Abstract
Cognitive deterioration and memory decline associated with the progression of Alzheimer’s disease (AD) primarily results from synaptic failure. However, current understanding of the upstream regulatory mechanisms controlling synaptic plasticity remains limited. Salt-inducible kinase 3 (SIK3) is central to the signal pathway and is involved in neuronal regulation of sleep duration in mice. We speculated that the SIK3 cascade signaling pathway might contribute to the pathogenesis of AD. Thus, the present study employed AD transgenic mouse models, Morris Water Maze, virus-mediated gene transfer, electrophysiology, co-immunoprecipitation, western blotting, quantitative polymerase chain reaction, immunofluorescence, ChIP-qPCR, Golgi-Cox staining and dendritic spine analysis to investigate this connection. Our results revealed that SIK3 mRNA/protein expression was significantly reduced in middle-aged AD transgenic mouse models and AD patients. Conditional deletion of SIK3 gene in dorsal hippocampal neurons of 5×FAD mice further accelerated cognitive deterioration and impaired synaptic plasticity. In hippocampal neuronal cultures, SIK3 formed a complex with HDAC4, directly phosphorylated HDAC4 and regulated its nuclear cytoplasmic shuttle. Overexpression of SIK3 could facilitate the expression of synaptic plasticity-related genes by directly repressing mef2c or involving the recruitment of histone deacetylase to promoter regions of target genes through regulation of p-HDAC4, and vice versa. Moreover, up-regulation of SLP-S, the truncated fragment of SIK3, in dorsal hippocampal neurons, restored the synaptic plasticity and alleviates the cognitive impairment in 5×FAD mice. Collectively, these findings revealed a novel and important role of SIK3-HDAC4 regulation of synaptic plasticity and propose a new target for therapeutic approaches of cognitive deficits associated with AD.
Subject terms: Epigenetics and plasticity, Hippocampus
Introduction
Alzheimer’s disease (AD) is the most common form of dementia and one of the greatest health challenges of this century, affecting approximately 50 million people worldwide. The main pathogenesis of AD is characterized by the presence of Aβ deposited plaques, neurofibrillary tangles formed by hyperphosphorylated tau, synaptic dysfunction and degeneration. In addition, synaptic loss is the major neurobiological substrate of cognitive dysfunction in AD [1–5]. Accordingly, it is imperative to explore the fundamental mechanisms that might restore synaptic function in the brain and promote the early diagnosis and treatment of AD.
Previous research on epigenetic markers showed histone modifications associated with aging in healthy individuals and those presenting with AD [6–11]. These modifications include the acetylation of lysine residues that leads to active gene transcription related to long-term synaptic plasticity and memory [12–15]. Histone acetylation is deposited by enzymes known as histone acetyltransferases (HAT), which are removed by histone deacetylases (HDACs). A previous study showed that HDAC2 functions in modulating synaptic plasticity, which in turn negatively regulates learning and memory [13]. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory [16], whereby HDAC4 haploinsufficiency is associated with mental problems in humans [17, 18]. Furthermore, increased levels of HDAC6 in AD mice models and patients contribute to disease pathology, while knocking out the protein in APP/PS1-21 mice rescued associative and spatial memory [19]. Moreover, the histone deacetylase inhibitors rescued acetylated histone levels associated with synaptic transmission genes and memory in different AD mice models [20, 21]. Notably, the specificity and potential side effects of HDAC inhibitors limit their clinical application [22]. In this respect, exploring the upstream regulatory genes involved in histone acetylation or specific molecular mechanisms of HDACs subtypes may represent a promising new therapeutic strategy for the restoration of synaptic plasticity in AD.
The salt-inducible kinase 3 (SIK3), a member of the AMP-activated protein kinase (AMPK) family, is ubiquitously expressed in various tissues. Type IIa HDACs are the substrates of SIK3 that have been identified. And SIK3-HDAC4/5 signaling cascade was highly required for hormonal signaling and metabolism in the peripheral tissues [23–25]. In the central nervous system (CNS), SIK3-HDAC4 signaling pathway positively regulates a subset of transcripts in excitatory neurons, increasing the duration of non-rapid eye movement sleep (NREMS) and depth in mice [26–28]. The aforementioned findings demonstrate that SIK3 plays vital functions in regulating cellular processes. However, the precise contribution of the SIK3 gene to the development of AD pathology remains unknown.
Here, we showed how the SIK3 signaling pathway regulates synaptic function using an in vivo model of 5×FAD transgenic mice. We first examined the expression profile of SIK3 in adult mouse brain regions and observed a significant decrease in the expression of SIK3 in middle-aged AD transgenic mice and AD patients. Moreover, the conditional deletion of SIK3 in the dorsal hippocampus impaired synaptic plasticity and cognitive function in middle-aged 5×FAD mice. We used hippocampal neuronal cultures to demonstrate that SIK3 regulates transcriptional activity of HDAC4 by disrupting its nucleocytoplasmic shuttling. We showed that HDAC4 regulate the expression of genes involved in synaptic plasticity through interfering with mef2c-dependent transcriptional activation and histone deacetylation. Finally, the SLP-S upregulation restored the synaptic plasticity and alleviated the cognitive impairment in 5×FAD mice. In summary, we propose a new therapeutic avenue that targets the SIK3 gene for the treatment of AD and a novel approach that specifically targets HDAC4 for controlling synaptic plasticity.
Methods and materials
All animal protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Fujian Medical University (IACUC FJMU 2022-0859) and followed the international guidelines for the ethical use of research animals.
See the Supplementary Methods and Materials for descriptions of the animal models, bioinformatics analysis, stereotaxic virus injection, electrophysiology, Morris Water Maze test, hippocampal neuronal cultures, quantitative polymerase chain reaction (qPCR), immunoblotting, Co-immunoprecipitation (Co-IP) assay, tissue and cellular immunofluorescence assays (IF), Aβ1-42 ELISA, ChIP-qPCR, Golgi-Cox staining and statistical analysis, as well as other details.
Results
SIK3 expression decreases in the middle-aged AD transgenic mouse models and AD patients
To understand the functional role of SIK3, we first examined the expression patterns of SIK3 in the mouse brain during development. Western blot analyses revealed that SIK3 was widely expressed in various brain regions (Supplementary Fig. S1A). The relative amount of SIK3 gradually increased after birth and reached its peak in adulthood (Supplementary Fig. S1B, C). Immunofluorescence and western blot assay indicated that SIK3 expression was essentially confined to neurons (Supplementary Fig. S1D, E). Collectively, SIK3 is abundantly expressed in the dorsal hippocampal neurons of the adult mouse.
