Abstract
BACKGROUND
Loneliness and isolation are associated with multiple cardiovascular diseases (CVDs), but there is a lack of research on whether they were causally linked. We conducted a Mendelian Randomization (MR) study to explore causal relationships between loneliness and isolation and multiple CVDs.
METHODS
Single nucleotide polymorphisms associated with loneliness and isolation were identified from a genome-wide association study (GWAS) of 455,364 individuals of European ancestry in the IEU GWAS database. Summary data for 15 CVDs were also obtained from the IEU GWAS database. We used three MR methods including inverse variance weighting, MR-Egger, and weighted median estimation to assess the causal effect of exposure on outcomes. Cochran's Q test and MR-Egger intercept test were used to evaluate the heterogeneity and pleiotropy.
RESULTS
MR analysis showed that loneliness and isolation were significantly associated with essential hypertension (OR = 1.07, 95% CI: 1.03–1.12), atherosclerotic heart disease (OR = 1.04; 95% CI: 1.02–1.06), myocardial infarction (OR = 1.02; 95% CI: 1–1.04) and angina (OR = 1.04; 95% CI =1.02–1.06). No heterogeneity and pleiotropy effects were found in this study.
CONCLUSIONS
Causal relationship of loneliness and isolation with CVDs were found in this study.
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide[1] and imposes a heavy economic burden.[2] To alleviate the substantial health and economic burden of CVDs, the identification and intervention of risk factors can improve prevention efforts. More and more people are at risk of loneliness in modern society due to social and demographic changes.[3] Loneliness and social isolation often lead to chronic stress in humans,[3,4] and they are independent risk factors for CVDs.[5] Loneliness, social isolation, and poor social support status predict CVDs events more than biological CVDs risk factors. According to a meta-analysis of 16 prospective longitudinal studies, loneliness and social isolation are associated with increased risk of coronary heart disease and stroke.[6] Loneliness and social isolation are associated with a variety of CVDs. Psychosocial stress is the third most modifiable risk factor for coronary heart disease and myocardial infarction, after lipids and smoking.[7,8] Results of longitudinal[9] and cross-sectional[10] studies suggest an association between elevated blood pressure and loneliness in older and middle-aged adults. There is ample evidence from animal studies that social isolation and social stress accelerate atherosclerosis.[11] However, loneliness and social isolation are associated with various social behaviors as well as health factors. Large amount of empirical evidence shows that loneliness predicts physical inactivity,[12] alcohol abuse,[13] smoking,[14] obesity,[15] high blood pressure[9] and depression.[16] A systematic review of Australians and New Zealanders showed that the associations between lack of social support and CVDs incidence were inconclusive.[17] There is a lack of research on whether there is a causal relationship between loneliness and social isolation on CVDs.
Mendelian randomization (MR) uses genetic variation as instrumental variables (IVs) to determine whether observational associations between risk factors and outcomes are consistent with causal effects.[18,19] MR relies on the natural random assortment of genetic variation during meiosis,[18] and individuals are naturally assigned at birth to inherit genetic variation affecting risk factors,[20] resulting in a random distribution of genetic variation in a population.[18] Because these genetic variants are generally not associated with confounding factors, the difference in outcomes between carriers and non-carriers could be attributed to differences in risk factors.[21] This minimizes potential methodological limitations, such as confounding and reverse causation,[20] and ultimately enables the inference of causal effects in the presence of unobserved confounding.[22]
The aim of this study was to assess whether loneliness and social isolation are causally related to CVDs and to further explore the types of CVDs affected. We conducted a two-sample MR study to explore the association of genetically predicted levels of loneliness and social isolation with 15 CVDs.
METHODS
Study Design
Single-nucleotide polymorphisms (SNPs) were selected as IVs for exposure and outcome from the genome-wide association study (GWAS) databases. MR analyses should fulfill the following three assumptions: (1) IVs are strongly associated with the exposure (loneliness and isolation), (2) IVs are not associated with confounders and (3) the IVs have effects on CVDs only through the exposure.
