany) was used as a substrate to determine the DEVDase activity. The amount of free aminomethylcoumarin was determined by using a fluorometer (Victor; Wallac, Freiburg, Germany) with a 390-nm excitation filter and a 460-nm emission filter. The specificity of the enzymatic reaction was assessed by using DEVD-CHO as an inhibitor for the in vitro reaction.
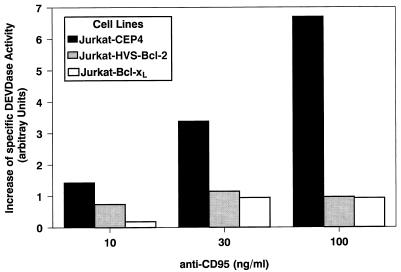
FIG. 7.
HVS-Bcl-2 and Bcl-xL block the appearance of caspase-3-like DEVDase activity. The different transfectants were treated with anti-CD95 at the indicated concentrations for 4 h or left untreated. The DEVDase activity in the cell lysates was measured by using DEVD-aminomethylcoumarin as a substrate. The specific DEVDase activity was calculated as the difference of DEVDase activity in the absence and presence of the inhibitor DEVD-CHO. The increase in the DEVDase specific activity after CD95 engagement was determined. The mean values for three individual cell clones expressing HVS-Bcl-2, of three clones expressing Bcl-xL, and of a control cell line are shown.
Western blots.
Bcl-xL expression was analyzed with a polyclonal rabbit antibody (Dianova, Hamburg, Germany). Poly(ADP-ribose) polymerase (PARP) was detected with MAb C2-10 (Pharmingen, Hamburg, Germany), cytochrome c was stained with MAb 7H8.2C12 (Pharmingen), and caspase-3 was analyzed with a MAb (C31720) from Transduction Laboratories (Lexington, Ky.). A lysis buffer consisting of 50 mM Tris-HCl (pH 8.0), 1% Triton X-100, 150 mM NaCl, 2 mM EDTA, 5 mM NaF, and 10 μg each of aprotinin and leupeptin (Sigma) per ml was used to analyze the expression of Bcl-xL. PARP and caspase-3 were detected in cell lysates prepared with 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and 50 mM Tris (pH 8.0). The cytoplasmic fraction, which was prepared as described above, was used to detect released cytochrome c. Proteins were separated under reducing conditions by SDS-polyacrylamide gel electrophoresis and electroblotted on Immobilon P membrane (Millipore, Eschborn, Germany). The blots were blocked with PBS containing 5% low-fat milk and 0.05% Tween 20. They were incubated with the primary Abs and then with a 1:1,000 dilution of peroxidase-conjugated goat anti-mouse F(ab)2 or a peroxidase-conjugated donkey anti-rabbit F(ab)2 fragment (Amersham, Braunschweig, Germany). Blots were developed by using the enhanced chemiluminescence Western blot detection system (Amersham).
Cell death detection and flow cytometry.
DNA fragmentation was detected essentially as described previously (23). Briefly, the cells were washed in PBS and incubated in a lysis buffer (1% Nonidet P-40, 100 mM EDTA, 50 mM Tris-HCl [pH 7.5]) for 10 s. The supernatant was obtained after centrifugation at 260 × g, and the lysis step was repeated with the pellet. These conditions result in an enrichment of DNA from cells undergoing apoptosis, because their nuclei are less resistant to detergent lysis. After centrifugation, the supernatants were pooled and SDS was added to a final concentration of 1%. Subsequently the material was digested with RNase A and subsequently with proteinase K (Boehringer Mannheim). The DNA was precipitated, washed with 70% ethanol, separated on an agarose gel, and stained with ethidium bromide.
Cell death was quantified by flow cytometry as follows. The cells were collected, washed once, incubated for at least 10 min in PBS containing 20 μg of propidium iodide (PI) per ml, and analyzed with a flow cytometer (FACStrak; Becton Dickinson, Heidelberg, Germany). Viable and dead cells were distinguished by both forward-scatter analysis and fluorescence caused by PI uptake. The specific cell death was calculated as 100 × (percent experimental cell death − percent spontaneous cell death in medium)/(100% − percent spontaneous cell death). Alternatively, cell death was quantified by a histone release enzyme-linked immunosorbent assay (cell death detection kit; Boehringer Mannheim). The histone released after treatment is delineated as the enrichment factor, which was calculated as described by the manufacturer, i.e., optical density after treatment/optical density of control cells.
