Abstract
Introduction
To assess the efficacy and safety of the combination of microblepharoexfoliation (MBE), intense pulse light (IPL) and meibomian gland expression (MGX) for treatment of meibomian gland dysfunction (MGD).
Methods
This was a prospective, parallel-control trial conducted from April 2022 to January 2023. Participants were assigned to receive either three sessions of MBE-IPL-MGX treatment and home-based therapy (treatment group) or home-based therapy alone (control group). Outcome measures were assessed at baseline and after 2-month follow-up.
Results
Seventy eyes of 70 patients were enrolled. MBE-IPL-MGX treatment achieved better improvements than home-based therapy in ocular surface disease index (OSDI) and symptom assessment in dry eye (SANDE) scores, noninvasive tear film break-up time (NIBUT), lipid layer grade (LLG), loss area meibomian gland (LAMG) and meibomian gland yielding secretion score (MGYSS). The mean differences between the two groups were as follows: OSDI (– 11.23 ± 4.68 points, P < 0.001), SANDE (– 24.63 ± 13.41 points, P < 0.001), NIBUT (1.3 ± 1.57 s, P = 0.033), LLG (0.4 ± 0.04 points, P = 0.003), LAMG (– 2.85 ± 1.69%, P = 0.023) and MGYSS (7.5 ± 2.32 points, P < 0.001). In addition, the increment (Δ) of MGYSS after MBE-IPL-MGX treatment was significantly higher in MGD grades 2 and 3 (all P < 0.001).
Conclusions
MBE-IPL-MGX treatment is an effective and well-tolerated procedure that improves dry eye symptoms and signs as well as meibomian gland secretions in patients with MGD. In addition, this treatment is recommended for MGD grades 2 and 3.
Keywords: Microblepharoexfoliation, Intense pulse light, Meibomian gland expression, Meibomian gland dysfunction, Dry eye disease
Key Summary Points
| Why carry out this study? |
| Dry eye disease (DED) therapies targeting specific mechanisms involved in meibomian gland dysfunction (MGD), such as microblepharoexfoliation (MBE), intense pulse light (IPL) and meibomian gland expression (MGX), have been shown to be safe and effective on an individual basis. However, the effects of combining these therapies do not appear to be sufficiently studied. |
| This study evaluates the efficacy and safety of MBE-IPL-MGX combination treatment in patients with DED owing to MGD. |
| What was learned from the study? |
| MBE-IPL-MGX combination treatment improves dry eye symptoms and signs as well as meibomian gland function compared to control group. |
| Our findings suggest that three sessions of this combined treatment are effective and safe for the treatment of MGD, especially in the most severe stages. |
Introduction
Dry eye disease (DED) is a chronic multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, which is accompanied by ocular symptoms caused by hyperosmolarity, ocular surface inflammation and neurosensory abnormalities [1, 2]. DED prevalence varies according to the definition used and the characteristics of the population studied, but it ranges from 5 to 50%, is more frequent in women and increases with age [3]. Currently, evidence suggests that all forms of DED have an evaporative component because without evaporation hyperosmolarity cannot occur [4].
Meibomian gland dysfunction (MGD) is the most common etiology of tear film evaporation, and it is characterized by the obstruction of meibomian glands due to terminal duct obstruction and/or changes in meibum quality and quantity [4, 5]. The management and therapy subcommittee of the Tear Film and Ocular Surface (TFOS) Dry Eye Workshop (DEWS) II recommended intense pulsed light (IPL) as a second-step therapy for MGD when education, lid hygiene, warm compresses and ocular lubrications do not achieve the desired effect [6]. This type of light therapy utilizes flash lamps to emit high-intensity, noncoherent, polychromatic light within the 500 to 1200 nm wavelength spectrum [7]. This targeted range excludes potentially harmful ultraviolet radiation below 500 nm [8]. In 2022, Toyos et al. [9] observed improvements in DED symptoms of patients with rosacea after IPL treatment. In addition, subsequent studies have affirmed the efficacy and safety of IPL in alleviating the symptoms and signs of DED associated with MGD [9–15]. IPL primarily operates through thermal action. The energy from IPL is absorbed by hemoglobin, leading to thrombosis in abnormal blood vessels [9]. This process decreases the levels of inflammatory mediators in the eyelids and meibomian glands, ultimately avoiding their dysfunction and enhancing meibum flow [16].
Combined or additional therapies have emerged in MGD treatment such as microblepharoexfoliation (MBE). MBE is a novel in-office treatment that works by exfoliating the eyelid margins to remove accumulated biofilm debris, epithelial keratinization, and capped meibomian glands, resulting in better meibum outflow [17]. Different studies have shown that MBE improves DED symptoms [18, 19], demodex blepharitis [18–21] and meibomian gland function [19, 20, 22]. In addition, meta-analyses recommend combining IPL with meibomian gland expression (MGX) compared to IPL or MGX alone [23–25]. However, to the best of our knowledge there is a lack of studies evaluating the benefits of the MBE, IPL and MGX combination.
Consequently, the purpose of the current study is to evaluate whether the combined treatment of MBE-IPL-MGX leads to an improvement of symptoms and signs in patients with DED due to MGD.
Methods
This prospective, monocentric, unmasked, parallel-control group study (NCT05857579) was approved by the clinical research ethics committee of the University of Murcia (ID: 4097/2022), adhered to the tenets of the Declaration of Helsinki and was performed at the Novovision Ophthalmology Clinic from April 2022 to January 2023. Informed consent was obtained from each patient before enrollment in the study.
Subjects
Patients with DED due to MGD attending Novovision Ophthalmology Clinic (Murcia, Spain) were enrolled. The inclusion criteria were as follows: (1) age ≥ 18 years old; (2) DED diagnosis according to DEWS II [26] meeting one of the following conditions: (2.1) ocular surface disease index (OSDI) score ≥ 13; (2.2) NIBUT < 10 s; and (2.3) ocular surface staining with > 5 or 9 corneal or conjunctival stains, respectively, and (3) MGD diagnosis according to the international workshop on MGD [27] meeting two of the following conditions: (3.1) irregularity of the eyelid margin or mucocutaneous junction; (3.2) vascularity of the eyelid margin; (3.3) plugged or capped meibomian gland orifices; (3.4) meibomian gland atrophy; (3.5) decreased meibum quality and quantity. Exclusion criteria included: (1) skin pathologies that prevent IPL treatment; (2) all corneal disorders that affect diagnostic tests, such as: (2.1) active corneal infections and (2.2) corneal dystrophies; (3) active ocular allergy; (4) pregnant or lactating women; (5) patients who did not understand or comprehend the informed consent. Systemic or ocular diseases, previous systemic or ocular treatments and ocular surgeries with > 6 months of postoperative evolution were not considered exclusion criteria to better reflect the patient population. Contact lens users were instructed not to wear their contact lenses 1 week before baseline and follow-up examinations.
