
Keywords: behavioral evaluations, gene knockout, human neuroblastoma cells (SH-SY5Y), human placental chorionic derived mesenchymal stem cells, interleukin-3, neonatal hypoxic-ischemic encephalopathy, nerve injury, oxygen-glucose deprivation, protein chip, small interfering RNA
Abstract
Neonatal hypoxic-ischemic encephalopathy is often associated with permanent cerebral palsy, neurosensory impairments, and cognitive deficits, and there is no effective treatment for complications related to hypoxic-ischemic encephalopathy. The therapeutic potential of human placental chorionic plate-derived mesenchymal stem cells for various diseases has been explored. However, the potential use of human placental chorionic plate-derived mesenchymal stem cells for the treatment of neonatal hypoxic-ischemic encephalopathy has not yet been investigated. In this study, we injected human placental chorionic plate-derived mesenchymal stem cells into the lateral ventricle of a neonatal hypoxic-ischemic encephalopathy rat model and observed significant improvements in both cognitive and motor function. Protein chip analysis showed that interleukin-3 expression was significantly elevated in neonatal hypoxic-ischemic encephalopathy model rats. Following transplantation of human placental chorionic plate-derived mesenchymal stem cells, interleukin-3 expression was downregulated. To further investigate the role of interleukin-3 in neonatal hypoxic-ischemic encephalopathy, we established an in vitro SH-SY5Y cell model of hypoxic-ischemic injury through oxygen-glucose deprivation and silenced interleukin-3 expression using small interfering RNA. We found that the activity and proliferation of SH-SY5Y cells subjected to oxygen-glucose deprivation were further suppressed by interleukin-3 knockdown. Furthermore, interleukin-3 knockout exacerbated neuronal damage and cognitive and motor function impairment in rat models of hypoxic-ischemic encephalopathy. The findings suggest that transplantation of hpcMSCs ameliorated behavioral impairments in a rat model of hypoxic-ischemic encephalopathy, and this effect was mediated by interleukin-3-dependent neurological function.
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a leading cause of neonatal mortality and severe neurological disability (Dan et al., 2022; Jiao et al., 2022; Xin et al., 2022; Zhang et al., 2023a). Neonates with birth asphyxia often experience difficulties in initiating and maintaining respirations, leading to lifelong cerebral palsy, neurosensory deficits, and cognitive impairments (Guan et al., 2023; Doycheva et al., 2013; Shetty, 2015; Huang et al., 2022). Numerous studies have investigated HI-related pathogenesis and have reported that excitatory amino acid release (Narayanamurthy et al., 2021), cellular proteolysis, reactive oxygen species generation, nitric oxide synthesis, and inflammation (Chen et al., 2022a) are involved in the mechanisms underlying HI injury.
HI injury induces changes at the molecular and cellular levels, including protein misfolding, aggregation, and destruction of organelles, and activates a series of apoptotic pathways, including the mitochondrial pathway, the extrinsic Fas receptor pathway, and the endoplasmic reticulum stress-induced pathway (Hua et al., 2017; Yu et al., 2022). A variety of therapies have been developed, such as xenon gas treatment (Sun et al., 2023), melatonin (Weiss et al., 2022), erythropoietin, and hypothermia (Wu et al., 2022), which have exhibited certain clinical efficacy against neurological deficits induced by HI injury. However, many surviving infants develop long-term complications such as cerebral palsy, which may render them unable to care for themselves even in adulthood (Dallera et al., 2022; Spiess et al., 2022). Therefore, exploring more effective therapeutic strategies is of vital urgency.
Stem cell-based therapies have emerged as a promising approach, with their ability to promote an anti-inflammatory environment and reduce the inflammatory response after injury, and have been successfully used for many years to treat various diseases (Gournay et al., 2022; Lozano Navarro et al., 2022; Chen et al., 2023; Mani et al., 2023; Menasché, 2023; Zhang et al., 2023b) with little controversy. A previous study reported that umbilical cord-derived mesenchymal stem cells (MSCs) and bone marrow-derived MSCs have the same biology (Han et al., 2012). Clinical studies have indicated that intravenous injection of umbilical cord stem cells and autologous cord blood cells reverse neurological dysfunction, alleviate cognitive disorders, and enhance emotional reaction and extrapyramidal function in neonates with HIE (Cotten et al., 2014; Xie et al., 2016).
The placenta, which is discarded after delivery, does not require an invasive acquisition process, and is more proliferative than traditional bone marrow MSCs. It has the advantages of a sufficient source, low immunogenicity, low viral contamination rate, and no social and ethical controversy. Fukuchi et al. (2004) obtained MSCs from mature placental leaflets, which can express several stem cell signature genes and tissue-specific genes. Bailo et al. (2004) isolated MSC-like cells from the amnion and chorion of mature placenta, and successfully transplanted the cells into neonatal rats and pigs after external expansion without any adverse reactions. Therefore, placenta-derived MSCs hold promise for clinical applications. Little is known about the role of human placental chorionic plate-derived MSCs (hpcMSCs) in HIE-induced long-term complications. In light of the lifelong and irreversible brain damage induced by HIE, there is an urgent need to investigate the efficacy of hpcMSCs in HIE-induced long-term dysfunction and explore the preliminary mechanism. Thus, in the present study we administered hpcMSCs to neonatal HIE model rats to investigate its effects. The therapeutic effect in rats will provide a theoretical basis for clinical application in the future.
Interleukin-3 (IL-3), an important member of the interleukin family, is also known as a multi-colony stimulating factor (Feng et al., 2022). It was reported that a cytokine mixture containing granulocyte-macrophage colony-stimulating factor and IL-3 improved traumatic brain injury and Parkinson's disease (Matsumoto et al., 2022). Previously, it was reported that IL-3 promoted neuronal survival in fimbria-fornix-transected rats (Kamegai et al., 1990) and prevented significant hippocampal CA1 neuron death and ischemia-induced learning disability (Wen et al., 1998). In addition, IL-3 was shown to trigger the induction of hematopoietic stem cells and various cell types originating from bone marrow (Edgar et al., 2022) and may be a neurite-promoting factor for cultured neurons of the superior cervical ganglion (Kannan et al., 1996). However, the role of IL-3 in HI and hpcMSC therapy after HIE is not yet known.
On the basis of the protective effects of MSCs and IL-3 in brain injury, the aim of this study was to explore the therapeutic effects of hpcMSCs and IL-3 on the long-term behavioral changes after hypoxic-ischemic injury in neonatal rats. In addition, we established an in vitro hypoxic-ischemic injury model by treating human neuroblastoma cells (SH-SY5Y) with oxygen-glucose deprivation (OGD) and silencing the expression of IL-3 using small interfering RNA (siRNA), and constructed IL-3 knockout (KO) rats to investigate the role of IL-3 in hypoxic-ischemic injury.
Methods
Animals
Ten pregnant female Sprague-Dawley rats at 20 days pregnant were provided by Department of Animal Zoology of Kunming Medical University (license No. SYXK (Dian) K2015-0002). IL-3 KO rats were constructed by clustered regularly interspaced short palindromic repeat-associated nuclease 9 (CRISPER/CAS9) technology Cyagen Biosciences (Guangzhou, China). After birth, the 7-day-old pups (weighing 12–15 g) were randomly assigned into five groups for in vivo experiments to investigate the therapeutic effects of hpcMSCs and IL-3 after HI injury: Sham group (wild type [WT] rats subjected to exposure of right carotid artery, n = 20), HI group (WT rats subjected to HI injury, n = 25), normal saline (NS) group (WT rats with HI injury subjected to right ventricle injection of NS, n = 5), stem cells group (WT rats with HI injury subjected to right ventricle injection of hpcMSCs, n = 5) and IL-3 KO group (IL-3 KO rats subjected to HI injury, n = 5). These rats were maintained with a 12-hour light/dark cycle at 21–25°C and humidity of 45–50%, with free access to food and water throughout the study. All experimental procedures, including animal care and testing, were approved by the Animal Care and Welfare Committee of Kunming Medical University in 2020 (approval No. kmmu2020001). All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (8th ed., National Research Council, 2011).