Changes in mitochondrial membrane potential were evaluated with rhodamine 123 (Molecular Probes, Leiden, The Netherlands). Rhodamine 123 is taken up by intact mitochondria and released upon permeability transition of the mitochondria (5, 20). Cells that had been treated to undergo apoptosis were incubated with rhodamine 123 for 30 min at 37°C, washed, incubated with PI, and then analyzed with the FACStrak.
Annexin-V binding to phosphatidylserine exposed on the outer part of the cell membrane was detected by incubating the cells with fluorescein isothiocyanate-conjugated annexin-V (Pharmingen) for 30 min at room temperature with a calcium-containing buffer as specified by the manufacturer. Then the solution was diluted 1:10 and measured by flow cytometry within the next 30 min.
RESULTS
Expression of HVS-Bcl-2 and Bcl-xL in stably transfected cells.
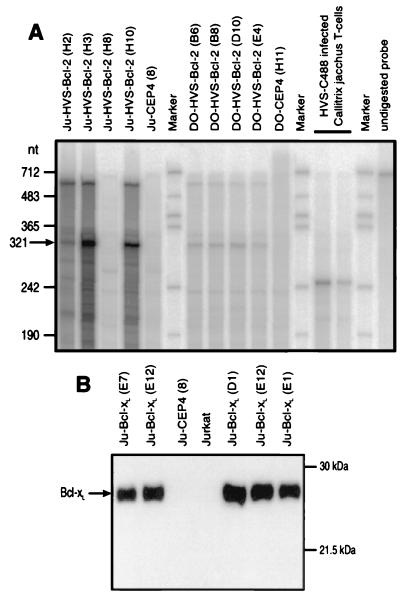
The expression of HVS-Bcl-2 in stably transfected cell lines was analyzed by RNase protection assays. Differences in the expression levels of different clones from Jurkat cells were observed. Strong expression of HVS-Bcl-2 was detected in clones Ju-H3, Ju-H10, and Ju-H2, with the highest level being found in clone Ju-H3 (Fig. 1A). The clones in Fig. 1 were used for the following experiments and compared with four clones that were transfected with the empty vector. Four HVS-transfected clones derived from the murine T-cell hybridoma line DO expressed HVS-Bcl-2 to a similar but lower degree (Fig. 1A). Western blot analysis confirmed the overexpression of Bcl-xL in transfected Jurkat cells (Fig. 1B). A low level of constitutively expressed Bcl-xL in wild-type Jurkat cells could also be detected under appropriate conditions (data not shown).
FIG. 1.
Expression of HVS-Bcl-2 and Bcl-xL in transfected cell lines. (A) Transcripts of HVS-Bcl-2 were detected by RNase protection. Different HVS- bcl-2-transfected clones derived from Jurkat cells (Ju) or DO cells were analyzed. Control cells contained the expression vector pCEP4 without insert. In cell lines transfected with HVS-bcl-2, the riboprobe of 700 nucleotides (nt) specifically protected a fragment of 321 nucleotides. This protected fragment of 321 nucleotides consists of 250 nucleotides of HVS-bcl-2, 48 nucleotides of pCR2.1, and 23 nucleotides of pCEP4. As a positive control, we used T-cell cultures from Callithrix jacchus that release infectious virus and transcribe HVS-bcl-2 (31). When RNA from these cells is used, the applied riboprobe protects a fragment of about 250 nucleotides, representing the calculated length of the viral transcript that is mirrored in the riboprobe. (B) The expression of Bcl-xL in transfected Jurkat cells was detected by Western blotting.
Protection from apoptosis induced by CD95 ligation, oxygen radicals, irradiation, and dexamethasone.
