Abstract
Introduction
The timely initiation of complementary feeding is essential to reduce infant mortality. In Ethiopia, 37.5 % of mothers did not initiate complementary feeding to their infants in time. However, previous studies could not identify the time to initiate complementary feeding among primipara mothers. Therefore, this study aims to identify the time to initiate complementary feeding and its predictors among primipara mothers with infants aged 6–12 months in the Awi zone, northwest Ethiopia.
Methods
A community-based retrospective follow-up study was conducted among 732 primipara mothers who had infants aged 6–12 months from January 1, 2022, to December 30, 2022. A multistage sampling technique was used to select study participants through questionnaires administered by interviewers. Data were entered into EPI-data 3.1 and exported to STATA 17 for further analysis. The Kaplan-Meier survival curve together with the log-rank test was used to assess the survival experience of the infant at specific times and to compare the survival of the infant in it between different categorical independent variables. Bivariable and multivariable Cox proportional hazard regression models were used to identify significant predictors. Model fitness was also assessed using the Schoenfield residual and the Cox-Snell global residual test. Statistical significance was declared at the p-value <0.05.
Result
The median time to initiate complementary feeding among primipara mother-infant pairs was 6 ± 2 months. The overall incidence rate of complementary feeding initiation before, at and after 6 months of age of the infant was 7.28 (95%CI: 6.44, 8.25), 41.41 (95%CI: 36.05, 47.56), and 42.97 (95%CI: 36.89, 50.05) per 100 person-month observations, respectively. Among those mothers who initiated complementary feeding for their infants, 249, 200, and 165 initiated complementary feeding before, at and after 6 months of age of the infants. Furthermore, the age (15–24 years) of mothers (AHR: 1.63, 95%CI: 1.16, 2.29), rich wealth (AHR: 1.35, 95%CI: 1.05, 1.75), and richest wealth (AHR: 1.43, 95%CI: 1.10, 1.84) were identified as statistically significant predictors of the time to initiate complementary feeding.
Conclusions
The median time to initiate complementary feeding among primipara mother-infant pairs was 6 months. The age of the mother and the wealth status of the household were found to be statistically independent predictors of the time to initiate complementary feeding. Therefore, community health professionals are better able to work on when to initiate complementary feeding to infants of rich and richest young primipara mothers.
Keywords: Complementary feeding, Primipara, Survival analysis, Awi zone, Ethiopia
1. Introduction
Complementary foods are solid, semisolid and smooth foods prepared at home or commercially that are given to children concomitant with breastfeeding [1]. In addition to breast milk, complementary feeding should be offered to infants/children aged 6 months–24 months that are nutritionally adequate meals [[2], [3], [4]]. The introduction of complementary feeding is the first significant proactive step towards development. It becomes a means of socialization after a succession of neurodevelopmental successes [2,3]. During the proper complementary feeding phase, it is possible to prevent stunting, wasting, micronutrient deficiencies, overweight, obesity, and non-communicable diseases related to diet [1].
The World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) recommended that complementary feeding should begin at the age of 6 months for the infant and continue up to breastfeeding cessation, at least 24 months [1,3]. UNICEF also revealed that children had a higher chance of surviving, growing, developing, and learning if they were fed enough nutritious foods in the right amounts at the right times in their development. Even in the face of disease, catastrophe, or crisis, they are better positioned to flourish [1]. Complementary feeding is particularly important for achieving three Sustainable Development Goals (SDGs): improving nutrition (SDG-2), reducing child mortality and the risk of noncommunicable diseases (SDG-3), and improving cognitive development and education (SDG-4) [5].
Complementing breast milk with meals before the age of 6 months is not needed and discouraged due to the possibility of contamination and the accompanying increased risk of infectious diseases. Because the frequency and intensity of sucking improve the production and release of breast milk, the early start of complementary feeding diminishes breast milk yield [1]. Furthermore, infants who initiate complementary feeding early have a six-fold increased risk of dying from respiratory infection or diarrhea [6,7]. After six months, breast milk is hardly sufficient to meet the infant's nutritional needs, so additional foods should be added to the child's diet [[1], [2], [3],8,9]. Complementary feeding practices that are implemented on time can help children survive, grow and develop, as well as prevent micronutrient deficiencies, morbidity, and obesity later in life [1,8,10]. Appropriate complementary feeding practices should be identified based on parity status; primiparity (only one birth) [11] and Multiparity mothers (numerous births after fetal viability, at least 28 weeks of pregnancy in Ethiopia) [12]. However, none of the studies in Ethiopia identified the timely initiation of complementary feeding according to parity status.
Although proper complementary feeding practices could save millions of lives every year [[13], [14], [15], [16]], the prevalence of undernutrition among children aged 6–24 years is high due to the introduction of breastmilk substitutes too early or too late and negatively impacts their growth as well as development [6,7]. In Ethiopia, still, the prevalence of wasting and stunting is high concomitantly with high neonatal and infant mortality [9,17]. This could be associated with the low proportion of timely initiation of complementary feeding. The proportion also varied in countries depending on local variables such as food availability, prosperity, and cultural variations [[18], [19], [20], [21], [22]].
The global overall impact of malnutrition on social, economic, medical, and developmental problems is long-lasting and serious for individuals, families, communities, and the country at large. Combating malnutrition in all its forms is one of the most challenging public health issues globally [23]. To accelerate this combating process, WHO and UNICEF jointly developed an indicator in 2021 for all children aged 0–23 months to improve children's feeding practices and further improve their health, and development of them [24].
Although complementary feeding is said to begin at 6 months of age, evidence shows that improper scheduling of complementary feeding practices remains a serious public health problem at a global and national level. One in every four children aged 6–8 months (27 %) did not receive solid, semi-solid, or soft food in 2020 globally. This inappropriate initiation time for complementary feeding can cause illness in children, as well as inadequate growth and development, and has been linked to 45 percent of childhood mortality [1,2,24,25].
Ethiopia is one of the developing countries with high childhood mortality. The country's latest infant mortality was predicted to be 43 deaths per 1000 live births, with the aggregate under-5 mortality rate being 55 deaths per 1000 live births. According to these data, 37 %, 21 %, and 7 % of children under five years of age are stunted, underweight, and wasted, respectively [17]. All of these conditions have been reduced if the timely initiation of complementary feeding for all infants was practiced.
Previous studies showed that different predictors such as sociodemographic, maternal, and obstetric variables contributed to the timely and non-scheduling of complementary feeding among all mothers of any party. But previous studies have not well studied the effect of these predictors on the time to initiate complementary feeding among primipara mothers separately [[17], [18], [19], [20],22,[26], [27], [28], [29], [30], [31], [32]].
Although countries, including Ethiopia, develop strategies and adopt WHO and UNICEF guidelines and indicators on the timely initiation of complementary feeding, the overall proportion of the timely initiation of complementary feeding is low [9]. The reason behind this figure was explored and quantified by including all mothers without parity specificity [18,19,28,33]. But studies abroad showed that a difference in parity has its contribution to the general well-being of child-caring and growth [34], including appropriate complementary feeding practices, specifically for timely initiation, which is not identified in previous studies.
In Ethiopia, although 62.5 % of mothers initiate complementary feeding in a timely manner, a higher proportion of stunting is still a challenge that could be 37.5 % of the untimely initiation of complementary feeding practices, which is not studied by parity difference or other factors related to nutrition identified by the previous studies [[8], [9], [10],[17], [18], [19], [20], [21], [22], [23], [24],30,31,35,36]. Similarly, in the region of Amhara, malnutrition is one of the major public health problems, including wasting, stunting and underweight with a respective burden of 9.8 %, 46.3 %, and 28.4 % [37]. In particular, in the Awi zone, only about 67 % of mothers initiated complementary feeding in a timely manner, which was based on all parity mothers [38].