Experimental Design
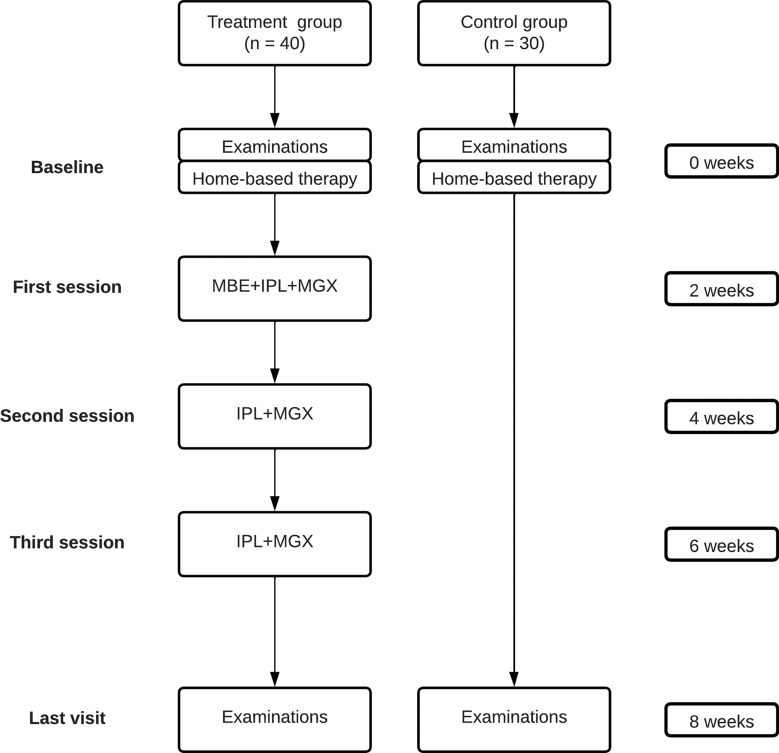
The experimental design of the study is presented in Fig. 1. Patients who met the inclusion criteria were classified into treatment and control groups by an independent investigator (unrelated to the study) in a non-randomized manner. All patients received home therapy based on Therapearl eye mask warming compress (Bausch & Lomb, Madrid, Spain) two times per day and Eyestil synfo eyedrops (Sifi Iberica SL, Madrid, Spain) four times per day during the study, including the follow-up period. Treatment group patients also underwent a series of three combined treatment sessions of MBE-IPL-MGX at 2-week intervals. All patients underwent a 2-month follow-up from baseline.
Fig. 1.
Flowchart of interventions and follow-up protocol for the control and treatment groups. Each patient received home-based therapy based on warming compress twice a day and artificial tears four times a day until the last visit. Treatment group patients also underwent a series of three combined treatment sessions of MBE, IPL and MGX at 2-week intervals. Clinical assessment was performed in both groups at baseline and 8 weeks after home-based therapy was prescribed
Clinical Assessment
Parameters were assessed with the S390L Firefly WDR slit-lamp (Shangai Mediworks Precision Instruments Co. Shangai, China), which includes a dry eye module designed to perform objective and non-invasive measures, which are automatically analyzed by an artificial intelligence (AI) identification system (Mediview R3.0 software), thus, ensuring the non-intervention of the observer in the evaluation of the measurements. To assess treatment efficacy, parameters were measured at baseline and the last visit. DED symptoms were assessed with the ocular surface disease index (OSDI) and symptoms assessment in dry eye (SANDE) questionnaires. Ocular surface measurement was performed by one examiner (ABS) in the sequence proposed by Ballesteros-Sánchez et al.[28] to best preserve the integrity of the tear film to avoid affecting the test results. One eye was randomly selected using an online randomizer program http://www.randomization.com. To evaluate treatment safety, adverse events (AEs) were reported.
Tear Film Stability and Volume
Tear film stability was automatically evaluated via detection of the first (F-NIBUT) and average noninvasive tear film break-up time (A-NIBUT) using a Placido disc. To assess the lipid layer grade (LLG), the lipid layer interferometric pattern was compared with the lipid layer thickness (LLT) grading scale template provided by the device, which has the following values: 1, LLT < 30 nm; 2, LLT of 30–60 nm; 3, LLT of 60–80 nm; 4, LLT > 80 nm. Regarding tear volume, tear meniscus height (TMH) and tear meniscus area (TMA) were also automatically assessed through focused image of the lower eyelids.
Ocular Hyperemia
Ocular hyperemia was assessed through complete picture of the ocular surface focused on the bulbar conjunctiva. Nasal ciliary hyperemia (NCIH), temporal ciliary hyperemia (TCIH), nasal conjunctival hyperemia (NCOH) and temporal conjunctival hyperemia (TCOH) were automatically analyzed with a value between 0% (no hyperemia) and 100% (the highest level of hyperemia).
Ocular Surface Staining
OSS was subjectively and invasively evaluated with the Oxford grading schema reported by Bron et al. [29]. Prior to assessing OSS, a single drop of unit dose saline was instilled onto a fluorescein impregnated strip. The lower right lid was then pulled down and the strip was tapped onto the lower tarsal conjunctiva. The same procedure was performed on the left. A cobalt-blue filter with yellow Kodak Wratten 12 barrier filter was used for better detection of fluorescein staining.
Meibomian Gland Analysis
Meibomian gland analysis was performed on the upper and lower eyelids using infrared light. The AI identification system automatically analyzed the meibomian glands, obtaining the upper loss area meibomian gland (U-LAMG) and lower loss area meibomian gland (L-LAMG) with a value between 0% (no glandular dropout) and 100% (the highest level of glandular dropout), and upper meibomian gland dysfunction grade (U-MGD grade) and lower meibomian gland dysfunction grade (L-MGD grade), which have the following values: 0, no MGD; 1, mild MGD; 2, moderate MGD; 3, severe MGD.
Meibomian gland secretion was assessed by MGX. Fifteen glands on the lower eyelids were evaluated. For each gland, the secretion had the following scores: 0, no secretion; 1, inspissated/toothpaste consistency; 2, cloudy liquid secretion; 3, clear liquid secretion. Then, three meibomian gland parameters were assessed: meibomian gland yielding secretion score (MGYSS) (range: 0–45), which was defined as the sum of the grades for all 15 glands, meibomian gland yielding clear secretion (MGYCS) (range: 0–15) and meibomian gland yielding liquid secretion (MGYLS) (range: 0–15) [30].
