1. CASE PRESENTATION
A 26‐year‐old man presented to the emergency department with abdominal pain and hematemesis 1.5 hours after ingesting 40 ounces (1183 mL) of 3% hydrogen peroxide. Physical examination demonstrated epigastric and right upper quadrant tenderness without respiratory, hemodynamic, or neurologic findings. Computed tomography (CT) of the abdomen revealed extensive portal venous gas (Figure 1), thickened gastric antrum and pylorus, and thickened, hypoenhanced duodenum concerning for caustic injury (Figure 2, arrow).
FIGURE 1.
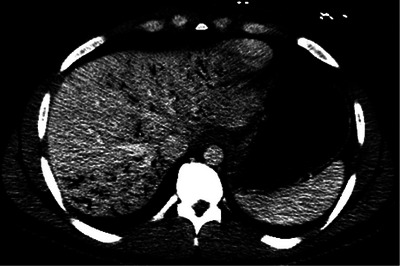
Axial view of computed tomography (CT) of abdomen, demonstrating extensive portal venous gas.
FIGURE 2.
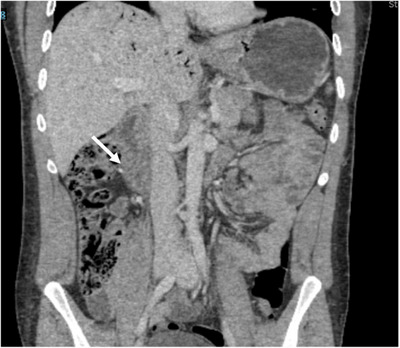
Coronal view of computed tomography (CT) of abdomen, demonstrating portal gas and a thickened, hypoenhanced duodenum (arrow).
2. DIAGNOSIS
2.1. Portal venous gas and caustic injury from hydrogen peroxide ingestion
Esophagogastroduodenoscopy showed stomach and duodenum erosions consistent with Zargar 2A caustic injury. His diet was advanced, and he tolerated enteral nutrition at discharge on hospital day 4.
Three percent hydrogen peroxide is commonly used in household applications; concentrations of ≥30% are used for textile and chemical production. 1 Toxicity results via two mechanisms. First, gas formation occurs when tissue catalase converts H2O2 to water and oxygen gas. One milliliter of 3% H2O2 liberates 10 mL O2, while 1 mL of 35% H2O2 liberates 115 mL O2, causing tissue injury from rapid expansion. 2 Management is supportive and includes hyperbaric oxygen for arterial air embolism that impedes cardiac output or cerebral blood flow. 2 Second, caustic injury results from the oxidizing effect of peroxide, reactive oxygen species, and lipid peroxidation. Gastrointestinal injuries and chemical colitis have been reported following ingestion of 35% H2O2. 3 , 4
Counts CJ, Greller HA, Calello DP, Meaden CW. A bad case of gas. JACEP Open. 2024;5::e13158. 10.1002/emp2.13158
REFERENCES
- 1. The National Institute for Occupational Safety and Health . Hydrogen peroxide. Centers for Disease Control and Prevention. 2019. Accessed October 31, 2023. https://www.cdc.gov/niosh/topics/hydrogen‐peroxide/default.html [Google Scholar]
- 2. Nelson LS, Howland M, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, eds. Goldfrank's Toxicologic Emergencies, 11e. McGraw Hill; 2019. [Google Scholar]
- 3. Watt BE, Proudfoot AT, Vale JA. Hydrogen peroxide poisoning. Toxicol Rev. 2004;23(1):51‐57. doi: 10.2165/00139709-200423010-00006 [DOI] [PubMed] [Google Scholar]
- 4. Alhasson H, Salama A, Alweis R. The dangers of ingesting antiseptics: hydrogen peroxide‐induced chemical colitis. Am J Med. 2021;134(2):206‐208. doi: 10.1016/j.amjmed.2020.06.027 [DOI] [PubMed] [Google Scholar]