HI model establishment
An HI model was established as described in our previously published study (Jiang et al., 2020). Briefly, 7-day-old newborn Sprague-Dawley rats (male and female) were anesthetized with 3% isoflurane inhalation (RWD Life Science Co., Ltd., Shenzhen, China). The right common carotid artery was exposed and blocked with an electrocoagulator (Spring Medical Beauty Equipment Co., Ltd., Wuhan, Hubei Province, China). After their body temperature (37°C) and state were stabilized, the pups were placed in a hypoxic chamber (8% O2, 92% N2) for 2 hours, with an airflow rate maintained at 1 L/min. The temperature (37°C), humidity (70%) and the body temperature of the pups in the hypoxic chamber were maintained constant throughout the experiment. At the end of the procedure, the pups were returned to their cage. Rats in the sham group underwent exposure of the right carotid artery without ischemic and hypoxic conditions. Neonatal HI rats had 5% mortality due to brain damage induced by HI.
Suspension preparation of hpcMSCs
HpcMSCs were provided and identified by the Yunnan Shunxi Stem Cell and Regenerative Medicine Research Center (Kunming, Yunnan Province, China; lot number: YNSX-KY20171201). After the cell culture flask was preheated in an incubator for 30 minutes, the cells were washed with 1 mL of 0.01 mM phosphate buffer solution (PBS) (Biosharp, Hefei, Anhui, China) once or twice, and then 1 mL 0.25% trypsin (Gibco, Grand Island, NY, USA) was added for digestion for 30 seconds to 3 minutes. The cells were observed under a microscope until they became spherical in shape and floated in the liquid.
Next, the trypsin suspension was aspirated and centrifuged for 8 minutes at 1118 × g. The supernatant was discarded, and 1 mL normal saline was added to resuspend the cells. Then, 80 μL high-glucose culture medium, 10 μL trypan blue (Biosharp), and 10 μL cell suspension were mixed in a 200-μL Eppendorf tube (Biosharp). The cell suspension was then extracted and cells were counted on the counter plate. After recentrifugation, the cell suspension was prepared into 2 × 105/5 μL, 30 μL of which was collected for the subsequent hpcMSC transplantation, and the remaining cells were used for passage.
Identification of hpcMSCs
HpcMSCs were resuspended in 0.25% trypsin (Gibco) and then seeded into a 96-well plate at a density of 4000 cells per well. After 48 hours of culturing, the cells were washed twice with 0.01 mM PBS and fixed with 4% paraformaldehyde (Biosharp) for 20 minutes. Subsequently, cells were washed three times with 0.01 mM PBS for 5 minutes each time, and then 100 μL of 5% goat serum (Solarbio, Beijing, China) with 0.3% Triton-X100 (Sigma, Shanghai, China) was added to each well at 37°C for 30 minutes. The primary antibodies CD45 (mouse, 1:100, Bioss, Beijing, China, Cat# bsm-33052M, RRID: AB_2939047) and CD90 (rabbit, 1:100, Bioss, Cat# bs-0778R, RRID: AB_10857837) or CD44 (rabbit, 1:100, Bioss, Cat# bs-2783R, RRID: AB_10854140) were incubated for 18 hours at 4°C. Afterwards, secondary antibodies Dylight 594 (goat anti-mouse IgG, 1:200, Abbkine, Wuhan, Hubei Province, China, Cat# A23410) and Dylight 488 (goat anti-rabbit IgG, 1:200, Abbkine, Cat# A23220, RRID: AB 2737289) were added for incubation at 37°C for 1 hour. The cells were sealed with 4′,6-diamidino-2-phenylindole (DAPI)-conjugated Antifade Mounting Medium (Beyotime, Shanghai, China) and observed at 400× under a fluorescence microscope (Leica, Wizzler, Hesse, Germany).
hpcMSCs injection
The neonatal HI rats received three injections of hpcMSCs 1, 3, and 10 days after HI in the same right lateral ventricle position. Rats were anesthetized with 3% isoflurane inhalation and subsequently placed on the brain stereotactic locator (RWD Life Science Co., Ltd). A 5 μL dose of hpcMSCs (2 × 105/5 μL) was slowly injected into the right lateral ventricle (4 mm depth at the following coordinates: 1.5 mm vertical to the front cymbal and 1.0 mm apart) at an injection rate of 1 μL/min (Xiong et al., 2020). The needle was maintained in the lateral ventricle for 2 minutes and then removed. The rats were then returned to their mother's cage for breeding. After hpcMSC transplantation, the immunosuppressant cyclosporin A (Novartis, Beijing, China) was intraperitoneally injected daily at a dose of 5 mg/kg.
Neurological evaluation
After the HI models were established, triphenyl tetrazolium chloride (TTC) staining was performed to verify the induced cerebral damage at 24 hours after HI. In addition, open field test, Morris water maze test, Y-maze test, rotarod test, and neurological severity score (NSS) (Xiong et al., 2020; Zhang et al., 2021), were performed to assess the cognition, motor ability and anxiety-like behavior of rats at 1 and 2 months after HI (Figure 1), which were conducted by scorers who were blind to the treatment of the rats.
Figure 1.

Timeline of hpcMSCs treatment study.
HI models were established in 7-day-old neonatal rats. Subsequently, hpcMSCs were transplanted into the HI rats at 1, 3 and 10 days after HI modeling through the lateral ventricle. The cognitive and locomotor function and anxiety-like behaviors were assessed at 1 and 2 months after hpcMSCs treatment by behavioral evaluations including open field test, Morris water maze test, Y-maze test, rotarod test and NSS. HI: Hypoxia-ischemia; hpcMSCs: human placental chorionic derived mesenchymal stem cells; NS: normal saline; NSS: neurological severity score; P: postnatal day.
TTC staining
The infarction volume was determined by TTC staining in accordance with the methods in our previous work (Xiong et al., 2020). At 24 hours after HI, rats were anesthetized with 3% isoflurane inhalation. Cerebral tissue was swiftly harvested, then sectioned into coronal sections at 2 mm thick using a freezing microtome (Seino Co., Ltd. Beijing, China). Subsequently, all slices were placed into an incubation chamber with 2% TTC solution (Sigma Co., St. Louis, MO, USA) at 37°C for 10 minutes. Afterwards, 4% paraformaldehyde (Biosharp) was used to fix the tissue. ImageJ Software V1.8 (National Institutes of Health, Bethesda, MD, USA) was used for tracking and analyzing the infarct ratio by indirect method: (contralateral hemisphere volume - non-ischemic ipsilateral hemisphere volume)/contralateral hemisphere volume. Brain swelling was determined by subtracting the total volume of the nonischemic hemisphere from that of the ischemic hemisphere.
Open field test
Briefly, rats were placed in a square field (100 cm × 100 cm × 40 cm) with appropriate light conditions and a video camera fixed over the field. Each rat was put in the center of the open field and allowed to explore freely for 10 minutes. Supermaze, a video tracking software system provided by XinRuan Information Technology Company (Shanghai, China), automatically recorded the number of grooming and rearing events and the time spent grooming and rearing, as well as the number of fecal particles over the 10-minute period to assess locomotor activity. The square field was cleaned with 70% ethanol after each experiment.
Morris water maze test
The Water Maze pool (RWD Life Science Co., Ltd) was manually divided into four equal parts with a platform placed in the middle of one of the quadrants. Ink was poured into the pool to conceal the small circular platform. Rats were placed at the center of the four quadrants. Each rat was trained for 90 seconds in the four quadrants per day, for a total of 5 days. During the training process, if the rats could not find the targeted platform in 90 seconds, they were guided by the laboratory staff. The latency to the target was recorded. After 5 days of training, the platform was removed on the 6th day and the rats were placed in the quadrant opposite to the original platform quadrant. The target crossings and the distance traveled in the target quadrant were recorded using the Smart 3.0 video tracking system (Panlab, Barcelona, Spain) to assess spatial memory.
Y-maze test
After the rats were fasted for 1 day, the average body weight was reduced to 85% of the original weight. The Y-maze device consisted of three arms: the initial, incorrect, and food arms (XinRuan Information Technology Company). During the adaptation period, the rats were allowed to explore the maze for 10 minutes (2–3 times for 1 day). During the training period, the door of the incorrect arm was closed, and food was placed in the food arm. Then, the rats were placed in the initial arm and allowed to find the food, with guidance if necessary. The training time was set to 10 minutes per day for 3 days. After training, the food and incorrect arms doors were opened. The rats were placed in the initial arm, and the time spent in each arm and the number of arm entries over 5 minutes were recorded. The accurate rate (food arm entries/total entries) and error rate (incorrect arm entries/total entries) were analyzed.
Rotarod test
Three days before the experiment, each group of rats underwent adaptive training. In brief, the rats were placed on a rotating rod (Jinan Yiyan Technology Co., LTD, Jinan, Shandong, China) to adapt to its movement and speed. The rats underwent training for 5–10 minutes per day for 3 days with a rotating speed set at 30–35 r/min. Eventually, the rotating speed was set to accelerate from 4 r/min to 40 r/min within 3 minutes, and the time the rats stayed on the rod was recorded. The test was conducted three times consecutively in a day and the longest time would be used for analysis.