Bioinformatics analysis revealed that the transcript levels of the SIK3 gene in the group of AD patients was significantly lower than controls (Fig. 1A). We then quantified the levels of SIK3 in the whole hippocampus of 5×FAD and APP/PS1 mice, which are the well-established transgenic models of AD. The results showed that the mRNA and protein levels of SIK3 had no significant changes at 2 months of age, but decreased significantly at 5 and/or 8 months old in 5×FAD mice (Fig. 1B, C). Western blotting results also showed a decline in the levels of SIK3 at both 6–7- and 12-month-old APP/PS1 mice compared to age-matched wild type (WT) mice (Fig. 1D). We further quantified hippocampal SIK3 level during aging. The analyses revealed that SIK3 protein levels were markedly decreased at 5-months of age and aggravated strikingly at 8 months of age in the 5×FAD mice (Fig. 1E), while there were no significant age-related changes in SIK3 protein levels in WT mice. After this, we used immunofluorescence to determine neuron-specific expression of SIK3 in 8-month-old 5×FAD mice. The relative SIK3 staining intensity on NeuN+ cells in the DG regions of 5×FAD mice was significantly lower than in the control group (Fig. 1F), and also found in the CA1, CA3 and cortex brain regions (Supplementary Fig. S2A). These results demonstrate that the expression of SIK3 is decreased in neurons of AD models in an age-dependent manner.
Fig. 1. SIK3 expression decreases in the middle-aged AD transgenic mouse models and AD patients.
A Bioinformation analysis showed a decreased expression of SIK3 in the temporal cortex of AD patients group compared with the control group. B Detection of SIK3 mRNA levels in the hippocampus of 2-,5- and 8-month-old WT or 5×FAD mice (n = 6–7 per group) measured by qPCR. C Representative immunoblots and quantitative analyses of SIK3 in the dorsal hippocampus of the 2-, 5- and 8-month-old WT or 5×FAD mice (n = 6–7 per group). D Representative immunoblots and quantitative analyses of SIK3 in the hippocampus of the 3-4, 6-7 and 12-month-old WT or APP/PS1 mice (n = 3 per group). E Representative immunoblots and quantitative analyses of SIK3 in the hippocampus of the 2-, 5- and 8-month-old WT or 5×FAD mice (n = 4 per group). *p < 0.05, 5-month-old 5×FAD versus 5-month-old WT mice, ****p < 0.0001, 8-month-old 5×FAD versus 8-month-old WT mice. #p < 0.05, 5-month-old 5×FAD mice versus 3-month-old 5×FAD mice, #### p < 0.0001, 8-month-old 5×FAD mice versus 3-month-old 5×FAD mice. F Doubled-labeled confocal immunofluorescent images showing the colocalization of SIK3 (red) and the neuronal marker NeuN (green; top) in the DG of 8-month-old WT or 5×FAD mice. Scale bar = 50 µm. And the quantification of SIK3 intensity on DG NeuN+ cells shown in the right panel (n = 3 for WT group and n = 3 for 5×FAD group). Data are expressed as mean ± SEM. Statistical significance was calculated by unpaired two-tailed t-test (A–D, F) and two-way ANOVA (E) followed by the Tukey’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Conditional deletion of SIK3 in dorsal hippocampus impairs cognitive function and synaptic plasticity in middle-aged 5×FAD mice
To knockdown SIK3 in the neurons, we injected the SIK3 knock-down adeno-associated virus (AAV) into the dorsal hippocampus of 6-month-old female and male WT mice and age/sex-matched 5×FAD mice. The three independent experiments, WB, qPCR and immunofluorescence, together showed that the knockdown efficiency of the virus was feasible (Supplementary Fig. S3A–D).
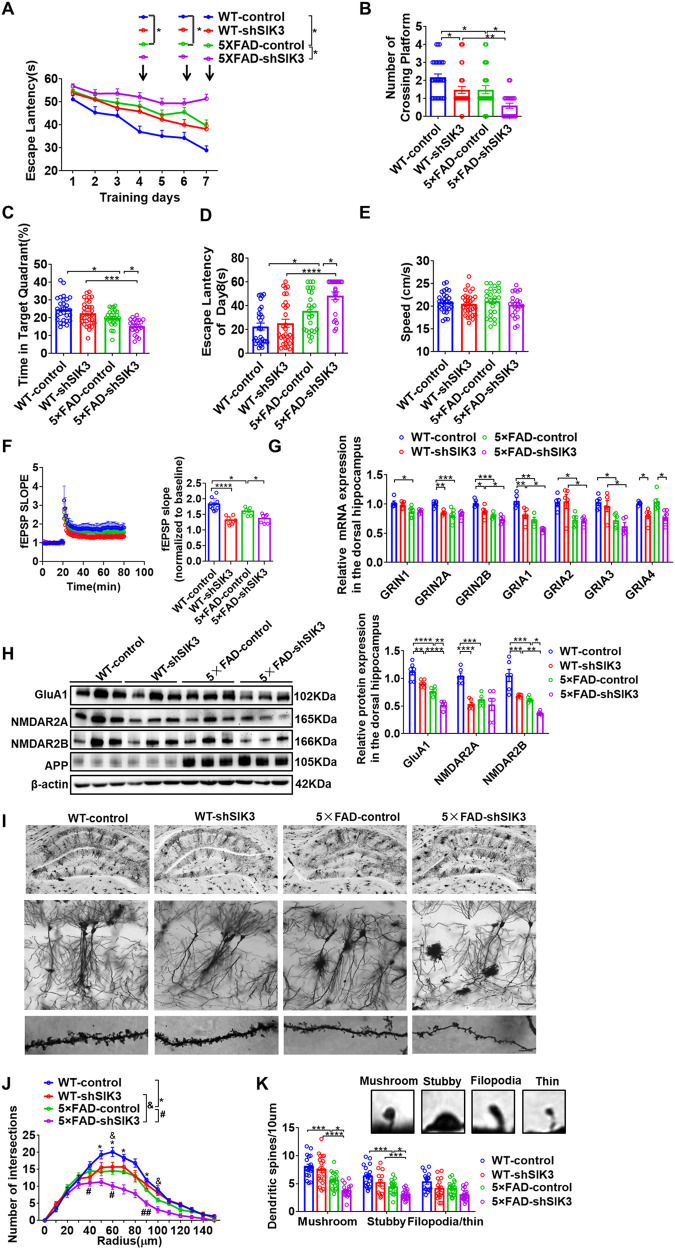
Morris Water Maze was used to evaluate the functional role of SIK3 in hippocampal-mediated spatial learning and memory. Firstly, mice were trained for 7 days with a hidden platform. During each trial, the escape latency was measured as an index of the spatial learning ability. 5×FAD-control mice showed much higher escape latencies on days 4, 6 and 7, compared to WT-control mice (Fig. 2A). We also found that the escape latencies in 5×FAD-control mice were significantly reduced at day 7 compared to 5×FAD-shSIK3 (Fig. 2A). In the probe test, the number of platform crossings in the 5×FAD-control and 5×FAD-shSIK3 groups were lower than those of the WT-control and WT-shSIK3 groups. Moreover, SIK3 knockdown showed a significantly decreased number of platform crossings, compared with control groups (Fig. 2B), while animals in the 5×FAD-control and 5×FAD-shSIK3 groups spent significantly more time in the target quadrant compared to WT-control and WT-shSIK3 groups. In addition, 5×FAD-shSIK3 mice also reported less time in the target quadrant compared to 5×FAD-control mice (Fig. 2C). Similarly, compared with the WT-control group, the escape latency was prolonged in 5×FAD-control, and further compromised in the 5×FAD-shSIK3 mice (Fig. 2D), with no noticeable changes in swimming speed between these groups (Fig. 2E). These results demonstrate that SIK3 knockdown results in impaired learning and memory in middle-aged 5×FAD mice.
