Abstract
Rationale & Objective
Older people with progressive chronic kidney disease (CKD) have complex health care needs. Geriatric evaluation preceding decision making for kidney replacement is recommended in guidelines, but implementation is lacking in routine care. We aimed to evaluate implementation of geriatric assessment in CKD care.
Study Design
Mixed methods implementation study.
Setting & Participants
Dutch nephrology centers were approached for implementation of geriatric assessment in patients aged ≥70 years and with an estimated glomerular filtration rate of ≤20 mL/min/1.73 m2.
Quality Improvement Activities/Exposure
We implemented a consensus-based nephrology-tailored geriatric assessment: a patient questionnaire and professionally administered test set comprising 16 instruments covering functional, cognitive, psychosocial, and somatic domains and patient-reported outcome measures.
Outcomes
We aimed for implementation in 10 centers and 200 patients. Implementation was evaluated by (i) perceived enablers and barriers of implementation, including integration in work routines (Normalization Measure Development Tool) and (ii) relevance of the instruments to routine care for the target population.
Analytical Approach
Variations in implementation practices were described based on field notes. The postimplementation survey among health care professionals was analyzed descriptively, using an explanatory qualitative approach for open-ended questions.
Results
Geriatric assessment was implemented in 10 centers among 191 patients. Survey respondents (n = 71, 88% response rate) identified determinants that facilitated implementation, ie, multidisciplinary collaboration (with geriatricians) -meetings and reports and execution of assessments by nurses. Barriers to implementation were patient illiteracy or language barrier, time constraints, and patient burden. Professionals considered geriatric assessment sufficiently integrated into work routines (mean, 6.7/10 ± 2.0 [SD]) but also subject to improvement. Likewise, the relevance of geriatric assessment for routine care was scored as 7.8/10 ± 1.2. The Clinical Frailty Score and Montreal Cognitive Assessment were perceived as the most relevant instruments.
Limitations
Selection bias of interventions’ early adopters may limit generalizability.
Conclusions
Geriatric assessment could successfully be integrated in CKD care and was perceived relevant to health care professionals.
Index words: Chronic kidney disease, feasibility studies, geriatric assessment, implementation science, older people, shared decision making
Plain-Language Summary
The number of older persons with kidney failure is increasing, many of whom have cognitive decline or are dependent on others for daily life tasks. These problems are often overlooked but relevant for future treatment choices, and they affect quality of life. We asked 10 health care centers to use tests and questionnaires to identify these issues, thus being able to offer additional support. We learned that it is possible to use these assessments in practice and that professionals found them relevant. Collaboration with geriatric departments was perceived valuable. However, there are also challenges, such as not having enough time and personnel and burden to patients. Understanding these possibilities and challenges is crucial for improving care for older patients with kidney failure.
Among the increasing population of older patients with kidney failure,1,2 unrecognized geriatric impairments are highly prevalent. Such impairments, including cognitive and functional decline, comorbid conditions, frailty, depression, and malnutrition,3, 4, 5 are associated with adverse health outcomes such as mortality, hospitalization, and reduced health-related quality of life.6,7 Understanding geriatric impairments can be valuable for decision making for kidney replacement therapy choices and for risk stratification. Therefore, Dutch and British guidelines recommend geriatric assessment for those at high risk of death or those identified as being frail, respectively.8,9 For this, clinicians have recognized the clinical and scientific value of a standardized set of instruments.7,10
In the absence of a gold standard for geriatric assessment, a group of Dutch health care professionals recently reached a consensus on a geriatric assessment tailored for nephrology care (nephrology-tailored geriatric assessment [NGA]).11 This set of instruments takes less than 1 hour to perform and could be routinely conducted by trained nephrology or geriatric nurses. In contrast to comprehensive geriatric assessment, this modified approach of geriatric assessment is therefore expected to be less challenging to embed in existing routines in nephrology care pathways. Furthermore, unlike short frailty screening instruments,12 the set would enable adequate recognition of geriatric impairments or frailty in the older chronic kidney disease (CKD) population. After NGA, if needed, a patient could be referred to a geriatrician for full comprehensive geriatric assessment, which includes a more extensive multidisciplinary diagnostic and treatment process that identifies medical, psychosocial, and functional impairments that may necessitate development of an integrated care plan.
Although some previous attempts at incorporating a geriatric assessment in nephrology populations have been reported,3,4,13, 14, 15, 16 consistent and widespread implementation of standardized geriatric assessment in routine nephrology care has yet to be achieved. Analysis of the process of implementation (including barriers and facilitators) may help to successfully incorporate this complex intervention in clinical practice.17 The current study, therefore, aimed to evaluate multicenter implementation of geriatric assessment in routine nephrology care.
Methods
Design
In this implementation study, we used a mixed methods approach, combining quantitative data collection with a partially qualitative postimplementation survey involving health care professionals.
Context
This study was part of the Pathway for Older Patients Reaching End-Stage Renal Disease (POLDER) initiative, aimed at designing, implementing, and evaluating geriatric assessment in Dutch nephrology clinics. In October 2017, we approached 16 Dutch university and non–university hospital–based nephrology centers on their interest in implementing geriatric assessment. This interest was evidenced either by partaking in previous studies involving geriatric assessment or by showing interest in the topic at conferences. Following an email survey to assess current practices,11 10 centers chose to participate, representing 18% of all Dutch nephrology centers. Although the geriatric assessment protocol was new for all participating centers, some had previously participated in studies using geriatric assessment in patients with CKD stage G4 or G5 (N = 3) or CKD G5 or G5D (N = 2).3,18 Additionally, 2 centers used geriatric assessment in their routine care on referral, whereas the remaining 3 centers had not previously used geriatric assessment in CKD care.
Intervention Description
Previously, we developed a consensus-based nephrology-tailored assessment (NGA).11 NGA consists of a patient questionnaire and professionally administered tests that can be completed within 1 hour by a trained nurse or medical specialist. NGA includes instruments covering various domains, including functional, cognitive, psychosocial, somatic, and patient-reported outcomes. Table 1 presents the included instruments19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 and by whom they were completed. NGA was developed to enhance routine care and research in older patients with advanced CKD, aiming to identify known and unknown geriatric impairments in older patients with CKD stage G4-G5. If needed, NGA could lead to appropriate supportive interventions (eg, physiotherapy, referral to geriatrician) and is beneficial to decision making in patients with kidney failure.
Table 1.