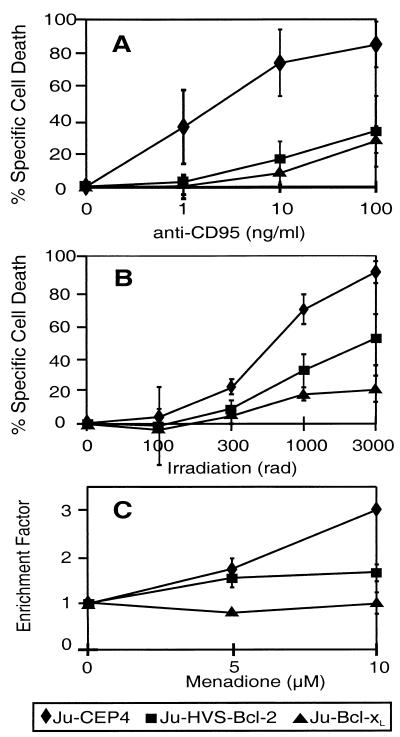
HVS-Bcl-2 and Bcl-xL protected Jurkat cells from CD95-mediated apoptosis. The results of one representative experiment of three with similar results are shown in Fig. 2A. This figure shows the mean percentages of cell death development after CD95 treatment of four different clones per transfected gene. When analyzing individual clones, we noted that there was a correlation between the expressed amount of HVS-Bcl-2 (Fig. 1) and the degree of protection from apoptosis. Upon treatment with 1 ng of anti-CD95 per ml, all tested HVS-Bcl-2-expressing clones were completely protected from cell death while 35% of the cells from four control clones died at this concentration of anti-CD95. Upon treatment with 10 ng of anti-CD95 per ml, clone Ju-H3, with the highest expression, showed only 2% cell death, clone H10 showed 17%, clone H2 showed 17%, clone H8 showed 31%, and control cells showed 74% specific cell death after 24 h.
FIG. 2.
HVS-Bcl-2 and Bcl-xL protect Jurkat cells (Ju) from apoptosis mediated by CD95 (A), irradiation (B), and menadione (C). Four different clones transfected with HVS-Bcl-2, four clones transfected with Bcl-xL, and four control clones were treated with anti-CD95 (A) or irradiated (B) at the indicated dosage. The specific cell death (mean ± standard deviation [SD]) was determined after 24 h (A) or 72 h (B) as described in Materials and Methods. The data for the individual cell lines expressing HVS-Bcl-2 or Bcl-xL and for the control cells were pooled for presentation in panels A and B. To determine the sensitivity to menadione-induced cell death, two different clones of each group were studied and the data were pooled (C). The development of apoptosis was quantified by a histone release enzyme-linked immunosorbent assay and the enrichment factor (mean ± SD) was calculated as described in Materials and Methods.
Irradiation and menadione-induced oxygen radicals triggered cell death in Jurkat cells. The kinetics of cell death development after these two treatments were different. The menadione-induced cell death was evident after 4 h, whereas the irradiation-induced cell death was observed after 48 to 72 h. HVS-Bcl-2 effectively protected Jurkat cells from cell death induced by irradiation as detected by measuring PI uptake (Fig. 2B). Protection from menadione-induced apoptosis was detected by measuring PI uptake (data not shown) and in a histone release assay (Fig. 2C). A similar degree of protection from this type of cell death was observed with cellular Bcl-xL (Fig. 2B and C).
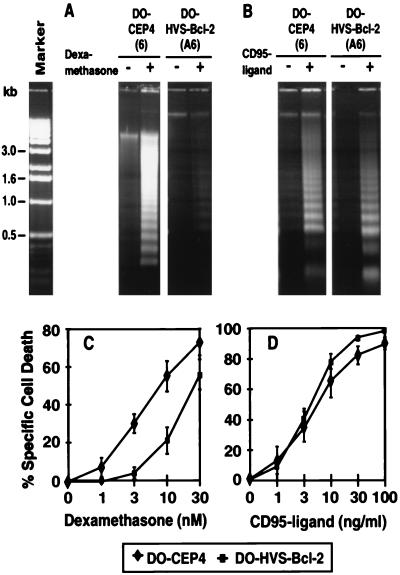
To gain further insight into the antiapoptotic properties of HVS-Bcl-2, a second lymphoid cell line, the murine T-cell hybridoma line DO, was transfected with HVS-Bcl-2. This cell line readily undergoes apoptosis upon treatment with dexamethasone. HVS-Bcl-2 partially protected these cells from dexamethasone-induced apoptosis as seen by reduced DNA fragmentation and by measurement of PI uptake (Fig. 3A and C). In contrast, the same clones were not protected from CD95-mediated cell death (Fig. 3B and D).
FIG. 3.