NSS
The NSS was used to evaluate performance in different tasks, including monoparesis/hemiparesis, straight walking, beam balancing, and reflex activity, as previously described (Xiong et al., 2020). Higher scores indicate more severe injury. All neurobehavioral assessments were conducted by three laboratories technicians who were blind to the experimental groups.
Tissue harvest
The rats were anesthetized with 3% isoflurane and perfused with precooled 0.9% NS (Biosharp) until the liver turned white. Then, the right cortex and hippocampus from sham and HI rats were obtained at 24 hours for protein chip analysis, and the brains from HI rats were fixed with 4% paraformaldehyde (Biosharp) for immunofluorescence staining. In addition, after neurological evaluation, the brains were removed and the right cortex and hippocampus were carefully dissected from the rats in the sham, HI, NS, and hpcMSCs groups at 2 months for real-time polymerase chain reaction (RT-PCR) to detect IL-3 expression.
Protein chip
The samples of right cortex and right hippocampus were transported on dry ice to Kangchen Bio-Tech Company (Shanghai, China) for protein chip analysis, as described in our previous study (Xiong et al., 2019).
RT-PCR
RT-PCR was performed as previously described (Xiong et al., 2020). Briefly, total RNA was extracted from the right cortex and hippocampus (30 mg) using Trizol reagent (Takara Bio Inc., Otsu, Japan) according to the manufacturer's protocol. Then, the total RNA was reverse-transcribed to cDNA using the Revert Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, MA, USA). Primer sequences were designed by Primer 5.0 software (Premier, San Francisco, CA, USA) for RT-PCR as follows: IL-3: forward, 5′-GGG ATA CCC ACC GTT TAA CCA-3′, reverse, 5′-AGG TTT ACT CTC CGA AAG CTC TT-3′; GAPDH: forward, 5′-TGA CTT CAA CAG CGA CAC CCA-3′, reverse, 5′-CAC CCT GTT GCT GTA GCC AAA-3′. Next, the reaction was proceeded in a DNA thermal cycler (Thermo Fisher Scientific) using the following protocol: one cycle of 95°C for 2 minutes, 40 cycles of 95°C for 15 seconds, annealing at 60°C for 40 seconds. Relative expression was calculated using the 2–ΔΔCt method with normalization to GAPDH.
Immunofluorescence staining
Frozen sections of the brain were used to determine the localization of IL-3 in the right and left cortex and hippocampus of HI rats at 24 hours by double immunofluorescence staining. The detailed procedures of immunofluorescence staining were described in our previously published study (Zhang et al., 2021). The sections were incubated with 5% goat serum (Solarbio) with 0.3% Triton X-100 (Sigma) for 2 hours at 25°C, then IL-3 (mouse, 1:200, GeneTex, SC, USA, Cat# GTX84295, RRID: AB_10728873) and neuron-specific nuclear protein (NeuN) (rabbit, 1:200, GeneTex, Cat# GTX132974, RRID: AB_2886793) or glial fibrillary acidic protein (GFAP) (rabbit, 1:500, GeneTex, Cat# GTX108711, RRID: AB_2037091) for 18 hours at 4°C. Then, secondary antibodies Dylight 594 (goat anti-mouse IgG, 1:200, Abbkine, Cat# A23410) and Dylight 488 (goat anti-rabbit IgG, 1:200, Abbkine, Cat# A23220, RRID: AB 2737289) were added for incubation at 25°C for 3 hours. The sections were sealed with DAPI-conjugated Antifade Mounting Medium (Beyotime) and observed at 200× under a fluorescence microscope.
Culture of SH-SY5Y cells
The method of cultivating SH-SY5Y cells has been described in detail in our previously published study (Zhang et al., 2021). The SH-SY5Y cell line was obtained from American Type Culture Collection (Rockville, MD, USA, Cat# CRL-2266, RRID: CVCL_0019). Briefly, frozen SH-SY5Y cells were taken from liquid nitrogen and quickly thawed into a 37°C water bath, then plated at a density of 5 × 105 cells/mL for cell culture at 37°C and 5% CO2. SH-SY5Y cells were passaged after seeding for 2 days, and then used for experimentation.
OGD
Briefly, SH-SY5Y cells were washed with 0.01 mmol/L PBS and then placed into the glucose-free medium (Gibco) at 37°C. Afterwards, the cells were subjected to hypoxia (Thermo Fisher Scientific) treatment (95% N2 and 5% CO2) at 37°C for 2 hours. Subsequently, the cells were placed into normal Dulbecco's Modified Eagle Medium with 95% O2 and 5% CO2, and incubated for 24 hours for reoxygenation.
IL-3 siRNA transfection
The siRNAs were designed to specifically inhibit IL-3 gene expression, along with one nonsense siRNA serving as a negative control (NC). They were provided by Ribobio Company (Guangzhou, Guangdong, China). Briefly, when the confluence of SH-SY5Y cells reached 80%, fresh medium including siRNA fragments 1 and 2 (F1 and F2) and CY3 using the riboFECTTM CP transfection reagents (Ribobio Company) was added to the SH-SY5Y cells. Then, the SH-SY5Y cells were randomly divided into normal group, Reagent group, NC group, F1 group, and F2 group. RT-PCR was used to detect the interference efficiency 3 days later, and the most efficient siRNA, F2, was selected for subsequent experiments. Images were obtained using a fluorescence microscope. Subsequently, SH-SY5Y cells were randomly divided into four groups (n = 5/group): normal, OGD for NC, reagent, and IL-3-si groups for the cell counting kit-8 (CCK-8) and methyl thiazolyl tetrazolium (MTT) assays to detect IL-3 after ODG in vitro. In addition, cell number was calculated by a low-power field (200 ×) under a fluorescence microscope.
CCK-8 detection
Cell viability was detected by CCK-8. Briefly, SH-SY5Y cells were seeded in 96-well plates with a density of 1 × 105 cells per well. Three days after transfection with IL-3 siRNA, the cells were treated with OGD for 24 hours, and then 10 μL of CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added to each well for 4 hours at 37°C. The optical density at 450 nm was measured using a Thermo Scientific Microplate Reader (Multlskan GO, Thermo Fisher Scientific) to detect cell viability.
MTT detection
The MTT assay was used to detect cell proliferation. SH-SY5Y cells were seeded in 96-well plates. Three days after transfection with IL-3 siRNA, the cells were subjected to OGD for 24 hours and then incubated in the serum-free medium containing 0.5 mg/mL MTT (Beyotime) for 4 hours at 37°C. Subsequently, 100 μL dimethyl sulfoxide (Biosharp) was added to each well. The optical density at 570 nm was measured using a Thermo Scientific Microplate Reader.
Genotype identification of IL-3 KO rats
For genotype identification, the tail tips were collected and marked with numbers for each rat (7–10 days). Then, DNA was extracted using the Transgen genomic DNA extraction kit (Thermo Fisher Scientific). PCR detection was conducted with the amplification primers: Rat IL3-F, 5′-AAC TCT TTG GAG GAC CAG AAC GA-3′; Rat IL3-R, 5′-AGA TGT GAA AAC GGA AGC AAG GCA -3′. Then, the final genotype (WT, IL-3 KO and heterozygote [HET]) detection was visualized using an agarose gel electrophoresis system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Sample sizes were estimated using MedSci Sample Size tools (version 6.2.7, Shanghai Meisi Medical Technology Co., Ltd, Shanghai, China) with a comparison of the mean of two independent samples: α = 0.05, power = 0.8. Statistical analysis was conducted using IBM SPSS Statistics for Windows (Version 19.0, IBM Corp., Armonk, NY, USA), and one-way analysis of variance with Tukey's post hoc test was performed for comparing multiple groups. Student's t-test was used for analyzing differences between two groups. GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was used for generating graphs. All data were presented as the primary data or mean ± standard deviation. Statistical differences were considered significant when P < 0.05.
Results
HI induces cerebral infarction
The right hemisphere of the HI rats exhibited obvious swelling and infarction relative to that of the sham rats (Figure 2A and B). Quantification confirmed the brain swelling and higher infarction ratio in the HI rats compared with the sham rats (P < 0.05; Figure 2C and D). These results confirmed the successful establishment of the HI model and demonstrated that HI induced cerebral infarction.
Figure 2.