Data Sources and Instruments
The samples size for loneliness and isolation phenotype as 455,364 (batch ID: ukb-b-8476). To ensure the number of instrumental variables was sufficient, a criterion of P-value less than 5 × 10-6 was used. Similar locus-wide significance levels are commonly used in other studies.[23,24] Clump analyses was used to exclude SNPs which have a strong linkage disequilibrium (r2 > 0.001) with lead SNP or near the lead SNP (genetic distance <10,000 kb).
We further eliminated SNPs which have a P-value less than 0.05 with CVDs, since these SNPs may have a direct effect on CVDs. We did not employ a traditional power calculation in this study. Instead, to ensure the robustness and validity of our genetic instruments and mitigate the potential for weak instrument bias, we adopted a strategy of selecting SNPs with F-statistics greater than 10. This threshold is in line with established standards in Mendelian Randomization (MR) research, aiming to guarantee sufficient instrument strength. F-statistics were calculated using a combination of variables including the effective allele frequency (EAF), minor allele frequency (MAF), and the proportion of variance explained (PVE) for each SNP. Specifically, we computed PVE for each SNP and then derived the F-statistic from the PVE, taking into account the sample size of the exposure GWAS. SNPs with ambiguous or palindromic effects were excluded through harmonization analysis. This rigorous selection process culminated in the identification of 81 strong and valid SNPs, which were then used as IVs for the exposure of loneliness and isolation (Supplementary Table S1). This approach ensures that our MR analyses are powered by robust and reliable genetic instruments, thereby enhancing the credibility of our findings.
This study employed a GWAS to analyze 15 CVD outcomes, encompassing 5 cerebrovascular diseases, 8 heart diseases, and 2 vascular diseases. The summary statistics for these CVD outcomes were sourced from the IEU GWAS database. As our study involves a diverse array of CVD outcomes, each already well-defined in prior research, we do not individually elaborate on their definitions here. For detailed information, readers are directed to public literature or the original data sets’ databases, such as the IEU GWAS database. Table 1 provides dataset numbers for deeper insights into the specific definitions of each CVD outcome. All ethical approvals and informed consent had been obtained from these original GWAS results.
Table 1. Characteristics of the GWAS datasets of CVDs.
| Outcome | Source | Cases | Controls | Sample size | Population | Dataset |
| MRC-IEU: Medical Research Council Integrative Epidemiology Unit; UKBB: UK Biobank; ISGC: International Stroke Genetics Consortium; CARDIoG-RAMplusC4D: Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics. | ||||||
| Cerebrovascular diseases | ||||||
| Stroke | MRC-IEU | 7,055 | 454,825 | 461,880 | European | ukb-b-8714 |
| Stroke | UKBB | 40,585 | 406,111 | 446,696 | European | ebi-a-GCST006906 |
| Ischemic stroke | UKBB | 34,217 | 406,111 | 440,328 | European | ebi-a-GCST006908 |
| Ischemic stroke | ISGC | 10,307 | 19,326 | 29,633 | Mixed | ieu-a-1108 |
| Ischemic stroke (small-vessel) | UKBB | 5,386 | 192,662 | 198,048 | European | ebi-a-GCST005841 |
| Lacunar stroke | UKBB | 7,338 | 225,258 | 232,596 | Mixed | ebi-a-GCST90014123 |
| Cardioembolic stroke | ISGC | 1,859 | 19,326 | 21,185 | Mixed | ieu-a-1109 |
| Heart diseases | ||||||
| Essential (primary) hypertension | MRC-IEU | 54,358 | 408,652 | 463,010 | European | ukb-b-12493 |