Fig. 2. Conditional deletion of SIK3 in dorsal hippocampus impairs cognitive function and synaptic plasticity in 5×FAD mice.
A–E WT-control, WT-shSIK3, 5×FAD-control and 5×FAD-shSIK3 mice were tested in the Morris Water Maze. Escape latency to the platform position during the training trails (1-7d) (A) and the probe (8d) trial (D). B The number of platform-position crossings. The percentage of time spent in the target quadrant (C) and the speed (E) in the probe trial (8d). n = 15 for male and n = 14 for female mice in WT-control group, n = 15 for male and n = 15 for female mice in WT-shSIK3 group, n = 15 for male and n = 10 for female mice in 5×FAD-control group, and n = 13 for male and n = 9 for female mice in 5×FAD-shSIK3 group. F Hippocampal CA1 LTP recordings from WT-control, WT-shSIK3, 5×FAD-control, and 5×FAD-shSIK3 groups. HFS high-frequency stimulation (100 Hz, 1 s). And fEPSP amplitude quantification during the last 10 min of LTP recording. WT-control (n = 4 mice, 10 slices), WT-shSIK3 (n = 4 mice,8 slices), 5×FAD-control (n = 4 mice, 7 slices), and 5×FAD –shSIK3 (n = 4 mice,7slices). G qPCR was used to detect NMDAR subunit (GRIN1/2 A/2B) and AMPAR subunit (GRIA1/2/3) mRNA levels in the dorsal hippocampus of WT-control, WT-shSIK3, 5×FAD-control and 5×FAD-shSIK3 groups. n = 6 per group. H Representative immunoblots and quantitative analyses of SIK3,GluA1,NMDAR2A and NMDAR2B in the dorsal hippocampus of WT-control, WT-shSIK3, 5×FAD-control and 5×FAD-shSIK3 groups (n = 6 per group). I Representative images of dendrites of CA1 pyramidal neurons from WT-control, WT-shSIK3, 5×FAD-control and 5×FAD-shSIK3 groups. Scale bar = 500 µm (upper), scale bar = 50 µm (middle), scale bar = 100 pixel (lower). J The sholl analysis of branch intersections number (n = 7 from 4 mice, respectively), *p < 0.05, WT-control versus 5×FAD-control. & p < 0.05, WT-shSIK3 versus 5×FAD-shSIK3. #p < 0.05, ##p < 0.01, 5×FAD-control versus 5×FAD-shSIK3. K Representative images of labeled spines for analysis from WT-control (19 neurons from 4 mice), WT-shSIK3 (20 neurons from 4 mice), 5×FAD-control (20 neurons from 4 mice) and 5×FAD-shSIK3 group (20 neurons from 4 mice). Mushroom, filopodia, thin, and stubby spines are identified based on structural measures. Numbers in parentheses indicate neurons/animals examined. Data are expressed as mean ± SEM. Statistical significance was calculated by two-way ANOVA (B–H, J, K), and three-way ANOVA (A) followed by the Tukey’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, #p < 0.05, ##p < 0.01, &p < 0.05.
To explore the effects of SIK3 on hippocampal synaptic plasticity, we first performed field recordings using hippocampus slices. In slices obtained from WT-control mice, a single train of HFS (100 Hz, 1 s) induced a robust LTP (50–60 min after HFS); whereas a significantly slower decay of LTP was observed in slices from 5×FAD-control mice. Compared with the two control groups, fEPSPs was compromised in the WT-shSIK3 and 5×FAD-shSIK3 mice respectively (Fig. 2F), suggesting SIK3 knockdown impaired the maintenance of LTP in both WT and 5×FAD mice.
Changes in N-methyl-D-aspartate receptors (NMDAR, GRIN1/2 A/2B) and AMPA-type ionotropic glutamate receptors (AMPAR, GRIA1/2/3) are often used to infer whether LTP had occurred in postsynapses, which are essential for synaptic plasticity and memory [29, 30]. Compared with the WT-control group, the 5×FAD-control group exhibited an evident reduction in the mRNA expression of GRIN1, GRIN2A, GRIN2B, GRIA1, GRIA2, and GRIA3, which were further decreased by SIK3 knockdown. We also found that the amount of GRIN2A, GRIN2B, GRIA1 and GRIA4 in WT-shSIK3 group was significantly lower than in the WT-control group (Fig. 2G). Similarly, the immunoblotting experiments indicated that GluA1, GRIN2A and GRIN2B were significantly reduced in the 5×FAD-control group, which were all further reduced by SIK3 knockdown. The protein level of GluA1 and GRIN2B in the 5×FAD-shSIK3 group was decreased in the hippocampus compared to the WT-shSIK3 group. We also found that the amount of GluA1, GRIN2A and GRIN2B in the WT-shSIK3 group was significantly lower than in the WT-GFP group (Fig. 2H). This indicates that SIK3 knockdown impaired the mRNA and protein levels of glutamate receptors in both WT and 5×FAD mice.
Synaptic function is closely related to the integrity of synaptic structure. Golgi-Cox staining was used to visualize individual CA1 pyramidal neurons (Fig. 2I), an area important for the storage of social memory [31]. Sholl analysis of CA1 pyramidal neurons revealed that the number of dendritic interactions decreased in 5×FAD-control mice compared to WT-control mice; while SIK3 knockdown significantly reduced the complexity compared to the 5×FAD-control group (Fig. 2J). Moreover, dendritic spines have been classified as mushroom, stubby, and filopodia/thin–shaped according to their morphology. We quantified these dendritic spines and found that the amount of mushroom and stubby in 5×FAD-control mice was significantly reduced compared to the WT-control mice respectively. SIK3 knockdown further reduced the amount of mushroom and stubby in 5×FAD-shSIK3 mice, but had no obvious effect on the amount of filopodia/thin. (Fig. 2K). Together, these results show that conditional deletion of SIK3 in dorsal hippocampus impaired synaptic plasticity.
We then examined the effect of SIK3 knockdown on the other pathological phenotype of AD, including amyloid and gliosis. The Aβ protein level determined by western blot and ELISA analysis, Aβ plaque (6E10) content measured by immunofluorescence staining were all prominent in the dorsal hippocampus of the 5×FAD-control mice, but no significant differences were observed between the control and shSIK3 mice (Supplementary Fig. S3E–G). The generation of Aβ is derived from APP processing. Compared with the control group, there was also no significant difference in the level of APP and BACE1 between the control and shSIK3 mice (Supplementary Fig. S3E). Furthermore, markers of activated astrocytes (GFAP) and microglia (CD68) were not different between the two groups (Supplementary Fig. S3E, F). These results indicate that the adverse effect of SIK3 knockdown on synaptic function was independent of the typical AD pathology.