Previous studies, which included all types of parity mothers, determined the timing of complementary feeding based on cross-sectional data, which did not show the temporal relationship in a better way. Thus, further determining the time to initiate complementary feeding using cohort design and survival analysis would show the time in a better way than the tried past. Therefore, determining the time to initiate complementary feeding considering primipara mothers specifically could generate new knowledge for scholars, healthcare administrators, and policy makers to develop a specific intervention for this specific population. Therefore, this study has aimed to identify the time to initiate complementary feeding and its predictors among primipara mothers who have infants aged 6–12 months in the Awi zone, northwest Ethiopia.
2. Methods and materials
2.1. Study setting
A community-based retrospective follow-up study was conducted in the Awi Zone, Amhara region, northwest Ethiopia. The follow-up period was from January 1, 2022 to December 30, 2022 among primipara mothers who have infants aged 6–12 months.
The Awi zone has a total population of 1,342,324, of whom 684,590 are females. Among the total population, 316,520 are women of reproductive age. It also has 41,800 under 12 months of age infants, 8913 are under 6 months and 32,887 are aged 6–12 months based on the data of the Awi zonal health information system.
2.2. Population and eligibility criteria
The source population was all primipara mothers who have infants aged 6–12 months in the Awi zone, whereas all primipara mothers who have infants aged 6–12 months in the Awi zone in the selected districts were the study populations. Those primipara mothers who have not initially breastfed at least once for the infant; who gave care other than their infants, and mothers who had the death of an infant (asking data on the deceased infant was unacceptable by the community culture) during the retrospective follow-up time were excluded from this study.
2.3. Sample size and sampling procedure
To calculate the sample size, critical values such as za/2 = 1.96 at a significant level of 0.05, z1-B = 80 % power, p1 = proportion of the population allocated to a non-exposure group, p2 = proportion of the population allocated to the exposure group, 1.67 hazard ratio and 0.625 as survival probability of the non-exposed group were taken from the previous literature [20].
Then the sample size for this study was determined using the log rank method of Stata 17 software (https://www.stata.com) by the command 'power log rank 0.625, hratio (1.67) power (80 %) wd prob (10 %)', withdrawal probability 10 % for non-response rate and design effect = 2 to obtain the minimum required sample size, which was 754 primipara mother-infant pairs.
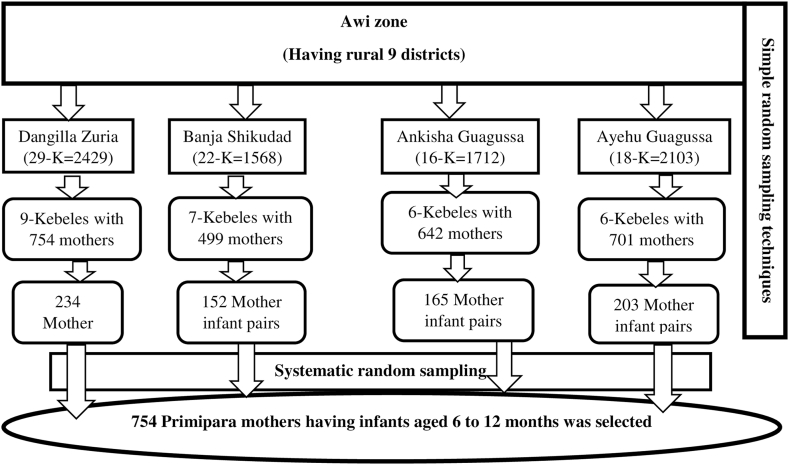
First, of the total of 9 rural districts in the Awi zone, 40 % of the districts (number = 4) and then 30 % of the kebeles (the lowest administrative unit in Ethiopian context) were selected using simple random sampling techniques to obtain primary and secondary sampling units, respectively. From the selected kebeles, using the data obtained from health posts, a household containing primipara mothers was selected and then interviewed. Then, the calculated sample size was allocated to the selected kebeles proportionally based on the size of the number of mother-infant pairs in the primipara, and was interviewed using a systematic random sampling technique at the third sampling interval. To obtain mothers having these infants the family folder and birth registration book at each health post of selected kebeles were used and then the data collectors and supervisors could have moved block by block of each selected household within the kebele until the sample size addressed (Fig. 1).
Fig. 1.
Schematic presentation of sampling procedures for the recruitment of primipara mothers who have infants aged 6–12 months in Awi zone, Northwest, Ethiopia, 2023.
2.4. Variables of the study
2.4.1. Dependent variables
Time to initiate complementary feeding in months.
2.4.2. Independent variables
Sociodemographic variables: Age of the mother, marital status, educational level of the mother, educational level of the father, wealth index, sex of the child, religion, family size and the mass media exposure.
Maternal and Obstetric related variables: ANC visit, PNC visit, birth type, place of delivery, mode of delivery, planned infant and birth preparedness.
2.5. Operational definitions
Primipara mother (PP): a mother who has born the first child alive, at least breastfed once for this infant, and was new to motherhood activities.
Incidence: the occurrence of early, on time and late initiation of complementary feeding during the study period and calculated as events per total person months (PM).
Event: when the mother initiates complementary feeding for the infant during the follow-up period during the study. It was based on the mothers' report and coded as '1'.
Censored: the mother did not initiate complementary feeding for the infant during the study period and was coded as “0”.
Survival time: the time from the start of breastfeeding (birth) to the start time of complementary feeding or censorship.
Birth preparedness: if the mother practiced at least three of the five components of birth preparedness [[39], [40], [41]], then considered 'Yes' otherwise 'No'.
Mass media exposure: if the household has a radio or television and listens at least once a week, it was considered as having “Yes” and otherwise “No” [9].
Educational status: classified as no formal education (includes being able to read and write, not being able to read and write), primary education (having at least a primary school certificate), and secondary and above (includes having at least a secondary school certificate). This category was based on previous works of literature [9].
Wealth index: an amalgamated indicator of a household's overall standard of living. Using readily available information on a household's ownership of specific assets, the wealth index was computed. Each household received a score determined by the proportion of assets they owned. Principal component analysis was used to determine these scores. Lastly, based on the wealth status score, the wealth of the household was divided into five categories: poorest (1), poor (2), middle (3), rich (4) and richest (5) [9,42].
2.5.1. Data collection tools, procedures, and quality control
Data were collected through personal interviews after the tools were developed from different published articles. Eight female B.Sc. Health professionals were involved as data collectors, four master health professionals as supervisors, and 28 community guiders were involved in the data collection process.
Data was collected when mothers could be easily accessed at home, such as on weekends or holidays in the community. The PP mother who could not be present during data collection time was further considered the next two holy or weekend days and then otherwise considered as non-response mothers.
Data quality was ensured by translating the questionnaire from English to Amharic and then back to English to see consistency using assistance experts in both the Amharic and English languages. Two-day training was provided to data collectors, community guiders, and supervisors. The questionnaire was pretested in 5 % of the mothers-infants pairs of none selected kebeles within the zone. The principal investigator and the supervisors conducted daily follow-ups during the entire data collection period. Each questionnaire was reviewed and verified for completeness by the supervisors and principal investigators on each day as soon as possible, and the necessary feedback was given to the data collectors before leaving the data collection site. The principal investigator and co-investigators managed the overall activity of the study.
