Neurodegenerative disorders represent a pervasive global health challenge, yet therapeutic options remain conspicuously limited. These disorders are inherently dynamic processes within the central nervous system, unfolding across distinct sub-stages: initial structural neuronal alterations (sub-stage 1), functional impairment (sub-stage 2), and culminating in neuronal death (sub-stage 3). Previous studies have revealed shared pathological features between amyotrophic lateral sclerosis (ALS) and Parkinson's disease (PD) (van Rheenen et al., 2021; Mantle and Hargreaves, 2022) including common genetic risk factors identified through genome-wide association studies (van Rheenen et al., 2021). Both disorders manifest similar neurodegenerative mechanisms, such as oligomer formation, aberrant protein accumulation, and protein misfolding-specifically, superoxide dismutase 1 in ALS and α-synuclein in PD. Mitochondrial dysfunction further serves as a common denominator in the pathogenesis of ALS and PD (Mantle and Hargreaves, 2022).
Early and accurate diagnosis is imperative to mitigate the rapid progression inherent to neurodegenerative diseases. Recent advancements in machine learning have shown promise in this regard. For instance, a convolutional neural network-long short-term memory model achieved a 99.42% accuracy in differentiating between ALS, PD, and Huntington's disease, as well as healthy controls, based on gait signal analysis (Amooei et al., 2023). Similar success has been reported in PD detection through speech signal analysis using neural networks and long short-term memories (Er et al., 2021). Moreover, convolutional neural network-long short-term memory outperformed traditional methods like support vector machines and standard deep neural networks in classifying these diseases based on speech data recorded during three different tasks such as spontaneous speech, diadochokinetic rate and sustained phoneme production (Mallela et al., 2020).
In our recent work, we employed state-space models to demonstrate the involvement of the ipsilateral motor network in voluntary movements (Ding et al., 2023). The dynamic nature of neurodegenerative processes involves instantaneous state transitions at each sub-stage. Correctly identifying and associating features from these critical states at varying temporal scales using non-linear state space models would uncover a unique and comprehensive framework to study neurodegeneration. The spatial and temporal features, when used to train convolutional neural networks, hold the potential for individualized patient outcome predictions. Such an approach would pave the way for personalized prognosis, selective lifestyle modifications, and targeted therapeutic interventions.
Concordantly, given the intricate complexity of neurodegenerative processes, a holistic, system-level biological approach is warranted. Such methodologies, both experimental and computational, offer great opportunities to uncover fundamental insights into neural network dynamics and their interactions. This is particularly important for the mechanism-based molecular signatures of disease hallmarks across varying pathological stages, potentially leading to personalized interventions such as lifestyle modifications and the possibility to direct patients towards new treatment concepts, aligning with the emerging paradigm of precision medicine (Morello et al., 2020).
This paper proposes a new framework that synergistically combines state-space modeling with deep learning techniques to enable individualized disease prediction and to identify optimal time windows for therapeutic options and lifestyle modifications. The unique contributions of this work can be summarized by four aspects, illustrated in Figure 1: (1) Identification of sub-stages that are common and distinct in neurodegenerative diseases; (2) Develop a state space model to extract state changes and factors driving neurodegeneration; (3) Use a deep learning neural network to identify critical windows for individualized disease prediction; (4) Ultimately, utilize the model to generate personalized prognosis, facilitating the selection of lifestyle modifications and potential therapeutic options.
Figure 1.
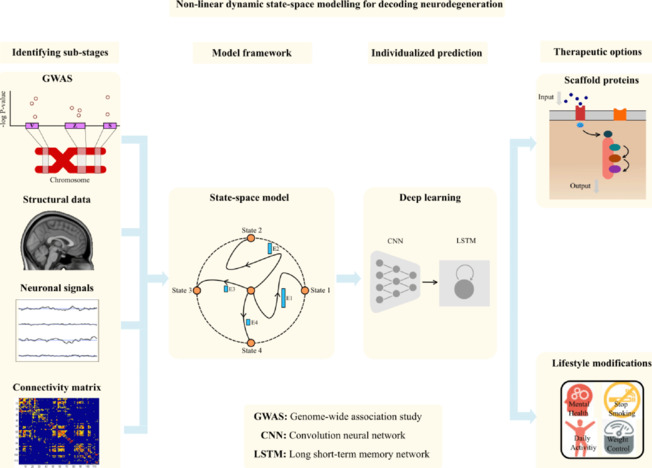
The four primary components of the proposed research framework.
