Abstract
C57BL/6 mice infected with mouse hepatitis virus strain JHM (MHV-JHM) develop a chronic demyelinating encephalomyelitis several weeks after inoculation. Previously, we showed that mutations in the immunodominant CD8 T-cell epitope (S-510-518) could be detected in nearly all samples of RNA and virus isolated from these mice. These mutations abrogated recognition by T cells harvested from the central nervous systems of infected mice in direct ex vivo cytotoxicity assays. These results suggested that cytotoxic T-lymphocyte (CTL) escape mutants contributed to virus amplification and the development of clinical disease in mice infected with wild-type virus. In the present study, the importance of these mutations was further evaluated by infecting naive mice with MHV-JHM variants isolated from infected mice and in which epitope S-510-518 was mutated. Compared to mice infected with wild-type virus, variant virus-infected animals showed higher mortality and morbidity manifested by decreased weight gain and neurological signs. Although a delay in the kinetics of virus clearance has been demonstrated in previous studies of CTL escape mutants, this is the first illustration of significant changes in clinical disease resulting from infection with viruses able to evade the CD8 T-cell immune response.
Cytotoxic T-lymphocyte (CTL) escape mutants have been identified in several viral infections and appear to be most important when the CTL response is strong and functionally monospecific (10, 19, 25). Such mutants have been detected in human patients infected with human immunodeficiency virus type 1 (HIV-1), hepatitis B virus, hepatitis C virus, and Epstein-Barr virus (3, 4, 10, 12, 18, 19, 25, 36, 38, 45, 47). CTL escape mutants have also been identified in animal models of viral infections. CTL escape mutants were first identified in mice transgenic for a T-cell receptor for lymphocytic choriomeningitis virus that were infected with this virus (37). In later studies, wild-type mice were infected with virus mutated in one or more of the immunodominant CTL epitopes (23, 29). Infection with mutated virus did not result in increased morbidity or mortality, although the kinetics of virus clearance was delayed. These studies in humans and experimental animals suggest but do not prove that CTL escape mutants result in disease progression or significantly change the outcome of the infection in question.
In previous reports, we showed that viral CTL escape mutants were selected in C57BL/6 (B6) mice infected with wild-type mouse hepatitis virus strain JHM (MHV-JHM) (34, 35). In the model used in those experiments, suckling B6 mice were infected intranasally with MHV-JHM at 10 days of age. To prevent the fatal acute encephalitis, pups were nursed by dams previously immunized against the virus. Mice infected with wild-type MHV-JHM remained asymptomatic during the early stages of the disease process, but 40 to 90% developed a chronic demyelinating disease with clinical signs of hind limb paralysis several weeks after inoculation (33). Virus was readily isolated from symptomatic mice but not from those that remained asymptomatic (33). Previously, we and others showed that two CD8 T-cell epitopes within the surface (S) glycoprotein of the virus were recognized by CTLs in B6 mice (2, 7). The more immunodominant of the two, encompassing amino acids 510 to 518 of the S glycoprotein (S-510-518 [CSLWNG PHL]), was mutated in nearly all samples of virus isolated from symptomatic mice (34). Epitope S-510-518 is encoded by a region of the S gene prone to deletion and single-base mutation (1, 7, 31, 40), and this may enhance the likelihood of development of CTL escape mutants. Mutations in residues 2 to 7 of the epitope have been detected, although only a single nucleotide change was usually present in the RNA isolated from an individual animal (34, 35). These mutations abrogated recognition by CTLs isolated from the central nervous systems (CNS) of MHV-infected mice and assayed in direct ex vivo cytotoxicity assays (34). Mutations were not detected in the RNA encoding the less dominant CTL epitope encompassing residues 598 to 605 of the S glycoprotein (S-598-605 [RCQI F ANI]) or in the RNA flanking either of these epitopes. Mutations arose at early times after infection and were only rarely detected in mice that did not develop clinical disease (34, 35). From these results, we concluded that the development of CTL escape mutants was necessary but not sufficient for the expression of clinical disease in B6 mice.
Although antiviral CD8 T cells are critical for clearance of MHV (11, 15, 46) and variant virus was most likely selected by escape from the immune response, it was not possible to determine definitively from those experiments whether CTL escape variants contributed to virus persistence or arose as a consequence of persistence. If CTL escape mutants are a major factor in virus persistence, infection of naive mice with MHV-JHM mutated in epitope S-510-518 (variant virus) under the same experimental conditions as described above could change the balance between the pathogen and host and thereby provides an excellent model for determining whether infection with CTL escape mutants can cause more rapid disease progression and a worse outcome.
MATERIALS AND METHODS
Animals.
MHV-seronegative 5- or 6-week-old B6 and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and Harlan Sprague Dawley (Indianapolis, Ind.). Mice were inoculated intranasally with 4 × 104 PFU of wild-type or variant virus. Animals were monitored daily for clinical disease, and weights were determined every 4 to 6 days until sacrifice.
Cells.
BALB/c 17Cl-1 cells were grown in Dulbecco modified Eagle medium supplemented with 5% fetal calf serum, 5% tryptose phosphate, and antibiotics. EL-4 (H-2b) and MC57 (H-2b) cells were grown in RPMI medium supplemented with 10% fetal calf serum and antibiotics.
Viruses.