| Essential (primary) hypertension | Neale Lab | 500 | 336,699 | 337,199 | European | ukb-a-531 |
| Atherosclerotic heart disease | MRC-IEU | 12,171 | 450,839 | 463,010 | European | ukb-b-1668 |
| Heart attack / Myocardial infarction | MRC-IEU | 10,616 | 452,317 | 462,933 | European | ukb-b-15829 |
| Heart attack / Myocardial infarction | Neale Lab | 7,735 | 329,424 | 337,159 | European | ukb-a-63 |
| Myocardial infarction | CARDIoG-RAMplusC4D | 43,676 | 128,199 | 171,875 | Mixed | ieu-a-798 |
| Acute myocardial infarction | Neale Lab | 3,927 | 333,272 | 337,199 | European | ukb-a-533 |
| Atrial fibrillation and flutter | MRC-IEU | 6,900 | 456,110 | 463,010 | European | ukb-b-6217 |
| Atrial fibrillation and flutter | Neale Lab | 3,818 | 333,381 | 337,199 | European | ukb-a-536 |
| Heart arrhythmia | MRC-IEU | 2,545 | 460,388 | 462,933 | European | ukb-b-3703 |
| Unstable angina | MRC-IEU | 1,543 | 461,467 | 463,010 | European | ukb-b-10756 |
| Unstable angina | Sakaue S et al., Nat Genet, 2021[25] |
9,481 | 446,987 | 456,468 | European | ebi-a-GCST90018932 |
| Unstable angina | Sakaue S et al., Nat Genet, 2021[25] |
5,891 | 146,214 | 152,105 | East Asian | ebi-a-GCST90018712 |
| Angina pectoris | MRC-IEU | 10,083 | 452,927 | 463,010 | European | ukb-b-15686 |
| Angina pectoris | Neale Lab | 4,837 | 332,362 | 337,199 | European | ukb-a-532 |
| Vessel diseases | ||||||
| Peripheral artery disease | Neale lab | 1,230 | 359,964 | 361,194 | European | ukb-d-I9_PAD |
| Venous thromboembolism | Neale lab | 4,620 | 356,574 | 361,194 | European | ukb-d-I9_VTE |
Statistical Analysis
R package TwoSampleMR was used to perform MR analyses. Three MR analysis methods were used to assess the causal effects of exposures on outcome, which were inverse variance weighted (IVW), MR-Egger, and weighted median estimate (WME). IVW incorporated the estimates of SNPs into meta-analysis, equivalent to a weighted regression of the outcome effect of SNPs on the exposure effect, with the intercept constrained to zero. If there were no horizontal pleiotropy then unbiased estimates could be obtained by using IVW. However, if there were SNPs that affect outcomes through causal pathways other than exposures, the assumptions will be violated. Therefore, we adopt MR-Egger and WME methods as complement to IVW. The MR-Egger regression allowed for horizontal pleiotropic effects. This method estimated the intercept and slope using a weighted regression model, where the slope reflected the causal effect of exposure on outcome and the intercept reflected horizontal pleiotropic effects. WME method was robust to up to 50% of the IVs being invalid, and could be less biased than the IVW method when there are outliers or invalid IVs. All MR methods have some limitations, so we conducted sensitivity analyses and used all three MR methods to assess the robustness of results. If the results of these methods were inconsistent, we prioritized IVW as the primary outcome.
MR estimates were expressed as OR and 95% confidence intervals (CIs). Cochran's Q test was used to check the heterogeneity of different genetic variants. MR-Egger intercept tests were used to evaluate the pleiotropy effect. Statistical significance was interpreted as P < 0.05.
RESULTS
MR Analysis
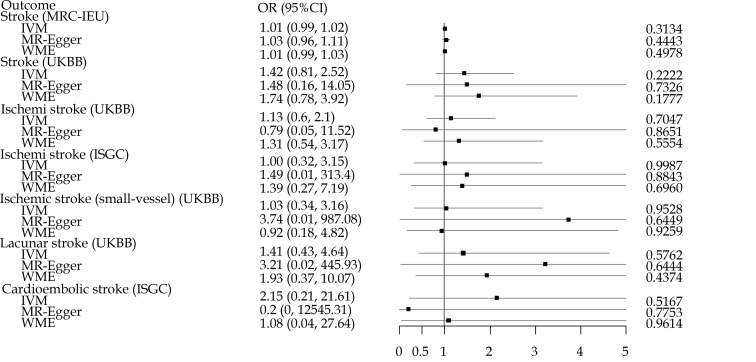
As shown in Figure 1, although most of ORs were higher than 1, the associations of loneliness and isolation instrumented by SNPs with cerebrovascular diseases were not significant in any datasets.