MBE-IPL-MGX Combined Treatment
MBE was performed with a 1.80-mm-diameter medical-grade diamond bur attached to a low-speed electrically driven hand piece (Karl Storz, St Louis, MO, USA) [31]. To ensure a well-tolerated procedure, topical application of 0.1% tetracaine hydrochloride and 0.4% oxybuprocaine hydrochloride (Novartis, Barcelona, Spain) was applied. After placing topical anesthetic, a corneal shield was used to protect the ocular surface, and a jojoba anesthetic ointment (JAO) (O'Brien Pharmacy, Kansas City, MO, USA) containing 8% lidocaine and 25% jojoba wax was placed on the lid margin. Patients underwent MBE on the upper and lower lid margin of both eyes at 500 rpm until complete removal of accumulated biofilm debris, epithelial keratinization or capped meibomian glands. MBE was performed only in the first combined treatment session. Immediately after MBE, JAO was cleaned with a cotton swab and IPL was performed.
IPL treatment was carried out with Thermaeye Plus (MDS Medical Technologies SL, Barcelona, Spain). The procedure began by applying an ultrasound gel (Carmado SL, Alicante, Spain) to the patient’s periocular areas and upper eyelids. In the periocular areas, six light pulses were applied: four light pulses on the skin below the lower eyelid (with handpiece placed horizontally in the first pass and second pass) and two light pulses on the canthal area (with handpiece placed vertically in the first and second pass). The parameters were as follows: (1) filter: 650 nm; (2) fluence: 8 j/cm2; (3) pulses: 2; (4) duration: 3 ms; (5) delay: 20 ms; (6) cooling: 70%. In the upper eyelids, four light pulses were applied: two light pulses in the first and second pass, respectively. The parameters were as follows: (1) filter: 650 nm; (2) fluence: 5 j/cm²; (3) pulses: 1; (4) duration: 3 ms; (5) cooling: 70% [32]. Fitzpatrick skin typing was assessed prior to IPL treatment [33]. IPL parameters were not adjusted for each patient because this device has been shown to be safe in all skin types on the Fitzpatrick scale [32].
Finally, the MGX was performed on both upper and lower eyelids of each eye with Collins forceps (Medi Instrument Inc, New York, NY, USA). After the first combined treatment session, patients were instructed to apply 0.5% dexamethasone sodium phosphate two times per day for 5 days. White Sun protection cream was recommended for the first 48 h in the IPL treatment area.
Statistical Analysis
Statistical analyses were performed with SPSS statistics software, version 26.0 (IBM Corp., Armonk, NY, USA). The sample size was estimated using the GRANMO calculator, version 7.12 (Municipal Institute of Medical Research, Barcelona, Spain). It was calculated based on assumed mean differences in F-NIBUT and MGYSS between the treatment and control groups at 2 months after the treatment onset, with values of 3.04 ± 3.86 and 19.75 ± 5.45, respectively. These assumed differences were based on the findings of a pilot study with 16 eyes of 8 patients in each group. With these assumptions, a sample size of 26 eyes per group would yield a power > 80% and a statistically significant paired difference of 95% confidence. Continuous variables were displayed as the mean ± standard deviation (SD) with interquartile ranges [IQRs], while ordinal categorical variables were expressed as frequencies (n) and percentages (%). After testing for normality and homogeneity of variance, the paired Student’s t-test (parametric) or Wilcoxon’s signed-rank test (nonparametric) was performed to compare intra-group clinical outcomes. Within each group, the increment (Δ) was calculated. It was defined as the change from the last visit (LV) to baseline (B) “Δ = LV – B.” Inter-group clinical outcomes were analyzed with the unpaired Student’s t-test (parametric) or Mann-Whitney’s U test (nonparametric). Between each group, the differences were calculated as “ΔTreatment group – ΔControl group.” The Pearson’s (parametric) or Spearman’s rho correlation coefficient (nonparametric) was used to analyze the correlations between the variables. Stepwise multiple linear regression analysis was performed to detect the influential factors in dry eye symptoms (ΔOSDI and ΔSANDE). In addition, one-way ANOVA (parametric) was performed to determine whether there were statistically significant differences in ΔMGYSS according to baseline L-MGD-grade. A post hoc analysis by Bonferroni’s test was carried out to determine statistically significant differences between baseline L-MGD grade. The level of significance was P < 0.05 for all comparisons.
Results
Patient characteristics are shown in Table 1. Seventy eyes of 70 patients, 14 (20%) men and 56 (80%) women with a mean age of 58.64 ± 12.9 [28 to 87] years, were enrolled in the study. No significant differences in demographic characteristics, systemic/ocular diseases and clinical parameters such as OSDI score and MGD grade were detected between the two groups at baseline. In addition, all patients completed the study.
Table 1.