MHV-JHM, used in all studies, was grown and titers were determined as previously described (33). MHV-JHM with mutations in epitope S-510-518 was harvested from chronically infected brains and spinal cords. Virus was plaque purified twice, and larger amounts were prepared by growth in 17Cl-1 cells. The presence of the desired mutations in S-510-518 was confirmed by sequencing as previously described (34). No mutations were detected in epitope S-598-605 or in the 200 nucleotides surrounding either epitope (34). Virus titers from infected brains and spinal cords were determined as previously described (33).
Recombinant vaccinia virus (VV) expressing the nucleocapsid (N) (VV-N), transmembrane (M) (VV-M), and S (VV-S) glycoproteins were constructed as described previously (28). A recombinant VV expressing the small membrane protein (E) (VV-E) was provided by J. Leibowitz, Texas A&M University. The N, M, and S genes were cloned behind the T7 promoter and required coinfection with vTF7.3 (kindly provided by B. Moss, National Institutes of Health) to provide T7 RNA polymerase. The E gene was under the control of an early-late VV promoter.
Isolation of mononuclear cells from the CNS.
Cells were isolated from the CNS of B6 mice as previously described (6). In brief, mice were perfused with phosphate-buffered saline, and brains were removed. Tissue was ground between frosted glass slides and triturated by vigorous pipetting in 5 ml of RPMI medium with 10% fetal calf serum. Following thorough dispersion of the tissue, Percoll (Pharmacia, Uppsala, Sweden) was added to a final concentration of 30%. The lysate was spun at 1,300 × g for 30 min at 4°C. The Percoll and lipid layers were aspirated, the cell pellet was washed twice, and the cells were counted.
Direct ex vivo cytoxicity assays with CNS-derived lymphocytes.
Mononuclear cells were harvested from the brains of B6 mice acutely infected with variant virus and analyzed in direct ex vivo cytotoxicity assays with EL-4 cells coated with the indicated peptides at a final concentration of 1 μM as previously described (7). The effector-to-target cell ratio was 50:1. Spontaneous release was <14%.
Histology and immunohistochemistry.
Mice were perfused with phosphate-buffered saline, and the brains and spinal cords were fixed in formalin prior to being embedded in paraffin. For histological examination, sections were cut, processed, and stained with luxol fast blue in order to detect areas of demyelination. For detection of viral antigen, sections were prepared, processed, and reacted with mouse monoclonal anti-MHV-JHM antibody (anti-N antibody 5B188.2, kindly provided by M. Buchmeier, Scripps Research Institute) as previously described (42). After incubation with primary antibodies, samples were incubated with biotinylated goat antimouse antibody (Jackson Immunoresearch Labs, West Grove, Pa.). Sections were then treated with avidin-conjugated horseradish peroxidase (Jackson Immunoresearch Labs), with 3,3′-diaminobenizidine as the final substrate. No staining was observed if CNS tissue from uninfected animals was processed with antibody to MHV-JHM or if irrelevant antibody was used as the primary antibody in the analysis of MHV-infected tissue.
Analysis of RNA by blot hybridization.
RNA was isolated from infected brains and spinal cords by the guanidinium isothiocyanate-cesium chloride method and analyzed by slot blot hybridization as previously described (32). Serial dilutions of each sample were analyzed, and radioactivity was counted by using a radioanalytic imaging detector (AMBIS [San Diego, Calif.] 4000). An average counts per minute per microgram was calculated for each RNA sample.
IFN-γ ELISPOT assays.
Gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed as described previously (48). Briefly, Immulon MaxiSorp plates (Nunc, Kamstrup, Denmark) were coated with 2 μg of anti-IFN-γ antibody (rat monoclonal R4-6A2; Pharmingen, San Diego, Calif.) per ml in 0.05 M carbonate buffer, pH 8.2. For antigen presentation, MC57 cells were either infected with VV-E or vTF7.3 or dually infected with vTF7.3 and VV-S, VV-N, or VV-M. MC57 cells express major histocompatibility complex (MHC) class I but not class II antigen and are useful stimulators for determining antigen-specific IFN-γ secretion by CD8 T cells. Previously, we showed by complement lysis prior to CTL assay that CD8 and not CD4 T cells responded to antigen presented by these cells (6). In each case, virus was used at a multiplicity of infection of 3. Mononuclear cells harvested from wild-type MHV-JHM- or variant MHV-JHM-infected mouse brains (2,500 to 50,000 cells/well) were mixed with irradiated, VV-infected MC57 cells and incubated for 36 h. ELISPOTs were developed by addition of polyclonal rabbit anti-mouse IFN-γ (kindly provided by J. Cowdery, University of Iowa). Following a 16-h incubation, samples were developed by sequential addition of alkaline phosphatase-conjugated donkey anti-rabbit immunoglobulin G (Jackson Immunoresearch Labs) and substrate (5-bromo-4-chloro-3-indoyl phosphate [Sigma, St. Louis, Mo.]) dissolved in 3% agarose in a phosphate-accepting 2-amino-2-methyl-1-propanol buffer prepared as previously described (41). ELISPOT-forming cells were directly counted under ×10 magnification with a dissecting microscope. Samples were analyzed by linear regression, and results were expressed as numbers of ELISPOTs per 100,000 cells.
Statistical analysis.
Statistical significance was determined as described in the figure legends. Analysis was performed with the help of the Biostatistics Core Facility at the University of Iowa.
RESULTS
Infection with variant virus results in increased mortality and decreased growth.
