Abstract
Background
Liver donation after cardiac death (DCD) makes up a small percentage of the organs used in transplantation and poses a higher risk of graft loss compared to donation after brain death (DBD); this is a result of ischemia reperfusion for which the exact injury mechanisms are currently not fully understood. However, reperfusion injury has been shown to lead to necrosis as well as apoptosis through oxidative stress and mitochondrial dysfunction. In this work, we propose that use of the pro-survival, anti-apoptotic CEPT cocktail in post-ischemia normothermic machine perfusion (NMP) may improve recovery in rat livers subjected to extended durations of warm ischemia.
Materials and Methods
Livers procured from male Lewis rats were subjected to 90 min of warm ischemia, followed by 6 h of NMP where they were treated either with the survival-enhancing anti-apoptotic cocktail (CEPT), the vehicle (DMSO) or the base media with no additives.
Results
The CEPT-treated group exhibited lower expression of hepatic injury biomarkers, and improvement in a range of hepatocellular symptoms associated with the hepatic parenchyma, biliary epithelium and the sinusoidal endothelium, including recovery of bile secretion and lowered vascular resistance.
Conclusions
This study's findings suggest apoptosis plays a more significant role in ischemia-reperfusion injury than previously understood, and provide useful insight for further investigation of the specific underlying mechanisms and development of novel treatment methods.
Keywords: Liver preservation, Ischemia-reperfusion injury, Machine perfusion, DCD, Marginal donors
1. Introduction
The growing use of novel ex-vivo methods of graft viability assessment such as normothermic machine perfusion (NMP) over standard static cold storage (SCS) has been shown to lead to an increase in overall graft survival as well as liver-recipient matching by expanding the transplantation criteria to include donation after cardiac death (DCD) [1,2]. However, despite the increased use of such marginal livers, there remains a notable gap between transplant demand and donor availability, which can potentially be narrowed by addressing the underlying mechanisms of injury in livers severely damaged by warm ischemia [3].
DCD donors are often rejected for transplantation and make up a small percentage of the donor pool due to a significantly higher risk of early graft dysfunction compared to donation after brain death (DBD) [4]. This is primarily a result of ischemia-reperfusion injury (IRI), a cascade of acute inflammatory responses initiated by injured hepatic cells upon restoration of circulation within the graft. While the mechanisms involved in this phenomenon are not fully understood, it is known that IRI triggers several cell death pathways of not only the inflammatory kind including necrosis, necroptosis, pyroptosis and ferroptosis, but also apoptosis [5], suggesting that intercepting cell death pathways may be a practical means of improving overall liver recovery following IRI. Additionally, hypoxic ischemia has been shown to result in heightened oxidative stress, causing critical damage to cellular components including DNA, proteins and lipids [6]; lack of oxygen delivery during ischemia leads to increased production and accumulation of reactive oxygen species (ROS), which can in turn trigger apoptosis and an arrest of the cell cycle upon reperfusion [7]. Previous studies have validated the potential of this hypothesis by demonstrating that inhibition of hepatocellular death through caspase inhibition results in lower expression of inflammatory molecules, amelioration of sinusoidal dysfunction and improved hepatocyte phenotype in chronically diseased rat livers [8]; however, the effects of such treatments on IRI in liver transplantation remains relatively understudied.
To explore the extent of apoptotic contribution to the effects of IRI in liver, we developed a protocol for recovery of male rat livers subjected to 90 min of warm ischemia (WI) at 37 °C, where during 6 h of post-ischemia NMP they were treated with CEPT, a survival-enhancing anti-apoptotic cocktail consisting of Chroman 1, Emricasan, Polyamines and Trans-ISRIB, previously shown to enhance the viability of human pluripotent stem cells (hPSCs) and the resulting differentiated cells [9]. Knowing that the effects of CEPT are currently understudied in the context of machine perfusion, we aim to specifically explore its efficacy in halting the exacerbation of cellular injury following ischemia-reperfusion and facilitating restoration of tissue functionality.
