Abstract
International guidelines recommend implantation of an implantable cardioverter-defibrillator (ICD) in non-ischaemic cardiomyopathy (NICM) patients with a left ventricular ejection fraction (LVEF) below 35% despite optimal medical therapy and a life expectancy of more than 1 year with good functional status. We propose refinement of these recommendations in patients with NICM, with careful consideration of additional risk parameters for both arrhythmic and non-arrhythmic death. These additional parameters include late gadolinium enhancement on cardiac magnetic resonance imaging and genetic testing for high-risk genetic variants to further assess arrhythmic risk, and age, comorbidities and sex for assessment of non-arrhythmic mortality risk. Moreover, several risk modifiers should be taken into account, such as concomitant arrhythmias that may affect LVEF (atrial fibrillation, premature ventricular beats) and resynchronisation therapy. Even though currently no valid cut-off values have been established, the proposed approach provides a more careful consideration of risks that may result in withholding ICD implantation in patients with low arrhythmic risk and substantial non-arrhythmic mortality risk.
Keywords: Non-ischaemic cardiomyopathy, Implantable cardioverter defibrillator, Mortality, Sudden cardiac death
Introduction
Heart failure with reduced ejection fraction (HFrEF) carries a significant mortality risk with progressive heart failure and arrhythmic events as predominant causes of death [1]. To prevent arrhythmic death, international guidelines recommend implantable cardioverter defibrillator (ICD) implantation in symptomatic patients with a left ventricular ejection fraction (LVEF) below 35%, despite optimal medical therapy (OMT) and with a life expectancy of more than 1 year [2, 3]. In the HFrEF population suffering from ischaemic cardiomyopathy (ICM), these guidelines are based on randomised trials that have consistently shown a survival benefit of ICD therapy [4–7]. For HFrEF patients with non-ischaemic cardiomyopathy (NICM), however, individual randomised trials have demonstrated varying results, and guideline recommendations are based on meta-analyses [3, 5–8].
Most of these randomised trials were performed over two decades ago. Since then, drug therapies for heart failure have improved and adherence to evidence-based medication has increased, resulting in a substantial decline in the incidence of both all-cause mortality and sudden cardiac death (SCD) [9]. This decrease in SCD reignited the discussion specifically on ICD eligibility for NICM patients and was further fuelled by the results of the contemporary DANISH trial, which showed no reduction in all-cause mortality after ICD implantation in NICM patients with HFrEF [8]. However, the DANISH trial has been criticised for mixing two treatment strategies, as the majority of enrolled patients received cardiac resynchronisation therapy (CRT), thus obscuring the results of ICD-only therapy. Indeed, other non-randomised contemporary ICD studies showed a significant benefit of ICD-only implantation in NICM patients, and similar rates of appropriate device therapy between ICM and NICM patients were observed [10–12].
The past two decades have provided new insights into risk stratification for SCD in the heart failure population. As NICM—by definition—includes all causes of cardiomyopathy other than ischaemic heart disease, each underlying aetiology may carry a different risk. Specific genetic variants carrying an increased arrhythmic risk, as well as more general arrhythmic risk stratifiers including late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR), and risk modifiers such as CRT have been identified. In addition, new insights have been obtained regarding competing risks (i.e. risk factors increasing the likelihood of non-arrhythmic death) against which ICD implantation will not protect. This obviates the need for a more individualised approach weighing arrhythmic risk, competing risks and possible risk modifiers to assess eligibility for ICD implantation in NICM patients. Based on these new insights, the present article aims to provide a framework to refine selection of NICM patients for ICD implantation eligibility beyond the current guidelines. This review will not discuss the use of prophylactic ICD implantation in patients with hypertrophic cardiomyopathy (HCM) or arrhythmogenic right ventricular cardiomyopathy (ARVC), as eligibility criteria for primary prevention therapy for these two NICM entities are not restricted to a LVEF < 35%. For primary prevention ICD therapy in HCM and ARVC we refer readers to the criteria in the ESC guidelines [3].
Optimal therapy prior to deciding on ICD implantation
As stated in the guidelines, patients should be on stable OMT, including a beta-blocker, angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) and mineralocorticoid receptor antagonist, before being evaluated for ICD implantation [2, 3]. Currently, however, OMT consists of more components than in the earlier guidelines, as the replacement of ACE inhibitor/ARB by angiotensin-receptor neprilysin inhibitors (ARNIs) and the addition of sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce symptoms, hospitalisation as well as mortality, and may lead to an increase in LVEF [9, 13].
