Key Clinical Message
A 46‐year‐old woman with congenitally corrected transposition of the great arteries (ccTGA) associated with dextrocardia, situs viscerus inversus, and left superior vena cava persistence presented with an incessant supraventricular tachycardia. Electrophysiological study was not conclusive in differential diagnosis of atrial tachycardia versus atypical atrioventricular (AV) nodal reentrant tachycardia, also due to the unconventional anatomy of the coronary sinus. By a comprehensive mapping of cardiac chambers, a double side slow‐pathway was localized in both atrial chambers and subsequently ablated by radiofrequency delivery without tachycardia changes. Aortic root and cusps were devoid of electrical activity. The muscular part of the sub‐pulmonary ventricle at the level of interatrial septum showed an earliest activation signal of −90 ms and ablation of this site was effective in abolish the tachycardia. This is the first case to report technical concerns of septal atrial tachycardia ablation in ccTGA associated with multiple anatomical malformations. Moreover, some peculiarities have been reported for the first time including the presence of double‐side AV nodal slow‐pathway and atypical localization of the tachycardia origin into the muscular part of the sub‐pulmonary ventricle instead of posterior pulmonary cusp.
Keywords: atrial tachycardia, catheter ablation, congenitally corrected transposition of great arteries, situs viscerus inversus
1. INTRODUCTION
Atrial tachycardia (AT) originating from the interatrial septum in patients with congenitally corrected transposition of the great arteries (ccTGA) have been previously reported. However, no cases of catheter ablation in patients with ccTGA and associated dextrocardia, situs viscerus inversus, and left superior vena cava persistence have been reported so far. Furthermore, several variants of the conduction system in these patients have been described. We present a unique case of catheter ablation of anterior interatrial septum AT from the muscular part of the sub‐pulmonary ventricle after ablation failure of double‐side slow pathway and left sided atrioventricular (AV) node. A 46‐year‐old woman with congenitally corrected L‐transposition of the great arteries (ccTGA) associated to dextrocardia, situs viscerus inversus, and left superior vena cava persistence presented with frequent palpitations. Her symptoms occurred sporadically over the past year, but had become nearly incessant over the past 2 months.
2. METHODS
The 12‐lead electrocardiograms (ECGs) with right precordial leads showed a regular supraventricular tachycardia with hidden P waves that sporadically appeared with a morphology similar to sinus ones during lengthening of RR cycle (slowing of tachycardia versus transient return to sinus rhythm) (Figure 1). The patient's palpitations were refractory to high‐dose beta‐blocker, and she was referred to the electrophysiology laboratory for evaluation. Preprocedural echocardiography and cardiac computer tomography confirmed the above reported cardiac malformations, characterized by double discordance between AV and ventriculo‐arterial position. Ventricular septal defect, subvalvular and/or valvular pulmonary stenosis, or abnormalities of the systemic (tricuspid) AV valve were excluded. Prior to the electrophysiological (EP) study, a decapolar catheter was advanced into the coronary sinus. An incessant supraventricular tachycardia with a cycle length of 370 ms and 1:1 conduction was easily induced by both programmed atrial pacing and atrial burst. Ventricular‐atrial (VA) interval was >70 ms, with concentric activation. Diagnostic maneuvers including ventricular overdrive pacing demonstrated constant VA conduction with brief AV dissociation at the end of the maneuver. Ventricular premature stimuli demonstrated absence of resetting of the tachycardia, with ventricular premature beats that pre excited the atria. Administration of ev adenosine caused transitory termination of the tachycardia. Since EP study was not conclusive in differential diagnosis between AT and atypical AV nodal reentrant tachycardia (AVNRT), electroanatomical mapping of the tachycardia was performed starting from the right atrium. A multielectrode catheter (PentaRay NAV, 2‐6‐2–mm spacing; Biosense Webster) was used. Activation mapping was performed using a stable intracardiac electrogram at the proximal coronary sinus as reference (approximately equal timing as the onset of the P wave). Right atrial (RA) activation map showed an earliest activation site (EAS) of −50 ms in the region of antero‐superior atrial septum. No His bundle signals were found. Through a 3.5‐mm, deflectable, irrigated catheter (NaviStar ThemoCool SmartTouch; Biosense Webster), radiofrequency (RF) applications using local impedance and force at 30W power were delivered with induction of several irritative junctional beats and constant retro‐conduction through fast‐pathway. However, the same tachycardia returned shortly afterward, and additional lesions at anterior RA septum did not affect the tachycardia. Through fluoroscopy‐guided transeptal puncture (using a Brockenbrough needle and a SL0 sheath, St Jude Medical), left atrial (LA) has been accessed and mapped demonstrating similar EAS in the region of antero‐superior atrial septum, in a mirror position to the EAS detected in RA. His bundle signal was found located in an antero‐inferior septal position. An RF protocol similar to previous was delivered and irritative junctional beats constantly retro‐conducted to the atrium were induced. Again, no changes in tachycardia occurred.
FIGURE 1.