The HI model is successfully established.
(A) Obvious swelling was observed the right hemisphere of HI rat brains compared with those of sham rats. The black box indicates the site of the swelling. Scale bar: 1 cm. (B) TTC staining of brain tissue showed the HI-induced infarct. Red arrows indicate the infarction. Scale bar: 1 cm. (C) Quantification of the brain swelling. (D) Quantification for the infarct ratio of brain tissue. Data are displayed as the mean ± SD (n = 5/group). *P < 0.05 (Student's t-test). HI: Hypoxia-ischemia; TTC: triphenyl tetrazolium chloride.
hpcMSCs transplantation attenuates HI-induced neurological dysfunction
Before transplanting hpcMSCs, we conducted immunofluorescence staining to identify the cells. The cells expressed CD90+CD44+CD45–, consistent with MSC characteristics (Figure 3A). Subsequently, the hpcMSCs were transplanted three times into the HI rats at 1, 3, and 10 days after HI. At 1 month after hpcMSCs transplantation, behavioral tests including open field test, Morris water maze, Y-maze test, rotarod test, and NSS were used to evaluate the neurological functions of rats. The open field test showed that the number of times grooming, the time spent grooming, and the number of fecal particles were significantly increased in the HI group compared with those in the sham group, and these measures were significantly decreased in the hpcMSCs group compared with the NS group (P < 0.05; Figure 3B). Additionally, the time spent rearing was reduced in HI rats compared with sham rats, and rearing time in hpcMSCs rats was greater than that in NS rats (P < 0.05; Figure 3B). The number of rearing times showed consistent results.
Figure 3.

Neurological evaluations of rats at 1 month after hpcMSCs transplantation.
(A) Immunofluorescence staining of hpcMSCs showed that the cells expressed CD90+/CD44+CD45−, consistent with mesenchymal stem cell characteristics. The green (Dylight 488) indicates CD90- or CD44-positive cells, red (Dylight 594) indicates CD45-positive cells, and blue represents DAPI (nuclei). Scale bars: 50 μm. (B) Quantification diagrams for number of grooming times, time spent grooming, number of feces, number of rearing times, and time spent rearing in the open field test. (C) Quantification diagrams for latency to target, number of target crossings, and distance traveled in target quadrant in the Morris water maze test. (D) The quantification diagrams for time spent in food arm, the number of food arm entries, accuracy, time spent in error arm, number of error arm entries, and the error rate in the Y-maze test. (E) The duration in the rotarod test. (F) The neurological severity scores. All data are presented as mean ± SD (n = 5/group). *P < 0.05 (one-way analysis of variance followed by Tukey's post hoc test). AL: Adjacent left quadrant; AR: adjacent right quadrant; DAPI: 4′,6-diamidino-2-phenylindole; HI: hypoxia-ischemia; hpcMSCs: human placental chorionic derived mesenchymal stem cells; NS: normal saline; O: opposite quadrant; T: target quadrant.
During the first 5 days of training in the Morris water maze, the latency to target in the HI group was significantly longer than that in the sham group, and a significant decrease was observed in the hpcMSCs treatment group relative to the NS group (P < 0.05; Figure 3C). After removing the target at 6 days, the distance traveled in the target quadrant and the number of target crossings of HI rats were both reduced compared with those in the sham group (P < 0.05). These measures were significantly increased in the hpcMSCs treatment group compared with the NS group (P < 0.05; Figure 3C).
Y-maze test results showed that the number of food arm entries, the time spent in the food arms, and the accuracy rate were significantly decreased in the HI group compared with the sham group, and these measures were increased in the hpcMSCs treatment group compared with those in the NS group (P < 0.05; Figure 3D). Similarly, the measures in the error arm were significantly decreased in the hpcMSCs treatment group (P < 0.05; Figure 3D). Additionally, the decreased rotarod test time in the HI group was reversed after hpcMSCs treatment (P < 0.05; Figure 3E). The NSS showed that the neurological dysfunctions caused by HI were alleviated with hpcMSCs treatment (P < 0.05; Figure 3F). The behavioral evaluations indicated that hpcMSCs transplantation significantly improved neurological function including cognitive, locomotor, and anxiety-like behaviors in HI rats at 1 month.
hpcMSCs transplantation alleviates HI-induced long-term neurological impairments in rats at 2 months
At 2 months after hpcMSC transplantation, we performed the behavioral tests again to further evaluate the long-term neurological function after hpcMSC treatment. Similar to the results at 1 month, the open field test showed that the number of times grooming, the time spent grooming, and the amount of feces were significantly increased in the HI group compared with the sham group, and were reduced after hpcMSC treatment (P < 0.05; Figure 4A). The time spent rearing and the number of times rearing were reduced in HI rats compared with sham rats, and hpcMSC treatment increased these measures (P < 0.05; Figure 4A). In Morris water maze, the latency in the sham and hpcMSC groups was significantly shorter than that in the HI and NS groups (P < 0.05; Figure 4B). After the target quadrant was removed, the distance traveled in the target quadrant and the number of target crossings of HI rats were both reduced compared with those in the sham group (P < 0.05), and these measures were significantly increased in the hpcMSCs treatment group compared with the NS group (P < 0.05; Figure 4B). The crossing number and distance traveled in the adjacent left or right quadrant, and the opposite quadrant did not show a statistically significant difference between groups (Figure 4B). The Y-maze test showed that the number of food arm entries, the time spent in the food arms, and the accuracy rate were significantly reduced in the HI group compared with the sham group, and were increased in the hpcMSCs treatment group compared with the NS group (P < 0.05; Figure 4C). These measures in the error arm were significantly decreased after hpcMSCs treatment (P < 0.05; Figure 4C). Moreover, the decreased rotarod test time in the HI group was reversed after hpcMSCs treatment (P < 0.05; Figure 4D). The NSS data showed that the neurological dysfunction caused by HI was alleviated with hpcMSCs treatment (P < 0.05; Figure 4E). These results further suggest that hpcMSC transplantation effectively promoted the recovery of long-term neurological dysfunction in HI rats at 2 months.
Figure 4.
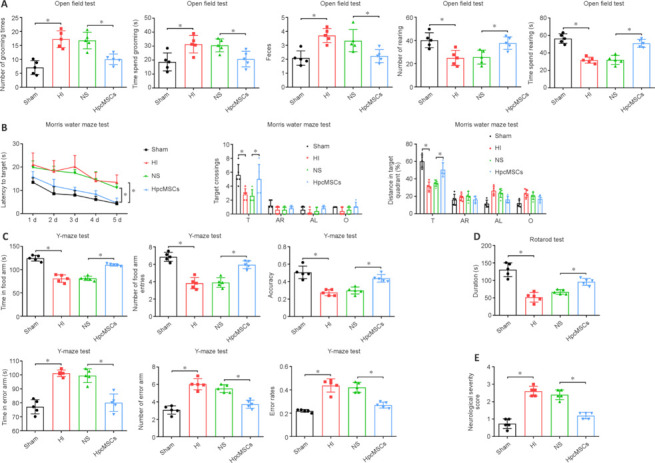
Neurological evaluations of rats at 2 months after hpcMSCs transplantation.
(A) Quantification diagrams for the number of times grooming, time spent grooming, number of feces, number of rearing, and the time spent rearing in the open field test. (B) Quantification diagrams for latency to target, number of target crossings, and distance traveled in target quadrant in the Morris water maze test. (C) Time spent in food arm, number of food arm entries, accuracy, time spent in error arm, number of error arm entries, and error rate in the Y-maze test. (D) The duration in the rotarod test. (E) The neurological severity scores. All data are presented as mean ± SD (n = 5/group). *P < 0.05 (one-way analysis of variance followed by Tukey's post hoc test). AL: Adjacent left quadrant; AR: adjacent right quadrant; HI: hypoxia-ischemia; hpcMSCs: human placental chorionic derived mesenchymal stem cells; NS: normal saline; O: opposite quadrant; T. target quadrant.
IL-3 expression in HI-induced brain is significantly reduced after hpcMSCs treatment
The proteomic data showed that growth hormone, IL-2, IL-3, and IL-4 were differentially expressed in the right cortex and hippocampus (Figure 5A and B), of which IL-3 showed the largest fold change (Figure 5C). This suggested that IL-3 may play an important role in HI. We next measured IL-3 mRNA levels in the injured cortex and hippocampus. In both the cortex and hippocampus, IL-3 expression was significantly increased after HI injury compared with that of the sham group (P < 0.05), and its levels were significantly reduced in the hpcMSC transplantation group (P < 0.05; Figure 5D). The results suggest that regulation of IL-3 may be involved in the therapeutic effect of hpcMSCs.
