ABSTRACT
Using a common protocol across seven countries in the European Union/European Economic Area, we estimated XBB.1.5 monovalent vaccine effectiveness (VE) against COVID‐19 hospitalisation and death in booster‐eligible ≥ 65‐year‐olds, during October–November 2023. We linked electronic records to construct retrospective cohorts and used Cox models to estimate adjusted hazard ratios and derive VE. VE for COVID‐19 hospitalisation and death was, respectively, 67% (95%CI: 58–74) and 67% (95%CI: 42–81) in 65‐ to 79‐year‐olds and 66% (95%CI: 57–73) and 72% (95%CI: 51–85) in ≥ 80‐year‐olds. Results indicate that periodic vaccination of individuals ≥ 65 years has an ongoing benefit and support current vaccination strategies in the EU/EEA.
Keywords: cohort design, COVID‐19, electronic health records, hospitalisation, multicountry study, SARS‐CoV‐2, vaccine effectiveness
1. Introduction
COVID‐19 vaccination in European Union/European Economic Area (EU/EEA) countries has been rolled out as a 2023 autumnal campaign targeting vulnerable populations, mostly in individuals aged ≥ 60 or ≥ 65 years and those with comorbidities. Periodic vaccination in target groups has been implemented to maintain protection against severe COVID‐19 outcomes due to waning of immunity and rapid decrease in vaccine effectiveness (VE) seen in recent vaccination campaigns [1, 2, 3, 4]. Adapted vaccines have been approved to match SARS‐CoV‐2 circulating variants and increase protection in periods of dominance of BA.4 or BA.5 and XBB.1.5 and have been used in the EU/EEA [1, 2, 3, 4, 5, 6, 7, 8, 9], although a new Omicron subvariant, BA.2.86, became dominant in EU/EEA countries around late November and early December 2023 [10].
Our objective was to estimate VE against COVID‐19 hospitalisation and death of XBB.1.5 monovalent vaccines administered as 2023 autumnal booster dose, during the period where there was generally a match between the vaccine composition and the dominant SARS‐CoV‐2 variant (October and November 2023).
2. Study Setting and Methods
Since October 2021, a multicountry COVID‐19 VE study using electronic health records (EHR) has been ongoing in EU/EEA countries under the Vaccine Effectiveness Burden and Impact Studies (VEBIS) project, funded by the European Centre of Disease Prevention and Control (ECDC). As of January 2024, seven countries participate in the VEBIS‐EHR network: Belgium, Denmark, Italy, Spain (Navarra), Norway, Portugal and the Netherlands.
Detailed methods and results have been previously published [11, 12, 13, 14]. Briefly, using a common protocol, retrospective fixed cohorts were constructed in participating countries using deterministic linkage of population and health databases. For the monitoring of the 2023/2024 season [12], we included individuals eligible to receive the autumnal booster vaccination at the beginning of the campaign (Table 1), specifically, those 65 years or older, with at least complete primary vaccination and who, in the last 90 days, had no vaccine dose administered and no documented SARS‐CoV‐2 infection. Individuals contributed person‐days to the ‘non‐exposed’ to the 2023 autumnal booster since the beginning of the study and up to the date of administration of the autumnal booster or the end of follow‐up. Individuals' person‐days started contributing to the 2023 autumnal booster ‘exposed’ group 14 days after receiving the booster. The first 14 days after receipt of the booster were dropped from the analysis, and consistently, the study period started 14 days after the start of the vaccination campaign in each country. Follow‐up in both groups ceased at the date of the outcome, date of death for any cause or end of the study period (25 November 2023).
TABLE 1.
Start of the COVID‐19 2023 autumnal vaccination campaign in participating countries.
| Study site | Date of start of the vaccination campaign |
|---|---|
| Belgium | 11 September 2023 |
| Denmark | 1 October 2023 |
| Italy | 27 September 2023 a |
| Navarra (Spain) | 16 October 2023 |
| The Netherlands | 2 October 2023 |
| Norway | 1 September 2023 |
| Portugal | 29 September 2023 |
Day of publication of the Ministerial Circular Law with the recommendations on which vaccines to use and to who it should be offered. The actual day of start varied across regions, with most starting the first week of October.