Instruments Included in the Nephrology-tailored Geriatric Assessment
| Nephrology-Tailored Geriatric Assessment: Domains and Instruments |
Type of Assessment |
||||
|---|---|---|---|---|---|
| Domain | Subdomain | Instrument | (I) Patient questionnaire | (II) Provider-administered test set | |
 |
Functional status | Activities of daily living | Katz Activities of Daily Living-619 | X | |
| Instrumental activities of daily living | Lawton Instrumental Activities of Daily Living20 | X | |||
| Handgrip strength | Handgrip strength | X | |||
| Fall risk assessment | One-year fall history, fear of falling | X | |||
 |
Cognitive status | Cognitive functioning | Montreal Cognitive Assessment21 | X | |
| 6-item Cognitive Impairment Test22 | X | ||||
| Letter Digit Substitution Test23 | X | ||||
 |
Psychological status/mood | Depression | Whooley-questions/Geriatric Depression Scale 15-item24,25 | X | |
| Optimism | Life Orientation Test-Revised26 | X | |||
 |
Patient-reported outcome measures | Health-related quality of life | 12-item Short Form Health Survey27 | X | |
| Symptoms | Dialysis Symptom Index28 | X | |||
 |
Somatic status | Clinical judgment | Surprise question29 | X | |
| Frailty | Clinical Frailty Score30 | X | |||
| Comorbid condition | Charlson Comorbidity Index31 | X | |||
| Polypharmacy | Polypharmacy (≥5 medications) | X | |||
| Nutritional status | Patient-Generated Subjective Global Assessment32 | X | X | ||
 |
Social | Caregiver burden | Self-perceived pressure from informal care-plus33 | Caregiver | |
Note: This nephrology-tailored geriatric assessment, and its consensus-based development, has been described in more detail by Voorend et al.11
Target Population
NGA was implemented among patients aged ≥70 years with an estimated glomerular filtration rate (eGFR) of ≤20 mL/min/1.73 m2 and the ability to read and understand the questionnaire. Patients were excluded in case of a history of dementia or lack of mental capacity assessed by a geriatrician.
Implementation Strategy
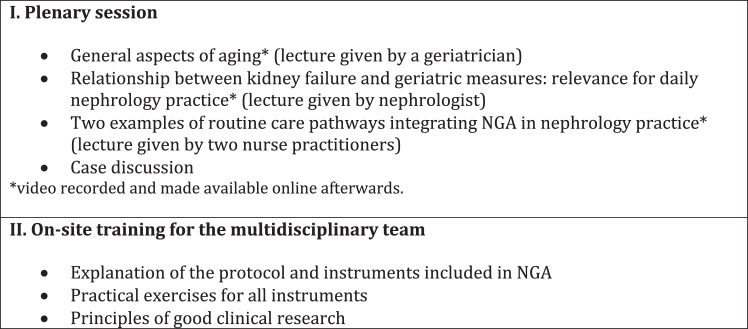
An educational program was developed to enhance awareness of geriatric impairments in the advanced CKD G4-G5 patient population and improve knowledge about geriatric assessment among involved health care professionals. The program existed of a plenary education and onsite training session, described in Figure 1. Participating centers had the flexibility to determine how they would embed the intervention in the care process. This encompassed decisions regarding who would conduct the NGA, the extent of geriatrics department’s involvement, and the organization of multidisciplinary team meetings for discussion of NGA outcomes and managing identified geriatric impairments. Additionally, optional digital entry of the patient questionnaires was offered, and a summary of NGA results was made available (after July 2020) for download after data entry in the online dashboard for data collection (see Supplementary File 1).
Figure 1.
Educational program as part of the implementation strategy.
Outcome
A priori, we aimed for 10 participating centers, each including 20 patients. We aimed to gain insights in variation of implementation practices, contextual changes that affected hospital participation and patient inclusion, and completeness of the NGA instruments.
Determinants of successful implementation were evaluated on 2 aspects. First, we assessed the presence of enablers and barriers to implementation and integration in work routines. A list of potential barriers and enablers was used as previously identified in a focus group study.34 To understand “integration in work routines” we employed the validated Dutch version of the Normalization Measure Development (NoMAD) tool,35,36 which has been previously used in the implementation of complex interventions in nephrology.37,38 Four processes of integration in work routines (ie, normalization) were assessed: sense making, cognitive participation, collective action, and reflexive monitoring (Table S1).39 Second, we investigated the perceived relevance of the NGA instruments to CKD care, specifically whether NGA achieved the intervention objective (enabling identification of known and unknown geriatric impairments), facilitated supportive interventions, and supported decision making for kidney failure treatment.
Data Collection
An overview of data collection is presented in Table 2. We collected data at the hospital level using field notes to capture information on the administration of the provider-administered test set, multidisciplinary team meetings, the involved disciplines, and any contextual changes that affected hospital participation or patient inclusion. At health care provider level, a postimplementation survey was conducted among partaking health care professionals. The survey (Supplementary File 2) included questions about respondents’ characteristics, the presence of enablers and barriers to implementation, the NoMAD tool, the perceived relevance of NGA practices and its individual components, and the intervention’s ideal target group. Additionally, explanatory open-ended questions probed for deeper understanding of implementation determinants. Data were collected on the number of patient inclusions, conducted NGAs, digital patient questionnaires entries, and instrument completeness. Patient characteristics were extracted from the database managed by Nefrovisie, the Dutch quality institute for nephrology.
Table 2.
Overview of Data Collected for Evaluation of NGA Implementation According to the Implementation Outcomes
| Aim | Implementation Outcome | Data Collected | Units or Score (Range) | Source |
|---|---|---|---|---|
| Feasibility of implementation | Hospital participation | Number of participating hospitals that implemented NGA practices | Percentage of aimed hospitals | Field notes (hospital level) |
Implemented components of the intervention were as follows:
|
Discipline Single or multiple visit Yes/no Disciplines - |
|||
| Patient inclusion |
|
Count Sex Age eGFR |
Database collection (patient level) | |
|
Percentage completed test sets and questionnaires Percentage complete instruments |
|||
| Determinants of (un)successful implementation | Enablers and barriers to implementation |
|
10-point Likert presence scale Top 3 given by respondent |
Postimplementation survey among partaking health care professionals |
| Integration in work routines | NoMAD tool:
|
0-10 visual analog scale; higher scores indicating more use. 5-point Likert scale (1-disagree to 5-agree). |
||
| Perceived relevance of the NGA | Agreement of relevance of NGA practices as a whole: Rating 3 aspects of the intervention objective, ie, NGA supports in the following:
|
10-point Likert scale: 0-“strongly disagree” to 10-“strongly agree” | ||
| Rating all 17 individual NGA instruments for their relevance to the intervention objective (see above) | 5-point Likert scale: 1 (not at all relevant) to 5 (very relevant) | |||
Appraisal of the target group by rating:
|
3 options: “too extended,” “sufficient,” or “too narrow” | |||
| Explanatory qualitative information | Understanding on determinants of implementation | Open questions | ||
| Additional information | Survey respondents’ characteristics | discipline, years’ experience in current function and department, affiliated hospital | ||
Abbreviations: eGFR, estimated glomerular filtration rate; NGA, nephrology-tailored geriatric assessment; NoMAD, Normalization MeAsure Development.