SIK3 forms a complex with HDAC4 to regulate its nucleocytoplasmic shuttling
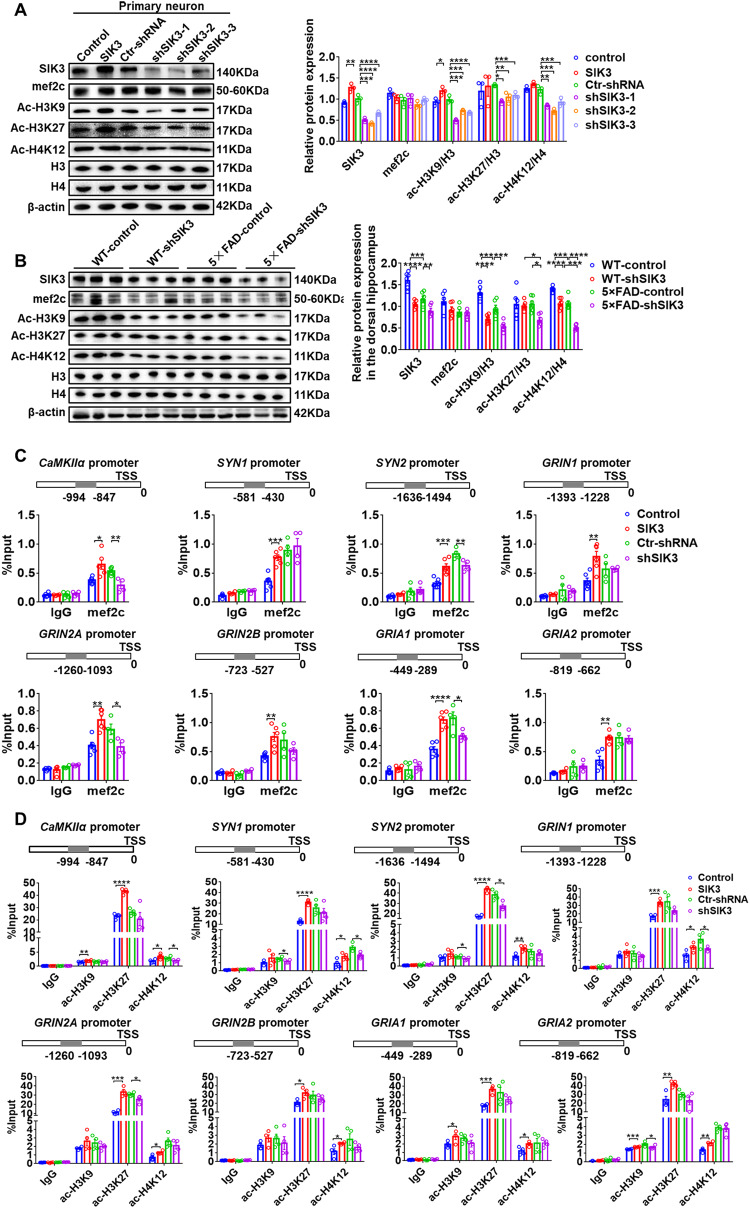
Given the crucial role of the SIK3 in the signal transduction pathway, we tested whether SIK3 related signaling is dysregulated in SIK3 knockdown mice and causally related to abnormal synaptic plasticity and function performance. A flag-tagged SIK3 overexpression lentivirus was transfected into hippocampal neuronal cultures and the overexpression efficiency was verified by western blotting (Fig. 3A). A significantly higher level of p-HDAC4/5/7 was detected in SIK3 overexpression cells compared to control cells (Fig. 3A); whereas the expression levels of p-PI3K, p-AKT, p-mTOR, p-S6K, p-GSK3β and p-P44/42 were all unchanged (Supplementary Fig. S4A). To evaluate how SIK3 regulates the phosphorylation of HDAC4/5/7, the Co-IP assays were performed to indicate that SIK3 could recruit 14-3-3 adaptor proteins and directly phosphorylate the HDAC4 at the conserved motifs (Fig. 3B), but not with HDAC5/7 protein (Supplementary Fig. S4B, C). The interaction between SIK3 and HDAC4 was further revealed by immunoprecipitation with anti-HDAC4 antibody (Fig. 3C) and 14–3–3 antibody (Fig. 3D), which was also confirmed in vivo using mouse brain lysate (Supplementary Fig. S4D–F). As expected, SIK3 knockdown substantially decreased the phosphorylation level of HDAC4 (Fig. 3E). The phosphorylation site of T221 is required for the kinase activity of SIK3. Alanine substitution of T221 of SIK3, kinase-inactivating mutation, completely reversed the effects of SIK3 overexpression on HDAC4 phosphorylation, which was also observed in the treatment of SIK3 inhibitors (Fig. 3F). That suggested that the phosphorylation-promoting activity of SIK3 was dependent on its kinase activity. The regulation of HDAC4 phosphorylation by SIK3 was further confirmed in 5×FAD and WT mice. Compared to the WT-control group, the 5×FAD-control group exhibited an evidently reduction in the expression of p-HDAC4, which was further decreased in SIK3 knockdown models. The expression of p-HDAC4 in 5×FAD-shSIK3 mice was much lower than that in WT-shSIK3 mice (Fig. 3G). Since HDAC4 phosphorylation promotes nucleocytoplasmic shuttling, immunofluorescence was used to detect the HDAC4 localization. When SIK3 was overexpressed, HDAC4 was mainly sequestrated in the cytoplasm, while the knockdown of SIK3 facilitated its transport from the cytoplasm to the nucleus (Fig. 3H). Subcellular fractionation showed decreased nuclear translocation of HDAC4 in SIK3 overexpression cells and increased nuclear localization in SIK3-knockdown cells (Fig. 3I). These results indicate that SIK3 regulates nucleocytoplasmic shuttling of HDAC4 by directly phosphorylation.
Fig. 3. SIK3 forms a complex with HDAC4 to regulate its nucleocytoplasmic shuttling.
A Representative immunoblots and quantitative analyses of SIK3, flag, p-HDAC4-5-7/HDAC4-5-7 in the SIK3 overexpression and control hippocampal neuronal cultures (n = 3 per group). B Co-immunoprecipitation of HDAC4 and 14-3-3 with flag antibody in the SIK3-flag and control cells. Hc IgG heavy chain, Lc IgG light chain. C Co-immunoprecipitation of SIK3 and 14-3-3 with HDAC4 antibody in the SIK3-flag overexpression and control cells. D Co-immunoprecipitation of SIK3 and HDAC4 with adaptor protein 14-3-3 antibody in the SIK3-flag overexpression and control cells. E Representative immunoblots and quantitative analyses of SIK3, p-HDAC4/HDAC4 in the Ctr-shRNA and shSIK3 cells (n = 3 per group). F Representative immunoblots and quantitative analyses of SIK3, p-HDAC4/HDAC4 in the control, SIK3 overexpression, SIK3T221A overexpression or SIK3 overexpression +SIK3 inhibitor and cells (n = 3 per group). G Representative immunoblots and quantitative analyses of SIK3, HDAC4, p-HDAC4 and APP in the dorsal hippocampus of WT-control,WT-shSIK3, 5×FAD-control and 5×FAD-shSIK3 mice (n = 6 per group). H The immunofluorescence of HDAC4 in the SIK3 overexpression or knockdown cells. Scale bar = 10 µm. n = 3. I Representative immunoblots and quantitative analyses of SIK3 and HDAC4 in the subcellular fractionation of SIK3 overexpression or knockdown groups. n = 3. Data are expressed as mean ± SEM. Statistical significance was calculated by unpaired two-tailed t-test (A, I), one-way ANOVA (E, F) and two-way ANOVA(G) followed by the Tukey’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
HDAC4 regulates the transcription of genes related to synaptic plasticity through transcription factors mef2c and histone acetylation at promoter sites
HDAC4 modulate the cellular processes through multiple transcription factors, among which the transcription factor mef2c has been regarded as established risk genes/loci for late-onset AD. We were thus interested in examining the effect of HDAC4 on mef2c expression. In hippocampal neuronal culture, we found no significant change in mef2c protein levels in either the SIK3 overexpression or the knockdown groups (Fig. 4A). Similarly, in vivo experiments showed no changes in mef2c protein levels in the shSIK3 group, regardless of the genotype (Fig. 4B). Therefore, ChIP-qPCR was utilized to detect the effects of SIK3 on the transcriptional activity of mef2c in hippocampal neuronal cultures. The results revealed that the significant increases in the special binding regions of calcium/calmodulin-activated protein kinase α (CaMKIIα), synapsins (SYN1/2), NMDAR and AMPAR subunits were occurred in the SIK3 overexpression group. We also found significant decreases in the special binding regions of CaMKIIα, SYN2, GRI2A and GRIA1 in the SIK3 knockdown group (Fig. 4C).