Hospitalisation due to COVID‐19 was defined as a hospital admission due to a severe acute respiratory infection with a SARS‐CoV‐2 positive test from 14 days before to 1 day after admission or with COVID‐19 as the main diagnosis in admission or discharge records, except in the Netherlands, where admissions with a positive SARS‐CoV‐2 test and missing or unknown reason for admission (about 50% of all admissions with a positive SARS‐CoV‐2 test) were also included.COVID‐19‐related death was defined as death for which COVID‐19 was recorded as the main cause (even with no positive SARS‐CoV‐2 test) or, if the cause was not available, laboratory‐confirmed SARS‐CoV‐2 infection with death in the 30 days after the positive test or symptom onset.
Cox regression with calendar time as the underlying scale was used to estimate vaccine hazard ratios and derived VE [HR; VE = (1 − HR) × 100] and 95% confidence intervals (95%CI), adjusting by 5‐year age‐groups, sex, region in the country, comorbidities and previous number of vaccine booster doses. Site‐specific estimates were pooled using a random‐effects meta‐analysis, given expected heterogeneity from study design and disease epidemiology. To estimate heterogeneity, we used the I2 index [15]. A fixed‐effects model was used as secondary analysis.
3. Results
In the group aged 65–79 years, 23.3 and 1.9 million person‐days were included in the non‐exposed and exposed groups, respectively; 5775 hospitalisations due to COVID‐19 and 386 COVID‐related deaths were included in the non‐exposed group versus 207 and 16 in the exposed. In the group aged ≥ 80 years, 9.5 and 1.0 million person‐days were included in the nonexposed and exposed groups, respectively; 8497 hospitalisations due to COVID‐19 and 1200 COVID‐related deaths were included in the nonexposed group versus 312 and 34 in the exposed. Individuals in the exposed group had more frequent comorbidities compared to the nonexposed (Table 2). As of November 2023, the autumnal boosters were mostly Pfizer mRNA XBB.1.5 monovalent vaccines (98%).
TABLE 2.
Descriptive characteristics by vaccination status, VEBIS‐EHR study, October to November 2023.
| Variable | Not vaccinated | Vaccinated | |||
|---|---|---|---|---|---|
| Count | % of cohort total | Count | % of cohort total | ||
| Sex | Female | 9,712,250 | 55% | 2,378,148 | 53% |
| Male | 7,901,263 | 45% | 2,118,470 | 47% | |
| Missing | 22 | 0% | < 5 | — | |
| Comorbidities | High‐risk comorbidities/immunocompromising | 479,967 | 3% | 393,205 | 9% |
| Medium‐risk comorbidities/non‐immunocompromising | 6,398,375 | 36% | 2,261,177 | 50% | |
| No comorbidity | 10,708,009 | 61% | 1,829,422 | 41% | |
| Missing | 27,184 | 0% | 12,815 | 0% | |
| Number of previous vaccine boosters | 0 | 1,745,023 | 10% | 16,981 | 0% |
| 1 | 8,386,032 | 48% | 226,135 | 5% | |
| 2 | 6,301,163 | 36% | 2,837,619 | 63% | |
| 3 | 1,177,481 | 7% | 1,413,806 | 31% | |
| 4 | 2472 | 0% | 16 | 0% | |
| 5 | 39 | 0% | — | 0% | |
| Missing | 1325 | 0% | 2062 | 0% | |
| Country of birth | Native | 13,243,192 | 75% | 1,397,411 | 31% |
| Non‐native | 508,094 | 3% | 39,082 | 1% | |
| Missing | 3,862,249 | 22% | 3,060,126 | 68% | |
| Nationality | National | 1,726,175 | 10% | 1,121,558 | 25% |
| Nonnational | 330,884 | 2% | 57,029 | 1% | |
| Missing | 15,556,476 | 88% | 3,318,032 | 74% | |
| Vaccine product | Pfizer (monovalent) | — | — | 61,332 | 1% |
| Moderna (monovalent) | — | — | 60 | 0% | |
| Pfizer (bivalent original/BA.1) | — | — | 4006 | 0% | |
| Moderna (bivalent original/BA.1) | — | — | 10 | 0% | |
| Pfizer (bivalent original/BA.4/BA.5) | — | — | 10,914 | 0% | |
| Moderna (bivalent original/BA.4/BA.5) | — | — | 116 | 0% | |
| Pfizer (XBB.1.5) | — | — | 4,370,556 | 97% | |
| Moderna (XBB.1.5) | — | — | 49,624 | 1% | |
| Novavax | — | — | — | 0% | |
| Other (all other types, including AZ) | — | — | < 5 | — | |
| Missing | — | — | — | 0% | |
Site‐specific and pooled VE estimates are shown in Figure 1 for hospitalisation due to COVID‐19 and in Figure 2 for COVID‐19–related death. There was certain heterogeneity across study sites, which was lower in the group 65–79, for both hospitalisation and death (38% and 0%, respectively), than for those aged ≥ 80 years (59% and 55%). Random‐effects pooled VE against hospitalisation due to COVID‐19 or COVID‐19–related death were, respectively, 67% (95%CI: 58–74) and 67% (95%CI: 42–81) in those aged 65–79 years and 66% (95%CI: 57–73) and 72% (95%CI: 51–85) in the group aged ≥80 years, though VE against death was based on three study sites.