2. Materials and methods
2.1. Experimental design
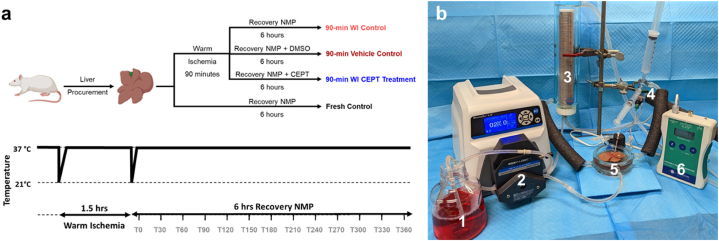
As illustrated in Fig. 1a, whole livers were procured from adult Lewis rats and underwent four conditions (n = 5 for each group): (1) Fresh Control: Immediate connection to machine perfusion device (2) 90-min warm ischemia (WI) CEPT Treatment (3) 90-min WI Vehicle (DMSO) Control (4) 90-min WI Control. The WI treatment was carried out identically for groups 2–4 through 1.5 h of storage in a bag of 0.9 % sodium chloride solution (Baxter, 2B1322Q), kept in a water bath set at 37 °C. All groups followed with 6 h of NMP at 37 °C using the machine perfusion setup shown in Fig. 1b. The CEPT cocktail or DMSO were added directly to the NMP base perfusate.
Fig. 1.
Experimental design. (a) Schematic illustration of the experimental design and groups (n = 5 per group) (b) Perfusion setup consisting of: 1. Perfusion media 2. Digital Peristaltic Pump 3. Oxygenation chamber 4. Bubble trap 5. Perfusion basin 6. Pressure monitor.
2.2. Perfusate preparation
Recovery perfusate was composed from a base of 500 mL Williams’ Medium E (with sodium bicarbonate, without L-glutamine, with phenol red) (Sigma-Aldrich, W1878) supplemented with 285.7143 nM polyethylene glycol 35,000 (PEG) (Sigma-Aldrich, 81,310), 61.1527 μM dexamethasone (water-soluble) (Sigma-Aldrich, D4902), 150.5344 μM bovine serum albumin (Sigma-Aldrich, A7906), 9.5227 mM sodium bicarbonate (Sigma-Aldrich), 10 U/mL sodium heparin (Pfizer Inc, 00409272002), and 40 U/mL penicillin-streptomycin (Gibco, 15140122) were added. On the day of experiment, 2 mM L-glutamine (Gibco, 25030081), 0.01 U/regular Insulin (Eli Lilly and Company, 0002821501), 25 μg/mL hydrocortisone sodium succinate (Pfizer, 00009001305) and 4.998 mM L-glutathione (Sigma-Aldrich, G4251) were added directly to perfusate before beginning perfusion. For the CEPT perfusate, 50 nM chroman 1 (MedChemExpress, HY-15392), 5 μM Emricasan (Selleck Chemical, S77755MG), 0.7 μM trans-ISRIB (Tocris Bioscience 5284), 40 ng/mL putrescine (Sigma-Aldrich, P5780), 4.5 ng/mL spermidine (Sigma-Aldrich, S0266), and 8 ng/mL spermine (Sigma-Aldrich, S4264), all suspended in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, 5.89569) were added directly to perfusate before beginning perfusion; the concentrations were identical to those of the original CEPT cocktail. For vehicle perfusate, 800 μL DMSO was added directly to perfusate before beginning perfusion.
2.3. Liver procurement
Livers from adult, male Lewis rats (250–300 g) (Charles River Laboratories, Boston MA, USA) were elected to be used for all experiments, after preliminary testing of the study design with female rats of the same strain and weight proved to be too damaging to the livers to allow for any recovery after the proposed ischemic duration, jeopardizing the unbiased comparability of results among different groups. In total, livers from 3 female rats underwent the experimental design, representative data for which can be found in Supplementary Fig. 1. The basis of this difference will be explored in future studies. All animals were socially housed in controlled, standard conditions (21 °C, 12-h light/dark cycle, 30–70 % humidity, mixed paper/cellulose bedding, pathogen-free HEPA filtered ventilated cages). The rats had unrestricted access to sterile water and chow, in accordance with National Research Council Guidelines. Rats were cared for by the Massachusetts General Hospital (MGH) Center for Comparative Medicine (CCM). The experimental protocol was approved by the Institutional Care and Use Committee (IACUC) of MGH (Protocol Number 2017N000227), and all experiments were carried out in accordance with guidelines established in said protocol.