In addition to OMT, efforts should be directed towards treatment of underlying causes of NICM, requiring a full diagnostic work-up prior to evaluation for ICD. Specific attention should be paid to the presence of types of supraventricular tachycardia, such as atrial fibrillation and atrial flutter, and frequent premature ventricular beats (PVBs), as these arrhythmias may be both the consequence and cause of NICM, and their ongoing presence may lead to further deterioration of cardiac function. PVBs are often a consequence of the cardiomyopathy. However, when one of these arrhythmias develops prior to or during the NICM disease process, further evaluation is mandatory. Generally, a PVB burden ≥ 10% is considered to be contributing to ventricular dysfunction [14]. LV dysfunction due to either of these arrhythmias may require rigorous attempts to preserve stable sinus rhythm, including antiarrhythmic medication and/or ablation [14, 15].
Ejection fraction and myocardial fibrosis
Among traditional risk factors, LVEF is the strongest independent predictor of SCD and the key parameter in guiding the decision regarding prophylactic ICD implantation [3]. However, its specificity for predicting SCD is limited, since LVEF is related to SCD as well as to cardiovascular death in general. Sensitivity for the occurrence of SCD is also poor, as approximately 6% of prophylactic ICD patients receive appropriate ICD shocks after 2 years of follow-up [11] whereas, in absolute terms, the majority of SCD events occur in individuals with a preserved LVEF [16].
In NICM patients, an additional risk factor more specific to ventricular arrhythmias may be obtained by CMR with LGE. The presence of myocardial fibrosis provides prognostic information, as it may facilitate re-entry tachyarrhythmia [17]. Approximately 45% of NICM patients have myocardial fibrosis present on LGE-CMR, typically with a septal midwall and/or subepicardial location [18]. Multiple studies have shown that the presence of myocardial fibrosis is associated with SCD and mortality, independent of LVEF [18–21]. Klem et al. showed in 1020 NICM patients with a LVEF < 50% that both myocardial fibrosis and LVEF < 35% were independently associated with mortality. However, myocardial fibrosis was strongly related to arrhythmic events, whereas LVEF was not [19]. These results are in line with a meta-analysis showing that midwall fibrosis was independently associated with ventricular arrhythmias [18]. Interestingly, a study evaluating NICM patients with a LVEF > 40%, therefore not considered eligible for ICD implantation, showed that NICM patients with LGE had a nine-fold higher risk of SCD compared to patients without LGE [20]. This is in line with another study showing that patients with a LVEF > 35% with myocardial fibrosis are more prone to arrhythmic events compared to patients with a LVEF between 21 and 35% without myocardial fibrosis [21]. Whereas in ICM the extent of transmural myocardial fibrosis is inversely correlated with the LV function [22], the correlation between the extent of myocardial fibrosis and LV function in most cases of NICM is less obvious. This might be an explanation for the strong correlation of LV dysfunction with arrhythmic events in ICM patients, whereas this correlation is less clear in NICM. As large and prospective studies strongly suggest myocardial fibrosis as a parameter to identify NICM patients at high risk of SCD, we propose that LGE-CMR be incorporated in the standard work-up for NICM, which is in line with the 2A recommendation of the current ESC guidelines [3]. Moreover, if LGE on CMR is absent and the additional evaluation, including genetic testing and cardiomyopathy substrate, shows a low arrhythmic risk, we propose that conservative treatment or CRT without a defibrillator is preferable. Nevertheless, randomised evidence is needed for further validation.