2.6. Data management and analysis
Epi-data 3.1 was used to verify, code and clean the data's consistency and completeness before it was exported to Stata 17 for additional analysis. Missing values are checked and assess whether there are any significant outliers. Characteristics of the cohort were described using descriptive statistics such as mean (standard deviation), median (interquartile range), frequency and proportions.
To evaluate the infants' survival experience during exclusive breastfeeding at particular times and to compare the survival status across various categorical independent variables, the Kaplan-Meier survival curve and the log-rank test were utilized. To verify the Cox proportional Hazard (PH) assumption, the Schoenfeld residual statistical test, the presence of a time-dependent covariate, and graphical approaches were used.
The Cox-Snell residuals global test was used to determine the adequacy of the model. The Breslow approach was applied to handle the tied failure observations. For each independent variable and outcome of interest, a bivariable Cox proportional hazards regression model was built to find potentially important variables with significant tests below 0.25 that would be taken into account in a multivariable Cox proportional hazards regression model. A model comprising each of the chosen variables was used to start the multivariable analysis, and a backward elimination regression technique was then used until the model was stable.
Finally, the median time to initiate complementary feeding was determined. The early or late incidence rate of complementary feeding was calculated as the number of events over the person months of follow-up. The result of the final model was expressed in terms of the hazard ratio (HR) with 95 % confidence intervals (CI) and interpreted accordingly. Statistical significance was declared at a p-value less than 0.05. The result was presented using tables, graphs, or text narration.
3. Results
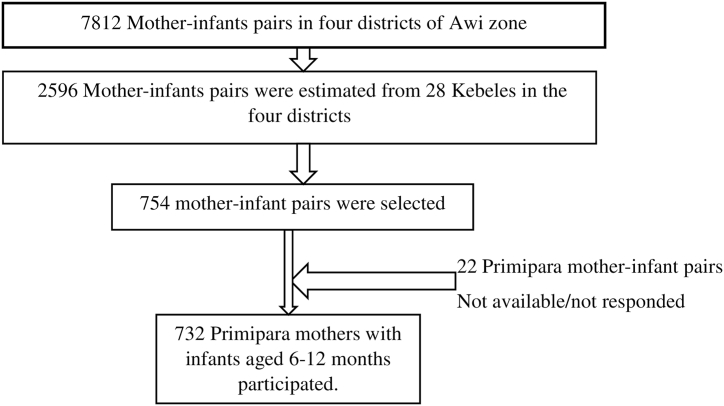
Of the 754 primipara mothers who had infants aged 6–12 months that were entered into the study, 22 of them did not respond during the data collection period and gave a response rate of 97 % (Fig. 2).
Fig. 2.
Final observations included in the analysis for time to initiate complementary feeding and its predictors among primipara mothers who have infants aged 6–12 months in Awi zone, Northwest, Ethiopia, 2023.
3.1. Sociodemographic characteristics of the participants
More than half of the primipara mothers who participated in this study were in the age category of 15–24 with an overall median ± interquartile range (IQR) of 24 ± 7 years. The minimum and maximum ages of the primipara mothers were 15 and 46 years, respectively. The median ± IQR age of the infants was 9 ± 2 months. Furthermore, more than 87 % of the primipara mothers were married, although there were 79 and 14 mothers who were single and divorced, respectively. The median ± IQR of family size of participants was 2 ± 1 with the minimum and maximum of 1 and 8, respectively. Around 73.36 % of mothers had no formal education. Of these, 231 (31.66 %) were unable to read and write. And 15 primipara mothers were educated at the college level (Table 1).
Table 1.
Sociodemographic characteristics of the participants among primipara mothers who had infants aged 6–12 months in the Awi Zone, northwest Ethiopia, 2023.
| Variables | Category | Event (%) | Censored (%) | Total (%) |
|---|---|---|---|---|
| Age of mothers in years | 15–24 | 323 (85.45) | 55 (14.55) | 378 (51.64) |
| 25–34 | 252 (82.08) | 55 (17.92) | 307 (41.94) | |
| ≥35 | 39 (82.98) | 8 (17.02) | 47 (6.42) | |
| Age of infants in months | 6–8 | 213 (76.62) | 65 (23.38) | 278 (37.98) |
| 9–12 | 401 (88.33) | 53 (11.67) | 454 (62.02) | |
| Marital status | Married | 536 (83.88) | 103 (16.12) | 639 (87.30) |
| Unmarried | 78 (83.87) | 15 (16.13) | 93 (12.70) | |
| Mothers' educational status | No formal education | 448 (83.43) | 89 (16.57) | 537 (73.36) |
| Primary Education | 84 (84.85) | 15 (15.15) | 99 (13.52) | |
| Secondary and above | 82 (85.42) | 14 (14.58) | 96 (13.11) | |
| Husbands' educational status | No formal education | 319 (83.51) | 63 (16.49) | 382 (59.78) |
| Primary Education | 144 (82.76) | 30 (17.24) | 174 (27.23) | |
| Secondary and above | 73 (87.95) | 10 (12.05) | 83 (12.99) | |
| Sex of infants | Male | 272 (82.93) | 56 (17.07) | 328 (44.81) |
| Female | 342 (84.65) | 62 (15.35) | 404 (55.19) | |
| Religion | Orthodox | 609 (83.88) | 117 (16.12) | 726 (99.18) |
| Muslim | 5 (83.33) | 1 (16.67) | 6 (0.82) | |
| Family size | <4 | 525 (83.60) | 103 (16.40) | 628 (85.79) |
| ≥4 | 89 (85.58) | 15 (14.42) | 104 (14.21) | |
| Mass media exposure | Yes | 295 (83.81) | 57 (16.19) | 352 (48.09) |
| No | 319 (83.95) | 61 (16.05) | 380 (51.91) | |
| Wealth index | Poorest | 116 (78.91) | 31 (21.09) | 147(20.08) |
| Poor | 128 (84.21) | 24 (15.79) | 152 (20.77) | |
| Middle | 125 (88.03) | 17 (11.97) | 142 (19.40) | |
| Rich | 124 (84.35) | 23 (15.65) | 147 (20.08) | |
| Richest | 121 (84.03) | 23 (15.97) | 144 (19.67) |
3.2. Maternal and obstetric characteristics of participants
The mean ± standard deviation (SD) number of antenatal care follow-up attended by primipara mothers who participated in this study was 3.42 ± 1.45. However, around 4.78 % of mothers did not follow-up on antenatal care. More than 63.68 % of primipara mothers were not ready to accept blood donation if bleeding and other health conditions occurred during labor and delivery. In addition, 38.65 % of participants were not prepared for transport to a health facility if labor had started at home. Approximately 33 % of the study participants were unable to list pregnancy danger signs. However, 122 (16.67 %) of the primipara mothers who participated in this study were delivered to their homes. Additionally, 181 (24.73 %) primipara mothers who participated in this study were delivered without a plan (Table 2).
Table 2.