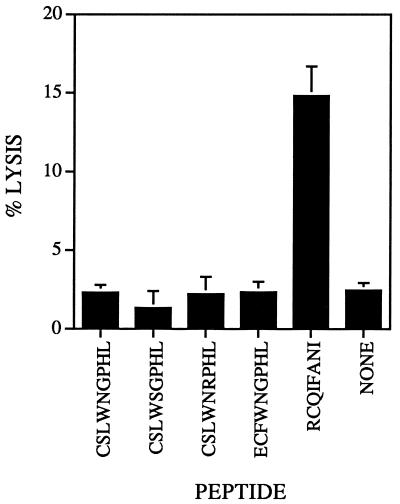
Virus mutated in epitope S-510-518 was isolated from four individual mice and prepared as described in Materials and Methods. The four isolates chosen for further study were mutated either in residues previously identified as the major or auxiliary anchors for binding to the MHC H-2Db molecule (CSLWSGPHL and CSRWNGPHL) or in a residue predicted to affect binding to the T-cell receptor (CSL W N R PHL) (17) or contained a three-nucleotide deletion (ECF W N G PHL). These mutations did not affect the ability of the virus to cause acute encephalitis in naive 6-week-old B6 mice (reference 32 and data not shown). Variant epitope S-510-518 was recognized only at high concentrations in CTL assays with lymphocytes derived from the CNS of mice with encephalitis caused by wild-type MHV-JHM (34). Acute infection with variant virus did not induce a CD8 T-cell response against either wild-type or variant epitope S-510-518 in direct ex vivo cytotoxicity assays with CNS-derived lymphocytes. As expected, epitope S-598-605, which is not mutated in variant virus, was still recognized in these assays (Fig. 1).
FIG. 1.
Recognition of epitope S-598-605 (RCQIFANI) but not wild-type (CSLWNGPHL) or variant (CSLWSGPHL, CSLWNRPHL, and ECFWNGPHL) S-510-518 epitopes in mice infected with variant virus. Lymphocytes harvested from the brains of mice with acute encephalitis were used in direct ex vivo cytotoxicity assays. Only peptide S-598-605 sensitized EL-4 cells for lysis above the background level (uncoated EL-4 cells [NONE]). Each peptide was analyzed in 3 to 14 independent experiments. The mean percent specific lysis for all experiments is shown. Bars show standard errors.
Next, the effect of these mutations on the ability of virus to persist was determined by infecting suckling mice nursed by dams immunized against MHV-JHM. Half of each litter was inoculated with wild-type virus and the other half was inoculated with variant virus to control for variability in the amount of protective antibody transmitted to the suckling mice. In preliminary experiments, we observed that mice infected with variant virus were often dead or moribund by 20 to 25 days postinoculation (p.i.). At this time, mice infected with wild-type virus are usually asymptomatic, although a few start to develop signs of hind limb paralysis. Therefore, to simplify the experimental design, all surviving mice, whether symptomatic or not, were sacrificed at 21 days p.i., and brains and spinal cords were analyzed for the presence of infectious virus or viral RNA and protein. In other models of MHV-induced disease, infectious virus is cleared by this time after inoculation (15, 16, 20, 21, 34).
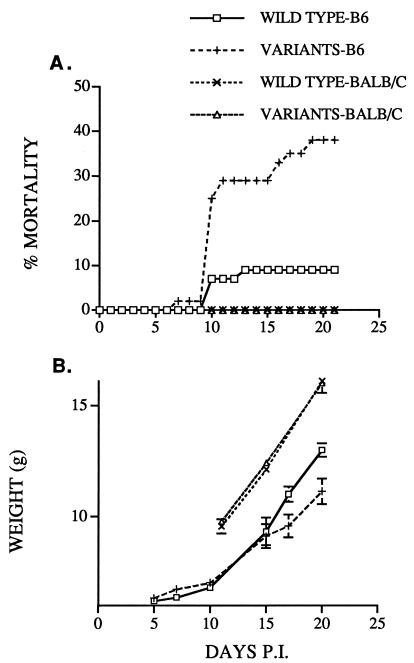
By 21 days p.i., significantly more mice infected with variant virus (38%) than mice infected with wild-type virus (9%) had died (Fig. 2A; Table 1). In addition, more of the variant virus-infected mice that survived to 21 days were symptomatic, with clinical signs of jitteriness, hind limb paresis, abnormal gait, and poor weight gain (Table 1). Pooled data for all of the mice infected with variant virus as well as data for mice infected with each variant virus are shown in Table 1. The weight gain for naive mice infected with wild-type or variant virus at 10 days of life is shown in Fig. 2B. Differences in growth became most apparent at 16 to 18 days p.i. By day 20, surviving mice infected with variant virus weighted 15% less than mice infected with wild-type virus (11.1 versus 13.0 g). As described above, this is the approximate time frame for virus clearance in other models of MHV-induced neurological disease, and differences would be evident at this time point if virus was cleared less efficiently in variant virus-infected mice.
FIG. 2.
Infection with variant virus results in increased mortality and morbidity. Suckling B6 mice were infected with wild-type MHV-JHM (45 mice) or variant virus (55 mice [17, 23, and 15 mice with virus encoding epitopes CSLWSGPHL, CSLWNRPHL, and ECFWNGPHL, respectively]). Also, suckling BALB/c mice were infected with wild-type virus (8 mice) or variant virus (11 mice [3 and 8 mice with virus encoding epitopes CSLWNRPHL and ECFWNGPHL, respectively]). Mice were nursed by dams previously immunized against MHV-JHM as described previously (33). All surviving mice were harvested at 21 days p.i. (A) Mice were monitored daily for mortality. The difference in survival between the two groups of B6 mice as analyzed by an accelerated-failure-time regression model with Weibull residual was statistically significant (P = 0.0069). (B) The same groups of mice were weighed every 4 to 6 days. Mean weights ± standard errors are shown. The pattern of weight gain diverged significantly (P ≤ 0.0001) between the two groups of B6 mice as determined by a mixed-model analysis of variance.