Risk associated with genetic background
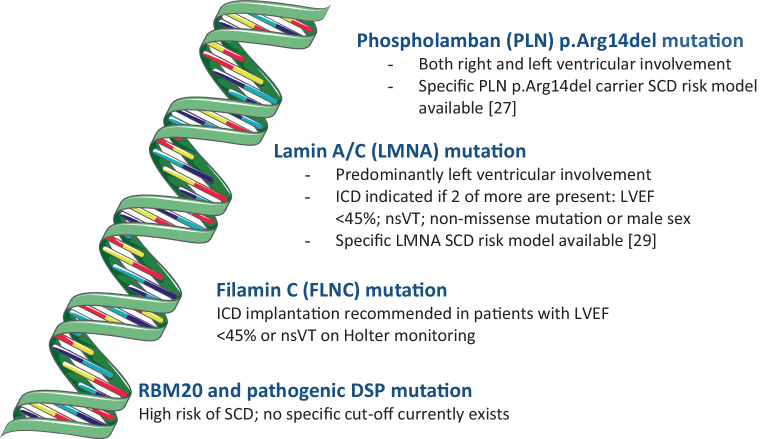
The terms NICM and dilated cardiomyopathy (DCM) are often used interchangeably. However, NICM includes different forms of cardiomyopathies, such as DCM, auto-immune cardiomyopathy, arrhythmia-induced cardiomyopathy etc. DCM is the most common subtype of NICM, and research and recommendations regarding genetic testing have, in contrast to other sections of the article, a specific focus on DCM patients. Approximately 17–25% of DCM cases have a hereditary cause and a pathogenic variant can be found [23]. More than 50 genes are identified to be monogenetically associated with DCM. The most frequent pathogenic variants are in genes coding for titin (TTN), lamin A/C (LMNA) and desmin. In the Netherlands, phospholamban (PLN) mutations are also a common cause because of the p.Arg14del Dutch founder mutation. In a historical cohort, 12% of Dutch DCM cases were found to harbour this PLN mutation [24]. Testing for genetic variants is generally recommended when the prevalence of a detectable pathogenic variant is sufficiently high to justify targeted genetic screening, e.g. in cases of familial DCM, and in patients with onset of disease at a young age without other predisposing factors for DCM [25]. Escobar-Lopez et al. developed a validated screening tool that can predict the probability for a positive genetic test in patients with DCM based on five clinical parameters [26]. It is important to acknowledge that there are gene- or even variant-specific effects. For example, the PLN p.Arg14del variant can lead to both right and left ventricular involvement, while pathogenic variants in LMNA are associated predominantly with left ventricular involvement and conduction abnormalities with or without a skeletal myopathy. Due to these gene- and variant-specific effects, the risk of SCD also varies per genotype. Currently, the LVEF criterion for ICD implantation has been specified for specific genetic backgrounds (Fig. 1). Indeed, for PLN p.Arg14del carriers a specific risk model estimating the risk of lethal arrhythmia was recently developed that can guide eligibility for ICD implantation [27]. In individuals with LMNA, two or more of the following are needed for ICD implantation to be recommended: LVEF < 45%, non-sustained ventricular tachycardia (VT), non-missense mutation or male sex [28, 29]. For filamin C, ICD implantation is recommended for patients with a LVEF < 45% or non-sustained VT on Holter monitoring [30, 31]. DCM patients with an RBM20 (RNA-binding motif protein 20) or pathogenic DSP (desmoplakin) mutation are also considered at high risk of SCD, but no specific cut-off currently exists [23]. In contrast, arrhythmic deaths do occur in TTN mutation carriers, but progressive heart failure predominates. In the future, more specific recommendations are expected for genetic DCM based on additional data on the genotype-phenotype relationship. Importantly, the online calculators for PLN p.Arg14del carriers and LMNA mutation carriers present a 5-year risk of ventricular arrhythmias. Although no specific cut-off values are specified regarding whether or not an ICD should be implanted in these specific patients, the European guideline committee for HCM considered a yearly risk of ≥ 1% of SCD acceptable for prophylactic ICD implantation [32].
Fig. 1.