Fig. 4. HDAC4 regulates the transcription of genes related to synaptic plasticity through mef2c and histone acetylation.
A Representative immunoblots and quantitative analyses of SIK3, mef2c, ac-H3K9, ac-H3K27, ac-H4K12, p-HDAC4/HDAC4, H3 and H4 in the SIK3 overexpression or knockdown groups. n = 3. B Representative immunoblots and quantitative analyses of SIK3, mef2c, ac-H3K9, ac-H3K27, ac-H4K12, p-HDAC4/HDAC4, H3 and H4 in WT- control, WT-shSIK3, 5×FAD-control and 5×FAD-shSIK3 mice (n = 3 per group). C ChIP-qPCR analyses of the enrichment of mef2c at CaMKIIα, SYN1, SYN2, GRIN1, GRI2A, GRI2B, GRIA1 and GRIA2 promoters in the SIK3 overexpression or knockdown cells. Rabbit IgG was used as the negative control. TSS, transcriptional start site. n = 4–6 per group. D ChIP-qPCR analyses of the enrichment of ac-H3K9, ac-H3K27 and ac-H4K12 at CaMKIIα, SYN1, SYN2, GRIN1, GRI2A, GRI2B, GRIA1 and GRIA2 promoters in the SIK3 overexpression or knockdown cells. Rabbit IgG was used as the negative control. TSS, transcriptional start site. n = 4–6 per group. Data are expressed as mean ± SEM. Statistical significance was calculated by unpaired two-tailed t-test (A, C, D), one-way ANOVA (A) and two-way ANOVA (B) followed by the Tukey’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
The catalytic activity of class IIa histonedeacetylase on acetylated lysines of histone tail peptides is very low when compared to Class I HDACs [32]. Nonetheless, they still exert a strong histone deacetylase activity, depending on the recruiting of different Class I HDACs, such as HDAC3. Accordingly, we examined the effects of SIK3 on the level of histone acetylation. In hippocampal neuronal cultures, western blot results showed that the levels of ac-H3K9/H3, ac-H3K27/H3 and ac-H4K12/H4 were decreased in SIK3 knockdown cells. And the overexpression of SIK3 increased the levels of ac-H3K9/H3 (Fig. 4A). Similarly, in vivo experiments showed that the amount of ac-H3K9/H3 and ac-H4K12/H4 were decreased in 5×FAD-control mice, compared to WT-control mice, while SIK3 knockdown revealed a further descend level of ac-H3K9/H3, ac-H3K27/H3 and ac-H4K12/H4 in the 5×FAD-shSIK3 group (Fig. 4B). In hippocampal neuronal cultures, the SIK3 group showed increased occupancy of ac-H3K9 at CaMKIIα, GRIA1, GRIA2 gene promoters; and enhanced enrichment of ac-H3K27 at CaMKIIα, SYN1, SYN2, GRIN1, GRI2A, GRI2B, GRIA1 and GRIA2 gene promoters; and elevated possession of ac-H4K12 at CaMKIIα, SYN1, SYN2, GRIN1, GRI2A, GRI2B, GRIA1 and GRIA2 gene promoters, compared to the control cells (Fig. 4D). In contrast, SIK3 knockdown mostly led to a decreased occupancy of ac-H3K9 at SYN1, SYN2 and GRIA2; a reduced enrichment of ac-H3K27 at SYN2 and GRI2A; and declined possession of ac-H4K12 at CaMKIIα, SYN1 and GRIN1 (Fig. 4D), which was consistent with downregulated mRNA expression of these genes in WT-shSIK3 and 5×FAD-shSIK3 mice (Fig. 2G and Supplementary Fig. S5A). The differential mRNA expression of other genes related to synaptic plasticity was also determined in vivo experiments (Supplementary Fig. S5B). Collectively, these data demonstrate that HDAC4 regulates the transcription of genes related to synaptic plasticity through the transcription factors mef2c or histone acetylation modification at promoter sites of target genes.