Figure 5.

Identification of differentially expressed proteins in the injured brain and expression of IL-3 after hpcMSC treatment.
(A) Protein chip profiles of differentially expressed proteins in the right cortex and hippocampus between sham and HI groups. Red represents high expression and green represents low expression. (B) The intersection of differentially expressed proteins from two tissues. (C) The fold change of three identified proteins with high fold change. (D) The mRNA expression of IL-3 in the cortex and hippocampus in sham, HI, NS and hpcMSCs transplantation groups. All data are presented as the primary data (C) or mean ± SD (D) (n = 5/group). *P < 0.05 (one-way analysis of variance followed by Tukey's post hoc test). HI: Hypoxia-ischemia; hpcMSC: human placental chorionic derived mesenchymal stem cell; IL-3: interleukin-3; NS: normal saline.
Downregulation of IL-3 inhibits the viability and growth of SH-SY5Y after OGD
Immunofluorescence staining showed that IL-3 was expressed in neurons (marked by NeuN) and astrocytes (marked by GFAP) of the ipsilateral cortex and hippocampus (Figure 6A and B). CY3-labeled SH-SY5Y cells indicated successful transfection with IL-3 siRNA (Figure 7A). RT-PCR showed that F2 significantly downregulated IL-3 expression and was the most efficient siRNA (P < 0.05; Figure 7B). Thus, F2 was selected for subsequent experiments. CCK-8 assay and MTT assay showed that cell viability and proliferation, respectively, were significantly reduced after IL-3 downregulation compared with those in the OGD + NC and OGD + reagent groups (P < 0.05; Figure 7C and D). Over time, the number of cells in the IL-3-si group was obviously less than that in the NC and reagent groups on days 1–6 (P < 0.05; Figure 7E).
Figure 6.
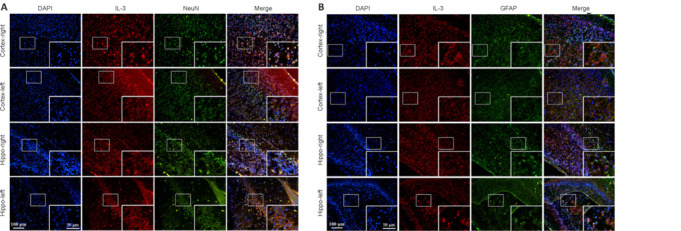
Double immunofluorescence staining of IL-3 and NeuN (A) or GFAP (B) in the right and left cortex and hippocampus.
(A) Double immunofluorescence staining of IL-3 and NeuN showed that the expression of IL-3 in neurons was obviously higher in the right cortex and hippocampus compared with that of the left cortex and hippocampus. The red (Dylight 594) indicates IL-3-positive cells, green (Dylight 488) indicates NeuN-positive cells (neurons), and blue represents DAPI (nuclei). Scale bars: 100 μm, 50 μm (enlarged image). (B) Double immunofluorescence staining of IL-3 and GFAP showed that the expression of IL-3 in astrocytes was obviously higher in the right cortex and hippocampus compared with that of the left cortex and hippocampus. Scale bars: 100 μm, 50 μm (enlarged image). The red (Dylight 594) indicates IL-3-positive cells, green (Dylight 488) indicates GFAP-positive cells (astrocytes), and blue represents DAPI (nuclei). DAPI: 4′,6-Diamidino-2-phenylindole; GFAP: glial fibrillary acidic protein; IL-3: interleukin-3; NeuN: neuron-specific nuclear protein.
Figure 7.
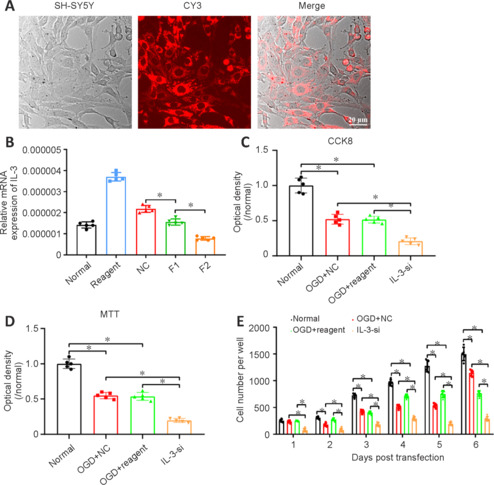
Downregulation of IL-3 impairs the viability and growth of SH-SY5Y cells after OGD.
(A) CY3 staining verified the successful transfection of IL-3 siRNAs in SH-SY5Y cells. Scale bar: 20 μm. (B) Relative mRNA expression of IL-3 in normal, reagent, NC, F1 and F2 groups. The lowest IL-3 expression was observed in the F2 group. (C–E) The cell viability detected by CCK-8 (C) and MTT (D) methods, and the number of cells (E) showed significantly reduced cell viability and growth after IL-3 downregulation compared with OGD + NC and OGD + reagent groups. All data are presented as the mean ± SD (n = 5/group). All procedures were conducted in triplicate and repeated at least three times. *P < 0.05 (one-way analysis of variance followed by Tukey's post hoc test). CCK-8: Cell counting kit-8; IL-3-si: interleukin-3 silencing; MTT: methyl thiazolyl tetrazolium; NC: negative control; OGD: oxygen-glucose deprivation.
Deletion of IL-3 exacerbates the behavioral dysfunction in HI rats
To further investigate the role of IL-3 in HI injury, we constructed IL-3 KO rats (Figure 8A and B). At 1 and 2 months after HI injury, the behavioral performance of rats was evaluated. In the open field test, HI IL-3 KO rats spent more time grooming and less time rearing than HI WT rats (P < 0.05; Figure 8C). In the Morris water maze test, the latency to target was significantly increased in HI rats compared with sham rats, and the latency was even longer in HI IL-3 KO rats compared with HI WT rats (P < 0.05; Figure 8D). The number of target crossings and the distance traveled in the target quadrant were markedly reduced after HI injury, and were reduced further in the HI IL-3 KO rats (P < 0.05; Figure 8D). In the Y-maze test, the number of food arm entries and the accuracy were progressively decreased in the sham, HI, and HI IL-3 KO rats (P < 0.05; Figure 8E). The number of error arm entries and the error rates showed the opposite trend in the three groups (P < 0.05; Figure 8E). Moreover, HI IL-3 KO rats had decreased rotarod test time compared with that of HI WT rats (P < 0.05; Figure 8F). The NSS showed that the neurological dysfunction caused by HI was exacerbated by IL-3 KO (P < 0.05; Figure 8G). At 2 months, the time spent grooming in IL-3 KO rats was longer than that of HI WT rats, and the rearing in these groups showed the opposite trend (Figure 8H). Similar results were also found in the water maze test, Y-maze test, rotarod test, and NSS (P < 0.05; Figure 8I–L). These findings indicated that the inhibition of IL-3 exacerbated the HI-induced long-term neurological dysfunction.
Figure 8.
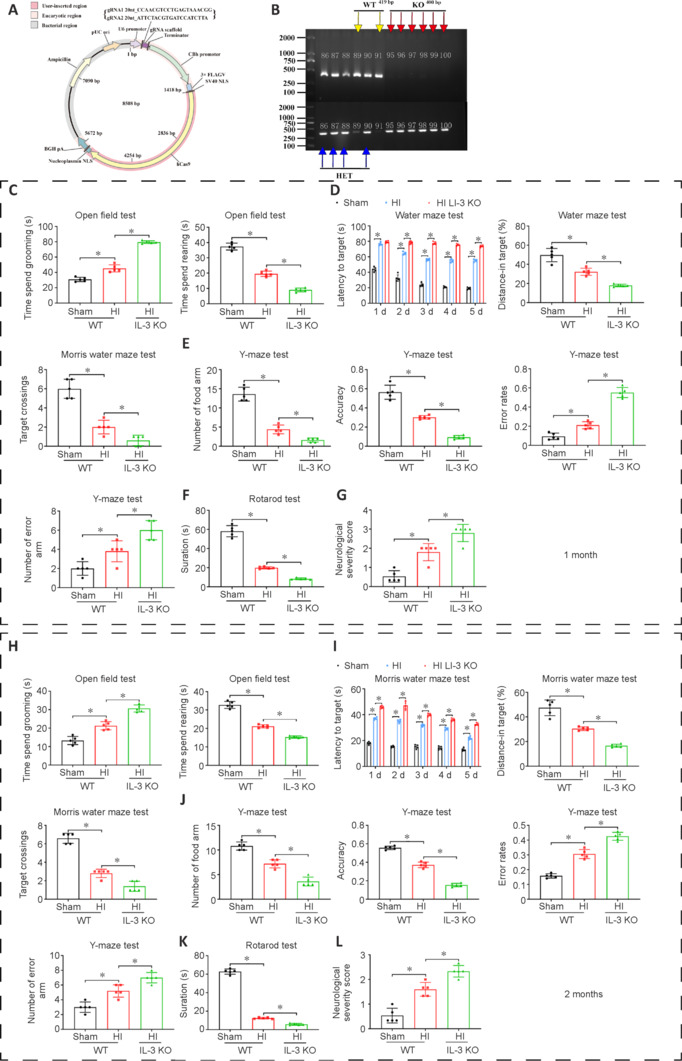
The effects of IL-3 KO on behavioral function of HI rats at 1 and 2 months after HI.