Specific genetic variants and the risk of sudden cardiac death (SCD). Currently, most non-ischaemic cardiomyopathy patients with a genetic variant are eligible for implantable cardioverter defibrillator (ICD) implantation if their left ventricular ejection fraction (LVEF) is ≤ 35%. However, some genetic variants have specific recommendations regarding ICD implantation. (Adapted from SMART—Servier Medical ART, Servier: https://smart.servier.com. nsVT non-sustained ventricular tachycardia)
Competing risks: the influence of comorbidities, age and sex
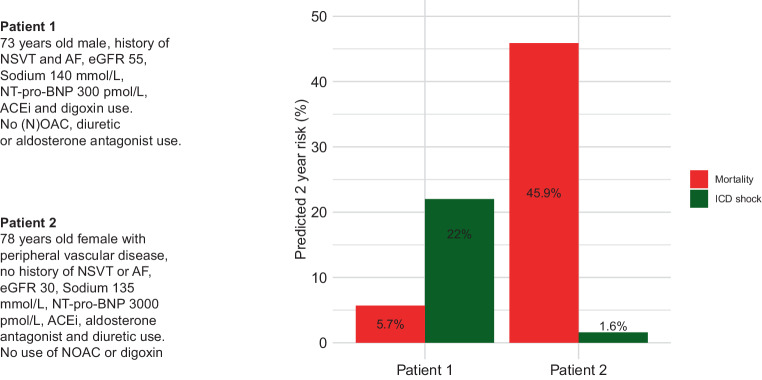
Efficacy of ICD implantation in patients at risk of arrhythmic death may be significantly modified by the risk of non-arrhythmic death. An ICD shock may prevent SCD; however, it does not necessarily prolong life if the chance of non-arrhythmic death in the near future is high. The importance of competing risks has been historically acknowledged in guidelines by stating that life expectancy should be more than 1 year. Presently, however, several validated models including demographic data (e.g. age and sex), as well as comorbidities and other risk markers (e.g. diabetes mellitus, renal failure, chronic obstructive pulmonary disease, N‑terminal pro-B-type natriuretic peptide level), are available for risk assessment in primary prevention ICD implantation and CRT implantation [12, 33]. For example, in the Dutch Do-IT study a validated online tool was developed for calculation of the risks of all-cause mortality and appropriate ICD shock [12]. Importantly, this study shows that risk stratification regarding appropriate device therapy remains challenging compared to predicting mortality, with poor performance for the ICD shock model (C statistic = 0.60) and good performance of the mortality model (C statistic = 0.74). Figure 2 shows two example cases in which the risk of appropriate ICD shock and all-cause mortality is predicted based on clinical parameters. Obviously, increasing age strongly affects mortality risk. A post hoc analysis of the DANISH trial showed that the rate of non-arrhythmic death doubles in NICM patients above the age of 70 years [34]. In line with the DANISH trial, several other studies have shown a decreased benefit of ICD implantation with increasing age [11, 33]. As a consequence, age should be incorporated in clinical decision making for ICD implantation.
Fig. 2.
Two sample cases in which the chance of all-cause mortality and implantable cardioverter defibrillator (ICD) shock can be used to determine if a patient will benefit from prophylactic ICD implantation. The Do-IT prediction models are used to estimate the individual risk of death and ICD shock. (Adapted from Verstraelen et al. [12] with permission. © 2021; Oxford, Academic. AF atrial fibrillation, ACEi angiotensin-converting enzyme inhibition, eGFR estimated glomerular filtration rate, (N)OAC (novel) oral anticoagulant drugs, nsVT non-sustained ventricular tachycardia, NT-pro-BNP N-terminal pro-B-type natriuretic peptide)
Along with age, comorbidities also strongly affect outcome. Almost 60% of HF patients have five or more chronic comorbidities, and this affects their functional status and mortality [35–37]. Studies have shown that, while in elderly patients with multiple comorbidities the benefit of ICD therapy is less clear [37–39], in elderly patients at high risk of SCD with a low burden of comorbidities ICD implantation may increase survival [39]. Importantly, the most recent cardiac pacing guidelines propose CRT‑D implantation based on individual risk assessment rather than a LVEF cut-off alone [40].
In addition to age and comorbidities, sex also stratifies risk. Male sex is associated with a higher all-cause mortality and higher incidence of SCD in ICM and NICM patients [6, 33, 41–43]. A meta-analysis of the pivotal randomised ICD trials including ICM and NICM patients showed that prophylactic ICD implantation is associated with a 25% reduction in mortality in men, whereas ICD implantation in women was not associated with a survival benefit [41]. This may partly be attributable to sex hormones affecting susceptibility for arrhythmia and a lower baseline risk of death in women [42, 44]. However, women have been underrepresented in randomised trials and differences in underlying causes of cardiomyopathy may also partly explain differences in outcome. In addition, female sex is associated with a greater response to CRT compared to males, resulting in improved survival rates and a lower incidence of SCD; yet further analysis suggests that this outcome may be attributable to differences in underlying cardiomyopathy and/or heart size rather than sex per se [45, 46]. As a consequence, incorporation of sex in risk assessment for SCD is less straightforward.