HVS-Bcl-2 blocks apoptosis mediated by dexamethasone but not by CD95 in DO cells. (A and C) DO cells transfected with HVS-Bcl-2 or control vector were treated with 10 nM dexamethasone and assessed for DNA fragmentation after 48 h (A). The development of cell death after dexamethasone treatment was quantified by measuring PI uptake after 48 h (C). Four clones transfected with HVS-Bcl-2 and five control clones were analyzed. The specific cell death (mean ± SD) is indicated (C). (B and D) To assess the sensitivity to CD95-mediated apoptosis, the same transfectants were treated with FLAG-CD95 ligand and anti-FLAG MAb. These cells were analyzed for DNA fragmentation 24 h after treatment with 10 ng of CD95 ligand per ml (B). Development of cell death (mean ± SD) was quantified by measuring PI uptake after 24 h, and the specific cell death (mean ± SD) is indicated (D).
Prevention of phosphatidylserine exposure.
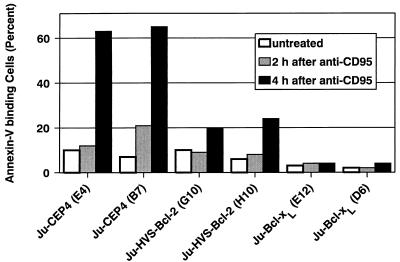
As an early event during apoptosis, cells expose phosphatidylserine on their outer cell membrane. When fluorescein isothiocyanate-conjugated annexin-V was used, the exposure of phosphatidylserine was detectable 2 to 4 h after CD95 ligation in control cells. In HVS-Bcl-2- and Bcl-xL-transfected cells, however, binding of annexin-V after CD95 ligation was considerably reduced (Fig. 4).
FIG. 4.
HVS-Bcl-2 and Bcl-xL block CD95-induced exposure of phosphatidylserine. The indicated Jurkat transfectants were treated with the CD95-specific MAb CH-11 at 100 ng/ml, and binding of annexin-V was determined at the indicated times after addition of the MAb.
Stabilization of mitochondria.
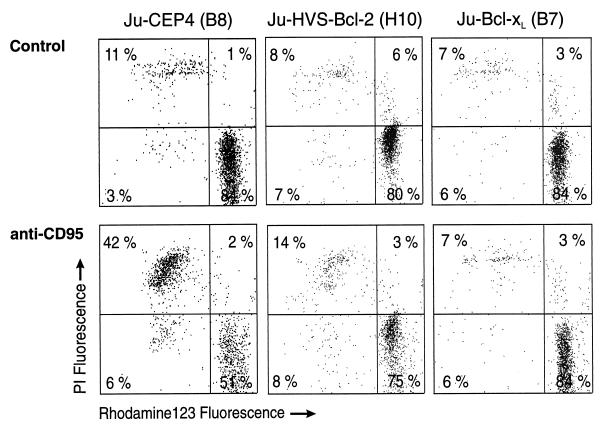
Two methods were applied to assess the integrity of mitochondria, namely, the retention of the fluorescent dye rhodamine 123 and the release of cytochrome c into the cytoplasm. Rhodamine 123 is released when the mitochondrial membrane potential is disturbed (5, 20). Jurkat cells transfected with HVS-Bcl-2, Bcl-xL, or control vector were treated with anti-CD95 and the maintenance of their mitochondrial membrane potential was assessed. Protection from apoptosis by HVS-Bcl-2 or Bcl-xL was associated with stabilization of mitochondria. Double staining with rhodamine 123 and PI showed that cells that exclude PI retain rhodamine 123 (Fig. 5). Likewise, HVS-Bcl-2- or Bcl-xL-induced protection from irradiation-induced apoptosis was associated with intact mitochondrial membrane potential, as seen by retention of rhodamine 123 in HVS-Bcl-2- and Bcl-xL-transfected cells (data not shown). Engagement of CD95 on Jurkat cells triggered a dose-dependent release of cytochrome c from mitochondria into the cytosol. The release of cytochrome c into the cytoplasm was inhibited by HVS-Bcl-2 and by Bcl-xL (Fig. 6).
FIG. 5.
HVS-Bcl-2 and Bcl-xL prevent mitochondrial damage. The indicated transfectants were cultured with anti-CD95 (10 ng/ml) or left untreated. After 16 h, the cells were stained with rhodamine 123 for 30 min at 37°C, washed, suspended in PBS containing PI, and analyzed by flow cytometry.
FIG. 6.