Maternal and obstetric characteristics of participants among primipara mothers who had infants aged 6–12 months in the Awi Zone, northwest Ethiopia, 2023.
| Variables | Category | Event (%) | Censored (%) | Total (%) |
|---|---|---|---|---|
| Antenatal care follow up | No | 27 (77.14) | 8 (22.86) | 35 (4.78) |
| <4 | 259 (84.92) | 46 (15.08) | 305 (41.67) | |
| ≥4 | 328 (83.67) | 64 (16.33) | 392 (53.55) | |
| Birth preparedness | Yes | 525 (84.54) | 96 (15.46) | 621 (84.84) |
| No | 89 (80.18) | 22 (19.82) | 111 (15.16) | |
| To receive Blood if any | Yes | 227 (85.98) | 37 (14.02) | 264 (36.31) |
| No | 382 (82.51) | 81 (17.49) | 463 (63.69) | |
| Transport plan for labor | Yes | 374 (83.86) | 72 (16.14) | 446 (61.35) |
| No | 235 (83.63) | 46 (16.37) | 281 (38.65) | |
| Place of delivery | Home | 96 (78.69) | 26 (21.31) | 122 (16.67) |
| Health center | 346 (83.57) | 68 (16.43) | 414 (56.56) | |
| Hospital | 172 (87.76) | 24 (12.24) | 196 (26.78) | |
| Mode of delivery | Vaginally normal | 502 (82.43) | 107 (17.57) | 609 (83.20) |
| Vaginally Instrumental | 81 (92.05) | 7 (7.95) | 88 (12.02) | |
| Cesarean section | 31 (88.57) | 4 (11.43) | 35 (4.78) | |
| Birth type | Single | 586 (84.32) | 109 (15.68) | 695 (94.95) |
| Twin | 28 (75.68) | 9 (24.32) | 37 (5.05) | |
| Birth plan | Yes | 468 (84.94) | 83 (15.06) | 551 (75.27) |
| No | 146 (80.66) | 35 (19.34) | 181 (24.73) | |
| Postnatal care follow up | Yes | 503 (84.82) | 90 (15.18) | 593 (81.01) |
| No | 111 (79.86) | 28 (20.14) | 139 (18.99) |
3.3. Survival status of infants with exclusive breastfeeding
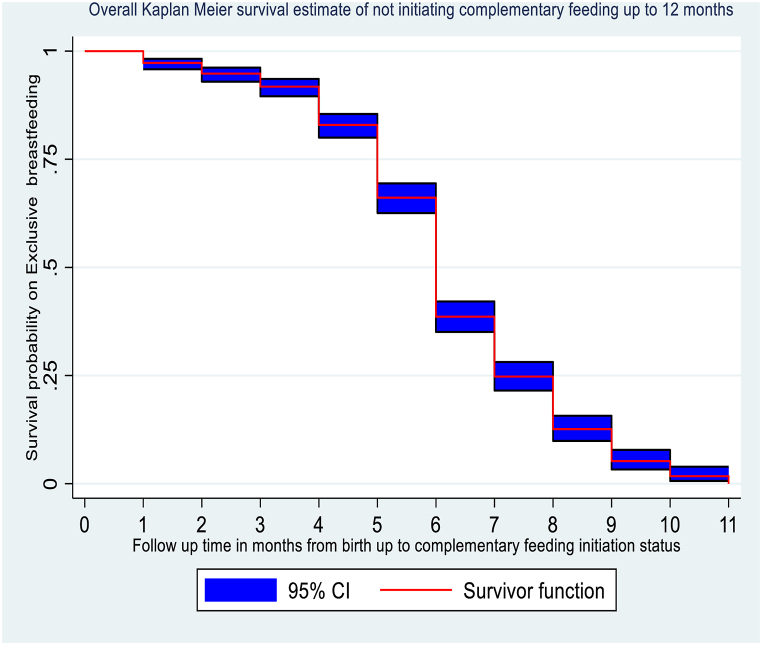
Among the 732 observations that followed, 118 (16.12 %) observations were censored at the end of the study. During the follow-up period, a total of 4284 mother-infant pair months of time risks were observed with a minimum follow-up time of 1 month and a maximum follow-up time of 11 months. The median follow-up time of observation was 6 months. Additionally, the median survival time for exclusive breastfeeding was 6 ± 2 months (interquartile range = 5, 7).
From the life table, the cumulative survival probabilities of not initiating complementary feeding up to the end of 5, 7, and 9 months were 0.66, 0.23, and 0.05, respectively. And the proportion of mothers who exclusively breastfed the infant until the end of 9 months was 5 % (95%CI: 3 %, 7 %) (Table 3).
Table 3.
Life table showing the probability of survival and failure of exclusive breastfeeding among primipara mothers who had infants aged 6–12 months in the Awi Zone, northwest Ethiopia, 2023.
| Time interval | The total number entered | Number of ICF* | Number Censored |
Probability of ICF* | SP* at the end of the interval | CSP* | 95 % CI** for CSP |
|---|---|---|---|---|---|---|---|
| 0–2 | 732 | 20 | 0 | 0.03 | 0.97 | 0.97 | 0.96, 0.98 |
| 2–4 | 712 | 40 | 0 | 0.06 | 0.94 | 0.92 | 0.90, 0.94 |
| 4–6 | 672 | 189 | 0 | 0.28 | 0.72 | 0.66 | 0.62, 0.69 |
| 6–8 | 483 | 281 | 98 | 0.65 | 0.35 | 0.23 | 0.20, 0.27 |
| 8–10 | 104 | 75 | 17 | 0.79 | 0.21 | 0.05 | 0.03, 0.07 |
| 10–12 | 12 | 9 | 3 | 0.86 | 0.14 | 0.007 | 0.001, 0.03 |
Footnote: ICF*: Initiating complementary feeding, SP*: Survival probability, CSP*: Cumulative survival probability, 95 % CI**: Confidence interval.
The overall graph of the Kaplan-Meier survivor function showed that there was a slow occurrence of events during the early (before 4 months) and late (after 9 months) follow-up periods. According to this graph, the survival status of infants with exclusive breastfeeding decreased rapidly at the mid of the follow-up period, which ranged from 5 to 8 months. Finally, the graph shows a slow decrease in the survival status of infants with exclusive breastfeeding to reach the cumulative survival probability of 0.7 % at the end of follow-up (Fig. 3).
Fig. 3.
Kaplan-Meier survival estimates of time to initiate complementary feeding among primipara mothers who had infants aged 6–12 months in the Awi Zone, Northwest Ethiopia, 2023.
3.3.1. Incidence and time to initiate complementary feeding
Of the total observations, 614 (83.88 %, 95%CI: 81.21, 86.55) mothers initiated complementary feeding for infants within the follow-up period with an overall incidence rate of 14.33 per 100 (95 % CI: 13.24, 15.51) person-month observations after 4284 life-risk follow-up months. The overall median time to initiate complementary feeding was 6 ± 2 months. Among those who initiated complementary feeding during the follow-up period, the median time to initiate complementary feeding was 6 months (IQR = 5–7, 95 % CI: 6, 6) months.
From the total events, the highest incidence of complementary feeding initiation was observed at the end of the 5th and 9th months of follow-up with the incidence rate of 29.72 (95 % CI: 26.66, 33.14) and 60.38 (95 % CI: 42.69, 85.38) per 100 person-month observations, respectively. Among those mothers who initiated complementary feeding for their infants, 249, 200, and 165 were before, at, and after 6 months of age of the infants. Furthermore, the incidence of complementary feeding initiation before, at, and after 6 months of follow-up was 7.29 (95%CI: 6.44, 8.25), 41.41 (95%CI: 36.05, 47.56), and 42.97 (95%CI: 36.89, 50.05) per 100 person-months observations, respectively.
3.3.2. Cox proportional hazard assumption test
Assumption tests were performed using graphical (ln-ln survival curve, observed vs. predicted survival curve) and statistical methods (Schoenfeld residuals test and time-varying covariate test). Variables that met at least two of the statistical tests were considered for cox proportional hazard regression, but the graphical methods are subjected to interpretation bias (Table 4, Table 5).
Table 4.