Nevertheless, physicians should estimate risks of both non-arrhythmic and arrhythmic death for each individual patient in order to assess eligibility for ICD implantation. For example, an elderly male NICM patient with several comorbidities, no significant LGE on CMR and no high-risk genetic substrate has a low arrhythmia risk and a substantial risk of non-arrhythmic mortality, and ICD implantation will not significantly improve life expectancy. In contrast, a young patient without comorbidities and with LGE on CMR has a substantial arrhythmic risk and relatively low mortality risk, and may therefore benefit from ICD implantation. Figure 3 depicts the interaction between the risk of arrhythmic death and non-arrhythmic death and can provide insight into the competing risks on a patient level.
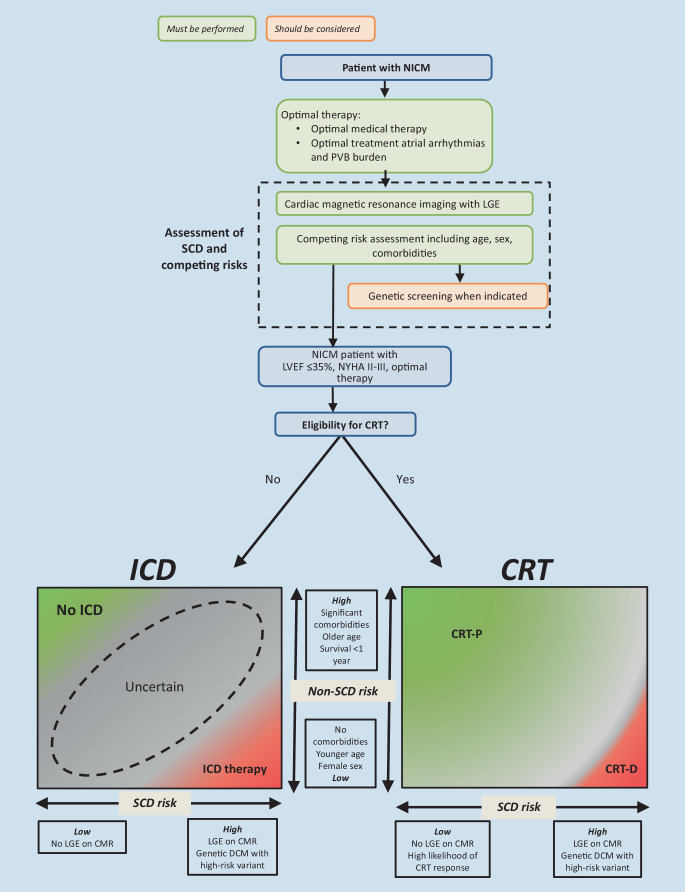
Fig. 3.
Suggested diagnostic work-up and optimisation of care for non-ischaemic cardiomyopathy (NICM) patients. See Fig. 1 and the guidelines for specific recommendations regarding implantable cardioverter defibrillator (ICD) recommendations for dilated cardiomyopathy (DCM) patients with specific genetic variants. (CMR cardiac magnetic resonance, CRT cardiac resynchronisation therapy, LGE late gadolinium enhancement, LVEF left ventricular ejection fraction, NYHA New York Heart Association, PVB premature ventricular beat, SCD sudden cardiac death)
Cardiac resynchronisation therapy
Approximately 35–50% of the NICM patient population eligible for ICD implantation have a ventricular conduction delay amenable to CRT [8, 12]. CRT may result in significant reverse remodelling and improvement of LVEF, and may consequently modify the mortality risk, including the risk of SCD [47]. As a consequence, the need for addition of a defibrillator to CRT therapy has been a subject of debate for a long time. No randomised controlled trials have been performed yet to directly compare the benefit of CRT with a defibrillator function (CRT-D) versus CRT with a pacemaker function (CRT-P) in NICM. However, two meta-analyses using subgroups of randomised and non-randomised studies have become available. These studies show that addition of a defibrillator to CRT therapy was not significantly associated with an overall decrease in all-cause mortality [48, 49]. In contrast, a recent subanalysis of the COMPANION study showed a significantly lower all-cause mortality in NICM patients receiving a CRT‑D compared to those receiving a CRT‑P [50]. It is noteworthy that only patients in New York Heart Association class III–IV were included in this study, with less than 70% of patients receiving ACE inhibitor and beta-blocker treatment, and that this post hoc analysis was relatively underpowered. In general, these findings support the opinion that in a substantial proportion of NICM patients eligible for CRT, a CRT‑P should be preferred over a CRT‑D. Specifically in patients with a high likelihood of CRT-induced reverse remodelling combined with a low estimated arrhythmic risk, such as older patients with a high comorbidity burden, CRT‑P should be considered [51]. The amount of reverse remodelling can be estimated based on patient characteristics and predicted using an easily available effect calculator [52], whereas the risk of SCD can be assessed using previously discussed predictors, including LGE-CMR. The clinical work-up prior to device implantation should not differ between CRT and ICD NICM patients, as LGE-CMR and genetic screening could provide guidance in deciding between CRT‑D and CRT‑P.