HVS-Bcl-2 and Bcl-xL block the release of cytochrome c into the cytosol. The indicated transfectants were treated for 4 h with the indicated concentrations of anti-CD95. Subsequently, the cytosolic fraction was prepared and analyzed by Western blotting for the presence of cytochrome c.
Inhibition of caspase-3-like activity.
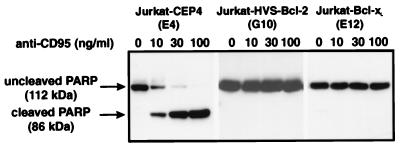
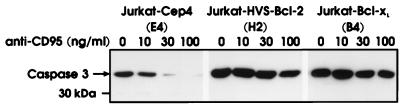
Treatment of Jurkat cells with anti-CD95 induced the activity of caspases recognizing the sequence DEVD. The CD95-mediated induction of this enzymatic activity was blocked by HVS-Bcl-2 and by Bcl-xL (Fig. 7). As an additional method for the detection of activation of caspases, we analyzed the cleavage of PARP, a natural substrate of caspase-3 and related caspases. CD95 ligation triggered the cleavage of PARP. This molecule has a molecular mass of 116 kDa, and its cleavage by caspase-3-related caspases leads to a 85-kDa fragment. The cleavage of PARP as a consequence of CD95 ligation was inhibited by HVS-Bcl-2 and by Bcl-xL (Fig. 8). To clarify whether the activity of caspase-3 was inhibited by HVS-Bcl-2, the level of the inactive 32-kDa proform of caspase-3 was determined by Western blotting. It is known that CD95 engagement triggers the cleavage of the inactive proform of caspase-3 to generate its active subunits (12). In control cells, CD95 engagement led to a dose-dependent disappearance of the 32-kDa form (Fig. 9). Upon the same treatments, HVS-Bcl-2- and Bcl-xL-transfected cells continued to show the uncleaved form at a similar level (Fig. 9). We noted that the applied MAb to caspase-3 gave only a very weak staining of small subunits of the active enzyme. Therefore, the disappearance of the 32-kDa proform was a more reliable marker of caspase-3 activation than was the generation of a small subunit.
FIG. 8.
HVS-Bcl-2 and Bcl-xL block the CD95-induced cleavage of PARP. The indicated transfectants had been treated with the indicated concentrations of anti-CD95 for 4 h. The cleavage of PARP was analyzed by Western blotting.
FIG. 9.
HVS-Bcl-2 and Bcl-xL prevent the CD95-induced cleavage of caspase-3. The indicated transfectants had been treated with different concentrations of anti-CD95 for 4 h. The content of the 32-kDa uncleaved proform of caspase-3 was analyzed by Western blotting.
DISCUSSION
Antiapoptotic spectrum of HVS-Bcl-2.
We found that HVS- Bcl-2 inhibits apoptosis triggered by radiation-induced DNA damage, by menadione (which gives rise to oxygen radicals), and by dexamethasone. HVS-Bcl-2 can also interfere with apoptosis mediated by the death receptor CD95. In the human T-cell leukemia line Jurkat, HVS-Bcl-2 effectively blocked CD95-mediated cell death. In contrast, in the murine T-cell hybridoma DO, HVS-Bcl-2 did not inhibit CD95-mediated apoptosis, although it conferred resistance to glucocorticoid-induced apoptosis in these cells.
Previous studies on the anti-apoptotic properties of cellular Bcl-2 family members have shown that Bcl-2 and Bcl-xL inhibited apoptosis induced by oxygen radicals, DNA damage, or growth factor or serum withdrawal in virtually all systems (32, 46). On the other hand, protection from apoptosis mediated by the death receptor CD95 has been observed in some cell types (5, 9) but not in others (26, 41). The observation that cellular and viral Bcl-2 family members protect against CD95-mediated cell death only in some cell types might be explained by different signaling cascades that are involved in CD95-mediated apoptosis. A well-understood signaling pathway used upon engagement of CD95 involves the recruitment of the adapter molecule FADD and subsequent activation of FLICE (caspase-8) (43). This pathway is inhibited by FLICE inhibitory proteins (FLIPs) that are encoded by HVS and other rhadinoviruses such as HHV-8, bovine herpesvirus 4, and equine herpesvirus 2 (2, 25, 57, 63). Recently, cellular FLIPs inhibiting CD95-mediated cell death have also been discovered (reviewed in reference 62). A second apoptotic pathway used upon CD95 engagement, which is mediated by Daxx, has been described (65). Daxx-mediated apoptosis could be blocked by Bcl-2, while the pathway that uses FLICE is not modulated by Bcl-2 (65). Taken together, this suggests that HVS-Bcl-2 and HVS-FLIP might be complementary in their ability to inhibit CD95-mediated apoptosis.