Livers were procured from male Lewis rats (250–300g; age 10–12 weeks; Charles River Laboratories, Wilmington, MA, USA). Donor rats were anaesthetized under 5 % isoflurane. The abdomen was opened via a transverse abdominal incision. Ligaments connecting the superior and inferior portions of the liver were dissected and the portal vein was exposed. The gastric and splenic branches of the portal vein, as well as the hepatic artery were ligated using 6-0 silk. The bile duct was partially dissected, cannulated using 24g tubing, and secured with 6-0 silk. The inferior vena cava was heparinized with 1 U/g using a 30-gauge insulin syringe. Following 5 min of heparin circulation, the portal vein was cannulated with a 16g cannula, followed by transection of the IVC. The cannula was connected to 16g tubing attached to a 50 mL syringe containing 1 mL heparin in 60 mL saline. The portal vein was hand flushed at 10 mL/min for 4 min, after which the remaining connective tissue was dissected, and the liver was removed from the body cavity. Following removal, the liver was flushed with the remaining 20 mL saline, immediately weighed, and connected to the perfusion system, keeping warm ischemic time below 5 min.
2.4. Normothermic machine perfusion
Perfusate circulation was carried out using a roller pump system (Masterflex L/S, Vernon Hills, IL) with two separate sets of tubing delivering perfusate into and out of the 500 mL perfusion reservoir. The system was consistently kept at a temperature of 37 °C via a water bath (PolyScience, Niles, Illinois, USA) continuously pumping heated water through the double-jacketed perfusion system components (Radnoti, Covina, CA, USA). Perfusate oxygen concentration was maintained within a close range of 500 mmHg using a 95 % O2/5 % CO2 gas cylinder (Airgas, Radnor, PA, USA). System pressure was zeroed, the liver was placed in the tissue bath and connected to the system. The flow rate was brought from 5 mL/min to 30 mL/min gradually, maintaining a maximum portal pressure of 5 mmHg. Outflow samples were collected every 30 min from the IVC, while inflow samples were collected from a port placed above the cannula perfusing the portal vein. The liver was weighed upon the end of perfusion to determine weight change. Biopsies of the left lateral lobe (LLL) and right medial lobe (RML) were carried out immediately after, with the LLL sample being snap frozen in liquid nitrogen for subsequent ATP analysis and the RML sample being formalin-fixed for histological analysis.
2.5. Liver viability assessment
Every 30 min, inflow and outflow perfusate samples were analyzed using a Siemens Rapidpoint 500 (Siemens, Munich, Germany). Liver performance metrics were analyzed to determine liver functionality during perfusion (pH, O2 consumption, lactate clearance, potassium). Following perfusion, hourly outflow samples were analyzed for hepatic injury markers (AST and ALT enzymes) using a Piccolo Xpress (Abaxis, Union City, CA, USA). Portal resistance was determined using pressure readings taken every half hour, and defined as pressure divided by flow, normalized to weight (g). Oxygen consumption was defined as portal inflow pO2 minus IVC outflow pO2. Weight change was defined as final weight minus initial weight divided by initial weight.
2.6. Histological analysis
Liver tissue samples were sectioned and stained with hematoxylin-eosin (H&E) and Terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) (Specialized Histopathology Services Core, MGH, Charlestown, MA, USA).
To quantify TUNEL, the Trainable Weka Segmentation add-on for Fiji [16] was utilized. Briefly, a TUNEL analysis classifier was trained using 2 classes: dead cells and background. To train the model, sample images were classified by hand with dark brown nuclei tagged as dead; and extracellular space and purple nuclei tagged as background. The classifier was then applied to 10 views per group. Following image classification, the classified images, and unclassified images were set to the same threshold, after which, particle analysis was performed with the same size threshold. Using the obtained counts, the ratio between live and dead cells was determined.
2.7. Caspase 3/7 activity assay
For caspase 3/7 activity measurements, livers were sacrificed at the end of each perfusion and 300–500 mg of the livers were flash-frozen in liquid nitrogen for −80 °C storage. On the day of measurement, the frozen livers were added to ice-cold PBS at 50 mg/mL and prepared for tissue homogenization (n = 3 per treatment group) using the gentleMACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). Then, equal volume of room-temperature PBS was added to the lysate to dilute to 25 mg/mL and 100 μL of the diluted lysate was treated with room-temperature Caspase-Glo 3/7 luminescent assay (Promega, Madison, Wisconsin, USA), adding 1:1 volume of caspase-glo to liver lysate in a 96-well white-bottom plate. The plate was mixed using a plate shaker set to 250 rpm for 30 s and then left to incubate at room temperature for 30 min. Following incubation, the luminescence was read using a plate reader.