Schoenfeld residuals test results for predictors in the multivariable cox proportional hazard regression model for time to initiate complementary feeding among primipara mothers who had infants aged 6–12 months in the Awi Zone, Northwest Ethiopia, 2023.
| Variable | Category | Chi-square value | P-value |
|---|---|---|---|
| Age of the mother | 15–24 | 0.79 | 0.3748 |
| 25–34 | 0.19 | 0.6639 | |
| ≥35 | – | – | |
| Marital status | Unmarried | 0.19 | 0.6621 |
| Married | – | – | |
| Birth Plan | Yes | 0.83 | 0.3626 |
| No | – | – | |
| Mass Media Exposure | Yes | 0.00 | 0.9627 |
| No | – | – | |
| Wealth Index | Poorest | – | – |
| Poor | 0.97 | 0.3245 | |
| Middle | 0.00 | 0.9649 | |
| Rich | 1.65 | 0.1989 | |
| Richest | 0.72 | 0.3946 | |
| Mode of delivery | SVD | – | – |
| CS/Instrumental | 0.43 | 0.5124 | |
| Place of delivery | Home | – | – |
| Health Institution | 0.41 | 0.5239 | |
| Postnatal Care | Yes | 0.05 | 0.8218 |
| No | – | – | |
| Global Ph. test | 8.43 | 0.7505 | |
Table 5.
Time varying covariate test results of predictors in the multivariable cox proportional hazard regression model for time to initiate complementary feeding among primipara mothers who had infants aged 6–12 months in the Awi Zone, Northwest Ethiopia, 2023.
| Variable | Category | P-value |
|---|---|---|
| Age of the mother | 15–24 | 0.314 |
| 25–34 | 0.550 | |
| ≥35 | – | |
| Marital status | Unmarried | 0.730 |
| Married | – | |
| Birth Plan | Yes | 0.412 |
| No | – | |
| Mass Media Exposure | Yes | 0.873 |
| No | – | |
| Wealth Index | Poorest | – |
| Poor | 0.315 | |
| Middle | 0.899 | |
| Rich | 0.185 | |
| Richest | 0.389 | |
| Mode of delivery | SVD | – |
| CS/Instrumental | 0.516 | |
| Place of delivery | Home | – |
| Health Institution | 0.559 | |
| Postnatal Care | Yes | 0.805 |
| No | – |
3.3.3. Predictors of time to initiate complementary feeding
From the bivariable cox-regression analysis, the age of the mother, the marital status of the mother, the educational status of the mother, the mass media exposure, wealth index, the antenatal care visit, post-natal care visit, mode of delivery, place of delivery, and birth preparedness were selected for the multivariable cox regression analysis at the p-value ≤0.25.
Then a complete multivariable cox regression analysis was performed using a stepwise and backward elimination process by including all potential predictors that had a p-value of ≤0.25 in the bivariable cox regression analysis. Consequently, eight variables were entered into multivariable Cox regression and, in the backward elimination process, four variables were removed from the model (Maternal age, wealth index, place of delivery, and postnatal care visit).
Lastly, two of the predictors were independent statistically significant predictors for the time to initiate complementary feeding during multivariable Cox proportional regression analysis at a 95 % confidence level. These variables were maternal age and wealth index (Table 6).
Table 6.
Bi-variable and multivariable Cox proportional hazard regression analysis of predictors for time to initiate complementary feeding among primipara mothers who had infants aged 6–12 months in the Awi Zone, northwest Ethiopia, 2023.
| Variables | Category | Complementary feeding initiation status |
Crude Hazard Ratio (95 % Confidence Interval) | Adjusted Hazard Ratio (95 % Confidence Interval) | P-value | |
|---|---|---|---|---|---|---|
| Event | Censored | |||||
| Age of the mother | 15–24 | 323 | 55 | 1.64 (1.17, 2.29) | 1.63 (1.16, 2.29) | 0.005 |
| 25–34 | 252 | 55 | 1.37 (0.97, 1.92) | 1.37 (0.98, 1.93) | 0.069 | |
| ≥35 | 39 | 8 | 1 | 1 | ||
| Place of delivery | Home | 95 | 27 | 1 | 1 | |
| Health Institution | 519 | 91 | 1.23 (0.98, 1.53) | 0.96 (0.60, 1.56) | 0.883 | |
| Postnatal Care | Yes | 504 | 89 | 1.23 (1.02, 1.52) | 1.21 (0.77, 1.90) | 0.421 |
| No | 110 | 29 | 1 | 1 | ||
| Wealth Index | Poorest | 116 | 31 | 1 | 1 | |
| Poor | 127 | 25 | 1.32 (1.02, 1.70) | 1.25 (0.97, 1.62) | 0.082 | |
| Middle | 125 | 17 | 1.32 (1.03, 1.71) | 1.28 (0.99, 1.65) | 0.060 | |
| Rich | 124 | 23 | 1.35 (1.04, 1.74) | 1.35 (1.05, 1.75) | 0.021 | |
| Richest | 122 | 22 | 1.43 (1.11, 1.85) | 1.42 (1.10, 1.84) | 0.008 | |
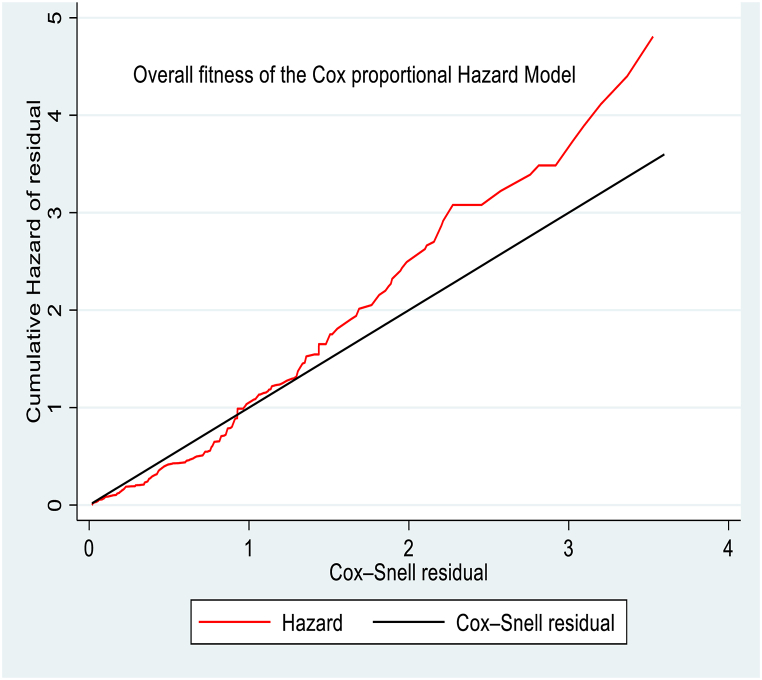
After fitting the multivariable Cox proportional hazard model, the overall adequacy of the model was checked using the Cox-Snell residuals graph. The graph of the cumulative residual hazard versus the Cox-Snell residual approximately made a straight line with slope one, so that the cumulative hazard lined a 450 to the reference line of the Cox-Snell residual, indicating that the cox proportional hazard model did not fit these data badly (Fig. 4).
Fig. 4.
Cox-Snell residual graph to assess the overall adequacy of the Cox proportional hazard model for time to initiate complementary feeding among primipara mothers who had infants aged 6–12 months in the Awi Zone, Northwest Ethiopia, 2023.
Furthermore, the general fitness of the Cox model was statistically checked using the global goodness of fit test, which gives P > chi2 = 0.7505. Therefore, the overall fitness of the model was adequate to interpret the variables in the model.