TABLE 1.
Summary of mortality and morbidity after infection with wild-type and variant MHV-JHMa
| Virus | No. of mice | No. (%)
|
||
|---|---|---|---|---|
| Dead | Symptomaticb | Asymptomaticb | ||
| Wild type | 45 | 4 (9) | 4 (9) | 37 (82) |
| Variants | ||||
| CSLWSGPHL | 17 | 4 (24) | 10 (58) | 3 (18) |
| CSLWRPHL | 23 | 10 (43) | 4 (19) | 9 (38) |
| ECFWNGPHL | 15 | 7 (47) | 7 (47) | 1 (6) |
| Totalc | 55 | 21 (38) | 21 (38) | 13 (24) |
A significant difference in outcome was observed between mice infected with wild-type virus and individual variants (for each, P < 0.001) and between mice infected with wild-type virus and the pooled group of variant viruses (P < 0.0001) when analyzed by Fisher’s exact test (two tailed).
All surviving mice were harvested at 21 days p.i.
Data for all mice infected with variant virus were pooled.
These differences in outcome occurred only in mice containing the MHC H-2Db allele. When the same experimental approach was used with BALB/c mice (H-2d), no difference in mortality or growth between mice infected with wild-type and variant viruses was observed (Fig. 2). Low levels of infectious virus could be isolated from the spinal cords of the same fraction of mice infected with either type of virus (4 of 8 mice infected with wild-type virus [geometric mean titer ± standard error = 2.73 ± 0.21] and 5 of 11 mice infected with variant virus [geometric mean titer ± standard error = 3.22 ± 0.17]).
Mice infected with variant virus develop a demyelinating encephalomyelitis.
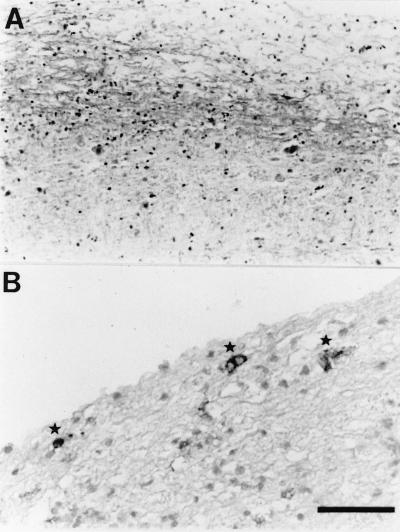
The hallmark of MHV-JHM-induced neurological disease is demyelination with concomitant inflammatory infiltration. To determine if similar pathological findings could be demonstrated in the CNS of mice infected with variant virus, brains and spinal cords were harvested and examined after staining for myelin with luxol fast blue. Large areas of demyelination were detected in the spinal cords of these animals, with extensive inflammatory infiltrates present in the vicinity of these lesions (Fig. 3A). Viral antigen was present in the white matter in areas with early signs of demyelination (Fig. 3B) and adjacent to regions of demyelination. Viral antigen was detected predominantly in the white matter, but a few MHV-positive cells were also identified in the gray matter of the spinal cord. These findings are indistinguishable from what is observed in mice with demyelination induced by wild-type virus and show that persistence of variant virus results in the same disease as occurs in mice infected with wild-type virus but in a higher percentage of animals and at earlier times p.i.
FIG. 3.
Infection with variant MHV-JHM results in a demyelinating encephalomyelitis. The brain and spinal cord from a mouse infected with virus encoding variant epitope CSLWSGPHL were harvested at 21 days p.i. The sample was fixed in formalin and embedded in paraffin. (A) Five-micrometer sections were cut, processed, and stained with luxol fast blue in order to detect areas of demyelination. (B) Sections (5 to 10 μm) were prepared and analyzed for viral antigen by using antibody to the N protein as described in Materials and Methods. Sections were lightly stained with hematoxylin after processing. Viral antigen (asterisks) is detected in the white matter adjacent to an area of demyelination. Bar, 100 μm (A) and 50 μm (B).
Virus is cleared more slowly in mice infected with variant virus.
The most likely explanation for these results is that variant MHV-JHM, which no longer encodes the immunodominant CTL epitope, is not cleared as efficiently as is wild-type virus. To determine if the increased mortality and morbidity described above correlated with a decrease in MHV clearance, virus titers in brains and spinal cords were measured at 21 days p.i. As shown in Table 2, virus was detected in the brains and the spinal cords of 48 and 74%, respectively, of mice infected with variant virus but in only 19 and 30%, respectively, of brains and spinal cords harvested from wild-type-infected mice. When only mice in which infectious virus could be detected were further analyzed, there were no significant differences in the titers of virus in the brain or spinal cord between mice infected with wild-type and variant viruses (Table 2).
TABLE 2.