Figure 1.
The impact of loneliness and isolation on cerebrovascular diseases using Mendelian randomization analysis.
To ensure that the images were recognizable, we truncated upper CI over 5.
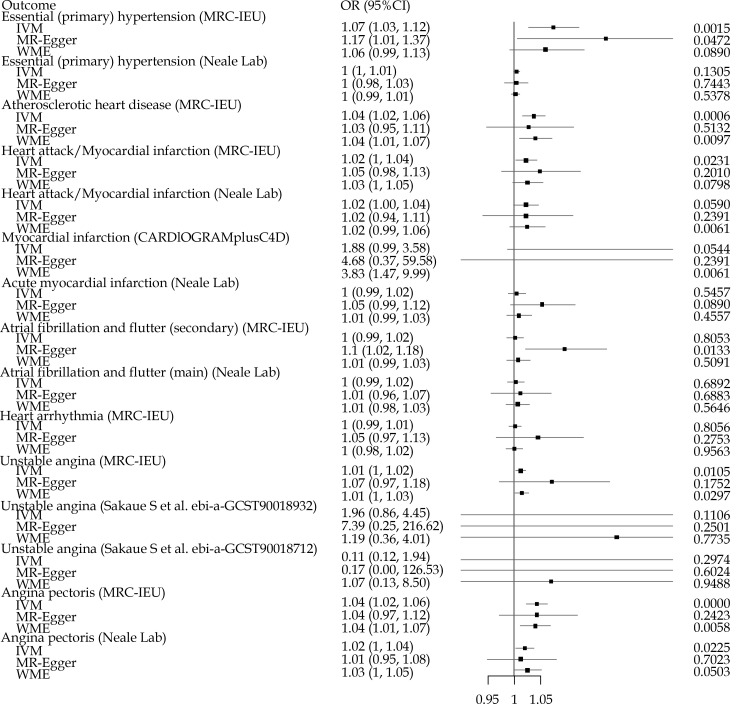
As shown in Figure 2, significant associations were found in essential hypertension, atherosclerotic heart disease, myocardial infarction, atrial fibrillation and flutter (secondary) and angina pectoris. For essential hypertension, significant association were found in MRC-IEU dataset, no matter when using the IVW or in using the MR-Egger method. However, similar results were not found in the Neale Lab dataset. For atherosclerotic heart disease, significant associations were found in using IVW method and WME method. For myocardial infarction, an association was found in MRC-IEU dataset using the IVW method. In CARDIoGRAMplusC4D dataset, only the WME method found associations. For atrial fibrillation and flutter (secondary), significant association was only found in using the MR-Egger method. For unstable angina, significant correlations were found using the IVW method and the WME method in the MRC-IEU dataset. However, no similar results were found in the data set of Sakaue, et al.[25] For angina pectoris, significant associations were found both in MRC-IEU dataset and Neale lab, no matter when using the IVW method or the WME method.
Figure 2.
The impact of loneliness and isolation on heart diseases.
Upper CI over 1.3 and lower CI below 0.9 were truncated.
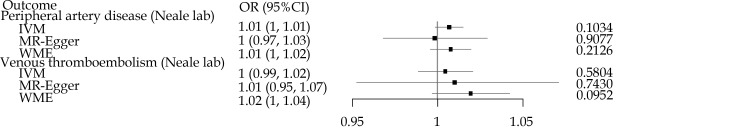
As shown in Figure 3, similar to the result of cerebrovascular diseases, no significant associations were found in any vessel diseases. For each of the CVDs affected by loneliness and isolation listed above, results obtained from different MR methods consistently pointed towards the same direction of effect, suggesting a stable causal association (Supplementary Table S2).