2.8. Statistical analysis and illustrations
Data are presented as mean ± SD, and differences are considered significant when p < 0.05. Statistical analysis was performed with Prism 8 software Version 9.1.2 (Graphpad Software, San Diego, CA, USA, graphpad.com). Two-way analysis of variance (ANOVA) was performed to compare time-dependent perfusion data, followed by Tukey's post-hoc test to examine statistical difference. Perfusion metrics were reported as means, with standard deviation as error bars. Statistical analysis was performed with Prism 8 software Version 9.1.2 (Graphpad Software, San Diego, CA, USA, graphpad.com) with a two-sided significance level of 0.05. Two-way analysis of variance (ANOVA) was performed to compare time-dependent perfusion data, followed by Tukey's post-hoc test to examine statistical difference. Perfusion metrics were reported as means, with standard deviation as error bars. All illustrations were created with Biorender (Toronto, ON, Canada).
3. Results
The experimental design and machine perfusion setup are outlined in Fig. 1a and b respectively.
3.1. Liver viability during recovery perfusion
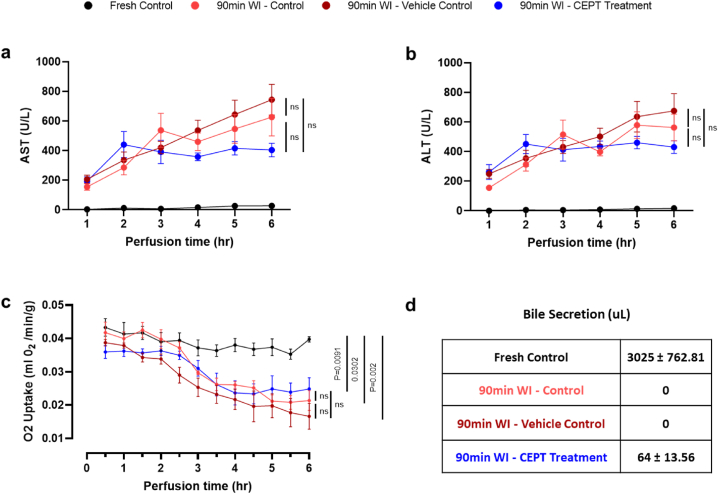
ALT and AST enzyme levels in the perfusate (Fig. 2a and b) serve as markers of hepatocellular injury. In CEPT-treated livers, AST levels (Fig. 2a) rose in the first 2 h of perfusion, but then decreased and stabilized. In contrast, the 90-min WI control and vehicle control livers exhibit an accumulation of AST for the entire duration of perfusion. ALT levels in the perfusate (Fig. 2b) follow a similar trend, although the difference among experimental groups was not as pronounced. The differences observed between the ischemic control groups and the CEPT treatment group were not found to be statistically significant (P > 0.05). Fresh control livers that did not undergo the additional 90-min warm ischemia maintained close-to-zero ALT and AST levels throughout the 6-h period of NMP.
Fig. 2.
Liver viability following 1.5 h of ex-vivo warm ischemia. Concentrations of (a) Aspartate aminotransferase (AST) and (b) Alanine transaminase in perfusate samples collected hourly from the IVC. Lower concentrations near the end of the 6-h NMP suggest higher rates of recovery from ischemic damage in livers treated with CEPT. (c) Rate of oxygen uptake in ex-vivo perfusion measured every 30 min and normalized to liver weight. (d) Total volume of bile produced during 6 h of NMP, compared across the 90-min WI control, 90-min WI vehicle control and 90-min WI CEPT-treated groups. Livers treated with CEPT secreted small amounts of bile, while there was no bile production by the two other control 90-min WI groups.
Oxygen uptake and bile secretion as markers of viability and function (Fig. 2c and d) are both severely affected by the extended duration of WI in the subjected groups. Oxygen uptake decreased in the WI Control, Vehicle Control and CEPT-treated groups, but remained relatively stable in fresh control livers. The two ischemic groups that were not treated with CEPT completely ceased to produce bile, while CEPT-treated livers still secreted a very small amount of bile (P < 0.0001).