Recommendations
There has been controversy over the usefulness and necessity of primary prevention ICD implantation in HFrEF patients with NICM. Guidelines recommend a decision based on risk assessment using LVEF, OMT and a life expectancy of more than 1 year. In recent decades, however, both the risk of SCD and life expectancy have been modified significantly by improvements in medical therapy. In addition, advances have been made in estimation of arrhythmic as well as non-arrhythmic risks. We therefore propose additional risk assessment refining current guidelines, carefully weighing arrhythmic risk versus non-arrhythmic risks of death for each individual patient (see Infobox 1). Figure 3 shows the proposed approach.
Infobox 1 Recommendations for further risk assessment
Verification of optimal therapy consisting of OMT (including ARNI and/or a SGLT2 inhibitor if possible) as well as optimisation of concomitant arrhythmias that might affect cardiac function.
Assessment of arrhythmic risk using LGE-CMR and genetic testing when appropriate.
Assessment of non-arrhythmic risk using age, comorbidities and sex.
Evaluation of possible risk modifiers, in particular CRT eligibility and its estimated effect size. In a substantial proportion of patients eligible for CRT, a CRT‑P should be preferred over CRT-D: specifically in patients with a high likelihood of CRT-induced reverse remodelling and a low arrhythmic risk.
Conclusion
This article aims to present a strategy beyond current guidelines for selection of NICM patients eligible for ICD implantation. As randomised evidence is limited, proposed strategies are largely based on observational studies and post hoc analyses. Although we feel that this provides a sufficient basis for the proposed approach, further studies—preferably randomised clinical trials or dedicated registries—are needed particularly for the patient group with intermediate risks.
Conflict of interest
D.A. Theuns has received research grants from Biotronik and Boston Scientific as well as consulting fees from Boston Scientific. K. Vernooy has received research grants from Medtronic, Abbott, Biosense Webster and Philips, is listed as a consultant for Boston Scientific, Abbott, Medtronic, Biosense Webster, Philips and Microport, is a member of the guideline committee of the AHA/HRS and is an associate editor of the Netherlands Heart Journal. A.A.M. Wilde is a member of the scientific advisory board ARMGO and Thryv Therapeutics. C.P. Allaart is president of the Netherlands Heart Rhythm Association, has received institutional research grants from Abbott, Biotronik and Medtronic as well as speaker fees from Abbott and Medtronic, and is an associate editor of the Netherlands Heart Journal. A.-L.C.J. van der Lingen, T.E. Verstraelen, L. van Erven, J.G. Meeder and A.H. Maass declare that they have no competing interests.