(A) The construction of IL-3 KO rats. (B) Genetic identification of IL-3 KO rats. Yellow arrows indicate WT, red arrows indicate KO, and blue arrows indicate HET. Numbers 86–91 and 95–100 represent the rat number. (C) The time spent grooming and rearing were measured during an open field test in the sham, HI and IL-3 KO groups at 1 month. (D) Quantifications of latency to target, number of target crossings, and distance traveled in target quadrant in the Morris water maze at 1 month. (E) Quantification diagrams for the number of food arm entries, accuracy, number of error arm entries, and error rate in Sham, HI and IL-3 KO groups during the Y-maze test at 1 month. (F) The duration on the rotarod in sham, HI and IL-3 KO groups at 1 month. (G) The neurological severity scores in these groups at 1 month. (H–L) Quantification of the same measures at 2 months after HI. All data are presented as mean ± SD (n = 5/group). *P < 0.05 (one-way analysis of variance followed by Tukey's post hoc test). HET: Heterozygote; HI: hypoxia-ischemia; IL-3: interleukin-3; KO: knockout; WT: wild type.
Discussion
In the present study, we established an HI model in 7-day-old neonatal rats. The hpcMSCs were transplanted three times into HI rats at 1, 3, and 10 days after HI. Behavioral testing at 1 and 2 months after hpcMSCs transplantation indicated that the HI rats receiving hpcMSCs transplantation had significant improvement in cognitive and locomotor function and anxiety-like behaviors compared with HI rats. Protein chip analysis showed high IL-3 expression in HI rats, and its expression was reduced after hpcMSC treatment. Furthermore, immunostaining showed that IL-3 was localized in neurons and astrocytes. To further verify the role of IL-3 in HI, an in vitro HI model was established by treating SH-SY5Y cells with OGD and inhibiting the expression of IL-3 using siRNA, and IL-3 knockout rats were established. The results showed that the inhibition of IL-3 exacerbated the OGD-induced neuronal damage and the long-term cognitive and motor impairment of HI rats.
HI-induced brain injury undergoes two stages: an acute phase and a long-term neurological dysfunction phase. Acute injury typically occurs soon after HI, involving secondary cell death, cytotoxic edema, progressive mitochondrial failure, delayed seizures, and cerebral palsy (Chen et al., 2022c; Fang et al., 2023). Cerebral palsy has been reported to occur even after hypothermia treatment, despite a decrease in the incidence of cerebral palsy in neonates with HIE (Pappas et al., 2015; Zhou et al., 2022; Robertsson Grossmann et al., 2023). In this study, 7-day-old Sprague-Dawley rats were subjected to an HI model of acute cerebral infarction and neurological impairment. Long-term behavioral experiments showed that HI rats exhibited significant cognitive, locomotor, and anxiety-like behavioral impairments compared with sham rats at 1 and 2 months after HI. These findings demonstrated that HI can lead to both acute and long-term neurological impairments. Although therapeutic hypothermia has been extensively studied and applied clinically, the incidence of death and long-term brain damage still cannot be avoided. Stem cells have provided a renewable source of replacement cells and tissues, offering the potential to combat diseases, and appear to be a promising strategy for nerve damage. Stem cell transplantation has been applied to treat chronic limb ischemia (Subramanian et al., 2011), late-stage heart failure (Madhusankar et al., 2007), leukemia, spinal cord injury (Chen et al., 2022d), and coronary heart disease (Chen et al., 2022b). Studies have already reported the therapeutic potential of stem cells in HI (Cotten et al., 2014; Xie et al., 2016), but the stability of these cells remains controversial.
Placental stem cells exhibit strong self-renewal potential, increased differentiation ability, immunosuppressive properties, high expansion rate, and minimal ethical or legal concerns, making them highly promising for application in regenerative medicine and prenatal gene therapy (Antoniadou and David, 2016). A previous study showed that hpcMSCs are a source of stem cells with the ability to differentiate towards osteogenic and neurogenic lineages (Fuchi et al., 2017). In our study, we cultured hpcMSCs in vitro and immunofluorescent staining indicated that the cells expressed CD44+CD90+CD45–, consistent with MSC characteristics. Our findings suggest that the cognitive, locomotor, and anxiety-like behaviors induced by HI were significantly ameliorated in rats receiving hpcMSCs treatment at 1 and 2 months after HI injury. This study is the first to demonstrate the therapeutic potential of hpcMSCs in the long-term behavioral impairments caused by HI.
To investigate the underlying mechanisms of hpcMSCs in HIE, protein chip analysis was performed on the right cortex and hippocampus from the sham and HI groups. The results showed that the relative expression of IL-3 in HI was significantly higher than that in the sham group, suggesting that IL-3 may play an important role in HI. Previously, it was reported that IL-3 mRNA was detected in primary cultured hippocampal neurons (but not in septal neurons) and in mixed glia cells by reverse transcription PCR (Konishi et al., 1994). However, in a transgenic mouse overexpressing IL-3, microglia cells were activated and white matter lesions were induced (Feng et al., 2022). Therefore, high expression or lack of IL-3 expression in the brain may lead to functional abnormalities in cells (Edgar et al., 2022). In this study, we detected upregulation of IL-3 in the cortex and hippocampus of rats subjected to HI injury. Furthermore, IL-3-positive cells, which were colocalized with neurons and astrocytes, were present in the hippocampus and cerebral cortex, although their numbers were relatively few. This evidence supports the existence of a significant functional link between the nervous and immune systems (Williams et al., 1981).
A previous study proposed that lymphoid tissues promote sympathetic neurite outgrowth, and this effect may be partially dependent on the presence of IL-3 (Kannan-Hayashi et al., 2008). Our findings showed that inhibition of IL-3 exacerbated neuronal damage induced by OGD. Consistently, our results showed that the knockout of IL-3 had a negative impact on the behavioral function in HI rats, suggesting that neurological dysfunction might be mediated by certain cytokines in the immune response. In this study, we found that IL-3 was increased in HI rats and slightly decreased after hpcMSCs treatment. However, the knockout of IL-3 exacerbated behavioral dysfunction in HI rats. This inconsistency might be due to the fact that IL-3 was increased after HI, and hpcMSCs treatment maintained IL-3 expression at normal levels and was therefore able to protect the neurological function in HI rats, whereas HI rats with IL-3 KO had very low levels of IL-3 and showed severe neurobehavioral impairment.
There are some limitations to consider. The present findings only preliminarily suggest that hpcMSCs significantly improve long-term neurological deficits induced by HI, and the survival, migration, and differentiation of hpcMSCs in the brain, as well as the formation of neural circuits, require further research and analysis. Moreover, in vitro and in vivo functional experiments of hpcMSCs regulated by IL-3 also require further exploration.
In summary, considering the therapeutic effect of hpcMSC transplantation in HI-induced long-term neurological impairments, hpcMSC-based therapy may have the potential to be used in clinical practice to prevent or treat HI in neonates at term or preterm. HpcMSC transplantation may alleviate HI-induced neurobehavioral impairments through the mediation of IL-3. The preclinical effect observed in rats provides a theoretical basis for the use of hpcMSCs in clinical patients in the future.
Additional file: Open peer review report 1 (85.9KB, pdf) .
Acknowledgments:
We show our great gratitude to Professor Qingjie Xia (West China Hospital of Sichuan University) for his technical guidance.
Funding Statement
Funding: This work was supported by the National Natural Science Foundation of China, No. 82001604; Guizhou Provincial Higher Education Science and Technology Innovation Team, No. [2023]072; Guizhou Province Distinguished Young Scientific and Technological Talent Program, No. YQK[2023]040; Guizhou Provincial Basic Research Program (Natural Science), No. ZK[2021]-368 (all to LXiong), and Zunyi City Innovative Talent Team Training Plan, No. [2022]-2.