Baseline characteristics
| Characteristics | Control group (n = 30) | Treatment group (n = 40) | P |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 60.10 (12.4) | 57.18 (13.34) | 0.353b |
| Sex, male/female (%) | 3 (10) / 27 (90) | 11 (27.5) / 29 (72.5) | 0.07c |
| Race, Caucasian (%) | 30 (100) | 40 (100) | - |
| Fitzpatrick skin type | |||
| I | 3 (7.5) | 5 (12.5) | 0.621c |
| II | 8 (26.7) | 11 (27.5) | 0.331c |
| III | 15 (50) | 22 (55) | 0.247c |
| IV | 4 (13.3) | 2 (5) | 0.784c |
| Related to DED | |||
| OSDI, mean ± SD | 43.46 (21.54) | 42.71 (21.43) | 0.885b |
| MGD grade, mean ± SD* | 2 (0.41) | 2.1 (0.44) | 0.099b |
| Contact lens wearer, n (%) | 13 (43.4) | 17 (42.5) | 0.944c |
| Refractive surgery, n (%) | 7 (23.3) | 6 (15) | 0.375c |
| Cataracts surgery, n (%) | 15 (50) | 15 (37.5) | 0.296c |
| Systemic disease, n (%) | |||
| Arterial hypertension | 7 (23.3) | 12 (30) | 0.535c |
| Diabetes mellitus | 1 (3.3) | 1 (2.5) | 0.836c |
| Rheumatoid arthritis | 7 (23.3) | 6 (15) | 0.375c |
| Rosacea | 2 (6.7) | 2 (5) | 0.766c |
| Sjögren's syndrome | 0 (0) | 1 (2.5) | 0.383c |
| Thyroid disease | 10 (33.3) | 7 (17.5) | 0.126c |
| Medications, n (%) | |||
| Antidepressants | 10 (33.3) | 9 (22.5) | 0.313c |
| Anxiolytics | 10 (33.3) | 9 (22.5) | 0.313c |
| Arterial hypertension drugs | 7 (23.3) | 12 (30) | 0.535c |
| Antihistamines | 1 (3.3) | 7 (17.5) | 0.065c |
| Antineoplastic drugs | 3 (10) | 1 (2.5) | 0.181c |
| Hormonal therapy | 4 (13.3) | 9 (22.5) | 0.329c |
| Diuretics | 8 (26.7) | 2 (5) | 0.355c |
| Isotretinoin | 1 (3.3) | 2 (5) | 0.733c |
DED dry eye disease, MGD meibomian gland dysfunction, OSDI ocular surface disease index (values from 0 to 100), SD standard deviation
aExpressed as the mean value of upper and lower eyelid MGD grade (values from 1 to 3)
bUnpaired t-test
cChi-squared test (χ2)
Efficacy of MBE-IPL-MGX Treatment
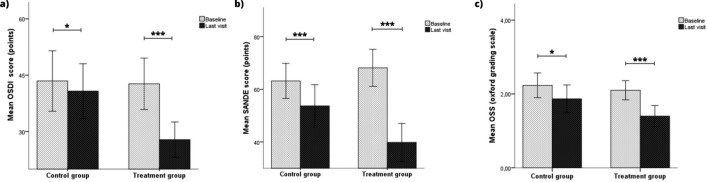
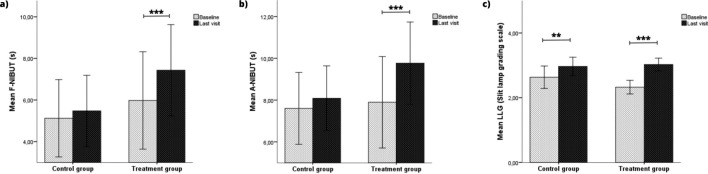
Regarding dry eye symptoms, OSDI and SANDE scores showed significant improvements in both groups (Fig. 2A and B). In addition, ΔOSDI and ΔSANDE scores were – 11.23 (P < 0.001) and – 24.63 (P < 0.001) points higher in the MBE-IPL-MGX group than in the control group, respectively. OSS also showed significant differences in both groups (Fig. 2C). However, ΔOSS achieved in the MBE-IPL-MGX group was not superior to the control group. Regarding tear film stability, F-NIBUT and A-NIBUT only achieved significant improvements in the MBE-IPL-MGX group (Fig. 3A and B) with an ΔF-NIBUT and ΔA-NIBUT of 1.1 (P = 0.047) and 1.4 s (P = 0.018) compared to the control group, respectively. LLG showed significant differences in both groups (Fig. 3C), but the MBE-IPL-MGX group achieved an ΔLLG of 0.4 points (P = 0.003) compared to the control group. Respecting tear volume, no significant differences were detected within and between groups in TMH and TMA. Similar results were reported for ocular hyperemia parameters (Table 2).
Fig. 2.
Symptoms and ocular surface outcomes within control (home-based therapy) and treatment (MBE-IPL-MGX) groups at 2-month follow-up. a Ocular surface disease index questionnaire (OSDI); b symptom assessment in dry Eye questionnaire (SANDE) and c ocular surface staining (OSS). *P < 0.05, ***P < 0.001
Fig. 3.
Tear film stability outcomes within control (home-based therapy) and treatment (MBE-IPL-MGX) groups at 2-month follow-up. a First noninvasive tear film break-up time (F-NIBUT); b average noninvasive tear film break-up time (A-NIBUT) and c lipid layer grade (LLG). **P < 0.01, ***P < 0.001
Table 2.
Intra-group clinical outcomes
| Characteristicsa | Control group | P | Treatment group | P | ||
|---|---|---|---|---|---|---|
| Baseline | Last visit (8th week) |
Baseline | Last visit (8th week) |
|||
| OSDI |
43.46 ± 21.54 [10.41 to 97.91] |
39.83 ± 19.44 [12.50 to 95.83] |
0.041c, d |
42.71 ± 21.43 [12.50 to 90] |
27.85 ± 14.7 [6.3 to 58.33] |
< 0.001c, d |
| SANDE |
62.95 ± 17.7 [24.5 to 100] |
59.25 ± 17.7 [10 to 98.9] |
< 0.001c, d |
68.15 ± 22 [8.94 to 100] |
39.82 ± 22.57 [8.94 to 88] |
< 0.001b, d |
| TMH (mm) |
0.19 ± 0.54 [0.08 to 0.28] |
0.20 ± 0.47 [0.10 to 0.28] |
0.104c |
0.20 ± 0.48 [0.13 to 0.32] |
0.21 ± 0.44 [0.12 to 0.30] |
0.297c |
| TMA (mm2) |
2.10 ± 0.51 [0.70 to 2.89] |
2.0 ± 0.53 [0.88 to 3.55] |
0.198c |
2.1 ± 0.56 [1.1 to 3.15] |
2.2 ± 0.61 [1.0 to 3.10] |
0.174c |
| F-NIBUT (s) |