Survey Sampling
Participants for the postimplementation survey were purposively sampled by asking each center’s contact person to list at least 5 colleagues from various disciplines involved in the care of older patients with CKD. The survey was disseminated after study inclusion closed in May 2021. Eighty-one professionals received a personal email invitation to participate in an online survey using Qualtrics software (Qualtrics, version 2021). Nonresponders received reminders 2 weeks later. We assured confidentiality through pseudonymized analyses of the data.
Data Analysis
Feasibility of implementation was assessed by analyzing per center the implemented components of the intervention, patient inclusion and completeness of NGA instruments. Demographic and clinical patient data, along with survey respondent characteristics and quantitative survey responses, were presented descriptively. To assess integration into routine work (ie, normalization), we used paired t tests to compare current and anticipated future use of NGA. Average construct scores were calculated for each participant, excluding “nonapplicable” items. Higher scores signified better-perceived implementation.37,39 A mean subconstruct score <4 indicated potential for improvement. The overall perceived relevance of the NGA set was appraised by the sample mean of the respondents’ judgment on 3 intervention objective achievement questions. A score of ≥7 was considered relevant (ie, successful in reaching the intervention objective). For the specific instruments, we strived for a rating of ≥4 (ie, “relevant” or “very relevant”) among 70% of the respondents. IBM SPSS statistics for Windows (version 25) was used for quantitative analysis.
Explanatory qualitative data analysis involved open-ended survey responses, initially coded by 2 authors independently (ie, project lead [CV] and an uninvolved author [LB]). Codes were discussed to ensure agreement on the final interpretations. We did not use qualitative research software. Triangulation of the quantitative and qualitative data was done by exploring (dis)agreements within and between hospitals in the survey responses and participants or centers with notable normalization levels.
The Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0)40 and Consensus-Based Checklist for Reporting of Survey Studies guided reporting of our results.41
Ethical Considerations
The POLDER study’s protocol was approved by the Medical Research Ethics Committee Leiden-Den Haag-Delft (reference NL65322.098.18) to facilitate patient data collection and analyses, and the study was conducted according to the principles of the Declaration of Helsinki and the Dutch Medical Research Involving Human Subjects Act. The POLDER study is registered in the Netherlands Trial Registry (trialsearch.who.int: NTR7310).
Results
Hospital Participation
Ten of the 16 initially approached centers implemented NGA practices, while 5 declined because of a lack of reimbursement and personnel shortages. Two nephrology units independently agreed to participate but later merged into one. Implementation started between October 2018 and March 2020, with the study start and inclusion period extended because of logistic and COVID-19–related challenges, and patient inclusion concluded in April 2021.
Geriatric tests were conducted by a nephrology nurse (practitioner) (N = 6 centers, 60%), a geriatrician or geriatric nurse (N = 3, 30%), or a research nurse (N = 1, 10%), see Table S2. Visits for the geriatric tests were combined with regular nephrology appointments at most centers (N = 9, 90%). Patient questionnaires were sent in advance of their visit for at-home completion or given afterward. Approximately 15% (n = 28) of patients digitally entered their questionnaires; this was done at 7 centers (ranging from 8%-38% per center). At 9 centers, outcomes were discussed in multidisciplinary team meetings with geriatric expertise involved in 7 settings. One center conducted meetings if geriatric impairments that needed further involvement of a geriatrician were detected.
Patient Inclusion and Characteristics
A total of 194 patients gave informed consent, with 3 unable to participate because of deteriorating health status (n = 2, 1%) or no-show (n = 1, 0.1%). NGA was performed in 191 patients. Participation varied between n = 10 and n = 30 per hospital. Most participants were men (n = 135, 71%). Median age was 77.5 (interquartile range, 74.3-81.9) years, and mean eGFR at the time of NGA was 15.0 ± 4.4 (SD) mL/min/1.73 m2.
Completeness of NGA
In total, 187 patients (98%) returned the patient questionnaire. All but one patient (n = 190, 99%) finalized the provider-administered test set. Table S3 reports completeness of the NGA instruments.
Somatic instruments were completed by all patients. Over 90% of the patients completed measures for activities of daily living, fall risk, 6-item Cognitive Impairment Test, Montreal Cognitive Assessment (MoCA), Letter Digit Substitution Test, and depressive mood. Measures of caregiver burden and nutritional status were conducted in 64% (n = 121) and 82% (n = 156) of the patients and were less often completed (60% and 66%, respectively).
Survey Respondents’ Characteristics
Of 81 invited health care professionals, 71 responded (88% response rate). Table 3 summarize the survey respondent characteristics, with a median of 6 professionals (range, 5-12) participating per hospital.
Table 3.
Survey Respondents’ Characteristics and Outcomes
| Survey Participant Characteristics | n = 71 |
|---|---|
| Respondents per hospital, median (range) | 6 (5-12) |
| Working experience (y), median (range) | |
| in current profession | 9 (1-30) |
| in current department | 8 (1-39) |
| Clinical role, n (%) | |
| Nephrologist | 27 (38) |
| Geriatrician | 10 (14) |
| Nurse practitionera | 10 (14) |
| Nurse (nephrology) | 11 (16) |
| Nurse (geriatrics/geriatrics-nephrology) | 2 (3) |
| Physician assistant (nephrology) | 1 (1) |
| Social worker | 7 (10) |
| Dietitian | 2 (3) |
| Research nurse | 1 (1) |
Including one respondent in training.
Enablers and Barriers to Implementation
The most frequently cited top 3 enablers for successful implementation included collaboration with geriatric department (n = 45, 76%), multidisciplinary meetings and reports (n = 39, 66%), assessment performed by nurses (practitioners) (n = 26, 44%), and discussion of purpose and outcomes of the test with patients (n = 26, 44%). Common barriers to implementation included patient illiteracy or non-Dutch speaking (46% of respondents’ top 3 barriers, n = 25), lack of time (n = 20, 37%), burden for patients (n = 20, 37%), and lack of patients’ willingness or eagerness to participate in NGA (n = 17, 31%). The open-ended questions showed concerns on patient burden: difficulty of NGA questions (Table S4, Q41), the toll (ie, time and burden) of hospital visits (Table S4, Q42), and the negative connotation of “geriatric assessment” (Table S4, Q43 and Q44). Table 4 presents the respondents’ agreement on the presence of implementation enablers and barriers. Interestingly, respondents reported that patients were often willing to participate in NGA (mean score as enabler, 6.9/10 ± 1.68), although this was frequently cited as a top 3 barrier.
Table 4.