Figure 3.
The impact of loneliness and isolation on vessel diseases with no truncation.
For a more comprehensive visualization of these results, detailed MR scatterplots for each cardiovascular outcome have been compiled in supplementary plots (Supplementary File). These supplementary plots, submitted alongside this manuscript, offer readers an in-depth graphical analysis of the individual CVD outcomes.
Sensitivity Analysis
We conducted sensitivity analyses by using Cochran's Q test and MR-Egger intercept tests. All P-values in the Cochran's Q test analysis were above 0.05, indicating no heterogeneity was observed. Similarly, all P-values for the MR-Egger intercepts were also above 0.05, indicating that no evidence of directional pleiotropy was observed (Table 2).
Table 2. Sensitivity analysis result with Cochran’s Q test and MR-Egger intercept.
| Outcome | Cochran Q test | MR-Egger | |||
| Q value | P-value | Intercept | P-value | ||
| Cerebrovascular diseases | |||||
| Stroke (MRC-IEU) | 49.3269 | 0.8357 | –0.0001 | 0.5683 | |
| Stroke (UKBB) | 56.1915 | 0.7156 | –0.0002 | 0.9716 | |
| Ischemic stroke (UKBB) | 51.3436 | 0.8308 | 0.0018 | 0.7910 | |
| Ischemic stroke (ISGC) | 39.1764 | 0.7161 | -0.0019 | 0.8819 | |
| Ischemic stroke (small-vessel) | 50.3819 | 0.6866 | –0.0062 | 0.6467 | |
| Lacunar stroke | 42.2953 | 0.9791 | –0.0042 | 0.7360 | |
| Cardioembolic stroke | 36.9359 | 0.7306 | 0.0115 | 0.6678 | |
| Heart diseases | |||||
| Essential (primary) hypertension (MRC-IEU) | 48.1453 | 0.5877 | -0.0005 | 0.2478 | |
| Essential (primary) hypertension (Neale Lab) | 61.6693 | 0.6610 | 0.0000 | 0.9620 | |
| Atherosclerotic heart disease | 46.8189 | 0.8533 | 0.0000 | 0.8134 | |
| Heart attack / Myocardial infarction (MRC-IEU) | 52.5110 | 0.6440 | –0.0001 | 0.4830 | |
| Heart attack / Myocardial infarction (Neale Lab) | 44.6690 | 0.8618 | 0.0000 | 0.9977 | |
| Myocardial infarction | 61.5354 | 0.5287 | –0.0047 | 0.4706 | |
| Acute myocardial infarction | 54.2596 | 0.6845 | -0.0002 | 0.1093 | |
| Atrial fibrillation and flutter (secondary) | 45.7735 | 0.8962 | –0.0004 | 0.0130 | |
| Atrial fibrillation and flutter (main) | 58.1225 | 0.7720 | 0.0000 | 0.7590 | |
| Heart arrhythmia | 49.3248 | 0.3417 | -0.0002 | 0.2857 | |
| Unstable angina (MRC-IEU) | 25.5225 | 0.9228 | –0.0002 | 0.2593 | |
| Unstable angina (Sakaue S et al. ebi-a-GCST90018932) | 45.3716 | 0.9541 | -0.0067 | 0.4290 | |
| Unstable angina (Sakaue S et al. ebi-a-GCST90018712) | 46.3483 | 0.6587 | 0.0051 | 0.7586 | |
| Angina pectoris (MRC-IEU) | 52.1029 | 0.6589 | 0.0000 | 0.9834 | |
| Angina pectoris (Neale Lab) | 49.6316 | 0.9065 | 0.0000 | 0.8159 | |
| Vessel diseases | |||||
| Peripheral artery disease | 54.4815 | 0.8208 | 0.0000 | 0.5723 | |
| Venous thromboembolism | 47.6172 | 0.9251 | 0.0000 | 0.8530 | |
DISCUSSION
This study utilized several MR methods to investigate the potential causal relationships between loneliness and isolation and CVDs prevalence. We provided causal evidence of positive associations of loneliness and isolation with essential hypertension, atherosclerotic heart disease and coronary artery disease (myocardial infarction, unstable angina, and angina pectoris).