Footnotes
K. Vernooy and A.A.M. Wilde are members of the European Reference Network for rare, low prevalence and complex diseases of the heart: ERN GUARD-Heart
References
- 1.Schultheiss HP, Fairweather D, Caforio ALP, et al. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5:32. doi: 10.1038/s41572-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonagh TA, Metra M, Adamo M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;2021(42):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 3.Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–1458. doi: 10.1161/01.CIR.0000012350.99718.AD. [DOI] [PubMed] [Google Scholar]
- 6.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 7.Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–1712. doi: 10.1016/S0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 8.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 9.Shen L, Jhund PS, Petrie MC, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 10.Schrage B, Uijl A, Benson L, et al. Association between use of primary-prevention implantable cardioverter-defibrillators and mortality in patients with heart failure: a prospective propensity score-matched analysis from the Swedish Heart Failure Registry. Circulation. 2019;140:1530–1539. doi: 10.1161/CIRCULATIONAHA.119.043012. [DOI] [PubMed] [Google Scholar]
- 11.Zabel M, Willems R, Lubinski A, et al. Clinical effectiveness of primary prevention implantable cardioverter-defibrillators: results of the EU-CERT-ICD controlled multicentre cohort study. Eur Heart J. 2020;41:3437–3447. doi: 10.1093/eurheartj/ehaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstraelen TE, van Barreveld M, van Dessel P, et al. Development and external validation of prediction models to predict implantable cardioverter-defibrillator efficacy in primary prevention of sudden cardiac death. Europace. 2021;23:887–897. doi: 10.1093/europace/euab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 14.Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2328–2344. doi: 10.1016/j.jacc.2019.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;2021(42):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 16.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, et al. Out-of-hospital cardiac arrest—the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/S0195-668X(03)00191-X. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KP, Walker R, Urie P, et al. Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;92:122–140. doi: 10.1172/JCI116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker MAJ, Cornel JH, van de Ven PM, et al. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging. 2018;11:1274–1284. doi: 10.1016/j.jcmg.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Klem I, Klein M, Khan M, et al. Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with nonischemic cardiomyopathy. Circulation. 2021;143:1343–1358. doi: 10.1161/CIRCULATIONAHA.120.048477. [DOI] [PubMed] [Google Scholar]
- 20.Halliday BP, Gulati A, Ali A, et al. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. 2017;135:2106–2115. doi: 10.1161/CIRCULATIONAHA.116.026910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Marco A, Brown PF, Bradley J, et al. Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;77:2890–2905. doi: 10.1016/j.jacc.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Rahimtoola SH, Dilsizian V, Kramer CM, et al. Chronic ischemic left ventricular dysfunction from pathophysiology to imaging and its integration into clinical practice. JACC Cardiovasc Imag. 2008;1:536–55. [DOI] [PMC free article] [PubMed]
- 23.Akhtar M, Elliott PM. Risk stratification for sudden cardiac death in non-ischaemic dilated cardiomyopathy. Curr Cardiol Rep. 2019;21:155. doi: 10.1007/s11886-019-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Zwaag PA, van Rijsingen IA, de Ruiter R, et al. Recurrent and founder mutations in the Netherlands-Phospholamban p.Arg14del mutation causes arrhythmogenic cardiomyopathy. Neth Heart J. 2013;21:286–293. doi: 10.1007/s12471-013-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charron P, Arad M, Arbustini E, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2010;31:2715–2726. doi: 10.1093/eurheartj/ehq271. [DOI] [PubMed] [Google Scholar]
- 26.Escobar-Lopez L, Ochoa JP, Royuela A, et al. Clinical risk score to predict pathogenic genotypes in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2022;80:1115–1126. doi: 10.1016/j.jacc.2022.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verstraelen TE, van Lint FHM, Bosman LP, et al. Prediction of ventricular arrhythmia in phospholamban p.Arg14del mutation carriers-reaching the frontiers of individual risk prediction. Eur Heart J. 2021;42:2842–2850. doi: 10.1093/eurheartj/ehab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rijsingen IA, Arbustini E, Elliott PM, et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59:493–500. doi: 10.1016/j.jacc.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 29.Wahbi K, Yaou BR, Gandjbakhch E, et al. Development and validation of a new risk prediction score for life-threatening ventricular tachyarrhythmias in laminopathies. Circulation. 2019;140:293–302. doi: 10.1161/CIRCULATIONAHA.118.039410. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz-Genga MF, Cuenca S, Dal Ferro M, et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68:2440–2451. doi: 10.1016/j.jacc.2016.09.927. [DOI] [PubMed] [Google Scholar]