Presence of Enablers and Barriers of Implementation of the NGA and Those Most Frequently Rated for Successful Implementation
| The Following Reasons May Have Led to Good or Less Good Implementation. In Your Experience, Were These Reasons Present In Your Hospital’s NGA Performance? | N | Agreement, mean ± SD | Presence | |||
|---|---|---|---|---|---|---|
| Enablers | Totally disagree (1) | Totally agree (10) | ||||
| ☺ | The purpose and results of the tests were discussed in detail with the patient | 48 | 6.8 (2.09) | 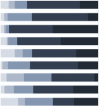 |
||
| ☹ | Patients were willing and available for the geriatric assessment | 56 | 6.9 (1.68) | |||
| ☺ | Good cooperation with geriatrics department | 58 | 8.1 (1.79) | |||
| ☺ | Multidisciplinary consultation and reports in which NGA outcomes and treatment policy were discussed | 65 | 7.7 (2.03) | |||
| Support from other disciplines (eg, dietitian, social worker) in the administration and interpretation of NGA | 62 | 6.6 (2.77) | ||||
| ☺ | Suitable (and trained) personnel were sufficiently available to administer the NGA | 53 | 7.2 (1.91) | |||
| The outpatient schedule was easy to adjust for NGA administration | 50 | 6.0 (2.04) | ||||
| Management supports the implementation of the NGA | 51 | 6.9 (2.22) | ||||
| The sum-score forms in the dashboard (available from July 2020) were helpful | 28 | 6.1 (2.81) | ||||
| Barriers | ||||||
| ☹ | The NGA is too much of a burden for many patients | 55 | 4.8 (1.81) | 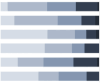 |
||
| ☹ | NGA was performed to a limited extent because many patients had low health literacy or because of a language barrier | 50 | 4.5 (1.88) | |||
| Reluctance in the Nephrology department to involve geriatrics/elderly care in routine care | 61 | 3.3 (2.35) | ||||
| Loss of geriatric knowledge and practical skills (for example because of team changes) | 47 | 3.3 (2.25) | ||||
| ☹ | Time constraints restricted carrying out the NGA | 46 | 4.7 (2.32) | |||
| Lack of budget is a reason to carry out NGA less often or adequately | 39 | 3.6 (2.28) | ||||
Notes: Both enablers and barriers are sorted by patient-related, multidisciplinary cooperation, and organizational aspects. Score range: 1 (totally disagree) to 10 (totally agree). Four enablers (☺) and 4 barriers (☹) that were most frequently in respondents’ top 3 for successful implementation. One enabler was mentioned as a determinant in the top 3 barriers for implementation.
Abbreviations: NGA, nephrology-tailored geriatric assessment.
Integration Into Work Routines
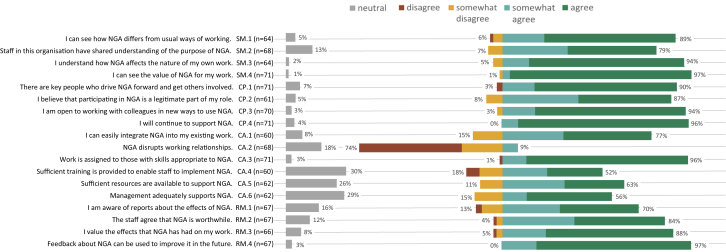
The general NoMAD questions indicated that NGA was not fully embedded at the time of the survey (mean, 6.9/10 ± 2.01 versus mean, 6.7 ± 1.42; P < 0.001). Figure 2 presents responses to each of the NoMAD questions. Although 97% (n = 69) recognized the potential value of NGA and 96% (n = 49) continued to support it, there were opportunities to enhance integration, according to 97% (n = 65). Table S4 and Figure S1 show the mean NoMAD (sub-)construct scores and qualitative analysis of the open-ended survey questions. Respondents found NGA meaningful (Sense Making mean score, 4.6 ± 0.59) and were committed to making it work (Cognitive Participation mean score, 4.6 ± 0.50). To a somewhat lesser extent, the respondents acknowledged the efforts of working together to make NGA work (Collective Action mean score, 4.1 ± 0.66) and appraised the effect of NGA (Reflexive Monitoring mean score, 4.3 ± 0.64). Concurrently, respondents recognized the potential improvement of sufficient training, resources, and knowledge about the effects of NGA, indicated by mean scores of <4 for the subconstructs of skill set workability, contextual integration, and systemization (Figure S1). The open-ended questions showed that nurses’ lack of time and availability hampered integration in routine work (Table S3, Q12), and suggested improvements such as reducing the number of tests (Table S3, Q36-Q39), and improvement of interdisciplinary cooperation (Table S3, Q28 and Q29).
Figure 2.
Response to statements on normalization of NGA. Figure bars show the percentage of respondents reporting their agreement with the NoMAD statements. The number of respondents lower than n = 71 bypassed the statement by indicating “not relevant for my role.” Questions relate to the constructs of the NPT framework, ie, SM: sense making, CP: cognitive participation, CA: collective action, and RM: reflexive monitoring. Abbreviations: NGA, nephrology-tailored geriatric assessment; NoMAD, Normalization measure development; NPT, normalization process theory.
Perceived Relevance of the NGA Instruments to Care of Patients With CKD
Health care professionals perceived NGA instruments as successful in reaching the intervention objective and its aimed effects, with an overall score of 7.8/10 ± 1.16. The relevance of each included instrument is shown quantitatively in Table 5 and qualitatively in Table S5. Providers rated the relevance of the MoCA and the Clinical Frailty Score as high (mean, 4.5/5 ± 0.60 and mean, 4.4 ± 0.69, respectively), while they appraised polypharmacy and the Life Orientation Test as less relevant (mean, 3.5 ± 0.98 and mean, 3.6 ± 0.79, respectively). Less than 70% of the users were convinced of the relevance of the latter 2 instruments and handgrip strength, Letter Digit Substitution Test, and the Patient Generated-Subjective Global Assessment.
Table 5.
Appraisal of Relevance of the Instruments Included in the NGA
|
Domain |
Instrument | Respondents (n) | Survey Outcomes |
 |
|||
|---|---|---|---|---|---|---|---|
| Relevance scorea mean (SD) | Not relevant n (%) | Neutral n (%) | Relevant n (%) | ||||
| Functional status | Activities of daily living (Katz ADL-6) | 59 | 4.2 (0.70) | 0 (0%) | 9 (15%) | 50 (85%) | 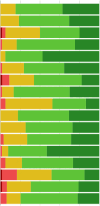 |
| Instrumental Activities of daily living (Lawton) | 59 | 4.1 (0.70) | 0 (0%) | 11 (19%) | 48 (81%) | ||
| Handgrip strength | 60 | 3.7 (0.88) | 3 (5%) | 21 (35%) | 36 (60%) | ||
| Fall risk assessment | 61 | 4.1 (0.68) | 1 (2%) | 9 (15%) | 51 (84%) | ||
| Cognitive functioning | Montreal Cognitive Assessment | 61 | 4.5 (0.60) | 0 (0%) | 3 (5%) | 58 (95%) | |
| 6-item Cognitive Impairment Test | 59 | 4.1 (0.80) | 1 (2%) | 13 (22%) | 45 (76%) | ||
| Letter Digit Substitution Test | 56 | 3.7 (0.90) | 5 (9%) | 14 (25%) | 37 (66%) | ||
| Psychological status/mood | Whooley-questions/Geriatric Depression Scale-15 | 60 | 4.2 (0.81) | 1 (2%) | 7 (12%) | 52 (87%) | |
| Life Orientation Test-Revised | 59 | 3.6 (0.79) | 3 (5%) | 28 (47%) | 28 (47%) | ||
| PROM’s | HRQoL: 12-item Short Form Health Survey | 62 | 4.1 (0.69) | 0 (0%) | 11 (18%) | 51 (82%) | |
| Dialysis Symptom Index | 59 | 4.0 (0.73) | 0 (0%) | 15 (25%) | 44 (75%) | ||
| Somatic status | Surprise question | 63 | 4.0 (0.81) | 2 (3%) | 15 (24%) | 46 (73%) | |
| Clinical Frailty Score, | 64 | 4.4 (0.69) | 1 (2%) | 4 (6%) | 59 (92%) | ||
| Charlson Comorbidity Index | 60 | 4.0 (0.80) | 3 (5%) | 10 (17%) | 47 (78%) | ||
| Polypharmacy | 60 | 3.5 (0.98) | 10 (17%) | 21 (35%) | 29 (48%) | ||
| Nutrition | Patient-Generated Subjective Global Assessment | 62 | 3.8 (0.88) | 4 (6%) | 15 (24%) | 43 (69%) | |
| Social | Caregiver burden: SPICC-plus | 64 | 4.0 (0.79) | 4 (6%) | 9 (14%) | 51 (80%) | |
Note: The percentages in the fifth to seventh columns do not add up to exactly 100% because of rounding differences.