3.2. Liver functionality
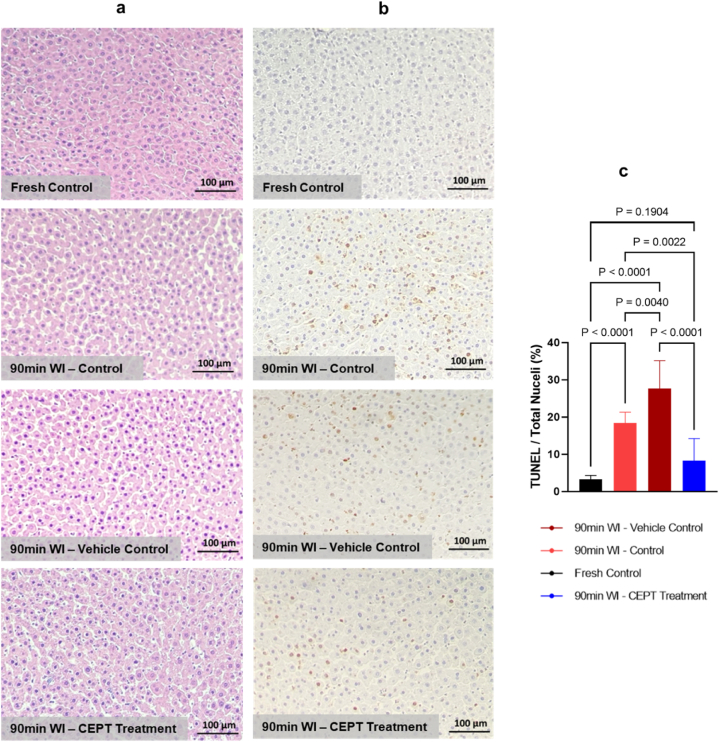
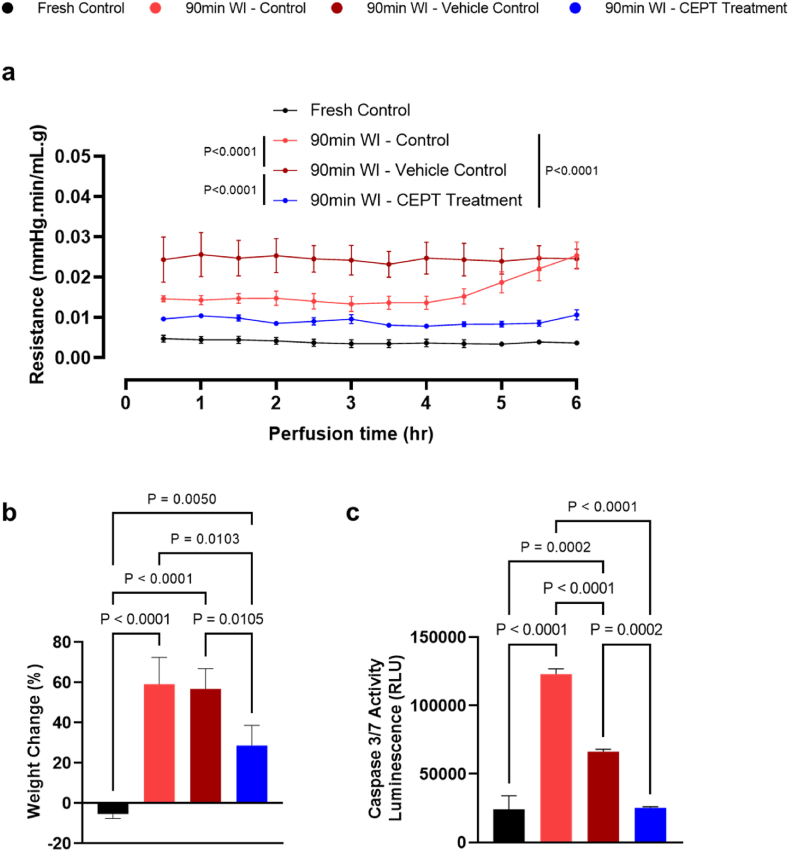
Illustrated in Fig. 3a and b, pathology slides were analyzed through H&E and TUNEL staining. While the ischemic groups little differences in overall morphology, the TUNEL staining results suggest that livers treated with CEPT show a significant reduction of apoptosis compared to both the vehicle and ischemia control groups, as quantified in Fig. 3c; as expected, the fresh control slides present little apoptosis. The CEPT-treated warm ischemic livers showed significantly and consistently lower resistance in the portal vein (Fig. 4a) compared to the two WI control groups (P < 0.0001), suggesting improved microvascular function following reperfusion; fresh control livers exhibited steadily low vascular resistance throughout the perfusion. As shown in Fig. 4b, both the 90-min WI control and vehicle control groups similarly experienced a significantly higher level of edema than the CEPT-treated group (P = 0.0103 and 0.0105 respectively), representing reduced microvascular damage and reaffirming the findings in Fig. 4a. As expected, fresh control livers showed a small amount of weight loss. Caspase 3/7 activity measured in hepatic tissue samples collected and flash frozen immediately following the 6-h NMP is shown in Fig. 4c. CEPT-treated livers had significantly lower levels of expression compared to the 90-min warm ischemia (P < 0.0001) and vehicle (P < 0.0002) control groups, with no statistical difference with the fresh control livers.