The SLP-S upregulation restores the synaptic plasticity and alleviates the cognitive impairment in middle-aged 5×FAD mice
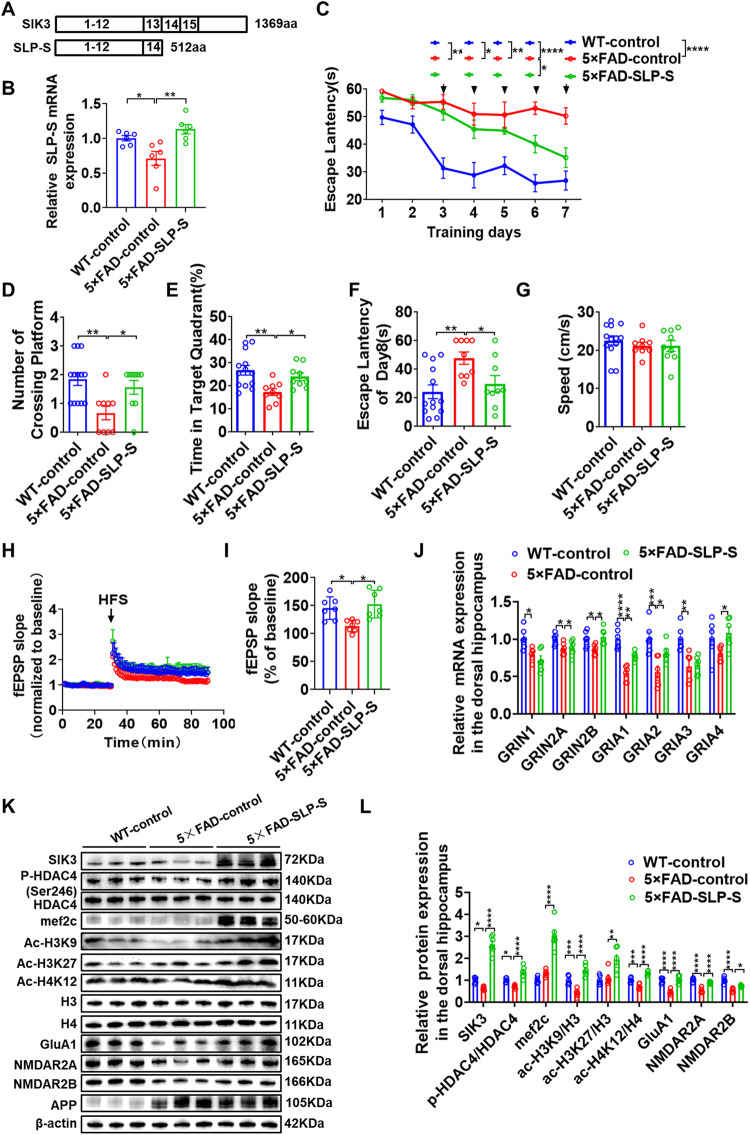
Lentiviral vector containing SIK3 was constructed because the large size of the SIK3 coding sequence (4180 base pairs) exceeds the packaging capacity of adeno-associated virus (AAV) vectors. Unfortunately, SIK3 was not successfully overexpressed in dorsal hippocampal neurons (Supplementary Fig. S6A, B). Therefore, we generated SLP-S fragment, which is essentially a truncated protein missing SIK3 exon 13 and half of the C-terminal of SIK3, and packaged it into an AAV vector (Fig. 5A) (Supplementary Fig. S6C) [33]. QPCR and Western blot analyses showed that SLP-S was obviously upregulated in the middle-aged 5×FAD mice (Fig. 5B, K). Next, we examined whether SLP-S upregulation can rescue the impaired spatial learning and memory in the MWM test. During the first 2 trained days, we found no significant differences between the four groups. However, 5×FAD-control mice showed much higher escape latencies from days 3, compared to WT-control mice (Fig. 5C). We also found that the escape latencies in 5×FAD-SLP-S mice were significantly reduced at day 6 compared to 5×FAD-control (Fig. 5C). In the probe test, the number of platform crossings in the WT-control and 5×FAD-SLP-S group were higher than those of the 5×FAD-control mice (Fig. 5D). Mice in the WT-control and 5×FAD-SLP-S groups spent significantly more time in the target quadrant compared to 5×FAD-control group (Fig. 5E). Similarly, compared with the 5×FAD-control group, the escape latency was prolonged in WT-control and 5×FAD-SLP-S groups (Fig. 5F), with no noticeable changes in swimming speed between these groups (Fig. 5G). These results indicate that SLP-S upregulation alleviates the cognitive impairment in the middle-aged 5×FAD mice.
Fig. 5. The SLP-S upregulation restores the synaptic plasticity and alleviates the cognitive impairment in 5×FAD mice.
A Schematic of different isoforms of SIK3 proteins. SLP-S, the truncated fragment of SIK3. B Overexpression efficiency of SLP-S was examined by qPCR. n = 3. C–G WT-control, 5×FAD-control and 5×FAD-SLP-S mice were tested in the Morris Water Maze. C Escape latency to the platform position during the training trails (1–7 d). D The number of platform-position crossings. E The percentage of time spent in the target quadrant and the speed (G) in the probe trial (8d). F Escape latency to the platform position during the training trails (1–7d) and the probe (8d) trial. n = 8 for male and n = 7 for female mice in WT-control group, n = 5 for male and n = 4 for female mice in 5×FAD-control group, n = 5 for male and n = 4 for female mice in 5×FAD-SLP-S group. H, I Hippocampal CA1 LTP recordings from WT-control, 5×FAD-control, and 5×FAD-SLP-S groups. HFS, high-frequency stimulation. WT-control (n = 3 mice, 7 slices), 5×FAD-control (n = 3 mice, 7 slices), and 5×FAD –SLP-S (n = 3 mice,7 slices). J qPCR was used to detect NMDAR subunit (GRIN1/2A/2B) and AMPAR subunit (GRIA1/2/3) mRNA levels in the dorsal hippocampus of WT-control, 5×FAD-control and 5×FAD-SLP-S groups. n = 6 per group. K, L Representative immunoblots and quantitative analyses of SIK3, mef2c, ac-H3K9, ac-H3K27, ac-H4K12, p-HDAC4/HDAC4, H3, H4, GluA1, NMDAR2A and NMDAR2B in the dorsal hippocampus of WT-control, 5×FAD-control and 5×FAD-SLP-S groups (n = 6 per group). Data are expressed as mean ± SEM. Statistical significance was calculated by one-way ANOVA (B, D–L), and three-way ANOVA (C) followed by the Tukey’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To explore the effects of SLP-S on hippocampal synaptic plasticity, we first performed field recordings using hippocampus slices. In slices obtained from WT-control mice, a significantly slower decay of LTP was observed in slices from 5×FAD-control mice (Fig. 5H). Nevertheless, fEPSPs was improved in the 5×FAD-SLP-S mice (Fig. 5I). Then we detected the mRNA and protein levels of the NMDAR and AMPAR subunits in the dorsal hippocampus region. Compared with the WT-control group, the 5×FAD-control group exhibited an evident reduction in the mRNA level of GRIN1, GRIN2A, GRIN2B, GRIA1, GRIA2, and GRIA3, which were elevated by SLP-S upregulation (Fig. 5J). The differential mRNA expression of other genes related to synaptic plasticity was also determined in vivo experiments (Supplementary Fig. S5C, D). Similarly, the immunoblotting experiments indicated that GluA1, GRIN2A and GRIN2B protein were significantly reduced in the 5×FAD-control group, which were all increased by SLP-S upregulation.
To test whether SLP-S upregulation restores the synaptic plasticity in the 5×FAD mice by modulating the HDAC4 activity, western blot was performed to determine the expression level of p-HDAC4, mef2c and histone acetylation (Fig. 5K). The amount of p-HDAC4, ac-H3K9 and ac-H4K12 in the 5×FAD-control group was significantly lower than in the WT-control group, which were all increased by SLP-S upregulation (Fig. 5L). This indicates that up-regulation of SLP-S, the truncated fragment of SIK3, restores the synaptic plasticity and alleviates the cognitive impairment in middle-aged 5×FAD mice by regulating the activity of HDAC4.
Discussion
The SIKs subfamily—SIK1, SIK2 and SIK3—are members of the AMPK family, the major regulator of energy homeostasis in cells and provides of body energy. The SIKs share sequence homology in their kinase domains, but their distribution are totally different. SIK2 is highly distributed in adipose and nerve cells, while SIK1 and SIK3 show a more general expression. The expression patterns of SIK3 in the mouse brain indicated that the SIK3 gene is relevant for brain development. In turn, SIK3 function/expression might be important for neurodegeneration disease and aging. We also found that the conditional deletion of SIK3 had no significant effect on SIK1 and SIK2 expression (Supplementary Fig. S3E), indicating that SIK3 functioned independently of these two genes. Hence, the role of SIK1 and SIK2 in the pathogenesis of AD also needs to be further studied.