Abbreviations: HRQoL, health-related quality of life; NGA, nephrology-tailored geriatric assessment; PROM, patient-reported outcome measure; SPICC, self-perceived pressure from informal care.
Scoring was done on a 5-point Likert scale: 1 (not at all relevant) to 5 (very relevant).
Perceived Relevance to the Target Group
Survey respondents had varying opinions on age and kidney function cutoffs for NGA practices. Cutoffs of >70 years and eGFR of <20 mL/min/1.73 m2 were too narrow for 24% (n = 16) and 18% (n = 12), good for 67% (n = 44) and 69% (n = 45), and too broad for 9% (n = 6) and 12% (n =8), respectively. Several respondents qualitatively addressed that NGA should be tailored to each patient’s situation, considering the progression of CKD, clinical judgment, and biological age rather than calendar age or kidney function.
Discussion
Our findings demonstrate the feasibility of implementing NGA in older patients with advanced CKD. Furthermore, they offer recommendations for extending guidance on how to integrate geriatric assessment in nephrology care. We identified determinants of successful implementation and barriers. Collaborative involvement from the geriatrics department, nurse practitioners, and multidisciplinary meetings enhanced implementation, while limited collective action, lack of time and personnel, and patient-related factors, such as burden and low literacy, hampered it. Integration of NGA into work routines could be enhanced because it was not yet fully integrated. Nonetheless, health care professionals recognized its relevance in care of patients with CKD.
Our findings contribute to the emergence of models for integrating geriatric care into hospital practices,42,43 gaining recognition for geriatric assessment as a predictive and rehabilitative instrument for older patients across various medical disciplines. Compared with oncology42 and acute care patients, care of patients with CKD involves longer-standing patient–provider relationships and generally longer-lasting decision trajectories. However, impairments, primarily cognitive problems, often go unnoticed in patients with CKD.3 Therefore, geriatric assessment should focus on detecting and managing such impairments and also on adapting how education and information on treatment selection is delivered. Similar to other medical fields, substantial implementation barriers were recognized, mainly concerning practicalities and resources such as time constraints and unavailability of health care personnel.42,44,45
Our study provides a practical example of the previously recognized need to integrate standardized geriatric assessment into routine nephrology practice.3,46, 47, 48, 49, 50 Our insights extend the paucity of guidance on the incorporation of geriatric assessment in nephrology care.13,51 Predominantly, collaboration with the geriatric department appears to be essential because it provides expertise for advice and referrals for management of identified impairments.13,15,16 Although the extent of geriatrics involvement may vary from overseeing all NGA practices to availability for referrals only, both are viable approaches.13
To improve integration of NGA in work routines, our results showed that collective action (ie, how people work together to make NGA practices work) deserves attention. Our study found that implementation often relied on one or a few key persons, which makes maintenance of NGA practices vulnerable. Evidence regarding the benefits and use of NGA would help to improve collective action. Related to the latter, discussion of carefully summarized results of NGA in multidisciplinary team meeting (including geriatric health care professionals) is essential.34,44,52 This will help to create awareness on the presence and relevance of detected cognitive and functional impairments and implications for treatment and supportive care.
A potential facilitator for implementation into routine nephrology care may be the integration of summarized NGA outcomes in electronic patient files. This may increase multidisciplinary use of recurring geriatric assessment and support its interpretation. In oncology, a tailored geriatric assessment summary with guided recommendations has facilitated and improved patient care,53 and web-based applications for geriatric assessment seem promising.54,55 Involvement of older (CKD) patients in future research and development of digital tools is desired.56
The negative effect of patient burden on implementation was noted, as in previous studies.34,42,57 Therefore, it is essential to integrate NGA in regular nephrology hospital visits and limit the frequency and duration of prolonged hospital visits. Adjustments to NGA practices for individual cases may be necessary and helpful, whereas refinement of the assessment by modifying the test set can also improve acceptance and implementation.
In absence of a gold standard,58 our test set was derived in a foregoing pragmatic consensus trajectory.11 If time for assessment practices is limited, some instruments could be considered for omission or substituted with shorter instruments, preferably only after the discriminative and predictive value of the tests is investigated in the CKD stage G4-G5 population. For example, polypharmacy was irrelevant to a substantial part of our NGA users, potentially because of overlap with repeated attention to this topic in routine medical care. The instrument on nutrition (Patient Generated-Subjective Global Assessment) showed considerable incompleteness and perceived irrelevance in our study, potentially because of inconvenient usage and overlapping practices of dieticians who already have a clearly defined role in multidisciplinary management of patients with CKD in routine care. In addition, the Life Orientation Test, Letter Digit Substitution Test, and handgrip strength were perceived less relevant to the professionals. Based on our evaluation and other literature,15,59, 60, 61, 62 both the Clinical Frailty Score30 and MoCA21 are a relatively new to outpatient nephrology care but may be promising instruments. The caregiver burden (Self-Perceived Pressure from Informal Care) questionnaire, though perceived relevant to professionals, reported considerable incompletion, which was to a large extent explained by those patients that did not need caregiver support.
Further development of NGA should focus on inclusiveness by incorporating linguistic and cultural factors.34,42,57 Although illiteracy was an exclusion criterion for the present study, apparently, health care professionals determined that assessment was still too elaborate for some patients. In our view, low health literacy as a barrier to implementation of geriatric assessment has been underreported in scientific studies.