Loneliness is associated with an elevation in total peripheral vascular resistance[10,26] and increases the risk of developing hypertension.[9,27] Increase in total peripheral resistance (TPR) is the main cause of elevated systolic blood pressure (SBP) in individuals up to 40 years of age, when arterial stiffness assumes a growing role.[28] TPR levels are chronically increased in young adults who are lonely relative to nonlonely individuals of the same age.[10,26] Loneliness also significantly increases likelihood of developing hypertension in elder adults.[29] By using the MRC-IEU data, we found that loneliness and isolation significantly increased the risk of essential hypertension, but the same situation was not observed in Neale Lab’s genetic data. This may due to the low proportion of cases in Neale Lab’s data (0.15%).
Social isolation induces oxidative stress in the brain and peripheral tissues.[11] In vasculature, oxidative stress increases vascular tone and promotes atherosclerosis.[30] In animal experiments, social isolation exacerbated atherosclerosis. This has been demonstrated in different models of atherosclerosis.[31] But the causal relationship between loneliness and isolation and atherosclerosis is unclear.[30] We confirmed a potential causal relationship between loneliness and atherosclerosis by MR analysis.
Lonely and isolated individuals are at increased risk of acute myocardial infarction.[32] Loneliness also linked to stroke and angina.[33] Our findings support previous research that loneliness and isolation promotes angina and myocardial infarction, but did not find an association between loneliness and isolation and stroke. Epidemiological studies have shown an association between loneliness and stroke,[7,34] but have not been able to prove a cause-and-effect relationship.
Although it is difficult to draw definitive conclusions, the likelihood of false positives or reverse causality in our study was very low due to the rigorous IV selection procedure and MR testing applied. Theoretically, if causality is evidenced by only one variant, the validity of the inference depends only on that variant. And our results do not depend on a variant, so it is stable.
We paid special attention to the selection of SNPs as instrumental variables. The adoption of F-statistics greater than 10 as a selection criterion was pivotal in minimizing weak instrument bias. This choice reflects our commitment to ensuring the reliability of our MR estimates, although it does not equate to a traditional power analysis. The incorporation of multiple variables like EAF, MAF, and PVE in calculating F-statistics demonstrates our comprehensive approach to SNP selection.
Our sensitivity analysis, addressing the potential sample overlap, used Cochran's Q test and MR-Egger intercept tests. The result (Table 2) shows no significant heterogeneity (all Cochran's Q test P-values above 0.05) and no evidence of directional pleiotropy (all MR-Egger intercept P-values above 0.05), suggesting that our causal estimates remain unbiased despite potential sample overlaps.
This study shows that genetically determined traits of loneliness and isolation are associated with a modest risk of some CVDs. According to the results, the associations between loneliness and isolation and CVDs were weak, with an OR of about 1. Due to the small effect size, these associations may not be clinically relevant. However, since several epidemiological studies have reported that loneliness and isolation are associated with the development of CVDs, improving loneliness and isolation to reduce CVDs risk remains attractive.