FIGURE 1.
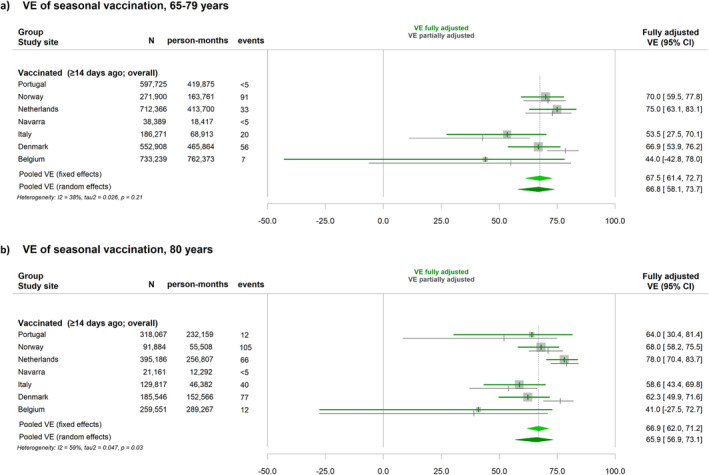
Vaccine effectiveness (VE) of 2023 autumnal monovalent XBB.1.5 vaccination against hospitalisation due to COVID‐19 in seven European VEBIS‐EHR study sites and pooled estimate using either fixed‐effects or random‐effects meta‐analysis. (a) In the group aged 65–79 years; (b) in the group aged 80 years and older, October to November 2023.
FIGURE 2.
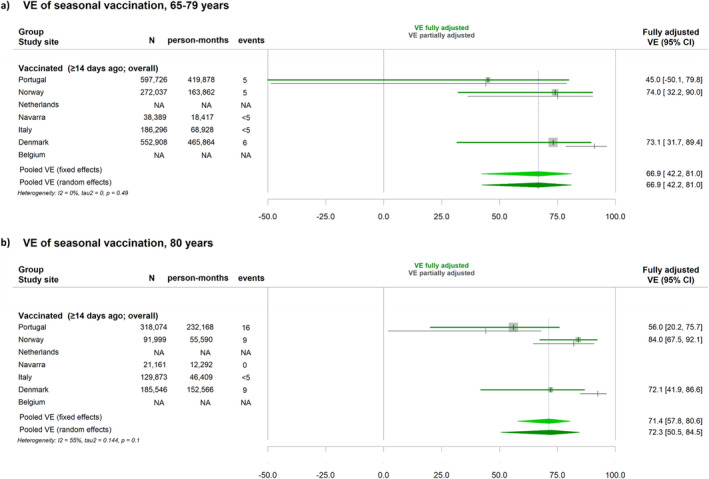
Vaccine effectiveness (VE) of 2023 autumnal monovalent XBB.1.5 vaccination against COVID‐19 related death in seven European VEBIS‐EHR study sites and pooled estimate using either fixed‐effects or random‐effects meta‐analysis. (a) In the group aged 65–79 years; (b) in the group aged 80 years and older, October to November 2023.
4. Discussion
Our estimates of VE with XBB.1.5 vaccines are comparable to others in this same period, when there was a match between the vaccine composition and the dominant SARS‐CoV‐2 variant [6]. Interestingly, because the VEBIS EHR monitoring network has been ongoing since October 2021, we are able to compare with the VE from the previous vaccination campaign, despite some changes in the methods and in the participating countries that have occurred in the network [11, 12, 13]. While, for previous boosters, we estimated VE according to the number of vaccine doses, for this autumnal dose, the network has transitioned to a seasonal approach, similar to the influenza VE monitoring framework [12]. Nevertheless, the VE as of November 2023 is comparable to the one estimated within this network in the 2022/23 between 1 October and 25 November, 2022, when estimated VE against hospitalisation due to COVID‐19 was, in those aged 65–79 years, 77% (95%CI: 66–83) for the second booster and, in the group aged ≥ 80 years, 76% (95%CI: 65–83) for the second booster and 71% (95%CI: 60–79) for the third booster [4]. Effectiveness was lower to the one estimated with a first booster dose in the autumn of 2021 [4]. Higher background immunity in the population and increased probability of selection of individuals with comorbidities in more recent vaccination campaigns may account, at least partially, for these differences.