Flexibility in adapting the abovementioned fixed parts of NGA according to organizational circumstances, resource constraints, and patient profile has proven advantageous for successful implementation.45,63
A strength of our study is that we are among the first to explore and report multicenter implementation of geriatric assessment in routine CKD care quantitatively, enriched by an explanatory qualitative analysis. Although NGA implementation was feasible for hospital participation, we fell short of our patient inclusion goal in half of the centers. This was due to incomplete integration of NGA practices, the effect of the COVID-19 pandemic, and scientific study formalities. However, the subsequent DIALOGICA (Dialysis or Not: Outcomes in Older Kidney Patients With Geriatric Assessment) study64 now involves 35 centers and >600 patients, showcasing widespread feasibility.65 Limitations of our study are that we did not structurally collect data on the management plan of identified geriatric impairments (eg, outcomes of multidisciplinary team meetings and follow-up of patients). We also did not include an economic component or use a randomized controlled or pre- or postsurvey design. This prevents us from drawing conclusions regarding efficacy, effectiveness, and efficiency of NGA practices. Furthermore, survey responders were committed to this topic, which may have led to bias toward positive outcomes. However, no financial incentives or inclusion fees were provided. Activities were organized in daily practices. Although some hospitals made use of local research departments for data entry and study-related logistics, this was outside the scope of the current implementation study but may have influenced implementation.
Future research should evaluate implementation of management of geriatric impairments and establish the efficacy of geriatric practices in nephrology,66 as was done in other medical fields.67,68 Also, the acceptability from patient perspective34 and the use of cross-cultural instruments57 needs further investigation.
In conclusion, geriatric assessment could successfully be integrated in CKD care and is perceived as relevant by partaking health care professionals.
Article Information
POLDER study group
Arjan van Alphen (Maasstad Hospital, Rotterdam), Noeleen Berkhout-Byrne (Leiden University Medical Center, Leiden), Fenna van Breda (Amsterdam University Medical Center, Amsterdam), Marjolijn van Buren (Haga Hospital, The Hague), Henk Boom (Reinier de Graaf Hospital, Delft), Willem Jan Bos (St. Antonius Hospital, Nieuwegein), Adry Diepenbroek (University Medical Center Groningen, Groningen), Marielle Emmelot-Vonk (University Medical Center Utrecht, Utrecht), Casper Franssen (University Medical Center Groningen, Groningen), Carlo Gaillard (University Medical Center Utrecht, Utrecht), Nel Groeneweg-Peeters (Reinier de Graaf Hospital, Delft), Bettie Hoekstra (Maasstad Hospital, Rotterdam), Nienke Hommes (Haaglanden Medical Center, The Hague), Francoise Hoornaar (St. Antonius Hospital, Nieuwegein), Hanneke Joosten (Maastricht University Medical Center +, Maastricht), Joep Lagro (Haga Hospital, The Hague), Elisabeth Litjens (Maastricht University Medical Center +, Maastricht), Femke Molenaar (University Medical Center Utrecht, Utrecht), Simon Mooijaart (Leiden University Medical Center), Aegida Neradova (Amsterdam University Medical Center, Dianet, Amsterdam), Mike Peters (University Medical Center Utrecht, Utrecht), Michelle Troost (Reinier de Graaf Hospital, Delft), Wilma Veldman (University Medical Center Groningen, Groningen), Carlijn Voorend (Leiden University Medical Center), Lidwien Westerbos (Amsterdam University Medical Center, Amsterdam), Carlijne Westerman-van der Wijden (Haaglanden Medical Center, The Hague), Judith Wierdsma (University Medical Center Utrecht, Utrecht)
Authors’ Full Names and Academic Degrees
Carlijn G.N. Voorend, MSc, Noeleen C. Berkhout-Byrne, MANP, Leti van Bodegom-Vos, PhD, Adry Diepenbroek, MANP, Casper F.M. Franssen, MD, PhD, Hanneke Joosten, MD, PhD, Simon P. Mooijaart, MD, PhD, Willem Jan W. Bos, MD, PhD, and Marjolijn van Buren, MD, PhD, on behalf of the POLDER study group
Authors’ Contributions
Research idea and study design: NB, AD, HJ, CF, SM, WB, and MB; data acquisition: CV, NB, and AD; data analysis/interpretation: CV, LB, SM, WB, and MB; statistical analysis: CV; supervision or mentorship: MB, WB, and SM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by the Dutch Kidney Foundation (grant number AD3P04); CV was supported by the Nephrosearch Foundation. The sponsors did not have any role in the study design; in the collection, analysis, and interpretation of the data; the writing of the report; and in the decision to submit the article for publication.
Financial Disclosure
CV was supported by Nephrosearch Foundation. WB received grant support from Zilveren Kruis during conduct of the study, outside of the submitted work. The remaining authors declare that they have no relevant financial interests.
Acknowledgments
We are grateful to all patients and health care professionals who participated in this study.
Peer Review
Received July 20, 2023. Evaluated by 4 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form January 26, 2024.
Footnotes
Complete author and article information provided before references.
Figure S1: Integration of NGA in routine care: visualization of the mixed methods approach using of normalization construct scores and open-ended questions
Item S1: Visualization of summary of individuals’ NGA results (file 1; put this title above the headline “Geriatric assessment scores and leave the logo in there).
Item S2: Post-implementation survey distributed among health-professionals; translated from Dutch
Table S1: Constructs of integration in work routines, assessed with the NoMAD tool (file 3)
Table S2: Implementation outcomes per center (file 3)
Table S3: Completeness of NGA instruments (file 3)
Table S4: Determinants of implementation identified in qualitative analysis (file 3)
Table S5: Outcomes of qualitative analysis on the relevance of each NGA instrument
Contributor Information
Carlijn G.N. Voorend, Email: c.g.n.voorend@lumc.nl.
POLDER study group:
Arjan van Alphen, Noeleen Berkhout-Byrne, Fenna van Breda, Marjolijn van Buren, Henk Boom, Willem Jan Bos, Adry Diepenbroek, Marielle Emmelot-Vonk, Casper Franssen, Carlo Gaillard, Nel Groeneweg-Peeters, Bettie Hoekstra, Nienke Hommes, Francoise Hoornaar, Hanneke Joosten, Joep Lagro, Elisabeth Litjens, Femke Molenaar, Simon Mooijaart, Aegida Neradova, Mike Peters, Michelle Troost, Wilma Veldman, Carlijn Voorend, Lidwien Westerbos, Carlijne Westerman-van der Wijden, and Judith Wierdsma
Supplementary Materials
Figure S1; Item S1-S2; Tables S1-S5.