Our study reveals the significant impact of social isolation and loneliness on CVD risk. This is consistent with a study that found loneliness was associated with a significantly increased risk of cardiovascular events.[34] Furthermore, according to another study, social isolation and loneliness may lead to an increase in multiple cardiovascular disease risk factors, such as high blood pressure, smoking, poor diet, and lack of physical activity. Increases in these risk factors further enhance the risk of cardiovascular disease.[35] Therefore, reducing social isolation and loneliness is not only a mental health issue but also key to cardiovascular health. In light of these findings, we recommend the inclusion of social and psychological factors in prevention and treatment strategies for cardiovascular disease. One study noted that factors such as social isolation and loneliness should be considered in clinical interventions for cardiovascular disease to improve treatment effectiveness and patient quality of life.[36] As mentioned previously, social isolation and loneliness affect cardiovascular health through biological mechanisms such as oxidative stress, further emphasizing the need for comprehensive interventions.[30] To reduce the negative impact of loneliness and social isolation on cardiovascular health, preventive measures such as increasing social participation and support networks are recommended. At the same time, healthcare professionals are advised to consider these social factors in clinical practice to improve cardiovascular health outcomes.[36]
This study's primary strength was the MR analysis, using multiple SNPs as IVs for traits of loneliness and social isolation, minimizing confounding and reverse causality, genetically predicting potential causal inferences of loneliness and social isolation on CVDs. In addition, we extensively assessed the causal relationship between loneliness and isolation and multiple CVDs. Data were drawn primarily from individuals of European ancestry, with SNP exposure and outcome estimates adjusted for the principal components of ancestry. Therefore, our results are unlikely to be significantly affected by population stratification bias. Sensitivity analysis indicated that the results were robust.
Our study also has some limitations. First, we did not have separate data for loneliness and social isolation, so we did not distinguish between the two. Some studies have shown that loneliness and social isolation play different roles in CVDs,[34,37] but both are related. Second, the limited population in this study somewhat weakens the generalizability of the findings to other populations (such as Asians, African-Americans, and others), resulting in low estimation precision and we may have missed weak associations. Finally, we were unable to assess potential nonlinear associations of loneliness and social isolation traits with CVDs due to lack of individual data.
Using large-scale human genetic data, this MR study found evidence of causal associations of increased loneliness and isolation levels with higher risk of CVDs, including essential hypertension, atherosclerotic heart disease, myocardial infarction, unstable angina, and angina pectoris. Further studies are needed to explore the underlying mechanisms between loneliness (isolation) and CVDs. Given the difficulty of preventing and treating CVDs, more attention should be paid to discovering modifiable risk factors to reduce the susceptibility to CVDs.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
References
- 1.Mendis S, Puska P, Norrving BE, et al. Global atlas on cardiovascular disease prevention and control. World Health Organization 2011.
- 2.Bloom DE, Cafiero E, Jané-Llopis E, et al. The global economic burden of noncommunicable diseases. Program on the Global Demography of Aging 2012.
- 3.Masi CM, Chen HY, Hawkley LC, et al A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011;15:219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPherson M, Smith-Lovin L, Brashears ME Social isolation in America: Changes in core discussion networks over two decades. Am Sociol Rev. 2006;71:353–375. doi: 10.1177/000312240607100301. [DOI] [Google Scholar]
- 5.Steptoe A, Kivimäki M Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–354. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- 6.Holt-Lunstad J, Smith TB Loneliness and social isolation as risk factors for CVD: implications for evidence-based patient care and scientific inquiry. Heart. 2016;102:987–989. doi: 10.1136/heartjnl-2015-309242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valtorta NK, Kanaan M, Gilbody S, et al Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102:1009–1016. doi: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosengren A, Hawken S, Ôunpuu S, et al Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. J Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 9.Hawkley LC, Thisted RA, Masi CM, et al Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychol Aging. 2010;25:132. doi: 10.1037/a0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacioppo JT, Hawkley LC, Crawford LE, et al Loneliness and health: Potential mechanisms. Psychosom Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Xia N, Li H Loneliness, social isolation, and cardiovascular health. Antioxid Redox Signal. 2018;28:837–851. doi: 10.1089/ars.2017.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pels F, Kleinert J Loneliness and physical activity: A systematic review. Int Rev Sport Exerc Psychol. 2016;9:231–260. doi: 10.1080/1750984X.2016.1177849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Åkerlind I, Hörnquist JO Loneliness and alcohol abuse: A review of evidences of an interplay. Soc Sci Med. 1992;34:405–414. doi: 10.1016/0277-9536(92)90300-F. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi LC, Steptoe A Social isolation, loneliness, and health behaviors at older ages: longitudinal cohort study. Ann Behav Med. 2018;52:582–593. doi: 10.1093/abm/kax033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauder W, Mummery K, Jones M, et al A comparison of health behaviors in lonely and non-lonely populations. Psychol Health Med. 2006;11:233–245. doi: 10.1080/13548500500266607. [DOI] [PubMed] [Google Scholar]
- 16.Heikkinen RL and Kauppinen M Depressive symptoms in late life: a 10-year follow-up. Arch Gerontol Geriatr. 2004;38:239–250. doi: 10.1016/j.archger.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Freak‐Poli R, Phyo AZ, Hu J, et al Are social isolation, lack of social support or loneliness risk factors for cardiovascular disease in Australia and New Zealand? A systematic review and meta‐analysis. Health Promot J Austr. 2022;33:278–315. doi: 10.1002/hpja.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey SG and Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003; 32: 1-22.