3.3.4. Interpretation of the proportional hazard cox regression analysis
The rate of initiation of complementary feeding was 1.63 times higher among primipara mothers whose age was 15–24 years than among primipara mothers whose age was >35 years (AHR: 1.63, 95%CI: 1.16, 2.29 p=0.005). Furthermore, the rate of initiation of complementary feeding was 1.35 times higher among primipara mothers whose wealth index category was rich than among primipara mothers whose category was the poorest (AHR: 1.35, 95%CI: 1.05, 1.75 p=0.021). Likewise, the rate of initiating complementary feeding was 1.43 times higher among primipara mothers whose wealth index category was richest than among primipara mothers whose category was the poorest (AHR: 1.43, 95%CI: 1.10, 1.84 p=0.008).
4. Discussion
The early or late introduction of complementary feeding increased the risk of diarrhea, respiratory infections, and infant death [43,44]. In Ethiopia, the time to initiate complementary feeding among primipara mothers was not well studied [9,22]. Therefore, this study assessed the incidence, the median time to initiate complementary feeding, and its predictors among primipara mothers in the Awi zone, northwest Ethiopia. According to this study, the mother's age (between 15 and 24 years old) and the household's level of wealth were found to be independent predictors of the time to initiate complementary feeding.
The findings of this study stated that the proportion of mothers who did not initiate complementary feeding up to the 6th month of follow-up was 66 % (95 % CI: 62 %, 69 %). This finding contrasts with the WHO and UNICEF recommendation, which stated that all infants should be exclusively breastfed until 6 months of age [1,2,4,43]. However, here only 66 % of infants did not initiate complementary feeding before 6 months of age. Furthermore, this study revealed that the failure probability of mothers before infants' age reached 6 months was 28 %. This implied that the rate at which the infants' probability of initiating complementary feeding before 6 months was 28 %.
The overall incidence rates of complementary feeding initiation before and after six months of infant age were 7.29 and 42.97 per 100 person-month observations, respectively. The actual figure of complementary feeding initiated before and after 6 months of age of the infant was 249 and 165, respectively. However, the incidence density of the initiation of complementary feeding calculated appeared low at an early age than at late age; it is due to the high observation time of risk, which was around 3417 and 384 months of mother-infant pairs.
Of the total mothers who initiated complementary feeding for the infant, around 40.55 % and 26.87 % were initiated before and after 6 months of age of the infant. These findings are higher than the results of the study carried out in Ethiopia, such as in Maychew district, West Gojjam and Mekele Town in all parity mothers [20,22,27]. The difference in findings may be associated with the difference in parity. The present study incorporated only primipara mothers, whereas previous studies included all mothers without consideration of parity. This implies that primipara mothers could not initiate complementary feeding in a timely manner than the general population. This may be an alerting input for the public officials and policy makers for designing parity-based interventions. Additionally, the finding of our study is higher than the finding of the study done in Dutch, which reported that 21.4 % of infants initiated complementary feeding before 4 months [45]. The difference may be associated with the study settings. Our study used a community-based retrospective follow-up study in which mothers were elicited to recall the time of the initiation of complementary feeding. While the Dutch study analyzed data already collected (a cluster randomized controlled trial-based study in the population for the primary prevention of overweight in young children (age 0–3 years) in the Netherlands) for other purposes [46]. But it is lower than the findings of the study conducted in Southwest Ethiopia, which revealed that around 59 % of mothers initiated complementary feeding before 6 months of age of the infant [28]. The reason behind this may be that the sample population for the study in south-west Ethiopia was infants aged 6 months to 2 years. As the child's age was older, the mother recalling the initiation time may be biased, which gave unreliable estimates. On the other hand, this finding is consistent with the findings of the study conducted in the town of Gondar and Finote Selam town [18,29]. Both studies revealed that 49.4 and 48.6 % of mothers started complementary feeding to their infants before 6 months. It may be associated with the application of similar strategies and recommendations about the timing of the initiation of complementary feeding in the study areas.
The overall median time to initiate complementary feeding was 6 months, which means that as pairs of mothers-infants were followed for 12 months, 50 % of the mothers’ survival time to exclusive breastfeeding of the infants was 6 months. This finding is in line with the findings of the study conducted in different parts of Ethiopia among any parity mothers [[18], [19], [20],22,28]. This could be due to the applicability of the strategies of WHO and UNICEF recommendations on the time to initiate complementary feeding similarly throughout the country (Ethiopia). This implied that both all parity and primipara mothers have a similar median time to initiate complementary feeding. However, this finding is lower than the report (6.7 months) of the study done in West Gojjam zone [27]. Therefore, our study was a retrospective study, there may be recall bias that underestimated the overall estimate compared to the findings of the West Gojjam randomized control trial. Additionally, this finding was higher than the reports of research carried out in China, Jiangyou (the median time was 4.5 months) [47]. The reason behind this difference is the data collection technique used, in which our study used an interviewer-based face-to-face interview. However, the study in China used a telephone interview.
In the current study, the rate of initiation of complementary feeding was higher among mothers whose age was 15–24 years than among mothers whose age was <35 years. This could be associated with the difference in knowledge and attitude of adolescent mothers and adult mothers with respect to the timely initiation of complementary feeding. In addition to that, mothers who recently gave birth to their first child care more for their babies than mothers who gave birth to their babies at an early age due to the cultural value of Ethiopia. The present study finding is in line with the study findings from two European countries, which reported that younger maternal age was a risk factor for the early introduction of complementary feeding [48]. However, this finding is in contrast to the findings of the study in South Ethiopia, which reported that mothers <30 years were a risk factor for initiating complementary feeding early [28]. The contrasted finding may be due to the age difference of the infants. This study included mothers who had infants aged 6–12 months. However, the South Ethiopia study included mothers who had children aged 6–24 months. The age of the infants from whom data were collected can affect the recall ability of the mother.
Additionally, the rate of initiation of complementary feeding was higher among mothers who lived in households with the rich and richest wealth than among mothers who lived in households with the poorest wealth status. This may be in the Ethiopian context, rich and richest families may give cow's milk and other foods to the infant to show that they can feed the infant better than the poor and poorest families [49]. It is inconsistent with the research reports from southern Ethiopia, which revealed that mothers in the middle wealth index class were 60 % less likely to initiate complementary feeding in time than mothers in the rich household class. Similarly, this study finding is opposite to the research reports done in Ethiopia at the national level, which stated that the rich wealth status was a factor in a timely initiation of complementary feeding [50]. This opposite finding may be associated with: our study includes mothers from similar cultures and feeding habits. However, the national-level study included mothers from all parts of Ethiopia, where culturally diversified peoples lived, where food habits were expected to vary. Furthermore, the finding of our study is inconsistent with the Brazilian study, which revealed that wealth status is inversely associated with the early introduction of other milks [51]. This difference may be due to the slight difference in the sample population. Our study included all mothers with infants in the rural community without considering socioeconomic status. However, the Brazilian study recruited urban mothers of low socioeconomic class.
5. Limitations of the study
Recall bias may cause an overestimate or underestimate of the actual time to initiate complementary feeding because this is a retrospective study. However, interviewers were trained in how to investigate and recall complementary feeding activities with the current infant, which helped to reduce recall bias by connecting practices to local conditions.
6. Conclusions and recommendations
In conclusion, the median time to initiate complementary feeding among primipara mother-infant pairs was 6 months. The overall survival probability that primipara mothers not initiating complementary feeding for the current infant until 6 months was 66 %. Furthermore, the overall incidence rates of complementary feeding initiation before, at, and after 6 months of age of the infants were 7.28, 41.41, and 42.97 per 100 person-months of observations, respectively. This study also identified that the age of the mother and the wealth status of the household were statistically independent predictors of the time to initiate complementary feeding.