References
- 1.Xie Y., Bowe B., Mokdad A.H., et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 2.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto N.A., van Loon I.N., Morpey M.I., et al. Geriatric assessment in elderly patients with end-stage kidney disease. Nephron. 2019;141(1):41–48. doi: 10.1159/000494222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novais T., Pongan E., Gervais F., et al. Pretransplant comprehensive geriatric assessment in older patients with advanced chronic kidney disease. Nephron. 2021;145(6):692–701. doi: 10.1159/000517342. [DOI] [PubMed] [Google Scholar]
- 5.Račić M., Petković N., Bogićević K., et al. Comprehensive geriatric assessment: comparison of elderly hemodialysis patients and primary care patients. Ren Fail. 2015;37(7):1126–1131. doi: 10.3109/0886022X.2015.1057459. [DOI] [PubMed] [Google Scholar]
- 6.van Loon I.N., Wouters T.R., Boereboom F.T., Bots M.L., Verhaar M.C., Hamaker M.E. The relevance of geriatric impairments in patients starting dialysis: a systematic review. Clin J Am Soc Nephrol. 2016;11(7):1245–1259. doi: 10.2215/CJN.06660615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallenberg M.H., Kleinveld H.A., Dekker F.W., et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD-a systematic review. Clin J Am Soc Nephrol. 2016;11(9):1624–1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nederlandse Internisten Vereniging / Federatie van Medisch Specialisten [Dutch Society of Internal Medicine / Dutch Association of Medical Specialists] Richtlijn Nierfunctievervangende behandeling [Guideline on Renal Replacement Therapy] Kennisinstituut van de Federatie van Medisch Specialisten. 2016 https://richtlijnendatabase.nl/richtlijn/nierfunctievervangende_behandeling/nierfunctievervangende_behandeling_-_startpagina.html [Google Scholar]
- 9.Turner G., Clegg A., British Geriatrics Society, Age UK, Royal College of General Practioners Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43(6):744–747. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 10.Oud F.M.M., de Rooij S.E.J.A., Arends A.J., et al. Meetinstrumenten bij kwetsbare ouderen: een pleidooi voor meer standaardisatie [Assessment instruments in frail older patients; a call for more standardisation] Ned Tijdschr Geneeskd. 2019;163:D3267. [in Dutch] [PubMed] [Google Scholar]
- 11.Voorend C.G.N., Joosten H., Berkhout-Byrne N.C., et al. Design of a consensus-based geriatric assessment tailored for older chronic kidney disease patients: results of a pragmatic approach. Eur Geriatr Med. 2021;12(5):931–942. doi: 10.1007/s41999-021-00498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Loon I.N., Goto N.A., Boereboom F.T.J., Bots M.L., Verhaar M.C., Hamaker M.E. Frailty screening tools for elderly patients incident to dialysis. Clin J Am Soc Nephrol. 2017;12(9):1480–1488. doi: 10.2215/CJN.11801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall R.K., Haines C., Gorbatkin S.M., et al. Incorporating geriatric assessment into a nephrology clinic: preliminary data from two models of care. J Am Geriatr Soc. 2016;64(10):2154–2158. doi: 10.1111/jgs.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown E.A., Farrington K. Geriatric assessment in advanced kidney disease. Clin J Am Soc Nephrol. 2019;14(7):1091–1093. doi: 10.2215/CJN.14771218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nixon A.C., Brown J., Brotherton A., et al. Implementation of a frailty screening programme and Geriatric Assessment Service in a nephrology centre: a quality improvement project. J Nephrol. 2021;34(4):1215–1224. doi: 10.1007/s40620-020-00878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulla A., Wright P.N., Ross L.E., et al. Proceedings From the Symposium on Kidney Disease in Older People: Royal Society of Medicine, London, January 19, 2017. Gerontol Geriatr Med. 2017;3 doi: 10.1177/2333721417736858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox S.T., Janda M., Hubbard R. Understanding how comprehensive geriatric assessment works: the importance of varied methodological approaches. Aging Clin Exp Res. 2023;35(2):417–423. doi: 10.1007/s40520-022-02305-7. [DOI] [PubMed] [Google Scholar]
- 18.Berkhout-Byrne N., Kallenberg M.H., Gaasbeek A., et al. The Cognitive decline in Older Patients with End stage renal disease (COPE) study – rationale and design. Curr Med Res Opin. 2017;33(11):2057–2064. doi: 10.1080/03007995.2017.1341404. [DOI] [PubMed] [Google Scholar]
- 19.Katz S., Ford A.B., Moskowitz R.W., Jackson B.A., Jaffe M.W. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 20.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 21.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Katzman R., Brown T., Fuld P., Peck A., Schechter R., Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 23.Jolles J., Houx P., van Boxtel M., Ponds R.W.H.M. The Maastricht Aging Study: Determinants of Cognitive Aging. In (vol 192), Maastricht, Neuropsych Publishers, 1995. https://breinweb.nl/maas/assets/maas_pb_intro.pdf
- 24.Whooley M.A., Avins A.L., Miranda J., Browner W.S. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12(7):439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yesavage J.A., Sheikh J.I. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165–173. [Google Scholar]
- 26.Scheier M.F., Carver C.S., Bridges M.W. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 27.Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Weisbord S.D., Fried L.F., Arnold R.M., et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 2004;27(3):226–240. doi: 10.1016/j.jpainsymman.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Moss A.H., Ganjoo J., Sharma S., et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384. doi: 10.2215/CJN.00940208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 32.Ottery F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(1 suppl):S15–S19. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 33.de Boer A.H., Oudijk D., Timmermans J.M., Pot A.M. Ervaren belasting door mantelzorg; constructie van de EDIZ-plus [Self perceived burden from informal care: construction of the EDIZ-plus] Tijdschr Gerontol Geriatr. 2012;43(2):77–88. doi: 10.1007/s12439-012-0010-4. [in Dutch] [DOI] [PubMed] [Google Scholar]
- 34.Voorend C.G.N., Berkhout-Byrne N.C., Meuleman Y., Mooijaart S.P., Bos W.J.W., van Buren M. Perspectives and experiences of patients and healthcare professionals with geriatric assessment in chronic kidney disease: a qualitative study. BMC Nephrol. 2021;22(1):9. doi: 10.1186/s12882-020-02206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vis C., Ruwaard J., Finch T., et al. Toward an objective assessment of implementation processes for innovations in health care: psychometric evaluation of the Normalization Measure Development (NoMAD) questionnaire among mental health care professionals. J Med Internet Res. 2019;21(2) doi: 10.2196/12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapley T., Girling M., Mair F.S., et al. Improving the normalization of complex interventions: part 1 - development of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT) BMC Med Res Methodol. 2018;18(1):133. doi: 10.1186/s12874-018-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott J., Finch T., Bevan M., et al. Acute kidney injury electronic alerts: mixed methods Normalisation Process Theory evaluation of their implementation into secondary care in England. BMJ Open. 2019;9(12) doi: 10.1136/bmjopen-2019-032925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsang J.Y., Blakeman T., Hegarty J., Humphreys J., Harvey G. Understanding the implementation of interventions to improve the management of chronic kidney disease in primary care: a rapid realist review. Implement Sci. 2016;11:47. doi: 10.1186/s13012-016-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finch T.L., Girling M., May C.R., et al. Improving the normalization of complex interventions: part 2 - validation of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT) BMC Med Res Methodol. 