In our analysis, HVS-Bcl-2 and cellular Bcl-xL inhibited a similar spectrum of apoptotic pathways. Previous studies comparing different cellular Bcl-2 family members with each other found that Bcl-2 and Bcl-xL are functionally similar (26) but may block cell death differentially in some systems (18, 52).
Possible biological role of HVS-Bcl-2.
Two kinds of apoptotic stimuli that eventually lead to apoptosis of virus-infected cells can be distinguished. First, the infected cell may undergo a cell-autonomous apoptosis occurring without attack by immune cells. In particular, replication of herpesviruses and alphaviruses (19) results in death of the infected cell. HVS-Bcl-2 interfered with apoptosis that occurs due to disturbances of the intracellular homeostasis. This indicates that a cell-autonomous apoptosis can be blocked by HVS-Bcl-2. This antiapoptotic activity may result in prolongation of the life span of the infected cell and consequently in higher viral replication. This is in line with the observation that HVS-Bcl-2 protects against apoptosis induced by heterologous infection (44). Second, in the host, virus-infected cells are attacked by cytotoxic T cells, natural killer cells, and macrophages. Cytotoxic T cells use two effector mechanisms to destroy their targets, the secretion of lytic granules and the CD95 ligand (1). HVS-Bcl-2 is able to block CD95-mediated cell death. This might attenuate the effect of cytotoxic T cells. Exposure of phosphatidylserine is an early cellular change during the development of apoptosis (38). HVS-Bcl-2 prevented the exposure of phosphatidylserine. Also, this might be beneficial for the virus, because exposure of phosphatidylserine on the surface of apoptotic cells triggers specific recognition and removal by phagocytes (15, 60). Whether protection from apoptosis is associated with prevention of phosphatidylserine exposure depends on the particular antiapoptotic strategy. Bcl-xL also interfered with phosphatidylserine exposure. Another antiapoptotic principle, blocking the activity of the p21-activated kinase 2, prevents cell death but not phosphatidylserine exposure (47).
Since lymphotropic herpesviruses like EBV, HHV-8, and HVS that code for a Bcl-2 homolog are potentially oncogenic, a role of the viral Bcl-2 in tumor formation might be expected. Prevention of apoptosis is frequently a component of tumor formation. Overexpression of cellular Bcl-2 due to a t(14;18) translocation leads to a follicular B-cell lymphoma (59). On the other hand, the expression pattern of HVS-Bcl-2 does not support a role of this protein in oncogenesis, since its expression is restricted to cultures with lytic replication: in human T cells transformed to stable growth by HVS, this virus persists episomally and only a limited number of genes are expressed (16, 30). HVS-bcl-2 is not transcribed in growth-transformed human T cells (31). These findings suggest that the main function of HVS-Bcl-2 is to prolong the life span of lytically infected cells, thereby increasing viral replication. Similar to HVS, the Bcl-2 homolog of HHV-8 is predominantly a lytic-cycle gene (6, 49). The EBV-encoded Bcl-2 is also expressed mainly during lytic replication, is dispensable for B-lymphocyte transformation in vitro (37), and is not expressed in posttransplantation lymphoproliferative disorders (42). Nevertheless, a role of herpesvirus Bcl-2 homologs in the initiation and development of certain tumors seems conceivable, since Bcl-2 may cooperate with cellular oncoproteins like c-myc and also viral oncoproteins like E1A to induce transformation (11).
Mode of action of HVS-Bcl-2.
We have analyzed the effects of HVS-Bcl-2 on the stability of mitochondria and on the activation of caspases. To assess the mitochondrial integrity, rhodamine 123, an indicator of mitochondrial membrane potential, was applied and the release of cytochrome c in the cytoplasm was determined. Both methods showed that the mitochondrial damage during the apoptotic process is blocked by HVS-Bcl-2. A disruption of mitochondrial integrity has been recognized as part of an apoptotic pathway induced by different cell death-inducing stimuli (36). Therefore, the stabilization of mitochondria by HVS-Bcl-2 is in line with the broad spectrum of antiapoptotic activity of HVS-Bcl-2.