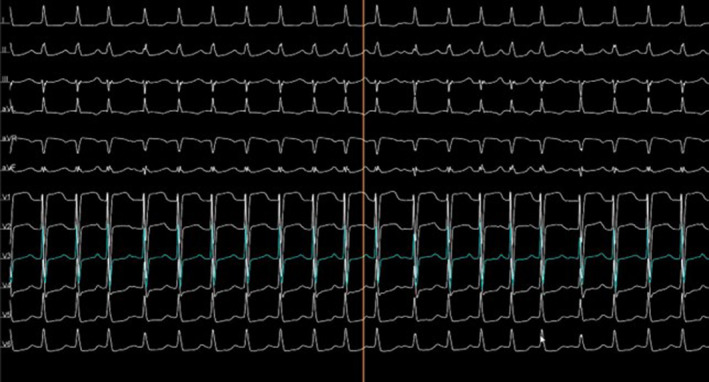
Electrocardiogram of the tachycardia.
3. CONCLUSION
Since double‐sided ablation of slow pathway had no effect on tachycardia, the suspected diagnosis oriented to anterior septum AT. Via retrograde aortic pathway, local activation map of aortic root was performed without evidence of local signals. Electroanatomic map also showed discrete distance between interatrial septum and posterior aortic root (Video S1). Therefore, right heart was approached again. Mapping catheter was then advanced through the sub‐pulmonary ventricle and mapping of the posterior, sub‐pulmonary cusp region evidenced a site of very EAS, characterized by QS at unipolar and presystolic (−90 ms), fragmented signal at bipolar map. RF delivery this site resulted in immediate termination of the AT within 4 s (Video S2). Additional lesions were delivered at the same site to consolidate the ablation (total RF ablation time of 3 min). The AT remained non inducible despite aggressive programmed stimulation, burst pacing, and isoproterenol infusion. Figure 2 illustrates critical parts of the procedure timeline. The patient was discharged home the following day with no recurrent arrhythmias over 1 year.
FIGURE 2.
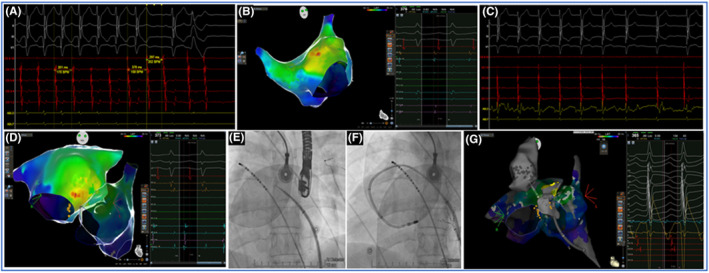
Timeline of the electrophysiological (EP) procedure. (Panel A) EP study: concentric coronary sinus activation and interruption of the tachycardia after adenosine bolus; (Panel B) Electronatomical map of the right atrium with earliest activation site (EAS) at the interatrial septum and without signals of conduction system; (Panel C) Irritative junctional rhythm during radiofrequency (RF) delivery at the EAS; (Panel D) Electronatomical map of the left atrium with EAS at the interatrial septum and with signals of conduction system; (Panel E) Fluoroscopic frame of transeptal puncture and radiological orientation of coronary sinus catheter; (Panel F) Fluoroscopic frame of ablation catheter position during RF delivery at the left side of interatrial septum; (Panel G) Earliest activation site at the sub‐pulmonary ventricle, site of tachycardia termination, and concomitant visualization of aortic root located anteriorly and with any signal of electrical activity.
4. DISCUSSION
This case report highlights some new concerns of patients with supraventricular arrhythmias affected by ccTGA: (1) The AV nodal slow pathway may be both right and left sided located, (2) the muscular part of the sub‐pulmonary ventricle may be the effective ablation site of an anterior interatrial septal AT instead of the posterior pulmonary sinus, (3) the persistence of left superior vena cava is related to an unconventional anatomical orientation of the coronary sinus (CS), which provide non negligible concerns in the reliability of the EP study and (4) aortic root is totally devoid of electrical signal due to the considerable distance from conduction system, (5) in cases of multiple associated malformations, an integrated use of imaging by fluoroscopy, electroanatomical mapping and if necessary intracardiac echocardiography may be helpful. Furthermore, two fundamental practical concerns of catheter ablation in patients with ccTGA are stressed: (1) the unique malformation of ccTGA makes the sub‐pulmonary ventricle a possible origin of AT instead of aorta, therefore excluding the need for retrograde aortic approach and (2) ccTGA patients often have altered anatomy of AV conduction system, therefore, electroanatomic map of both atria is usually suggested. By electroanatomical mapping we demonstrated the close anatomic relationship between the sub‐pulmonary ventricle and the interatrial septum. EAS of this site was significantly earlier than the atrial septal ones. So far, four previous reports have highlighted the origin of septal AT from posterior pulmonary valve synus in patients with ccTGA. 1 , 2 , 3 , 4 In these small case series, an EAS from −40 to −80 ms was localized into the pulmonary valve sinus. Double potentials were also noted. Compared with these patients, the origin of the AT of our case was more inferior and with marked earlier EAS. Although the arrhythmia showed a “focal” activation pattern, the termination with the use of adenosine favored a micro‐re‐entrant or triggered activity mechanism. To the best of our knowledge, this is the first case which report a double‐sided slow‐pathway ablation at antero‐superior atrial septum which failed to terminate an adenosine‐sensitive tachycardia in a patient with ccTGA. Interesting, EAS recorded from atrial septum coincided with slow‐pathway fibers but not with the anatomical origin of the tachycardia. Recently, the superior slow pathway, a variant of the slow pathway originating from the compact node and extending superiorly outside of Koch's triangle, has been reported both in the general population 5 and in a ccTGA patient. 6 Another report of unconventional location of the slow pathway in patients with ccTGA have been described, but with unsuccessful ablation of the tachycardia despite bipolar ablation. 7 The AV conduction system variability in ccTGA has been well described and numerous variants have been recognized such as twin AV nodes and His extension anterior to membranous septum, 8 , 9 Of particular interest, in our case we did not find conduction system signals beyond LA despite an extensive mapping of the atrial chambers and sub‐pulmonary ventricle. The absence of His bundle signals, which are fundamental landmarks during a tachycardia mapping, significantly increased the complexity of the procedure.