2018;18(1):135. doi: 10.1186/s12874-018-0591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogrinc G., Davies L., Goodman D., Batalden P., Davidoff F., Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma A., Minh Duc N.T., Luu Lam Thang T., et al. A consensus-based Checklist for Reporting of Survey Studies (CROSS) J Gen Intern Med. 2021;36(10):3179–3187. doi: 10.1007/s11606-021-06737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sattar S., Alibhai S.M., Wildiers H., Puts M.T. How to implement a geriatric assessment in your clinical practice. Oncologist. 2014;19(10):1056–1068. doi: 10.1634/theoncologist.2014-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker S.G., McLeod A., McCue P., et al. New horizons in comprehensive geriatric assessment. Age Ageing. 2017;46(5):713–721. doi: 10.1093/ageing/afx104. [DOI] [PubMed] [Google Scholar]
- 44.McKenzie G.A.G., Bullock A.F., Greenley S.L., Lind M.J., Johnson M.J., Pearson M. Implementation of geriatric assessment in oncology settings: a systematic realist review. J Geriatr Oncol. 2021;12(1):22–33. doi: 10.1016/j.jgo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Kenis C., Heeren P., Decoster L., et al. A Belgian survey on geriatric assessment in oncology focusing on large-scale implementation and related barriers and facilitators. J Nutr Health Aging. 2016;20(1):60–70. doi: 10.1007/s12603-016-0677-2. [DOI] [PubMed] [Google Scholar]
- 46.Parlevliet J.L., Buurman B.M., Pannekeet M.M., et al. Systematic comprehensive geriatric assessment in elderly patients on chronic dialysis: a cross-sectional comparative and feasibility study. BMC Nephrol. 2012;13:30. doi: 10.1186/1471-2369-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown E.A., Bargman J.M., Li P.K. Managing older patients on peritoneal dialysis. Perit Dial Int. 2015;35(6):609–611. doi: 10.3747/pdi.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Loon I.N., Boereboom F.T., Bots M.L., Verhaar M.C., Hamaker M.E. A national survey on the decision-making process of dialysis initiation in elderly patients. Neth J Med. 2015;73(5):227–235. [PubMed] [Google Scholar]
- 49.Aucella F., Brunori G., Dalmartello M., et al. Assessment of the geriatric competence and perceived needs of Italian nephrologists: an internet survey. J Nephrol. 2016;29(3):385–390. doi: 10.1007/s40620-015-0232-y. [DOI] [PubMed] [Google Scholar]
- 50.Hall R., Rutledge J., Colón-Emeric C., Fish L.J. Unmet needs of older adults receiving in-center hemodialysis: a qualitative needs assessment. Kidney Med. 2020;2(5):543–551.e1. doi: 10.1016/j.xkme.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall R.K., McAdams-DeMarco M.A. Breaking the cycle of functional decline in older dialysis patients. Semin Dial. 2018;31(5):462–467. doi: 10.1111/sdi.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chadborn N.H., Goodman C., Zubair M., et al. Role of comprehensive geriatric assessment in healthcare of older people in UK care homes: realist review. BMJ Open. 2019;9(4) doi: 10.1136/bmjopen-2018-026921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohile S.G., Epstein R.M., Hurria A., et al. Communication with older patients with cancer using geriatric assessment: a cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020;6(2):196–204. doi: 10.1001/jamaoncol.2019.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurria A., Akiba C., Kim J., et al. Reliability, validity, and feasibility of a computer-based geriatric assessment for older adults with cancer. J Oncol Pract. 2016;12(12):e1025–e1034. doi: 10.1200/JOP.2016.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang R., Low H., McDonald A., Park G., Song X. Web-based software applications for frailty assessment in older adults: a scoping review of current status with insights into future development. BMC Geriatr. 2021;21(1):723. doi: 10.1186/s12877-021-02660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mannheim I., Schwartz E., Xi W., et al. Inclusion of older adults in the research and design of digital technology. Int J Environm Res Public Health. 2019;16(19):3718. doi: 10.3390/ijerph16193718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franzen S., European Consortium on Cross-Cultural Neuropsychology (ECCroN) Watermeyer T.J., et al. Cross-cultural neuropsychological assessment in Europe: position statement of the European Consortium on Cross-Cultural Neuropsychology (ECCroN) Clin Neuropsychol. 2022;36(3):546–557. doi: 10.1080/13854046.2021.1981456. [DOI] [PubMed] [Google Scholar]
- 58.Farrington K., Covic A., Nistor I., et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrol Dial Transplant. 2017;32(1):9–16. doi: 10.1093/ndt/gfw411. [DOI] [PubMed] [Google Scholar]
- 59.Kumarasinghe A.P., Chakera A., Chan K., et al. Incorporating the Clinical Frailty Scale into routine outpatient nephrology practice: an observational study of feasibility and associations. Intern Med J. 2021;51(8):1269–1277. doi: 10.1111/imj.14892. [DOI] [PubMed] [Google Scholar]
- 60.Drew D.A., Tighiouart H., Rollins J., et al. Evaluation of screening tests for cognitive impairment in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2020;31(4):855–864. doi: 10.1681/ASN.2019100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiffin-Richards F.E., Costa A.S., Holschbach B., et al. The Montreal Cognitive Assessment (MoCA) - a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S.H., Cho A., Min Y.K., Lee Y.K., Jung S. Comparison of the montreal cognitive assessment and the mini-mental state examination as screening tests in hemodialysis patients without symptoms. Ren Fail. 2018;40(1):323–330. doi: 10.1080/0886022X.2018.1455589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.To T.H.M., Soo W.K., Lane H., et al. Utilisation of geriatric assessment in oncology - a survey of Australian medical oncologists. J Geriatr Oncol. 2019;10(2):216–221. doi: 10.1016/j.jgo.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 64.van Oevelen M., Abrahams A.C., Bos W.J.W., et al. DIALysis or not: Outcomes in older kidney patients with GerIatriC Assessment (DIALOGICA): rationale and design. BMC Nephrol. 2021;22(1):39. doi: 10.1186/s12882-021-02235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trailbureau Zorgevaluatie Leading the Change DIALOGICA study. 2023. https://www.zorgevaluatienederland.nl/evaluations/dialogica
- 66.Logan B., Viecelli A.K., Johnson D.W., et al. Study protocol for The GOAL Trial: comprehensive geriatric assessment for frail older people with chronic kidney disease to increase attainment of patient-identified goals-a cluster randomised controlled trial. Trials. 2023;24(1):365. doi: 10.1186/s13063-023-07363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamaker M., Lund C., Te Molder M., et al. Geriatric assessment in the management of older patients with cancer - a systematic review (update) J Geriatr Oncol. 2022;13(6):761–777. doi: 10.1016/j.jgo.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Eamer G., Taheri A., Chen S.S., et al. Comprehensive geriatric assessment for older people admitted to a surgical service. Cochrane Database Syst Rev. 2018;1(1) doi: 10.1002/14651858.CD012485.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Item S1-S2; Tables S1-S5.