- 19.Burgess S and Thompson SG. Mendelian randomization: methods for using genetic variants in causal estimation. CRC Press 2015 Mar 6.
- 20.Emdin CA, Khera AV, Kathiresan S Mendelian randomization. JAMA. 2017;318:1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 21.Sanderson E, Glymour MM, Holmes MV, et al Mendelian randomization. Nat Rev Methods Primers. 2022;2:6. doi: 10.1038/s43586-021-00092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang K, Wang P, Xu Z, et al Causal effects of gut microbiome on systemic lupus erythematosus: a two-sample mendelian randomization study. Front Immunol. 2021;12:667097. doi: 10.3389/fimmu.2021.667097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang Y, Zhang C, Wang J, et al Identification of host gene-microbiome associations in colorectal cancer patients using mendelian randomization. J Transl Med. 2023;21:535. doi: 10.1186/s12967-023-04335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaue S, Kanai M, Tanigawa Y, et al A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53:1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PubMed] [Google Scholar]
- 25.Hawkley LC, Burleson MH, Berntson GG, et al Loneliness in everyday life: cardiovascular activity, psychosocial context, and health behaviors. J Pers Soc Psychol. 2003;85:105. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Hawkley LC, Masi CM, Berry JD, et al Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21:152. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- 27.Franklin SS, Gustin IV W, Wong ND, et al Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.CIR.96.1.308. [DOI] [PubMed] [Google Scholar]
- 28.Momtaz YA, Hamid TA, Yusoff S, et al Loneliness as a risk factor for hypertension in later life. J Aging Health. 2012;24:696–710. doi: 10.1177/0898264311431305. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Xia N The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020;37:101585. doi: 10.1016/j.redox.2020.101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shively CA, Clarkson TB, Kaplan JR Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77:69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 31.Hakulinen C, Pulkki-Råback L, Virtanen M, et al Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479054 men and women. Heart. 2018;104:1536–1542. doi: 10.1136/heartjnl-2017-312663. [DOI] [PubMed] [Google Scholar]
- 32.Pengpid S, Peltzer K Associations of loneliness with poor physical health, poor mental health and health risk behaviours among a nationally representative community‐dwelling sample of middle‐aged and older adults in India. Int J Geriatr Psychiatry. 2021;36:1722–1731. doi: 10.1002/gps.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bu F, Zaninotto P, Fancourt D Longitudinal associations between loneliness, social isolation and cardiovascular events. Heart. 2020;106:1394–1399. doi: 10.1136/heartjnl-2020-316614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallée A Association between Social Isolation and Loneliness with Estimated Atherosclerotic Cardiovascular Disease Risk in a UK Biobank Population-Based Study. Int J Environ Res Public Health. 2023;20:2869. doi: 10.3390/ijerph20042869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma T, Padala PR, Mehta JL Loneliness and social isolation: determinants of cardiovascular outcomes. Curr Cardiol Rev. 2021;17:37–44. doi: 10.2174/1573403X17666210129101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freak-Poli R, Ryan J, Neumann JT, et al Social isolation, social support and loneliness as predictors of cardiovascular disease incidence and mortality. BMC Geriatr. 2021;21:1–14. doi: 10.1186/s12877-020-01943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.