Based on these findings, the authors recommended that it is better to conduct a more qualitative and prospective interventional cohort study to clearly identify the relationship between age and wealth status in the introduction of complementary feeding among primipara mothers. In addition, the health department was better able to strengthen the activity of community health workers with respect to the creation of awareness and the promotion of complementary feeding initiation through home and family-based interventions among primipara mothers. Furthermore, community health professionals should work better on when to start complementary feeding for infants for young primipara mothers. It is also better to strengthen the promotion of breast milk as an inexpensive and irreplaceable food for infants until the age of 6 months for those primipara mothers who have a better wealth status.
Ethical approval and informed consent
The Ethics Review Committee of the Medicine and Health Sciences College of Injibara University provided the ethical approval letter (Ref. No.: IN/U/C/M/H/S/326/09). Following approval, a formal letter of cooperation was sent to the corresponding organizations. According to the recommendation letter of the college, permission was given by the relevant administrative health offices. Codes were used instead of personal identification in the questionnaires. Written informed consent was obtained from the primipara mothers and occasionally the head of the family was taken into account. Information security was preserved by being password protected and only accessible by the principal investigator.
Consent for publication
Not applicable.
Data availability statement
All data are included in the manuscript and are available in Injibara University main library documentation room. However, it doesn't have accession number yet and also not found online.
CRediT authorship contribution statement
Tilahun Degu Tsega: Writing – original draft, Methodology, Formal analysis, Conceptualization. Tamiru Alene: Writing – review & editing, Supervision, Methodology. Yeneneh Negesse Kebede: Writing – review & editing, Supervision, Methodology. Abebaw Molla Kebede: Writing – review & editing, Supervision, Methodology. Mekuanint Asmare Yizengaw: Visualization, Supervision, Formal analysis. Tadesse Miretie Dessie: Writing – review & editing, Supervision, Methodology. Tewodros Worku: Visualization, Supervision, Formal analysis. Bezawit Adane: Supervision, Software, Data curation. Melaku Yalew: Validation, Supervision, Software, Formal analysis, Data curation. Yitaysh Damitie: Validation, Software, Methodology, Data curation. Sileshi Berihun Delele: Writing – review & editing, Visualization, Methodology. Almaw Genet: Writing – review & editing, Visualization, Methodology. Animut Takele Telayneh: Writing – review & editing, Visualization, Methodology. Kefale Mitiku Haylu: Writing – original draft, Methodology, Formal analysis, Conceptualization. Zewdu Bishaw Aynalem: Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All authors acknowledge Injibara University for being as source of the finance for the data collectors, community guiders and Supervisors for this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29663.
Contributor Information
Tilahun Degu Tsega, Email: tilahund2121@gmail.com.
Tamiru Alene, Email: tamirualene1212@gmail.com.
Yeneneh Negesse Kebede, Email: hebneyan@gmail.com.
Abebaw Molla Kebede, Email: abebawtsehay1@gmail.com.
Mekuanint Asmare Yizengaw, Email: mekuastt2005@gmail.com.
Tadesse Miretie Dessie, Email: tadessemiretie19@gmai.com.
Tewodros Worku, Email: tedyworku44@gmail.com.
Bezawit Adane, Email: adanebeza1987@gmail.com.
Melaku Yalew, Email: meleya7@gmail.com.
Yitaysh Damitie, Email: yitutile@gmail.com.
Sileshi Berihun Delele, Email: sileshberihun@gmail.com.
Almaw Genet, Email: yeshiwas690@gmail.com.
Animut Takele Telayneh, Email: animuttakele@gmail.com.
Kefale Mitiku Haylu, Email: kefale21mtkl@gmail.com.
Zewdu Bishaw Aynalem, Email: zest7@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.United Nations Children’s Fund (UNICEF) UNICEF Programming Guidance. UNICEF; New York: 2020. Improving young Children's diets during the complementary feeding period. [Google Scholar]
- 2.Were F.N., Lifschitz C.J.A.o.N. vol. 73. 2018. pp. 20–25. (And Metabolism, Complementary Feeding: Beyond Nutrition). [DOI] [PubMed] [Google Scholar]
- 3.WHO and UNICEF . WHO; Geneva: 2003. Global Strategy for Infant and Young Child Feeding; p. 37. [Google Scholar]
- 4.World Health Organization . In: Complementary Feeding, Family Foods for Breastfeeding Children. Development D.o.N.f.H.a., editor. WHO; 2000. p. 56. [Google Scholar]
- 5.Habtewold T.D., et al. Breast and complementary feeding in Ethiopia: new national evidence from systematic review and meta-analyses of studies in the past 10 years. 2019;58(7):2565–2595. doi: 10.1007/s00394-018-1817-8. [DOI] [PubMed] [Google Scholar]
- 6.Radford A., Rickitt C., Williams A. Breast feeding: the baby friendly initiative: unicef's baby friendly initiative is making great progress in UK. BMJ Br. Med. J. (Clin. Res. Ed.) 1998;317(7169):1385. [PMC free article] [PubMed] [Google Scholar]
- 7.Radford A. Unicef is crucial in promoting and supporting breast feeding. BMJ. 2001;322(7285):555. [PMC free article] [PubMed] [Google Scholar]
- 8.Nita Bhandari R.C. Infant and young child feeding guideline. Proc Indian Natn Sci Acad. 2016;82(5):1507–1517. [Google Scholar]
- 9.Central Statistical Agency and ICF. Ethiopian Demographic and health survey, 2016, addis abeba, Ethiopia, the DHS program. CSA and ICF: Rockville, Maryland, USA. 2016:551. [Google Scholar]
- 10.Christine Prell B.K. Breastfeeding and Complementary-recommendations on infant nutrition. Deutsches Ärzteblatt International. 2016;113:435–444. doi: 10.3238/arztebl.2016.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.F. Gary Cunningham Kenneth J. Leveno Steven L. Bloom, C.Y.S.J.S.D. McGraw-Hill Education; New York: 2019. Barbara L. Hoffman Brian M. Casey, Jeanne S. Sheffield, Williams Obstetrics 24 Ed. 2014. 1376. [Google Scholar]
- 12.Assegid M. Addis Abeba: Ethiopian Public Health Training Initiative. 2003. Obstetric and gynecological nursing lecture notes for nursing students by carter center; p. 336. [Google Scholar]
- 13.Yeneabat T., Belachew T., Haile M. Determinants of cessation of exclusive breastfeeding in ankesha guagusa woreda, Awi zone, northwest Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. 2014;14(1):1–12. doi: 10.1186/1471-2393-14-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicoll A., Williams A. Breast feeding. Arch. Dis. Child. 2002;87(2):91–92. doi: 10.1136/adc.87.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mané N.B., et al. Early breastfeeding cessation in rural Senegal: causes, modes, and consequences. Am. J. Public Health. 2006;96(1):139–144. doi: 10.2105/AJPH.2004.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chudasama R., Patel P., Kavishwar A. Factors associated with duration of exclusive breastfeeding. Internet J. Pediatr. Neonatol. 2008;9(1):1–6. [Google Scholar]
- 17.Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF . EPHI and ICF; Rockville, Maryland, USA: 2019. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. [Google Scholar]
- 18.Bazezew K., Worku W., Abebe Z. Timely initiation of complementary feeding practices in gondar town northwest Ethiopia: a cross-sectional study. Ecol. Food Nutr. 2020;59(3):329–341. doi: 10.1080/03670244.2020.1733994. [DOI] [PubMed] [Google Scholar]