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Data availability statement: The data are available from the corresponding author on reasonable request.
Open peer reviewer: Paula Izquierdo-Altarejos, Príncipe Felipe Research Center Foundation, Spain.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editoirs: McCollum L, Song LP; T-Editor: Jia Y
References
- Antoniadou E, David AL. Placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:13–29. doi: 10.1016/j.bpobgyn.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, Albertini A, Zorzi F, Cavagnini A, Candotti F, Wengler GS, Parolini O. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- Chen HR, Chen CW, Kuo YM, Chen B, Kuan IS, Huang H, Lee J, Anthony N, Kuan CY, Sun YY. Monocytes promote acute neuroinflammation and become pathological microglia in neonatal hypoxic-ischemic brain injury. Theranostics. 2022a;12:512–529. doi: 10.7150/thno.64033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Ning X, Li W, Pan Y, Wang L, Li H, Fan X, Zhang J, Luo T, Wu Y, Ou C, Chen M. Fabrication of Tβ4-Exosome-releasing artificial stem cells for myocardial infarction therapy by improving coronary collateralization. Bioact Mater. 2022b;14:416–429. doi: 10.1016/j.bioactmat.2022.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Xia Y, Zhang L, Xu T, Yi Y, Chen J, Liu Z, Yang L, Chen S, Zhou X, Chen X, Wu H, Liu J. Loading neural stem cells on hydrogel scaffold improves cell retention rate and promotes functional recovery in traumatic brain injury. Mater Today Bio. 2023;19:100606. doi: 10.1016/j.mtbio.2023.100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang J, Wu Y, Tucker R, Baird GL, Domonoske R, Barrios-Anderson A, Lim YP, Bath K, Walsh EG, Stonestreet BS. Inter-alpha inhibitor proteins ameliorate brain injury and improve behavioral outcomes in a sex-dependent manner after exposure to neonatal hypoxia ischemia in newborn and young adult rats. Neurotherapeutics. 2022c;19:528–549. doi: 10.1007/s13311-022-01217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang H, Fan C, Zhuang Y, Yang W, Chen Y, Shen H, Xiao Z, Zhao Y, Li X, Dai J. Adhesive, stretchable, and spatiotemporal delivery fibrous hydrogels harness endogenous neural stem/progenitor cells for spinal cord injury repair. ACS Nano. 2022d;16:1986–1998. doi: 10.1021/acsnano.1c06892. [DOI] [PubMed] [Google Scholar]
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164:973–979.e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallera G, Skopec M, Battersby C, Barlow J, Harris M. Review of a frugal cooling mattress to induce therapeutic hypothermia for treatment of hypoxic-ischaemic encephalopathy in the UK NHS. Global Health. 2022;18:43. doi: 10.1186/s12992-022-00833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan QQ, Ma Z, Tan YX, Visar B, Chen L. AQP4 knockout promotes neurite outgrowth via upregulating GAP43 expression in infant rats with hypoxic-ischemic brain injury. ibrain. 2022;8:324–337. doi: 10.1002/ibra.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doycheva D, Shih G, Chen H, Applegate R, Zhang JH, Tang J. Granulocyte-colony stimulating factor in combination with stem cell factor confers greater neuroprotection after hypoxic-ischemic brain damage in the neonatal rats than a solitary treatment. Transl Stroke Res. 2013;4:171–178. doi: 10.1007/s12975-012-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, Michaels YS, Zandstra PW. Multi-objective optimization reveals time- and dose-dependent inflammatory cytokine-mediated regulation of human stem cell derived T-cell development. NPJ Regen Med. 2022;7:11. doi: 10.1038/s41536-022-00210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Liu J, Zhang Z, Li Y, Zhu J, Lin Z. Chloroquine protects hypoxia/ischemia-induced neonatal brain injury in rats by mitigating blood-brain barrier disruption. ACS Chem Neurosci. 2023;14:1764–1773. doi: 10.1021/acschemneuro.2c00650. [DOI] [PubMed] [Google Scholar]
- Feng L, Li C, Zeng LW, Gao D, Sun YH, Zhong L, Lin H, Shu HB, Li S. MARCH3 negatively regulates IL-3-triggered inflammatory response by mediating K48-linked polyubiquitination and degradation of IL-3Rα. Signal Transduct Target Ther. 2022;7:21. doi: 10.1038/s41392-021-00834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchi N, Miura K, Doi H, Li TS, Masuzaki H. Feasibility of placenta-derived mesenchymal stem cells as a tool for studying pregnancy-related disorders. Sci Rep. 2017;7:46220. doi: 10.1038/srep46220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- Gournay V, Vallet N, Peux V, Vera K, Bordenave J, Lambert M, Corneau A, Michonneau D, Peffault de Latour R, Caillat-Zucman S, Socié G, Chevalier MF. Immune landscape after allo-HSCT: TIGIT- and CD161-expressing CD4 T cells are associated with subsequent leukemia relapse. Blood. 2022;140:1305–1321. doi: 10.1182/blood.2022015522. [DOI] [PubMed] [Google Scholar]
- Guan YH, Zhou HS, Luo BY, Hussain S, Xiong LL. Research progress of neonatal hypoxic-ischemic encephalopathy in nonhuman primate models. Ibrain. 2023;9:183–194. doi: 10.1002/ibra.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZB, Yang ZX, Chi Y, Wang YW, Wang T, Ji YR, Yang P, Meng L, Han ZC. Comparative study of biological characteristics of human umbilical cord and placental chorionic villous mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:692–696. [PubMed] [Google Scholar]
- Hua C, Ju WN, Jin H, Sun X, Zhao G. Molecular chaperones and hypoxic-ischemic encephalopathy. Neural Regen Res. 2017;12:153–160. doi: 10.4103/1673-5374.199008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, U KP, Yang F, Ji Z, Lin J, Weng Z, Tsang LL, Merson TD, Ruan YC, Wan C, Li G, Jiang X. Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic-ischaemic brain damage. Theranostics. 2022;12:143–166. doi: 10.7150/thno.57234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Bai X, Li TT, Al-Hawwas M, Jin Y, Zou Y, Hu Y, Liu LY, Zhang Y, Liu Q, Yang H, Ma J, Wang TH, Liu J, Xiong LL. COX5A over-expression protects cortical neurons from hypoxic ischemic injury in neonatal rats associated with TPI up-regulation. BMC Neurosci. 2020;21:18. doi: 10.1186/s12868-020-00565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Sun YT, Chen NF, Zhou LN, Guan X, Wang JY, Wei WJ, Han C, Jiang XL, Wang YC, Zou W, Liu J. Human umbilical cord-derived mesenchymal stem cells promote repair of neonatal brain injury caused by hypoxia/ischemia in rats. Neural Regen Res. 2022;17:2518–2525. doi: 10.4103/1673-5374.339002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamegai M, Niijima K, Kunishita T, Nishizawa M, Ogawa M, Araki M, Ueki A, Konishi Y, Tabira T. Interleukin 3 as a trophic factor for central cholinergic neurons in vitro and in vivo. Neuron. 1990;4:429–436. doi: 10.1016/0896-6273(90)90055-k. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Bienenstock J, Ohta M, Stanisz AM, Stead RH. Nerve growth factor and cytokines mediate lymphoid tissue-induced neurite outgrowth from mouse superior cervical ganglia in vitro. J Immunol. 1996;157:313–320. [PubMed] [Google Scholar]
- Kannan-Hayashi Y, Okamura K, Hattori S, Kuwamura M, Higuchi E, Terayama H, Moriyama M, Mukamoto M, Okada M, Ohsugi Y, Nakamura Y. Neuritogenic effects of T cell-derived IL-3 on mouse splenic sympathetic neurons in vivo. J Immunol. 2008;180:4227–4234. doi: 10.4049/jimmunol.180.6.4227. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Kamegai M, Takahashi K, Kunishita T, Tabira T. Production of interleukin-3 by murine central nervous system neurons. Neurosci Lett. 1994;182:271–274. doi: 10.1016/0304-3940(94)90814-1. [DOI] [PubMed] [Google Scholar]