The similarity between the current VE estimates and the ones from previous years is in agreement with other results from the VEBIS‐EHR network, which indicate that time since vaccination seems more relevant for protection than the number of previous COVID‐19 vaccine boosters [4, 14]. This supports the recommendation of having offered a new dose of COVID‐19 vaccines before a period of expected high burden of disease, such as that of the respiratory winter season, regardless of total number of previous vaccine boosters received. Also, our results confirm that periodic vaccination of individuals aged 65 years or older has an ongoing benefit, and support the current vaccination strategies in the EU/EEA countries. Ongoing monitoring of COVID‐19 VE in the coming months is needed to determine if the advent of the BA.2.86 variant has impacted the protection conferred by the XBB.1.5 boosters and the duration of protection by time since vaccination. Stable multicountry networks provide unique opportunities for ongoing monitoring of COVID‐19 vaccines VE and are key to inform public health recommendations.
Author Contributions
Susana Monge: Conceptualization; Investigation; Funding acquisition; Writing – original draft; Methodology; Visualization; Supervision. James Humphreys: Conceptualization; Writing – review and editing; Validation; Methodology; Visualization; Formal analysis; Data curation. Nathalie Nicolay: Conceptualization; Investigation; Funding acquisition; Writing – review and editing; Methodology; Project administration; Supervision; Resources. Toon Braeye: Writing – review and editing; Formal analysis; Methodology; Data curation; Validation. Izaak Van Evercooren: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Christian Holm Hansen: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Hanne‐Dorthe Emborg: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Chiara Sacco: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Alberto Mateo‐Urdiales: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Jesús Castilla: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Iván Martínez‐Baz: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Brechje de Gier: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Susan Hahné: Methodology; validation; Writing – review and editing; Formal analysis; Data curation. Hinta Meijerink: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Anja Bråthen Kristoffersen: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Ausenda Machado: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Patricia Soares: Methodology; Validation; Writing – review and editing; Formal analysis; Data curation. Anthony Nardone: Conceptualization; Funding acquisition; Writing – review and editing; Methodology; Project administration; Supervision. Sabrina Bacci: Project administration; Methodology; Writing – review and editing; Conceptualization; Funding acquisition; Supervision; Resources. Esther Kissling: Conceptualization; Investigation; Methodology; Writing – review and editing; Project administration; Supervision. Baltazar Nunes: Supervision; Project administration; Methodology; Conceptualization; Investigation; Writing – original draft; Visualization. VEBIS‐EHR working group: Data curation; Formal analysis; Software.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/irv.13292.
Acknowledgements
The authors would like to thank all the people from the study sites involved in the data collection and for producing estimates of VE, as without their work these results would not be available for the scientific community and the public.
VEBIS‐Lot 4 Working Group: Alexis Sentís, Mario Fontán‐Vela, Joris Van Loenhout, Pierre Hubin, Ida Rask Moustsen‐Helms, Massimo Fabiani, Daniele Petrone, Patrizio Pezzotti, Itziar Casado, Aitziber Echeverria, Camino Trobajo‐Sanmartín, Stijn Andeweg, Diana Lucas.
Funding: All the public health organisations involved received funding from the European Centre for Disease Prevention and Control (ECDC) implementing Framework Contract ECDC/2021/018 ‘Vaccine effectiveness and impact of COVID‐19 vaccines through routinely collected exposure and outcome using health registries’ (RS/2022/DTS/24104). In Portugal, this work was also supported by FCT – Fundação para a Ciência e Tecnologia, I.P. by project reference CEECINST/00049/2021/CP2817/CT0001 and DOI identifier 10.54499/CEECINST/00049/2021/CP2817/CT0001.
The author Chiara Sacco is a fellow of the ECDC Fellowship Programme, supported financially by the European Centre for Disease Prevention and Control. The views and opinions expressed herein do not state or reflect those of ECDC. ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn.
For a complete list of the VEBIS‐EHR Working Group, see the Acknowledgments section.
Data Availability Statement
Authors cannot share the data used for this study, which should be requested to the data owner institutions following their respective procedures.