Memory and learning deficits are highly correlated with aberrant synaptic plasticity. LTP results could reflect changes in the strength of the synaptic connection, which was a manifestation of functional synaptic plasticity. In our results, SIK3 knockdown had no significant effect on the behavioral measures of WT mice, but it was observed to impair the maintenance of LTP in WT mice. The decline in SIK3 expression maybe positively correlated with impaired spatial cognition. The hyper-reduced SIK3 levels in AD mice, which already start off with lower SIK3 levels, were required to disrupt memory, so SIK3 knockdown in WT mice may not have additional reduced behavioral impact. This also may indicate that SIK3 may be a mechanism already contributing to the reduced LTP in WT mice, but how SIK3 induces the production of LTP will be studied in the future.
The activation of SIK3 involves the regulation of several signaling pathways in the physiological process. SIK3 activated PI3K-AKT-mTOR signaling transduction pathways linked to breast and colorectal cancer growth and apoptosis [34, 35]. SIK2/3 induced adipogenesis/lipogenesis and inflammatory adipocytokines secretion in adipocytes via activating mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and AKT-mTOR pathways [36]. Our previous work demonstrated that SIK represses gastric tumorigenesis by inhibiting AKT/GSK3β/β-catenin signaling [37]. SIK3-HDAC4 is involved in several physiological and pathological processes, such as acute myeloid leukemia progression, skeletal development, and sleep quantity and depth [26, 27, 38, 39]. Moreover, these classical AKT/mTOR/GSK3β/MAPK/HDACs signaling pathways have all been implicated in synaptic plasticity [16, 40–43]. We therefore tested whether those SIK3 related signaling is dysregulated in cells SIK3 overexpressing or having the gene knockdown. Our results revealed that SIK3 increased the phosphorylation levels of HDAC4, with Co-IP further demonstrating that HDAC4 was a direct target of SIK3.
Previous study indicated that HDAC4 was a transcriptional repressor that regulates a group of “synaptic” genes [16]. Based on our experiments, ChIP-qPCR analysis demonstrated a slight increase in HDAC4 binding to “synaptic” genes (data not shown). Hence, we hypothesize that HDAC4 may not regulate transcription directly but through the inhibition of other transcription factors. HDAC4 modulates the activities of multiple transcriptional factors, including MEF2, CREB, RUNX2 and FOXO [44–49]. Mef2c have been identified or confirmed in GWAS analysis, and are since regarded as established risk genes/loci for late-onset AD [50, 51]. We were thus interested in examining the key regulatory activity of mef2c. And then we screened the promoter regions (0 to −2000bp relative to TSS) of target genes with a putative mef2c consensus binding motif (TTTAAAATAG or CTATAAATAG), including CaMKIIα, SYN, NMDAR and AMPAR subunits. We utilized several ChIP-qPCR experiments to reveal that mef2c specifically binds to the promoter regions of target genes, which is consistent with previous studies [52, 53]. The other mechanism of HDAC4 regulation of cellular processes is recruiting HDAC3.ChIP-qPCR analysis was also performed to evaluate the enrichment of histone acetylation on the promoter regions of genes related to synaptic plasticity. In conclusion, we report for the first time that SIK3-HDAC4 regulates the expression of genes related to synaptic plasticity via direct transcriptional repression and/or involving histone acetylation modifications at promoter regions of targeted genes.
Our data have shown that SIK3 expression was decreased in the middle-aged AD transgenic mouse models. Moreover, SIK3 protein levels in the 5×FAD mice were markedly decreased during age, while no significant changes were observed in WT mice. Therefore, we only carried out the SLP-S overexpression in 5×FAD mice, but not in WT mice, to explore whether it had a rescue effect on memory. To verify whether SIK3 was also required for memory in healthy mice, we performed the knockdown of SIK3 in WT mice.
These findings confirm an unidentified and important mechanism of SIK3-HDAC4 in the brain of AD mice and have implications for drug discovery (Supplementary Fig. S6D). Further characterization of its effects, particularly in the development of small molecules of SIK3 activators, will shed light onto the mechanisms of action that support its therapeutic potential for treating neurodegenerative diseases.
Supplementary information
Acknowledgements
We thank Professor Qinghua Liu from National Institute of Biological Sciences, Beijing, China for providing the SLP-S plasmid. We thank Guanghao Liu from Department of Bioinformatics, Fujian Medical University for bioinformation analysis.
Author contributions
XD performed the experiments and wrote the manuscript; AL performed electrophysiological recording of brain slices and data analysis; LZ designed and validated viruses. QZ performed the breeding of mice; LC, YW, HL, and WG completed virus injection, animal behavior and behavioral analysis; JZ and XC conceived and designed the project, and prepared and revised the manuscript; all authors read and commented on the manuscript.
Funding
This work was supported by grants to Xiaoman Dai from the National Natural Science Foundation of China (No.82101481), the Excellent Young Scholars Cultivation Project of Fujian Medical University Union Hospital (No.2022XH032), the Health and Family Planning Commission of Fujian Province (No.2021GGA012), the Science and Technology Program of Fujian Province (No. 2022J01250). This work was also supported by grants to Xiaochun Chen from the National Natural Science Foundation of China (No. U21A20362).
Data availability
The authors declare that all data supporting the findings of this study are available in this article and its Supplementary information files. Further inquiries can be directed to the corresponding author.
Competing interests
The authors declare no competing interests.
Consent for publication
All authors read and approved the final manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaoman Dai, Anlan Lin.
Contributor Information
Jing Zhang, Email: drzj@fjmu.edu.cn, Email: drzj@163.com.
Xiaochun Chen, Email: chenxc998@fjmu.edu.cn, Email: chenxc998@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01775-1.