Virus titers in mice surviving to 21 days p.i.a
| Virus(es) | No. of mice | No. (%) with virus detected in:
|
Virus titerb in:
|
||
|---|---|---|---|---|---|
| Brain | Spinal cord | Brain | Spinal cord | ||
| Wild type | 37 | 7 (19) | 11 (30) | 3.89 ± 0.32 | 3.61 ± 0.38 |
| Variants | 31 | 15 (48) | 23 (74) | 4.04 ± 0.16 | 3.89 ± 0.25 |
When wild-type virus- and variant virus-infected samples were compared, a significant difference was observed in the percentages of mice with virus detectable in the brain (P < 0.05) and spinal cord (P < 0.001) when analyzed by Fisher’s exact test (two tailed). No statistical difference in the level of titer (log transformed) was detected, however, when only those animals with virus detected were analyzed by t tests.
Virus titers are expressed as geometric mean titers (log10 PFU/gram ± standard error. Only samples positive for virus are included in these calculations.
MHV-JHM shows a specific tropism for the CNS, but other closely related strains of MHV, such as MHV-A59, also infect the liver. To determine if the loss of epitope S-510-518 changed viral tropism, livers, lungs, kidneys, and hearts were assayed for infectious virus. No virus could be detected in any of these organs from three moribund variant virus-infected mice harvested at 21 days p.i.
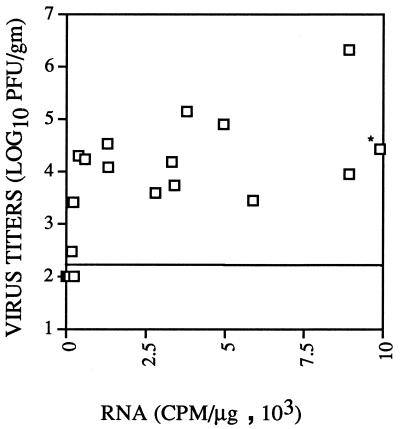
Although the presence of infectious virus is the best measure of virus persistence, high-level expression of viral RNA and protein in the absence of infectious virus could also result in clinical and pathological disease. This occurs in human patients with subacute sclerosing panencephalitis caused by a persistent measles virus infection (13). To determine if high levels of viral RNA could be detected in MHV-infected mice in the absence of infectious virus, viral RNA in the spinal cord was quantitated by slot blot analysis and compared to the virus titer in 29 animals. As shown in Fig. 4, mice with higher titers of infectious virus in general had higher levels of viral RNA, indicating that the presence of virus correlated with the viral burden in these animals. Some mice with detectable infectious virus had low levels of viral RNA, but in no animals were high levels of viral RNA detected in the absence of infectious virus. This correlation was true for mice infected with either wild-type or variant virus. These results show that the detection of infectious virus was a valid measure of virus persistence in these MHV-infected mice.
FIG. 4.
Levels of infectious virus and viral RNA in the spinal cords of infected mice are correlated. Levels of infectious virus and viral RNA were measured as described in Materials and Methods. Fifteen mice infected with wild-type MHV-JHM, six mice infected with the epitope CSLWSGPHL variant, and eight mice infected with the epitope ECFWNGPHL variant were used in these analyses. The limit of detection, 160 PFU/g of tissue, is indicated by the horizontal line. Infectious virus could not be detected in 14 mice (shown as a single square below the line of detection). Each sample of RNA was analyzed in two independent experiments, and the average of the two is shown. RNA levels were quantitated by using a radioanalytic imaging system. The RNA level in the sample marked with an asterisk was 4.49 × 104 cpm/μg.
No additional CTL epitopes are recognized in mice infected with variant virus.
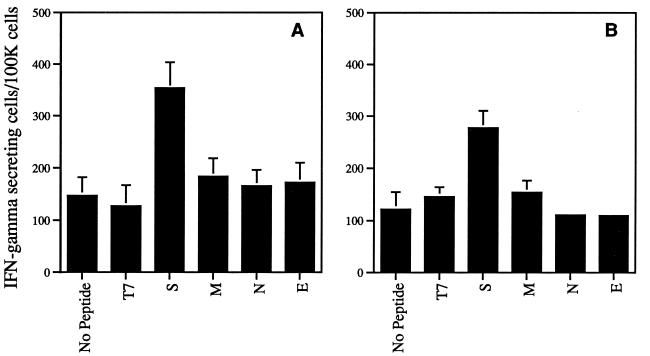
In humans infected with HIV-1, additional CTL epitopes are recognized after selection of mutations at a dominant CTL epitope occurs (25). Similarly, CTLs from mice infected with lymphocytic choriomenigitis virus in which all three CTL epitopes are mutated recognize a novel CTL epitope (23). To determine if additional CTL epitopes were recognized in mice infected with variant MHV-JHM, CNS-derived lymphocytes from mice with acute encephalitis were analyzed in direct ex vivo assays with target cells infected with recombinant VV expressing the four MHV structural proteins (S, N, M, or E). Samples were analyzed both for cytotoxicity and for IFN-γ secretory activity by using an ELISPOT assay. With both assays, only the S glycoprotein was recognized in mice infected with variant virus. The data for the IFN-γ ELISPOT assay are shown in Fig. 5. To determine further if additional epitopes were recognized by cells harvested from these mice, a panel of S-specific peptides matching the consensus motif for binding to the H-2Db and H-2Kb molecules (7) was analyzed in IFN-γ ELISPOT assays. In these assays, only S-598-605 stimulated cells to secrete IFN-γ (data not shown). These data suggest that no novel CTL epitopes are recognized in mice infected with variant virus.
FIG. 5.