The first block is identifying the sub-stages using multimodal data such as genetics, structural magnetic resonance images, neuronal signals, or connectivity matrix. The second block is the model framework using the state space model for extracting the features namely the state and causality changes. The extracted features will be fed in the third block for the individualized prediction and finally therapeutic option depicted in the fourth block. Created with Inkscape software.
Identification of critical sub-stages: Neurodegenerative diseases, for which no definitive cure exists, comprise a diverse spectrum of disorders characterized by progressive neuronal dysfunction and death within the central nervous system. The etiology of neurodegeneration is inherently complex, dynamic, and influenced by a confluence of both micro-level factors, such as genetics, protein, single-cell, and electrophysiology, and macro-level elements, including systemic conditions, environmental exposures, and lifestyle choices (Bertram and Hampel, 2011). This multifactorial landscape contributes to disease susceptibility and disrupts the homeostatic equilibrium of neural networks.
Predominant neurodegenerative disorders, including ALS and Parkinson's, and their respective disease subtypes, often exhibit paradoxical profiles owing to their commonly overlapping profiles resulting from their closely allying multifactorial nature. Yet, even within a disease category, inter-individual variability exists in the distinct clinical and pathological features. A hallmark of the pathophysiology of these diseases is the abnormal accumulation of proteins, implicated in neuronal injury and death. Intriguingly, some neurodegenerative diseases share common proteinopathies, such as Tau pathology in both ALS and Parkinson's diseases, with varying degrees of aggregation demarcating different disease stages.
Current investigations into the fundamental mechanisms and molecular signatures of neurodegeneration are constrained, particularly in the context of mechanism-based classification and tailored interventions. Despite the inherent non-linearity in disease progression, molecular signatures of neurodegeneration tend to spread across brain regions in a stereotypical manner. Recent studies have also elucidated variations in neural network characteristics at different disease stages and in association with distinct neurodegenerative factors (Koirala et al., 2017; Muthuraman et al., 2018). Therefore, accurate modeling of these distinct factors could lead to reliable characterization of disease stages and accurate predictions of the intrinsic processes that culminate in neurodegeneration.
Building a state-space model framework: The state-space model serves as a robust mathematical framework for conceptualizing the brain as a dynamic system, characterized by intricate regional interactions. In this context, each point in a multidimensional space represents a specific configuration of the brain at a given time. Here, the spatial dimensions correspond to distinct brain regions, while the states are indicative of various disease stages. State space modeling provides a formalized approach to representing a system through its inputs, outputs, and internal states. These state variables are governed by difference equations, which can be conveniently expressed in matrix form in the case of linear systems. In state space modeling, we can estimate the dynamical state changes (disease stages) and simultaneously estimate the causality (network interplay) (Muthuraman et al., 2018).
Individualized prediction: The changes between different stages of neurodegenerative diseases, as captured by state-space models, can be effectively used as input variables to build a convolutional neural network (ConvNets) (LeCun et al., 2015). ConvNets, a specialized form of deep learning algorithm, are designed to mimic certain features of natural systems, using them as inductive biases, namely shared weights, pooling of data, and use of many layers to extract complex features from the input data. Depending on the data modality, these features can be represented in various array formats: 1D for signals, 2D for images, and 3D for volumetric images. The architecture of ConvNets typically comprises two foundational layers in its initial stages: convolutional and pooling layers. Outputs from these layers undergo a non-linear transformation via, e.g., a rectified linear function, which offers advantages in terms of computational efficiency and suitability for unsupervised learning. The pooling layer serves to consolidate similar features and render the model invariant to small shifts and distortions. ConvNets have gained prominence for image segmentation, particularly for connectomics (Sengur et al., 2017), owing to their ability to excel in prediction tasks and improved computational efficiency due to advances in GPU computing. These networks can be individually trained for each neurodegenerative disease, enabling the subsequent application of the trained model for data-driven, individualized data-driven patient prediction.