Multiple connections exist between disruption of mitochondria and activation of caspases (5, 55). Loss of mitochondrial membrane potential has been implicated in the release of cytochrome c, which then participates in triggering apoptosis (34, 66). In cell extracts, an apoptotic program could be induced by the addition of dATP (34). Three protein factors, designated apoptotic protease-activating factors (Apaf-1, Apaf-2, and Apaf-3), were found to be necessary and sufficient to reconstitute dATP-dependent caspase-3 activation in such cell extracts (68). Apaf-1 is the mammalian homolog of CED-4, Apaf-2 is cytochrome c, and Apaf-3 has been identified as caspase-9 (33). According to these findings, caspase-9 binds to Apaf-1 in a cytochrome c- and dATP-dependent fashion and becomes activated under these conditions (33). The active caspase-9 then cleaves and activates caspase-3. Caspase-3 and related caspases play major roles in the execution of nuclear apoptosis (13, 48) and also in extranuclear changes such as exposure of phosphatidylserine (24). Caspase-3 and related caspases cleave the DNA repair enzyme PARP (45). Caspase-3 recognizes the sequence DEVD (22). To determine whether HVS-Bcl-2 modulates the activation of caspase-3 and related caspases, three different methods were used. First, DEVDase activity induced upon engagement of CD95 was quantified in cell extracts. The induction of this enzymatic activity was reduced by HVS-Bcl-2. Second, cleavage of PARP upon CD95 engagement was determined and found to be blocked by HVS-Bcl-2. Third, the activation of caspase-3 was monitored by determining the level of the inactive 32-kDa precursor form of caspase-3. In control cells, CD95 engagement induced the cleavage and disappearance of the 32-kDa form of caspase-3. After the same treatments, in HVS-Bcl-2-transfected cells, no loss of the uncleaved caspase-3 was detected. Together, these experiments indicate that HVS-Bcl-2 acts upstream of caspase-3. This is consistent with our observation that HVS-Bcl-2 blocks phosphatidylserine exposure (see above), since caspase-3 is involved in phosphatidylserine externalization (24). In our assays, HVS-Bcl-2 and Bcl-xL were similar with respect to the stabilization of mitochondria and inhibition of caspase-3-like activity. This is in line with recent studies indicating that Bcl-2 and Bcl-xL interfere with the activation of caspases (8, 51), regulate the membrane potential of mitochondria, and inhibit cytosolic cytochrome c accumulation (20, 28, 29, 64). Bcl-xL has also been reported to maintain cell viability after the activation of caspases (5).
In conclusion, HVS-Bcl-2 protects against diverse apoptotic stimuli, stabilizes mitochondria, and acts upstream of the generation of caspase-3 like activity.
ACKNOWLEDGMENTS
We thank L. Boise and C. Thompson for the Bcl-xL cDNA.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 466), the Bayerische Forschungsstiftung, the Bundesministerium für Bildung und Forschung, and the EU (Shared Cost Action Project on Immunoregulatory Aspects of T Cell Autoimmunity in Multiple Sclerosis).