- Lozano Navarro LV, Chen X, Giratá Viviescas LT, Ardila-Roa AK, Luna-Gonzalez ML, Sossa CL, Arango-Rodríguez ML. Mesenchymal stem cells for critical limb ischemia: their function, mechanism, and therapeutic potential. Stem Cell Res Ther. 2022;13:345. doi: 10.1186/s13287-022-03043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusankar N, Vaidyanathan K, Rajesh V, Prasad G, Kirtivasan V, Naveen A, Abraham S, Cherian K. Use of bone marrow derived stem cells in patients with cardiovascular disorders. J Stem Cells Regen Med. 2007;3:28–29. [PubMed] [Google Scholar]
- Mani KK, El-Hakim Y, Branyan TE, Samiya N, Pandey S, Grimaldo MT, Habbal A, Wertz A, Sohrabji F. Intestinal epithelial stem cell transplants as a novel therapy for cerebrovascular stroke. Brain Behav Immun. 2023;107:345–360. doi: 10.1016/j.bbi.2022.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Choudhury ME, Takeda H, Sato A, Kihara N, Mikami K, Inoue A, Yano H, Watanabe H, Kumon Y, Kunieda T, Tanaka J. Microglial re-modeling contributes to recovery from ischemic injury of rat brain: a study using a cytokine mixture containing granulocyte-macrophage colony-stimulating factor and interleukin-3. Front Neurosci. 2022;16:941363. doi: 10.3389/fnins.2022.941363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasché P. Mesenchymal stromal cell therapy for heart failure: never stop DREAMing. J Am Coll Cardiol. 2023;81:864–866. doi: 10.1016/j.jacc.2022.12.019. [DOI] [PubMed] [Google Scholar]
- Narayanamurthy R, Yang JJ, Yager JY, Unsworth LD. Drug delivery platforms for neonatal brain injury. J Control Release. 2021;330:765–787. doi: 10.1016/j.jconrel.2020.12.056. [DOI] [PubMed] [Google Scholar]
- National Research Council . Washington, DC, USA: National Academies Press; 2011. Guide for the Care and Use of Laboratory Animals, 8th edition. [Google Scholar]
- Pappas A, Shankaran S, McDonald SA, Vohr BR, Hintz SR, Ehrenkranz RA, Tyson JE, Yolton K, Das A, Bara R, Hammond J, Higgins RD. Cognitive outcomes after neonatal encephalopathy. Pediatrics. 2015;135:e624–634. doi: 10.1542/peds.2014-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsson Grossmann K, Eriksson Westblad M, Blennow M, Lindström K. Outcome at early school age and adolescence after hypothermia-treated hypoxic-ischaemic encephalopathy: an observational, population-based study. Arch Dis Child Fetal Neonatal Ed. 2023;108:295–301. doi: 10.1136/archdischild-2022-324418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty J. Neonatal seizures in hypoxic-ischaemic encephalopathy--risks and benefits of anticonvulsant therapy. Dev Med Child Neurol 57 Suppl. 2015;3:40–43. doi: 10.1111/dmcn.12724. [DOI] [PubMed] [Google Scholar]
- Spiess DA, Campos RMP, Conde L, Didwischus N, Boltze J, Mendez-Otero R, Pimentel-Coelho PM. Subacute AMD3100 treatment is not efficient in neonatal hypoxic-ischemic rats. Stroke. 2022;53:586–594. doi: 10.1161/STROKEAHA.120.033768. [DOI] [PubMed] [Google Scholar]
- Subrammaniyan R, Amalorpavanathan J, Shankar R, Rajkumar M, Baskar S, Manjunath SR, Senthilkumar R, Murugan P, Srinivasan VR, Abraham S. Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy. 2011;13:993–999. doi: 10.3109/14653249.2011.579961. [DOI] [PubMed] [Google Scholar]
- Sun M, An Z, Wei H, Li T, Qin M, Liu Y, Jiang H. Xenon attenuates hypoxic-ischemic brain damage by inhibiting autophagy in neonatal rats. Neuroreport. 2023;34:273–279. doi: 10.1097/WNR.0000000000001888. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Carloni S, Vanzolini T, Coppari S, Balduini W, Buonocore G, Longini M, Perrone S, Sura L, Mohammadi A, Rocchi MBL, Negrini M, Melandri D, Albertini MC. Human-rat integrated microRNAs profiling identified a new neonatal cerebral hypoxic-ischemic pathway melatonin-sensitive. J Pineal Res. 2022;73:e12818. doi: 10.1111/jpi.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TC, Tanaka J, Peng H, Desaki J, Matsuda S, Maeda N, Fujita H, Sato K, Sakanaka M. Interleukin 3 prevents delayed neuronal death in the hippocampal CA1 field. J Exp Med. 1998;188:635–649. doi: 10.1084/jem.188.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Peterson RG, Shea PA, Schmedtje JF, Bauer DC, Felten DL. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull. 1981;6:83–94. doi: 10.1016/s0361-9230(81)80072-x. [DOI] [PubMed] [Google Scholar]
- Wu YW, Comstock BA, Gonzalez FF, Mayock DE, Goodman AM, Maitre NL, Chang T, Van Meurs KP, Lampland AL, Bendel-Stenzel E, Mathur AM, Wu TW, Riley D, Mietzsch U, Chalak L, Flibotte J, Weitkamp JH, Ahmad KA, Yanowitz TD, Baserga M, et al. Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N Engl J Med. 2022;387:148–159. doi: 10.1056/NEJMoa2119660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Gu P, Wang W, Dong C, Zhang L, Zhang J, Liu H, Qiu F, Han R, Zhang Z, Yan B. Therapeutic effects of human umbilical cord mesenchymal stem cells transplantation on hypoxic ischemic encephalopathy. Am J Transl Res. 2016;8:3241–3250. [PMC free article] [PubMed] [Google Scholar]
- Xin DQ, Zhao YJ, Li TT, Ke HF, Gai CC, Guo XF, Chen WQ, Liu DX, Wang Z. The delivery of miR-21a-5p by extracellular vesicles induces microglial polarization via the STAT3 pathway following hypoxia-ischemia in neonatal mice. Neural Regen Res. 2022;17:2238–2246. doi: 10.4103/1673-5374.336871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong LL, Xue LL, Jiang Y, Ma Z, Jin Y, Wang YC, Wang YY, Xia QJ, Zhang Y, Hu Q, Liu J, Wang TH. Suppression of PDGF induces neuronal apoptosis after neonatal cerebral hypoxia and ischemia by inhibiting P-PI3K and P-AKT signaling pathways. Brain Res. 20191719:77–88. doi: 10.1016/j.brainres.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Xiong LL, Xue LL, Du RL, Zhou HL, Tan YX, Ma Z, Jin Y, Zhang ZB, Xu Y, Hu Q, Bobrovskaya L, Zhou XF, Liu J, Wang TH. Vi4-miR-185-5p-Igfbp3 network protects the brain from neonatal hypoxic ischemic injury via promoting neuron survival and suppressing the cell apoptosis. Front Cell Dev Biol. 2020;8:529544. doi: 10.3389/fcell.2020.529544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Doycheva DM, Gamdzyk M, Gao Y, Guo Y, Travis ZD, Tang J, Chen WX, Zhang JH. BMS-470539 attenuates oxidative stress and neuronal apoptosis via MC1R/cAMP/PKA/Nurr1 signaling pathway in a neonatal hypoxic-ischemic rat model. Oxid Med Cell Longev. 2022;2022:4054938. doi: 10.1155/2022/4054938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TL, Zhang ZW, Lin W, Lin XR, Lin KX, Fang MC, Zhu JH, Guo XL, Lin ZL. Reperfusion after hypoxia-ischemia exacerbates brain injury with compensatory activation of the anti- ferroptosis system: based on a novel rat model. Neural Regen Res. 2023a;18:2229–2236. doi: 10.4103/1673-5374.369117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shang J, Yang Q, Dai Z, Liang Y, Lai C, Feng T, Zhong D, Zou H, Sun L, Su Y, Yan S, Chen J, Yao Y, Shi Y, Huang X. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnology. 2023b;21:29. doi: 10.1186/s12951-023-01788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZB, Xiong LL, Xue LL, Deng YP, Du RL, Hu Q, Xu Y, Yang SJ, Wang TH. MiR-127-3p targeting CISD1 regulates autophagy in hypoxic-ischemic cortex. Cell Death Dis. 2021;12:279. doi: 10.1038/s41419-021-03541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KQ, Dhillon SK, Bennet L, Gunn AJ, Davidson JO. Targeting persistent neuroinflammation after hypoxic-ischemic encephalopathy-is exendin-4 the answer? Int J Mol Sci. 2022;23:10191. doi: 10.3390/ijms231710191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