5.12 ± 4.98 [0.10 to 16.73] |
5.5 ± 4.59 [0.35 to 16.28] |
0.069b |
5.97 ± 7.32 [0.06 to 20.53] |
7.43 ± 6.86 [0.07 to 19.74] |
< 0.001b, d |
| A-NIBUT (s) |
7.60 ± 4.60 [1.54 to 18] |
8.10 ± 4.16 [2.56 to 18.6] |
0.066b |
7.9 ± 6.84 [0.17 to 21] |
9.78 ± 6.2 [0.33 to 20.87] |
< 0.001b, d |
| LLG |
2.63 ± 0.93 [1 to 4] |
2.97 ± 0.76 [2 to 4] |
0.002c, d |
2.32 ± 0.66 [1 to 4] |
3.02 ± 0.51 [2 to 4] |
< 0.001b, d |
| U-LAMG (%) |
41.23 ± 20.54 [14 to 97] |
42.73 ± 19.65 [18 to 99] |
0.083c |
41.87 ± 17.47 [10 to 89] |
39.97 ± 17.1 [17 to 89] |
0.071c |
| L-LAMG (%) |
51.13 ± 20.36 [25 to 89] |
52.53 ± 18.9 [20 to 91] |
0.228b |
58.58 ± 19.87 [13 to 90] |
56.7 ± 18.73 [25 to 92] |
0.098c |
| U-MGD grade |
1.7 ± 0.65 [1 to 3] |
1.63 ± 0.72 [1 to 3] |
0.414b |
1.85 ± 0.58 [1 to 3] |
1.75 ± 0.63 [1 to 3] |
0.102b |
| L-MGD grade |
2.03 ± 0.76 [1 to 3] |
1.9 ± 0.84 [1 to 3] |
0.102c |
2.38 ± 0.63 [1 to 3] |
2.3 ± 0.65 [1 to 3] |
0.180c |
| MGYSS |
19.97 ± 7.58 [6 to 36] |
20 ± 7.35 [6 to 34] |
0.880c |
19.02 ± 7.96 [4 to 40] |
26.6 ± 6.23 [15 to 40] |
< 0.001c, d |
| MGYLS |
7.2 ± 3.59 [4 to 15] |
7.7 ± 3.63 [2 to 15] |
0.060c |
5.73 ± 4.17 [0 to 15] |
10.43 ± 3 [5 to 15] |
< 0.001b, d |
| MGYCS |
1.1 ± 1.65 [0 to 6] |
1.3 ± 1.34 [0 to 4] |
0.198b |
1.27 ± 2.04 [0 to 10] |
2.88 ± 2 [0 to 10] |
< 0.001b, d |
| NCIH (%) |
9.4 ± 5.8 [1.6 to 34.5] |
8.5 ± 3.9 [1.7 to 22] |
0.125b |
8.8 ± 4 [4.3 to 22.1] |
8.4 ± 3.1 [3.50 to 18.60] |
0.167b |
| TCIH (%) |
8.5 ± 3.6 [2.6 to 15.5] |
8.2 ± 3.5 [1.6 to 16.3] |
0.309c |
9.3 ± 3.4 [3.9 to 17.3] |
9.5 ± 2.8 [4.4 to 14.7] |
0.518c |
| NCOH (%) |
10.1 ± 5.56 [2.9 to 31.1] |
9.4 ± 4.28 [3.4 to 25.6] |
0.214b |
10.81 ± 3.2 [6.4 to 20.4] |
10.33 ± 2.65 [5.1 to 18.3] |
0.199b |
| TCOH (%) |
8.97 ± 2.3 [4.3 to 16.3] |
8.87 ± 2.74 [4.2 to 14.6] |
0.704c |
11.1 ± 3.24 [6.1 to 18.70] |
10.5 ± 2.8 [5.3 to 16] |
0.223c |
| OSS |
2.23 ± 0.89 [1 to 4] |
1.87 ± 1 [0 to 3] |
0.027b, d |
2.1 ± 0.81 [1 to 4] |
1.4 ± 0.9 [0 to 3] |
< 0.001b, d |
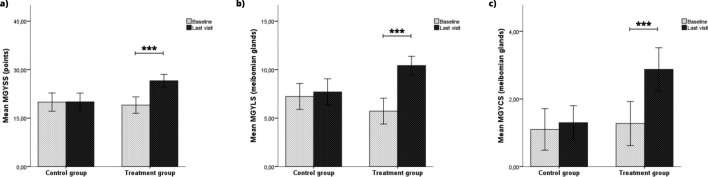
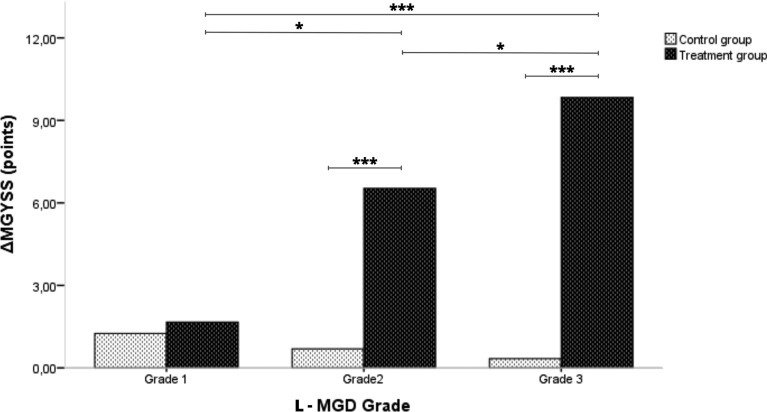
Regarding meibomian gland analysis, no significant differences in LAMG and MGD grade were found in the two groups. In addition, there were also no significant differences in ΔU-MGD and ΔL-MGD grade between the two groups. However, ΔU-LAMG and ΔL-LAMG showed an improvement of – 3.4% (P = 0.017) and – 2.3% (P = 0.038) in the MBE-IPL-MGX group compared to the control group, respectively. Regarding meibomian gland secretion, MGYSS, MGYLS and MGYCS only showed significant improvements in the MBE-IPL-MGX group (Fig. 4A–C). ΔMGYSS, ΔMGYLS and ΔMGYCS achieved an improvement of 7.5 points, 4.2 glands and 1.4 glands (all P < 0.001) in the MBE-IPL-MGX group compared to the control group, respectively. Furthermore, ΔMGYSS according to L-MGD grade only showed significant differences in the MBE-IPL-MGX group (P < 0.05 for all comparisons) (Fig. 5). ΔMGYSS showed no significant differences in L-MGD grade 1 between the two groups, while significant differences were found in L-MGD grades 2 (P < 0.001) and 3 (P < 0.001).
Fig. 4.
Meibomian gland secretion outcomes within control (home-based therapy) and treatment (MBE-IPL-MGX) groups at 2-month follow-up. a Meibomian gland yielding secretion score (MGYSS); b meibomian gland yielding clear secretion (MGYCS) and c meibomian gland yielding liquid secretion (MGYLS). ***P < 0.001
Fig. 5.
Outcomes of meibomian gland yielding secretion score increment (ΔMGYSS) in the control (home-based therapy) and treatment (MBE-IPL-MGX) groups according to baseline lower meibomian gland dysfunction grade (L-MGD grade). *P < 0.05, ***P < 0.001
Regarding single correlations, ΔTMH (r = 0.41, P = 0.009) was significantly correlated with ΔOSDI score in the MBE-IPL-MGX group, while ΔMGYCS (ρ = 0.39, P = 0.034) and ΔTCIH (r = – 0.40, P = 0.028) were significantly correlated with ΔSANDE score in the control group. Multiple correlations showed that ΔTMH (95% CI 29.17 to 194.31, β = 0.41, P = 0.009) had the strongest association with ΔOSDI score in the MBE-IPL-MGX group (R2 = 0.17, P = 0.009). In addition, ΔMGYCS (95% CI 6.34 to 15.13, β = 0.68, P < 0.001), ΔTCOH (95% CI – 5.40 to – 0.81, β = – 0.36, P = 0.010), ΔA-NIBUT (95% CI 0.51 to 4.80, β = 0.34, P = 0.017) and ΔTCIH (95% CI – 4.35 to – 0.04, β = – 0.27, P = 0.046) also had an association with ΔSANDE score in the control group (R2 = 0.61, P < 0.001).