- 19.Andualem A., et al. Timely initiation of complementary feeding and associated factors among mothers of children aged 6-24 Months in Dessie referral hospital, Northeast Ethiopia, 2019. J Nutr Metab. 2020;2020 doi: 10.1155/2020/6756202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reda EB, Teferra AS, Gebregziabher MG. Time to initiate complementary feeding and associated factors among mothers with children aged 6–24 months in Tahtay Maichew district, northern Ethiopia. BMC Res Notes. 2019;12(1):1–8. doi: 10.1186/s13104-019-4061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sisay W., Edris M., Tariku A. Determinants of timely initiation of complementary feeding among mothers with children aged 6-23 months in Lalibela District, Northeast Ethiopia, 2015. BMC Publ. Health. 2016;16(1):884. doi: 10.1186/s12889-016-3566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shumey A., Demissie M., Berhane Y. Timely initiation of complementary feeding and associated factors among children aged 6 to 12 months in Northern Ethiopia: an institution-based cross-sectional study. BMC Publ. Health. 2013;13:1050. doi: 10.1186/1471-2458-13-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . 2022. WHO Fact Sheets on Complementary Feeding and Malnutrition; p. 5. [Google Scholar]
- 24.WHO and UNICEF . 2021. Indicators for Assessing Infant and Young Child Feeding Practices Definitions and Measurement Methods; p. 122. [Google Scholar]
- 25.UNICEF . 2021. UNICEF Programming Guide–Infant and Young Child Feeding. [Google Scholar]
- 26.Abdurahman A.A., et al. Magnitude and determinants of complementary feeding practices in Ethiopia: a systematic review and meta-analysis. 2019;5(7) doi: 10.1016/j.heliyon.2019.e01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abiyu C., Belachew T. Effect of complementary feeding behavior change communication delivered through community-level actors on the time of initiation of complementary foods in rural communities of West Gojjam zone, Northwest Ethiopia: a cluster-randomized controlled trial. BMC Pediatr. 2020;20(1):509. doi: 10.1186/s12887-020-02396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agedew E., et al. Early initiation of complementary feeding and associated factors among 6 months to 2 years young children. 2014;4(6):314. [Google Scholar]
- 29.Hailu D., Tilahun A., Dagnew Y. Complementary feeding practice and its determinants among mothers with children 6 to 23 months of age in Finote Selam, Ethiopia. Pan Afr Med J. 2021;40:14. doi: 10.11604/pamj.2021.40.14.27411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed S., et al. Prevalence of initiation of complementary feeding at 6 months of age and associated factors among mothers of children aged 6 to 24 months in Addis Ababa, Ethiopia. 2018;4(1):1–7. doi: 10.1186/s40795-018-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neves R.O., et al. vol. 25. 2020. pp. 4593–4600. (Can Parity Influence Infant Feeding in the First Six Months of Life?). [DOI] [PubMed] [Google Scholar]
- 32.Ogunlesi T.A., et al. Determinants of timely initiation of complementary feeding among children aged 6-24 months in Sagamu, Nigeria. Niger. J. Clin. Pract. 2014;17(6):785–790. doi: 10.4103/1119-3077.144399. [DOI] [PubMed] [Google Scholar]
- 33.Chane T., et al. Initiation of complementary feeding and associated factors among children of age 6-23 months in Sodo town, Southern Ethiopia: cross-sectional study. 2018;9(4):7240. doi: 10.4081/pr.2017.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christian P., et al. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015;39(5):361–372. doi: 10.1053/j.semperi.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Atimati AO. Adam VY.Infant and young child feeding practices and nutritional status of children (0-24 months) in egor local government area of Edo State, Nigeria. Nigerian J. Nutr. Sci. 2019;40(1) [Google Scholar]
- 36.Basnet S., et al. Reasons for early or late initiation of complementary feeding: a study in Pokhara. 2015;3(4A):69–75. [Google Scholar]
- 37.Mengesha D.K., Merkeb Y. 2020. Prevalence of Malnutrition and Associated Risk Factors Among Children under Five Years of Age in Amhara Region, Ethiopia: Evidence from 2016 Ethiopian Demographic and Health Survey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.belete Ferede A., Bikes G.A., gebregyorgis Gebremichael T. Northwest Ethiopia: Community-Based Cross-Sectional Study. 2019. Appropriate Complementary Feeding Practice and associated factors among mothers with children age 6-23 months in Faggeta-Lekoma District. [Google Scholar]
- 39.Maternal J.J.C.f.c.p. Johns Hopkins, Bloomberg school of Public Health; 2004. Family Care International, Neonatal Health: Monitoring Birth Preparedness and Complication Readiness, Tools and Indicators for Maternal and Newborn Health. [Google Scholar]
- 40.Tadesse T, Makeda S. Knowledge of obstetric danger signs and birth preparedness practices among pregnant women in rural communities of eastern Ethiopia. Int. J. Nurs. Midwifery. 2016;8(1):1–11. [Google Scholar]
- 41.Wudu M.A., Tsegaye T.B. Birth preparedness and complication readiness and associated factors among recently delivered mothers in mizan-aman town, Southwest Ethiopia, 2019. Int J Womens Health. 2021;13:177–187. doi: 10.2147/IJWH.S279201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woldeamanuel B.T. Trends and factors associated to early initiation of breastfeeding, exclusive breastfeeding and duration of breastfeeding in Ethiopia: evidence from the Ethiopia Demographic and Health Survey 2016. Int. Breastfeed. J. 2020;15(1):1–13. doi: 10.1186/s13006-019-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization . 2020. Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: the Revised Baby-Friendly Hospital Initiative: 2018 Implementation Guidance: Frequently Asked Questions. [Google Scholar]
- 44.Lamberti L.M., et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. 2013;13:1–8. doi: 10.1186/1471-2458-13-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., et al. Factors associated with early introduction of complementary feeding and consumption of non-recommended foods among Dutch infants: the BeeBOFT study. 2019;19(1):1–12. doi: 10.1186/s12889-019-6722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raat H., et al. Primary prevention of overweight in preschool children, the BeeBOFT study (breastfeeding, breakfast daily, outside playing, few sweet drinks, less TV viewing): design of a cluster randomized controlled trial. 2013;13(1):1–11. doi: 10.1186/1471-2458-13-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang L., Lee A.H., Binns C.W.J.P.i. Predictors of early introduction of complementary feeding: longitudinal study. 2015;57(1):126–130. doi: 10.1111/ped.12421. [DOI] [PubMed] [Google Scholar]
- 48.Zielinska M.A., et al. Factors influencing the age of complementary feeding—a cross-sectional study from two European countries. 2019;16(20):3799. doi: 10.3390/ijerph16203799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsega T.D., et al. Time to breastfeeding cessation and its predictors among mothers who have children aged two to three years in Gozamin district, Northwest Ethiopia: a retrospective follow-up study. 2022;17(1) doi: 10.1371/journal.pone.0262583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilano G., Sako S., Gilano K.J.S.R. Determinants of timely initiation of complementary feeding among children aged 6–23 months in Ethiopia. 2022;12(1) doi: 10.1038/s41598-022-21992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maciel B., et al. Infant feeding practices and determinant variables for early complementary feeding in the first 8 months of life: results from the Brazilian MAL-ED cohort site. 2018;21(13):2462–2470. doi: 10.1017/S136898001800099X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and are available in Injibara University main library documentation room. However, it doesn't have accession number yet and also not found online.