IFN-γ secretion in response to VV-expressed MHV proteins by lymphocytes isolated from brains of mice with MHV-induced acute encephalitis. Four mice infected with wild-type MHV-JHM (A) and six mice infected with variant virus (four with the epitope CSRWNGPHL variant and two with the epitope CSLWSGPHL variant) (B) were used in these studies. The number of IFN-γ-secreting cells was determined by an ELISPOT assay as described in Materials and Methods. VV-infected MC57 cells expressing T7 RNA polymerase alone (T7) were used as a negative control for VV-infected MC57 cells expressing viral S, M, N, or E proteins. The data shown are the mean number of IFN-γ-secreting spots per 100,000 cells and standard error for each sample.
Mutations in epitope S-598-605 are not selected in mice persistently infected with variant virus.
Mutations in epitope S-510-518 but not epitope S-598-605 are selected in mice persistently infected with wild-type MHV-JHM. Since only S-598-605 is recognized by CTLs in mice acutely infected with variant virus and this epitope is also located in the hypervariable region of the S protein (7, 13, 31), it was possible that mutations in this epitope could be selected in persistently infected mice. To determine if such mutations were selected, infectious virus was isolated from six mice infected with variant virus (two each with CSLWSGPHL, CSLWNRPHL, and ECFWNGPHL), and the region encompassing this epitope was sequenced as described previously (34). Only wild-type sequence was detected, showing that further escape from CTL surveillance did not occur in these mice. As expected, sequence analysis of the RNA encompassing epitope S-510-518 revealed the presence of neither additional mutations nor reversion to wild-type sequence.
Above, we showed that low levels of infectious virus could be isolated at 21 days p.i. from BALB/c mice infected with wild-type or variant virus. In these mice, mutated epitope S-510-518 should confer no selective advantage, and reversion to the wild-type sequence would be predicted to occur. However, sequence analysis of virus isolated from five BALB/c mice infected with variant virus revealed the presence of only mutated epitope S-510-518, suggesting that, in fact, virus containing wild-type epitope S-510-518 did not have a significant selective advantage.
DISCUSSION
The unique finding in the present study is a clear demonstration of increased mortality and morbidity attributable to the loss of CTL recognition. MHV-JHM is a virulent strain of virus that causes a fatal acute encephalitis in the absence of experimental intervention (e.g., infection with attenuated virus or passive infusion of antiviral antibodies or T cells [16, 21]). The demyelinating disease observed in survivors is, in large part, immune mediated (15, 44). Thus, the outcome of a given infection is determined by the balance between the viral infection and the host immune response. Infection with MHV-JHM mutated in epitope S-510-518, which is able to avoid the dominant CTL response in B6 mice, decreases the kinetics of virus clearance and thereby changes the balance between the virus and the host and, consequently, the outcome of the infection. These results are consistent with our previous studies showing that the outgrowth of CTL escape mutants correlated with virus amplification and the development of clinical disease after infection with wild-type virus (34).
Of note is that the mechanism of MHV-induced demyelination has not been completely determined. Both CD4 and CD8 T cells are believed to be effector cells in this process (15, 16), and if MHV-induced demyelination is similar to that caused by Theiler’s encephalomyelitis virus, CD4 T cells are most important (26, 43). Also, CD8 T cells recognizing epitope S-598-605 were detected in variant virus-infected mice (Fig. 1). Thus, it is not surprising that demyelination occurs to the same extent in mice infected with wild-type virus and with virus lacking the immunodominant CD8 T-cell epitope.
Although the presence of CTL escape mutants has been clearly demonstrated in previous studies, it has been difficult to demonstrate their biological significance. First, CTL escape mutants have been demonstrated most commonly in viral infections of humans (19, 25). In most viral infections of humans, the CTL response is polyclonal and not focused on a single epitope. A monospecific CTL response is relatively uncommon in a genetically diverse population, and by extension, the frequency of CTL mutants developing in a large population is relatively low. However, such mutations have been reported. In New Guinea, a large fraction of the population express the HLA-A11 allele, and Epstein-Barr virus isolated from this population is mutated in an amino acid critical for recognition by virus-specific CTLs (9). Second, there is often a shift to subdominant CTL epitopes after the CTL response to a dominant epitope is evaded (epitope spreading) (22, 27). This was recently demonstrated by Borrow et al. in a study of a patient acutely infected with HIV-1 in which CTL escape mutants arose very early after infection (5). The virus load did not increase acutely, presumably because the response to new CTL epitopes was able to contain the infection. The shift to subdominant epitopes may result, however, in less effective control of the infection (30), and in the patient reported by Borrow et al. (5), the response to subdominant CTL epitopes was subsequently unable to prevent disease progression. Similarly, the response to epitope S-598-605 is unable to control the infection in mice persistently infected with MHV-JHM (34). Of note is that we have been unable thus far to detect a CTL response to any additional epitopes in mice infected with variant virus (Fig. 5).
While mortality and morbidity are increased after infection with MHV mutated in epitope S-510-518, virus is still cleared in a minority of animals, and these mice remain asymptomatic. In marked contrast, when mice in which MHC class I function is disrupted genetically [β2-microglobulin (−/−) mice] are infected with MHV in the model described above, no suckling mice survive the acute encephalitis. This is true even when large amounts of neutralizing antibody are delivered passively to each suckling mouse (unpublished observations). In addition, β2-microglobulin (−/−) mice are very susceptible to infection with attenuated strains of MHV-JHM and with the closely related strain MHV-A59 (11, 15). Thus, the antiviral CD8 T-cell response is critical for virus clearance.