References
- 1. Andersson N. W., Thiesson E. M., Baum U., et al., “Comparative Effectiveness of Bivalent BA.4‐5 and BA.1 mRNA Booster Vaccines Among Adults Aged ≥50 Years in Nordic Countries: Nationwide Cohort Study,” BMJ 382 (2023): e075286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antunes L., Mazagatos C., Martínez‐Baz I., et al., “Effectiveness of the Adapted Bivalent mRNA COVID‐19 Vaccines Against Hospitalisation in Individuals Aged ≥ 60 Years During the Omicron XBB Lineage‐Predominant Period: VEBIS SARI VE Network, Europe, February to August, 2023,” Eurosurveillance 29 (2024): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fabiani M., Mateo‐Urdiales A., Sacco C., et al., “Relative Effectiveness of Bivalent Original/Omicron BA.4‐5 mRNA Vaccine in Preventing Severe COVID‐19 in Persons 60 Years and Above During SARS‐CoV‐2 Omicron XBB.1.5 and Other XBB Sublineages Circulation, Italy, April to June 2023,” Eurosurveillance 28 (2023): 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontán‐Vela M., Kissling E., Nicolay N., et al., “Relative Vaccine Effectiveness Against COVID‐19 Hospitalisation in Persons Aged ≥ 65 Years: Results From a VEBIS Network, Europe, October 2021 to July 2023,” Eurosurveillance 29, no. 1 (2024): 2300670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kislaya I., Machado A., Magalhães S., et al., “COVID‐19 mRNA Vaccine Effectiveness (Second and First Booster Dose) Against Hospitalisation and Death during Omicron BA.5 Circulation: Cohort Study Based on Electronic Health Records, Portugal, May to July 2022,” Eurosurveillance 27, no. 37 (2022): 2200697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Werkhoven C. H., Valk A. W., Smagge B., et al., “Early COVID‐19 Vaccine Effectiveness of XBB.1.5 Vaccine Against Hospitalisation and Admission to Intensive Care, the Netherlands, 9 October to 5 December 2023,” Eurosurveillance 29, no. 1 (2024): 2300703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mateo‐Urdiales A., Sacco C., Fotakis E. A., et al., “Relative Effectiveness of Monovalent and Bivalent mRNA Boosters in Preventing Severe COVID‐19 Due to Omicron BA.5 Infection up to 4 Months Post‐Administration in People Aged 60 Years or Older in Italy: A Retrospective Matched Cohort Study,” The Lancet Infectious Diseases 23, no. 12 (2023): 1349–1359. [DOI] [PubMed] [Google Scholar]
- 8. Fabiani M., Mateo‐Urdiales A., Sacco C., et al., “Protection Against Severe COVID‐19 After Second Booster Dose of Adapted Bivalent (Original/Omicron BA.4‐5) mRNA Vaccine in Persons ≥ 60 Years, by Time since Infection, Italy, 12 September to 11 December 2022,” Eurosurveillance 28, no. 8 (2023): 2300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.“ECDC‐EMA Statement on Updating COVID‐19 Vaccines Composition for New SARS‐CoV‐2 Virus Variants,” Emergency Task Force, 7 June 2023. [Internet], accessed February 13, 2024, https://www.ecdc.europa.eu/sites/default/files/documents/covid‐19‐vaccines‐composition‐variants‐statement‐ECDC‐EMA_0.pdf.
- 10.“European Respiratory Virus Surveillance Summary (ERVISS) [Internet]”, accessed February 13, 2024, https://erviss.org/.
- 11. European Centre for Disease Prevention and Control , Pilot Protocol for a COVID‐19 Vaccine Effectiveness Study Using Health Data Registries (Stockholm: ECDC, 2022). [Google Scholar]
- 12. European Centre for Disease Prevention and Control , Protocol for a COVID‐19 Vaccine Effectiveness Study Using Health Data Registries, v.2.0 (Stockholm: ECDC, 2024). [Google Scholar]
- 13. European Centre for Disease Prevention and Control , Protocol for a COVID‐19 Vaccine Effectiveness Study Using Health Data Registries (Stockholm: ECDC, 2023). [Google Scholar]
- 14. European Centre for Disease Prevention and Control , Interim Analysis of COVID‐19 Vaccine Effectiveness against Hospitalisation and Death Using Electronic Health Records in Six European Countries (Stockholm: ECDC, 2023). [Google Scholar]
- 15. Higgins J. P. and Thompson S. G., “Quantifying Heterogeneity in a Meta‐Analysis,” Statistics in Medicine 21, no. 11 (2002): 1539–1558, 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors cannot share the data used for this study, which should be requested to the data owner institutions following their respective procedures.