Lifestyle modifications and therapeutic options: Once the individualized prediction model has been established and the various sub-stages of neurodegeneration have been delineated, targeted windows for therapeutic interventions can be identified. These windows can be fine-tuned on daily life activities, which serve as modifiable factors influencing the efficacy of therapeutic responses. Such lifestyle adjustments may encompass increasing daily physical activity and managing hypertension, optimizing vitamin levels, and mitigating smoking. Additionally, the model enables the identification of specific spatial-temporal patterns that characterize the interplay between cortical and subcortical networks. These identified patterns can be used in therapeutic trials. As a result, the predictive model is able to isolate precise time frames that are most conducive for targeted treatments.
In summary, we have presented a comprehensive methodology that integrates state space modeling with deep learning models to achieve precise disease prognosis in neurodegenerative conditions. This approach facilitates the identification of optimal time windows for implementing therapeutic interventions and lifestyle modifications. The recommendations from this proposed algorithm have great potential to reduce the effect of neurodegenerative diseases in patients.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Project-ID 424778381-TRR 295 and the Fondazione Grigioni per il Morbo di Parkinson (to MM).
Footnotes
C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
References
- Amooei E, Sharifi A, Manthouri M. Early diagnosis of neurodegenerative diseases using CNN-LSTM and wavelet transform. J Healthc Inform Res. 2023;7:104–124. doi: 10.1007/s41666-023-00130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Hampel H. The role of genetics for biomarker development in neurodegeneration. Prog Neurobiol. 2011;95:501–504. doi: 10.1016/j.pneurobio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Ding H, Seusing N, Nasseroleslami B, Anwar AR, Strauss S, Lotze M, Grothe M, Groppa S, Muthuraman M. The role of ipsilateral motor network in upper limb movement. Front Physiol. 2023;14:1199338. doi: 10.3389/fphys.2023.1199338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Er MB, Isik E, Isik I. Parkinson's detection based on combined CNN and LSTM using enhanced speech signals with Variational mode decomposition. Biomed Signal Process Control. 2021;70:103006. [Google Scholar]
- Koirala N, Fleischer V, Glaser M, Zeuner KE, Deuschl G, Volkmann J, Muthuraman M, Groppa S. Frontal lobe connectivity and network community characteristics are associated with the outcome of subthalamic nucleus deep brain stimulation in patients with Parkinson's disease. Brain Topogr. 2017;6:017–0597. doi: 10.1007/s10548-017-0597-4. [DOI] [PubMed] [Google Scholar]
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- Mallela J, Illa A, Suhas B, Udupa S, Belur Y, Atchayaram N, Yadav R, Reddy P, Gope D, Ghosh PK. Voice based classification of patients with amyotrophic lateral sclerosis, Parkinson's disease and healthy controls with CNN-LSTM using transfer learning. ICASSP 2020-2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Barcelona, Spain. 2020:6784–6788. doi: 10.1109/ICASSP40776.2020.9053682. [Google Scholar]
- Mantle D, Hargreaves IP. Mitochondrial dysfunction and neurodegenerative disorders: role of nutritional supplementation. Int J Mol Sci. 2022;23:12603. doi: 10.3390/ijms232012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello G, Salomone S, D'Agata V, Conforti FL, Cavallaro S. From multi-omics approaches to precision medicine in amyotrophic lateral sclerosis. Front Neurosci. 2020;14:577755. doi: 10.3389/fnins.2020.577755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraman M, Raethjen J, Koirala N, Anwar AR, Mideksa KG, Elble R, Groppa S, Deuschl G. Cerebello-cortical network fingerprints differ among essential, Parkinson and mimicked tremors. Brain. 2018;141:1770–1781. doi: 10.1093/brain/awy098. [DOI] [PubMed] [Google Scholar]
- Sengur A, Akbulut Y, Guo Y, Bajaj V. Classification of amyotrophic lateral sclerosis disease based on convolutional neural network and reinforcement sample learning algorithm. Health Inf Sci Syst. 2017;5:9. doi: 10.1007/s13755-017-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rheenen W, van der Spek RAA, Bakker MK, van Vugt JJFA, Hop PJ, Zwamborn RAJ, de Klein N, Westra HJ, Bakker OB, Deelen P, Shireby G, Hannon E, Moisse M, Baird D, Restuadi R, Dolzhenko E, Dekker AM, Gawor K, Westeneng HJ, Tazelaar GHP, et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat Genet. 2021;53:1636–1648. doi: 10.1038/s41588-021-00973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