REFERENCES
- 1.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 2.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boise L H, Gonzalez Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 5.Boise L H, Thompson C B. Bcl-x(L) can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng E H-Y, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Nature. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 9.Chiou S K, Tseng C C, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 12.Dubrez L, Savoy I, Hamman A, Solary E. Pivotal role of a DEVD-sensitive step in etoposide-induced and Fas-mediated apoptotic pathways. EMBO J. 1996;15:5504–5512. [PMC free article] [PubMed] [Google Scholar]
- 13.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 14.Ensser A, Pflanz R, Fleckenstein B. Primary structure of the alcelaphine herpesvirus 1 genome. J Virol. 1997;71:6517–6525. doi: 10.1128/jvi.71.9.6517-6525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 16.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fickenscher H, Fleckenstein B. Growth-transformation of human T cells. Methods Microbiol. 1998;25:573–603. [Google Scholar]
- 18.Gottschalk A R, Boise L H, Thompson C B, Quintans J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci USA. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin D E, Hardwick J M. Regulators of apoptosis on the road to persistent alphavirus infection. Annu Rev Microbiol. 1997;51:565–592. doi: 10.1146/annurev.micro.51.1.565. [DOI] [PubMed] [Google Scholar]
- 20.Heiden M G V, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 21.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkart P A. ICE family proteases: mediators of all apoptotic cell death? Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann M, Lorenz H M, Voll R, Grunke M, Woith W, Kalden J R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, Yamamoto K, Sasada M. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998;187:587–600. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu S, Vincenz C, Buller M, Dixit V M. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 26.Huang D C, Cory S, Strasser A. Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene. 1997;14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- 27.Huang D C S, Adams J M, Cory S. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 1998;17:1029–1039. doi: 10.1093/emboj/17.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan Z M, Saxena S, Weichselbaum R, Nalin C, Kufe D. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluck R M, Bossy Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 30.Knappe A, Hiller C, Thurau M, Wittmann S, Hofmann H, Fleckenstein B, Fickenscher H. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J Virol. 1997;71:9124–9133. doi: 10.1128/jvi.71.12.9124-9133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraft M S, Henning G, Fickenscher H, Lengenfelder D, Tschopp J, Fleckenstein B, Meinl E. Herpesvirus saimiri transforms human T cells to stable growth without inducing resistance to apoptosis. J Virol. 1998;72:3138–3145. doi: 10.1128/jvi.72.4.3138-3145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;6:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti P, Castedo M, Susin S A, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchini A, Tomkinson B, Cohen J I, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bc12, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meinl E, Fickenscher H, Hoch R M, de Waal Malefyt R, ’t Hart B A, Wekerle H, Hohlfeld R, Fleckenstein B. Growth transformation of antigen-specific T cell lines from rhesus monkeys by herpesvirus saimiri. Virology. 1997;229:175–182. doi: 10.1006/viro.1996.8427. [DOI] [PubMed] [Google Scholar]
- 40.Meinl E, Hohlfeld R, Wekerle H, Fleckenstein B. Immortalization of human T cells by herpesvirus saimiri. Immunol Today. 1995;16:55–58. doi: 10.1016/0167-5699(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 41.Memon S A, Moreno M B, Petrak D, Zacharchuk C M. Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 42.Murray P G, Swinnen L J, Constandinou C M, Pyle J M, Carr T J, Hardwick J M, Ambinder R F. BCL-2 but not its Epstein-Barr virus-encoded homologue, BHRF1, is commonly expressed in posttransplantation lymphoproliferative disorders. Blood. 1996;87:706–711. [PubMed] [Google Scholar]
- 43.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 44.Nava V E, Cheng E H-Y, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 46.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 47.Rudel T, Bokoch G M. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 48.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 49.Sarid R, Sato T, Bohenzky R A, Russo J J, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 50.Schirm S, Müller I, Desrosiers R C, Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984;49:938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene. 1996;12:2251–2257. [PubMed] [Google Scholar]
- 52.Simonian P L, Grillot D A, Nunez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–1216. [PubMed] [Google Scholar]
- 53.Smith C A. A novel viral homologue of Bcl-2 and CED-9. Trends Cell Biol. 1995;5:344. doi: 10.1016/s0962-8924(00)89061-3. [DOI] [PubMed] [Google Scholar]
- 54.Stamminger T, Fickenscher H, Fleckenstein B. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J Gen Virol. 1990;71:105–113. doi: 10.1099/0022-1317-71-1-105. [DOI] [PubMed] [Google Scholar]
- 55.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schröter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 58.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 59.Tsujimoto Y, Cossman J, Jaffe E, Croce C M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 60.Verhoven B, Schlegel R A, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K, Dal Canto A J D, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallach D. Placing death under control. Nature. 1997;388:123–126. doi: 10.1038/40516. [DOI] [PubMed] [Google Scholar]
- 63.Wang G H, Bertin J, Wang Y, Martin D A, Wang J, Tomaselli K J, Armstrong R C, Cohen J I. Bovine herpesvirus 4 BORFE2 protein inhibits Fas- and tumor necrosis factor receptor 1-induced apoptosis and contains death effector domains shared with other gamma-2 herpesviruses. J Virol. 1997;71:8928–8932. doi: 10.1128/jvi.71.11.8928-8932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 65.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu W, Cowie A, Wasfy G W, Penn L Z, Leber B, Andrews D W. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]
- 68.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]