Fig. 3.
Morphology of ischemic hepatic tissue after 6 h of CEPT treatment. (a) Light microscopy images of liver tissue collected at the end of NMP (20× magnification) (b) Light microscopy images of TUNEL-stained liver tissue show a reduction in DNA fragmentation after treatment of ischemic livers with CEPT (20× magnification). (c) Ratio of TUNEL-positive to total nuclei, quantified using 10 representative images per group.
Fig. 4.
Treatment with CEPT may improve endothelial dysfunction following warm ischemia-reperfusion injury. (a) Vascular resistance of the portal vein. CEPT-treated livers exhibited the lowest vascular resistance among all warm ischemic groups. (b) Liver weight change after 6 h of NMP. CEPT-treated livers show the lowest amount of weight gain on average among all warm ischemic groups, suggesting lower endothelial damage and edema. (c) Caspase 3/7 activity measured in flash-frozen liver samples collected at the end of NMP. Similar to the fresh control group, CEPT-treated livers exhibited significantly lower caspase activity than both the 90-min WI control and 90-min WI vehicle control livers.
4. Discussion
Pan-caspase inhibition through Emricasan treatment was previously shown to mitigate inflammatory responses in discarded human livers in an NMP setting [10], setting a promising precedent for targeting non-inflammatory apoptotic pathways as a means of improving marginal organ function; this is supported by the existing knowledge of the oxidative stress caused by hypoxic ischemia, and its direct role in regulation of apoptosis. The goal of our study was to test a biochemically similar yet more comprehensive approach in the context of ischemia reperfusion injury in livers, by applying DCD-like conditions; considering the extended duration of ischemia used in our experimental design, our findings are most relevant to DCD livers in categories 1 (dead on arrival) or 2 (unsuccessful cardiopulmonary resuscitation) under the Maastricht classification. Using extensive high-throughput screening methods, a recent study developed a small molecule cocktail abbreviated as CEPT, with Emricasan as one of the components, which was optimized to improve viability of human pluripotent stem cells (hPSCs) and their progeny [9]; despite the clear difference between the functionality of hPSCs and liver cell types, the CEPT cocktail's general mechanisms of action targeting promotion of cell growth, protein synthesis and stabilization of cellular structures led us to hypothesize that it could prove effective against reperfusion injury in warm-ischemic livers. We have shown that treatment of warm ischemic rat livers with the CEPT cocktail can result in amelioration of ischemia-reperfusion injury by demonstrating improvement of endothelial dysfunction symptoms, lower expression of hepatocellular injury markers and partial recovery of bile production.
Qualitative histological assessment of hepatic tissue (involving >30 visual fields per experimental group) after 6 h of recovery NMP reveals slight morphological improvement after CEPT treatment, namely reduction of hepatocyte shrinkage, a characteristic phenotype associated with apoptotic hepatic tissue [11]. Along with the lower number of TUNEL-stained nuclei and significantly reduced levels of Caspase 3/7 expression in CEPT-treated tissue, this confirms effective hepatocellular uptake and biochemical impact of the cocktail under our perfusion conditions and duration (expression at the mRNA level remains unaffected as shown in Supplementary Fig. 2). This is further validated by a comparison of ALT/AST concentration trends across the experimental groups that, although not statistically significant, points to an improved recovery in CEPT-treated livers; over the course of the 6-h NMP both ALT and AST concentrations in the CEPT-treated group stabilize, contrasted by a relatively consistent rise in the warm ischemia and vehicle controls. The extended duration of ischemia in our study design leads to severe damage in the biliary epithelium, as is evident by the complete cessation of bile secretion in the warm ischemic control groups and supported by the literature [12], however the slight recovery of bile production in the CEPT-treated livers could mean that the interference of apoptotic pathways prevent further cascading of injuries not only to hepatocytes, but also cholangiocytes, whose populations are known to drop by an acute increase in apoptosis following reperfusion [13]. This hypothesis is backed by our pre-existing knowledge that prolonged periods of warm ischemia cause aggravated biliary injury through initiation of apoptosis in bile duct epithelium [14].