- 31.Gigli M, Stolfo D, Graw SL, et al. Phenotypic expression, natural history, and risk stratification of cardiomyopathy caused by filamin C truncating variants. Circulation. 2021;144:1600–1611. doi: 10.1161/CIRCULATIONAHA.121.053521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Authors/Task Force m, Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79. [DOI] [PubMed]
- 33.Bilchick KC, Wang Y, Cheng A, et al. Seattle Heart Failure and Proportional Risk Models predict benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol. 2017;69:2606–2618. doi: 10.1016/j.jacc.2017.03.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elming MB, Nielsen JC, Haarbo J, et al. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation. 2017;136:1772–1780. doi: 10.1161/CIRCULATIONAHA.117.028829. [DOI] [PubMed] [Google Scholar]
- 35.Lee CS, Chien CV, Bidwell JT, et al. Comorbidity profiles and inpatient outcomes during hospitalization for heart failure: an analysis of the U.S. Nationwide inpatient sample. BMC Cardiovasc Disord. 2014;14:73. doi: 10.1186/1471-2261-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–143. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruwald AC, Vinther M, Gislason GH, et al. The impact of co-morbidity burden on appropriate implantable cardioverter defibrillator therapy and all-cause mortality: insight from Danish nationwide clinical registers. Eur J Heart Fail. 2017;19:377–386. doi: 10.1002/ejhf.685. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail. 2014;2:623–629. doi: 10.1016/j.jchf.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barra S, Providencia R, Paiva L, et al. Implantable cardioverter-defibrillators in the elderly: rationale and specific age-related considerations. Europace. 2015;17:174–186. doi: 10.1093/europace/euu296. [DOI] [PubMed] [Google Scholar]
- 40.Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 41.Barra S, Providencia R, Boveda S, et al. Do women benefit equally as men from the primary prevention implantable cardioverter-defibrillator? Europace. 2018;20:897–901. doi: 10.1093/europace/eux203. [DOI] [PubMed] [Google Scholar]
- 42.Russo AM, Poole JE, Mark DB, et al. Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. J Cardiovasc Electrophysiol. 2008;19:720–724. doi: 10.1111/j.1540-8167.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 43.van der Lingen ACJ, Theuns D, Rijnierse MT, et al. Sex-specific differences in outcome and risk stratification of ventricular arrhythmias in implantable cardioverter defibrillator patients. Esc Heart Fail. 2021;8:3726–3736. doi: 10.1002/ehf2.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tadros R, Ton AT, Fiset C, Nattel S. Sex differences in cardiac electrophysiology and clinical arrhythmias: epidemiology, therapeutics, and mechanisms. Can J Cardiol. 2014;30:783–792. doi: 10.1016/j.cjca.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 45.de Waard D, Manlucu J, Gillis AM, et al. Cardiac resynchronization in women: a substudy of the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial. JACC Clin Electrophysiol. 2019;5:1036–1044. doi: 10.1016/j.jacep.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Zweerink A, Friedman DJ, Klem I, et al. Size matters: normalization of QRS duration to left ventricular dimension improves prediction of long-term cardiac resynchronization therapy outcome. Circ Arrhythm Electrophysiol. 2018;11:e006767. doi: 10.1161/CIRCEP.118.006767. [DOI] [PubMed] [Google Scholar]
- 47.Killu AM, Mazo A, Grupper A, et al. Super-response to cardiac resynchronization therapy reduces appropriate implantable cardioverter defibrillator therapy. Europace. 2018;20:1303–1311. doi: 10.1093/europace/eux235. [DOI] [PubMed] [Google Scholar]
- 48.Patel D, Kumar A, Black-Maier E, et al. Cardiac resynchronization therapy with or without defibrillation in patients with nonischemic cardiomyopathy a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2021;14:e008991. doi: 10.1161/CIRCEP.120.008991. [DOI] [PubMed] [Google Scholar]
- 49.Theuns DA, Verstraelen TE, van der Lingen ACJ, et al. Implantable defibrillator therapy and mortality in patients with non-ischaemic dilated cardiomyopathy: an updated meta-analysis and effect on Dutch clinical practice by the Task Force of the Dutch Society of Cardiology. Neth Heart J. 2023;31:89–99. doi: 10.1007/s12471-022-01718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doran B, Mei C, Varosy PD, et al. The addition of a defibrillator to resynchronization therapy decreases mortality in patients with nonischemic cardiomyopathy. JACC Heart Fail. 2021;9:439–449. doi: 10.1016/j.jchf.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Mullens W, Auricchio A, Martens P, et al. Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care. Europace. 2021;23:1324–1342. doi: 10.1093/europace/euaa411. [DOI] [PubMed] [Google Scholar]
- 52.Maass AH, Vernooy K, Wijers SC, et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. Europace. 2018;20:e1–e10. doi: 10.1093/europace/euw445. [DOI] [PubMed] [Google Scholar]