Safety of MBE-IPL-MGX Treatment
In the treatment group, eyelid margin discomfort (n = 13), eyelash loss density (n = 5) and cutaneous erythema (n = 4) were reported after MBE-IPL-MGX combination. Eyelid margin discomfort and cutaneous erythema were resolved in all patients with the postoperative treatment. Eyelash density was completely recovered before the last visit. Regarding home-based therapy, no AEs were reported in the two groups.
Discussion
Several studies have reported the individual efficacy of MBE [18–20, 22], IPL [9–15] and MGX [34] in patients with DED due to MGD. To the best of our knowledge, this is the first prospective, controlled study to report that three sessions of MBE-IPL-MGX treatment significantly improved dry eye symptoms and signs compared to home-based therapy in patients with MGD. A total of 19 parameters related to DED were assessed after a 2-month follow-up period. MBE-IPL-MGX treatment significantly improved OSDI and SANDE scores, F-NIBUT, A-NIBUT, LLG, OSS, MGYSS, MGYLS and MGYCS at 8 weeks from baseline. Although home-based therapy also significantly improved OSDI and SANDE scores, LLG and OSS, the effects of MBE-IPL-MGX treatment on these parameters were significantly higher except for OSS. This may be due to the treatment group patients showing more severe OSS values compared to those in the control group.
The relationship between dry eye signs and symptoms is still unclear [35–37]. In this study, significant correlations were found between dry eye signs and symptoms in both groups. However, dry eye signs only were able to predict 38.9% of dry eye symptoms. This lack of correlation may have implications for monitoring the response to treatment, which demonstrates that DED is a complex condition [38]. There is evidence that IPL improves meibomian gland function [39–41]. However, its effect on the lacrimal gland remains unclear [42, 43], which may explain why no significant improvements in TMH and TMA were found in this study. Some studies have reported significant improvement in LAMG after IPL-MGX treatment [44, 45]. In this study, U-LAMG and L-LAMG also achieved an improvement in the MBE-IPL-MGX group, which may have been due to the decrease in tarsal conjunctival inflammation after MBE-IPL-MGX treatment, allowing the observation of glandular areas that were not visible before. However, these improvements were not significant, which suggests that long-term MBE-IPL-MGX treatment effects on LAMG need to be analyzed, as well as setting the number of sessions required. Regarding meibomian gland secretion, ΔMGYSS showed no significant differences in L-MGD grade 1 between the two groups, while significant differences were found in L-MGD grades 2 and 3. This suggests that home-based therapy may be recommended in MGD grade 1 as indicated by the management and therapy subcommittee of the TFOS DEWS II [46], while MBE-IPL-MGX treatment should be recommended in more severe MGD grades. Significant improvements in ocular hyperemia have also been reported after IPL-MGX treatment [35]. In this study, ocular hyperemia parameters showed non-significant improvement after MBE-IPL-MGX treatment. This may be because the mean ocular hyperemia was 10 ± 2.83% in the treatment group, which could be considered normal [47]. Overall, it seems that the results reported in this study are slightly superior to some RCTs that perform IPL-MGX treatment [45, 48]. This may be due to combining MBE with IPL and MGX. MBE removes the epithelial keratinization and debris accumulated in the eyelid margin that prevents the meibum outflow onto the ocular surface [17]. However, IPL energy absorbed by hemoglobin and Demodex’s exoskeleton reduces the concentration of inflammatory and microbial mediators in the eyelid and meibomian glands [49–51], thus preventing their dysfunction [52]. Therefore, MBE improves expressed meibum quantity, while IPL improves expressed meibum quality. In addition, MGX prevents meibomian gland obstruction [34]. Altogether, this leads to an improvement of the lipid layer of the tear film, which increases NIBUT, reduces OSS and thus improves dry eye symptoms.
Limitations
This study has limitations that merit consideration. First, the absence of randomization in assigning patients to the treatment and control groups is the main limitation of the study. Although an investigator unrelated to the study conducted the classification, the lack of randomization introduces selection bias. Moreover, the considerable disparity in the treatments administered to both groups complicates the masking of the study. Second, the number of enrolled patients may not be sufficiently large to determine the efficacy and safety of MBE-IPL-MGX treatment. The relatively small sample size increases the risk of Type II errors and limits the generalizability of the findings. However, it is important to mention the independence of the observer's participation in the evaluation of the measurements since they were analyzed automatically by the AI identification system included in the S390L Firefly WDR slit-lamp. Third, although the evaluation took place 8 weeks after the initial visit, it should be mentioned that the MBE-IPL-MGX group received a treatment based on dexamethasone for 5 days after the first session, which may also have influenced the results. Fourth, the MBE effects in combined IPL-MGX treatment could not be determined because the control group did not receive IPL-MGX treatment. Therefore, it is possible that the efficacy of the treatment is due solely to the combination of IPL and MGX, which is widely accepted as a treatment for DED. In addition, MBE was performed with a medical-grade diamond bur in this study. However, MBE is usually performed with a medical-grade microsponge, making the effects not comparable. Therefore, future research should evaluate the effects of different MBE devices on clinical outcomes, which would allow a better understanding of the efficacy and safety of each technique. In addition, there is also a need for larger, well-designed, strictly blinded, randomized clinical trials evaluating the long-term effects of MBE within the context of combined IPL-MGX treatment.
Conclusions
In conclusion, this study has shown that MBE-IPL-MGX combination treatment improves dry eye symptoms and signs, as well as meibomian gland function, reporting minimal AEs in patients with MGD. Although further studies are needed, our findings suggest that three sessions of this combined treatment are effective and safe for the treatment of MGD and should be recommended for MGD grades 2 and 3.
Author Contribution
Antonio Ballesteros-Sánchez: Conceptualization, methodology, formal analysis, investigation, writing—original draft preparation, visualization. José-María Sánchez-González: Data curation, writing—review & editing, supervision, project administration. Beatriz Gargallo-Martínez: Data curation, writing—review & editing, supervision, project administration. Ramón Gutiérrez-Ortega: Validation, supervision, project administration.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
The authors, Antonio Ballesteros-Sánchez, José-María Sánchez-González, Ramón Gutiérrez-Ortega and Beatriz Gargallo-Martínez, declare that they have no conflict of interest relevant to the content of this article. The authors confirm that there have been no changes in their affiliations during or after the completion of the manuscript. The affiliations listed on the title page accurately reflect their associations at the time of the study.
Ethical Approval
This prospective, monocentric, unmasked, parallel-control group study (NCT05857579) was approved by the clinical research ethics committee of the University of Murcia (ID: 4097/2022), adhered to the tenets of the Declaration of Helsinki, and was performed at the Novovision Ophthalmology Clinic from April 2022 to January 2023. Informed consent was obtained from each patient before enrollment in the study.