While it has been demonstrated that pan-caspase inhibition leads to amelioration of portal hypertension in chronically diseased livers, cirrhosis in specific [8], its efficacy had not been previously tested in livers subjected to ischemic damage. Interestingly, vascular resistance in the portal vein was a major area of difference across our experimental groups. CEPT-treated livers expressed lower portal resistance throughout the NMP period than both other ischemic groups, while the vehicle control sustained the highest; following the same trend, weight gain was lowest when the ischemic liver was treated with CEPT, proposing a highly likely connection between the effects of the anti-apoptotic cocktail and preservation of endothelial integrity in reperfusion. This can be explained by a previously unexpected hypothesis, that despite signs of recovery in the liver parenchyma, CEPT may have a more direct, profound, and fast-acting effect on the hepatic sinusoids. Furthermore, considering that primary hepatocytes account for the majority of hepatic tissue by population (60–65 %) [15] and therefore its highest metabolic demand, this can provide a rationale for the absence of any significant recovery in overall oxygen consumption of warm ischemic livers when treated with CEPT.
Future studies are required to further explore the conclusions drawn from our findings and establish a detailed map of hepatic and apoptosis-specific biomarkers affected by anti-apoptotic treatment during reperfusion; in particular, immunohistochemical staining of endothelial markers in conjunction with apoptosis markers downstream of caspase activation will shed more light on the pronounced effects of CEPT on vascular integrity. Furthermore, the use of anti-apoptotic agents in combination with anti-oxidative stress treatments such as ROS scavengers is a promising approach that needs to be optimized and studied for potential adverse effects.
5. Conclusions
Our data shows an overall improvement in viability and function of ischemic male rat livers following reperfusion as a direct result of exposure to the anti-apoptotic, survival-enhancing CEPT cocktail. While further biochemical and genetic analyses are required to map out the extent of CEPT's effects on various hepatic cell types, and the metabolic, signaling and biosynthetic pathways being impacted by it, apoptosis appears to play a more substantive role in IRI than previously understood. Moreover, the goal of this study was not to elucidate the mechanism of action of each CEPT component in liver cells, but to investigate whether its previously shown effects are translatable in hepatic ischemia. Therefore, further work is required to investigate therapeutic targets, and explore alternative components for optimization in liver.
Ethics statement
The experimental protocol was approved by the Institutional Care and Use Committee (IACUC) of MGH (Protocol Number 2017N000227), and all experiments were carried out in accordance with guidelines established in said protocol.
Funding
This material is partially based upon work supported by the National Science Foundation under Grant No. EEC 1941543. Support from the US National Institutes of Health is gratefully acknowledged for the following awards: R01DK114506, R01DK096075, R01EB028782.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data associated with our study have not been deposited into a publicly available repository.
CRediT authorship contribution statement
Mohammadreza Mojoudi: Writing – original draft, Visualization, Investigation, Data curation, Conceptualization. McLean S. Taggart: Investigation, Formal analysis, Data curation. Anil Kharga: Investigation, Formal analysis. Huyun Chen: Investigation, Formal analysis. Antonia T. Dinicu: Investigation, Formal analysis. Benjamin T. Wilks: Conceptualization. James F. Markmann: Supervision. Mehmet Toner: Writing – review & editing, Supervision, Funding acquisition. Shannon N. Tessier: Writing – review & editing, Supervision. Heidi Yeh: Supervision, Funding acquisition. Korkut Uygun: Writing – review & editing, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Korkut Uygun reports financial support was provided by Massachusetts General Hospital. Korkut Uygun reports a relationship with National Institutes of Health that includes: funding grants. Some authors declare competing interests. Drs. Uygun, Tessier, Yeh and Toner have patent applications relevant to this study. Drs. Uygun, Tessier and Toner have a financial interest in and serve on the Scientific Advisory Board for Sylvatica Biotech Inc., a company focused on developing high subzero organ preservation technology. Competing interests for MGH investigators are managed by the MGH and MGB in accordance with their conflict-of-interest policies. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29519.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Markmann J.F., Abouljoud M.S., Ghobrial R.M., Bhati C.S., Pelletier S.J., Lu A.D., Ottmann S., Klair T., Eymard C., Roll G.R., Magliocca J., Pruett T.L., Reyes J., Black S.M., Marsh C.L., Schnickel G., Kinkhabwala M., Florman S.S., Merani S., Demetris A.J.…MacConmara M.P. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: the OCS liver PROTECT randomized clinical trial. JAMA surgery. 2022;157(3):189–198. doi: 10.1001/jamasurg.2021.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mergental H., Laing R.W., Kirkham A.J., Perera M.T.P.R., Boteon Y.L., Attard J., Barton D., Curbishley S., Wilkhu M., Neil D.A.H., Hübscher S.G., Muiesan P., Isaac J.R., Roberts K.J., Abradelo M., Schlegel A., Ferguson J., Cilliers H., Bion J., Adams D.H.…Mirza D.F. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020;11(1):2939. doi: 10.1038/s41467-020-16251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellini M.I., Bonaccorsi Riani E., Giorgakis E., Kaisar M.E., Patrono D., Weissenbacher A. Organ reconditioning and machine perfusion in transplantation. Transpl. Int. 2023 Jan 12;36 doi: 10.3389/ti.2023.11100. PMID: 36713115; PMCID: PMC9876970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque O., Yuan Q., Uygun K., Markmann J.F. Evolving utilization of donation after circulatory death livers in liver transplantation: the day of DCD has come. Clin. Transplant. 2021;35(3) doi: 10.1111/ctr.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirao H., Nakamura K., Kupiec-Weglinski J.W. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat. Rev. Gastroenterol. Hepatol. 2022;19(4):239–256. doi: 10.1038/s41575-021-00549-8. [DOI] [PubMed] [Google Scholar]
- 6.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20(25):8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kung-Chun Chiu D., Pui-Wah Tse A., Law C.T., Ming-Jing Xu I., Lee D., Chen M., Kit-Ho Lai R., Wai-Hin Yuen V., Wing-Sum Cheu J., Wai-Hung Ho D., Wong C.M., Zhang H., Oi-Lin Ng I., Chak-Lui Wong C. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019;10(12):934. doi: 10.1038/s41419-019-2155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gracia-Sancho J., Manicardi N., Ortega-Ribera M., Maeso-Díaz R., Guixé-Muntet S., Fernández-Iglesias A., Hide D., García-Calderó H., Boyer-Díaz Z., Contreras P.C., Spada A., Bosch J. Emricasan ameliorates portal hypertension and liver fibrosis in cirrhotic rats through a hepatocyte-mediated paracrine mechanism. Hepatology communications. 2019;3(7):987–1000. doi: 10.1002/hep4.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Tristan C.A., Chen L., Jovanovic V.M., Malley C., Chu P.H., Ryu S., Deng T., Ormanoglu P., Tao D., Fang Y., Slamecka J., Hong H., LeClair C.A., Michael S., Austin C.P., Simeonov A., Singeç I. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat. Methods. 2021;18(5):528–541. doi: 10.1038/s41592-021-01126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raigani S., Santiago J., Ohman A., Heaney M., Baptista S., Coe T.M., de Vries R.J., Rosales I., Shih A., Markmann J.F., Gruppuso P., Uygun K., Sanders J., Yeh H. Pan-caspase inhibition during normothermic machine perfusion of discarded livers mitigates ex situ innate immune responses. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.940094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhi H., Guicciardi M.E., Gores G.J. Hepatocyte death: a clear and present danger. Physiol. Rev. 2010;90(3):1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolboom H., Pouw R.E., Izamis M.L., Milwid J.M., Sharma N., Soto-Gutierrez A., Nahmias Y., Uygun K., Berthiaume F., Yarmush M.L. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 2009;87(2):170–177. doi: 10.1097/TP.0b013e318192df6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W.H., Ye Q.F., Xia S.S. Apoptosis and proliferation of intrahepatic bile duct after ischemia-reperfusion injury. Hepatobiliary Pancreat. Dis. Int. : HBPD INT. 2004;3(3):428–432. [PubMed] [Google Scholar]
- 14.Zhu X.H., Pan J.P., Wu Y.F., Ding Y.T. Effects of warm ischemia time on biliary injury in rat liver transplantation. World J. Gastroenterol. 2012;18(43):6308–6314. doi: 10.3748/wjg.v18.i43.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bale S.S., Geerts S., Jindal R., Yarmush M.L. Isolation and co-culture of rat parenchymal and non-parenchymal liver cells to evaluate cellular interactions and response. Sci. Rep. 2016;6 doi: 10.1038/srep25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindelin J., Arganda-Carreras I., Frise E., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data associated with our study have not been deposited into a publicly available repository.