In variant virus-infected mice that clear the infection, clearance may be mediated by CD8 T cells recognizing the less immunodominant CTL epitope, epitope S-598-605 (Fig. 1). Epitope S-598-605 is also likely to be the target for antiviral CTLs in mice infected with MHV-A59, since epitope S-510-518 is deleted in this virus (7, 24, 31). Although the precursor frequency for CD8 T cells recognizing S-598-605 in mice immunized with wild-type virus is similar to that for CD8 T cells recognizing epitope S-510-518 (8), peptide S-598-605 is 50- to 200-fold less potent at sensitizing targets for lysis (7). This relative lack of potency may explain why MHV mutated in epitope S-510-518 is able to persist in most mice in the presence of a CTL response to epitope S-598-605. This lack of potency may also explain why virus mutated in epitope S-598-605 does not develop. CTL escape mutants develop peferentially when the T-cell response is strong (10, 39), and the response to the epitope may be too weak to select for viral mutants. In support of this, ex vivo proliferation of CTLs responsive to epitope S-598-605 can be detected only when stimulators are coated with peptide S-598-605 and not when antigen is presented endogenously (8), suggesting that the ligand density for these CTLs on most cells is too low for recognition.
Alternatively, clearance of variant virus may be mediated by components of the immune system other than CD8 T cells. For example, anti-MHV cytotoxic CD4 T cells have been demonstrated in B6 mice infected with MHV-A59 (14). Although antiviral cytotoxic CD4 T cells have not been demonstrated in mice infected with MHV-JHM, these cells could well contribute to clearance in animals infected with variant virus.
ACKNOWLEDGMENTS
We thank G. Wu, M. Dailey, J. Harty, and M. Stoltzfus for critical review of the manuscript.
This research was supported in part by grants from the National Institutes of Health (NS 36592) and from the National Multiple Sclerosis Society (RG2864-A-2).
REFERENCES
- 1.Banner L, Keck J G, Lai M M C. A clustering of RNA recombination sites adjacent to a hypervariable region of the peplomer gene of murine coronavirus. Virology. 1990;175:548–555. doi: 10.1016/0042-6822(90)90439-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann C C, Yao Q, Lin M, Stohlman S A. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted CTL response. J Gen Virol. 1996;77:315–325. doi: 10.1099/0022-1317-77-2-315. [DOI] [PubMed] [Google Scholar]
- 3.Bertoletti A, Costanzo A, Chisari F V, Levero M, Artini M, Sette A, Penna A, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J Exp Med. 1994;180:933–943. doi: 10.1084/jem.180.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Wei X, Horwitz M, Peffer N, Meyers H, Nelson J A, Gairin J, Hahn B, Oldstone M B A, Shaw G. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape mutants. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 6.Castro R F, Evans G D, Jaszewski A, Perlman S. Coronavirus-induced demyelination occurs in the presence of virus-specific cytotoxic T cells. Virology. 1994;200:733–743. doi: 10.1006/viro.1994.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro R F, Perlman S. CD8+ T-cell epitopes within the surface glycoprotein of a neurotropic coronavirus and correlation with pathogenicity. J Virol. 1995;69:8127–8131. doi: 10.1128/jvi.69.12.8127-8131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro R F, Perlman S. Differential antigen recognition by T cells from the spleen and central nervous system of coronavirus-infected mice. Virology. 1996;222:247–251. doi: 10.1006/viro.1996.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Campos-Lima P O, Levitsky V, Brooks J, Lee S P, Hu L, Rickinson A B, Masucci M G. T cell responses and virus evolution: loss of HLA A11-restricted CTL epitopes in Epstein-Barr virus isolates from highly A11-positive populations by selective mutation of anchor residues. J Exp Med. 1994;179:1297–1305. doi: 10.1084/jem.179.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco A, Ferrari C, Sette A, Chisari F V. Viral mutations, TCR antagonism and escape from the immune response. Curr Opin Immunol. 1995;7:524–531. doi: 10.1016/0952-7915(95)80098-0. [DOI] [PubMed] [Google Scholar]
- 11.Gombold J, Sutherland R, Lavi E, Paterson Y, Weiss S R. Mouse hepatitis virus A59-induced demyelination can occur in the absence of CD8+ T cells. Microb Pathog. 1995;18:211–221. doi: 10.1016/S0882-4010(95)90058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulder P, Phillips R, Colbert R, McAdam S, Ogg G, Nowak M, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 13.Hall W, Choppin P W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis. N Engl J Med. 1981;304:1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- 14.Heemskerk M, Schoemaker H, Spaan W, Boog C. Predominance of MHC class II-restricted CD4+ cytotoxic T cells against mouse hepatitis virus A59. Immunology. 1995;84:521–527. [PMC free article] [PubMed] [Google Scholar]
- 15.Houtman J J, Fleming J O. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol. 1996;2:101–110. doi: 10.3109/13550289609146543. [DOI] [PubMed] [Google Scholar]
- 16.Houtman J J, Fleming J O. Pathogenesis of mouse hepatitis virus-induced demyelination. J Neurovirol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- 17.Hudrisier D, Mazarguil H, Oldstone M B A, Gairin J E. Relative implication of peptide residues in binding to major histocompatibility complex class I H-2Db: application to the design of high-affinity, allele-specific peptides. Mol Immunol. 1995;32:895–907. doi: 10.1016/0161-5890(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 18.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A, Glangrande P, Phillips R E, McMichael A J. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 gag variants. Nature. 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 19.Koup R. Virus escape from CTL recognition. J Exp Med. 1994;180:779–782. doi: 10.1084/jem.180.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyuwa S, Stohlman S A. Pathogenesis of a neurotropic murine coronavirus, strain JHM in the central nervous system of mice. Semin Virol. 1990;1:273–280. [Google Scholar]