References
- 1.John A, Reddy PH. Synaptic basis of Alzheimer’s disease: focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res Rev. 2021;65:101208. doi: 10.1016/j.arr.2020.101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Styr B, Slutsky I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat Neurosci. 2018;21:463–73. doi: 10.1038/s41593-018-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM. Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci. 2017;40:347–57. doi: 10.1016/j.tins.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RR, Wood MA. How the epigenome integrates information and reshapes the synapse. Nat Rev Neurosci. 2019;20:133–47. doi: 10.1038/s41583-019-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Kaang BK. Epigenetic regulation and chromatin remodeling in learning and memory. Exp Mol Med. 2017;49:e281. doi: 10.1038/emm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9:477. doi: 10.1038/s41467-017-02794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustos FJ, Ampuero E, Jury N, Aguilar R, Falahi F, Toledo J, et al. Epigenetic editing of the Dlg4/PSD95 gene improves cognition in aged and Alzheimer’s disease mice. Brain. 2017;140:3252–68. doi: 10.1093/brain/awx272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halder R, Hennion M, Vidal RO, Shomroni O, Rahman RU, Rajput A, et al. DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nat Neurosci. 2016;19:102–10. doi: 10.1038/nn.4194. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy AJ, Rahn EJ, Paulukaitis BS, Savell KE, Kordasiewicz HB, Wang J, et al. Tcf4 regulates synaptic plasticity, DNA methylation, and memory function. Cell Rep. 2016;16:2666–85. doi: 10.1016/j.celrep.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penney J, Tsai LH. Histone deacetylases in memory and cognition. Sci Signal. 2014;7:re12. doi: 10.1126/scisignal.aaa0069. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhang J, Li D, He C, He K, Xue T, et al. Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory. Neuron. 2021;109:957–70.e958. doi: 10.1016/j.neuron.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Sando R, 3rd, Gounko N, Pieraut S, Liao L, Yates J, 3rd, Maximov A. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell. 2012;151:821–34. doi: 10.1016/j.cell.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler PG, Huang D, Dai Z. Haploinsufficiency of HDAC4 does not cause intellectual disability in all affected individuals. Am J Med Genet A. 2014;164a:1826–9. doi: 10.1002/ajmg.a.36542. [DOI] [PubMed] [Google Scholar]
- 18.Williams SR, Aldred MA, Der Kaloustian VM, Halal F, Gowans G, McLeod DR, et al. Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. Am J Hum Genet. 2010;87:219–28. doi: 10.1016/j.ajhg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schlüter OM, Bradke F, et al. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner FF, Zhang YL, Fass DM, Joseph N, Gale JP, Weïwer M, et al. Kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci. 2015;6:804–15. doi: 10.1039/c4sc02130d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis YI, Fà M, Ashraf H, Zhang H, Staniszewski A, Latchman DS, et al. Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J Alzheimers Dis. 2009;18:131–9. doi: 10.3233/JAD-2009-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Liu Q. Age- and disease-related memory decline: epigenetic biomarker and treatment. Sci Bull (Beijing) 2023;68:1719–21. doi: 10.1016/j.scib.2023.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Nishimori S, Wein MN, Kronenberg HM. PTHrP targets salt-inducible kinases, HDAC4 and HDAC5, to repress chondrocyte hypertrophy in the growth plate. Bone. 2021;142:115709. doi: 10.1016/j.bone.2020.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi F, de Fatima Silva F, Liu D, Patel HU, Xu J, Zhang W, et al. Salt-inducible kinase inhibition promotes the adipocyte thermogenic program and adipose tissue browning. Mol Metab. 2023;74:101753. doi: 10.1016/j.molmet.2023.101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, et al. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou R, Wang G, Li Q, Meng F, Liu C, Gan R, et al. A signalling pathway for transcriptional regulation of sleep amount in mice. Nature. 2022;612:519–27. doi: 10.1038/s41586-022-05510-6. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Hotta-Hirashima N, Asano F, Kitazono T, Iwasaki K, Nakata S, et al. Kinase signalling in excitatory neurons regulates sleep quantity and depth. Nature. 2022;612:512–8. doi: 10.1038/s41586-022-05450-1. [DOI] [PubMed] [Google Scholar]
- 28.Asano F, Kim SJ, Fujiyama T, Miyoshi C, Hotta-Hirashima N, Asama N, et al. SIK3-HDAC4 in the suprachiasmatic nucleus regulates the timing of arousal at the dark onset and circadian period in mice. Proc Natl Acad Sci USA. 2023;120:e2218209120. doi: 10.1073/pnas.2218209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herring BE, Nicoll RA. Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu Rev Physiol. 2016;78:351–65. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- 30.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 31.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–41. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci USA. 2007;104:17335–40. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Zhou R, Wang G, Guo Y, Gao X, Zhou S, et al. Regulation of sleep quantity and intensity by long and short isoforms of SLEEPY kinase. Sleep. 2022;45:zsac198. [DOI] [PubMed]
- 34.Ponnusamy L, Kothandan G, Manoharan R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165897. doi: 10.1016/j.bbadis.2020.165897. [DOI] [PubMed] [Google Scholar]
- 35.Yan P, Wang Y, Meng X, Yang H, Liu Z, Qian J, et al. Whole exome sequencing of ulcerative colitis-associated colorectal cancer based on novel somatic mutations identified in Chinese patients. Inflamm Bowel Dis. 2019;25:1293–301. doi: 10.1093/ibd/izz020. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Sorn SR, Lee Y, Kang I. Salt induces adipogenesis/lipogenesis and inflammatory adipocytokines secretion in adipocytes. Int J Mol Sci. 2019;20:160. [DOI] [PMC free article] [PubMed]
- 37.Dai XM, Zhang YH, Lin XH, Huang XX, Zhang Y, Xue CR, et al. SIK2 represses AKT/GSK3β/β-catenin signaling and suppresses gastric cancer by inhibiting autophagic degradation of protein phosphatases. Mol Oncol. 2021;15:228–45. doi: 10.1002/1878-0261.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarumoto Y, Lin S, Wang J, Milazzo JP, Xu Y, Lu B, et al. Salt-inducible kinase inhibition suppresses acute myeloid leukemia progression in vivo. Blood. 2020;135:56–70. doi: 10.1182/blood.2019001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasagawa S, Takemori H, Uebi T, Ikegami D, Hiramatsu K, Ikegawa S, et al. SIK3 is essential for chondrocyte hypertrophy during skeletal development in mice. Development. 2012;139:1153–63. doi: 10.1242/dev.072652. [DOI] [PubMed] [Google Scholar]
- 40.O’ Neill C. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol. 2013;48:647–53. doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 42.Narvaes RF, Furini CRG. Role of Wnt signaling in synaptic plasticity and memory. Neurobiol Learn Mem. 2022;187:107558. doi: 10.1016/j.nlm.2021.107558. [DOI] [PubMed] [Google Scholar]
- 43.Perry S, Kiragasi B, Dickman D, Ray A. The role of histone deacetylase 6 in synaptic plasticity and memory. Cell Rep. 2017;18:1337–45. doi: 10.1016/j.celrep.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanchard FJ, Collins B, Cyran SA, Hancock DH, Taylor MV, Blau J. The transcription factor Mef2 is required for normal circadian behavior in Drosophila. J Neurosci. 2010;30:5855–65. doi: 10.1523/JNEUROSCI.2688-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivachenko A, Li Y, Abruzzi KC, Rosbash M. The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior. Neuron. 2013;79:281–92. doi: 10.1016/j.neuron.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Z, Zhang Z, Guo L, Wei X, Zhang Y, Wang X, et al. The role of histone deacetylase 4 during chondrocyte hypertrophy and endochondral bone development. Bone Joint Res. 2020;9:82–89. doi: 10.1302/2046-3758.92.BJR-2019-0172.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013;9:319–25. doi: 10.1038/nchembio.1223. [DOI] [PubMed] [Google Scholar]
- 49.Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN, Jin YH, et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem. 2006;281:16502–11. doi: 10.1074/jbc.M512494200. [DOI] [PubMed] [Google Scholar]
- 50.Verheijen J, Sleegers K. Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet. 2018;34:434–47. doi: 10.1016/j.tig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Barker SJ, Raju RM, Milman NEP, Wang J, Davila-Velderrain J, Gunter-Rahman F, et al. MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci Transl Med. 2021;13:eabd7695. doi: 10.1126/scitranslmed.abd7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaudhary R, Agarwal V, Kaushik AS, Rehman M. Involvement of myocyte enhancer factor 2c in the pathogenesis of autism spectrum disorder. Heliyon. 2021;7:e06854. doi: 10.1016/j.heliyon.2021.e06854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Q, Telese F. Genome-wide epigenetic analysis of MEF2A and MEF2C transcription factors in mouse cortical neurons. Commun Integr Biol. 2015;8:e1087624. doi: 10.1080/19420889.2015.1087624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available in this article and its Supplementary information files. Further inquiries can be directed to the corresponding author.