References
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, et al. New Perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15:65–76. doi: 10.1016/j.jtos.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf [Internet]. 2017 [cited 2022 Oct 15];15:438–510. Available from: https://pubmed.ncbi.nlm.nih.gov/28736340/
- 5.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmol Vis Sci. 2011;52:1938. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones L, Downie LE, Korb D, Benitez-del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf [Internet]. 2017 [cited 2022 Oct 15];15:575–628. Available from: https://pubmed.ncbi.nlm.nih.gov/28736343/ [DOI] [PubMed]
- 7.Babilas P, Schreml S, Szeimies RM, Landthaler M. Intense pulsed light (IPL): a review. Lasers Surg Med. 2010;42:93–104. doi: 10.1002/lsm.20877. [DOI] [PubMed] [Google Scholar]
- 8.Ash C, Town G, Whittall R, Tooze L, Phillips J. Lasers and intense pulsed light (IPL) association with cancerous lesions. Lasers Med Sci. 2017;32:1927–1933. doi: 10.1007/s10103-017-2310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg [Internet]. 2015 [cited 2022 Oct 15];33:41–6. Available from: https://pubmed.ncbi.nlm.nih.gov/25594770/ [DOI] [PMC free article] [PubMed]
- 10.Craig JP, Chen YH, Turnbull PRK. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci [Internet]. 2015 [cited 2022 Jul 24];56:1965–70. Available from: https://pubmed.ncbi.nlm.nih.gov/25678687/ [DOI] [PubMed]
- 11.Li D, Lin S Bin, Cheng B. Intense pulsed light treatment for meibomian gland dysfunction in Skin Types III/IV. Photobiomodul Photomed Laser Surg [Internet]. 2019 [cited 2022 Apr 9];37:70–6. Available from: https://pubmed.ncbi.nlm.nih.gov/31050931/ [DOI] [PubMed]
- 12.Vigo L, Giannaccare G, Sebastiani S, Pellegrini M, Carones F. Intense pulsed light for the treatment of dry eye owing to meibomian gland dysfunction. Journal of Visualized Experiments [Internet]. 2019 [cited 2022 Apr 9];2019. Available from: https://pubmed.ncbi.nlm.nih.gov/30985743/ [DOI] [PubMed]
- 13.Li D, Lin S Bin, Zhang MZ, Cheng B. Preliminary Assessment of Intense Pulsed Light Treatment on the Upper Eyelids for Meibomian Gland Dysfunction. Photobiomodul Photomed Laser Surg [Internet]. 2020 [cited 2022 Oct 15];38:249–54. Available from: https://pubmed.ncbi.nlm.nih.gov/32301670/ [DOI] [PubMed]
- 14.Yan S, Wu Y. Efficacy and safety of intense pulsed light therapy for dry eye caused by meibomian gland dysfunction: A randomised trial. Ann Palliat Med. 2021;10:7857–7865. doi: 10.21037/apm-21-1303. [DOI] [PubMed] [Google Scholar]
- 15.Zarei-Ghanavati S, Hassanzadeh S, Azimi Khorasani A, Ehsaei A, Bakhtiari E. Efficacy of five-flash intense pulsed light therapy technique in patients with meibomian gland dysfunction. Clin Exp Optom. 2022;105:687–693. doi: 10.1080/08164622.2021.1976595. [DOI] [PubMed] [Google Scholar]
- 16.Giannaccare G, Taroni L, Senni C, Scorcia V. Intense pulsed light therapy in the treatment of meibomian gland dysfunction: Current perspectives. Clin Optom (Auckl) 2019;11:113–126. doi: 10.2147/OPTO.S217639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballesteros-Sánchez A, Gargallo-Martínez B, Gutiérrez-Ortega R, Sánchez-González J-M. Eyelid Exfoliation Treatment Efficacy and Safety in Dry Eye Disease, Blepharitis, and Contact Lens Discomfort Patients: A Systematic Review. Asia-Pacific Journal of Ophthalmology [Internet]. 2023;12:315–25. Available from: https://journals.lww.com/10.1097/APO.0000000000000607 [DOI] [PubMed]
- 18.Murphy O, O’Dwyer V, Lloyd-McKernan A. The efficacy of tea tree face wash, 1, 2-Octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Cont Lens Anterior Eye. 2018;41:77–82. doi: 10.1016/j.clae.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad-Rabei H, Arabi A, Shahraki T, Rezaee-Alam Z, Baradaran-Rafii A. Role of Blepharoexfoliation in Demodex Blepharitis: A Randomized Comparative Study. Cornea [Internet]. 2022;Publish Ah. Available from: https://journals.lww.com/corneajrnl/Fulltext/9900/Role_of_Blepharoexfoliation_in_Demodex.25.aspx [DOI] [PubMed]
- 20.Siddireddy JS, Tan J, Vijay AK, Willcox MDP. The Effect of Microblepharon Exfoliation on Clinical Correlates of Contact Lens Discomfort. Optom Vis Sci [Internet]. 2019 [cited 2022 Sep 25];96:187–99. Available from: https://pubmed.ncbi.nlm.nih.gov/30801507/ [DOI] [PubMed]
- 21.Epstein IJ, Rosenberg E, Stuber R, Choi MB, Donnenfeld ED, Perry HD. Double-Masked and Unmasked Prospective Study of Terpinen-4-ol Lid Scrubs With Microblepharoexfoliation for the Treatment of Demodex Blepharitis. Cornea. 2020;39:408–416. doi: 10.1097/ICO.0000000000002243. [DOI] [PubMed] [Google Scholar]
- 22.Moon SY, Han SA, Kwon HJ, Park SY, Lee JH, Chung HS, et al. Effects of lid debris debridement combined with meibomian gland expression on the ocular surface MMP-9 levels and clinical outcomes in moderate and severe meibomian gland dysfunction. BMC Ophthalmol [Internet]. 2021 [cited 2022 Oct 27];21. Available from: /pmc/articles/PMC8040198/ [DOI] [PMC free article] [PubMed]
- 23.Sambhi RDS, Sambhi GDS, Mather R, Malvankar-Mehta MS. Intense pulsed light therapy with meibomian gland expression for dry eye disease. Can J Ophthalmol. 2020;55:189–198. doi: 10.1016/j.jcjo.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Leng X, Shi M, Liu X, Cui J, Sun H, Lu X. Intense pulsed light for meibomian gland dysfunction: a systematic review and meta-analysis. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2021;259. [DOI] [PubMed]
- 25.Miao S, Yan R, Jia Y, Pan Z. Effect of Intense Pulsed Light Therapy in Dry Eye Disease Caused by Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. Eye Contact Lens. 2022; [DOI] [PubMed]