In conclusion, in patients with these characteristics, a careful mapping of all structures in close proximity to interatrial septum is crucial to identify the origin of the AT as well as to appreciate the variability in the conduction system.
AUTHOR CONTRIBUTIONS
Alberto Preda: Writing – original draft. Alessio Testoni: Methodology. Matteo Baroni: Software. Patrizio Mazzone: Supervision. Lorenzo Gigli: Resources; validation; writing – review and editing.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Supporting information
Video S1
Video S2
Data S1
ACKNOWLEDGMENTS
None.
Preda A, Testoni A, Baroni M, Mazzone P, Gigli L. Atrial tachycardia ablation through the sub‐pulmonary ventricle in a patient with multiple malformations associated with congenitally corrected transposition of the great arteries and double‐sided slow‐pathway. Clin Case Rep. 2024;12:e8745. doi: 10.1002/ccr3.8745
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request to the corresponding author.
REFERENCES
- 1. Cheung CC, Kim E, Tseng ZH, et al. Atrial tachycardia ablation at the pulmonic valve in a patient with congenitally corrected transposition of great arteries. JACC Clin Electrophysiol. 2021;7:1473‐1481. doi: 10.1016/j.jacep.2021.06.017 [DOI] [PubMed] [Google Scholar]
- 2. Guo XG, Liao Z, Sun Q, et al. Mapping and ablation of anteroseptal atrial tachycardia in patients with congenitally corrected transposition of the great arteries: implication of pulmonary sinus cusps. Europace. 2017;19:2015‐2022. doi: 10.1093/europace/euw281 [DOI] [PubMed] [Google Scholar]
- 3. Jiang CX, Long DY, du X, et al. Atrial tachycardia eliminated at the ventricular side in patients with congenitally corrected transposition of the great arteries: electrophysiological findings and anatomical concerns. Heart Rhythm. 2020;17:1337‐1345. doi: 10.1016/j.hrthm.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 4. Roca‐Luque I, Rivas N, Francisco J, et al. Para‐Hisian atrial tachycardia ablation in a patient with congenitally corrected transposition of great vessels. HeartRhythm Case Rep. 2017;3:340‐343. doi: 10.1016/j.hrcr.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaneko Y, Naito S, Okishige K, et al. Atypical fast‐slow atrioventricular nodal reentrant tachycardia incorporating a “superior” slow pathway. Circulation. 2016;133:114‐123. doi: 10.1161/CIRCULATIONAHA.115.018443 [DOI] [PubMed] [Google Scholar]
- 6. Kawada S, Nishii N, Asada S, Nakagawa K, Morita H, Ito H. Successful ablation of a superior fast‐slow atrioventricular reentrant tachycardia in a patient with congenitally corrected transposition of great arteries. HeartRhythm Case Rep. 2021;7:698‐701. doi: 10.1016/j.hrcr.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Upadhyay S, Marie Valente A, Triedman JK, Walsh EP. Catheter ablation for atrioventricular nodal reentrant tachycardia in patients with congenital heart disease. Heart Rhythm. 2016;13:1228‐1237. doi: 10.1016/j.hrthm.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 8. Anderson RH, Becker AE, Arnold R, Wilkinson JL. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911‐923. doi: 10.1161/01.cir.50.5.911 [DOI] [PubMed] [Google Scholar]
- 9. Baruteau AE, Abrams DJ, Ho SY, Thambo JB, McLeod CJ, Shah MJ. Cardiac conduction system in congenitally corrected transposition of the great arteries and its clinical relevance. J Am Heart Assoc. 2017;6:e007759. doi: 10.1161/jaha.117.007759 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Data S1
Data Availability Statement
The data that support the findings of this study are available on request to the corresponding author.