- 21.Lane T E, Buchmeier M J. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann P, Forsthuber T, Miller A, Sercarz E E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 23.Lewicki H, Von Herrath M, Evans C, Whitton J L, Oldstone M. CTL escape viral variants. II. Biologic activity in vivo. Virology. 1995;211:443–450. doi: 10.1006/viro.1995.1426. [DOI] [PubMed] [Google Scholar]
- 24.Luytjes W, Sturman L S, Bredenbeek P J, Charite J, van der Zeijst B A M, Horzinek M C, Spaan W J M. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology. 1987;161:479–487. doi: 10.1016/0042-6822(87)90142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael A J, Phillips R E. Escape of human immunodeficiency virus from immune control. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 26.Miller S D, Karpus W J. The immunopathogenesis and regulation of T-cell-mediated demyelinating diseases. Immunol Today. 1994;15:356–361. doi: 10.1016/0167-5699(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 27.Miller S D, Vanderlugt C, Begolka W, Pao W, Yauch R, Neville K, Katz-Levy Y, Carrizosa A, Kim B. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 28.Mobley J, Evans G, Dailey M O, Perlman S. Immune response to a murine coronavirus: identification of a homing receptor-negative CD4+ T cell subset that responds to viral glycoproteins. Virology. 1992;187:443–452. doi: 10.1016/0042-6822(92)90446-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskophidis D, Zinkernagel R M. Immunobiology of cytotoxic T-cell escape mutants of lymphocytic choriomeningitis virus. J Virol. 1995;69:2187–2193. doi: 10.1128/jvi.69.4.2187-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak M A, May R M, Phillips R E, Rowland-Jones S, Lalloo D G, McAdam S, Klenerman P, Koppe B, Sigmund K, Bangham C R M, McMichael A J. Antigenic oscillations and shifting immunodominance in HIV-1 infections. Nature. 1995;375:606–611. doi: 10.1038/375606a0. [DOI] [PubMed] [Google Scholar]
- 31.Parker S E, Gallagher T M, Buchmeier M J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989;173:664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perlman S, Jacobsen G, Olson A L, Afifi A. Identification of the spinal cord as a major site of persistence during chronic infection with a murine coronavirus. Virology. 1990;175:418–426. doi: 10.1016/0042-6822(90)90426-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman S, Schelper R, Bolger E, Ries D. Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb Pathog. 1987;2:185–194. doi: 10.1016/0882-4010(87)90020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pewe L, Wu G, Barnett E M, Castro R, Perlman S. Cytotoxic T cell-resistant variants are selected in a virus-induced demyelinating disease. Immunity. 1996;5:253–262. doi: 10.1016/s1074-7613(00)80320-9. [DOI] [PubMed] [Google Scholar]
- 35.Pewe L, Xue S, Perlman S. Cytotoxic T-cell-resistant variants arise at early times after infection in C57BL/6 but not in SCID mice infected with a neurotropic coronavirus. J Virol. 1997;71:7640–7647. doi: 10.1128/jvi.71.10.7640-7647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips R E, Rowland-Jones S, Nixon D F, Gotch F M, Edwards J P, Ogunlesi A O, Elvin J G, Rothbard J A, Bangham C R M, Rizza C R, McMichael A J. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 37.Pircher H, Moskophidis D, Rohrer U, Burki K, Hengartner H, Zinkernagel R. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 38.Price D, Goulder P, Klenerman P, Sewell A, Easterbrook P, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehermann B, Pasquinelli C, Mosier S, Chisari F. Hepatitis B virus (HBV) sequence variation in cytotoxic T lymphocyte epitopes is not common in patients with chronic HBV infection. J Clin Invest. 1995;96:1527–1534. doi: 10.1172/JCI118191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe C L, Baker S C, Nathan M J, Fleming J O. Evolution of mouse hepatitis virus: detection and characterization of S1 deletion variants during persistent infection. J Virol. 1997;71:2959–2967. doi: 10.1128/jvi.71.4.2959-2969.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedgwick J D, Holt P G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;57:301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 42.Sun N, Grzybicki D, Castro R, Murphy S, Perlman S. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213:482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunoda I, Fujinami R S. Two models of multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:672–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Stohlman S A, Fleming J O. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol. 1990;30:31–41. doi: 10.1016/0165-5728(90)90050-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiner A, Erickson A, Kanospon J, Crawford K, Muchmore E, Hughes A, Houghton M, Walker C M. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci USA. 1995;92:2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson J S, Stohlman S A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolinsky S M, Korber B, Neumann A U, Daniels M, Kunstman K, Whetsell A, Furtado M, Cao Y, Ho D, Safrit J, Koup R. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 48.Xue S, Perlman S. Antigen specificity of CD4 T cell response in the central nervous system of mice infected with mouse hepatitis virus. Virology. 1997;238:68–78. doi: 10.1006/viro.1997.8819. [DOI] [PubMed] [Google Scholar]