- 26.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Ian Pearce E, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballesteros-Sánchez A, Gargallo-Martínez B, Gutiérrez-Ortega R, Sánchez-González J-M. Intra-observer repeatability assessment of the S390L Firefly WDR slit lamp in patients with dry eye disease: Objective, automated and non-invasive measures. Eye Contact Lens. 2023;Publish Ah. [DOI] [PubMed]
- 29.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Li J, Hu M, Zhao Y, Lin X, Chen Y, et al. Comparison of two intense pulsed light patterns for treating patients with meibomian gland dysfunction. Int Ophthalmol. 2020;40:1695–1705. doi: 10.1007/s10792-020-01337-0. [DOI] [PubMed] [Google Scholar]
- 31.Reidy JJ, Paulus MP, Gona S. Recurrent erosions of the cornea: epidemiology and treatment. Cornea. 2000;19:767–771. doi: 10.1097/00003226-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Vergés C, Salgado-Borges J, Ribot FM de. Prospective evaluation of a new intense pulsed light, thermaeye plus, in the treatment of dry eye disease due to meibomian gland dysfunction. J Optom [Internet]. 2021 [cited 2023 Apr 9];14:103. Available from: /pmc/articles/PMC8093543/ [DOI] [PMC free article] [PubMed]
- 33.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.1988.01670060015008. [DOI] [PubMed] [Google Scholar]
- 34.Kaiserman I, Rabina G, Mimouni M, Sadi Optom NB, Duvdevan N, Levartovsky S, et al. The Effect of Therapeutic Meibomian Glands Expression on Evaporative Dry Eye: A Prospective Randomized Controlled Trial. Curr Eye Res. 2021;46:195–201. doi: 10.1080/02713683.2020.1789663. [DOI] [PubMed] [Google Scholar]
- 35.Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom. 2018;101:23–33. doi: 10.1111/cxo.12541. [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Yu S, He X, Yang L, Wu Y, Qin G, et al. Tear film interferometry assessment after intense pulsed light in dry eye disease: A randomized, single masked, sham-controlled study. Cont Lens Anterior Eye. 2022;45. [DOI] [PubMed]
- 37.Xie L, Chen S, Hong J, Jin X, Chen W, Rong B, et al. The lack of correlation between symptoms and signs in patients with meibomian gland dysfunction: a secondary analysis of the multicenter, randomized controlled trial. BMC Ophthalmol. 2022;22. [DOI] [PMC free article] [PubMed]
- 38.Bartlett JD, Keith MS, Sudharshan L, Snedecor SJ. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;9:1719–1730. doi: 10.2147/OPTH.S89700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vora GK, Gupta PK. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr Opin Ophthalmol. 2015;26:314–318. doi: 10.1097/ICU.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 40.Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017;11:1167–1173. doi: 10.2147/OPTH.S139894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wladis EJ, Aakalu VK, Foster JA, Freitag SK, Sobel RK, Tao JP, et al. Intense pulsed light for meibomian gland disease: a report by the american academy of ophthalmology. Ophthalmology. 2020;127:1227–1233. doi: 10.1016/j.ophtha.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Di Marino M, Conigliaro P, Aiello F, Valeri C, Giannini C, Mancino R, et al. Combined Low-Level Light Therapy and Intense Pulsed Light Therapy for the Treatment of Dry Eye in Patients with Sjögren’s Syndrome. J Ophthalmol. 2021;2021:2023246. doi: 10.1155/2021/2023246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huo Y, Wan Q, Hou X, Zhang Z, Zhao J, Wu Z, et al. Therapeutic Effect of Intense Pulsed Light in Patients with Sjögren’s Syndrome Related Dry Eye. J Clin Med. 2022;11. [DOI] [PMC free article] [PubMed]
- 44.Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocular Surface. 2019;17:104–110. doi: 10.1016/j.jtos.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Yan X, Hong J, Jin X, Chen W, Rong B, Feng Y, et al. The Efficacy of Intense Pulsed Light Combined With Meibomian Gland Expression for the Treatment of Dry Eye Disease Due to Meibomian Gland Dysfunction: A Multicenter, Randomized Controlled Trial. Eye Contact Lens [Internet]. 2021 [cited 2022 Jul 24];47:45–53. Available from: https://pubmed.ncbi.nlm.nih.gov/32452923/ [DOI] [PMC free article] [PubMed]
- 46.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II Report Executive Summary. Ocul Surf [Internet]. 2017 [cited 2022 Nov 26];15:802–12. Available from: https://pubmed.ncbi.nlm.nih.gov/28797892/ [DOI] [PubMed]
- 47.Murphy PJ, Lau JSC, Sim MML, Woods RL. How red is a white eye? Clinical grading of normal conjunctival hyperaemia. Eye (Lond) 2007;21:633–638. doi: 10.1038/sj.eye.6702295. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Li J, Wu Y, Lin X, Deng X, Yun-e Z. Comparative Evaluation in Intense Pulsed Light Therapy Combined with or without Meibomian Gland Expression for the Treatment of Meibomian Gland Dysfunction. Curr Eye Res. 2021;46:1125–1131. doi: 10.1080/02713683.2020.1867750. [DOI] [PubMed] [Google Scholar]
- 49.Papageorgiou P, Clayton W, Norwood S, Chopra S, Rustin M. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol [Internet]. 2008 [cited 2022 Apr 23];159:628–32. Available from: https://pubmed.ncbi.nlm.nih.gov/18565174/ [DOI] [PubMed]
- 50.Cheng S nan, Jiang F gang, Chen H, Gao H, Huang Y kan. Intense Pulsed Light Therapy for Patients with Meibomian Gland Dysfunction and Ocular Demodex Infestation. Curr Med Sci. 2019;39:800–9. [DOI] [PubMed]
- 51.Fishman HA, Periman LM, Shah AA. Real-Time Video Microscopy of In Vitro Demodex Death by Intense Pulsed Light. Photobiomodul Photomed Laser Surg. 2020;38:472–476. doi: 10.1089/photob.2019.4737. [DOI] [PubMed] [Google Scholar]
- 52.Giannaccare G, Taroni L, Senni C, Scorcia V. Intense Pulsed Light Therapy In The Treatment Of Meibomian Gland Dysfunction: Current Perspectives. Clin Optom. 2019;11:113–26. ense Pulsed Light Therapy In The Treatment Of Meibomian Gland Dysfunction: C. Clin Optom (Auckl). 2019;11:113–26 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.